Abstract
Background
Tobacco and alcohol are well-established risk factors for numerous cancers, yet their relationship to biliary tract cancers remains unclear.
Methods
We pooled data from 26 prospective studies to evaluate associations of cigarette smoking and alcohol consumption with biliary tract cancer risk. Study-specific hazard ratios (HRs) and 95% confidence intervals (CIs) for associations with smoking and alcohol consumption were calculated. Random-effects meta-analysis produced summary estimates. All statistical tests were two-sided.
Results
Over a period of 38 369 156 person-years of follow-up, 1391 gallbladder, 758 intrahepatic bile duct, 1208 extrahepatic bile duct, and 623 ampulla of Vater cancer cases were identified. Ever, former, and current smoking were associated with increased extrahepatic bile duct and ampulla of Vater cancers risk (eg, current vs never smokers HR = 1.69, 95% CI = 1.34 to 2.13 and 2.22, 95% CI = 1.69 to 2.92, respectively), with dose-response effects for smoking pack-years, duration, and intensity (all Ptrend < .01). Current smoking and smoking intensity were also associated with intrahepatic bile duct cancer (eg, >40 cigarettes per day vs never smokers HR = 2.15, 95 % CI = 1.15 to 4.00; Ptrend = .001). No convincing association was observed between smoking and gallbladder cancer. Alcohol consumption was only associated with intrahepatic bile duct cancer, with increased risk for individuals consuming five or more vs zero drinks per day (HR = 2.35, 95%CI = 1.46 to 3.78; Ptrend = .04). There was evidence of statistical heterogeneity among several cancer sites, particularly between gallbladder cancer and the other biliary tract cancers.
Conclusions
Smoking appears to increase the risk of developing all biliary tract cancers except gallbladder cancer. Alcohol may increase the risk of intrahepatic bile duct cancer. Findings highlight etiologic heterogeneity across the biliary tract.
Biliary tract cancers, which include cancers of the gallbladder, intrahepatic bile duct, extrahepatic bile duct, and ampulla of Vater, are relatively rare but highly fatal malignancies (1–3). Although incidence rates of biliary tract cancers are low globally, they are substantially higher in certain geographic regions and among some ethnic and racial subgroups (1,2,4). Increasing incidence rates of intrahepatic and extrahepatic bile duct cancers have also been reported in the United States (1,5). Yet, because of the rarity of biliary tract cancers and a paucity of published data, the etiology of these malignancies remains poorly understood.
Tobacco and alcohol are established risk factors for several cancers (6) and have been classified as group 1 carcinogens by the International Agency for Research on Cancer (7). Tobacco smoking is the leading cause of cancer worldwide. Alcohol consumption accounts for a smaller proportion of new cancer cases; however, the global prevalence of alcohol use is high, with approximately 38% of the world’s population age 15 years or older regularly consuming alcohol (7,8).
Despite a large body of tobacco- and alcohol-related research, the relationship of these exposures to biliary tract cancers remains unclear. Prior studies investigating associations of tobacco smoking and alcohol consumption with biliary tract cancer risk have yielded inconsistent or inconclusive results (6,9–22). Most prior studies have been relatively small, retrospective case-control studies, limiting their ability to detect modest associations and to perform analyses individually by anatomic site. Prior meta-analyses relied primarily on case-control data and almost exclusively evaluated ever vs never smoking and alcohol consumption (23–28). With the exception of one pooled analysis of intrahepatic bile duct cancer (29), previous meta-analyses have relied on published literature and have often been limited by publication biases and an inability to control for important confounders (23–28).
To address these limitations, we pooled individual-level data from 26 prospective cohort studies and trials to evaluate associations of cigarette smoking and alcohol consumption with primary biliary tract cancer risk. We evaluated all associations separately by anatomic cancer.
Methods
Study Population
We used data from the Biliary Tract Cancers Pooling Project (BiTCaPP), which includes prospective information from more than 2.8 million participants. Studies were identified for potential inclusion via the National Cancer Institute Cancer Cohort Consortium and individual research collaborations. Eligible studies were prospective studies with at least one biliary tract cancer case. Deidentified individual-level datasets were requested electronically and successfully received from all studies. Study-specific datasets were then harmonized (ie, formatted uniformly across studies to allow for inferential equivalence) (30) and compiled in a pooled dataset. All data used in this analysis were checked for consistency and completeness using logical queries and comparisons with published data. No major data quality issues were identified.
Each study received ethical approval from its respective institutional review board and all study participants provided written informed consent. BiTCaPP was exempted from additional ethical review by the National Cancer Institute’s Office of Human Subjects Research.
We included in this analysis 26 BiTCaPP studies with information both on cigarette smoking and alcohol consumption. Details on the included studies are described in Table 1. Briefly, this sample included 21 prospective cohort studies (31–51), four randomized control trials (52–55), and one cancer screening trial (56). Studies were conducted in North America (n = 16) (31–33,35,37,38,41–44,48,49,53–56), Europe (n = 5) (34,36,50–52), Asia (n = 4) (39,45–47), and Australia (n = 1) (40).
Table 1.
Summary of studies included in the smoking and alcohol analyses of the Biliary Tract Cancers Pooling Project
| Study (abbreviation) | Period | No. in baseline sample* | Age at baseline, median (IQR), y | Female sex, † % | Current smokers, ‡ % | Alcoholic drinks per day, § median (IQR) | Gallstones, ‖ % | Cholecystectomy, ¶ % | No. of biliary tract cancer cases |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GBC | IHBDC | EHBDC | AVC | |||||||||
| Agricultural Health Study (AgHealth) (31) | 1993–2013 | 89 009 | 45.0 (37.0–56.0) | 37.7 | 14.5 | 0.1 (0.0–0.6) | — | — | 23 | 19 | 18 | 11 |
| Adventist Health Study-2 (AHS-2) (32) | 2002–2015 | 95 942 | 57.0 (47.0–69.0) | 64.9 | 1.1 | 0.4 (0.1–0.7) | 3.3 | — | 16 | 9 | 11 | 7 |
| Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (ATBC) (52) | 1985–2010 | 29 127 | 57.0 (53.0–61.0) | 0 | 100 | 1.0 (0.4–2.0) | 5.6 | 4.9 | 17 | 38 | 42 | 16 |
| Breast Cancer Detection Demonstration Project (BCDDP) (33) | 1980–1998 | 47 224 | 61.5 (56.0–67.7) | 100 | 12.5 | 0.2 (0.1–0.7) | — | — | 12 | 6 | 7 | 8 |
| Cohort of Swedish Men (COSM) (34) | 1998–2008 | 45 801 | 59.5 (52.5–68.5) | 0 | 25.0 | 0.7 (0.3–1.4) | 11.7 | — | 13 | 6 | 15 | 3 |
| Cancer Prevention Study II Nutrition Cohort (CPS-II NC) (35) | 1992–2011 | 155 077 | 63.0 (58.0–68.0) | 52.6 | 8.9 | 0.6 (0.2–1.3) | 11.6 | 12.7 | 70 | 54 | 57 | 36 |
| European Prospective Investigation into Cancer and Nutrition (EPIC) (36) | 1992–2010 | 490 466 | 51.5 (45.1–58.3) | 70.0 | 22.9 | 0.5 (0.1–1.2) | 6.3 | — | 134 | 119 | 111 | 86 |
| Health Professionals Follow-Up Study (HPFS) (37) | 1986–2012 | 51 375 | 54.0 (45.0–62.0) | 0 | 10.0 | 0.7 (0.3–1.4) | — | 3.2 | 11 | 8 | 23 | 10 |
| Iowa Women’s Health Study (IWHS) (38) | 1986–2013 | 37 977 | 61.0 (58.0–65.0) | 100 | 14.8 | 0.3 (0.1–0.8) | — | — | 69 | 14 | 30 | 12 |
| Japan Public Health Center-based prospective Study 1 and 2 (JPHC) (39) | 1990–2011 | 99 595 | 52.9 (46.2–58.9) | 52.4 | 28.1 | 1.8 (0.8–3.4) | 2.9 | — | 172 | 120 | 199 | 38 |
| Melbourne Collaborative Cohort Study (MCCS) (40) | 1990–2009 | 39 992 | 55.5 (47.6–62.8) | 58.8 | 11.4 | 0.8 (0.3–1.6) | 9.1 | 7.6 | 35 | 20 | 22 | 6 |
| Multiethnic Cohort Study (MEC) (41) | 1993–2010 | 187 414 | 60.0 (52.0–67.0) | 54.5 | 16.5 | 0.5 (0.1–1.6) | 6.7 | 6.4 | 110 | 60 | 117 | 64 |
| Nurses’ Health Study (NHS) (42) | 1980–2010 | 100 667 | 47.0 (41.0–53.0) | 100 | 29.0 | 0.2 (0.1–0.8) | 1.6 | 7.7 | 54 | 17 | 34 | 18 |
| National Institutes of Health-American Association of Retired Persons Diet and Health Study (NIH-AARP) (43) | 1995–2011 | 556 036 | 63.0 (58.0–67.0) | 40.2 | 12.5 | 0.3 (0.1–1.2) | 9.6 | 14.2 | 218 | 134 | 242 | 151 |
| New York University Women’s Health Study (NYUWHS) (44) | 1985–2007 | 13 350 | 51.0 (43.0–58.0) | 100 | 18.1 | 0.6 (0.3–1.2) | 5.0 | — | 7 | 6 | 9 | 0 |
| Physicians’ Health Study (PHS) (53) | 1982–2009 | 28 426 | 54.3 (47.6–61.4) | 0 | 9.3 | 0.4 (0.1–1.0) | 3.7 | — | 7 | 9 | 9 | 7 |
| Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO) (56) | 1993–2009 | 116 583 | 65.7 (61.0–70.0) | 50.8 | 9.5 | 0.3 (0.1–1.1) | 11.6 | — | 34 | 13 | 31 | 24 |
| Radiation Effects Research Foundation Life Span Study (RERF) (45) | 1950–2005 | 51 302 | 51.4 (41.5–62.0) | 61.4 | 31.1 | 0.1 (0.0–0.1) | — | — | 148 | — | 120 | 25 |
| Singapore Chinese Health Study (SCHS) (46) | 1993–2008 | 61 320 | 55.0 (49.0–62.0) | 55.5 | 19.7 | 0.5 (0.5–0.5) | — | — | 29 | 26 | 14 | 22 |
| Shanghai Cohort Study (SCS) (47) | 1986–2012 | 18 075 | 56.0 (52.0–60.0) | 0 | 50.5 | 1.5 (0.5–1.5) | — | — | 15 | 13 | 21 | 15 |
| Sister Study (SISTER) (48) | 2003–2012 | 47 798 | 55.4 (48.9–62.0) | 100 | 8.1 | 0.2 (0.0–0.7) | 14.5 | 12.8 | 4 | 4 | 0 | 4 |
| Swedish Mammography Cohort (SMC) (51) | 1998–2008 | 37 151 | 60.5 (54.5–69.5) | 100 | 23.2 | 0.3 (0.1–0.6) | 19.9 | — | 42 | 3 | 6 | 1 |
| VITamins and Lifestyle Study (VITAL) (49) | 2000–2009 | 77 011 | 61.0 (55.0–68.0) | 51.9 | 8.4 | 0.5 (0.1–1.2) | — | — | 16 | 15 | 15 | 5 |
| Women’s Health Initiative (WHI) (54) | 1993–2014 | 161 081 | 63.0 (57.0–69.0) | 100 | 7.0 | 0.3 (0.1–0.8) | 16.3 | 12.9 | 113 | 32 | 50 | 42 |
| Women’s Health Study (WHS) (55) | 1992–2010 | 39 788 | 53.0 (49.0–59.0) | 100 | 13.1 | 0.3 (0.1–0.9) | 9.9 | — | 10 | 7 | 1 | 11 |
| Women’s Lifestyle and Health Study (WLHS) (50) | 1991–2011 | 47 392 | 40.0 (35.0–45.0) | 100 | 21.3 | 0.2 (0.1–0.4) | — | — | 12 | 6 | 4 | 1 |
| Total | 2 724 982 | 58.0 (51.0–65.0) | 59.5 | 16.9 | 0.4 (0.1–1.1) | 8.9 | 10.5 | 1391 | 758 | 1208 | 623 | |
No. in baseline sample: Baseline sample size is calculated after excluding individuals who were younger than 18 years, had missing entry or exit age, had prior cancer diagnoses other than nonmelanoma skin cancer at study entry, had biliary tract cancers of undefined and/or unknown site or overlapping lesions, and/or had missing biliary tract cancer status. —indicates the study did not report this variable; AVC = ampulla of Vater cancer; EHBDC = extrahepatic bile duct cancer; GBC = gallbladder cancer; IHBDC = intrahepatic bile duct cancer; IQR = interquartile range.
Variables are missing for 38 participants (0.001%).
Variables are missing for 54 426 participants (2.0%).
Variables are missing for 118 605 participants (4.4%).
Variables are missing for 162 690 participants (7.2%).
Variables are missing for 327 588 participants (24.7%).
We excluded individuals younger than 18 years (n = 130) and/or for whom entry or exit age was missing (n = 5044). We also excluded participants with prior cancer diagnoses other than nonmelanoma skin cancer at study entry (n = 61 232), biliary tract cancers of undefined and/or unknown site or overlapping lesions (n = 329), and participants with missing biliary tract cancer status (n = 11). The final analytic sample thus included 2 724 982 individuals.
Ascertainment of Outcomes
Outcomes of interest for this study were primary biliary tract cancers occurring as incident, first cancers. Each study provided data on primary biliary tract cancers by anatomic site using International Classification of Disease codes (Supplementary Table 1, available online). Cancer diagnoses were ascertained via linkage to a local, state, provincial, or national cancer registry (Agricultural Health Study [AgHealth]; Adventist Health Study-2 [AHS-2]; Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study [ATBC]; Cohort of Swedish Men [COSM]; Iowa Women’s Health Study [IWHS]; Melbourne Collaborative Cohort Study [MCCS]; Multiethnic Cohort Study [MEC]; National Institutes of Health-American Association of Retired Persons Diet and Health Study [NIH-AARP]; Radiation Effects Research Foundation Life Span Study [RERF]; Singapore Chinese Health Study [SCHS]; Swedish Mammography Cohort [SMC]; VITamins and Lifestyle Study [VITAL]; Women’s Lifestyle and Health Study [WLHS]); self-report verified by medical record, pathology report, cancer registry linkage, or death certificate (Health Professionals Follow-Up Study [HPFS]; Nurses’ Health Study [NHS]; Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial [PLCO]; Physicians’ Health Study [PHS]; Sister Study [SISTER]; Women’s Health Initiative [WHI]; Women's Health Study [WHS]); or a combination of methods (Breast Cancer Detection Demonstration Project [BCDDP]; Cancer Prevention Study II Nutrition Cohort [CPS-II NC]; European Prospective Investigation into Cancer and Nutrition [EPIC]; Japan Public Health Center-based prospective Study 1 and 2 [JPHC]; New York University Women’s Health Study [NYUWHS]; Shanghai Cohort Study [SCS]).
Ascertainment of Exposures
All studies provided self-reported data on demographic and epidemiologic characteristics, including data on smoking and alcohol, which was obtained via questionnaires and/or in-person interviews. We assessed self-reported ever vs never smoking, smoking status (never, former, current), duration of smoking (years), smoking intensity (cigarettes per day), and smoking pack-years. Detailed information on the ascertainment of smoking information for each study is provided in Supplementary Table 2 (available online). Briefly, all 26 studies provided information on ever smoking and smoking status; however, one study (ATBC) included only current smokers by design and thus was excluded from the smoking analyses. Most studies defined ever smoking as “regular smoking” (n = 7) or having smoked at least 100 cigarettes (n = 7). Current smoking was usually defined as any cigarette smoking reported at the time of data collection (n = 23). A total of 25 and 21 studies provided information on cigarette smoking duration and intensity, respectively. For 15 studies, smoking duration was calculated by subtracting the age at initiation of smoking from either the age at which the subject quit (former smokers) or the age at baseline (current smokers). The remaining 10 studies collected self-reported total years smoked at baseline. Nine studies reported smoking duration excluding intermittent periods of cessation. For smoking intensity, studies provided either the average number of cigarettes smoked per day over the lifetime smoking period (n = 12) or the average smoked per day at the time of data collection or last smoking period (n = 9). We calculated smoking pack-years (cigarettes per day divided by 20, multiplied by duration in years) for 21 studies.
For all 26 studies, we assessed self-reported number of alcoholic drinks consumed per day (Supplementary Table 3, available online). Studies provided either grams of alcohol (n = 22) or alcoholic drinks (n = 4) consumed per day. For the purposes of these analyses, one alcoholic drink was defined as 14 g of ethanol (57). Most studies (n = 17) reported average alcohol consumption over the past 12 months.
Ever smoking and smoking status were analyzed categorically, with never smokers as the reference group. Smoking and alcohol dose variables were analyzed 1) categorically using a priori cut points (smoking pack-years: never smokers [reference], >0–20, >20–40, >40; smoking duration [years] and intensity [cigarettes per day]: never smokers [referent], >0–10, >10–20, >20–40, >40; alcoholic drinks per day: 0 [referent], >0–0.5, >0.5–1, 1–<3, 3–<5, ≥5) and 2) continuously (analyzed per 10-unit increase [smoking variables] or per one drink [alcohol]).
Statistical Analysis
We assessed associations of cigarette smoking and alcohol consumption with biliary tract cancer risk using Cox proportional hazards regression with age as the timescale and left truncation at baseline. Visual and statistical examination of the scaled Schoenfeld residuals for the main exposures (smoking status and alcoholic drinks per day [categorical]) in the pooled dataset showed no evidence of proportional hazards assumption violation (58). All global P values for potential violations of the proportional hazards assumption for the multivariable models as a whole were greater than .10. Participant follow-up time began at baseline and ended at the time of first primary cancer diagnosis (other than nonmelanoma skin cancer), loss to follow-up, death, or study-specific end date, whichever occurred first.
We performed our primary analyses using a two-stage approach. In the first stage, we calculated site- and study-specific hazard ratios (HRs) and 95% confidence intervals (CIs) adjusted for a priori confounders identified based on directed acyclic graphs: sex (male, female), race (white, black, Asian and Pacific Islander, other), educational attainment (<high school graduate, high school graduate, some college or post-high school training), body mass index in kg/m2 (<18.5, 18.5–<25, 25–<30, ≥30), history of diabetes (ever vs never diagnosed), and birth cohort (1870–1899, 1900–1909, 1910–1919, 1920–1929, 1930–1939, 1940–1949, 1950–1959, 1960–1982). Smoking models were additionally adjusted for alcoholic drinks per day (0, >0–0.5, >0.5–1, 1–<3, ≥3), and alcohol models were additionally adjusted for smoking status (never, former, current). Results were not materially altered after excluding body mass index and diabetes, which could be potential confounders or causal mediators depending on temporal relationships (data not shown). In the second stage, we performed a random-effects meta-analysis of the study-specific estimates and assessed statistical heterogeneity between studies using the I2 statistic (59). We also repeated these analyses using a one-stage approach, calculating hazard ratios in the aggregate, pooled dataset and stratifying the baseline hazard by study. We evaluated heterogeneity by biliary tract cancer site in the pooled dataset using a data duplication method for competing risks, testing for heterogeneity via the Wald test (60). Heterogeneity analyses were conducted for the associations with smoking status and alcoholic drinks per day (categorical).
We performed several prespecified sensitivity analyses in the pooled dataset. We analyzed smoking and alcohol dose-response variables restricting to ever smokers and individuals who consumed more than 0 alcoholic drinks per day, with the lowest exposure group as the reference group. For smoking intensity and duration, we also conducted analyses adjusting for smoking pack-years (categorical) to assess if associations with intensity or duration persisted after accounting for the effect of total exposure (61). We evaluated potential multiplicative effect-measure modification by sex and race for the associations with smoking status and alcoholic drinks consumed per day (categorical). Effect-measure modification was assessed using likelihood ratio tests comparing models with and without a multiplicative term (62). In a subset of 17 studies with information on prior gallstone diagnoses (Table 1), we also compared risk estimates with and without adjustment for history of gallstones to explore whether the associations with smoking and alcohol might be mediated by gallstones, a key risk factor for biliary tract cancers (63). Finally, for all analyses of gallbladder cancer, we compared overall risk estimates with estimates that were generated after restricting to participants who had no history of cholecystectomy (ie, only individuals who were at risk for gallbladder cancer at baseline). This analysis was restricted to nine studies that provided information on cholecystectomies (Table 1).
Analyses were performed in SAS software version 9.4 (SAS Institute Inc, Cary, NC), Stata software version 15.0 (StataCorp LLC, College Station, TX), and R software version 3.5.1 (R Development Core Team, Vienna, Austria). Participants with missing data were case-deleted. All statistical tests were two-sided and P values less than .05 were considered statistically significant.
Results
Of the 2 724 982 people in the BiTCaPP sample, 1391 (0.05%) developed gallbladder cancer, 758 (0.03%) developed intrahepatic bile duct cancer, 1208 (0.04%) developed extrahepatic bile duct cancer, and 623 (0.02%) developed ampulla of Vater cancer over a total of 38 369 156 person-years of follow-up. The median time to diagnosis of any biliary tract cancer was 10.6 years (interquartile range [IQR] = 5.6–14.5 years), and the median age at biliary tract cancer diagnosis was 71.8 years (IQR = 65.0–78.5 years), with no substantial variations by anatomic site.
Characteristics of the participants and studies are shown in Table 1. The percentage of current smokers ranged from 1.1% in AHS-2 to 50.5% in SCS (in ATBC, 100% of participants were current smokers by design), whereas median alcoholic drinks per day ranged from 0.1 in AgHealth and the RERF to 1.8 in the JPHC.
Summary risk estimates for the associations of cigarette smoking and alcohol consumption with biliary tract cancer risk are shown in Tables 2 and 3. There was low to moderate between-study heterogeneity for most risk estimates, with 66% of I2 lower than 10%, and 91% lower than 40%.
Table 2.
Adjusted hazard ratios and 95% confidence intervals for associations of cigarette smoking with biliary tract cancer risk by anatomic site in the Biliary Tract Cancers Pooling Project–summary risk estimates from the random-effects meta-analysis*
| Cigarette smoking | No. of noncases†,‡ | Gallbladder cancer |
Intrahepatic bile duct cancer |
Extrahepatic bile duct cancer |
Ampulla of Vater cancer |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of cases‡ | HR (95% CI) | I2, % | No. of cases‡ | HR (95% CI) | I2, % | No. of cases‡ | HR (95% CI) | I2, % | No. of cases‡ | HR (95% CI) | I2, % | ||
| Ever smoking | |||||||||||||
| No | 1 084 270 | 596 | (Referent) | — | 263 | (Referent) | — | 348 | (Referent) | — | 189 | (Referent) | — |
| Yes | 1 191 039 | 493 | 1.02 (0.89 to 1.17) | 0.0 | 315 | 1.11 (0.93 to 1.33) | 0.0 | 541 | 1.38 (1.17 to 1.62) | 6.1 | 317 | 1.52 (1.24 to 1.86) | 0.0 |
| Smoking status | |||||||||||||
| Never smoker | 1 084 270 | 596 | (Referent) | — | 263 | (Referent) | — | 348 | (Referent) | — | 189 | (Referent) | — |
| Former smoker | 827 061 | 332 | 0.98 (0.84 to 1.14) | 0.0 | 211 | 1.06 (0.87 to 1.30) | 0.0 | 351 | 1.29 (1.09 to 1.53) | 0.0 | 209 | 1.38 (1.11 to 1.72) | 0.0 |
| Current smoker | 362 313 | 161 | 1.18 (0.98 to 1.43) | 0.0 | 104 | 1.30 (1.00 to 1.69) | 0.0 | 190 | 1.69 (1.34 to 2.13) | 15.1 | 108 | 2.22 (1.69 to 2.92) | 0.0 |
| Smoking pack-years | |||||||||||||
| Never smoker | 984 603 | 545 | (Referent) | — | 246 | (Referent) | — | 333 | (Referent) | — | 174 | (Referent) | — |
| >0–20 | 385 482 | 165 | 0.95 (0.79 to 1.14) | 0.0 | 78 | 0.89 (0.67 to 1.19) | 0.0 | 146 | 1.16 (0.93 to 1.45) | 4.0 | 94 | 1.41 (1.07 to 1.85) | 0.0 |
| >20–40 | 223 798 | 112 | 1.19 (0.93 to 1.51) | 10.6 | 76 | 1.35 (0.97 to 1.89) | 16.2 | 126 | 1.53 (1.12 to 2.10) | 38.4 | 72 | 1.84 (1.27 to 2.66) | 22.0 |
| >40 | 148 402 | 68 | 1.16 (0.88 to 1.52) | 0.0 | 48 | 1.29 (0.92 to 1.81) | 0.0 | 93 | 1.76 (1.24 to 2.51) | 37.7 | 46 | 1.79 (1.11 to 2.90) | 30.8 |
| P trend § | .39 | .08 | <.001 | .005 | |||||||||
| Continuous per 10 pack-years | — | — | 1.03 (0.99 to 1.07) | 0.0 | — | 1.06 (1.02 to 1.11) | 3.1 | — | 1.10 (1.05 to 1.16) | 39.5 | — | 1.09 (1.02 to 1.16) | 36.6 |
| Smoking duration (years) | |||||||||||||
| Never smoker | 1 070 496 | 594 | (Referent) | — | 258 | (Referent) | — | 345 | (Referent) | — | 187 | (Referent) | — |
| >0–10 | 164 873 | 56 | 1.07 (0.80 to 1.43) | 0.0 | 41 | 1.46 (1.02 to 2.09) | 0.0 | 51 | 1.46 (1.07 to 1.99) | 0.0 | 30 | 1.53 (1.01 to 2.31) | 0.0 |
| >10–20 | 194 455 | 57 | 0.93 (0.67 to 1.30) | 15.2 | 39 | 1.23 (0.85 to 1.79) | 0.0 | 56 | 1.20 (0.88 to 1.64) | 0.0 | 37 | 1.81 (1.18 to 2.76) | 6.5 |
| >20–40 | 501 925 | 227 | 1.11 (0.94 to 1.32) | 0.0 | 128 | 1.06 (0.82 to 1.36) | 5.9 | 225 | 1.29 (1.03 to 1.62) | 20.5 | 126 | 1.57 (1.15 to 2.14) | 20.3 |
| >40 | 127 3389 | 64 | 1.06 (0.72 to 1.55) | 44.1 | 41 | 1.29 (0.89 to 1.87) | 3.2 | 93 | 1.96 (1.47 to 2.63) | 13.5 | 55 | 2.18 (1.56 to 3.06) | 0.0 |
| P trend § | .86 | .57 | .008 | <.001 | |||||||||
| Continuous per 10 years | — | — | 1.00 (0.96 to 1.05) | 7.1 | — | 1.02 (0.94 to 1.10) | 25.9 | — | 1.10 (1.02 to 1.19) | 47.8 | — | 1.14 (1.07 to 1.21) | 0.0 |
| Smoking intensity (cigarettes/day) | |||||||||||||
| Never smoker | 984 603 | 545 | (Referent) | — | 246 | (Referent) | — | 333 | (Referent) | — | 174 | (Referent) | — |
| >0–10 | 316 037 | 166 | 1.06 (0.88 to 1.28) | 0.0 | 60 | 0.86 (0.61 to 1.22) | 13.1 | 128 | 1.20 (0.97 to 1.49) | 0.0 | 87 | 1.49 (1.14 to 1.96) | 0.0 |
| >10–20 | 336 669 | 151 | 1.13 (0.93 to 1.37) | 0.0 | 108 | 1.33 (1.03 to 1.72) | 0.0 | 185 | 1.62 (1.21 to 2.17) | 41.6 | 97 | 1.87 (1.20 to 2.91) | 50.8 |
| >20–40 | 230 576 | 91 | 1.13 (0.88 to 1.45) | 0.0 | 77 | 1.46 (1.09 to 1.95) | 0.0 | 135 | 1.74 (1.39 to 2.19) | 0.0 | 78 | 1.76 (1.29 to 2.40) | 0.0 |
| >40 | 46 656 | 17 | 1.75 (1.00 to 3.06) | 8.0 | 19 | 2.15 (1.15 to 4.00) | 17.7 | 24 | 3.00 (1.17 to 7.73) | 60.4 | 13 | 2.02 (0.92 to 4.43) | 17.9 |
| P trend § | .34 | .001 | <.001 | .001 | |||||||||
| Continuous per 10 cigarettes | — | — | 1.04 (0.98 to 1.10) | 0.0 | — | 1.12 (1.05 to 1.20) | 0.0 | — | 1.17 (1.10 to 1.24) | 11.1 | — | 1.13 (1.05 to 1.21) | 0.0 |
Adjusted for sex (male, female), race (white, black, Asian and Pacific Islander, other), education (less than high school graduate, high school graduate, some college or post-high school training), body mass index in kg/m2 (<18.5, 18.5–<25, 25–<30, ≥30), history of diabetes (ever vs never diagnosed), birth cohort (1870–1899, 1900–1909, 1910–1919, 1920–1929, 1930–1939, 1940–1949, 1950–1959, 1960–1982), and alcoholic drinks per day (0, >0–0.5, >0.5–1, 1–<3, ≥3). CI = confidence interval; HR= hazard ratio.
No. of noncases: The same noncase group was used for all analyses, except for analyses of intrahepatic bile duct cancer. The Radiation Effects Research Foundation Life Span Study (RERF) did not provide information on intrahepatic bile duct cancer diagnoses, so this study was excluded from these analyses and the maximum noncase group was thus n = 2 267 501.
No. of noncases and No. of cases: Some counts do not add to totals because of missing data. Numbers represent the maximum possible number of participants included in each analysis.
P trend: Two-sided P value for the ordinal variable, calculated using the Wald test.
Table 3.
Adjusted hazard ratios and 95% confidence intervals for associations of alcohol consumption with biliary tract cancer risk by anatomic site in the Biliary Tract Cancers Pooling Project–summary risk estimates from the random-effects meta-analysis*
| Alcohol consumption | No. of noncases†,‡ | Gallbladder cancer |
Intrahepatic bile duct cancer |
Extrahepatic bile duct cancer |
Ampulla of Vater cancer |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of cases‡ | HR (95% CI) | I2, % | No. of cases‡ | HR (95% CI) | I2, % | No. of cases‡ | HR (95% CI) | I2, % | No. of cases‡ | HR (95% CI) | I2, % | ||
| Alcoholic drinks/day | |||||||||||||
| 0 | 716 188 | 456 | (Referent) | 230 | (Referent) | 326 | (Referent) | 161 | (Referent) | ||||
| >0–0.5 | 865 648 | 408 | 1.07 (0.91 to 1.26) | 0.0 | 173 | 0.79 (0.62 to 1.00) | 0.0 | 292 | 0.87 (0.68 to 1.12) | 30.8 | 181 | 1.08 (0.80 to 1.45) | 13.7 |
| >0.5–<1 | 269 152 | 99 | 1.10 (0.87 to 1.39) | 0.0 | 59 | 0.91 (0.65 to 1.26) | 0.0 | 103 | 1.14 (0.82 to 1.58) | 28.4 | 52 | 0.99 (0.69 to 1.41) | 0.0 |
| 1–<3 | 338 965 | 112 | 0.94 (0.74 to 1.21) | 0.0 | 95 | 0.98 (0.73 to 1.31) | 0.0 | 140 | 1.08 (0.74 to 1.58) | 48.3 | 95 | 1.33 (0.99 to 1.80) | 0.0 |
| 3–<5 | 68 509 | 20 | 1.16 (0.69 to 1.94) | 0.0 | 32 | 1.25 (0.77 to 2.02) | 8.5 | 45 | 1.82 (0.98 to 3.39) | 57.2 | 18 | 1.16 (0.66 to 2.01) | 0.0 |
| ≥5 | 42 166 | 9 | 2.39 (0.63 to 9.12) | 64.9 | 24 | 2.35 (1.46 to 3.78) | 0.0 | 22 | 1.02 (0.64 to 1.62) | 0.0 | 14 | 1.59 (0.85 to 2.98) | 0.0 |
| P trend § | .31 | .04 | .84 | .35 | |||||||||
| Continuous per 1 drink | — | — | 0.98 (0.92 to 1.05) | 12.0 | — | 1.03 (1.01 to 1.06) | 0.0 | — | 1.03 (0.98 to 1.08) | 25.3 | — | 1.00 (0.95 to 1.04) | 0.0 |
Adjusted for sex (male, female), race (white, black, Asian and Pacific Islander, other), education (less than high school graduate, high school graduate, some college or post-high school training), body mass index in kg per m2 (<18.5, 18.5–<25, 25–<30, ≥30), history of diabetes (ever vs never diagnosed), birth cohort (1870–1899, 1900–1909, 1910–1919, 1920–1929, 1930–1939, 1940–1949, 1950–1959, 1960–1982), and smoking status (never, former, current). CI = confidence interval; HR = hazard ratio.
No. of noncases: The same noncase group was used for all analyses, except for analyses of intrahepatic bile duct cancer. The Radiation Effects Research Foundation Life Span Study (RERF) did not provide information on intrahepatic bile duct cancer diagnoses, so this study was excluded from these analyses and the maximum noncase group was thus n = 2 267 501.
No. of noncases and No. of cases: Some counts do not add to totals because of missing data. Numbers represent the maximum possible number of participants included in each analysis.
P trend: Two-sided P value for the ordinal variable, calculated using the Wald test.
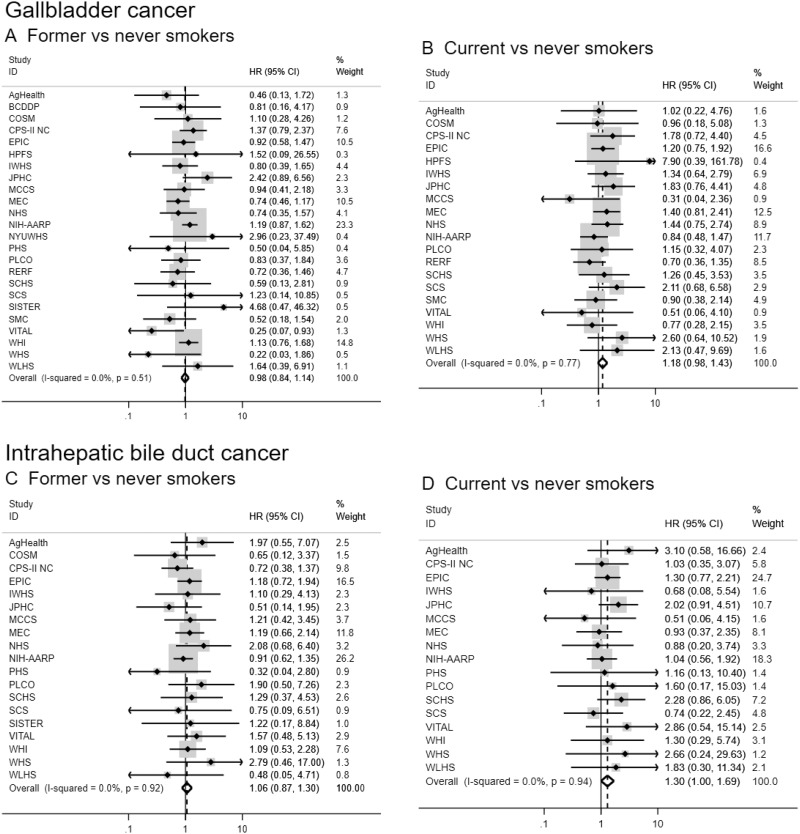
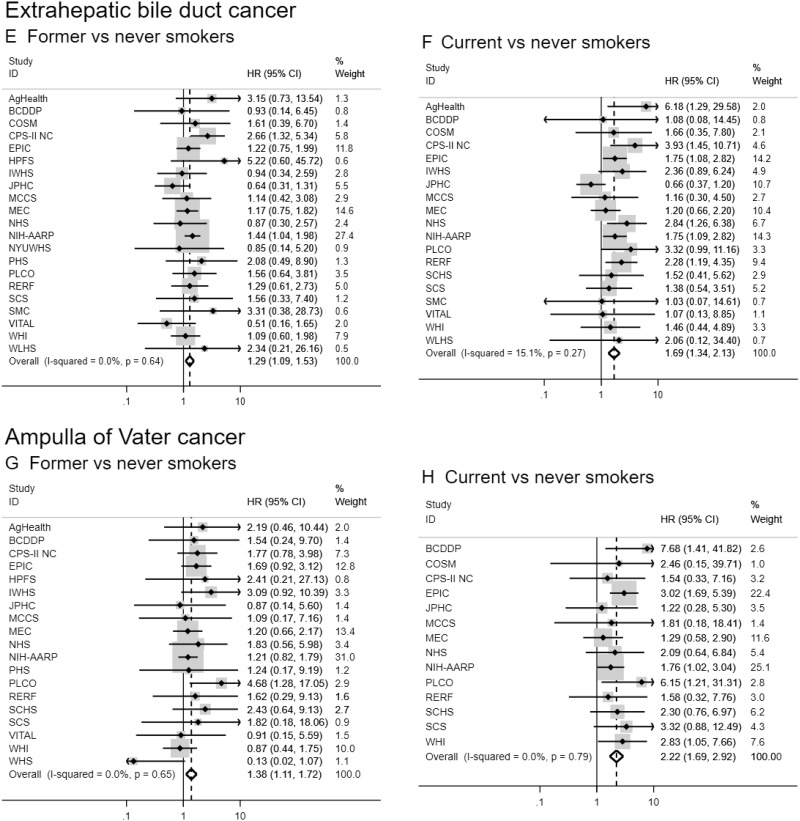
Ever, former, and current smokers were at increased risk of extrahepatic bile duct and ampulla of Vater cancers when compared never smokers (HR for current vs never smokers = 1.69, 95% CI = 1.34 to 2.13 and 2.22, 95% CI = 1.69 to 2.92, respectively; Table 2 and Figure 1). Current smokers were also at increased risk of intrahepatic bile duct cancer (HR = 1.30 , 95% CI = 1.00 to 1.69). Increasing levels of smoking pack-years, duration, and intensity were associated with extrahepatic bile duct and ampulla of Vater cancers (eg, HR = 1.96, 95% CI = 1.47 to 2.63, and HR = 2.18, 95% CI = 1.56 to 3.06, for smoking duration >40 years vs never smokers, respectively). In contrast, only smoking intensity was consistently associated with intrahepatic bile duct cancer risk (eg, HR = 2.15, 95% CI = 1.15 to 4.00, for >40 cigarettes per day vs never smokers). We observed no convincing evidence of an association between cigarette smoking and gallbladder cancer risk. Although a few estimates were suggestive of an increased risk (eg, HR = 1.75, 95% CI = 1.00 to 3.06, for >40 cigarettes per day vs never smokers), there was no consistent pattern or evidence of a dose-response relationship, and some estimates were based on a small number of cases (eg, <20).
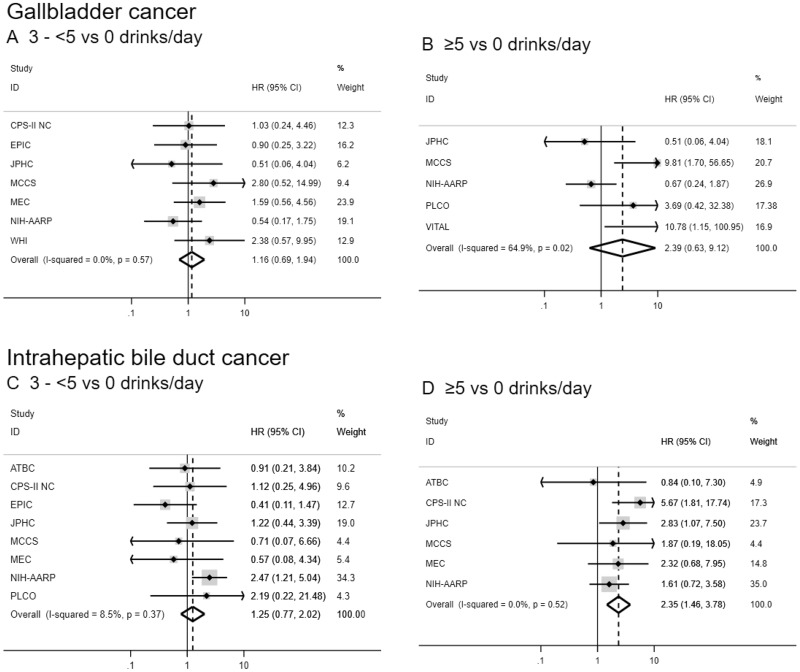
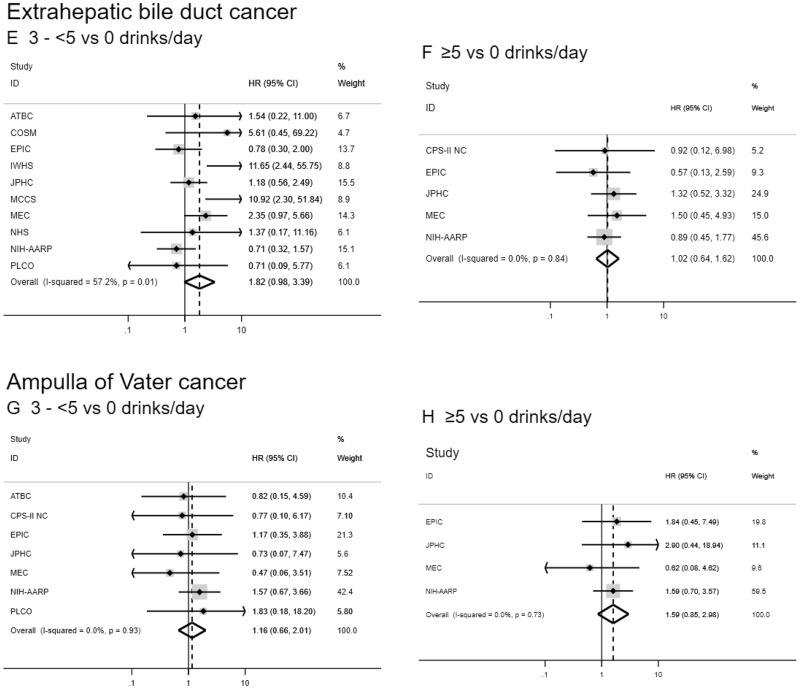
Figure 2.
Forest plots for associations between alcohol consumption (drinks per day) and biliary tract cancer risk by anatomic site in the Biliary Tract Cancers Pooling Project. Hazard ratios for three to fewer than five vs zero drinks per day (A, C, E, G) and five or more vs zero drinks per day (B, D, F, H) are adjusted for sex (male, female), race (white, black, Asian and Pacific Islander, other), education (less than high school graduate, high school graduate, some college or post-high school training), body mass index in kg per m2 (<18.5, 18.5–<25, 25–<30, ≥30), diabetes (ever vs never diagnosed), birth cohort (1870–1899, 1900–1909, 1910–1919, 1920–1929, 1930–1939, 1940–1949, 1950–1959, 1960–1982), and cigarette smoking status (never, former, current). Small black-filled diamonds represent the point estimates for each study. Horizontal lines represent 95% confidence intervals; if ending in an arrow, the interval transcends the region plotted. % weight describes the weight (inverse variance) each study contributed to the summary hazard ratio. Study weight is also represented by the shaded gray region around each study-specific point estimate. I2 is the percentage of variation due to between-study heterogeneity. Summary hazard ratios (dotted lines) and 95% confidence intervals (hollow diamonds) were estimated via random-effects meta-analysis. All statistical tests were two-sided. P values were calculated using the Wald test. Some additional studies collected information on alcoholic drinks per day but could not contribute to this meta-analysis because they did not have a sufficient number of biliary tract cancer patients consuming three to fewer than five or five or more drinks per day. ATBC = Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; CI = confidence interval; COSM = Cohort of Swedish Men; CPS-II NC = Cancer Prevention Study II Nutrition Cohort; EPIC = European Prospective Investigation into Cancer and Nutrition; HR = hazard ratio; IWHS = Iowa Women’s Health Study; JPHC = Japan Public Health Center-based prospective Study 1 and 2; MCCS = Melbourne Collaborative Cohort Study; MEC = Multiethnic Cohort Study; NHS = Nurses’ Health Study; NIH-AARP = National Institutes of Health-American Association of Retired Persons Diet and Health Study; PLCO = Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; VITAL = VITamins and Lifestyle Study; WHI = Women’s Health Initiative.
Figure 1.
Forest plots for associations between cigarette smoking status and biliary tract cancer risk by anatomic site in the Biliary Tract Cancers Pooling Project. Hazard ratios for former vs never smoking (A, C, E, G) and current vs never smoking (B, D, F, H) are adjusted for sex (male, female), race (white, black, Asian and Pacific Islander, other), education (less than high school graduate, high school graduate, some college or post-high school training), body mass index in kg per m2 (<18.5, 18.5–<25, 25–<30, ≥30), diabetes (ever vs never diagnosed), birth cohort (1870–1899, 1900–1909, 1910–1919, 1920–1929, 1930–1939, 1940–1949, 1950–1959, 1960–1982), and alcoholic drinks per day (0, >0–0.5, >0.5–1, 1–<3, ≥3). Small black-filled diamonds represent the point estimates for each study. Horizontal lines represent 95% confidence intervals; if ending in an arrow, the interval transcends the region plotted. % weight describes the weight (inverse variance) each study contributed to the summary hazard ratio. Study weight is also represented by the shaded gray region around each study-specific point estimate. I2 is the percentage of variation due to between-study heterogeneity. Summary hazard ratios (dotted lines) and 95% confidence intervals (hollow diamonds) were estimated via random-effects meta-analysis. All statistical tests were two-sided. P values were calculated using the Wald test. Some additional studies collected information on cigarette smoking status but could not contribute to this meta-analysis because they did not have a sufficient number of biliary tract cancer patients who were former or current smokers. AgHealth = Agricultural Health Study; BCDDP = Breast Cancer Detection Demonstration Project; CI = confidence interval; COSM = Cohort of Swedish Men; CPS–II NC = Cancer Prevention Study II Nutrition Cohort; EPIC = European Prospective Investigation into Cancer and Nutrition; HR = hazard ratio; HPFS = Health Professionals Follow–Up Study; IWHS = Iowa Women’s Health Study; JPHC = Japan Public Health Center-based prospective Study 1 and 2; MCCS = Melbourne Collaborative Cohort Study; MEC = Multiethnic Cohort Study; NHS = Nurses’ Health Study; NIH-AARP = National Institutes of Health-American Association of Retired Persons Diet and Health Study; NYUWHS = New York University Women’s Health Study; PHS = Physicians’ Health Study; PLCO = Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; RERF = Radiation Effects Research Foundation Life Span Study; SCHS = Singapore Chinese Health Study; SCS = Shanghai Cohort Study; SISTER = Sister Study; SMC = Swedish Mammography Cohort; VITAL = VITamins and Lifestyle Study; WHI = Women’s Health Initiative; WHS = Women’s Health Study; WLHS = Women’s Lifestyle and Health Study.
Results for smoking dose variables were fairly consistent when analyzed continuously, although effect sizes were smaller given these analyses evaluated the average impact of a 10-unit increase and assumed a linear relationship. In addition, smoking pack-years was associated with intrahepatic bile duct cancer when analyzed continuously, which may suggest a loss of power in the categorical analysis (given marginal associations observed in high categories) or a chance finding. We saw evidence for a dose-response relationship of smoking pack-years, duration, and intensity with extrahepatic bile duct and ampulla of Vater cancers (all Ptrend < .01). There was also evidence of a dose-response relationship between smoking intensity and intrahepatic bile duct cancer (Ptrend = .001). When we analyzed associations using the aggregate, pooled dataset, results were consistent, although most associations were attenuated (Supplementary Table 4, available online). Risk estimates restricted to ever smokers were also generally consistent (Supplementary Table 5, available online), as were the estimates for smoking intensity and duration when additionally adjusted for smoking pack-years (Supplementary Table 6, available online).
Drinkers who reported drinking at least five alcoholic drinks per day were at increased risk of intrahepatic bile duct cancer compared with participants who consumed zero alcoholic drinks per day (HR = 2.35, 95% CI = 1.46 to 3.78), and there was evidence of a dose-response trend (Ptrend = .04; Table 3 and Figure 2). Individuals consuming three to fewer than five drinks per day were at marginally increased risk of extrahepatic bile duct cancer (HR = 1.82, 95% CI = 0.98 to 3.39); however, there was no evidence of a dose-response relationship (Ptrend = .84), there was high between-study heterogeneity (I2 = 57.2%), and these results were not robust to sensitivity analyses. No associations between alcohol consumption and gallbladder or ampulla of Vater cancer were observed. In the pooled dataset, the pattern of results was similar, but some of the associations did not reach statistical significance (Supplementary Table 4, available online). Results were similar when we analyzed drinks per day as a continuous variable (Table 3). When restricting to individuals who consumed alcohol, the pattern of results was consistent (Supplementary Table 5, available online).
For the associations with smoking status and alcoholic drinks per day, there was evidence of potential heterogeneity between several biliary tract cancers, particularly gallbladder cancer (all Pheterogeneity < .001 for comparisons with gallbladder cancer; alcoholic drinks per day Pheterogeneity = .03 for ampulla of Vater vs intrahepatic bile duct cancer; smoking status Pheterogeneity = .08 for ampulla of Vater vs intrahepatic bile duct cancer; and smoking status Pheterogeneity = .09 for extrahepatic bile duct cancer vs intrahepatic bile duct cancer). There was little evidence of heterogeneity between ampulla of Vater and extrahepatic bile duct cancer (smoking status Pheterogeneity = .77; alcoholic drinks per day Pheterogeneity = .22). For the associations with alcoholic drinks per day, there was also little evidence of heterogeneity between extrahepatic and intrahepatic bile duct cancers (Pheterogeneity = .33).
There was evidence of possible multiplicative effect-measure modification by race for the association of smoking status with extrahepatic bile duct cancer (likelihood ratio test P = .05) and intrahepatic bile duct cancer (likelihood ratio test P = .09). For extrahepatic bile duct cancer, the associations with smoking status appeared to be stronger for whites and blacks than for Asians and Pacific Islanders, and there was limited evidence of an association with individuals of other races (Supplementary Table 7, available online). However, sample sizes were small for some comparisons (eg, <40 cases). For intrahepatic bile duct cancer, the association with current smoking was strongest for Asians and Pacific Islanders and individuals of other races. Race was not a multiplicative effect modifier of the associations between alcohol consumption and biliary tract cancer risk (all likelihood ratio test P ≥ .98). There was also no evidence of multiplicative effect-measure modification by sex at any cancer site (all likelihood ratio test P ≥ .38).
When we additionally adjusted for history of gallstones in 17 studies, risk estimates were not materially altered (Supplementary Table 8, available online). In nine studies, we restricted the pooled analyses of gallbladder cancer to individuals without a prior cholecystectomy. As shown in Supplementary Table 9 (available online), results were consistent after restriction, although some risk estimates for cigarette smoking were slightly stronger among those without a prior cholecystectomy.
Discussion
In this large, prospective study, we evaluated associations of cigarette smoking and alcohol consumption with the risk of biliary tract cancers by anatomic site. Cigarette smoking was associated with approximately 1.3- to 3.0-fold higher risks of intrahepatic bile duct, extrahepatic bile duct, and ampulla of Vater cancers. Associations with smoking were strongest and most consistent with extrahepatic bile duct and ampulla of Vater cancers, whereas associations with intrahepatic bile duct cancer appeared to be more modest and most apparent at higher levels of smoking intensity. In contrast, there was no convincing evidence of an association between cigarette smoking and gallbladder cancer. High levels of alcohol consumption (ie, ≥5 alcoholic drinks per day) were associated with a 2.4-fold higher risk of intrahepatic bile duct cancer, but no associations were observed with other biliary tract cancers. These findings highlight etiologic heterogeneity across the biliary tract.
Prior studies investigating the associations of cigarette smoking and alcohol consumption with biliary tract cancer risk have been largely inconclusive or conflicting. Relatively limited research exists on the association of tobacco smoking with intrahepatic bile duct cancer, and both null (10,24) and positive (9,16,18,19,28,29) associations have been reported. For extrahepatic bile duct cancer, some prior studies have reported positive associations with tobacco smoking (11,12,18,25,28), but other studies have reported null (9,10,17) or inverse associations (15). Reasons for the inconsistencies in these associations may include the small sample sizes of some prior studies (eg, <100 cases), variability in study design, or inability to control for important confounders. Regarding associations with ampulla of Vater cancer, ever smoking was associated with increased risk in one prior case-control study; however, risk estimates were based on a small number of cases (<50) (11). We have demonstrated this association in a well-powered prospective study and have additionally shown associations with smoking pack-years, intensity, and duration.
Our findings are consistent with the proven carcinogenicity of tobacco smoke (7) and the well-established associations between cigarette smoking and other cancers, including cancers of the lung, bladder, liver, pancreas, and stomach (6). Mechanisms for the association between smoking and intrahepatic bile duct, extrahepatic bile duct, and ampulla of Vater cancers likely include direct or indirect toxic effects of carcinogens (eg, polycyclic aromatic hydrocarbons, N-nitrosamines, aromatic amines, and volatile organics) (64), some of which may be concentrated in bile (65), and/or cell-mediated and humoral immunological changes (eg, T-cell unresponsiveness and modulations in the production of proinflammatory cytokines) (66). We observed moderate to high between-study heterogeneity for some associations, particularly associations with extrahepatic bile duct cancer. One possible explanation for this heterogeneity is that extrahepatic bile duct cancer diagnosis criteria may vary among study populations, and adjudication of rare cancers is a challenge. Other potential sources of heterogeneity include etiologic differences across different populations and/or variations in study design and smoking ascertainment.
In some studies, tobacco smoking has been associated with an increased risk of gallbladder cancer incidence (12,26) and mortality (14), yet other studies have observed no association (20–22). Overall, evidence for a clear pattern in these associations is still lacking, and there may be preferential reporting of positive associations (6). In our analyses, we observed no convincing association between cigarette smoking and gallbladder cancer risk. We observed a few marginal associations, and risk estimates tended to be slightly stronger when restricted to individuals without a prior cholecystectomy, suggesting a possible attenuation of gallbladder cancer risk estimates by the presence of people with cholecystectomy. However, marginal associations were weak and tended to be inconsistent. We also observed no evidence of a dose-response relationship with smoking pack-years, duration, or intensity. Taken together, our results do not provide strong evidence for an association between cigarette smoking and gallbladder cancer risk.
Studies investigating the association between alcohol consumption and biliary tract cancer risk have also been mixed. Positive (10,23,24,29) as well as null (11,15,19,23,25) associations of alcohol consumption with extrahepatic and intrahepatic bile duct cancer risk have been reported, and the International Agency for Research on Cancer has deemed the epidemiologic evidence for an association insufficient (6). Null associations between alcohol consumption and ampulla of Vater cancer have been reported by prior studies (11,23), which is consistent with our findings. Associations between alcohol consumption and gallbladder cancer, however, have often been contradictory (12–14,23,27). These contradictory findings may be partially attributed to the potential protective effects of alcohol intake on gallstone formation (67) due to inhibition of gallbladder water absorption and alterations in the composition of biliary lipids (68). Nonetheless, in the present study, our results were robust to additional adjustment for history of gallstones.
We observed positive associations between high levels of alcohol consumption and intrahepatic bile duct cancer risk. Possible mechanisms for the positive association with alcohol consumption include genotoxic effects of acetaldehyde, production of reactive oxygen and nitrogen species, DNA methylation, reduced immune surveillance, and inflammatory responses (69). In the present study, alcohol consumption was not associated with gallbladder, extrahepatic bile duct, or ampulla of Vater cancers. It is possible that we did not have sufficient statistical power to detect associations at the highest levels of alcohol consumption, where there were fewer than 25 cases and estimates were based on a subset of studies with a sufficient number of high consumers. It is also possible that alcohol consumption is not an important risk factor for these cancers.
There was evidence of effect-measure modification by race for the association of smoking status with extrahepatic and intrahepatic bile duct cancers. Results suggested that the strength of the associations may vary by race (eg, the association between smoking status and extrahepatic bile duct cancer was stronger among whites and blacks than among other racial groups, whereas the association with intrahepatic bile duct cancer was strongest among Asians and Pacific Islanders and individuals of other races). Although we were underpowered to detect associations in some subgroups, these variations in associations by race for some but not all anatomic sites point to potential etiologic differences among biliary tract cancers despite their anatomic proximity.
Gallstones is one of the primary known risk factors for biliary tract cancers (63), and it may act as a mediator in the associations we observed. Nevertheless, when we additionally adjusted for history of gallstones, there were no appreciable changes in risk estimates, suggesting that the associations we observed were likely not mediated by gallstones.
Strengths of our study include its prospective design, diversity of included studies, large sample size, and long follow-up period, which provided unprecedented statistical power to assess associations with rare biliary tract cancer by anatomic site. Meta-analyses of individual participant data offer several advantages over meta-analyses of the published literature (70). For example, by pooling individual-level data, we were able to standardize adjustments for multiple potential confounders, mitigate publication bias, and decrease the variability in effect estimates that is caused by inconsistencies in analytic modeling. For two-thirds of the associations assessed, we observed low heterogeneity (I2 < 10%) and a similar pattern of risk estimates across studies that differed substantially in design, population, and geographic location, lending greater confidence to these findings. The robustness of our findings to multiple sensitivity analyses also lends greater credence to these associations.
Our study also has several limitations. Smoking and alcohol data were provided at a single timepoint for most studies, so we were unable to assess changes in these exposures over the course of follow-up, and approaches to measuring these exposures varied across studies. Data on cigarette smoking and alcohol consumption were ascertained via self-report and may be subject to under- or misreporting. Regarding alcohol exposure, definitions of standard drink measures and sizes often vary substantially among and within countries (71), which may have introduced some misclassification in the assessment of alcoholic drinks consumed per day. We were also unable to assess duration of drinking, total alcohol exposure, or differences by beverage type. In addition, findings for heavy alcohol consumption should be interpreted with caution given modest sample sizes and few studies contributing to the highest exposure categories. There may be residual or unmeasured confounding, particularly from other lifestyle factors (eg, diet or physical activity). Finally, there may have been some histological misclassification of cancers.
In this pooled analysis of 26 studies, we assessed associations between two potentially modifiable lifestyle factors and biliary tract cancer risk in a well-powered, prospective study. Our findings provide evidence that smoking is associated with intrahepatic bile duct, extrahepatic bile duct, and ampulla of Vater cancers, suggesting that tobacco may be a risk factor for even more cancers than previously appreciated. High levels of alcohol consumption were also associated with intrahepatic bile duct cancer risk. Findings suggest etiologic heterogeneity across the biliary tract, particularly for gallbladder cancer, underscoring the importance of analyzing biliary tract cancers individually by anatomic site to understand the unique factors contributing to each of these malignancies. More broadly, our findings provide insight into the etiology of these rare, understudied cancers and support ongoing public health efforts to mitigate cigarette smoking and heavy alcohol consumption.
Funding
AgHealth: This study was funded by the Intramural Program of the National Institutes of Health, National Cancer Institute (Z01 P010119). and the National Institute of Environmental Health Sciences (Z01 ES 049030–11).
AHS-2: Project support was obtained from National Cancer Institute grant 1U01CA152939.
ATBC: The ATBC Study is supported by the Intramural Research Program of the US National Cancer Institute, National Institutes of Health, and by US Public Health Service contract HHSN261201500005C from the National Cancer Institute, Department of Health and Human Services.
BCDDP: The BCDDP Follow-up Study was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
COSM: This cohort is supported by the Swedish Research Council (Research Infrastructure SIMPLER), the Swedish Cancer Foundation, and by Strategic Funds from Karolinska Institutet, Stockholm, Sweden.
CPS-II NC: The American Cancer Society funds the creation, maintenance, and updating of the Cancer Prevention Study-II cohort.
EPIC: The coordination of the EPIC is financially supported by the European Commission (DG-SANCO) and the International Agency for Research on Cancer. The national cohorts are supported by Danish Cancer Society, Denmark; Ligue Contre le Cancer, France; Institut Gustave Roussy, France; Mutuelle Générale de l’Education Nationale, France; Institut National de la Santé et de la Recherche Médicale, France; Deutsche Krebshilfe, Germany; Deutsches Krebsforschungszentrum and Federal Ministry of Education and Research, Germany; Hellenic Health Foundation, Greece; Italian Association for Research on Cancer; National Research Council, Italy; Dutch Ministry of Public Health, Welfare and Sports, the Netherlands; Netherlands Cancer Registry, the Netherlands; LK Research Funds, the Netherlands; Dutch Prevention Funds, the Netherlands; Dutch ZON (Zorg Onderzoek Nederland), the Netherlands; World Cancer Research Fund, London, UK; Statistics Netherlands, the Netherlands; European Research Council, Norway; Health Research Fund, Regional Governments of Andalucía, Asturias, Basque Country, Murcia (project no. 6236) and Navarra, ISCIII RETIC (RD06/0020/0091), Spain; Swedish Cancer Society, Sweden; Swedish Scientific Council, Sweden; Regional Government of Skåne and Västerbotten, Sweden; Cancer Research, UK; Medical Research Council, UK; Stroke Association, UK; British Heart Foundation, UK; Department of Health, Food Standards Agency, UK; and Wellcome Trust, UK.
HPFS: This work was supported by grants from the National Institutes of Health (UM1 CA167552, P01 CA55075), the Entertainment Industry Foundation, and the National Colorectal Cancer Research Alliance. HPFS would like to thank the participants and staff of the HPFS for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, and WY. The authors assume full responsibility for analyses and interpretation of these data.
IWHS: IWHS was funded by a grant from the National Cancer Institute (R01 CA39742).
JPHC: This work was supported by the National Cancer Center Research and Development Fund (since 2011) and a grant-in-aid from Cancer Research (1989–2010) from the Ministry of Health, Labor, and Welfare of Japan.
MCCS: MCCS receives core funding from Cancer Council Victoria and is additionally supported by grants from the Australian National Health and Medical Research Council (209057, 251533, 396414, and 504715).
MEC: This work was supported by the National Institutes of Health (P01 CA33619 and U01 CA164973).
NHS: Data used in this study were supported by an infrastructure grant (UM1 CA186107) and a program project grant that funds cancer research (P01 CA87969). NHS would like to thank the participants and staff of the NHS for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, and WY. The authors assume full responsibility for analyses and interpretation of these data.
NIH-AARP: This study was supported (in part) by the Intramural Research Program of the National Institutes of Health, National Cancer Institute. Cancer incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, GA. Cancer incidence data from California were collected by the California Cancer Registry, California Department of Public Health’s Cancer Surveillance and Research Branch, Sacramento, CA. Cancer incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Community Health Administration, Lansing, MI. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System (Miami, FL) under contract with the Florida Department of Health, Tallahassee, FL. The views expressed herein are solely those of the authors and do not necessarily reflect those of the Florida Cancer Data System or the Florida Department of Health. Cancer incidence data from Louisiana were collected by the Louisiana Tumor Registry, Louisiana State University Health Sciences Center School of Public Health, New Orleans, LA. Cancer incidence data from New Jersey were collected by the New Jersey State Cancer Registry, Rutgers Cancer Institute of New Jersey, New Brunswick, NJ. Cancer incidence data from North Carolina were collected by the North Carolina Central Cancer Registry, Raleigh, NC. Cancer incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, PA. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services, Phoenix, AZ. Cancer incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services, Austin, TX. Cancer incidence data from Nevada were collected by the Nevada Central Cancer Registry, Division of Public and Behavioral Health, State of Nevada Department of Health and Human Services, Carson City, NV. The study authors are indebted to the participants in the NIH-AARP Diet and Health Study for their outstanding cooperation. Additional acknowledgments can be found https://dietandhealth.cancer.gov/acknowledgement.html.
NYUWHS: The NYUWHS is supported by grants UM1 CA182934 and P30 CA16087 from the National Cancer Institute and by grant P30 ES000260 from the National Institute of Environmental Health Sciences.
PHS: PHS is supported by grants from the National Cancer Institute (CA-34933, CA-40360, and CA-097193) and from the National Heart, Lung, and Blood Institute (HL-26490 and HL-34595), National Institutes of Health, Bethesda, MD.
PLCO: The PLCO Cancer Screening Trial is supported by contracts from the National Cancer Institute.
RERF: The Radiation Effects Research Foundation (RERF), Hiroshima and Nagasaki, Japan, is a public interest foundation funded by the Japanese Ministry of Health, Labour and Welfare and the US Department of Energy (DOE). The research was also funded in part through DOE award DE-HS0000031 to the National Academy of Sciences. This publication was supported by RERF Research Protocol A2-13. The views of the authors do not necessarily reflect those of the two governments.
SCHS: This study is supported by the National Cancer Institute (R01CA080205, R01CA144034, and UM1CA182876).
SCS: The Shanghai Cohort Study is supported by the National Cancer Institute (R01CA043092, R01CA144034, and UM1CA182876).
SISTER: The Sister Study is supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (ZO1-ES-044005).
SMC: This cohort is supported by the Swedish Research Council (Research Infrastructure SIMPLER), the Swedish Cancer Foundation, and by Strategic Funds from Karolinska Institutet, Stockholm, Sweden.
VITAL: The VITAL study was supported by the National Institutes of Health grant K05-CA154337 (National Cancer Institute and Office of Dietary Supplements).
WHI: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services through contracts, HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A listing of WHI investigators can be found at http://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Short%20List.pdf.
WHS: WHS was supported by grants CA047988, HL043851, HL080467, and HL099355.
WLHS: The WLHS project was supported by the Swedish Research Council (grant number 521–2011-295) and a Distinguished Professor Award at Karolinska Institutet to Hans-Olov Adami, grant number 2368/10–221.
Notes
Affiliations of authors: Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (EEM, SSJ, JLP, ALVD, DA, GA, LEBF, ABdG, NDF, PH, CMK, LML, MPP, CS, RS, RSS, SJW, BZ, KAM, JK); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (HOA); Department of Health Management and Health Economics, University of Oslo, Oslo, Norway (HOA); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (JEB, FG, IML, HDS); Division of Preventive Medicine, Department of Medicine, Brigham and Women’s Hospital, Boston, MA (JEB, JMG, IML, HDS); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital, Boston, MA (ATC, FG, XZ); Division of Gastroenterology, Department of Medicine, Massachusetts General Hospital, Boston, MA (ATC, TGS); Clinical and Translational Epidemiology Unit, Massachusetts General Hospital, and Harvard Medical School, Boston, MA (ATC, TGS); Department of Population Health and Perlmutter Cancer Center, New York University School of Medicine, New York, NY (YC, AZJ); School of Public Health, Loma Linda University, Loma Linda, CA (GEF, SFK); Department of Epidemiology, Shanghai Cancer Institute, Shanghai, China (YTG); Behavioral and Epidemiology Research Group, American Cancer Society, Inc, Atlanta, GA (SMG, PTC); Boston Veteran Affairs Healthcare System, Boston, MA (JMG); Cancer Epidemiology Division, Cancer Council Victoria, Melbourne, Australia (GGG, RLM); Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Parkville, Victoria, Australia (GGG, RLM); Precision Medicine, School of Clinical Sciences at Monash Health, Monash University, Clayton, Victoria, Australia (GGG); Department of Epidemiology, Radiation Effects Research Foundation, Hiroshima, Japan (EJG); Section of Nutrition and Metabolism, International Agency for Research on Cancer, World Health Organization, Lyon, France (MJ); Health Services and Systems Research, Duke-NUS Medical School, Singapore (WPK); Saw Swee Hock School of Public Health, National University of Singapore, Singapore (WPK); Unit of Nutritional Epidemiology, Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden (SCL, AW); Department of Epidemiology and Biostatistics, School of Public Health, Indiana University Bloomington, Bloomington, IN (JL); Department of Preventive Medicine, Keck School of Medicine and Norris Comprehensive Cancer Center, University of Southern California, Los Angeles, CA (KRM); Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA (MLN, UP); Epidemiology Branch, National Institute of Environmental Health Sciences, Research Triangle Park, NC (KMO, DPS); Department of Epidemiology, School of Public Health, University of Washington, Seattle, WA (UP); Department of Pediatrics, University of Minnesota, Minneapolis, MN (JNP); Exercise and Nutrition Sciences, Milken Institute School of Public Health, George Washington University, Washington, DC (KR); Epidemiology and Prevention Group, Center for Public Health Sciences, National Cancer Center, Tokyo, Japan (NS, ST); UPMC Hillman Cancer Center, University of Pittsburgh, Pittsburgh, PA (RW, JMY); International Agency for Research on Cancer, World Health Organization, Lyon, France (EW); Department of Epidemiology, University of Washington, Seattle, WA (EWh); Department of Surgical Sciences, Uppsala University, Uppsala, Sweden (AW); Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA (JMY).
The funders had no role in the design of the study, the collection, analysis, and interpretation of the data, writing of the manuscript, or the decision to submit it for publication.
Potential competing interests: None.
Author contributions: Study concept and design (all authors); acquisition of data (all authors); statistical analysis (McGee, Jackson, Zhu, Koshiol); interpretation of data (McGee, Koshiol); drafting of manuscript (McGee); critical review and approval of final manuscript (all authors).
Disclaimer
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
Supplementary Material
References
- 1. Castro FA, Koshiol J, Hsing AW, et al. Biliary tract cancer incidence in the United States—demographic and temporal variations by anatomic site. Int J Cancer. 2013;133(7):1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hsing A, Rashid A, Devesa S, et al. Biliary tract cancer In: Schottenfeld D, Fraumeni JF Jr eds. Cancer Epidemiology and Prevention. 3rd ed. New York: Oxford University Press, Inc; 2006:787–800. [Google Scholar]
- 3. Njei B. Changing pattern of epidemiology in intrahepatic cholangiocarcinoma. Hepatology. 2014;60(3):1107–1108. [DOI] [PubMed] [Google Scholar]
- 4. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. [DOI] [PubMed] [Google Scholar]
- 5. Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33(6):1353–1357. [DOI] [PubMed] [Google Scholar]
- 6.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Personal habits and indoor combustions. Volume 100 E. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt E):1–538. [PMC free article] [PubMed] [Google Scholar]
- 7. Secretan B, Straif K, Baan R, et al. A review of human carcinogens–Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10(11):1033–1034. [DOI] [PubMed] [Google Scholar]
- 8. Praud D, Rota M, Rehm J, et al. Cancer incidence and mortality attributable to alcohol consumption. Int J Cancer. 2016;138(6):1380–1387. [DOI] [PubMed] [Google Scholar]
- 9. Welzel TM, Graubard BI, El–Serag HB, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. 2007;5(10):1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shaib YH, El-Serag HB, Nooka AK, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol. 2007;102(5):1016–1021. [DOI] [PubMed] [Google Scholar]
- 11. Chow WH, McLaughlin JK, Menck HR, et al. Risk factors for extrahepatic bile duct cancers: Los Angeles County, California (USA). Cancer Causes Control. 1994;5(3):267–272. [DOI] [PubMed] [Google Scholar]
- 12. Grainge MJ, West J, Solaymani-Dodaran M, et al. The antecedents of biliary cancer: a primary care case–control study in the United Kingdom. Br J Cancer. 2009;100(1):178–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ozasa K. Alcohol use and mortality in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC). Asian Pac J Cancer Prev. 2007;8(suppl):81–88. [PubMed] [Google Scholar]
- 14. Yagyu K, Kikuchi S, Obata Y, et al. Cigarette smoking, alcohol drinking and the risk of gallbladder cancer death: a prospective cohort study in Japan. Int J Cancer. 2008;122(4):924–929. [DOI] [PubMed] [Google Scholar]
- 15. Yen S, Hsieh CC, MacMahon B.. Extrahepatic bile duct cancer and smoking, beverage consumption, past medical history, and oral-contraceptive use. Cancer. 1987;59(12):2112–2116. [DOI] [PubMed] [Google Scholar]
- 16. Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248(1):84–96. [DOI] [PubMed] [Google Scholar]
- 17. Ahrens W, Timmer A, Vyberg M, et al. Risk factors for extrahepatic biliary tract carcinoma in men: medical conditions and lifestyle: results from a European Multicentre Case-Control Study . Eur J Gastroenterol Hepatol. 2007;19(8):623–630. [DOI] [PubMed] [Google Scholar]
- 18. Petrick JL, Yang B, Altekruse SF, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based study in SEER-Medicare. PLoS One. 2017;12(10):e0186643.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Makiuchi T, Sobue T, Kitamura T, et al. Smoking, alcohol consumption, and risks for biliary tract cancer and intrahepatic bile duct cancer. J Epidemiol .2019;29(5):180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hsing AW, Zhang M, Rashid A, et al. Hepatitis B and C virus infection and the risk of biliary tract cancer: a population-based study in China. Int J Cancer 2008;122(8):1849–1853. [DOI] [PubMed] [Google Scholar]
- 21. Nakadaira H, Lang I, Szentirmay Z, et al. A case-control study of gallbladder cancer in Hungary. Asian Pac J Cancer Prev. 2009;10(5):833–836. [PubMed] [Google Scholar]
- 22. Zatonski WA, La Vecchia C, Przewozniak K, et al. Risk factors for gallbladder cancer: a Polish case-control study. Int J Cancer. 1992;51(5):707–711. [DOI] [PubMed] [Google Scholar]
- 23. Li Y, Yang H, Cao J.. Association between alcohol consumption and cancers in the Chinese population–a systematic review and meta-analysis. PLoS One. 2011;6(4):e18776.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palmer WC, Patel T.. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57(1):69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ye XH, Huai JP, Ding J, et al. Smoking, alcohol consumption, and the risk of extrahepatic cholangiocarcinoma: a meta-analysis. World J Gastroenterol. 2013;19(46):8780.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wenbin D, Zhuo C, Zhibing M, et al. The effect of smoking on the risk of gallbladder cancer: a meta-analysis of observational studies. Eur J Gastroenterol Hepatol. 2013;25(3):373–379. [DOI] [PubMed] [Google Scholar]
- 27. Bagnardi V, Rota M, Botteri E, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer. 2015;112(3):580–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang Y, You L, Xie W, et al. Smoking and risk of cholangiocarcinoma: a systematic review and meta-analysis. Oncotarget. 2017;8(59):100570–100581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petrick JL, Campbell PT, Koshiol J, et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: the Liver Cancer Pooling Project. Br J Cancer. 2018;118(7):1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fortier I, Doiron D, Burton P, et al. Invited commentary: consolidating data harmonization—how to obtain quality and applicability? Am J Epidemiol. 2011;174(3):261–264. [DOI] [PubMed] [Google Scholar]
- 31. Alavanja MC, Sandler DP, McMaster SB, et al. The Agricultural Health Study. Environ Health Perspect. 1996;104(4):362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Butler TL, Fraser GE, Beeson WL, et al. Cohort profile: the Adventist Health Study-2 (AHS-2). Int J Epidemiol. 2008;37(2):260–265. [DOI] [PubMed] [Google Scholar]
- 33. Schairer C, Lubin J, Troisi R, et al. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA. 2000;283(4):485–491. [DOI] [PubMed] [Google Scholar]
- 34. Orsini N, Bellocco R, Bottai M, et al. Combined effects of obesity and physical activity in predicting mortality among men. J Intern Med. 2008;264(5):442–451. [DOI] [PubMed] [Google Scholar]
- 35. Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94(9):2490–2501. [DOI] [PubMed] [Google Scholar]
- 36. Riboli E, Hunt KJ, Slimani N, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5(6b):1113–1124. [DOI] [PubMed] [Google Scholar]
- 37. Grobbee DE, Rimm EB, Giovannucci E, et al. Coffee, caffeine, and cardiovascular disease in men. N Engl J Med. 1990;323(15):1026–1032. [DOI] [PubMed] [Google Scholar]
- 38. Folsom AR, Kaye SA, Sellers TA, et al. Body fat distribution and 5-year risk of death in older women. JAMA. 1993;269(4):483–487. [PubMed] [Google Scholar]
- 39. Tsugane S, Sawada N.. The JPHC study: design and some findings on the typical Japanese diet. Jpn J Clin Oncol. 2014;44(9):777–782. [DOI] [PubMed] [Google Scholar]
- 40. Giles GG, English DR.. The Melbourne Collaborative Cohort Study. IARC Sci Publ. 2002;156:69–70. [PubMed] [Google Scholar]
- 41. Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151(4):346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Belanger CF, Hennekens CH, Rosner B, et al. The Nurses’ Health Study. Am J Nurs. 1978;78(6):1039–1040. [PubMed] [Google Scholar]
- 43. Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119–1125. [DOI] [PubMed] [Google Scholar]
- 44. Toniolo PG, Levitz M, Zeleniuch-Jacquotte A, et al. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J Natl Cancer Inst. 1995;87(3):190–197. [DOI] [PubMed] [Google Scholar]
- 45. Beebe GW, Ishida M, Jablon S.. Studies of the mortality of A-bomb survivors. I. Plan of study and mortality in the medical subsample (selection 1), 1950-1958. Radiat Res. 1962;16:253–280. [PubMed] [Google Scholar]
- 46. Hankin JH, Stram DO, Arakawa K, et al. Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer. 2001;39(2):187–195. [DOI] [PubMed] [Google Scholar]
- 47. Ross RK, Yuan JM, Yu MC, et al. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet. 1992;339(8799):943–946. [DOI] [PubMed] [Google Scholar]
- 48. Sandler DP, Hodgson ME, Deming-Halverson SL, et al. The Sister Study Cohort: baseline methods and participant characteristics. Environ Health Perspect. 2017;125(12):127003.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. White E, Patterson RE, Kristal AR, et al. VITamins and Lifestyle cohort study: study design and characteristics of supplement users. Am J Epidemiol. 2004;159(1):83–93. [DOI] [PubMed] [Google Scholar]
- 50. Kumle M, Weiderpass E, Braaten T, et al. Use of oral contraceptives and breast cancer risk. The Norwegian-Swedish Women’s Lifestyle and Health Cohort Study. Cancer Epidemiol Biomarkers Prev. 2002;11(11):1375–1381. [PubMed] [Google Scholar]
- 51. Wolk A, Larsson SC, Johansson J, et al. Long-term fatty fish consumption and renal cell carcinoma incidence in women. JAMA. 2006;296(11):1371–1376. [DOI] [PubMed] [Google Scholar]
- 52. The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. The ATBC Cancer Prevention Study Group. Ann Epidemiol. 1994;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- 53. Sesso HD, Gaziano JM, VanDenburgh M, et al. Comparison of baseline characteristics and mortality experience of participants and nonparticipants in a randomized clinical trial. Control Clin Trials. 2002;23(6):686–702. [DOI] [PubMed] [Google Scholar]
- 54. Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(suppl 9):S5–S17. [DOI] [PubMed] [Google Scholar]
- 55. Rexrode KM, Lee IM, Cook NR, et al. Baseline characteristics of participants in the Women’s Health Study. J Womens Health Gend Based Med. 2000;9(1):19–27. [DOI] [PubMed] [Google Scholar]
- 56. Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21(suppl 6):273s–309s. [DOI] [PubMed] [Google Scholar]
- 57.National Institute on Alcohol Abuse and Alcoholism. What is a standard drink? https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink. Accessed July 21, 2018.
- 58. Grambsch PM, Therneau TM.. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- 59. Higgins JP, Thompson SG.. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 60. Pierce DA, Preston DL.. Joint analysis of site-specific cancer risks for the atomic bomb survivors. Radiat Res. 1993;134(2):134–142. [PubMed] [Google Scholar]
- 61. Lubin JH, Alavanja MCR, Caporaso N, et al. Cigarette smoking and cancer risk: modeling total exposure and intensity. Am J Epidemiol. 2007;166(4):479–489. [DOI] [PubMed] [Google Scholar]
- 62. Rothman KJ, Greenland S, Lash TL.. Modern Epidemiology. 3rd ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 63. Hsing AW, Gao YT, Han TQ, et al. Gallstones and the risk of biliary tract cancer: a population-based study in China. Br J Cancer. 2007;97(11):1577.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3(10):733–744. [DOI] [PubMed] [Google Scholar]
- 65. Schulze J, Richter E, Binder U, et al. Biliary excretion of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in the rat. Carcinogenesis. 1992;13(11):1961–1965. [DOI] [PubMed] [Google Scholar]
- 66. Sopori ML, Kozak W.. Immunomodulatory effects of cigarette smoke. J Neuroimmunol. 1998;83(1–2):148–156. [DOI] [PubMed] [Google Scholar]
- 67. Wang J, Duan X, Li B, et al. Alcohol consumption and risk of gallstone disease: a meta-analysis. Eur J Gastroenterol Hepatol. 2017;29(4):e19–e28. [DOI] [PubMed] [Google Scholar]
- 68. Kurtin WE, Schwesinger WH, Stewart RM.. Effect of dietary ethanol on gallbladder absorption and cholesterol gallstone formation in the prairie dog. Am J Surg. 1991;161(4):470–474. [DOI] [PubMed] [Google Scholar]
- 69. Boffetta P, Hashibe M.. Alcohol and cancer. Lancet Oncol. 2006;7(2):149–156. [DOI] [PubMed] [Google Scholar]
- 70. Blettner M, Sauerbrei W, Schlehofer B, et al. Traditional reviews, meta-analyses and pooled analyses in epidemiology. Int J Epidemiol. 1999;28(1):1–9. [DOI] [PubMed] [Google Scholar]
- 71. Dawson DA. Defining risk drinking. Alcohol Res Health. 2011;34(2):144–156. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.