Abstract
Accumulating evidence has demonstrated that microRNAs are associated with malignant biological behaviour, including tumorigenesis, cancer progression and metastasis via the regulation of target gene expression. Our previous study demonstrated that programmed cell death protein 4 (PDCD4), which is a tumour suppressor gene, is a target of microRNA-21 (miR-21), which affects the proliferation and transformation capabilities of renal cell carcinoma (RCC) cells. However, the role of miR-21 in the molecular mechanism underlying the migration, invasion and angiogenesis of RCC remains poorly understood. The effects of miR-21 on the invasion, migra tion and angiogenesis of RCC cells was determined through meta-analysis and regulation of miR-21 expression in vitro. After searching several databases, 6 articles including a total of 473 patients met the eligibility criteria for this analysis. The combined results of the meta-analysis revealed that increased miR-21 expression was significantly associated with adverse prognosis in patients with RCC, with a pooled hazard ratio estimate of 1.740. In in vitro experiments, we demonstrated that a miR-21 inhibitor decreased the number of migrating and invading A498 and 786-O RCC cells, along with a decrease in PDCD4, c-Jun, matrix metalloproteinase (MMP)2 and MMP9 expression. Additionally, inhibition of miR-21 was revealed to reduce tube formation and tube junctions in the endothelial cell line HMEC-1 by affecting the expression of angiotensin-1 and vascular endothelial growth factor A, whereas PDCD4 small interfering RNA exerted opposite effects on the same cells. Overall, these findings, along with evidence-based molecular biology, demonstrated that miR-21 expression promoted the migration, invasion and angiogenic abilities of RCC cells by directly targeting the PDCD4/c-Jun signalling pathway. The results may help elucidate the molecular mechanism under lying the development and progression of RCC and provide a promising target for microRNA-based therapy.
Keywords: microRNA-21, renal cell carcinoma, programmed cell death protein 4, angiogenesis, meta-analysis
Introduction
Renal cell carcinoma (RCC) is a major cause of cancer-associated mortality and one of the most common types of urological cancer worldwide alongside prostate and bladder cancer (1). An estimated 65,240 newly diagnosed RCC cases and >14,970 cases of RCC-associated mortality were reported in the USA in 2018 (2). Although the diagnosis and treatment strategies for RCC are continuously improving, the proportion of patients who present with metastases at initial diagnosis is 20-30% (3-5). Additionally, one-third of patients with localized RCC may experience recurrence or progression to metastatic disease following curative tumour resection (6,7). For metastatic RCC, the therapeutic efficacy of radiotherapy and chemotherapy is low, leading to a poor prognosis and a 5-year survival rate of <10% (8,9). At present, several well-known risk factors, including patient-specific (smoking, obesity, hypertension, etc.) and tumour-specific (TNM stage, Eastern Cooperative Oncology Group performance status, etc.) factors, are widely used for RCC prognosis (7,10,11). However, the complex and heterogeneous characteristics of RCC may affect the value and accuracy of these predictors. Therefore, it is important to identify and understand the alterations in the cancer in order to determine the clinical significance of biological markers and develop targeted therapeutics (10).
Previous molecular studies have revealed that abnormal expression of microRNAs (miRNAs) is involved in tumorigenesis (12-14). miRNAs are endogenous non-coding molecules 18-25 nucleotides in length, which regulate the processes of cellular homeostasis and tumorigenesis, including cell proliferation and apoptosis, through specific binding to the 3′-untranslated region (UTR) of target mRNAs (14,15). Abnormal expression of miRNAs has been reported in most tumours; in breast cancer, miR-30 has been identified to directly target multiple bone metastasis-associated genes, including cadherin 11, which affects tumour cell osteomimicry, and integrin subunit a 5, which affects invasiveness, thereby inhibiting cancer cell invasion, osteomimicry, and bone destruction (16). Similarly, miR-125b may act as a tumour suppressor by inducing cellular senescence and apoptosis during hepatocellular carcinogenesis by directly targeting the 3′-UTR of sirtuin 6 (17). However, in previous study, microRNA (miR)-21 was identified to promote cellular hyperplasia in numerous types of cancers including thyroid (18), colorectal (19) and pancreatic cancer (20) and contribute to malignant cell transformation. Additionally, it downregulated the expression of the tumour suppressor programmed cell death protein 4 (PDCD4) protein, which was consistent with the findings of fluorescence microscopic evaluation (21). The aim of the present study was to investigate the potential role of miR-21 in the angiogenesis, invasiveness and progression of RCC cells, and to elucidate the underlying mechanism.
Materials and methods
Search strategy
A search was performed in the PubMed (ncbi.nlm.nih.gov/pubmed/), Medline (ovid.com/) and Cochrane electronic databases (cochranelibrary.com) and the reference lists of the identified articles, in order to identify relevant studies in these databases that were published between 1994 and 2018. In addition to electronic searches of original papers, we also reviewed the abstracts that were published in major academic conferences (European Society of Urology, American Urological Association, Asian Society of Urology, American Society of Clinical Oncology, European Society for Medical Oncology and others). The search terms included 'microRNA', 'miR-21', 'renal cell carcinoma', 'kidney cancer', 'prognosis', 'mortality', 'recurrence', 'progression' and 'relapse'. There were no language restrictions. Studies were considered eligible if: i) They reported an effect measure [i.e., hazard ratios (HRs), Kaplan-Meier survival curves or log-rank P-values] of miR-21 expression on overall survival (OS), cancer-specific survival (CSS), recurrence free survival, progression-free survival (PFS) or metastasis-free survival; ii) miR-21 expression was evaluated in primary kidney cancer exhibiting histological homogeneity; iii) the search was limited to human subjects; and iv) the study was a cohort or case-control study. Reviews, abstracts, non-clinical studies and duplicate publications were excluded in the present study.
Data extraction
The following data were extracted from each study (22-28) where available: Last name of first author, publication year, origin of study population, patient number, RCC stage, miR-21 detection method, cut-off value, patient outcomes, follow-up, effect assessments [such as relative risk (RR), HR or odds ratio (OR) with a corresponding 95% confidence interval (CI)] of mortality and overall assessment of potential bias. When studies reported only Kaplan-Meier survival curves or a log-rank P-value, HR and 95% CI were calculated using the method described by Tierney et al (29), with any discrepancies regarding evaluation of RCC stage, miR-21 detection method, cut-off value and patient outcomes resolved through discussion based on evaluation of inclusion and exclusion criteria.
Cell culture and transfection
Human renal carcinoma 786-O and A498 cell lines, and human micro vessel endothelial (HMEC-1) cells were obtained from the American Tissue Culture Collection and grown in DMEM (Thermo Fisher Scientific, Inc.) supplemented with 15% FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin at 37°C with 5% CO2. HMEC-1 cells were grown in the same culture medium, which was additionally supplemented with 10 ng/l vascular endothelial growth factor (VEGF; Beyotime Institute of Biotechnology).
For the cell transfection assay, the cells were first seeded into a 6-well plate, and when they had grown to ~50% confluence, Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) was used to transfect the cells with a final concentration of 100 nM miR-21 inhibitor (5′-UCAACAUCAGUCUGAUAAGCUA-3′) or miR-21 mimics (sense, 5′-UAGCUUAUCAGACUGAUGUUGA-3′; antisense, 5′-AACAUCAGUCUGAUAAGCUAUU-3′) to specifically inhibit or upregulate miR-21 expression, miR-21 inhibitor negative control (5′-CAGUACUUUUGUGUAGUACAA-3′) and miR-21 mimics negative control (sense, 5′-UUCUCCGAACGUGUCACGUTT-3′; antisense, 5′-ACGUGACACGUUCGGAGAATT-3′), PDCD4 small interfering RNA (siRNA) (sense, 5′-GUGCAUCCGUACUCCCAAA-3′; antisense, 5′-UUUGGGAGUACGGAUGCAC-3′), c-Jun siRNA (sense, 5′-GAAAGUCAUGAACCACGUUTT-3′; antisense, 5′-UAGUAAGAGAGGCUAUCCCTT-3′) and scrambled siRNA negative control (NC) (sense, 5′-UUCUCCGAACGUGUCACGUTT-3′; antisense, 5′-ACGUGACACGYYCGGAGAATT-3′), which were purchased from Shanghai GenePharma Co., Ltd. Total RNA or protein was extracted after 36 or 48 h and used for further experiments.
Cell migration assay
Transwell chambers with 8-µm pore filters were used to assess the migratory ability of 786-O and A498 cells. After 24 or 36 h of transfection, cells with different transfection treatments were trypsinized, and ~5×104 cells were seeded in the upper chamber in serum-free DMEM. DMEM supplemented with 15% FBS as a chemoattractant was added to the lower chamber. Following incubation for 24 h at 37°C, cotton swabs were used to remove the non-migratory cells. Subsequently, the cells that had migrated to the bottom of the membrane were fixed with 95% ethanol for 20 min at room temperature, and 1% eosin was used for staining for 15 min at room temperature, followed by washing with PBS. The number of the stained cells were counted by Image-Pro Plus v6.0 software (National Institutes of Health) using an inverted light microscope at a ×200 magnification.
Cell invasion assay
For the cell invasion assay, 5×104 transfected cells were added to the top chamber using the same procedure as the cell migration assay. However, the upper chambers were pre-coated with 40 µg Matrigel (BD Biosciences) at 37°C for 30 min at a dilution of 1:8. Subsequently, the cells were cultured for 48 h in a humidified incubator at 37°C, and cotton swabs were used to remove non-invading cells. The invading cells were then fixed, stained and visualized as aforementioned in cell migration assay section.
Tube formation assay
Serum-free DMEM and pre-cooled melted Matrigel were mixed at a ratio of 1:1. The Matrigel mixture (50 µl) was coated onto a 96-well plate, and polymerized for 1 h at 37°C. Subsequently, 2×103 HMEC-1 cells were seeded on the surface of the Matrigel into each well and incubated at 37°C. Tube formation was observed using an inverted light microscope at a magnification of ×100 after 1, 2, 6, 12 and 24 h. For quantification, the extent of tube formation was assessed by determining the number and branching points of the tube formations.
Western blot analysis
Cell Extraction buffer (Thermo Fisher Scientific, Inc.) was used to lyse the cells to extract protein, and the bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc.) was used to quantify the protein. Protein (30 µg) was then separated using 10% SDS-PAGE and transferred to PVDF membranes (EMD Millipore). The membranes were blocked with 5% non-fat milk at room temperature for 2 h, followed by incubation overnight at 4°C with the relevant primary antibodies against PDCD4 (1:1,000; Abcam; cat. no. ab51495), c-Jun (1:1,000; Abcam; cat. no. ab31419), phosphorylated (p-)c-Jun (1:1,000; Abcam; cat. no. ab32385), matrix metalloproteinase (MMP)2 (1:1,000; ProteinTech Group, Inc.; cat. no. 10373-2-AP), MMP9 (1:800; ProteinTech Group, Inc.; cat. no. 10375-2-AP), angiotensin (ANG)-1 (1:1,000; ProteinTech Group, Inc.; cat. no. 23302-1-AP), vascular endothelial growth factor (VEGF)A (1:2,000; ProteinTech Group, Inc.; cat. no. 66828-1-lg) and GAPDH (1:4,000; Santa Cruz Biotechnology, Inc.; cat. no. sc-47724). The PVDF membranes were washed with TBS with 0.1% Tween-20 and incubated for 1 h at room temperature with a horseradish peroxidase conjugated anti-mouse or rabbit secondary antibody (1:3,000; ProteinTech Group, Inc.; cat. nos. SA00001-1 and SA00001-2). An ECL kit (Advansta, Inc.) and Image Lab software 4.0 (Bio-Rad Laboratories, Inc.) were used to detect the protein levels and band intensities.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was used to extract total RNA from A498 and 786-O cells according to the manufacturer's protocol. miR-21 expression was detected with the NCode EXPRESS SYBR-GreenER miRNA RT-qPCR kit (Invitrogen; Thermo Fisher Scientific, Inc.) and normalized to the expression of U6 snRNA. c-Jun mRNA expression was detected with the SYBR PrimeScript RT-PCR kit II (Takara Bio, Inc.) and normalized to the expression levels of GAPDH. The reverse transcription temperature protocol was as follows: 42°C for 30 min and 85°C for 5 sec. The thermocycling conditions for qPCR were as follows: 95°C for 10 min; followed by 40 cycles of 95°C for 10 sec and 58°C for 45 sec. (Invitrogen; Thermo Fisher Scientific, Inc.). The relative expression level was calculated using the 2−ΔΔCq method (30). The PCR primers were as follows: miR-21 forward, 5′-TAGCTTATCAGACTGATG-3′ and reverse, 5′-TGGTGTCGTGGAGTCG-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′; c-Jun forward, 5′-ATCCTGAAACAGAGCATGAC-3′ and reverse, 5′-TTGCTGGACTGGATTATCA-3′; and GAPDH forward, 5′-TCAACGACCACTTTGTCAAGCTCA-3′ and reverse, 5′-GCTGGTGGTCCAGGGGTCTTACT-3′.
Chromatin immunoprecipitation (ChIP) assay
To examine whether c-Jun or p-c-Jun [containing activator protein 1 (AP-1) binding site] directly interacted with the promoter region of miR-21, ChIP assays were performed using A498 and 786-O cells. In brief, the cells were crosslinked with 1% formaldehyde for 10 min at room temperature. Subsequently, the cells were lysed with cell lysis buffer and then the cell lysates were sonicated 10-15 times for 10 sec each time. The sonicated lysates were then incubated with normal immunoglobulin G (1:1,000; Abcam; cat. no. ab171870), c-Jun (1:1,000; Abcam; cat. no. ab31419) or p-c-Jun (1:1,000; Abcam; cat. no. ab32385) overnight at 4°C, followed by immunoprecipitation with protein A/G agarose beads. The samples were reverse-cross-linked at 65°C for 4 h, and the immunoprecipitated DNA fragments were extracted with a QiAquick PCR Purification Kit (Qiagen, Inc.; cat. no. 28104). DNA was quantified by PCR, using the following primers: Forward, 5′-GCCTCCCAAGTTTGCTAATG-3′ and reverse, 5′-TGTACTCTGGTATGGCACAAAGA-3′, in a 1% agarose gel with the DNA fluorescent dye GelStain (1:10,000; cat. no. GS101; Beijing Transgen Biotech Co., Ltd.). The DNA bands were observed using a TGel Image System (OSE-470P; Tiangen Biotech Co., Ltd.) The thermocycling conditions were: 10 min at 95°C; followed by 40 cycles of 30 sec at 95°C, 30 sec at 60°C and 30 sec at 72°C.
Data analysis
Stata v13.0 software (StataCorp LP) was used to perform the complete data meta-analysis. The effects of the factors of interest were evaluated with HR/RR/OR estimates and 95% CIs. To test heterogeneity, the χ2 and I2 tests were used. In each analysis, the potential bias was assessed by a funnel plot and Egger's test. SPSS version 20.0 (IBM, Corp.) was used to perform the statistical analyses. Values are presented as the mean ± standard error of the mean of three independent experiments. Mann-Whitney U test or Student's t-test were used to analyse the differences between two groups, and a one-way ANOVA with a post-hoc Tukey's test was used to compare multiple groups. P<0.05 was considered to indicate a statistically significant difference.
Results
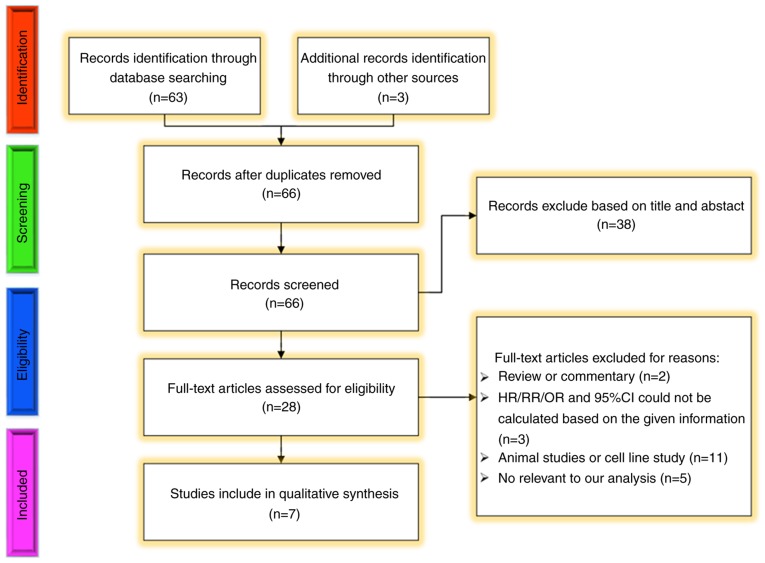
Identification and eligibility of studies included in the meta-analysis
Table I summarizes the main characteristics of the seven relevant cohort studies (22-28). These studies were published between 2012 and 2018. Of the included studies, three were performed in North America, three in Central Europe and one in East Asia. The sample size of the studies ranged between 36 and 121 patients (total, n=473). Patients with pT1-pT3 RCC and T3 RCC were enrolled respectively in three studies and one study included patients with all stages of RCC. The stage of patients RCC patients was unknown in two of the studies. The detection method in six of the studies was RT-qPCR and in one study, it was qPCR.
Table I.
Main characteristics of the seven included cohort studies.
| Author, year | Origin of populations | Number of patients | Stage | Sample type | Detection method | Cut-off value | Outcome | Follow up (Month) | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|
| Lokeshwar et al, 2018 | USA | 75 | pT0-pT4 | Frozen tissue | qPCR | ROC curve | OS MFS | 23 | (22) |
| Kowalczyk et al, 2016 | Poland | 56 | pT1-pT3 | Frozen tissue | RT-qPCR (TaqMan) | - | OS | 28.8 | (23) |
| Tang and Hu, 2015 | China | 45 | - | Frozen tissue | RT-qPCR (SYBR) | X-tile algorithm | CSS | 58.4 | (24) |
| Vergho et al, 2014 | Germany | 103 | pT1-pT3 | Frozen tissue | RT-qPCR (TaqMan) | ROC curve | CSS | 68 | (25) |
| Vergho et al, 2014 | Germany | 37 | T3 | FFPE | RT-qPCR (TaqMan) | ROC curve | CSS | 152 | (26) |
| Faragalla et al, 2012 | Canada | 121 | pT1-pT3 | FFPE | RT-qPCR (TaqMan) | X-tile algorithm | OS DFS | 50 | (27) |
| Zaman et al, 2012 | USA | 36 | - | FFPE | RT-qPCR (TaqMan) | - | OS | - | (28) |
ROC, receiver operating curve; RT-qPCR, reverse transcription-quantitative PCR; qPCR, quantitative PCR; OS, overall survival; CSS, cancer-specific survival; MFS, metastasis-free survival; DFS, disease-free survival; FFPE, formalin-fixed and paraffin-embedded.
Meta-analysis of the effect of miR-21 expression on RCC prognosis and patient survival
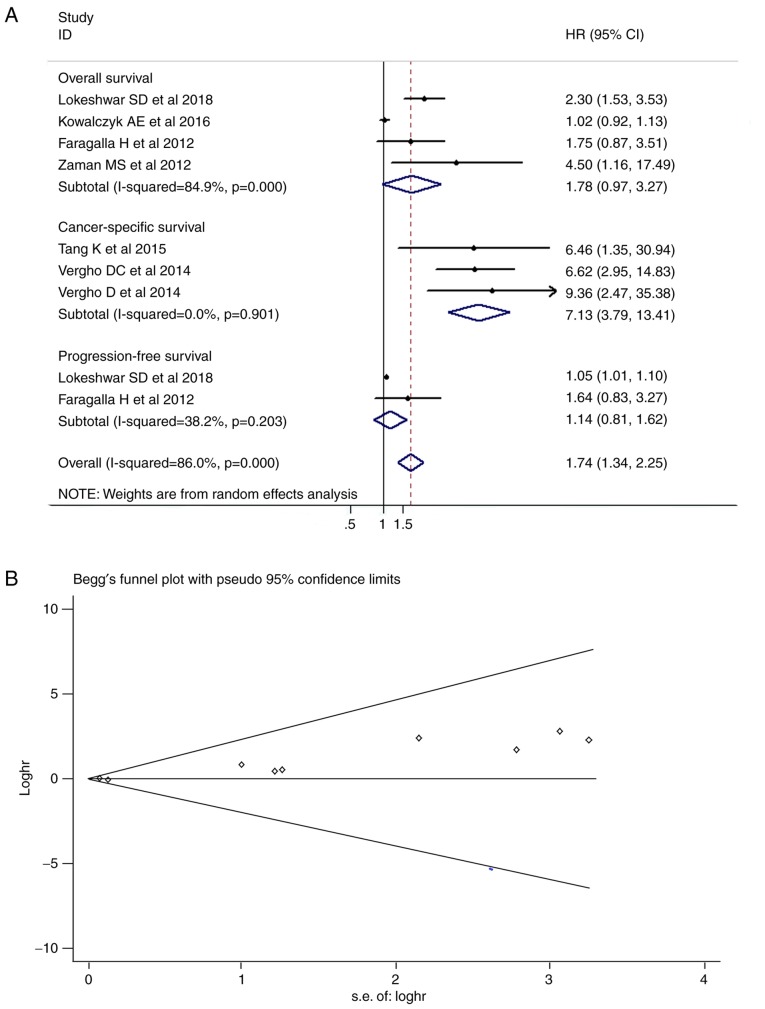
Among the seven included studies, which contained a total of 473 patients with survival data, three studies reported an association between miR-21 expression and CSS in RCC, and the remaining studies investigated OS. Due to heterogeneity, a random-effects model was used to calculate the pooled HR value for the survival and progression data (Fig. 1). The combined HR was calculated as 1.74 (95% CI, 1.34-2.25; P<0.001) in Fig. 2A, suggesting that increased expression levels of miR-21 were significantly associated with adverse prognosis in the pooled patient group. In the subgroup analysis, the expression levels of miR-21 were significantly associated with CSS, with a pooled HR estimate of 7.13 (95% CI, 3.79-13.41; P<0.001). However, non-significant decreases in OS and PFS were observed in patients with RCC with high miR-21 expression (P=0.063 and P=0.443, respec tively). The Begg's funnel plot revealed asymmetry (Fig. 2B), which is typically associated with publication bias. For the Egger's regression asymmetry test, the P-value was 0.002, indicating publication bias.
Figure 1.
Methodological flow diagram of the systematic review process. Among these, other sources included potentially eligible articles or abstracts which were published in major academic conferences (European Society of Urology, American Urological Association, Asian Society of Urology, American Society of Clinical Oncology, European Society for Medical Oncology amongst others). HR, hazard ratio; RR, risk ratio, OR, odds ratio; CI, confidence interval.
Figure 2.
Meta-analysis of miR-21 expression and survival of patients with RCC. (A) Forest plot and (B) funnel plot of the association between the miR-21 expression and survival of patients with RCC. Squares represent HR in each trial. The horizontal line crossing the square indicates the 95% CI. Diamonds represent the predicted pooled effect beneath the Mantel-Haenszel random-effects model. Visual inspection of the Begg's funnel plot identified slight asymmetry. HR, hazard ratio; CI, confidence interval; s.e., standard error.
miR-21 regulates the migration, invasion and angiogenic abilities of A498 and 786-O cells by targeting PDCD4
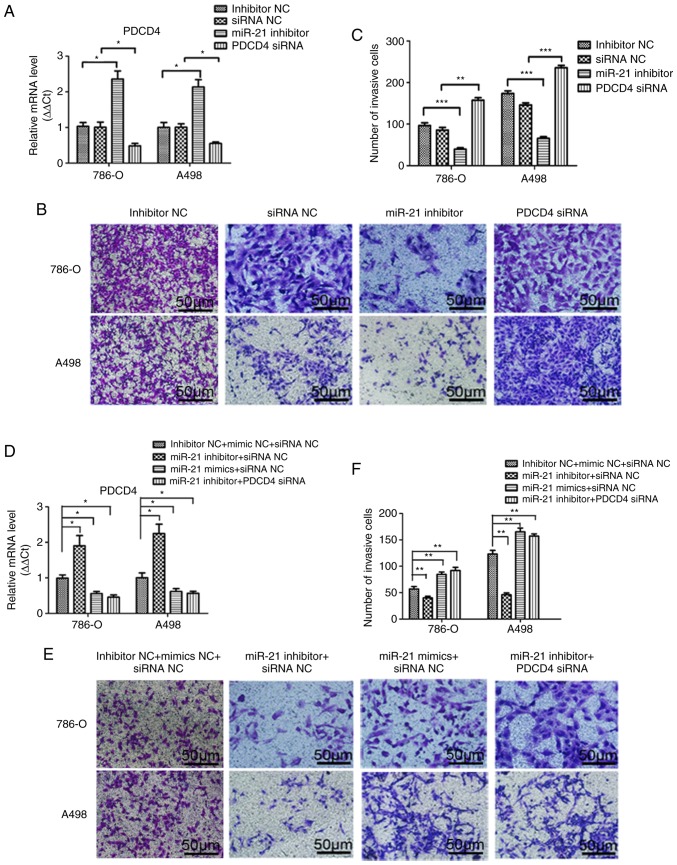
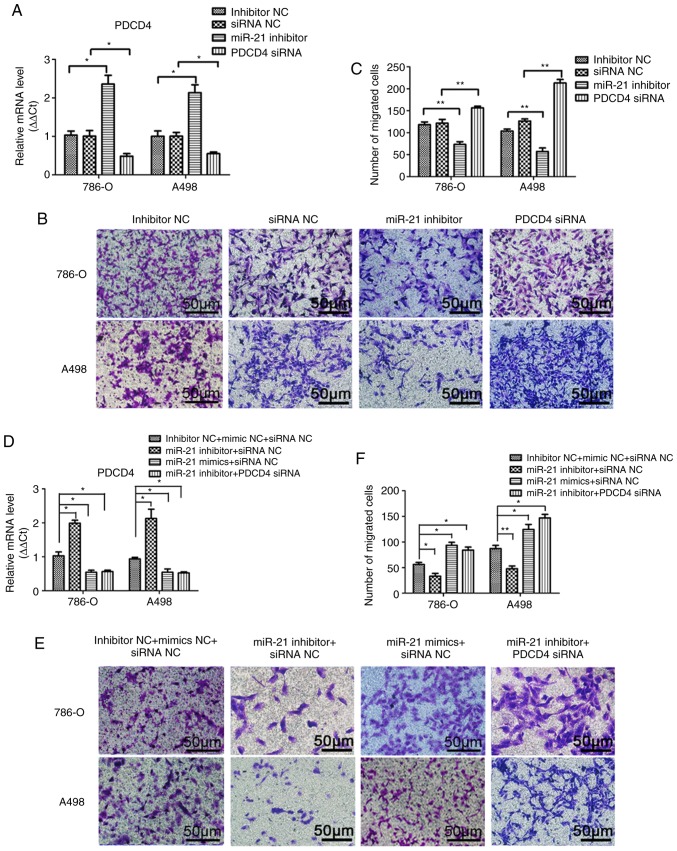
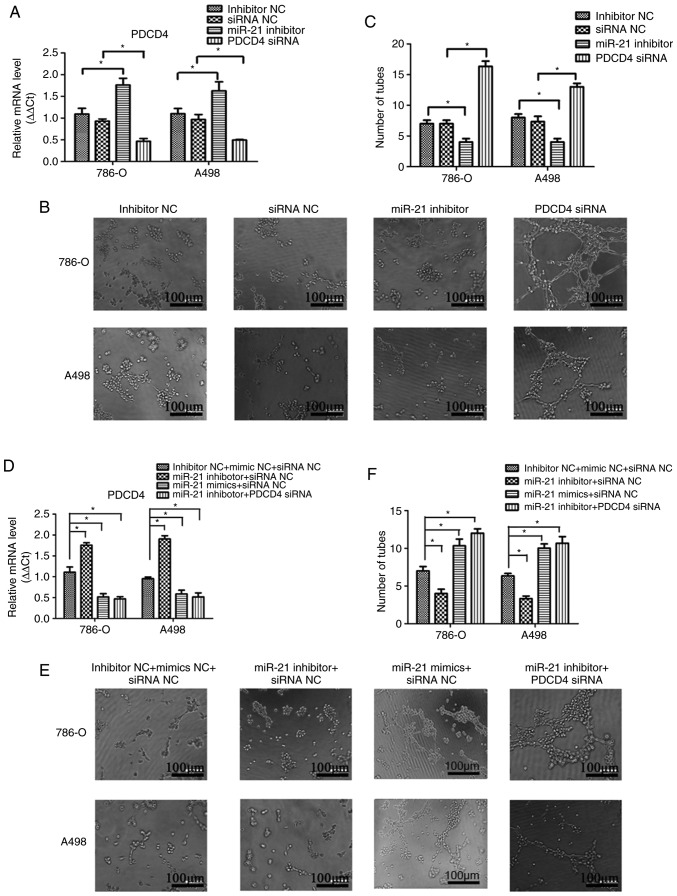
A Transwell assay was performed to investigate the effects of miR-21 on RCC cell migration and invasion. As shown in Figs. 3B and C, and 4B and C, notable differences were observed in the migration and invasion abilities among the different groups of A498 or 786-O cells following transfection. Transfection efficiency was verified by qPCR (Figs. 3A and 4A). Compared with the miRNA inhibitor NC and siRNA NC groups, the migration and invasion abilities were significantly decreased in the miR-21 inhibitor group, and significantly increased in the group transfected with PDCD4 siRNA. To elucidate the effect of the supernatant of A498 or 786-O cells on the endothelial cell line HMEC-1, a tube formation assay in Matrigel was performed, and the results are presented in Fig. 5. The transfection efficiency was verified by qPCR (Fig. 5A). Similarly, by inhibiting miR-21 expression, the number of tubes and tube junctions in the miR-21 inhibitor group was significantly decreased, whereas downregulating PDCD4 increased the angiogenic ability (Fig. 5B and C). Additionally, the migration, invasion and tube formation abilities in the miR-21 inhibitor + siRNA NC group were lower compared with those in the inhibitor NC + siRNA NC and the miR-21 inhibitor + PDCD4 siRNA groups, whereas the decrease in these abilities was reversed in the miR-21 mimics + siRNA NC group (Figs. 3E and F; 4E and F and 5E and F). These findings indicated that miR-21 may regulate the migration, invasion and angiogenic abilities of RCC cells through PDCD4.
Figure 3.
miR-21 promotes the migration of A498 and 786-O cells through PDCD4 regulation. (A) Transfection efficiency was verified by quantitative PCR after transfection with miR-21 inhibitor, PDCD4 siRNA, miRNA inhibitor NC or siRNA NC for 48 h. (B) Migratory ability was observed using a Transwell assay after transfection with miR-21 inhibitor, PDCD4 siRNA, miRNA inhibitor NC or siRNA NC. (C) The number of migrating cells were calculated. (D) Transfection efficiency was verified by quantitative PCR after transfection with combined miR-21 inhibitor, miR-21 mimics, PDCD4 siRNA and the respective NCs. (E) Migratory ability was observed using a Transwell assay after transfection with combined miR-21 inhibitor, miR-21 mimics, PDCD4 siRNA and respective NCs. (F) The number of migrating cells were calculated. Data are presented as the mean ± standard error of the mean. *P<0.05; **P<0.01; ***P<0.001. PDCD4, programmed cell death protein 4; miRNA, microRNA; siRNA, small interfering RNA; NC, negative control.
Figure 4.
Effect on the invasion of A498 and 786-O cells by miR-21 expression through PDCD4. (A) Transfection efficiency was verified by quantitative PCR after transfected with miR-21 inhibitor, PDCD4 siRNA, miRNA inhibitor NC or siRNA NC for 48 h. (B) The invasion ability was observed by Transwell assay after transfected with miR-21 inhibitor, PDCD4 siRNA, miRNA inhibitor NC and siRNA NC. (C) Number of invading cells were calculated. (D) Transfection efficiency was verified by quantitative PCR after transfected with combined miR-21 inhibitor, miR-21 mimics, PDCD4 siRNA and respective NC. (E) The invasion ability was observed by Transwell assay after transfected with combined miR-21 inhibitor, miR-21 mimics, PDCD4 siRNA and respective NC. (F) Number of invading cells were calculated. Data are presented as the mean ± standard error of the mean. *P<0.05; **P<0.01. PDCD4, programmed cell death protein 4; miRNA, microRNA; siRNA, small interfering RNA; NC, negative control.
Figure 5.
miR-21 promotes the angiogenic ability of A498 and 786-O cells through targeting PDCD4. (A) Transfection efficiency was determined by quantitative PCR after transfection with miR-21 inhibitor, PDCD4 siRNA, miRNA inhibitor NC or siRNA NC. (B) Conditioned medium from 786-O and A498 cells transfected with miR-21 inhibitor, PDCD4 siRNA, miRNA inhibitor NC and siRNA NC was used to overlay HMEC-1 cells seeded on a bed of Matrigel for 6 h in an endothelial cell tube formation assays. (C) Number of tubes and tube junctions were calculated. (D) Transfection efficiency was verified by quantitative PCR after transfected with combined miR-21 inhibitor, miR-21 mimics, PDCD4 siRNA and respective NC. (E) Conditioned medium from 786-O and A498 cells transfected with miR-21 inhibitor, miR-21 mimics, PDCD4 siRNA and respective NC was used to overlay HMEC-1 cells seeded on a bed of Matrigel for 6 h in endothelial cell tube formation assays. (F) Number of tubes and tube junctions were calculated. Data are presented as the mean ± standard error of the mean. *P<0.05. PDCD4, programmed cell death protein 4; miRNA, microRNA; siRNA, small interfering RNA; NC, negative control.
miR-21 regulates AP-1 signalling in RCC cells via PDCD4
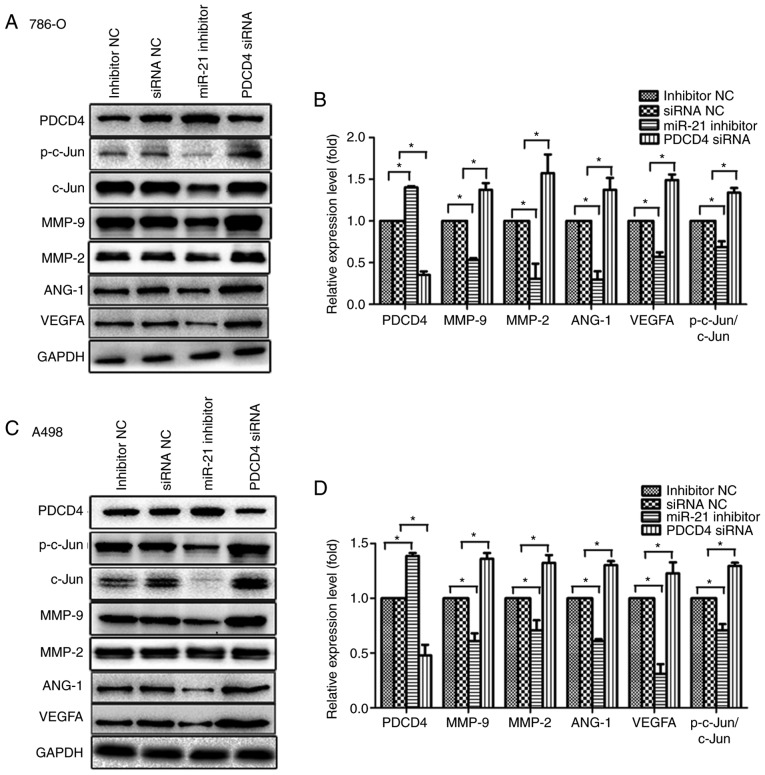
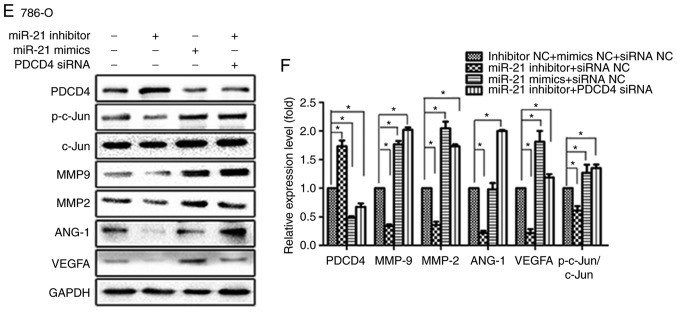
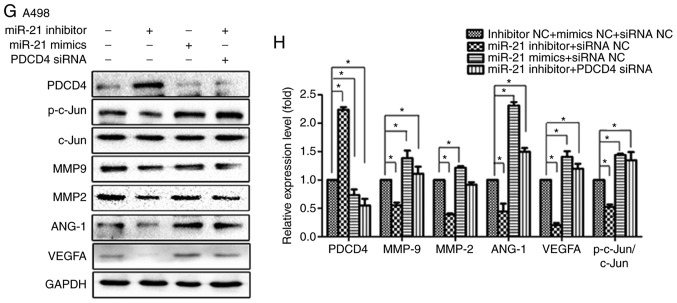
To elucidate the mechanism underlying the effect of miR-21 on the migration, invasion and angiogenesis of RCC cells by regulating the PDCD4/AP-1 signalling pathway, A498 and 786-O cells were temporarily transfected with miR-21 inhibitor, PDCD4 siRNA, inhibitor NC or siRNA NC. The results demonstrated that miR-21 inhibition decreased the protein levels of p-c-Jun and total c-Jun in A498 and 786-O cells, and inhibited the expression of downstream molecules, including MMP2, MMP9, ANG-1 and VEGFA (Fig. 6A D). Furthermore, the present study investigated the ability of PDCD4 to regulate the miR-21-mediated inhibition of MMP2, MMP9, ANG-1 and VEGFA protein expression. miR-21 inhibition induced downregulation of MMP2, MMP9, ANG-1, VEGFA and p-c-Jun levels in A498 and 786-O cells was rescued by PDCD4 siRNA (Fig. 6E G), and miR-21 mimics exhibited the same effects as PDCD4 siRNA.
Figure 6.
Suppression of PDCD4 expression, activation of c-Jun and expression of downstream signalling molecules, including MMP2, MMP9, ANG-1 and VEGFA, with increased miR-21 expression. (A) Transfection of 786-O cells with miRNA inhibitor NC, siRNA NC, miR-21 inhibitor or PDCD4 siRNA. Proteins were extracted and analysed by western blotting. (B) Relative protein expression by scanning densitometry was calculated. (C) Transfection of A498 cells with miRNA inhibitor NC, siRNA NC, miR-21 inhibitor or PDCD4 siRNA. Proteins were extracted and analysed by western blotting. (D) Relative protein expression by scanning densitometry was calculated. (E) Transfection of 786-O cells with a combination of miR-21 inhibitor, miR-21 mimics, PDCD4 siRNA and respective NC. Total protein was extracted and analysed by western blotting. (F) Relative protein expression by scanning densitometry was calculated. Suppression of PDCD4 expression, activation of c-Jun and expression of downstream signalling molecules, including MMP2, MMP9, ANG-1 and VEGFA, with increased miR-21 expression. (G) Transfection of A498 cells with a combination of miR-21 inhibitor, miR-21 mimics, PDCD4 siRNA and respective NC. Total protein was extracted and analysed by western blotting. (H) Relative protein expression by scanning densitometry was calculated. Data are presented as the mean ± standard error of the mean. *P<0.05. PDCD4, programmed cell death protein 4; miRNA, microRNA; siRNA, small interfering RNA; NC, negative control; VEGF, vascular endothelial growth factor; MMP, matrix metalloproteinase.
Transcription of miR-21 is activated by c-Jun
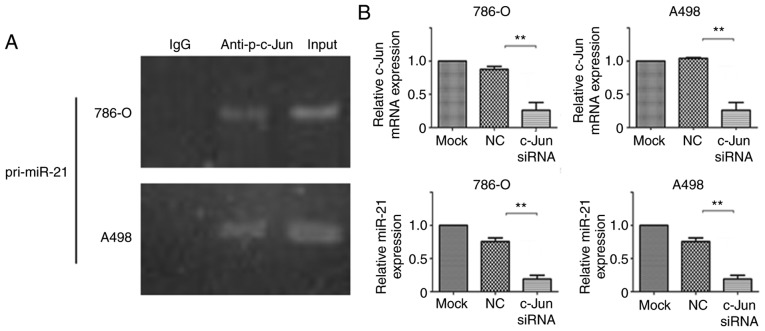
It has been previously demonstrated that c-Jun motivates gene transcription by attaching to its binding sites within the promoter regions of genes (31,32). To examine whether c-Jun directly interacts with the promoter region of miR-21, the pri-miR-21 promoter regions associated with p-c-Jun were assessed by ChIP assay. The results (Fig. 7A) demonstrated that p-c-Jun binds specifically to the pri-miR-21 promoter region in A498 and 786-O cells. To assess the control of miR-21 expression by c-Jun in RCC, A498 and 786-O cells were transfected with c-Jun siRNA and the expression levels of miR-21 were subsequently determined. As shown in Fig. 7B, the expression levels of miR-21 were significantly decreased in cells following transfection with c-Jun siRNA compared with in control cells, indicating that c-Jun may activate miR-21 transcription.
Figure 7.
Transcription of miR-21 is activated by p-c-Jun. (A) Chromatin immunoprecipitation analysis demonstrated that p-c-Jun binds specifically to the pri-miR-21 promoter region. PCR amplification of the region containing the p-c-Jun recognition sequence in the pri-miR-21 DNA. (B) Inhibition of miR-21 expression by a decrease in c-Jun expression in A498 and 786-O cells. Transfection of A498 and 786-O cells with mock, NC or c-Jun siRNA for 24 h. c-Jun and miR-21 expression levels were examined using reverse transcription-quantitative PCR analysis and normalized to GAPDH and U6 snRNA expression, respectively. Data are presented as the mean ± standard error of the mean. **P<0.01. p-, phospho; miRNA, microRNA; siRNA, small interfering RNA; NC, negative control; IgG, immunoglobulin.
Discussion
Several studies have indicated that high miR-21 expression in patients with RCC is associated with an unfavourable prognosis, including poorer metastasis-free survival, OS and disease-specific mortality (33,34). However, Kowalczyk et al (23) identified dissimilar outcomes regarding miR 2l expression and its prognostic value in RCC. A survey of 56 patients with RCC undergoing radical nephrectomy revealed that high levels of miR-21 expression were not an independent predictor of OS (23). Therefore, the prognostic implications of miR-21 in patients with RCC are inconsistent. On one hand, specific race/sex/age-associated factors may be responsible for these differences (35). Delfino et al (36) reported that four miRNAs, including ebv-miR-bhrf1-1, hsa-miR-565, hsa-miR-137 and hsa-miR-512-3p, are associated with OS and PFS in glioblastoma. On the other hand, different sample types and lack of a unified cut-off value for miR-21 may affect the results and produce statistical heterogeneity (37,38). Frozen or formalin-fixed tissues and paraffin-embedded tissues are the sources for total RNA extraction. However, RNA degradation caused by formalin fixation may affect subsequent quantitative analyses (37,38). Kakimoto et al (39) revealed that the mean read length of RNAs from formalin-fixed and paraffin-embedded (FFPE) tissue is shorter compared with that from the matched refrigerated sample, demonstrating that longer RNA is segmented into smaller RNA, resulting in an increase in total reading count in FFPE samples. Finally, certain quantitative methods for miRNAs are based on RT-qPCR, including TaqMan and SYBR. TaqMan's advanced miRNA assays can translate all miRNAs into cDNA in the same tube. As TaqMan analysis is occasionally restricted by the efficiency of the additional enzymatic steps required, exceptive reagents, including enzymatic stem loop probes and locked nucleic acid modified primers, which may reduce nonspecific ligation of probes and interference of precursor miRNA, are required (40,41). Androvic et al (42) used a two-tailed RT-qPCR approach, which uses SYBR Green to achieve the efficiency of a poly-tail-based approach.
miR-21 is frequently overexpressed in cancer, acting as an oncogene and tumour prognostic marker (43,44). In patients with pancreatic cancer, overexpression of miR-21 is associated with a low OS rate and a HR of 2.01 (45). In gastric cancer, Ren et al (46) reported the association between miR-21 and lymph node metastasis and suggested that the expression of miR-21 may be applied to predict lymph node metastasis. Additionally, miR-21 exerts an effect on the molecular and cellular biology of multiple types of tumours, including the following aspects: i) Promoting malignant biological behaviour. In hepatocellular carcinoma, the overexpression of miR-21 can enhance the liver cancer stem cell phenotype and promote invasion, migration and tumorigenesis (47). Similarly, Qi et al (48) reported that miR-21 expression promotes the growth of gastric cancer cells by targeting prostaglandin E2 to control the PTEN/AKT signalling pathway. ii) Regulating the drug resistance of tumours. By targeting HMG box transcription factor 1, a transcriptional repressor 513 amino acid residues in length, miR-21 markedly affects drug sensitivity and invasion of drug-resistant lung adenocarcinoma cells (49). In epithelial ovarian cancer, miR-21 may enhance resistance of epithelial cancer cells, and chemoresistance to cisplatin may be improved through downregulation of PTEN (50). iii) Participating in intercellular communication through vesicular miRNAs. As mediators of carcinogenesis, extracellular vesicles are responsible for the communication between the cells of the tumour microenvironment (51). Samsonov et al (52) reported that miR-21 and miR-18Ia-Sp are expressed in the exosomes of patients with thyroid cancer (TC). This comparative assessment may contribute to distinguishing between papillary and follicular types of TC with 100% sensitivity and 77% specificity (52). Finally, in gastric cancer, proliferation of BGC-823 cells may be caused by the release of a small RNA-21 inhibitor from macrophages (53).
It has been widely reported that miR-21 is involved in the regulation of the aggressiveness of several diseases, such as hepatic fibrosis in chronic hepatitis C virus (54), myeloid leukaemia (55) and hypertensive kidney injury (56). As miR-21 is expressed aberrantly in rheumatoid arthritis (RA), an inhibitor targeting miR-21 may reduce the invasive ability of RA-derived fibroblast-like synoviocytes (FLSs) by inhibiting the transforming growth factor β1/Smad4/7 signalling pathway and altering the expression of MMPs, thereby suppressing the invasiveness of FLSs (57). In addi tion to benign diseases, miR-21 is overexpressed in a variety of diverse malignancies and is associated with metastasis of tumour cells as follows: i) As an upstream promoter, miR-21 affects the expression or biological function of downstream genes associated with tumour suppression, including PTEN, which negatively regulates the PI3K/AKT signalling pathway in the oral squamous cell carcinoma SCC15 and SCC25 cell lines (58); Snail1, which is implicated in epithelial-mesenchymal transition (EMT) by directly suppressing the level of E-cadherin in salivary adenoid cystic carcinoma (59); and PDCD4, which activates the expression of AKT, IKKβ and mTORC1, which are necessary for the migration and inva sion of RCC cells (60). Additionally, Bera et al (60) reported that there is a positive feedback loop among miR-21 level, phosphorylated IKKβ and NF κB activation, as PDCD4 has been demonstrated to negatively affect the phosphorylation and activation of IKKβ and NF κB. Furthermore, the present study revealed that miR-21 resulted in variations in the expression levels of MMPs by targeting the PDCD4/c-Jun signalling pathway, which is involved in the metastasis of renal cancer. ii) As a downstream tumour promoting miRNA, the levels or target genes of miR-21 are regulated by upstream oncogenes, including Sox2, which not only positively regulates miR21 associated migration/invasion signalling in glioma cells, but also induces EMT of laryngeal cancer by activation of Wnt/β catenin signalling (61). As an upstream regulator of miR-21, p-STAT3 may regulate the metastatic capacity of hepatocellular carcinoma cells by targeting miR-21, which increases expression of cysteine rich proteins with kazal motifs and PDCD4 (62). iii) As endocytosis of exosomes of target cancer cells contributes to the intracellular release of vesicular contents, exosomal miR-21 can increase the ability of invasion potential and aggressive phenotype of ovarian cancer cells through upregulation of MMP1, which is transferred by cell-cell communication (63).
Angiogenesis is not only a key developmental process, such as ocular neovascularization, it is also essential in the pathological processes of various diseases including cardiovascular diseases (64), osteoarthritis (65) and diabetic peripheral neuropathy (66,67). Therefore, research regarding the role of miR-21 in angiogenesis is currently focused mainly on haematological tumours. In diffuse large B-cell lymphoma, Zheng et al (68) demonstrated a direct link between miR-21 and tumour angiogenesis in lymphoma. miR-21 can increase the interaction of endothelial cells with Treg cells. Subsequently, after enhancing the expression of inducible T cell costimulator (ICOS) on Treg cells, miR-21 prompts tumour angiogenesis via ICOS/inducible T cell costimulator ligand pathway signalling, which promotes disease progression and chemoresistance of B-cell lymphoma (68). In acute monocytic leukaemia (AML), the expression levels of miR-21 and VEGF in the peripheral blood monocytes of the patients was higher compared with that in healthy controls. Being the direct target of miR-21, the level of interleukin 12 (IL-12) in the supernatant of THP-1 cells is increased following transfection with miR-21 mimic (69). Additionally, IL-12 may induce VEGF expression and angiogenic ability in human umbilical vein endothelial cells, which suggests that miR-21 may possess pro-angiogenic properties in human AML (69). At present, there is little research on the role of miR-21 in the process of solid tumour angiogenesis. In colorectal cancer, miR-21 is overexpressed and affects cell cycle progression, apoptosis and viability of colon cancer cells (70). Song and Rossi (70) identified an anti-miR-21 that targets miR-21 to inhibit genes by post transcriptional or transcriptional gene silencing. Since anti-miR-21 and pri-miR30 exhibit homology between anti-miR-21 and the 3′ end of pri-miR30, anti-miR-21 may reduce the expression of miR30, which affects vessel number and length, by inhibition of angiogenic pathways (70). Therefore, anti-miR-21 may be a useful curative strategy by regulating the process of angiogenesis in colon cancer. In the present study, the effects of the supernatant from A498 or 786-O cells on the endothelial HMEC-1 cell line were investigated, and it was observed that the quantity of formed tubes and tube junctions in the miR-21 inhibitor group was significantly decreased, whereas that in the PDCD4 siRNA group was significantly increased, compared with the respective control groups. To the best of our knowledge, this constitutes the first evidence that miR-21 expression may promoted the ability of HMEC-1 cells to become organised into tubular networks by directly targeting the PDCD4/c-Jun signalling pathway and regulating the level of ANG-1 and VEGFA, indicating a direct association between miR-21 and tumour angiogenesis and progression in RCC.
There are several miR-21 target genes. PDCD4 has been recognized as a protein expressed during the processes of apoptosis and tumour suppression. However, only few studies have specifically demonstrated that there is an interaction between miR-21 and PDCD4 in RCC (71). As AP-1 is a transcription activating heterodimer composed of c-Jun and c-Fos, studies have demonstrated (72-74) that AP-1 is associated with invasion and metastasis of tumours by regulating MMP2 or MMP9, and with angiogenesis by controlling ANG-1 or VEGFA. Under the influence of miR-21, the protein levels of downstream molecules of the PDCD4 AP-1 signalling pathway, including MMP2, MMP9, ANG-1 and VEGFA, were decreased. Additionally, in A498 and 786-O cells, the miR-21 inhibition induced downregulation of MMP2, MMP9, ANG-1, VEGFA and p-c-Jun expression was reversed by PDCD4 siRNA. Among the targets (Fig. 8), ANG-1 usually promotes the interaction between endothelial and perivascular cells to obtain a stable vasculature (75,76). VEGFA serves a key role in the growth of new vessels and has become a promising target for anti-angiogenesis based tumour therapy (77,78). As MMPs have been initially identified as proteases that act on the extracellular matrix, the overexpression of MMP2 and MMP9 has been associated with aggressive behaviour of tumours and metastasis (79-81).
Figure 8.
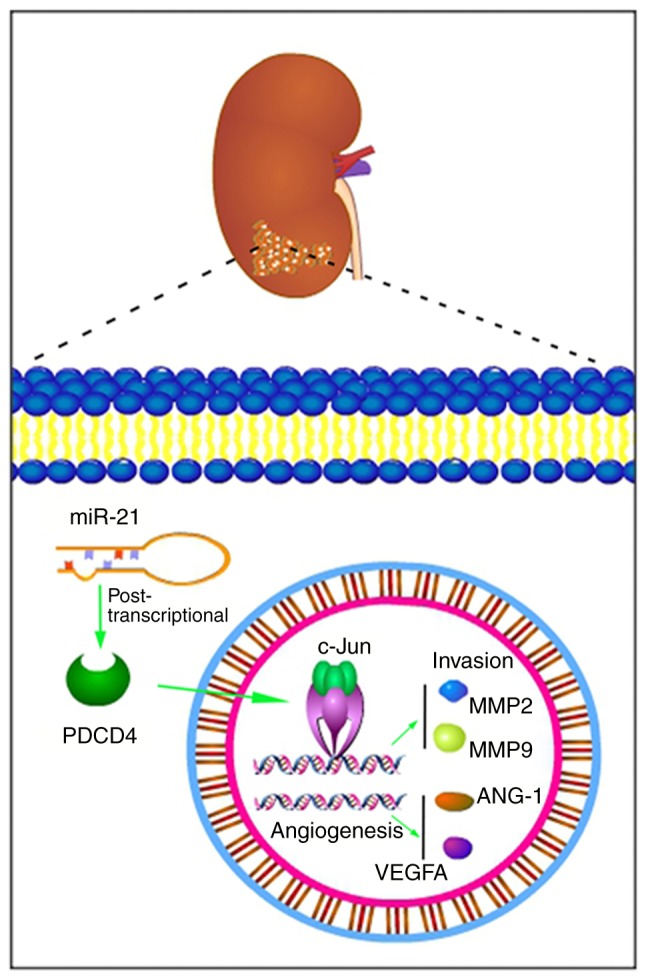
Schematic diagram of the roles of miR-21 and the PDCD4/c-Jun signalling pathway in renal cell carcinoma. The overexpression of miR-21 and the reduced PDCD4 expression, leading to the activation of c-Jun, further increased the levels of MMP2, MMP9, ANG-1 and VEGFA, which promoted tumour cell migration, invasion and angiogenesis. PDCD4, programmed cell death protein 4; miR, microRNA; VEGF, vascular endothelial growth factor; MMP, matrix metalloproteinase.
In summary, the present study attempted to combine evidence-based medicine and molecular biology and demonstrated that increased miR-21 levels were significantly associated with adverse prognosis in patients with RCC. Additionally, the migration, invasion and angiogenic abilities of RCC cells were markedly affected by the expression of miR-21 through direct targeting of the PDCD4/c-Jun signalling pathway, indicating that miR-21 may be of value as a therapeutic target for RCC.
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the National Natural Science Foundation of China (grant no. 31800787, grant no. 81572505 and grant no. 81972831), the Natural Science Foundation of Liaoning Province (grant no. LQ2017025), the Doctoral Research Startup Foundation of Liaoning Province (grant no. 20180540020) and the Medical Scientific Research Project of Dalian City (grant no. 1812038).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
BF, YYJ, HSZ, TJL and XCL conceived and designed the study and wrote the manuscript. BF, YYJ and HSZ performed the experiments. BF, RZ, MS and MFS collected the data. YYJ, WW and XGW analysed the data. HSZ, WKL, NY and QW interpreted the data. BF, HSZ, TJL and XCL reviewed the manuscript. All authors have read and approved the final version of this manuscript for publication.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bhatt JR, Finelli A. Landmarks in the diagnosis and treatment of renal cell carcinoma. Nat Rev Urol. 2014;11:517–525. doi: 10.1038/nrurol.2014.194. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Stahl M, Wilke H, Schmoll HJ, Schöber C, Diedrich H, Casper J, Freund M, Poliwoda H. A phase II study of high dose tamoxifen in progressive, metastatic renal cell carcinoma. Ann Oncol. 1992;3:167–168. doi: 10.1093/oxfordjournals.annonc.a058136. [DOI] [PubMed] [Google Scholar]
- 4.Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal cell carcinoma. N Engl J Med. 2017;376:354–366. doi: 10.1056/NEJMra1601333. [DOI] [PubMed] [Google Scholar]
- 5.Dabestani S, Marconi L, Hofmann F, Stewart F, Lam TB, Canfield SE, Staehler M, Powles T, Ljungberg B, Bex A. Local treatments for metastases of renal cell carcinoma: A systematic review. Lancet Oncol. 2014;15:e549-e561. doi: 10.1016/S1470-2045(14)70235-9. [DOI] [PubMed] [Google Scholar]
- 6.Qu L, Wang ZL, Chen Q, Li YM, He HW, Hsieh JJ, Xue S, Wu ZJ, Liu B, Tang H, et al. Prognostic value of a long non-coding RNA signature in localized clear cell renal cell carcinoma. Eur Urol. 2018;74:756–763. doi: 10.1016/j.eururo.2018.07.032. [DOI] [PubMed] [Google Scholar]
- 7.Sekar RR, Patil D, Baum Y, Pearl J, Bausum A, Bilen MA, Kucuk O, Harris WB, Carthon BC, Alemozaffar M, et al. A novel preoperative inflammatory marker prognostic score in patients with localized and metastatic renal cell carcinoma. Asian J Urol. 2017;4:230–238. doi: 10.1016/j.ajur.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escudier B, Motzer RJ, Sharma P, Wagstaff J, Plimack ER, Hammers HJ, Donskov F, Gurney H, Sosman JA, Zalewski PG, et al. Treatment beyond progression in patients with advanced renal cell carcinoma treated with nivolumab in checkmate 025. Eur Urol. 2017;72:368–376. doi: 10.1016/j.eururo.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 9.Kroeger N, Stenzl A, Burchardt M, Bedke J. Adjuvant treatment of high-risk renal cell carcinoma: Leaving the det. Eur Urol. 2017;71:695–696. doi: 10.1016/j.eururo.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 10.Leibovich BC, Lohse CM, Cheville JC, Zaid HB, Boorjian SA, Frank I, Thompson RH, Parker WP. Predicting oncologic outcomes in renal cell carcinoma after surgery. Eur Urol. 2018;73:772–780. doi: 10.1016/j.eururo.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Verbiest A, Couchy G, Job S, Caruana L, Lerut E, Oyen R, de Reyniès A, Tosco L, Joniau S, Van Poppel H, et al. Molecular subtypes of clear-cell renal cell carcinoma are prognostic for outcome after complete metastasectomy. Eur Urol. 2018;74:474–480. doi: 10.1016/j.eururo.2018.01.042. [DOI] [PubMed] [Google Scholar]
- 12.Hydbring P, Wang Y, Fassl A, Li X, Matia V, Otto T, Choi YJ, Sweeney KE, Suski JM, Yin H, et al. Cell-cycle-targeting MicroRNAs as therapeutic tools against refractory cancers. Cancer Cell. 2017;31:576–590.e8. doi: 10.1016/j.ccell.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang HY, Barbi J, Wu CY, Zheng Y, Vignali PD, Wu X, Tao JH, Park BV, Bandara S, Novack L, et al. MicroRNA-17 modulates regulatory T cell function by targeting Co-regulators of the Foxp3 transcription factor. Immunity. 2016;45:83–93. doi: 10.1016/j.immuni.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin XJ, Chong Y, Guo ZW, Xie C, Yang XJ, Zhang Q, Li SP, Xiong Y, Yuan Y, Min J, et al. A serum microRNA classifier for early detection of hepatocellular carcinoma: A multicentre, retrospective, longitudinal biomarker identification study with a nested case-control study. Lancet Oncol. 2015;16:804–815. doi: 10.1016/S1470-2045(15)00048-0. [DOI] [PubMed] [Google Scholar]
- 15.Iqbal MA, Arora S, Prakasam G, Calin GA, Syed MA. MicroRNA in lung cancer: Role, mechanisms, pathways and therapeutic relevance. Mol Aspects Med. 2018 Aug 18; doi: 10.1016/j.mam.2018.07.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Croset M, Pantano F, Kan CWS, Bonnelye E, Descotes F, Alix-Panabières C, Lecellier CH, Bachelier R, Allioli N, Hong SS, et al. miRNA-30 family members inhibit breast cancer invasion, osteomimicry, and bone destruction by directly targeting multiple bone metastasis-associated genes. Cancer Res. 2018;78:5259–5273. doi: 10.1158/0008-5472.CAN-17-3058. [DOI] [PubMed] [Google Scholar]
- 17.Song S, Yang Y, Liu M, Liu B, Yang X, Yu M, Qi H, Ren M, Wang Z, Zou J, et al. miR-125b attenuates human hepatocellular carcinoma malignancy through targeting SIRT6. Am J Cancer Res. 2018;8:993–1007. [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, Zhang S, Wang M, Dong S, Wang Y, Liu S, Lu T, Fu Y, Wang X, Chen G. Downregulated miR-21 mediates matrine-induced apoptosis via the PTEN/Akt signaling pathway in FTC 133 human follicular thyroid cancer cells. Oncol Lett. 2019;18:3553–3560. doi: 10.3892/ol.2019.10693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dino P, D'Anna C, Sangiorgi C, Di Sano C, Di Vincenzo S, Ferraro M, Pace E. Cigarette smoke extract modulates E-Cadherin, Claudin-1 and miR-21 and promotes cancer invasiveness in human colorectal adenocarcinoma cells. Toxicol Lett. 2019;317:102–109. doi: 10.1016/j.toxlet.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Sun J, Jiang Z, Li Y, Wang K, Chen X, Liu G. Downregulation of miR-21 inhibits the malignant phenotype of pancreatic cancer cells by targeting VHL. Onco Targets Ther. 2019;12:7215–7226. doi: 10.2147/OTT.S211535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Xin S, He Z, Che X, Wang J, Xiao X, Chen J, Song X. microRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor PDCD4 and promotes cell transformation, proliferation, and metastasis in renal cell carcinoma. Cell Physiol Biochem. 2014;33:1631–1642. doi: 10.1159/000362946. [DOI] [PubMed] [Google Scholar]
- 22.Lokeshwar SD, Talukder A, Yates TJ, Hennig MJP, Garcia Roig M, Lahorewala SS, Mullani NN, Klaassen Z, Kava BR, Manoharan M, et al. Molecular characterization of renal cell carcinoma: A potential three MicroRNA prognostic signature. Cancer Epidemiol Biomarkers Prev. 2018;27:464–472. doi: 10.1158/1055-9965.EPI-17-0700. [DOI] [PubMed] [Google Scholar]
- 23.Kowalczyk AE, Krazinski BE, Godlewski J, Grzegrzolka J, Kiewisz J, Kwiatkowski P, Sliwinska-Jewsiewicka A, Dziegiel P, Kmiec Z. SATB1 is down-regulated in clear cell renal cell carcinoma and correlates with miR-21-5p overexpression and poor prognosis. Cancer Genomics Proteomics. 2016;13:209–217. [PubMed] [Google Scholar]
- 24.Tang K, Xu H. Prognostic value of meta-signature miRNAs in renal cell carcinoma: An integrated miRNA expression profiling analysis. Sci Rep. 2015;5:10272. doi: 10.1038/srep10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vergho D, Kneitz S, Rosenwald A, Scherer C, Spahn M, Burger M, Riedmiller H, Kneitz B. Combination of expression levels of miR-21 and miR-126 is associated with cancer-specific survival in clear cell renal cell carcinoma. BMC Cancer. 2014;14:25. doi: 10.1186/1471-2407-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vergho DC, Kneitz S, Kalogirou C, Burger M, Krebs M, Rosenwald A, Spahn M, Löser A, Kocot A, Riedmiller H, Kneitz B. Impact of miR-21, miR-126 and miR-221 as prognostic factors of clear cell renal cell carcinoma with tumor thrombus of the inferior vena cava. PLoS One. 2014;9:e109877. doi: 10.1371/journal.pone.0109877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faragalla H, Youssef YM, Scorilas A, Khalil B, White NM, Mejia-Guerrero S, Khella H, Jewett MA, Evans A, Lichner Z, et al. The clinical utility of miR-21 as a diagnostic and prognostic marker for renal cell carcinoma. J Mol Diagn. 2012;14:385–392. doi: 10.1016/j.jmoldx.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Zaman MS, Shahryari V, Deng G, Thamminana S, Saini S, Majid S, Chang I, Hirata H, Ueno K, Yamamura S, et al. Up regulation of microRNA-21 correlates with lower kidney cancer survival. PLoS One. 2012;7:e31060. doi: 10.1371/journal.pone.0031060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Zhou J, Wang KC, Wu W, Subramaniam S, Shyy JY, Chiu JJ, Li JY, Chien S. microRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci USA. 2011;108:10355–10360. doi: 10.1073/pnas.1107052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Echevarría-Vargas IM, Valiyeva F, Vivas-Mejía PE. Upregulation of miR-21 in cisplatin resistant ovarian cancer via JNK-1/c-Jun pathway. PLoS One. 2014;9:e97094. doi: 10.1371/journal.pone.0097094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlsson J, Christiansen J, Davidsson S, Giunchi F, Fiorentino M, Sundqvist P. The potential role of miR 126, miR-21 and miR 10b as prognostic biomarkers in renal cell carcinoma. Oncol Lett. 2019;17:4566–4574. doi: 10.3892/ol.2019.10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Gu Y, Shen W. microRNA-21 functions as an oncogene and promotes cell proliferation and invasion via TIMP3 in renal cancer. Eur Rev Med Pharmacol Sci. 2017;21:4566–4576. [PubMed] [Google Scholar]
- 35.Fan B, Zhang H, Jin H, Gai Y, Wang H, Zong H, Jin M, Yang H, Wan S, Zhu J, et al. Is overexpression of Ki-67 a prognostic biomarker of upper tract urinary carcinoma? A retrospective cohort study and meta-analysis. Cell Physiol Biochem. 2016;40:1613–1625. doi: 10.1159/000453211. [DOI] [PubMed] [Google Scholar]
- 36.Delfino KR, Serão NV, Southey BR, Rodriguez-Zas SL. Therapy-, gender- and race-specific microRNA markers, target genes and networks related to glioblastoma recurrence and survival. Cancer Genomics Proteomics. 2011;8:173–183. [PMC free article] [PubMed] [Google Scholar]
- 37.Czachorowski MJ, Amaral AF, Montes Moreno S, Lloreta J, Carrato A, Tardón A, Morente MM, Kogevinas M, Real FX, Malats N, SBC/EPICURO investigators Cyclooxygenase-2 expression in bladder cancer and patient prognosis: Results from a large clinical cohort and meta-analysis. PLoS One. 2012;7:e45025. doi: 10.1371/journal.pone.0045025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ku JH, Byun SS, Jeong H, Kwak C, Kim HH, Lee SE. The role of p53 on survival of upper urinary tract urothelial carcinoma: A systematic review and meta-analysis. Clin Genitourin Cancer. 2013;11:221–228. doi: 10.1016/j.clgc.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Kakimoto Y, Tanaka M, Kamiguchi H, Ochiai E, Osawa M. MicroRNA stability in FFPE tissue samples: Dependence on GC content. PLoS One. 2016;11:e0163125. doi: 10.1371/journal.pone.0163125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wyman SK, Parkin RK, Mitchell PS, Fritz BR, O'Briant K, Godwin AK, Urban N, Drescher CW, Knudsen BS, Tewari M. Repertoire of microRNAs in epithelial ovarian cancer as determined by next generation sequencing of small RNA cDNA libraries. PLoS One. 2009;4:e5311. doi: 10.1371/journal.pone.0005311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stratmann J, Wang CJ, Gnosa S, Wallin A, Hinselwood D, Sun XF, Zhang H. Dicer and miRNA in relation to clinicopathological variables in colorectal cancer patients. BMC Cancer. 2011;11:345. doi: 10.1186/1471-2407-11-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Androvic P, Valihrach L, Elling J, Sjoback R, Kubista M. Two-tailed RT-qPCR: A novel method for highly accurate miRNA quantification. Nucleic Acids Res. 2017;45:e144. doi: 10.1093/nar/gkx588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macfarlane LA, Murphy PR. MicroRNA: Biogenesis, function and role in cancer. Curr Genomics. 2010;11:537–561. doi: 10.2174/138920210793175895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nassar FJ, Nasr R, Talhouk R. MicroRNAs as biomarkers for early breast cancer diagnosis, prognosis and therapy prediction. Pharmacol Ther. 2017;172:34–49. doi: 10.1016/j.pharmthera.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Negoi I, Hostiuc S, Sartelli M, Negoi RI, Beuran M. microRNA-21 as a prognostic biomarker in patients with pancreatic cancer - A systematic review and meta-analysis. Am J Surg. 2017;214:515–524. doi: 10.1016/j.amjsurg.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 46.Ren J, Kuang TH, Chen J, Yang JW, Liu YX. The diagnostic and prognostic values of microRNA-21 in patients with gastric cancer: A meta-analysis. Eur Rev Med Pharmacol Sci. 2017;21:120–130. [PubMed] [Google Scholar]
- 47.Jiang J, Yang P, Guo Z, Yang R, Yang H, Yang F, Li L, Xiang B. Overexpression of microRNA-21 strengthens stem cell-like characteristics in a hepatocellular carcinoma cell line. World J Surg Oncol. 2016;14:278. doi: 10.1186/s12957-016-1028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qi R, Wang DT, Xing LF, Wu ZJ. miRNA-21 promotes gastric cancer growth by adjusting prostaglandin E2. Eur Rev Med Pharmacol Sci. 2018;22:1929–1936. doi: 10.26355/eurrev_201804_14717. [DOI] [PubMed] [Google Scholar]
- 49.Su C, Cheng X, Li Y, Han Y, Song X, Yu D, Cao X, Liu Z. miR-21 improves invasion and migration of drug resistant lung adenocarcinoma cancer cell and transformation of EMT through targeting HBP1. Cancer Med. 2018;7:2485–2503. doi: 10.1002/cam4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu X, Chen Y, Tian R, Li J, Li H, Lv T, Yao Q. miRNA-21 enhances chemoresistance to cisplatin in epithelial ovarian cancer by negatively regulating PTEN. Oncol Lett. 2017;14:1807–1810. doi: 10.3892/ol.2017.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno Bueno G, Hergueta Redondo M, Williams C, García-Santos G, Ghajar C, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samsonov R, Burdakov V, Shtam T, Radzhabovа Z, Vasilyev D, Tsyrlina E, Titov S, Ivanov M, Berstein L, Filatov M, et al. Plasma exosomal miR-21 and miR-181a differentiates follicular from papillary thyroid cancer. Tumour Biol. 2016;37:12011–12021. doi: 10.1007/s13277-016-5065-3. [DOI] [PubMed] [Google Scholar]
- 53.Wang JJ, Wang ZY, Chen R, Xiong J, Yao YL, Wu JH, Li GX. Macrophage-secreted exosomes delivering miRNA-21 inhibitor can regulate BGC-823 cell proliferation. Asian Pac J Cancer Prev. 2015;16:4203–4209. doi: 10.7314/APJCP.2015.16.10.4203. [DOI] [PubMed] [Google Scholar]
- 54.Besheer T, Elalfy H, Abd El-Maksoud M, Abd El-Razek A, Taman S, Zalata K, Elkashef W, Zaghloul H, Elshahawy H, Raafat D, et al. Diffusion weighted magnetic resonance imaging and micro-RNA in the diagnosis of hepatic fibrosis in chronic hepatitis C virus. World J Gastroenterol. 2019;25:1366–1377. doi: 10.3748/wjg.v25.i11.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Panagal M, S R SK, P S, M B, M K, Gopinathe V, Sivakumare P, Sekar D. MicroRNA21 and the various types of myeloid leukemia. Cancer Gene Ther. 2018;25:161–166. doi: 10.1038/s41417-018-0025-2. [DOI] [PubMed] [Google Scholar]
- 56.Chen C, Lu C, Qian Y, Li H, Tan Y, Cai L, Weng H. Urinary miR-21 as a potential biomarker of hypertensive kidney injury and fibrosis. Sci Rep. 2017;7:17737. doi: 10.1038/s41598-017-18175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiong G, Huang Z, Jiang H, Pan Z, Xie J, Wang S. Inhibition of microRNA-21 decreases the invasiveness of fibroblast-like synoviocytes in rheumatoid arthritis via TGFβ/Smads signaling pathway. Iran J Basic Med Sci. 2016;19:787–793. [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng Y, Xie J, Jiang F, Li Y, Chang G, Ma H. Inhibition of miR-21 promotes cell apoptosis in oral squamous cell carcinoma by upregulating PTEN. Oncol Rep. 2018;40:2798–2805. doi: 10.3892/or.2018.6663. [DOI] [PubMed] [Google Scholar]
- 59.Yan F, Wang C, Li T, Cai W, Sun J. Role of miR-21 in the growth and metastasis of human salivary adenoid cystic carcinoma. Mol Med Rep. 2018;17:4237–4244. doi: 10.3892/mmr.2018.8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bera A, Das F, Ghosh-Choudhury N, Kasinath BS, Abboud HE, Choudhury GG. microRNA-21 induced dissociation of PDCD4 from rictor contributes to Akt-IKKβ-mTORC1 axis to regulate renal cancer cell invasion. Exp Cell Res. 2014;328:99–117. doi: 10.1016/j.yexcr.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo G, Luo W, Sun X, Lin J, Wang M, Zhang Y, Luo W, Zhang Y. microRNA-21 promotes migration and invasion of glioma cells via activation of Sox2 and β-catenin signaling. Mol Med Rep. 2017;15:187–193. doi: 10.3892/mmr.2016.5971. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Zhang N, Duan WD, Leng JJ, Zhou L, Wang X, Xu YZ, Wang XD, Zhang AQ, Dong JH. STAT3 regulates the migration and invasion of a stem-like subpopulation through microRNA-21 and multiple targets in hepatocellular carcinoma. Oncol Rep. 2015;33:1493–1498. doi: 10.3892/or.2015.3710. [DOI] [PubMed] [Google Scholar]
- 63.Au Yeung CL, Co NN, Tsuruga T, Yeung TL, Kwan SY, Leung CS, Li Y, Lu ES, Kwan K, Wong KK, et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun. 2016;7:11150. doi: 10.1038/ncomms11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang L, Jia Q, Xinnong C, Xie Y, Yang Y, Zhang A, Liu R, Zhuo Y, Zhang J. Role of cardiac progenitor cell-derived exosome-mediated microRNA-210 in cardiovascular disease. J Cell Mol Med. 2019;23:7124–7131. doi: 10.1111/jcmm.14562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie W, Su W, Xia H, Wang Z, Su C, Su B. Synovial Fluid microRNA-210 as a potential biomarker for early prediction of osteoarthritis. Biomed Res Int. 2019;2019:7165406. doi: 10.1155/2019/7165406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhuang Y, Peng H, Mastej V, Chen W. MicroRNA regulation of endothelial junction proteins and clinical consequence. Mediators Inflamm. 2016;2016:5078627. doi: 10.1155/2016/5078627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Merrigan SL, Kennedy BN. Vitamin D receptor agonists regulate ocular developmental angiogenesis and modulate expression of dre-miR-21 and VEGF. Br J Pharmacol. 2017;174:2636–2651. doi: 10.1111/bph.13875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng Z, Xu PP, Wang L, Zhao HJ, Weng XQ, Zhong HJ, Qu B, Xiong J, Zhao Y, Wang XF, et al. MiR21 sensitized B-lymphoma cells to ABT-199 via ICOS/ICOSL-mediated interaction of Treg cells with endothelial cells. J Exp Clin Cancer Res. 2017;36:82. doi: 10.1186/s13046-017-0551-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.He XP, Chen P, Yang K, Liu B, Zhang Y, Wang F, Guo Z, Liu XD, Lou JX, Chen HR. Overexpression of miR-21 is involved in acute monocytic leukemia-associated angiogenesis by targeting IL-12. Mol Med Rep. 2018;18:4122–4128. doi: 10.3892/mmr.2018.9357. [DOI] [PubMed] [Google Scholar]
- 70.Song MS, Rossi JJ. The anti-miR21 antagomir, a therapeutic tool for colorectal cancer, has a potential synergistic effect by perturbing an angiogenesis associated miR30. Front Genet. 2014;4:301. doi: 10.3389/fgene.2013.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yuan H, Xin S, Huang Y, Bao Y, Jiang H, Zhou L, Ren X, Li L, Wang Q, Zhang J. Downregulation of PDCD4 by miR-21 suppresses tumor transformation and proliferation in a nude mouse renal cancer model. Oncol Lett. 2017;14:3371–3378. doi: 10.3892/ol.2017.6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suman P, Godbole G, Thakur R, Morales-Prieto DM, Modi DN, Markert UR, Gupta SK. AP-1 transcription factors, mucin-type molecules and MMPs regulate the IL-11 mediated invasiveness of JEG 3 and HTR 8/SVneo trophoblastic cells. PLoS One. 2012;7:e29745. doi: 10.1371/journal.pone.0029745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hwang YP, Yun HJ, Choi JH, Han EH, Kim HG, Song GY, Kwon KI, Jeong TC, Jeong HG. Suppression of EGF-induced tumor cell migration and matrix metalloproteinase-9 expression by capsaicin via the inhibition of EGFR-mediated FAK/Akt, PKC/Raf/ERK, p38 MAPK, and AP-1 signaling. Mol Nutr Food Res. 2011;55:594–605. doi: 10.1002/mnfr.201000292. [DOI] [PubMed] [Google Scholar]
- 74.Chen Q, Chen P, Pang X, Hu Y, Zhang Y. Adrenomedullin up regulates the expression of vascular endothelial growth factor in epithelial ovarian carcinoma cells via JNK/AP-1 pathway. Int J Gynecol Cancer. 2015;25:953–960. doi: 10.1097/IGC.0000000000000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cossutta M, Darche M, Carpentier G, Houppe C, Ponzo M, Raineri F, Vallée B, Gilles ME, Villain D, Picard E, et al. Weibel-palade bodies orchestrate pericytes during angiogenesis. Arterioscler Thromb Vasc Biol. 2019;39:1843–1858. doi: 10.1161/ATVBAHA.119.313021. [DOI] [PubMed] [Google Scholar]
- 76.Pellegrinelli V, Rouault C, Veyrie N, Clément K, Lacasa D. Endothelial cells from visceral adipose tissue disrupt adipocyte functions in a three-dimensional setting: Partial rescue by angiopoietin-1. Diabetes. 2014;63:535–549. doi: 10.2337/db13-0537. [DOI] [PubMed] [Google Scholar]
- 77.Brudno Y, Ennett Shepard AB, Chen RR, Aizenberg M, Mooney DJ. Enhancing microvascular formation and vessel maturation through temporal control over multiple pro-angiogenic and pro maturation factors. Biomaterials. 2013;34:9201–9209. doi: 10.1016/j.biomaterials.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khan JA, Maki RG, Ravi V. Pathologic angiogenesis of malignant vascular sarcomas: Implications for treatment. J Clin Oncol. 2018;36:194–201. doi: 10.1200/JCO.2017.74.9812. [DOI] [PubMed] [Google Scholar]
- 79.Liu Z, Ivanoff A, Klominek J. Expression and activity of matrix metalloproteases in human malignant mesothelioma cell lines. Int J Cancer. 2001;91:638–643. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1102>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 80.Hirano H, Tsuji M, Kizaki T, Sashikata T, Yoshi Y, Okada Y, Mori H. Expression of matrix metalloproteinases, tissue inhibitors of metalloproteinase, collagens, and Ki67 antigen in pleural malignant mesothelioma: An immunohistochemical and electron microscopic study. Med Electron Microsc. 2002;35:16–23. doi: 10.1007/s007950200002. [DOI] [PubMed] [Google Scholar]
- 81.Zhu L, Kate P, Torchilin VP. Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting. ACS Nano. 2012;6:3491–3498. doi: 10.1021/nn300524f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.