Abstract
Drug-induced nephrotoxicity is responsible for 20% to 60% of cases of acute kidney injury in hospitalized patients and is associated with increased morbidity and mortality in both children and adults. Antimicrobials are one of the most common classes of medications prescribed globally and also among the most common causes of nephrotoxicity. A broad range of antimicrobial agents have been associated with nephrotoxicity, but the features of kidney injury vary based on the agent, its mechanism of injury and the site of toxicity within the kidney. Distinguishing nephrotoxicity caused by an antimicrobial agent from other potential inciting factors is important to facilitate both early recognition of drug toxicity and prompt cessation of an offending drug, as well as to avoid unnecessary discontinuation of an innocuous therapy. This review will detail the different types of antimicrobial-induced nephrotoxicity: acute tubular necrosis, acute interstitial nephritis and obstructive nephropathy. It will also describe the mechanism of injury caused by specific antimicrobial agents and classes (vancomycin, aminoglycosides, polymyxins, antivirals, amphotericin B), highlight the toxicodynamics of these drugs and provide guidance on administration or monitoring practices that can mitigate toxicity, when known. Particular attention will be paid to paediatric patients, when applicable, in whom nephrotoxin exposure is an often-underappreciated cause of kidney injury.
Introduction
The kidney is a major organ of drug excretion and, thus, is exposed to high concentrations of potentially toxic medications. Drug-induced nephrotoxicity is a common and potentially serious complication of medication administration that occurs in both inpatient and outpatient settings. While the terms acute kidney injury (AKI) and nephrotoxicity are often interchanged, AKI specifically refers to a reduction in kidney function [i.e. glomerular filtration rate (GFR)], but nephrotoxicity more broadly encompasses the spectrum of medication- or toxin-induced kidney damage. Kidney injury must be substantial to affect traditional serum biomarkers, with 30%–50% parenchymal damage necessary before changes in creatinine can be detected.1
A broad range of medications have been associated with nephrotoxicity including various antimicrobial, antihypertensive, chemotherapeutic, immunosuppressant and anti-inflammatory agents, among others. Nephrotoxic medication exposure significantly contributes to AKI development in critically ill children, as well as in children cared for on general paediatric wards.2,3 Anywhere from 20% to 60% of AKI in hospitalized patients is attributed to drug toxicity.4–6 In non-critically ill children, AKI develops in roughly a quarter of those children administered nephrotoxins7 and is associated with greater hospital costs and longer length of stay.2
Antimicrobials are one of the most commonly prescribed drug classes in children. In a global point prevalence study in 2012, 37% of hospitalized children across 226 hospitals were receiving antimicrobials on the survey date, including 61% of paediatric ICU patients.8 Although lifesaving and often critical, many antibiotics are unfortunately also nephrotoxic. It is well described that several antimicrobial classes and agents have potential to cause nephrotoxicity,9 and the frequency of toxicity varies based on the properties of the individual agent, as well as the physiological status and underlying condition of the patient receiving the drug.10 It is often difficult to tease out the relative contribution of antimicrobial exposure to AKI in hospitalized patients, since patients requiring antimicrobials are often sick (e.g. haemodynamically unstable), have underlying comorbidities and receive other potentially nephrotoxic drugs. Nevertheless, as a result of their frequent use, antimicrobials account for a large proportion of nephrotoxic medication exposures in hospitalized patients of all ages.2,3,11
The purpose of this review is to describe the mechanisms by which selected antimicrobials result in nephrotoxicity, highlighting the most common antimicrobial classes and agents to cause kidney injury in children. While AKI is most often multifactorial, it is important for clinicians to recognize the high-risk antimicrobials and strategies that may be employed in children to minimize toxicity. Alternatively, it is also imperative for clinicians to recognize when toxicity is not attributable to specific agents to avoid unnecessary medication changes. Understanding how antimicrobials induce kidney injury will support conscientious prescribing and therapeutic monitoring.
Mechanisms of nephrotoxicity
Drug-induced nephrotoxicity is classified as either dose dependent or dose independent.12,13 Dose-dependent toxicities are predictable and related to the main pharmacological effect of the drug (type A reactions). For most drugs that cause type A reactions, AKI is linked to the degree of drug exposure over time and the toxicodynamic parameters associated with nephrotoxicity are either the drug’s AUC or the peak concentration (Cmax). Other agents, such as aminoglycosides, cause toxicity via drug accumulation and the trough (Cmin) is more closely associated with renal injury. Dose-dependent toxicities can generally be mitigated by dose reductions, but sometimes cessation of therapy is necessary.
Dose-independent toxicities, known as type B reactions, are idiosyncratic, occur at any time during therapy and are highly variable from patient to patient. Hypersensitivity reactions are the most common dose-independent side effects. In the case of nephrotoxic AKI, drug exposure should precede changes in renal function, when characterized by changes in serum creatinine and/or a reduction in urine output, by at least 24 h, to be considered plausibly responsible.12,14
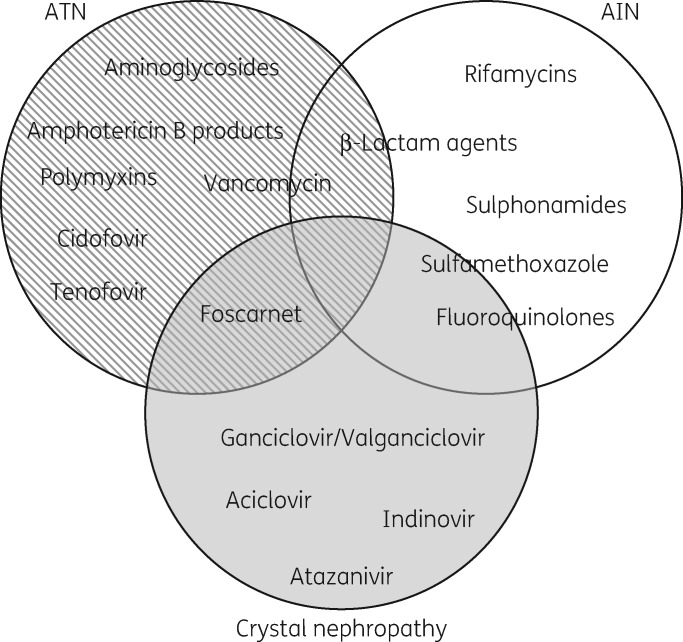
The mechanisms by which antimicrobials cause nephrotoxicity vary across classes and agents (Table 1) and several agents can cause multiple types of injury (Figure 1). Nephrotoxic effects can be categorized by the type of damage induced and resulting clinical presentation of the injury.10,15 Acute tubular necrosis (ATN; tubuloepithelial injury), acute interstitial nephritis (AIN; tubulointerstitial disease) and crystal (obstructive) nephropathy are the primary means by which antimicrobials cause nephrotoxicity.13 The distinguishing features of these types of nephrotoxic effects are summarized in Table 2. Osmotic nephrosis and chronic interstitial nephritis are untoward effects of some medications, but rarely of antimicrobials, and will not be discussed in detail in this review.
Table 1.
Antimicrobials and their mechanisms of kidney injury
| Agent | Mechanism(s) of kidney injury | Proposed approaches to minimize toxicity |
|---|---|---|
| Antibacterials | ||
| aminoglycosidesa |
|
|
| β-lactam agents |
|
|
| rifamycins |
|
|
| polymyxins (colistin, polymyxin B)a |
|
|
| sulphonamides |
|
|
| vancomycina |
|
|
| Antifungals | ||
| amphotericin B productsa |
|
|
| Antivirals | ||
| aciclovira, valaciclovir |
|
|
| atazanavir |
|
|
| cidofovira |
|
|
| foscarneta |
|
|
| ganciclovir, valganciclovir |
|
|
| indinavir |
|
|
| tenofovir |
|
|
Incidence of nephrotoxicity in children >10%.
Figure 1.
Diagram of the primary types of kidney injury caused by specific antimicrobial agents. Specific agents that have been reported to inflict multiple types of injury are displayed as overlapping.
Table 2.
Features of drug-induced nephrotoxicity
| ATN | AIN | Crystal nephropathy | |
|---|---|---|---|
| Mechanism of injury | direct cytotoxicity on tubular epithelial cells, most often proximal tubules | immunologically mediated damage to the interstitium | precipitation of drug as crystals causes damage to the tubular epithelium and/or tubular obstruction |
| Dose dependence | yes | no | yes; may also be infusion rate-dependent |
| Time course | days | usually 7–14 days, but can be sooner in previously sensitized individuals | any time during treatment; can occur as soon as following a single dose |
| Clinical features |
|
|
|
| Antimicrobials most commonly implicated |
|
|
|
ATN
Tubuloepithelial injury results from the direct cytotoxic effects of drugs on proximal and/or distal tubule epithelial cells (Figure 2a).15 Because this type of toxicity is dose dependent, it occurs along a spectrum from membrane or organelle damage to complete cell death and necrosis.16 The term ATN is commonly used to describe tubuloepithelial injury, although actual cell necrosis is infrequent.15,17 Aminoglycosides, vancomycin and amphotericin B are the most common antimicrobials to cause ATN, yet all elicit tubular damage through unique mechanisms (see below).
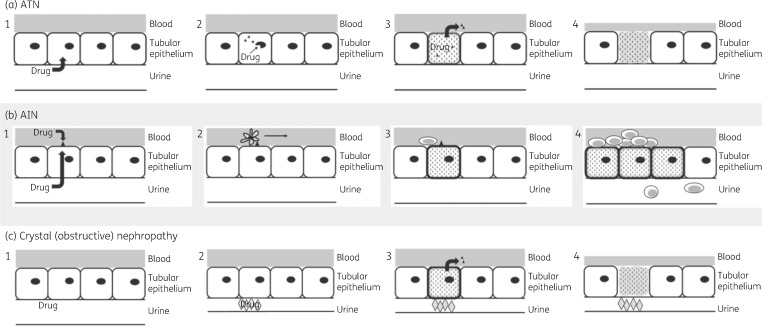
Figure 2.
Mechanisms of antimicrobial-induced nephrotoxicity. (a) ATN: begins with endocytosis of drug from the urine into tubular epithelial cells (a1). Once inside the cell, the drug causes damage to cell organelles (a2). This initiates the process of cellular apoptosis and death, and release of systemic inflammatory signals (a3). Renal blood flow is then reduced (a4) as a result of tubuloglomerular feedback mechanisms. (b) AIN: antigen from either freely filtered drug or drug that is circulating in the blood is deposited on the basement membrane (b1). The antigen is recognized by dendritic cells (b2), which induce a T cell-mediated immune response (b3) and interstitial inflammation with pyuria (b4). (c) Crystal (obstructive) nephropathy: drug is filtered into the urine (c1). When the urine becomes supersaturated with drug, as in the setting of decreased urine flow, the drug precipitates (often as crystals) and obstructs the tubular lumen (c2). This leads to the release of inflammatory signals into the blood (c3), as well as induction of cellular apoptosis and reduced renal blood flow via tubuloglomerular feedback mechanisms (c4).
ATN is the most common form of drug-induced kidney injury and clinically manifests as a rise in creatinine with or without oliguria, an increased fractional excretion of sodium, microscopic haematuria and/or mild proteinuria.15 Urinary biomarkers of tubular injury (KIM-1, NGAL and several others) are elevated, often prior to detectable changes in serum creatinine, but are not routinely measured clinically at the current time.14 Tubular injury leads to a reduction in renal function via a complex tubuloglomerular feedback loop: tubular cell damage/death causes spilling of cellular components, which obstruct tubules, and impairs tubular reabsorption.18 This causes excess water and electrolytes to be delivered to the distal nephron, increasing the hydrostatic pressure on the distal nephron and triggering compensatory vascular feedback mechanisms that reduce renal blood flow and glomerular filtration in efforts to limit the fluid and electrolyte losses.19,20 In the setting of ongoing or severe tubular injury, oxidative stress and inflammation increase cellular damage, as well as the glomerular and vascular effects, potentiating ATN and leading to a further reduction in GFR.
AIN
AIN is characterized by tubular and interstitial inflammation that results from a non-IgE-mediated hypersensitivity reaction (Figure 2b).21,22 Medications are the primary cause of AIN and antibiotics account for roughly one-third of drug-induced AIN cases;23 penicillins, cephalosporins and sulphonamides are most often implicated. AIN is a much less frequent cause of nephrotoxic AKI in children than ATN, but may be responsible for up to 25% of unexplained AKI.24 Following administration, drugs become immunogenic and induce a lymphocytic, cell-mediated inflammatory response that is accompanied by systemic signs of inflammation including fever (most patients) and rash (<50% of cases); peripheral eosinophilia is classically described in cases of AIN but rarely present. AIN generally develops after prolonged exposure to the drug (2–3 weeks), but can occur earlier in patients previously exposed to the offending agent.16
Drug-induced AIN presents as non-oliguric AKI with laboratory abnormalities including elevated serum creatinine, sterile pyuria, microscopic haematuria and tubular proteinuria, which consists of low molecular weight proteins (i.e. cystatin C, β-2-microglobulin, haemoglobin) rather than the larger proteins lost in glomerular diseases, such as albumin or immunoglobulins.16,24 Eosinophils can be found in the urine in cases of AIN, although eosinophiluria is neither sensitive nor specific for AIN.25 Kidney biopsy is required for diagnosis, which demonstrates characteristic histopathological changes including interstitial inflammation (predominantly lymphocytic, ±eosinophils), interstitial oedema and fibrosis, and tubulitis.16,24,26 Because AIN is an immune-mediated process, corticosteroids are commonly used as treatment, particularly in patients who fail to improve following discontinuation of the offending agent.26
Crystal (obstructive) nephropathy
Some antimicrobials precipitate as crystals in the urinary system, causing damage to the tubular epithelium and obstruction of renal tubules (Figure 2c).27 This most often manifests as AKI, but chronic kidney disease can develop, depending on the rapidity and extent of crystal formation.28 Volume depletion is the major risk factor for crystal nephropathy, resulting in supersaturation of the urine and crystal formation in renal tubules; metabolic derangements and urinary pH may also predispose patients to crystal formation.21,29 Crystal-induced tubule cell damage stimulates inflammation and necrosis, as described with ATN above, while obstruction of the tubular lumen, if significant, can affect the hydrostatic pressure within the kidney and promote the release of signals that decrease GFR. Antivirals, including aciclovir, indinavir and ganciclovir, are the antimicrobial agents most often associated with crystal nephropathy, which may develop following as little as a single dose of medication.30–32 There have also been reports of sulfamethoxazole and fluoroquinolones causing crystaluria.33–35 Dose reduction or slowing the rate of infusion, along with administration of intravenous fluids, may decrease the risk of crystal formation by promoting urine flow and limiting supersaturation.
Haemodynamically mediated kidney injury
Glomerular filtration is regulated via a complex balance of afferent and efferent blood flow through the glomerulus. Medications that reduce afferent blood flow (non-steroidal anti-inflammatory drugs, COX-2 inhibitors, calcineurin inhibitors) or increase efferent blood flow (angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers) alter the hydrostatic pressure in glomerular capillaries and glomerular filtration.5,10 An inability to regulate the balance of afferent and efferent blood flow in patients receiving these drugs can lead to renal hypoperfusion or ischaemia, causing ATN, particularly in the setting of other haemodynamic insults, such as sepsis. Although no antimicrobials directly cause nephrotoxicity in this manner, patients receiving medications that affect renal blood flow (i.e. transplant recipients on calcineurin inhibitors) may be unable to compensate for the nephrotoxic insults caused by antimicrobials, potentiating their toxic effects.
Specific medications/medication classes and mechanisms of nephrotoxicity
Vancomycin
Vancomycin is a glycopeptide antibiotic developed in the early 1950s.36 It mainly acts by prevention of cell-wall biosynthesis of Gram-positive bacteria and is the first choice for the treatment of MRSA infections,37,38 but its use is limited by adverse effects, most notably nephrotoxicity.39 In a recent meta-analysis of eight paediatric studies,40 renal toxicity was reported in 12.7% of vancomycin recipients (range across studies: 2.4%–24.3%), although there was variability in the AKI definitions used and patient populations studied. Vancomycin causes biopsy-proven ATN and AIN in both adult and paediatric patients.41–46 AIN is mediated by an immunological reaction to vancomycin,47 while two mechanisms have been suggested for ATN: vancomycin-induced direct oxidative stress and obstructive cast formation in proximal tubule cells.48,49
Oxidative stress is an imbalance between free radicals and antioxidants within cells that leads to mitochondrial dysfunction and cellular apoptosis. Vancomycin has been shown to stimulate oxidative phosphorylation in cultured cells and produce oxygen free radicals.50,51 The free radicals induce lipid peroxidation and the superoxides produced cause depolarization of mitochondrial membrane potential with the release of cytochrome c and activation of downstream caspases involved in apoptotic cell death.49,52
Cast formation is another important mechanism for vancomycin-induced kidney injury. Uromodulin may interact with nanospheric vancomycin aggregates leading to tubular cast formation and subsequent ATN.48 Luque et al.48 detected vancomycin casts in nine patients with ATN and reproduced the obstructive vancomycin-associated cast nephropathy in mice. Recent preclinical data suggest that the vancomycin accumulation in proximal tubule cells is due to apical reabsorption at the brush border membrane via dehydropeptidase and megalin,52,53 and that cilastatin inhibited vancomycin cellular uptake and reduced apoptosis of porcine renal proximal tubular epithelial cells in culture.53
In the clinical setting, vancomycin-associated nephrotoxicity is defined by increases in traditional markers of AKI such as creatinine and blood urea nitrogen and has been reported to occur at a median of 6–7 days into therapy.54–56 In children, higher vancomycin troughs (i.e. those ≥15 mg/L) are associated with >2.5-fold more AKI in the non-critical care population and >3.5-fold more toxicity in the paediatric ICU.40 Current vancomycin dosing guidelines focus on measurement of trough concentrations;57 however, more recent data from our laboratory suggest that nephrotoxicity is more directly related to Cmax or total plasma exposure (AUC0–24) rather than troughs (Cmin).58,59 In adults, a 24 h AUC threshold of 650 mg·h/L has been reported60 and a randomized trial of AUC- versus trough-driven vancomycin dosing demonstrated decreased nephrotoxicity among the AUC-guided therapy group.61 Paediatric data support an AUC0–24-toxicity threshold of 800 mg·h/L.62 No studies have directly compared AUC-toxicity thresholds in adult and paediatric patients but adults may be more susceptible to the nephrotoxic effects of vancomycin than children (i.e. have decreased renal reserve), resulting in a lower observed AUC-toxicity cut-off.
Vancomycin efficacy is also AUC dependent but new data suggest that efficacy is similar even with higher exposures.63,64 As a result, AUC-directed therapy may become more commonplace in children.65 These findings are translating to the clinic as pharmacists are now monitoring vancomycin AUC:MIC concentrations in adult and paediatric patients.66 Future dosing schemes may benefit from prolonging the infusion, although these studies have not yet been conducted in paediatric patients.67
Recently, numerous paediatric studies have reported an increased risk of AKI from combination therapy with vancomycin plus piperacillin/tazobactam compared with vancomycin alone or vancomycin plus cephalosporins or carbapenems. This finding has been established in adult patients as well. While the mechanism responsible for the increased AKI risk has not been elucidated, avoidance of this specific combination is generally recommended.
Aminoglycosides
Aminoglycoside antibiotics inhibit bacterial protein synthesis by binding to the 30S ribosomal subunit. They were first introduced in the 1940s and continue to play an important role in the treatment of Gram-negative infections in both adult and paediatric patients. Aminoglycoside-induced kidney injury has been well described and studies show that up to 33% of children exposed to aminoglycosides will develop AKI.68 Despite known toxicity, aminoglycosides continue to be a mainstay of therapy due to their bactericidal activity and the increasingly prevalent resistance of Gram-negative bacteria to β-lactam agents.19,69
Aminoglycoside-induced kidney injury occurs when the drug accumulates within the proximal tubule epithelial cells of the renal cortex, leading to direct cytotoxicity.70 After glomerular filtration, a portion of the drug binds to an endocytic receptor, megalin, located on the apical surface of the proximal tubule epithelial cell, and is endocytosed.71 Expression of megalin is directly related to the degree of drug accumulation, as it is the principle receptor for aminoglycoside uptake in the kidney.72 Following endocytosis, the drug traffics through the endosomal compartment and accumulates principally within lysosomes and then interacts with membrane phospholipids causing damage73,74 in a process called phospholipidosis.75 Drug is released into the cytosol, damages mitochondria and causes release of cytochrome c, activation of caspase-3 and induction of apoptosis.74 Cell damage causes spilling of cellular components, which obstruct tubules,18 impairs the excretory function of the nephron, increases the hydrostatic pressure and leads to proteinuria, enzymuria and loss of water and electrolytes in the urine.76 In turn, glomerular filtration is reduced via the tubuloglomerular feedback mechanism.19
Traditionally, aminoglycosides are dosed multiple times per day; however, extended-interval dosing may mitigate kidney injury. Larger doses, given at extended intervals (i.e. once daily), optimize peak serum concentrations and the bactericidal killing of aminoglycosides.77 Adult studies have found once-daily dosing to be equally efficacious, with lower rates of both ototoxicity and nephrotoxicity.78–81 A meta-analysis of 24 paediatric randomized clinical trials found no significant differences in clinical failure or microbiological failure when comparing multiple-daily to extended-interval dosing.82 The primary pooled nephrotoxicity outcome rates were similar between once-daily and multiple-daily dosing, as evidenced by any increase in serum creatinine levels or decrease in CLCR. However, pooled secondary nephrotoxicity rates, based on urinary excretion of proteins or phospholipids, were significantly lower in the once-daily [3/69 cases (4.3%)] versus multiple-daily [11/69 cases (15.9%)] (P = 0.03) dosing arms.
Aminoglycosides require close therapeutic drug monitoring (TDM) in order to mitigate potential toxicities, including kidney injury. Peaks and troughs are most often measured during conventional, multiple-daily dosing regimens. However, these provide less informative data during extended-interval dosing regimens as a goal of extended-interval dosing is to ensure a trough below the level of quantification before re-dosing. Measurement of two concentrations during the post-distribution phase (i.e. at ≥1 and 6–9 h after the end of infusion) can promote estimation of the duration that plasma concentrations fall below the limits of quantification and confirm adequate drug clearance prior to administration of the next dose.83,84
Polymyxins
The polymyxins, a group of polypeptide antibiotics first discovered in 1947, demonstrate significant activity against Gram-negative pathogens.85,86 In the 1970s, reports on renal and neurological adverse effects led to the gradual withdrawal of the polymyxins from clinical practice as newer antimicrobial agents with improved toxicity profiles were introduced.87,88 However, recent progression of antimicrobial resistance, coupled with development of few new agents, have brought the polymyxins back into clinical use as a last line of defence.89,90 The polymyxins consist of five chemically different compounds, i.e. polymyxins A–E.86 Only polymyxin B and colistin (polymyxin E) have demonstrated clinical effectiveness in the treatment of Gram-negative infections.91 Structurally, polymyxin B is similar to colistin but differs in one amino acid.86 Colistin is the most widely used polymyxin in children and is clinically available as colistin sulphate and colistimethate sodium (CMS);92–94 colistin sulphate is more potent and toxic. Both polymyxin B and colistin can be rapidly bactericidal by disruption of the bacterial cell membrane,85,86,95 ultimately causing bacterial cell content leakage and cell death.85,91,95
The polymyxins cause renal toxicity that often limits clinical treatment.93–95 Most studies in paediatric patients describe rates of nephrotoxicity between 3% and 10%; however, incidences over 20% have been reported.92,96–98 Given the narrow therapeutic window and severity of nephrotoxicity, dose escalation of the polymyxins for resistant infections is often not advisable.98–100
Renal toxicity of polymyxins is a complex process. First, administration of polymyxins appears to induce renal vasoconstriction, sensitizing proximal tubule cells to direct cytotoxic effects of the drug.101 Drug accumulation in proximal tubule cells is potentially driven by apical reabsorption at the brush border membrane via megalin-mediated endocytosis;102,103 oxidative stress subsequently plays an important role in the development of renal toxicity.103–105 Ultimately, drug accumulation within cells leads to organelle damage, increased membrane permeability, cell lysis and ATN.99,100 Preclinical data indicate that accumulation of polymyxins may be a saturable, non-passive process, as with aminoglycosides.106
Dosing strategies for the polymyxins are based both on pharmacokinetic (PK) and pharmacodynamic (PD) properties. The PK for colistin and polymyxin differ substantially. CMS is excreted renally while polymyxin B and colistin are eliminated via non-renal mechanisms.107 Thus, FDA recommendations exist for children to reduce the dose of CMS in the setting of renal failure.108 The microbiological PD activity of the polymyxins is best described by the AUC:MIC ratio,109 but fewer data exist on their toxicodynamics and whether renal toxicity is linked to Cmax or overall AUC. Therefore, it is unclear whether daily doses of colistin or polymyxin B should be fractionated into smaller aliquots or given via continuous infusion. Abdelraouf et al.106 conducted in vitro and in vivo studies that suggest that multiple-daily dosing of polymyxin B resulted in higher tissue accumulation and renal toxicity when compared with the equivalent once-daily dosing. This study may have important implications for dosing polymyxin B in paediatric patients; however, more data are needed. If toxicity occurs via a saturable mechanism, larger and fewer doses should result in less toxicity.106,110,111
Antivirals
Aciclovir [9-(2-hydroxyethoxymethyl)guanine] is an acyclic nucleoside in the class of nucleoside analogues.112 It is a substrate and specific inhibitor of herpesvirus DNA polymerase, blocking DNA synthesis, and is effective against herpes simplex virus type 1 and 2 and varicella-zoster virus infections, as well as several other viruses. Aciclovir is primarily excreted via both glomerular filtration and tubular excretion and is eliminated mostly as unchanged drug.112 One paediatric cohort study described an AKI incidence of 35% in children treated with intravenous aciclovir,113 although few paediatric studies have evaluated the incidence of AKI from this drug.
The mechanism of nephrotoxicity most often described in aciclovir therapy is crystal nephropathy. Aciclovir has low urine solubility and may precipitate or crystallize in tubular lumens causing tubular obstruction,114 particularly in the setting of low urine output. Use of high doses or administration via rapid intravenous bolus may further contribute to crystallization in the tubules. Also, crystal nephropathy can develop following a single dose of medication.113 It is therefore recommended to administer aciclovir as a slower infusion rather than a rapid bolus and to avoid excessively high dosages when possible. It is also paramount to achieve and maintain adequate hydration throughout the course of treatment, including at initiation, to limit the potential for crystal nephropathy.114
Direct tubular toxicity is another important mechanism for aciclovir-induced nephrotoxicity. Preclinical models in rats have shown a dose-dependent elevation in urinary N-acetyl-β-d-glucosaminidase activity, which is a marker of renal tubular damage.115In vitro models are also consistent with direct injury to proximal tubular cells by aciclovir, possibly through aciclovir aldehyde, an intermediate metabolite that is produced in tubular cells.116 These preclinical data are supported by case series of paediatric patients treated with aciclovir who demonstrated nephrotoxicity: renal biopsies in three patients showed tubulointerstitial nephritis or tubular epithelial damage and loss of proximal–distal tubular differentiation without intratubular crystals.117,118
Foscarnet is another intravenous antiviral agent with notable nephrotoxic potential,114 particularly in immunocompromised children,119 in whom it is used primarily to treat cytomegalovirus disease. A pyrophosphate analogue, foscarnet is eliminated via a combination of glomerular filtration and tubular secretion with minimal tubular reabsorption.120 ATN of proximal tubule cells is its most common form of nephrotoxicity, although several other types of kidney injury with this agent have been described.121,122 Aggressive hydration with intravenous fluids throughout the treatment course appears to mitigate a significant portion of nephrotoxicity from foscarnet.121,123
Amphotericin B
Amphotericin B is a polyene antifungal with activity against a wide spectrum of fungal infections. It exerts its fungicidal activity by binding to the ergosterol of the lipid bilayer of the fungi and disrupting membrane permeability, leading to a loss of anions and glucose.124 While active against most invasive fungal infections, it also produces serious infusion-related adverse effects, most notably dose-limiting nephrotoxicity.125 Systemic imidazole and triazole antifungals have replaced amphotericin B as first-line treatment for many invasive fungal infections due to their efficacy and improved safety profiles.126,127 However, amphotericin B is still utilized for life-threatening invasive fungal infections due to its broad spectrum of fungicidal activity.126,128
Clinically, amphotericin B-induced renal impairment manifests as increased levels of blood urea nitrogen and creatinine, electrolyte wasting, and a reduction in GFR of up to 40%–80%.129,130 The mechanism of amphotericin B-induced nephrotoxicity has not been clearly defined, but it may be produced by a variety of mechanisms. One proposed mechanism of nephrotoxicity involves changes in cell permeability. Amphotericin B disrupts the fungal cell membrane by binding to ergosterol, which is structurally similar to cholesterol in mammalian cells. Therefore, amphotericin B may disrupt renal cell membranes to create transmembrane pores, thereby causing an electrolyte imbalance.131 These pores cause a cascade of events whereby sodium enters the cells causing depolarization, voltage-gated calcium channels are triggered, allowing calcium to enter, and cell contraction is instigated. Multiple studies have shown that calcium channel blockers prevent afferent arteriole vasoconstriction, which supports this hypothesis.132,133 Another hypothesis involves the direct vasoconstriction of the afferent arteriole of the glomerulus by amphotericin B.133 This direct vasoconstriction can be attenuated by salt loading: an increased sodium concentration triggers the release of atrial natriuretic peptide and nitric oxide in the endothelium, thus inducing vasodilation, and has been shown to be clinically effective in preventing amphotericin B-induced nephrotoxicity.134,135 Another possible mechanism is apoptosis of renal tubular epithelial and interstitial cells.136 In the study by Varlam et al.,136 renal cell lines from rats, dogs and pigs all demonstrated apoptosis and necrosis in a dose-dependent manner. These in vitro results were supported by in vivo studies in rats where dose-dependent toxicities and side effects were replicated and then attenuated when amphotericin B was administered concomitantly with the anti-apoptotic agent recombinant human insulin-like growth factor-1.
Aside from the reduction in renal blood flow and GFR, amphotericin B-induced nephrotoxicity also impairs the ability to acidify and concentrate urine. The aforementioned transmembrane pores created by amphotericin B explain poor urine acidification.137,138 In a study by Kim et al.,139 rats administered amphotericin B exhibited a reduction in aquaporin-2 expression and its regulator, adenylyl cyclase, as well as increased serum creatinine levels, high urinary flow rates and a markedly reduced urine osmolality, which is also observed in humans. These results suggest that the reduction in aquaporin-2, which is primarily expressed in the collecting ducts, is responsible for polyuria associated with amphotericin B administration.
The formulation of amphotericin B may also contribute to nephrotoxicity. In order to minimize the nephrotoxicity observed with traditional amphotericin B deoxycholate, new formulations have inserted the amphotericin B into liposomal structures.140 All three lipid formulations of the drug [amphotericin B colloidal dispersion (ABCD), amphotericin B lipid complex (ABLC), liposomal amphotericin B] distribute well into tissues and have reduced kidney accumulation relative to amphotericin B deoxycholate. When amphotericin B is complexed with lipids, amphotericin B concentrates in phagocytes and is distributed to sites of inflammation.141 As a result, less free amphotericin B is circulating, which reduces the overall side-effect profile.140,142 Deoxycholate, which was added to amphotericin in the conventional formulation to improve solubility, is also nephrotoxic in itself.143 Liposomal formulations also selectively target and bind to high-density lipoproteins of fungal organisms instead of mammalian cells.144–146 While efficacy is similar between the formulations, the rate of nephrotoxicity with amphotericin B deoxycholate is between 12% and 50%, which is markedly higher than rates of the liposomal formulations, which range from 9% to 25%.147 While some studies have reported decreased nephrotoxicity of continuous infusion compared with standard infusions (2–6 h),148 the data are generally conflicting and do not universally support this practice.134,135 Administration of supplemental intravenous sodium has been associated with decreased toxicity of amphotericin B deoxycholate in premature infants,149 although not fully studied in other paediatric populations.
Future directions
As detailed above, most antimicrobials elicit kidney injury by causing ATN in a dose-dependent manner. When toxicodynamic endpoints are defined, such as for vancomycin, strong consideration should be given to implementation of effective TDM that aims to minimize toxicity risks. Reliance upon vancomycin troughs, for instance, is an inadequate approach to prevent vancomycin-associated nephrotoxicity in children, given that vancomycin displays AUC-dependent toxicity and estimation of AUCs from troughs is poor.61 The use of bedside decision-support software can allow clinicians to estimate AUC more reliably using Bayesian approaches and derive personalized dosing regimens that achieve both effective and safe drug concentrations, not only for vancomycin but for all drugs in which clinical sampling can be performed. In addition, studies are needed to determine whether alternative administration strategies (i.e. continuous infusions for vancomycin) can mitigate drugs’ toxic effects in paediatric patients.
Clinicians should also recognize that traditional biomarkers of kidney injury, blood urea nitrogen and serum creatinine, are insensitive and non-specific in children. Reliance on changes in these biomarkers will only detect patients who have already sustained significant injury. This reactive approach does not prevent AKI and is not a reliable tactic to mitigate toxicity in children receiving nephrotoxic medications. While monitoring of traditional biomarkers remains the standard of care, largely due to cost, availability and interpretability, more sensitive markers of toxicity have been identified that more directly relate to the site of injury within the nephron (Figure S1, available as Supplementary data at JAC Online). Use of these sensitive urinary biomarkers may allow clinicians to recognize toxicity prior to the onset of significant damage and promote preemptive dose adjustments or medication changes. Both the US FDA and EMA have issued letters of support for KIM-1 and osteopontin.150,151 In addition, KIM-1, clusterin and cystatin C have already been qualified for preclinical toxicological evaluations by the FDA, the EMA and the Pharmaceutical and Medical Devices Agency in Japan.97 However, at the time of writing, there are no FDA-approved urinary biomarker tests available for clinical use in children. Additional studies will be needed to bring these tests into the paediatric TDM arena.
Conclusions
Antimicrobials are an important cause of AKI in children. Through direct cytotoxic effects, indirectly via immune-mediated mechanisms or via perpetuation of other concurrent nephrotoxic insults, antimicrobial administration can lead to a clinically meaningful impairment of renal function in paediatric patients. While nephrotoxicity may be unavoidable or unpredictable, such as when the mechanism is AIN, clinicians can utilize knowledge of the toxico-dynamics of antimicrobial agents to develop personalized regimens that reduce the likelihood of toxicity for patients. When alternative agents are not feasible, close monitoring of kidney function, urine output and hydration status is imperative in high-risk patients—those receiving multiple other nephrotoxic medications, with underlying kidney disease or past AKI, or haemodynamic instability (i.e. impaired renal perfusion). Dosing/administration strategies known to minimize nephrotoxicity (i.e. once-daily aminoglycosides, AUC-targeted vancomycin, liposomal formulations of amphotericin B, concurrent hydration for aciclovir and foscarnet) should also be implemented routinely in high-risk patients.
Antimicrobials are vital to the preservation of health and the prevention of disease in people of all ages. They are, understandably, one of the most commonly prescribed classes of medications in both inpatient and ambulatory care settings; however, nephrotoxicity is a pertinent and often predictable adverse effect of many antimicrobial agents. Clinicians need to identify high-risk patients and implement strategies to allay toxicity or, at a minimum, detect it early. Periodic measurement of creatinine alone is insufficient to ensure safe administration of these drugs and awareness of patients’ urine output/hydration status, haemodynamics and concurrent medications is important. TDM should also be used, when available, to deliver effective and safe doses that target known PK/PD endpoints.
Funding
This review was carried out as part of our routine work.
K. J. D. is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number K23HD091365. N. R. Z. is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number 1K99HD096123.
Transparency declarations
K. J. D. has received research support from Merck & Co., Inc. and Pfizer, Inc. unrelated to the current work. G. M. P. has received research support from Merck & Co., Inc. unrelated to the current work. J. L. has received research support from Merck & Co., Inc. unrelated to the current work. S. L. G. serves as a consultant for Bioporto, Inc. and receives royalties from Vigilanz, Inc. for an electronic medical record-based nephrotoxic medication-associated AKI application. M. H. S. has served as a consultant for Achaogen, Inc. and reports having research grants from Merck & Co., Inc. and Nevakar, Inc. unrelated to the current work. A. F. Z. has received research support from the Eunice Kennedy Shriver National Institute of Child Health & Human Development and the National Heart, Lung and Blood Institute of the NIH, the US Department of Defense, Ilera Healthcare and Zelda Therapeutics unrelated to the current work. All other authors: none to declare.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the agencies mentioned above.
Supplementary Material
References
- 1. Duarte CG, Preuss HG.. Assessment of renal function–glomerular and tubular. Clin Lab Med 1993; 13: 33–52. [PubMed] [Google Scholar]
- 2. Moffett BS, Goldstein SL.. Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-ill children. Clin J Am Soc Nephrol 2011; 6: 856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Slater MB, Gruneir A, Rochon PA. et al. Identifying high-risk medications associated with acute kidney injury in critically ill patients: a pharmacoepidemiologic evaluation. Paediatr Drugs 2017; 19: 59–67. [DOI] [PubMed] [Google Scholar]
- 4. Khalili H, Bairami S, Kargar M.. Antibiotics induced acute kidney injury: incidence, risk factors, onset time and outcome. Acta Med Iran 2013; 51: 871–8. [PubMed] [Google Scholar]
- 5. Taber S, Pasko D.. The epidemiology of drug-induced disorders: the kidney. Expert Opin Drug Saf 2008; 7: 679–90. [DOI] [PubMed] [Google Scholar]
- 6. Mehta RL, Pascual MT, Soroko S. et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int 2004; 66: 1613–21. [DOI] [PubMed] [Google Scholar]
- 7. Goldstein SL, Kirkendall E, Nguyen H. et al. Electronic health record identification of nephrotoxin exposure and associated acute kidney injury. Pediatrics 2013; 132: e756–67. [DOI] [PubMed] [Google Scholar]
- 8. Versporten A, Bielicki J, Drapier N. et al. The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) point prevalence survey: developing hospital-quality indicators of antibiotic prescribing for children. J Antimicrob Chemother 2016; 71: 1106–17. [DOI] [PubMed] [Google Scholar]
- 9. Choudhury D, Ahmed Z.. Drug-associated renal dysfunction and injury. Nat Clin Pract Nephrol 2006; 2: 80–91. [DOI] [PubMed] [Google Scholar]
- 10. Nolin TD, Himmelfarb J.. Mechanisms of drug-induced nephrotoxicity. Handb Exp Pharmacol 2010; 196: 111–30. [DOI] [PubMed] [Google Scholar]
- 11. Hsieh EM, Hornik CP, Clark RH. et al. Medication use in the neonatal intensive care unit. Am J Perinatol 2014; 31: 811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mehta RL, Awdishu L, Davenport A. et al. Phenotype standardization for drug-induced kidney disease. Kidney Int 2015; 88: 226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pannu N, Nadim MK.. An overview of drug-induced acute kidney injury. Crit Care Med 2008; 36 Suppl 4: S216–23. [DOI] [PubMed] [Google Scholar]
- 14. Fuchs TC, Hewitt P.. Biomarkers for drug-induced renal damage and nephrotoxicity—an overview for applied toxicology. AAPS J 2011; 13: 615–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. John R, Herzenberg AM.. Renal toxicity of therapeutic drugs. J Clin Pathol 2009; 62: 505–15. [DOI] [PubMed] [Google Scholar]
- 16. Markowitz GS, Perazella MA.. Drug-induced renal failure: a focus on tubulointerstitial disease. Clin Chim Acta 2005; 351: 31–47. [DOI] [PubMed] [Google Scholar]
- 17. George CRP. The rise and fall of acute tubular necrosis - an exercise in medical semiotics. G Ital Nefrol 2018; 35: 138–42. [PubMed] [Google Scholar]
- 18. Neugarten J, Aynedjian HS, Bank N.. Role of tubular obstruction in acute renal failure due to gentamicin. Kidney Int 1983; 24: 330–5. [DOI] [PubMed] [Google Scholar]
- 19. Lopez-Novoa JM, Quiros Y, Vicente L. et al. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int 2011; 79: 33–45. [DOI] [PubMed] [Google Scholar]
- 20. Vallon V. Tubuloglomerular feedback and the control of glomerular filtration rate. News Physiol Sci 2003; 18: 169–74. [DOI] [PubMed] [Google Scholar]
- 21. Bartoli E. Adverse effects of drugs on the kidney. Eur J Intern Med 2016; 28: 1–8. [DOI] [PubMed] [Google Scholar]
- 22. Krishnan N, Perazella MA.. Drug-induced acute interstitial nephritis: pathology, pathogenesis, and treatment. Iran J Kidney Dis 2015; 9: 3–13. [PubMed] [Google Scholar]
- 23. Baker RJ, Pusey CD.. The changing profile of acute tubulointerstitial nephritis. Nephrol Dial Transplant 2004; 19: 8–11. [DOI] [PubMed] [Google Scholar]
- 24. Perazella MA. Diagnosing drug-induced AIN in the hospitalized patient: a challenge for the clinician. Clin Nephrol 2014; 81: 381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Muriithi AK, Nasr SH, Leung N.. Utility of urine eosinophils in the diagnosis of acute interstitial nephritis. Clin J Am Soc Nephrol 2013; 8: 1857–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moledina DG, Perazella MA.. Drug-induced acute interstitial nephritis. Clin J Am Soc Nephrol 2017; 12: 2046–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghane Shahrbaf F, Assadi F.. Drug-induced renal disorders. J Renal Inj Prev 2015; 4: 57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mulay SR, Anders HJ.. Crystal nephropathies: mechanisms of crystal-induced kidney injury. Nat Rev Nephrol 2017; 13: 226–40. [DOI] [PubMed] [Google Scholar]
- 29. Mulay SR, Shi C, Ma X. et al. Novel insights into crystal-induced kidney injury. Kidney Dis (Basel) 2018; 4: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fleischer R, Johnson M.. Acyclovir nephrotoxicity: a case report highlighting the importance of prevention, detection, and treatment of acyclovir-induced nephropathy. Case Rep Med 2010; 2010: 602783.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Izzedine H, Harris M, Perazella MA.. The nephrotoxic effects of HAART. Nat Rev Nephrol 2009; 5: 563–73. [DOI] [PubMed] [Google Scholar]
- 32. Reilly RF, Tray K, Perazella MA.. Indinavir nephropathy revisited: a pattern of insidious renal failure with identifiable risk factors. Am J Kidney Dis 2001; 38: E23.. [DOI] [PubMed] [Google Scholar]
- 33. Stratta P, Lazzarich E, Canavese C. et al. Ciprofloxacin crystal nephropathy. Am J Kidney Dis 2007; 50: 330–5. [DOI] [PubMed] [Google Scholar]
- 34. Khan M, Ortega LM, Bagwan N. et al. Crystal-induced acute kidney injury due to ciprofloxacin. J Nephropathol 2015; 4: 29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goli R, Mukku KK, Raju SB. et al. Acute ciprofloxacin-induced crystal nephropathy with granulomatous interstitial nephritis. Indian J Nephrol 2017; 27: 231–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Levine DP. Vancomycin: a history. Clin Infect Dis 2006; 42 Suppl 1: S5–12. [DOI] [PubMed] [Google Scholar]
- 37. Watanakunakorn C. Mode of action and in-vitro activity of vancomycin. J Antimicrob Chemother 1984; 14 Suppl D: 7–18. [DOI] [PubMed] [Google Scholar]
- 38. Rodvold KA, McConeghy KW.. Methicillin-resistant Staphylococcus aureus therapy: past, present, and future. Clin Infect Dis 2014; 58 Suppl 1: S20–7. [DOI] [PubMed] [Google Scholar]
- 39. Bruniera FR, Ferreira FM, Saviolli LR. et al. The use of vancomycin with its therapeutic and adverse effects: a review. Eur Rev Med Pharmacol Sci 2015; 19: 694–700. [PubMed] [Google Scholar]
- 40. Fiorito TM, Luther MK, Dennehy PH. et al. Nephrotoxicity with vancomycin in the pediatric population: a systematic review and meta-analysis. Pediatr Infect Dis J 2018; 37: 654–61. [DOI] [PubMed] [Google Scholar]
- 41. Shah-Khan F, Scheetz MH, Ghossein C.. Biopsy-proven acute tubular necrosis due to vancomycin toxicity. Int J Nephrol 2011; 2011: 436856.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sawada A, Kawanishi K, Morikawa S. et al. Biopsy-proven vancomycin-induced acute kidney injury: a case report and literature review. BMC Nephrol 2018; 19: 72.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bergman MM, Glew RH, Ebert TH.. Acute interstitial nephritis associated with vancomycin therapy. Arch Intern Med 1988; 148: 2139–40. [PubMed] [Google Scholar]
- 44. Wicklow BA, Ogborn MR, Gibson IW. et al. Biopsy-proven acute tubular necrosis in a child attributed to vancomycin intoxication. Pediatr Nephrol 2006; 21: 1194–6. [DOI] [PubMed] [Google Scholar]
- 45. Wu CY, Wang JS, Chiou YH. et al. Biopsy proven acute tubular necrosis associated with vancomycin in a child: case report and literature review. Ren Fail 2007; 29: 1059–61. [DOI] [PubMed] [Google Scholar]
- 46. Stidham T, Reiter PD, Ford DM. et al. Successful utilization of high-flux hemodialysis for treatment of vancomycin toxicity in a child. Case Rep Pediatr 2011; 2011: 678724.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Azar R, Bakhache E, Boldron A.. [Acute interstitial nephropathy induced by vancomycin]. Nephrologie 1996; 17: 327–8. [PubMed] [Google Scholar]
- 48. Luque Y, Louis K, Jouanneau C. et al. Vancomycin-associated cast nephropathy. J Am Soc Nephrol 2017; 28: 1723–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Arimura Y, Yano T, Hirano M. et al. Mitochondrial superoxide production contributes to vancomycin-induced renal tubular cell apoptosis. Free Radic Biol Med 2012; 52: 1865–73. [DOI] [PubMed] [Google Scholar]
- 50. King DW, Smith MA.. Proliferative responses observed following vancomycin treatment in renal proximal tubule epithelial cells. Toxicol In Vitro 2004; 18: 797–803. [DOI] [PubMed] [Google Scholar]
- 51. Nishino Y, Takemura S, Minamiyama Y. et al. Targeting superoxide dismutase to renal proximal tubule cells attenuates vancomycin-induced nephrotoxicity in rats. Free Radic Res 2003; 37: 373–9. [DOI] [PubMed] [Google Scholar]
- 52. Humanes B, Jado JC, Camano S. et al. Protective effects of cilastatin against vancomycin-induced nephrotoxicity. Biomed Res Int 2015; 2015: 704382.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hori Y, Aoki N, Kuwahara S. et al. Megalin blockade with cilastatin suppresses drug-induced nephrotoxicity. J Am Soc Nephrol 2017; 28: 1783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lodise TP, Patel N, Lomaestro BM. et al. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 2009; 49: 507–14. [DOI] [PubMed] [Google Scholar]
- 55. Wunderink RG, Niederman MS, Kollef MH. et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 2012; 54: 621–9. [DOI] [PubMed] [Google Scholar]
- 56. Minejima E, Choi J, Beringer P. et al. Applying new diagnostic criteria for acute kidney injury to facilitate early identification of nephrotoxicity in vancomycin-treated patients. Antimicrob Agents Chemother 2011; 55: 3278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu C, Bayer A, Cosgrove SE. et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52: 285–92. [DOI] [PubMed] [Google Scholar]
- 58. O’Donnell JN, Rhodes NJ, Lodise TP. et al. 24-Hour pharmacokinetic relationships for vancomycin and novel urinary biomarkers of acute kidney injury. Antimicrob Agents Chemother 2017; 61: e00416–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rhodes NJ, Prozialeck WC, Lodise TP. et al. Evaluation of vancomycin exposures associated with elevations in novel urinary biomarkers of acute kidney injury in vancomycin-treated rats. Antimicrob Agents Chemother 2016; 60: 5742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Aljefri DM, Avedissian SN, Rhodes NJ. et al. Vancomycin area under the curve and acute kidney injury: a meta-analysis. Clin Infect Dis 2019; doi:10.1093/cid/ciz051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Neely MN, Kato L, Youn G. et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother 2018; 62: e02042–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Le J, Ny P, Capparelli E. et al. Pharmacodynamic characteristics of nephrotoxicity associated with vancomycin use in children. J Pediatric Infect Dis Soc 2015; 4: e109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lodise TP Jr, Rosenkranz SL, Finnemeyer M. et al. The Emperor’s New Clothes: prospective observational evaluation of the association between the day 2 vancomycin exposure and failure rates among adult hospitalized patients with MRSA bloodstream infections (PROVIDE). Open Forum Infect Dis 2017; 4 Suppl 1: S30–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liang X, Fan Y, Yang M. et al. A prospective multicenter clinical observational study on vancomycin efficiency and safety with therapeutic drug monitoring. Clin Infect Dis 2018; 67 Suppl 2: S249–55. [DOI] [PubMed] [Google Scholar]
- 65. Patel K, Crumby AS, Maples HD.. Balancing vancomycin efficacy and nephrotoxicity: should we be aiming for trough or AUC/MIC? Paediatr Drugs 2015; 17: 97–103. [DOI] [PubMed] [Google Scholar]
- 66. Pettit RS, Peters SJ, McDade EJ. et al. Vancomycin dosing and monitoring in the treatment of cystic fibrosis: results of a national practice survey. J Pediatr Pharmacol Ther 2017; 22: 406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gwee A, Cranswick N, Metz D. et al. Neonatal vancomycin continuous infusion: still a confusion? Pediatr Infect Dis J 2014; 33: 600–5. [DOI] [PubMed] [Google Scholar]
- 68. McWilliam SJ, Antoine DJ, Smyth RL. et al. Aminoglycoside-induced nephrotoxicity in children. Pediatr Nephrol 2017; 32: 2015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Edson RS, Terrell CL.. The aminoglycosides. Mayo Clin Proc 1999; 74: 519–28. [DOI] [PubMed] [Google Scholar]
- 70. Rougier F, Claude D, Maurin M. et al. Aminoglycoside nephrotoxicity. Curr Drug Targets Infect Disord 2004; 4: 153–62. [DOI] [PubMed] [Google Scholar]
- 71. Moestrup SK, Cui S, Vorum H. et al. Evidence that epithelial glycoprotein 330/megalin mediates uptake of polybasic drugs. J Clin Invest 1995; 96: 1404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schmitz C, Hilpert J, Jacobsen C. et al. Megalin deficiency offers protection from renal aminoglycoside accumulation. J Biol Chem 2002; 277: 618–22. [DOI] [PubMed] [Google Scholar]
- 73. Silverblatt FJ, Kuehn C.. Autoradiography of gentamicin uptake by the rat proximal tubule cell. Kidney Int 1979; 15: 335–45. [DOI] [PubMed] [Google Scholar]
- 74. Servais H, Van Der Smissen P, Thirion G. et al. Gentamicin-induced apoptosis in LLC-PK1 cells: involvement of lysosomes and mitochondria. Toxicol Appl Pharmacol 2005; 206: 321–33. [DOI] [PubMed] [Google Scholar]
- 75. De Broe ME, Paulus GJ, Verpooten GA. et al. Early effects of gentamicin, tobramycin, and amikacin on the human kidney. Kidney Int 1984; 25: 643–52. [DOI] [PubMed] [Google Scholar]
- 76. Banday AA, Farooq N, Priyamvada S. et al. Time dependent effects of gentamicin on the enzymes of carbohydrate metabolism, brush border membrane and oxidative stress in rat kidney tissues. Life Sci 2008; 82: 450–9. [DOI] [PubMed] [Google Scholar]
- 77. Jenh AM, Tamma PD, Milstone AM.. Extended-interval aminoglycoside dosing in pediatrics. Pediatr Infect Dis J 2011; 30: 338–9. [DOI] [PubMed] [Google Scholar]
- 78. Nicolau DP, Freeman CD, Belliveau PP. et al. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother 1995; 39: 650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nordstrom L, Ringberg H, Cronberg S. et al. Does administration of an aminoglycoside in a single daily dose affect its efficacy and toxicity? J Antimicrob Chemother 1990; 25: 159–73. [DOI] [PubMed] [Google Scholar]
- 80. Prins JM, Buller HR, Kuijper EJ. et al. Once versus thrice daily gentamicin in patients with serious infections. Lancet 1993; 341: 335–9. [DOI] [PubMed] [Google Scholar]
- 81. Gilbert DN. Once-daily aminoglycoside therapy. Antimicrob Agents Chemother 1991; 35: 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Contopoulos-Ioannidis DG, Giotis ND, Baliatsa DV. et al. Extended-interval aminoglycoside administration for children: a meta-analysis. Pediatrics 2004; 114: e111–8. [DOI] [PubMed] [Google Scholar]
- 83. Wong G, Sime FB, Lipman J. et al. How do we use therapeutic drug monitoring to improve outcomes from severe infections in critically ill patients? BMC Infect Dis 2014; 14: 288.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Coulthard KP, Peckham DG, Conway SP. et al. Therapeutic drug monitoring of once daily tobramycin in cystic fibrosis—caution with trough concentrations. J Cyst Fibros 2007; 6: 125–30. [DOI] [PubMed] [Google Scholar]
- 85. Yahav D, Farbman L, Leibovici L. et al. Colistin: new lessons on an old antibiotic. Clin Microbiol Infect 2012; 18: 18–29. [DOI] [PubMed] [Google Scholar]
- 86. Falagas ME, Kasiakou SK.. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis 2005; 40: 1333–41. [DOI] [PubMed] [Google Scholar]
- 87. Koch-Weser J, Sidel VW, Federman EB. et al. Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy. Ann Intern Med 1970; 72: 857–68. [DOI] [PubMed] [Google Scholar]
- 88. Tamma PD, Newland JG, Pannaraj PS. et al. The use of intravenous colistin among children in the United States: results from a multicenter, case series. Pediatr Infect Dis J 2013; 32: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Evans ME, Feola DJ, Rapp RP.. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann Pharmacother 1999; 33: 960–7. [DOI] [PubMed] [Google Scholar]
- 90. Thomas TA, Broun EC, Abildskov KM. et al. High performance liquid chromatography-mass spectrometry assay for polymyxin B1 and B2 in human plasma. Ther Drug Monit 2012; 34: 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Voigt LM, Zammit KT.. Colistin and polymyxin B In: Cohen H, ed. Casebook in Clinical Pharmacokinetics and Drug Dosing. New York, NY, USA: McGraw-Hill Education, 2015; 87–96. [Google Scholar]
- 92. Kumar PP, Giri SR, Shaikh FA. et al. Safety and efficacy of intravenous colistin in children. Indian Pediatr 2015; 52: 129–30. [DOI] [PubMed] [Google Scholar]
- 93. Karbuz A, Ozdemir H, Yaman A. et al. The use of colistin in critically ill children in a pediatric intensive care unit. Pediatr Infect Dis J 2014; 33: e19–24. [DOI] [PubMed] [Google Scholar]
- 94. Antachopoulos C, Iosifidis E.. Colistin use in neonates and children with infections due to carbapenem-resistant bacteria. Pediatr Infect Dis J 2017; 36: 905–7. [DOI] [PubMed] [Google Scholar]
- 95. Zavascki AP, Goldani LZ, Li J. et al. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antimicrob Chemother 2007; 60: 1206–15. [DOI] [PubMed] [Google Scholar]
- 96. Iosifidis E, Antachopoulos C, Ioannidou M. et al. Colistin administration to pediatric and neonatal patients. Eur J Pediatr 2010; 169: 867–74. [DOI] [PubMed] [Google Scholar]
- 97. Kapoor K, Jajoo M, Dublish S. et al. Intravenous colistin for multidrug-resistant gram-negative infections in critically ill pediatric patients. Pediatr Crit Care Med 2013; 14: e268–72. [DOI] [PubMed] [Google Scholar]
- 98. Falagas ME, Vouloumanou EK, Rafailidis PI.. Systemic colistin use in children without cystic fibrosis: a systematic review of the literature. Int J Antimicrob Agents 2009; 33: 503.e501–13. [DOI] [PubMed] [Google Scholar]
- 99. Justo JA, Bosso JA.. Adverse reactions associated with systemic polymyxin therapy. Pharmacotherapy 2015; 35: 28–33. [DOI] [PubMed] [Google Scholar]
- 100. Kelesidis T, Falagas ME.. The safety of polymyxin antibiotics. Expert Opin Drug Saf 2015; 14: 1687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Vattimo Mde F, Watanabe M, da Fonseca CD. et al. Polymyxin B nephrotoxicity: from organ to cell damage. PLoS One 2016; 11: e0161057.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Suzuki T, Yamaguchi H, Ogura J. et al. Megalin contributes to kidney accumulation and nephrotoxicity of colistin. Antimicrob Agents Chemother 2013; 57: 6319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zavascki AP, Nation RL.. Nephrotoxicity of polymyxins: is there any difference between colistimethate and polymyxin B? Antimicrob Agents Chemother 2017; 61: e02319–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Yousef JM, Chen G, Hill PA. et al. Ascorbic acid protects against the nephrotoxicity and apoptosis caused by colistin and affects its pharmacokinetics. J Antimicrob Chemother 2012; 67: 452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yousef JM, Chen G, Hill PA. et al. Melatonin attenuates colistin-induced nephrotoxicity in rats. Antimicrob Agents Chemother 2011; 55: 4044–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Abdelraouf K, Braggs KH, Yin T. et al. Characterization of polymyxin B-induced nephrotoxicity: implications for dosing regimen design. Antimicrob Agents Chemother 2012; 56: 4625–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Nation RL, Velkov T, Li J.. Colistin and polymyxin B: peas in a pod, or chalk and cheese?. Clin Infect Dis 2014; 59: 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.FDA. Coly-Mycin® M Parenteral (Colistimethate for Injection, USP) 2017.
- 109. Landersdorfer CB, Nation RL.. Colistin: how should it be dosed for the critically ill? Semin Respir Crit Care Med 2015; 36: 126–35. [DOI] [PubMed] [Google Scholar]
- 110. Miglis C, Rhodes NJ, Avedissian SN. et al. Population pharmacokinetics of polymyxin B in acutely ill adult patients. Antimicrob Agents Chemother 2018; 62: e01475–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sandri AM, Landersdorfer CB, Jacob J. et al. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis 2013; 57: 524–31. [DOI] [PubMed] [Google Scholar]
- 112. Gnann JW Jr, Barton NH, Whitley RJ.. Acyclovir: mechanism of action, pharmacokinetics, safety and clinical applications. Pharmacotherapy 1983; 3: 275–83. [DOI] [PubMed] [Google Scholar]
- 113. Rao S, Abzug MJ, Carosone-Link P. et al. Intravenous acyclovir and renal dysfunction in children: a matched case control study. J Pediatr 2015; 166: 1462–8.e1–4. [DOI] [PubMed] [Google Scholar]
- 114. Izzedine H, Launay-Vacher V, Deray G.. Antiviral drug-induced nephrotoxicity. Am J Kidney Dis 2005; 45: 804–17. [DOI] [PubMed] [Google Scholar]
- 115. Xing W, Gu L, Zhang X. et al. A metabolic profiling analysis of the nephrotoxicity of acyclovir in rats using ultra performance liquid chromatography/mass spectrometry. Environ Toxicol Pharmacol 2016; 46: 234–40. [DOI] [PubMed] [Google Scholar]
- 116. Gunness P, Aleksa K, Bend J. et al. Acyclovir-induced nephrotoxicity: the role of the acyclovir aldehyde metabolite. Transl Res 2011; 158: 290–301. [DOI] [PubMed] [Google Scholar]
- 117. Ahmad T, Simmonds M, McIver AG. et al. Reversible renal failure in renal transplant patients receiving oral acyclovir prophylaxis. Pediatr Nephrol 1994; 8: 489–91. [DOI] [PubMed] [Google Scholar]
- 118. Vomiero G, Carpenter B, Robb I. et al. Combination of ceftriaxone and acyclovir—an underestimated nephrotoxic potential? Pediatr Nephrol 2002; 17: 633–7. [DOI] [PubMed] [Google Scholar]
- 119. Didsbury MS, Mackie FE, Kennedy SE.. A systematic review of acute kidney injury in pediatric allogeneic hematopoietic stem cell recipients. Pediatr Transplant 2015; 19: 460–70. [DOI] [PubMed] [Google Scholar]
- 120. Lietman PS. Clinical pharmacology: foscarnet. Am J Med 1992; 92: 8S–11S. [DOI] [PubMed] [Google Scholar]
- 121. Jayaweera DT. Minimising the dosage-limiting toxicities of foscarnet induction therapy. Drug Saf 1997; 16: 258–66. [DOI] [PubMed] [Google Scholar]
- 122. Justrabo E, Zanetta G, Martin L. et al. Irreversible glomerular lesions induced by crystal precipitation in a renal transplant after foscarnet therapy for cytomegalovirus infection. Histopathology 1999; 34: 365–9. [DOI] [PubMed] [Google Scholar]
- 123. Cheung TW, Jayaweera DT, Pearce D. et al. Safety of oral versus intravenous hydration during induction therapy with intravenous foscarnet in AIDS patients with cytomegalovirus infections. Int J STD AIDS 2000; 11: 640–7. [DOI] [PubMed] [Google Scholar]
- 124. Weissmann G, Sessa G.. The action of polyene antibiotics on phospholipid-cholesterol structures. J Biol Chem 1967; 242: 616–25. [PubMed] [Google Scholar]
- 125. Ostrosky-Zeichner L, Marr KA, Rex JH. et al. Amphotericin B: time for a new “gold standard”. Clin Infect Dis 2003; 37: 415–25. [DOI] [PubMed] [Google Scholar]
- 126. Maertens JA. History of the development of azole derivatives. Clin Microbiol Infect 2004; 10 Suppl 1: 1–10. [DOI] [PubMed] [Google Scholar]
- 127. Herbrecht R, Denning DW, Patterson TF. et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 2002; 347: 408–15. [DOI] [PubMed] [Google Scholar]
- 128. Nett JE, Andes DR.. Antifungal agents: spectrum of activity, pharmacology, and clinical indications. Infect Dis Clin North Am 2016; 30: 51–83. [DOI] [PubMed] [Google Scholar]
- 129. Branch RA. Prevention of amphotericin B-induced renal impairment. A review on the use of sodium supplementation. Arch Intern Med 1988; 148: 2389–94. [PubMed] [Google Scholar]
- 130. Medoff G, Kobayashi GS.. Strategies in the treatment of systemic fungal infections. N Engl J Med 1980; 302: 145–55. [DOI] [PubMed] [Google Scholar]
- 131. Grela E, Wieczor M, Luchowski R. et al. Mechanism of binding of antifungal antibiotic amphotericin B to lipid membranes: an insight from combined single-membrane imaging, microspectroscopy, and molecular dynamics. Mol Pharm 2018; 15: 4202–13. [DOI] [PubMed] [Google Scholar]
- 132. Sabra R, Branch RA.. Mechanisms of amphotericin B-induced decrease in glomerular filtration rate in rats. Antimicrob Agents Chemother 1991; 35: 2509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Sawaya BP, Weihprecht H, Campbell WR. et al. Direct vasoconstriction as a possible cause for amphotericin B-induced nephrotoxicity in rats. J Clin Invest 1991; 87: 2097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sawaya BP, Briggs JP, Schnermann J.. Amphotericin B nephrotoxicity: the adverse consequences of altered membrane properties. J Am Soc Nephrol 1995; 6: 154–64. [DOI] [PubMed] [Google Scholar]
- 135. Karimzadeh I, Farsaei S, Khalili H. et al. Are salt loading and prolonging infusion period effective in prevention of amphotericin B-induced nephrotoxicity? Expert Opin Drug Saf 2012; 11: 969–83. [DOI] [PubMed] [Google Scholar]
- 136. Varlam DE, Siddiq MM, Parton LA. et al. Apoptosis contributes to amphotericin B-induced nephrotoxicity. Antimicrob Agents Chemother 2001; 45: 679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Finn JT, Cohen LH, Steinmetz PR.. Acidifying defect induced by amphotericin B: comparison of bicarbonate and hydrogen ion permeabilities. Kidney Int 1977; 11: 261–6. [DOI] [PubMed] [Google Scholar]
- 138. Stinebaugh BJ, Schloeder FX, Tam SC. et al. Pathogenesis of distal renal tubular acidosis. Kidney Int 1981; 19: 1–7. [DOI] [PubMed] [Google Scholar]
- 139. Kim SW, Yeum CH, Kim S. et al. Amphotericin B decreases adenylyl cyclase activity and aquaporin-2 expression in rat kidney. J Lab Clin Med 2001; 138: 243–9. [DOI] [PubMed] [Google Scholar]
- 140. Hiemenz JW, Walsh TJ.. Lipid formulations of amphotericin B: recent progress and future directions. Clin Infect Dis 1996; 22 Suppl 2: S133–44. [DOI] [PubMed] [Google Scholar]
- 141. Mehta RT, McQueen TJ, Keyhani A. et al. Phagocyte transport as mechanism for enhanced therapeutic activity of liposomal amphotericin B. Chemotherapy 1994; 40: 256–64. [DOI] [PubMed] [Google Scholar]
- 142. Szoka FC Jr, Milholland D, Barza M.. Effect of lipid composition and liposome size on toxicity and in vitro fungicidal activity of liposome-intercalated amphotericin B. Antimicrob Agents Chemother 1987; 31: 421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zager RA, Bredl CR, Schimpf BA.. Direct amphotericin B-mediated tubular toxicity: assessments of selected cytoprotective agents. Kidney Int 1992; 41: 1588–94. [DOI] [PubMed] [Google Scholar]
- 144. Karimzadeh I, Khalili H, Farsaei S. et al. Role of diuretics and lipid formulations in the prevention of amphotericin B-induced nephrotoxicity. Eur J Clin Pharmacol 2013; 69: 1351–68. [DOI] [PubMed] [Google Scholar]
- 145. Mehta R, Lopez-Berestein G, Hopfer R. et al. Liposomal amphotericin B is toxic to fungal cells but not to mammalian cells. Biochim Biophys Acta 1984; 770: 230–4. [DOI] [PubMed] [Google Scholar]
- 146. Wasan KM, Rosenblum MG, Cheung L. et al. Influence of lipoproteins on renal cytotoxicity and antifungal activity of amphotericin B. Antimicrob Agents Chemother 1994; 38: 223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Steimbach LM, Tonin FS, Virtuoso S. et al. Efficacy and safety of amphotericin B lipid-based formulations—a systematic review and meta-analysis. Mycoses 2017; 60: 146–54. [DOI] [PubMed] [Google Scholar]
- 148. Peleg AY, Woods ML.. Continuous and 4 h infusion of amphotericin B: a comparative study involving high-risk haematology patients. J Antimicrob Chemother 2004; 54: 803–8. [DOI] [PubMed] [Google Scholar]
- 149. Bes DF, Rosanova MT, Sberna N. et al. Deoxycholate amphotericin B and nephrotoxicity in the pediatric setting. Pediatr Infect Dis J 2014; 33: e198–206. [DOI] [PubMed] [Google Scholar]
- 150. The Drug Induced Kidney Injury Work Package of Innovative Medicines Initiative SAFE-T Consortium https://c-path.org/wp-content/uploads/2018/01/DIKI-DataSummary_final.pdf. 2017.
- 151. Predictive Safety Testing Consortium https://c-path.org/programs/pstc/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.