Abstract
Patient: Male, 70
Final Diagnosis: Metachronous pancreatic metastasis
Symptoms: None
Medication: —
Clinical Procedure: Surgery
Specialty: Gastroenterology and Hepatology
Objective:
Rare disease
Background:
Pancreatic metastasis from colorectal cancer is rare and can masquerade as primary pancreatic cancer.
Case Report:
A 70-year-old male was diagnosed with advanced rectal cancer with multiple liver metastases. After neoadjuvant chemotherapy, he underwent radical surgery for the primary tumor and hepatectomy for multiple liver metastases. Adjuvant chemotherapies and additional surgeries were subsequently required for recurrences in the liver, lung, and lymph nodes. A diffuse hypovascular nodule in the pancreatic head and a solitary liver metastasis were detected 2.5 years after the initial surgery and he accordingly underwent further chemotherapy. However, the pancreatic tumor progressed, invading the pancreatic duct and biliary tract. Obstructive jaundice finally prompted discontinuation of chemotherapy and he underwent biliary drainage. His diffuse and hypovascular tumor was clinically and radiographically diagnosed as a primary pancreatic cancer. Pancreatic resection for the pancreatic tumor and hepatectomy for the liver metastasis were performed 4.2 years after the initial surgery, achieving radiographic and surgical curative resection. Pathological examination of the surgical specimen resulted in a definitive diagnosis of metachronous pancreatic metastasis from his primary rectal cancer. Despite further chemotherapy, his general condition worsened; however, he remains alive 5.4 years after the initial surgery, with best supportive care.
Conclusions:
Pancreatic metastasis originating from rectal cancer can masquerade as primary pancreatic cancer clinically and radiologically. Multimodality treatment is mandatory for metastatic colorectal cancer. Aggressive surgeries for pancreatic metastasis should be considered if curative resection appears possible radiographically and/or intraoperatively.
MeSH Keywords: Colorectal Neoplasms, Neoplasm Metastasis, Pancreas, Rectal Neoplasms
Background
There are various types of primary cancers of the pancreas, some of which are rare [1]. Invasive ductal carcinoma, the most common type of primary pancreatic cancer [1], usually shows as a diffuse and hypovascular nodule in imaging studies. Infiltrating carcinoma is often accompanied by invasions of the blood vessels, biliary tract, and pancreatic duct. Obstructive jaundice and associated pancreatitis frequently complicate invasive tumors located in the pancreatic head [1].
Metastases to the pancreas account for approximately 2% of pancreatic neoplasms [2]. Pancreatic metastases from colorectal cancer are rare [3–14], and acceptable outcomes can be achieved by curative resection in carefully selected patients [5,6,8,10,12–15]. Treatment guidelines recommend aggressive resection of hematogenous metastases (i.e., lung or liver metastases) [16,17] if radiographic and/or surgical R0 (i.e., radiographic/surgical R0 resection, according to the Japanese Classification of Colorectal, Appendiceal and Anal carcinoma [18]) is accomplished [5,7–10,12,13,15–17]. Whether pancreatic metastases occur via hematogenous pathways is controversial [7–10,12]. Aggressive surgery should be a component of multimodality treatment of metastatic colorectal cancer [5,6,8–10,12–17].
Here, we report a rare and misdiagnosed case of pancreatic metastasis originating from rectal cancer. Imaging findings resembled those of primary pancreatic cancer. We also discuss the role of surgical resection in treatment of this rare metastasis.
Case Report
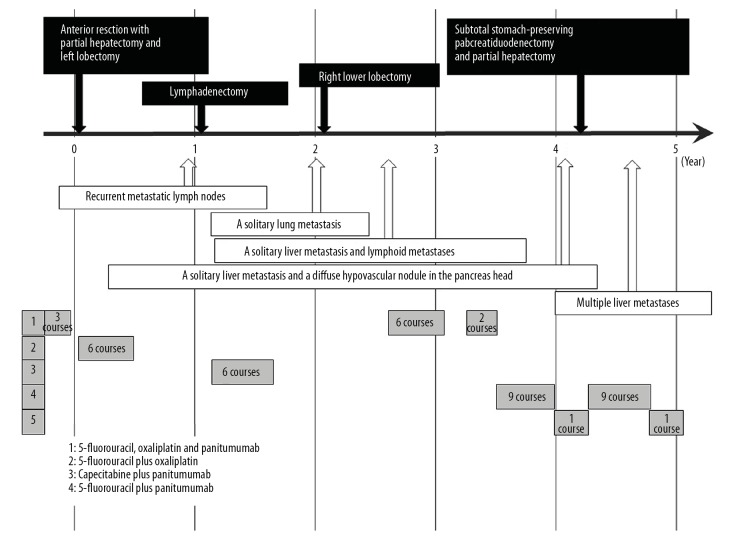
A 70-year-old male visited our hospital with symptoms of bowel obstruction. Colorectal endoscopy, pathological diagnosis based on biopsy specimen, and enhanced computed tomography were performed as initial diagnostic workup. Detailed examination resulted in a clinical diagnosis of advanced rectal cancer with multiple liver metastases. He underwent neoadjuvant chemotherapy with 3 courses of 5-fluorouracil plus oxaliplatin followed by 2 courses of panitumumab followed by laparoscopic low anterior resection with intentional lymphadenectomy. Partial hepatectomy and left lobectomy were also performed for multiple liver metastases. According to imaging studies and surgical findings, curative resection was achieved (i.e., radiographic/surgical R0 resection [18]). The final pathological diagnosis was T4aN1aM1a stage IVA (TNM classification [19]) well-differentiated tubular adenocarcinoma. Adjuvant chemotherapy with 6 courses of 5-fluorouracil plus oxaliplatin was completed. One year after the initial surgery, lymphadenectomy for recurrent metastatic lymph nodes was required, achieving radiographic/surgical R0 [18]; thereafter, further chemotherapy with capecitabine plus panitumumab was administered. Subsequently, right lower lobectomy was performed for a solitary lung metastasis and chemotherapy discontinued for 7 months because radiographic/surgical R0 [18] had been achieved. A solitary liver metastasis and lymphoid metastases were then detected; these resolved radiographically after recommencing chemotherapy with 5-fluorouracil, oxaliplatin and panitumumab. This was discontinued once radiographic R0 [18] had again been achieved, and then resumed after detection of another solitary liver metastasis and lymphoid metastasis. Oxaliplatin was omitted from his regimen because of refractory peripheral neuropathy. Four cycles of chemotherapy with 5-fluorouracil plus leucovorin were administered, achieving radiological resolution of these metastases (radiographic R0 [18]). At 2.5 years after the initial surgery, a further solitary liver metastasis and a diffuse hypovascular nodule in the pancreatic head were detected (Figure 1). Further chemotherapy with 5-fluorouracil, irinotecan and bevacizumab was administered for 1.6 years, and then discontinued because of development of progressive obstructive jaundice caused by the pancreatic tumor. Radiographic and ultrasonography details of this pancreatic neoplasm are shown in Figures 2–4. The tumor was in the pancreatic head, hypovascular, and 21×19 mm in size (Figures 1, 2). Diffuse invasion of the pancreatic duct and biliary tract resulted in obstruction and remarkable dilation of the distal pancreatic duct, intrahepatic bile ducts, and extrahepatic biliary tract (Figures 3, 4). Biliary drainage was achieved via an endoscopic nasobiliary drainage tube (Figure 4). Pathological examination of a biopsy specimen resulted in a diagnosis of moderately-differentiated tubular adenocarcinoma. It was difficult to make a definitive diagnosis of our patient’s locally advanced pancreatic tumor; however, we concluded on the basis of the clinical and radiographic evidence that it was a primary pancreatic cancer rather than a metachronous metastasis from the original rectal cancer. At 4.2 years after the initial surgery, subtotal stomach-preserving pancreaticoduodenectomy for the pancreatic tumor and partial hepatectomy for the solitary liver metastasis were performed, achieving radiographic/surgical R0 resection [18]. Pathological examination revealed that the pancreatic tumor was similar to the original rectal cancer (Figure 5); furthermore, the pancreatic neoplasm lacked the typical pathological features (e.g., not a tubular adenocarcinoma) of primary pancreatic cancer. Immunohistological studies revealed negativity for cytokeratin 7 and positivity for cytokeratin 20 and caudal type homeobox transcription factor 2 (Figure 6). These findings resulted in a definitive pathological diagnosis of metachronous pancreatic metastasis originating from our patient’s primary rectal cancer. A total of 6 courses of chemotherapy with 5-fluorouracil, panitumumab and leucovorin were given; however, multiple liver metastases appeared. His general condition worsened despite further chemotherapy with 5-fluorouracil, panitumumab and leucovorin and with 5-fluorouracil, irinotecan and bevacizumab. Although a regimen switch to trifluridine and tipiracil hydrochloride was considered, he opted for best supportive care at 4.8 years after the initial surgery. Carcinomatous ascites has been well controlled, and he remains alive 5.4 years after the initial surgery. Time course of this patient (e.g., surgical resections and chemotherapy regimens) was summarized in Figure 7.
Figure 1.
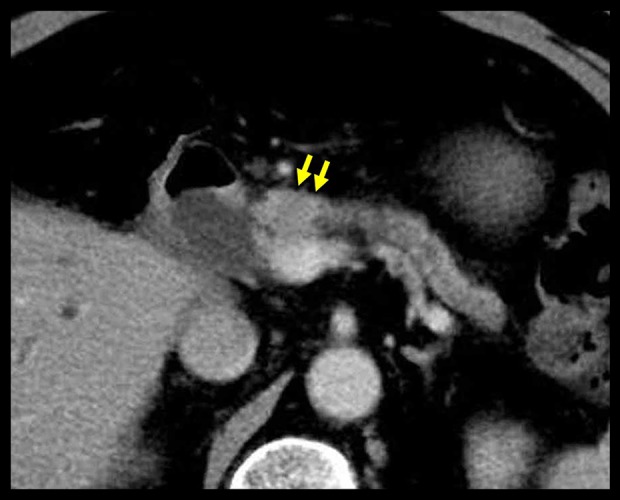
Findings on dynamic computed tomography (CT). At 2.5 years after the initial surgery, a diffuse and low-density nodule (yellow arrows) was detected in the pancreatic head. This hypovascular nodule shows no enhancement on dynamic CT. The distal pancreatic duct is dilated and there is evidence of associated pancreatitis.
Figure 2.
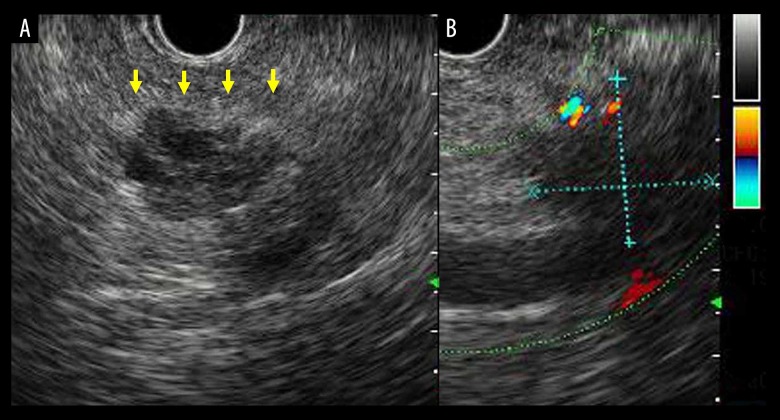
Findings on endoscopic ultrasonography. The low echoic and diffuse nodule (A) (yellow arrows) was 21×19 mm in size and Doppler ultrasonography (B) revealed that it was hypovascular.
Figure 3.
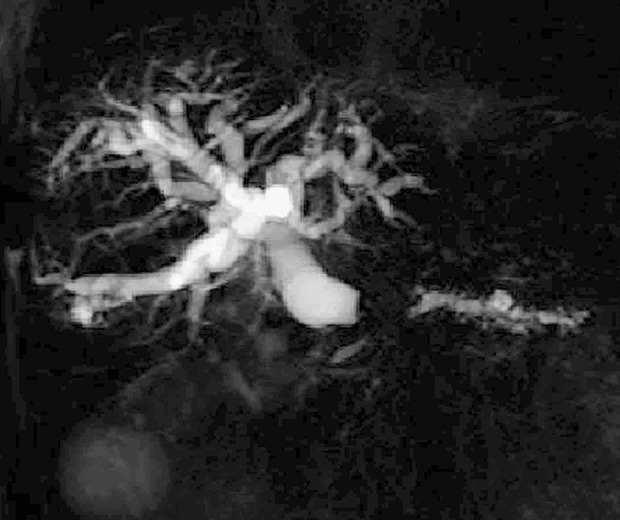
The finding on magnetic resonance imaging. The finding on magnetic resonance cholangiopancreatography is shown. The distal pancreatic duct, intrahepatic bile ducts, and extrahepatic biliary tract are remarkably dilated as a result of obstruction in the pancreatic head.
Figure 4.
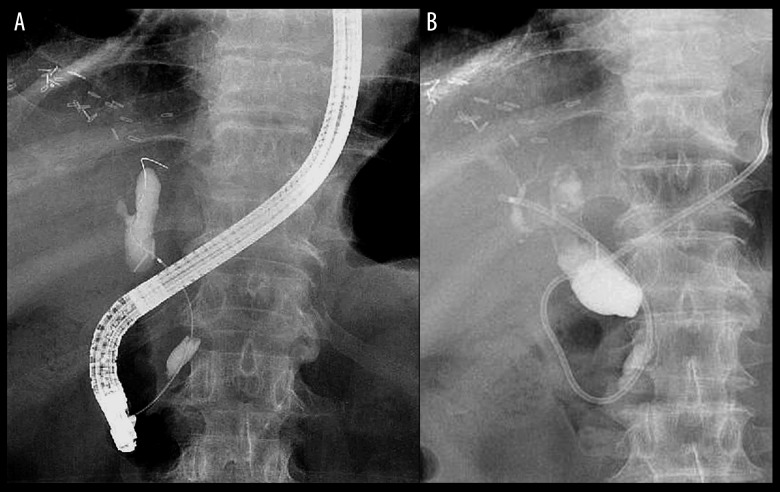
The finding on endoscopic retrograde cholangiopancreatography and biliary drainage by nasobiliary drainage tube. The finding on endoscopic retrograde cholangiopancreatography is shown (A). The resultant obstructive jaundice was progressive and biliary drainage was required. A nasobiliary drainage tube was placed endoscopically (B).
Figure 5.
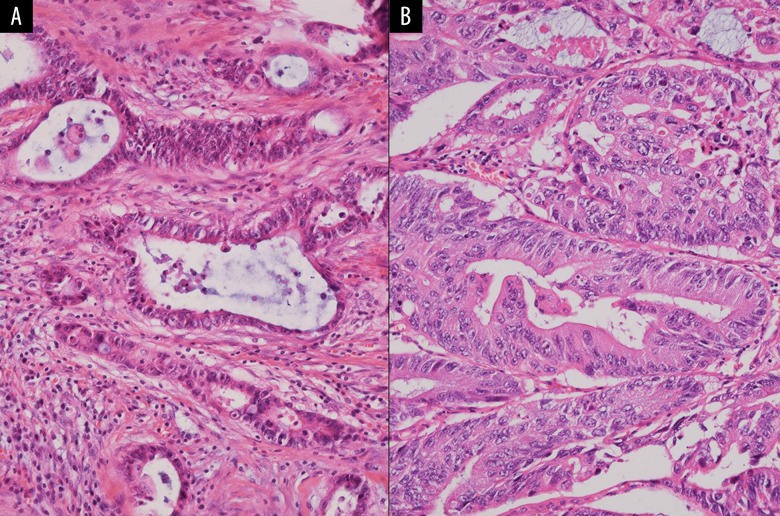
Histopathological assessment. Pathological findings on hematoxylin-eosin stained sections are shown (100×). The pathological features of the rectal cancer (A) and pancreatic neoplasm (B) are similar.
Figure 6.
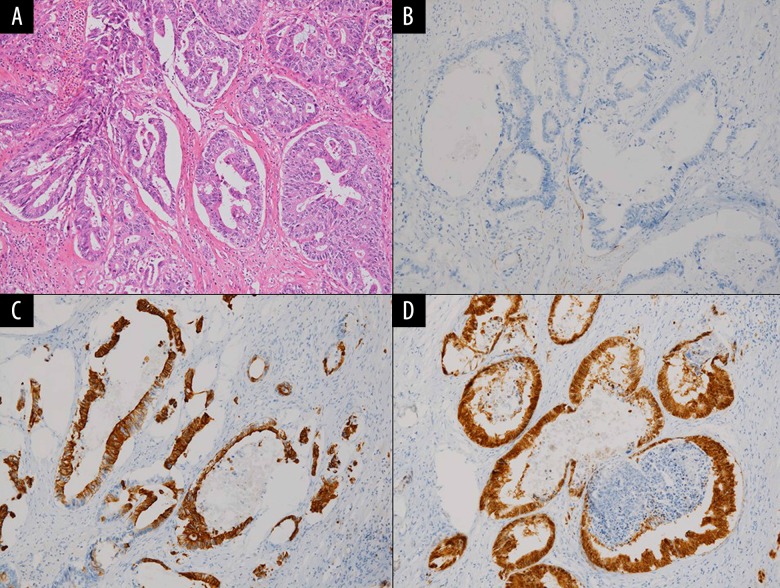
Immunohistological studies. Pathological finding on hematoxylin-eosin staining (A) and immunohistological findings on cytokeratin 7 (B), cytokeratin 20 (C), and caudal type homeobox transcription factor 2 (D) in the pancreatic tumor were shown (20×).
Figure 7.
Time course of this patient. Time course of this patient (e.g., surgical resections and chemotherapy regimens) was summarized.
Discussion
The primary cancers that are most frequently responsible for pancreatic metastases are reportedly renal cell carcinoma (54.3–83.3%) and rectal cancer (7.5–16.7%) [2,9,12,20]. The median interval between resection of the primary cancer and development of pancreatic metastases is relatively longer, being reportedly 32.5–157 months [5,7–9,20]. Pancreatic metastases appearing long after the initial surgery (>5 years) have been documented [4]. However, T1 colorectal cancers according to TNM classification [19] (i.e., oncological depth within submucosal layer) rarely cause pancreatic metastases [9].
Accurate preoperative diagnosis of pancreatic metastasis rather than a primary pancreatic cancer is difficult [7–10,12]. Cytological examination of a fine needle aspirate or pathological examination of a biopsy specimen obtained under endoscopic ultrasonography guidance is useful for preoperative diagnosis [4,8,9,20,21]. In our case, although pathological assessment of a biopsy specimen suggested moderately-differentiated tubular adenocarcinoma, it was not possible to make a definite preoperative diagnosis of pancreatic metastasis originating from rectal cancer, which was regrettable given the impact the diagnosis made on subsequent management. Unfortunately, the clinical features, particularly the findings on imaging studies (e.g., obstructive jaundice with biliary dilatation and associated pancreatitis with dilated pancreatic duct), misled us into diagnosing primary pancreatic cancer. It must always be kept in mind that a pancreatic metastasis may closely resemble the more common primary pancreatic tumor. Only 1.3% of pancreatic metastases originate from colorectal cancer [7] and the pancreas is a very rare site for metastases from colorectal cancer [3–13]; thus, the statistical unlikelihood of metastasis was a factor in our preoperative misdiagnosis.
The usefulness of immunohistological staining for cytokeratin 7, cytokeratin 20, and caudal type homeobox transcription factor 2 is well-documented [4,7,9,14]. Negativity for cytokeratin 7 and positivity for cytokeratin 20 favor colorectal cancer over primary pancreatic cancer [7,9]; however, immunohistological assessment is not practicable as an intraoperative diagnostic tool. Although thyroid transcription factor 1 specifically expresses in most thyroid tumors and in a significant subset of pulmonary neoplasms [22], combinations of cytokeratin 7, cytokeratin 20, and caudal type homeobox transcription factor 2 are not specific for colorectal cancer.
Optimal treatment for pancreatic metastasis has not yet been established [5,8,10,15,20]. Pancreatic resection may confer a survival benefit on patients with pancreatic metastases [2,5–10,12–15,20], the 5-year survival rate after this procedure reportedly being approximately 30% [23]. Cases of successfully resected pancreatic metastases from colorectal cancer after multiple resections of metastatic lesions have been reported [2,5–10,12–14,24]. Thus, patients who are fit for surgery and free of metastases in other organs should be considered good candidates for pancreatic resection [2,5–10,12–14,24].
The role of intentional lymphadenectomy in patients with pancreatic metastases is controversial [10,12,23]; pancreatic metastasis may be accompanied by lymphoid metastases in regional lymph nodes [8,23]. The mortality and morbidity rates for pancreatic resections including pancreaticoduodenectomy for pancreatic metastasis are reportedly 1.4% and 48.3%, respectively [2]. Simple pancreatic resection without intentional dissection of lymph nodes and nerve plexus is currently considered safe and feasible [25]; intentional lymphadenectomy increases the risk of pancreatic leakage [25].
Pancreatic cancer is a solid cancer with a poor prognosis [1]; pancreatic metastases also have a poor prognosis (8.7 months) [10]. In the absence of a definitive diagnosis of pancreatic metastasis, it is not possible to select the optimal chemotherapy regimen [13,26]. From the viewpoint of therapeutic strategy, surgical resection of a pancreatic mass is justified not only to make a definitive diagnosis [8,13,26] but also to improve survival [2,5,6,8–10,12–15,20]. The overall survival of patients with stage IV colorectal cancer is approximately 9 months if chemotherapy is not administered after primary resection [17]. Multimodality therapy is crucial for stage IV colorectal cancer [16,17]. As in our case, aggressive treatment aimed at achieving radiographic and/or surgical R0 should be repeatedly administered to patients with colorectal cancer when they develop recurrences and metastases.
Conclusions
Metachronous pancreatic metastasis originating from rectal cancer can masquerade clinically and radiographically as a primary pancreatic cancer. The possibility of a rare, easily misdiagnosed metastatic tumor in the pancreas must always be kept in mind. Aggressive surgery for pancreatic metastases is justified, not only to make a definitive diagnosis to inform selection of subsequent chemotherapy but also to improve survival, even in the presence of recurrences and metastases provided radiographic and/or surgical curative resection can be accomplished.
Acknowledgments
We are grateful to Yoshihiro Yamamoto and Masayuki Shintaku (Department of Pathology, Shiga General Hospital, Moriyama, Japan) for their assistance with the histopathological and immunopathological assessments.
Footnotes
Conflicts of interest
None.
References:
- 1.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 2.Huang Q, Zhou H, Liu C, et al. Surgical resection for metastatic tumors in the pancreas: A single-center experience and systematic review. Ann Surg Oncol. 2019;26:1649–56. doi: 10.1245/s10434-019-07258-2. [DOI] [PubMed] [Google Scholar]
- 3.Greve E, Dumas O, Fumex F, et al. Solitary pancreatic metastasis four years after curative treatment for rectal carcinoma. Gastroenterol Clin Biol. 2008;32:258–60. doi: 10.1016/j.gcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Sano I, Katanuma A, Yane K, et al. Pancreatic metastasis from rectal cancer that was diagnosed by endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) Intern Med. 2017;56:301–5. doi: 10.2169/internalmedicine.56.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sperti C, Pasquali C, Berselli M, et al. Metastasis to the pancreas from colorectal cancer: Is there a place for pancreatic resection? Dis Colon Rectum. 2009;52:1154–59. doi: 10.1007/DCR.0b013e31819f7397. [DOI] [PubMed] [Google Scholar]
- 6.Hino H, Kagawa H, Kinugasa Y, et al. Long-term survival with surgery for metachronous retroperitoneal lymph node and pancreatic metastases after curative resection of rectal cancer: a case report. Surg Case Rep. 2016;2:49. doi: 10.1186/s40792-016-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamaguchi T, Takii Y, Maruyama S, et al. [Four cases of resectable metastatic pancreatic cancer from colorectal cancers] The Japanese Journal of Gastroenterological Surgery. 2012;45:740–48. [in Japanese] [Google Scholar]
- 8.Okumura S, Yoshimura T, Fujita K. [A case of metachronous metastasis to the pancreas from rectal cancer] Journal of the Japan Pancreas Society. 2014;29:253–62. [in Japanese] [Google Scholar]
- 9.Sakon M, Hamada J, Sekino Y, et al. [A case of metachronous metastasis of the pancreas from rectal cancer] Journal of the Japan Pancreas Society. 2008;23:748–54. [in Japanese] [Google Scholar]
- 10.Ishigure K, Kawase Y, Kanazumi N, et al. [Report of three resected cases of pancreatic metastatic tumors] The Japanese Journal of Gastroenterological Surgery. 2000;33:1686–90. [in Japanses] [Google Scholar]
- 11.Bachmann J, Michalski CW, Bergmann F, et al. Metastasis of rectal adenocarcinoma to the pancreas. Two case reports and a review of the literature. JOP. 2007;8:214–22. [PubMed] [Google Scholar]
- 12.Tatsuzawa Y, Kurokawa M, Mochiki Y, et al. [A case of pancreatic metastasis from colorectal cancer] The Japanese Journal of Gastroenterological Surgery. 2001;34:132–36. [Google Scholar]
- 13.Lee CW, Wu RC, Hsu JT, et al. Isolated pancreatic metastasis from rectal cancer: A case report and review of literature. World J Surg Oncol. 2010;8:26. doi: 10.1186/1477-7819-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsubara N, Baba H, Okamoto A, et al. Rectal cancer metastasis to the head of the pancreas treated with pancreaticoduodenectomy. J Hepatobiliary Pancreat Surg. 2007;14:590–94. doi: 10.1007/s00534-007-1219-4. [DOI] [PubMed] [Google Scholar]
- 15.You DD, Choi DW, Choi SH, et al. Surgical resection of metastasis to the pancreas. J Korean Surg Soc. 2011;80:278–82. doi: 10.4174/jkss.2011.80.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lintoiu-Ursut B, Tulin A, Constantinoiu S. Recurrence after hepatic resection in colorectal cancer liver metastasis – Review article. J Med Life. 2015;8:12–14. [PMC free article] [PubMed] [Google Scholar]
- 17.Japanese Society for Cancer of the Colon and Rectum . JSCCR guidelines 2019 for the treatment of colorectal cancer. Tokyo: Kanehara; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Japanese Society for Cancer of the Colon and Rectum . Japanese classification of colorectal, appendiceal and anal carcinoma. 9th edition. Tokyo: Kanehara; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Union for International Cancer Control . TNM classification of malignant tumors. 8th edition. New York: Wiley Blackwell; 2017. [Google Scholar]
- 20.Endo Y, Noda H, Watanabe F, et al. A retrospective analysis of preoperative evaluation and surgical resection for metastatic tumors of the pancreas. Indian J Surg Oncol. 2019;10:251–57. doi: 10.1007/s13193-019-00905-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hussain T, Salamat A, Farooq MA, et al. Indications for endoscopic ultrasound and diagnosis on fine-needle aspiration and cytology. J Coll Physicians Surg Pak. 2009;19:223–27. [PubMed] [Google Scholar]
- 22.Aversa S, Bellan C. TTF1 expression in pulmonary metastatic rectal adenocarcinoma. Case Rep Gastrointest Med. 2018;2018:6405125. doi: 10.1155/2018/6405125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reddy S, Wolfgang CL. The role of surgery in the management of isolated metastases to the pancreas. Lancet Oncol. 2009;10:287–93. doi: 10.1016/S1470-2045(09)70065-8. [DOI] [PubMed] [Google Scholar]
- 24.Saito M, Teranishi F, Shibata T, et al. [A case of resected pancreatic metastasis of rectal cancer after multiple resections of metastatic lesions] Gan To Kagaku Ryoho. 2019;46:327–29. [in Japanese] [PubMed] [Google Scholar]
- 25.Hori T, Yamamoto H, Harada H, et al. Inferior pancreaticoduodenal artery aneurysm related with groove pancreatitis persistently repeated hemosuccus pancreaticus even after coil embolization. Am J Case Rep. 2019;20:567–74. doi: 10.12659/AJCR.914832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yabe N, Murai S, Ozawa H, et al. [A case of unresectable advanced rectal cancer with a pancreatic tumor that was successfully treated with FOLFIRINOX] Gan To Kagaku Ryoho. 2016;43:2468–70. [PubMed] [Google Scholar]