Abstract
Unc-5 Netrin Receptor C (UNC5C) is a netrin-1 dependence receptor that mediates the induction of apoptosis in the absence of netrin-1. The present study found that UNC5C is heterogeneously expressed in breast cancer cell lines. By knocking down UNC5C in SK-BR-3 and ZR-75-30 cells and overexpressing UNC5c in MDA-MB-231 cells, it was demonstrated that UNC5C exerts an inhibitory effect on the growth and metastasis of breast cancer cells. The mechanism involved a UNC5C-knockdown-induced enhancement of matrix metalloproteinase (MMP)3, MMP7, MMP9 and MMP10 expression via activation of the PI3K/AKT, ERK and p38 MAPK signaling pathways. Notably, UNC5C directly interacted with integrin α6, which is involved in the growth and metastasis of breast cancer cells. Additionally, UNC5C-knockdown enhanced the phosphorylation of FAK and SRC, which are key kinases in the netrin-1/Unc5C and netrin-1/integrin α6/β4 signaling pathways. This suggests that netrin-1 functions as an integrator for both the netrin-1/Unc5C and netrin-1/integrin α6/β4 signaling pathways. UNC5C-knockdown potentiated netrin-1/integrin α6/β4 signaling. Given that UNC5C-knockdown inhibited integrin-liked protein kinase phosphorylation at Thr-173, at least in SK-BR-3 cells, this may be an inhibitory phosphorylation site rather than activating phosphorylation site for relaying integrin signaling.
Keywords: breast cancer, integrin α6, metastasis, matrix metalloproteinase, Unc-5 Netrin Receptor C
Introduction
Axon guidance molecules modulate the growth and migration of neurons during development of the neural system. These molecules are classified according to their genetic and biochemical properties into four highly conserved families; netrins, slits, semaphorins and ephrins (1). Unc-5 Netrin Receptor (UNC5)C is one of seven cognate receptors, including neogenin, deleted in colorectal cancer (DCC), UNC5A, UNC5B, UNC5C, UNC5D and adenosine A2b receptor, for netrin-1 (NTN1), which mediates the directed extension and migration of axons during neural development (2). UNC5C mediates the repellent response to NTN1. UNC5C-deletion in mice disrupts the long-range dorsal guidance of inferior olivary and pontine axons after crossing the midline (3). UNC5C is also involved in tumor progression (4). Thiebault et al (5) used a full-length complementary DNA (cDNA) probe that recognizes UNC5A, UNC5B and UNC5C mRNA, and found that UNC5A, UNC5B and UNC5C were significantly downregulated in 93, 88, 49, 48, 68 and 74% of colorectal, ovarian, breast, uterine, gastric and lung cancer samples, respectively, indicating that UNC5A, UNC5B and UNC5C are potential tumor suppressor genes. UNC5 receptors are members of the dependence receptor family, which elicit an apoptotic signal in the absence of their ligand, NTN1, instead of being inactive (5). The loss of function of UNC5C typically occurs in early stages of colorectal cancer (6), and inherited UNC5C mutations can inhibit cell apoptosis and increase the risk of colorectal cancer (7). UNC5A, UNC5B and UNC5C are downregulated in colorectal cancer by 48, 27 and 74–77%, respectively (5), suggesting that UNC5C plays an important inhibitory role in colorectal cancer. In addition to the loss of heterozygosity, several studies have attributed UNC5C downregulation to the abnormal methylation of its promoter (5,8).
UNC5 receptors perform functions through interactions with other axon guidance molecule receptors. For example, UNC5B was shown to interact with the netrin-4 receptor neogenin (9) or roundabout guidance receptor 4 (10) to inhibit angiogenesis. UNC5B also interacts with DCC to convert NTN1-induced growth cone attraction to repulsion (11). However, the impact of these interactions on tumor progression remains unknown.
Breast cancer is the most malignant type of cancer in females, and it is difficult to treat due to its high rates of recurrence and mortality. Breast cancer alone accounted for 11.6% of all cancer cases and 6.6% of all cancer-associated mortalities among females in 2018 (12). Metastasis is the leading cause of mortality in breast cancer patients (13). Most studies on the role of UNC5C in tumorigenesis have focused on colorectal cancer (7,8,14). To the best of our knowledge, the function and mechanism of UNC5C in breast cancer have not been widely reported. Fitamant et al (15) found that metastatic breast cancer expresses NTN1 as a mechanism by which breast cancer cells escape apoptosis.
The present study investigated the effects of UNC5C on cell growth and metastasis both in vitro and in vivo by knocking down and overexpressing UNC5C in breast cancer cell lines. It was identified that UNC5C interacted with the integrin α6 subunit and UNC5C-knockdown enhanced the growth and metastasis of breast cancer cells, which was likely partially attributable to the upregulation of matrix metalloproteinase (MMP)9 expression via the NTN1/integrin α6/β4 signaling pathway. These findings confirm the inhibitory effects of UNC5C on breast cancer cell viability and metastasis.
Materials and methods
Cell lines and reagents
The SK-BR-3, ZR-75-30 and MDA-MB-231 breast cancer cell lines were purchased from the American Type Culture Collection. 293T, MCF-10a and MCF-7 cells were purchased from the Cell Resource Center, Institute of Life Sciences, Chinese Academy of Science. All cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM, HyClone; GE Healthcare) with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). All cells were incubated in a 5% CO2 humidified atmosphere at 37°C. The lentivirus vector pll3.7 for UNC5C-knockdown and packaging plasmid pHR were obtained from Addgene, Inc. pVSVG was obtained from Invitrogen; Thermo Fisher Scientific, Inc. The pbobi plasmids for UNC5C (UNC5C full length gene sequence ID: 8633) and integrin α6 subunit (ITGA6 full length gene sequence ID: 3655) overexpression were provided by Professor Jiahuai Han (Xiamen University, Xiamen, China). The mitogen activated protein kinase (MAPK) inhibitors SB203580, U0126 and LY294002 were purchased from Merck KGaA. Breast tissue chips that contained 30 pairs of tumor and adjacent tissues (cat. no. HBre-Duc060CS-02) were purchased from Shanghai Outdo Biotech Co., Ltd.
Antibodies
Anti-UNC5C (cat. no. ab106949) and integrin α6 (cat. no. ab20142 for immunoprecipitation and ab181551 for western blotting) antibodies were purchased from Abcam. Anti-phosphorylated (p)-Akt (cat. no. 4060; Ser473), Akt (cat. no. 4685), p-p38 MAPK (clone D3F9; cat. no. 4511), p38 MAPK (clone D13E1; cat.no. 8690), p-ERK1/2 (Thr202/Tyr204; cat. no. 9101), ERK1/2 (cat. no. 9102), Src (cloneL4A1; cat. no. 2110), p-Src (Tyr416; cat. no. 2101), FAK (cat. no. 3285) and p-FAK (clone D20B1; Tyr397; cat. no. 8556) antibodies were purchased from Cell Signaling Technology, Inc. Anti-α-tubulin (cat. no. T6199) and Flag (cat. no. F3040) antibodies were purchased from Sigma-Aldrich; Merck KGaA. Anti-myc antibody (cat. no. HT101) was purchased from Transgene SA. Anti-integrin-linked kinase (ILK; clone E-2, cat. no. sc-137221) and p-ILK antibodies (Thr173; cat. no. sc-130196) were purchased from Santa Cruz Biotechnology, Inc. Goat anti-mouse horseradish peroxidase (HRP)-conjugated immunoglobulin G (IgG; cat. no. G-21040) and goat anti-rabbit HRP-conjugated IgG (cat. no. A16096) were purchased from Pierce; Thermo Fisher Scientific, Inc.
Establishment of stable cell lines
The stable cell lines with UNC5C-knockdown or overexpression were established as previously described (16). Briefly, virus stocks were prepared by co-transfecting pll3.7 (for UNC5C mRNA knockdown) or pbobi (cMyc tag; for UNC5C mRNA overexpression) with two packaging plasmids (pHR and pVSVG) into 293T cells. For UNC5C-knockdown, SK-BR-3 and ZR-75–30 cells were infected with pll3.7 in the presence of polybrene (8 µg/ml; Sigma-Aldrich; Merck KGaA) for 2 days. Infected cells were screened by treatment with G418 (1,500 µg/ml; Sigma-Aldrich; Merck KGaA) for 1 week. The targeted sequences for UNC5C-knockdown were as follows: shRNA1, 5'-TCTTGGATTGCAAGACGAGG-3'; and shRNA2, 5'-CAAAGTCACGATGATTCTTC-3'. Empty plasmid was used as a control (shCtrl). For UNC5C overexpression (UNC5C full length gene sequence ID: 8633), MDA-MB-231 cells were infected with pbobi in the presence of polybrene, and screened by treatment with puromycin (2.5 µg/ml; Sigma-Aldrich; Merck KGaA) for 1 week. After the screened cells were maintained in culture for 1 week UNC5C-knockdown and overexpression efficiency were determined by western blotting.
Western blotting and immunoprecipitation
The MCF-10a, MCF-7, SK-BR-3, ZR-75–30 and MDA-MB-231 breast cancer cells were lysed with RIPA buffer (cat. no. P0013B; Beyotime Institute of Biotechnology) that contained a protease inhibitor (cat. no. 04906845001; Roche Diagnostics). Isolated proteins were quantified using the Pierce™ BCA Protein assay kit (cat. no. 23225; Thermo Fisher Scientific, Inc.). Protein samples (30 µg) were fractionated by 7.5% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (EMD Millipore). After blocking in 5% milk for 1 h at room temperature, membranes were incubated at 4°C overnight with primary antibodies at a dilution of 1:1,000 for anti-phosphorylated (p)-Akt, Akt, p-p38 MAPK, p38 MAPK, p-ERK1/2, ERK1/2, Src, p-Src, FAK, p-FAK, Unc5C and integrin α6, and at a dilution of 1:500 for anti-integrin-linked kinase and p-ILK, The membranes were then washed and incubated with the appropriate HRP-conjugated secondary antibody (1:5,000) for 1 h at room temperature. The bound antibody was developed using an enhanced chemiluminescence system (Bio-Rad Laboratories, Inc.). The density of the protein bands was analyzed by ImageJ software (version 1.52a; National Institutes of Health). For immunoprecipitation, the cells were rinsed three times with ice-cold PBS, NP-40 buffer (cat. no. P0013F; Beyotime Institute of Biotechnology) that contained phenylmethylsulfonyl fluoride (cat. no. M145-5; Amresco, LLC) was then added to the cells. The cell lysates were centrifuged at 12,000 × g for 20 min at 4°C. The supernatants were incubated with the primary antibodies anti-myc, anti-Flag, anti-Unc5C and anti-integrin α6 (all 1:500) at 4°C for 2 h, and then incubated with protein G agarose beads (cat. no. 11719416001; Roche Diagnostics) overnight at 4°C. After centrifugation and aspirating the supernatants, the beads were washed 3–6 times, followed by routine western blot analysis. Non-specific normal IgG (1:200; cat. no. ab200699; Abcam) was used as a negative control.
Oncomine analysis
The raw data of Unc5C mRNA expression in ductal breast carcinoma and normal breast tissues were extracted from three datasets in the Oncomine database (https://www.oncomine.org/resource/login.html), including the Perou et al (17), Sørlie et al (18) and Richardson et al (19) datasets. The threshold in the analysis was set at P<1x10−4, fold change >2 and gene rank in top 10%. The raw data were re-plotted using GraphPad Prism software (version 6; GraphPad Software, Inc.).
Immunohistochemistry
The breast tissue chips with different clinicopathological stages (grade II–III) were deparaffinized and incubated in citrate buffer for antigen retrieval for ≥4 h. The grades were determined according to the guidelines of the Chinese Society of Clinical Oncology: Breast Cancer (20). After washing three times with PBS, the chips were blocked with 3% H2O2 in PBS for 10 min at room temperature, followed by three washes with PBS. The chips were blocked by TNB blocking buffer at room temperature for 1–2 h and then incubated with anti-UNC5C antibody (cat no. ab106949; Abcam; 1:500) overnight at 4°C. After washing with TNT buffer, the sections were incubated with the appropriate HRP-conjugated secondary antibody for 1 h at room temperature. After a 10–30 min wash with DAB solution and mounting, the slides were visualized under a light microscope (magnification, ×40, ×200 or ×400) and images were captured. The analysis was performed as described previously (21), with minor modifications. The UNC5C-positive staining intensity was scored as: 0, negative; 1, weak; 2, moderate; and 3, strong. The scoring for the UNC5C stained area was: 0, <5%; 1, 5–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100% of stained tumor cells. The final staining score was calculated by multiplying the staining intensity score by the staining area score, with a range between 0 and 12.
Quantitative PCR (qPCR) and semi-qPCR
RNA isolation from SK-BR-3 and MDA-MB-231 cells was performed using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was prepared using the ReverTra Ace RT-qPCR kit (Toyobo Life Science) with 1 µg RNA per reaction. RT was performed at 37°C for 15 min followed by 98°C for 5 min to inactivate reverse transcriptase activity. qPCR was performed with the Thunderbird SYBR qPCR mix (Toyobo Life Science) and the thermocycling conditions were as previously described (22). The primers used are listed in Table SI. Relative MMP gene expression was calculated using the 2-ΔΔCq method (23), normalized to GAPDH and compared with the MMP3 level.
For semi-qPCR, the amplification conditions were the following: Initial denaturation at 95°C for 1 min, followed by 38 cycles of 95°C for 30 sec, 58°C for 30 sec and 72°C for 1 min for MMPs, and 23 cycles under the same conditions for GAPDH. Taq polymerase (Takara Bio, Inc.) was used as the DNA polymerase. Each condition was run in triplicate with the primers listed in Table SI. Amplified products were resolved by 1% agarose gel electrophoresis and visualized by 4S green plus (Sangon Biotech Co., Ltd.), and then photographed with a Bio-Imaging system (DNR Bio Imaging Systems). The band intensity of each lane was analyzed by ImageJ software (version 1.52a; National Institutes of Health) as described previously (24).
Enzyme-linked immunosorbent assay (ELISA)
A total of 1×106 cells/well were seeded in 6-well microplates. The culture medium was replaced with serum-free fresh DMEM (2 ml/well) 8 h later. After 24 h, supernatants were collected and centrifuged at 200 × g for 5 min at room temperature to remove cellular debris. Secreted NTN1 in the supernatants was measured using an ELISA kit (cat. no. KTE62214; Abbkine Scientific Co., Ltd.) according to the manufacturer's protocol. The final optical density of each well was read at 450 nm using a microplate reader (Multiscan MK3; Thermo Fisher Scientific, Inc.).
Tumor xenograft and metastasis
Six-week old nude (BALB/c) mice (n=40, 18–22 g, 15 males and 25 females) were purchased from SLRC Laboratory Company and maintained at the Laboratory Animal Center, Xiamen University (Xiamen, China). The mice were housed in plastic cages and maintained in a climate-controlled animal room (23±1°C; 55±5% humidified atmosphere) with a 12/12 h light-dark cycle. Food and water were freely available. The protocols for the xenograft and metastasis experiments were approved by the Animal Ethics Committee of Xiamen University (approval no. XMULAC20120030). For the xenograft experiment, plasmid pll3.7 transfected-SK-BR-3 (Unc5C-shCtrl, Unc5C-shRNA1 and Unc5C-shRNA2) and pbobi transfected-MDA-MB-231 (GFP and Unc5C) cells were detached and resuspended in serum-free medium. Subsequently, 5.0×106 cells were subcutaneously injected in a volume of 200 µl into the flank of 6-week-old female nude BALB/c mice (n≥4/group), which were maintained in a specific-pathogen-free environment. The mice were palpated every 3 days to monitor tumorigenesis. Tumor growth was recorded using a Vernier caliper, and the volume of the tumors was calculated according to the following formula: 0.52 × width2 × length. The humane endpoint was set when the tumor diameters of any mouse exceeded 12 mm, at which point the experiment was terminated. After 4 weeks, all of the mice were euthanized by CO2 exposure at a flow rate of 1.2 l/min, which displaces 20% of the cage volume per minute. Death of the mice was verified by persistent unconsciousness and no breathing. The resulting tumors were completely dissected and weighed.
For the analysis of lung metastasis, male nude BALB/c mice were divided into three groups (n=5). Plasmid pll3.7 transfected-SK-BR-3 cells (2.0×106) were injected in a volume of 100 µl PBS into each mouse via the tail vein. The body weight of the mice was monitored every 3 days. A 20% reduction of body weight was defined as the humane endpoint. After 8 weeks, all of the mice were euthanized by CO2 exposure at a flow rate of 1.2 l/min, which displaced 20% of the cage volume per minute. Death of the mice was verified by persistent unconsciousness and no breathing. Lung tissues were harvested and washed twice with PBS. A cytomegalovirus-enhanced green fluorescent protein reporter cassette was included in the pll3.7 vector to monitor expression, thus allowing evaluation of the metastasis of cancer cells by examining GFP-labeled metastatic tumor nodules in all lung lobes. The lung tissues were stored in 4% paraformaldehyde at room temperature for 24 h, embedded in paraffin and cut into 5-µm thick sections. The sections were finally stained with hematoxylin for 3 min and eosin for 2 min at room temperature, and visualized under a light microscope (magnification, ×200).
MTT assay
SK-BR-3, ZR-75–30 and MDA-MB-231 cells were seeded in 96-well plates at a density of 3.0×103/well. MTT solution (10 µl; Beyotime Institute of Biotechnology) was then added to each well every 24 h, and the cells were incubated at 37°C for 4 h with 5% CO2. The MTT solution was then removed and replaced with 150 µl DMSO. The plate was further incubated at 37°C for 15 min with 5% CO2 and agitated on an orbital shaker for 10 min. The optical density of the wells was read at a wavelength of 560 nm with a 630 nm reference filter.
Tumor cell invasion assay
The tumor cell invasion assay was performed as described previously (16). Briefly, the invasion chambers (8-µm; EMD Millipore) were coated with 20 µl diluted Matrigel (BD Biosciences) at 37°C for 30 min and inserted into a 24-well plate. Cells were added to the top chambers at 1×104 cells/well. The upper chamber was filled with 200 µl DMEM that contained 5% FBS, and the lower chamber was filled with 500 µl DMEM that contained 20% FBS. After incubation for 24 h, non-invading cells were removed from the upper chamber with a cotton swab, and invading cells were fixed with PAF for 10 min at room temperature and stained using 1% crystal violet for 5 min at room temperature (cat. no. C0775; Sigma-Aldrich; Merck KGaA). Subsequently, the cells were visualized under an inverted microscope (magnification, ×20). The number of cells was counted and calculated using ImagePro Plus 6.0 software (Media Cybernetics, Inc.). Three identical replicates were performed for each condition.
Statistical analysis
The statistical analysis was performed using SPSS software (version 20; IBM Corp.). Unless otherwise indicated, data are presented as the mean ± standard error of the mean. No samples or animals were excluded from the analysis. Comparisons were performed using Student's t-test for the comparison of two groups or one-way analysis of variance followed by Tukey's post hoc test for the comparison of multiple groups. P<0.05 was considered to indicate a statistically significant difference.
Results
UNC5C is heterogeneously expressed in breast cancer cell lines
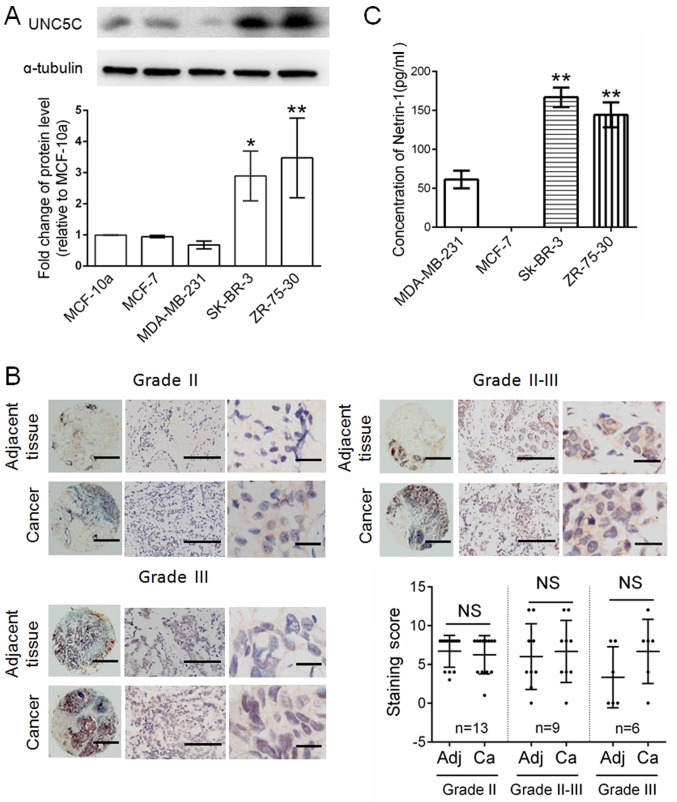
UNC5C expression was detected by western blotting in various commercially available breast cancer cell lines. The normal breast cell line MCF-10a was used as a control. As presented in Fig. 1A, UNC5C was detected in MCF-10a, MCF-7, SK-BR-3 and ZR-75–30 cells, with the highest UNC5C expression in ZR-75–30 cells and the lowest expression in MDA-MB-231 cells. The MCF-7 and ZR-75-30 cells are derived from ductal breast carcinoma. MDA-MB-231 and SK-BR-3 are derived from breast adenocarcinoma. The present results indicate that the heterogeneity of UNC5C expression in various cell lines may depend on the specific type of cell line. To confirm the results that were found in MCF-7 and ZR-75-30 cells, UNC5C expression in ductal breast carcinoma was further examined by Oncomine data-mining analysis and the immunostaining of breast tissue chips. The Oncomine data indicated no significant differences between normal tissues and ductal breast carcinoma (17–19) (Fig. S1). Breast tissue chips from normal tissue and ductal breast carcinoma were separately stained with UNC5C antibodies. UNC5C protein was widely expressed in most ductal breast carcinoma and adjacent normal tissues at different clinical stages, and no significant difference was observed between them (Fig. 1B). UNC5 receptors are NTN1 dependence receptors (5). Therefore, ELISA was used to detect the relative expression of NTN1 in culture media of the aforementioned breast cancer cell lines. NTN1 expression in SK-BR-3 and ZR-75-30 cells, which express a relatively high level of UNC5C, was significantly higher than in MDA-MB-231 cells and not detected in MCF7 cells (Fig. 1C). NTN1 expression appears to be positively associated with the UNC5C receptor.
Figure 1.
UNC5C is heterogeneously expressed in clinical samples and breast cancer cell lines. (A) Detection of relative UNC5C expression in MCF-10a, MCF-7, SK-BR-3, ZR-75–30 and MDA-MB-231 breast cancer cell lines by western blotting. The expression of UNC5C was first normalized to α-tubulin and then to the value in the MCF-10a normal breast cell line. *P<0.05, **P<0.01 vs. MCF-10a. (B) Detection of UNC5C expression in breast tissue microarrays at different clinical stages by immunostaining. Left panels, whole tissue chip (magnification, ×40; scale bar, 200 µm). Middle panels, part of the tissue chip (magnification, ×200; scale bar, 100 µm). Right panels, part of the tissue chip (magnification, ×400; scale bar, 50 µm). Significant differences were determined by paired Student's t-test. (C) Detection of relative NTN1 expression in the culture medium of breast cancer cell lines by ELISA. **P<0.01 vs. MDA-MD-231. Data are expressed as the mean ± standard deviation. NS, not significant; Adj, adjacent tissues; Ca, cancer; UNC5C, Unc-5 Netrin Receptor C.
UNC5C inhibits the viability and invasion of breast cancer cells
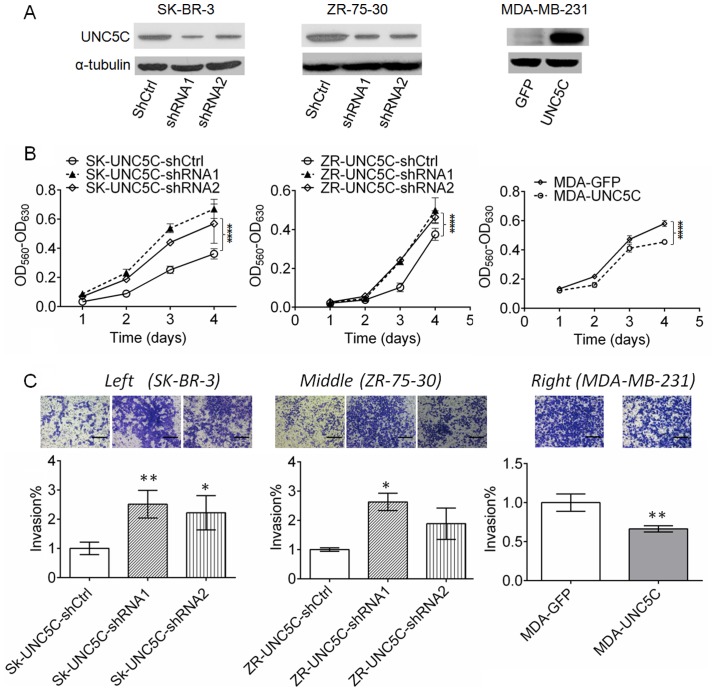
To evaluate the function of UNC5C in breast cancer cells, UNC5C-knockdown experiments were performed in SK-BR-3 and ZR-75-30 cells, and UNC5C-overexpression experiments were performed with MDA-MB-231 cells. Two RNA sequences were designed and inserted into the pll3.7 vector to knockdown UNC5C expression in SK-BR-3 and ZR-75-30 cells. An empty control vector was used as a control. Plasmid pbobi that encoded the full-length human UNC5C with a cMyc tag sequence was transfected into MDA-MB-231 cells. The plasmid with the GFP coding sequence was used as a control. As shown in Fig. 2A, UNC5C expression was effectively downregulated in SK-BR-3 and ZR-75-30 cells, whereas UNC5C was markedly overexpressed in MDA-MB-231 cells. To examine the effects of UNC5C expression on NTN1, the concentration of NTN1 in culture media of these breast cancer cells was detected by ELISA. The results demonstrated that UNC5C-knockdown increased the concentration of NTN1, and UNC5C-overexpression decreased NTN1 concentrations; however, these changes were not statistically significant for cells transfected with SK-UNC5C-shRNA1 or pbobi overexpression vector (Fig. S2). Notably, NTN1 expression was not assessed by western blotting or RT-qPCR, which may be a limitation of the present study.
Figure 2.
UNC5C inhibits the viability and invasion of breast cancer cells. (A) UNC5C-knockdown reduced UNC5C protein expression in SK-BR-3 and ZR-75–30 cells, and UNC5C-overexpression enhanced UNC5C protein expression in MDA-MB-231 cells. (B) MTT assay. UNC5C-knockdown promoted viability of the breast cancer cell lines SK-BR-3 and ZR-75-30. UNC5C-overexpression inhibited viability of the breast cancer line MDA-MB-231. ****P<0.0001. (C) UNC5C-knockdown in SK-BR-3 and ZR-75-30 cells leads to enhanced invasion, while UNC5C-overexpression in MDA-MB-231 cells leads to reduced invasion of breast cancer cells in the Transwell assay. Scale bar, 50 µm. *P<0.05, **P<0.01 vs. control cells. Data are expressed as the mean ± standard deviation. UNC5C, Unc-5 Netrin Receptor C; sh, short hairpin; Ctrl, control; OD, optical density.
The downregulation of UNC5C significantly enhanced the viability of SK-BR-3 cells (P<0.001) and ZR-75-30 cells (P<0.001). By contrast, the viability of MDA-MB-231 breast cancer cells was significantly suppressed by UNC5C-overexpression (P<0.001). These results indicate that UNC5C inhibits the viability of breast cancer cells (Fig. 2B). To examine the effects of UNC5C on tumor invasion, the Matrigel coated-Transwell assay was conducted. The number of cells that passed through the Matrigel matrix was significantly increased following UNC5C knockdown (P<0.01 for SK-BR-3-shRNA1 and P<0.05 for ZR-75-30-shRNA1) and reduced by UNC5C overexpression (P<0.01), suggesting that UNC5C suppresses tumor cell invasion (Fig. 2C).
UNC5C suppresses the growth and metastasis of breast cancer cells in vivo
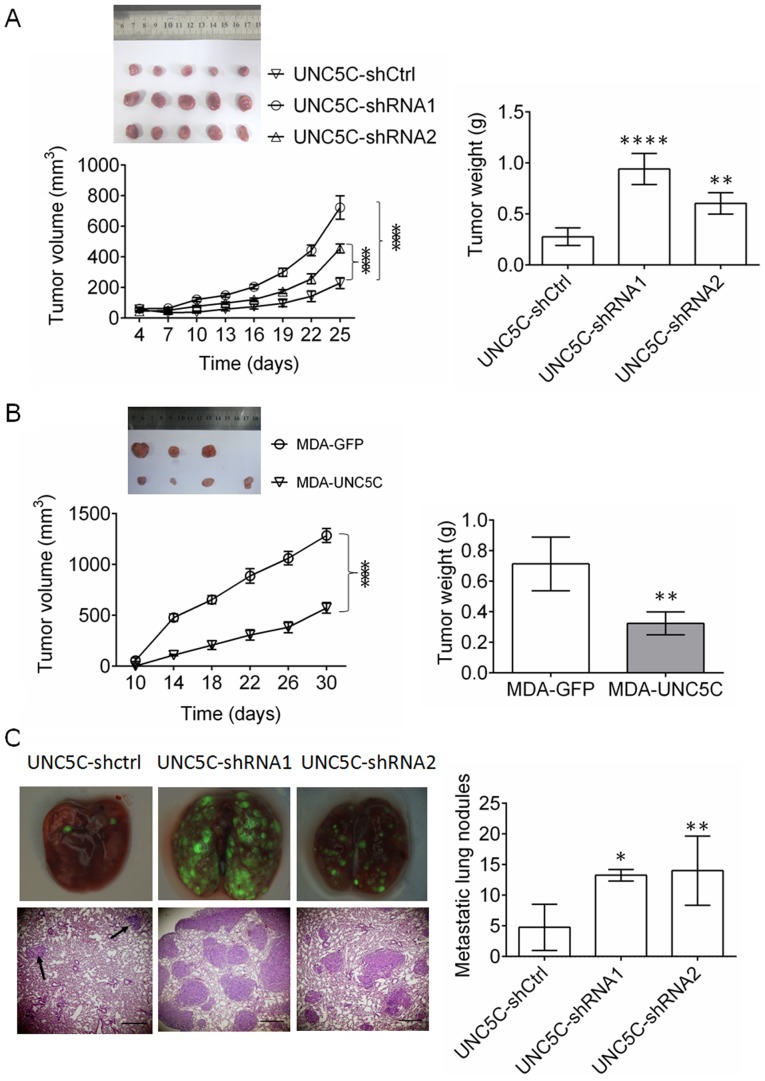
To confirm the effects of UNC5C on tumor growth in vivo, a subcutaneous tumor xenograft assay was performed with SK-BR-3 and MDA-MB-231 cells. As presented in Fig. 3A, UNC5C-knockdown in SK-BR-3 cells significantly enhanced tumor growth (P<0.001 for tumor volume and P<0.01 for final tumor weight; n=5). By contrast, UNC5C-overexpression (Fig. 3B) resulted in the opposite effect in MDA-MB-231 cells (P<0.001 for tumor volume and P<0.01 for final tumor weight; n=4).
Figure 3.
UNC5C suppresses the growth and metastasis of breast cancer in vivo. For the tumor growth assay, (A) SK-BR-3 cells (n=5) or (B) MDA-MB-231 cells (n=4) were inoculated subcutaneously in the right flanks of 4-week-old nude BALB/c mice. The volume of the resulting tumors was measured every 3 or 4 days according to the formula: Volume = width2 × length × 0.52. One month later, the resulting tumors were completely dissected and weighed. The maximum diameter of the tumor that formed at the end of the experiments was 9.2 mm in the SK-UNC5C-Ctrl group, 12.4 mm in the SK-UNC5C-shRNA1 group, 12.3 mm in the SK-UNC5C-shRNA2 group, 12.2 mm in the MDA-MB-231-GFP group and 10.2 mm in the MDA-MB-231-Unc5C group. (C) For tumor metastasis, SK-BR-3 cells were injected intravenously. Metastatic tumor nodules in all lung lobes were photographed under a fluorescence microscope and counted in the paraffin sections. Magnification of upper panels, ×40. Magnification of lower panels, ×200. The data are expressed as the mean ± standard deviation. *P<0.05, **P<0.01, ****P<0.0001 vs. UNC5C-shCtrl or MDA-GFP. UNC5C, Unc-5 Netrin Receptor C; sh, short hairpin; Ctrl, control.
To investigate the effects of UNC5C on tumor metastasis in vivo, SB-BR-3 cells were injected in the tail vein in mice. UNC5C-knockdown in SK-BR-3 cells significantly promoted tumor metastasis (P<0.05 for shRNA1 and P<0.01 for shRNA2; n=5; Fig. 3C). These findings confirm that UNC5C also inhibits the growth and metastasis of breast cancer cells in vivo.
UNC5C suppresses the expression of MMPs in breast cancer cells
For tumor invasion and metastasis, tumor cells must traverse the basement membrane that lines the basal face of tumor cells, which contains collagen type IV as a major structural component (25). Secreted MMPs that can degrade collagen type IV, including MMP2, MMP3, MMP7, MMP9 and MMP10, of which MMP2 and MMP9 are the most efficient (26), play critical roles in tumor invasion and metastasis. MMP3, MMP7, MMP9 and MMP10 expression was upregulated by UNC5C-knockdown in SK-BR-3 cells and downregulated by UNC5C-overexpression in MDA-MB-231 cells. However, no difference in MMP2 expression was detected in either UNC5C-knockdown or -overexpressing cells (Fig. 4A). Western blotting further confirmed MMP2 and MMP9 expression at the protein level (Fig. 4B). These findings indicated that UNC5C inhibits the invasion and metastasis of breast cancer cells, which is associated with suppression of the expression and activity of MMP3, MMP7, MMP9 and MMP10.
Figure 4.
UNC5C suppresses MMP expression in breast cancer cells and inhibits the phosphorylation of AKT, MAPK/ERK and MAPK/p38. (A) Semi-quantitative PCR analysis. UNC5C-knockdown in SK-BR-3 cells enhanced and overexpression in MDA-MB-231 cells inhibited the expression of MMP3, MMP7, MMP9 and MMP10, but not MMP2. (B) Western blotting. UNC5C-knockdown upregulated MMP9 expression, but not MMP2. UNC5C-overexpression inhibited MMP9 expression at the protein level. (C) MMP9 expression in SK-BR-3 cells was markedly suppressed when the PI3K/AKT, ERK and p38 signaling pathways were blocked. (D) UNC5C-knockdown enhanced the phosphorylation of AKT, ERK1/2 and p38. UNC5C, Unc-5 Netrin Receptor C; sh, short hairpin; Ctrl, control; MMP, matrix metalloproteinase; p, phosphorylated.
UNC5C suppresses the phosphorylation of PI3K/AKT, ERK and p38 MAPK
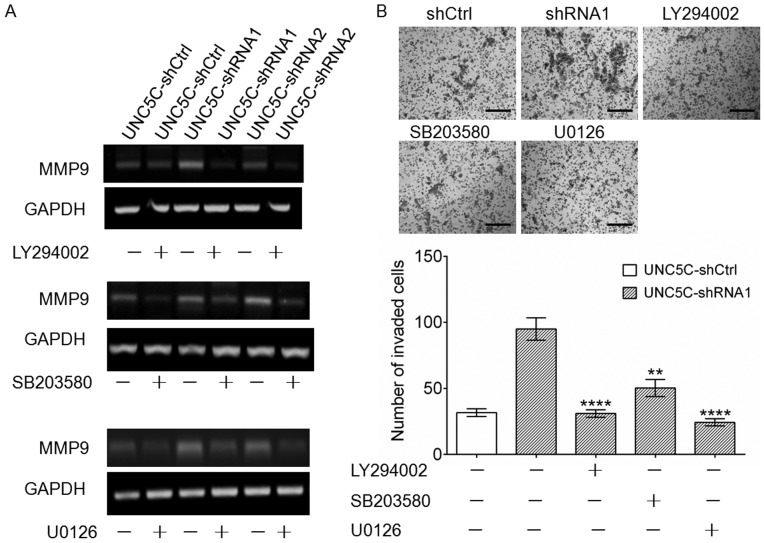
Based on the findings that MMP9 expression is maintained at a higher level than MMP3, MMP7 and MMP10 (Fig. S3) and that MMP9, not MMP2, is affected by UNC5C (Fig. 4A and B), we focused on MMP9 to further investigate the effects of UNC5C on breast cancer cell invasion and metastasis. Several studies have shown that the activation of PI3K/AKT (27), ERK (28) and p38 MAPK (29) is important for the regulation of MMP expression in breast cancer cells. To examine the possibility that MMP9 expression is regulated by the PI3K/AKT, ERK or p38 MAPK signaling pathways, PI3K (LY294002), ERK (U0126) and p38 MAPK (SB203580) inhibitors were used to investigate their influence on MMP9 expression. As shown in Fig. 4C, MMP9 expression was markedly suppressed when the PI3K/AKT, ERK and p38 MAPK signaling pathways were blocked. Notably, these inhibitors antagonized the upregulation of MMP9 (Figs. 5A and S4) and increased invasion ability (Fig. 5B) that was induced by UNC5C-knockdown. These results, combined with the findings that phosphorylation levels of AKT, ERK and p38 MAPK increased after UNC5C-knockdown in SK-BR-3 cells and decreased after UNC5C-overexpression in MDA-MB-231 cells (Fig. 4D), suggest that UNC5C likely exerts inhibitory effects on breast cancer metastasis at least partially by inhibiting MMP9 expression through the PI3K/AKT, ERK and p38 MAPK signaling pathways.
Figure 5.
Effects of PI3K/AKT, ERK and p38 MAPK inhibitors on MMP9 expression and the invasion of SK-BR-3 cells. (A) The upregulation of MMP9 induced by UNC5C-knockdown was decreased by the inhibitors. (B) The UNC5C-knockdown-induced increase in invasion was antagonized by the inhibitors. Scale bar, 50 µm. Significant differences were determined by one-way ANOVA followed by Tukey's post hoc test. **P<0.01, ****P<0.0001 vs. no inhibitors. UNC5C, Unc-5 Netrin Receptor C; sh, short hairpin; Ctrl, control; MMP, matrix metalloproteinase.
UNC5C inhibits the growth and metastasis of breast cancer cells through interactions with integrin α6
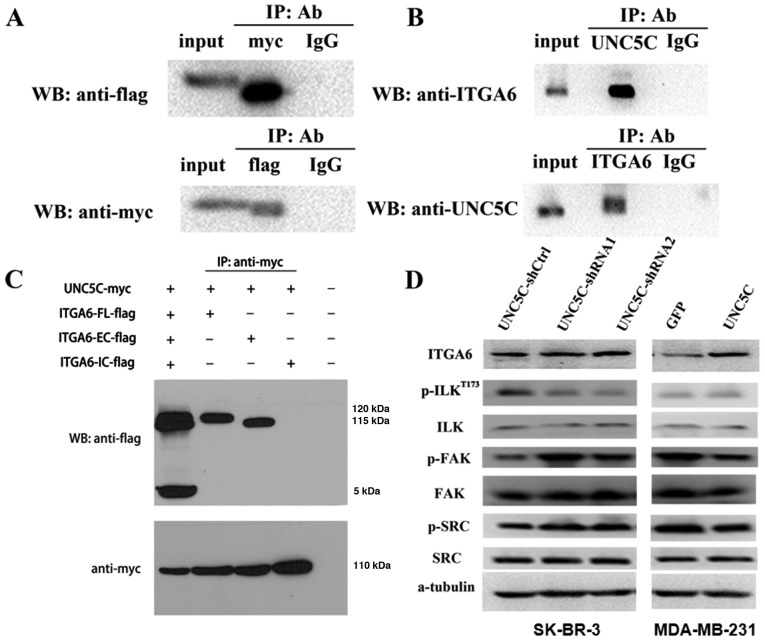
A notable characteristic of NTN1 receptors is their mutual interactions (9–11). Integrins comprise a family of receptors that usually mediate cell to extracellular matrix (ECM) adhesion and migration. Integrin signaling pathways play crucial roles in regulation of MMP expression in various cancer types (30–32). NTN1 has been reported to promote epithelial cell adhesion and migration during development of the pancreas by binding to integrin α6/β4 and integrin α3β1, which have been identified as NTN1 receptors (33). Integrin α6/β4 is normally expressed in the breast epithelium and upregulated in invasive breast cancer (34). One possibility is that UNC5C may regulate metastasis in breast cancer through interactions with integrin α6/β4 signaling pathways. Therefore, the present study examined interactions between UNC5C and integrin α6. Immunoprecipitation demonstrated that UNC5C interacted with integrin α6 both exogenously (Fig. 6A) and endogenously (Fig. 6B). UNC5C also interacted with integrin α6 through extracellular domains (Fig. 6C). The effects of UNC5C on integrin α6 expression were then examined and the results showed that UNC5C-knockdown did not affect integrin α6 expression in SK-BR-3 cells. Unexpectedly, UNC5C-overexpression in MDA-MB-231 cells enhanced integrin α6 expression (Figs. 6D and S5).
Figure 6.
UNC5C activates integrin α6-associated signaling through interactions with integrin α6. (A) UNC5C interacts with integrin α6 exogenously. 293T cells were co-transfected with a UNC5C-Myc plasmid and integrin α6-Flag plasmid. Immunoprecipitation was performed with MYC and Flag antibody separately. (B) Immunoprecipitation was performed with antibodies against UNC5C and integrin α6 in SK-BR-3 cells for the endogenous interaction analysis. (C) UNC5C interacts with integrin α6 through an extracellular domain. 293T cells were transfected with UNC5C-Myc, integrin α6-FL-Flag, integrin α6-EC-Flag and integrin α6-IC-Flag in the indicated combinations. Immunoprecipitation was performed to precipitate MYC. (D) Western blotting. UNC5C-knockdown exerted no significant effects on integrin α6 in SK-BR-3 cells, decreased ILK phosphorylation, and increased FAK and SRC phosphorylation. Whereas UNC5C-overexpression upregulated integrin α6 expression, increased ILK phosphorylation and decreased FAK and SRC phos-phorylation. FL, full length; EC, extracellular; IC, intracellular; WB, western blot; UNC5C, Unc-5 Netrin Receptor C; ITGA6, integrin α6; p, phosphorylated; ILK, integrin-linked kinase; sh, short hairpin; Ctrl, control; IP, immunoprecipitate; Ab, antibody.
ILK, FAK and SRC kinases play critical roles in integrin signaling (35,36). To investigate the effects of UNC5C on integrin signaling, the present study examined the phosphorylation of ILK, FAK and SRC kinases. The results showed that ILK phosphorylation at Thr-173 decreased following UNC5C-knockdown in SK-BR-3 cells. UNC5C-overexpression in MDA-MB-231 cells increased the phosphorylation of ILK, although no significant difference was found. By contrast, opposite results were revealed for FAK and SRC phosphorylation (Fig. 6D and S5).
Discussion
A unique characteristic of dependence receptors is their ability to perform dual opposing roles by activating opposite signaling pathways. In the presence of their cognate ligand, they trigger the activation of signaling pathways that are involved in cell survival, migration and differentiation. In the absence of the ligand, the receptors elicit apoptosis-inducing signals (37). The heterogeneous expression of UNC5C was observed in various breast cancer cell lines in the present study (Fig. 1A). The results were consistent with Thiebault et al (5), who examined UNC5C expression in 53 breast cancer samples and corresponding normal samples. They found a ≥2-fold lower expression in 49% of breast tumors, indicating the heterogeneity of UNC5C expression in breast cancer samples. For cancer cells without or with low levels of the cognate ligand NTN1, such as the MCF-7 and MDA-MB-231 cell lines, to avoid the induction of apoptosis, UNC5C expression was downregulated or silenced, supposedly through promoter methylation or the loss of heterogeneity. Conversely, for cells that expressed NTN1, such as the SK-BR-3 and ZR-75-30 cell lines, the NTN1/Unc5C signaling pathway transduced anti-apoptotic signals for survival or other unknown aspects of cancer. Thus, dependence receptors, such as UNC5C, are also termed 'conditional tumor suppressors'. Indeed, the NTN1/Unc5 signaling pathway transmits signals for cancer survival, which may be a self-rescuing reaction of cancer cells that are affected by chemotherapy (38). Several chemotherapeutic agents, such as doxorubicin, 5-fluorouracil, paclitaxel and cisplatin, trigger an increase in the expression of NTN1 and its receptors, such as DCC and UNC5, in various human cancer cell lines (38).
No significant difference was found between clinical normal tissues and ductal breast carcinoma in the present study. This would be expected because both normal and abnormal physiological activities may need, depending on the tissue context, the 'conditional tumor suppressor' function of UNC5C to eliminate unnecessary cells in the absence of NTN1, and also need survival signals that are elicited by the NTN1/UNC5C pathway (Fig. 1B) to control the number of cells in tissues or to regulate directed migration, which usually occurs during neural development.
Interference with NTN1 was reported to induce apoptosis and inhibit metastasis in various cancer types (15,39). Unexpectedly, UNC5C-knockdown in SK-BR-3 and ZR-75-30 cells that expressed NTN1 enhanced tumor growth and metastasis both in vitro (Fig. 2) and in vivo (Fig. 3), indicating that NTN1 and UNC5C exert opposite effects on tumor growth and metastasis. One unresolved issue is why UNC5C-knockdown enhanced tumor growth and metastasis in SK-BR-3 and ZR-75-30 cells even in the presence of NTN1 (Figs. 1C and 2). It can be hypothesized that NTN1 mediates a complex signaling process that integrates both anti-apoptotic and survival signals, leading to the survival and metastasis of cancer cells. NTN1 binding may limit the pro-apoptotic role of UNC5C, and UNC5C-knockdown fully releases the survival signal that is elicited by NTN1, thus enhancing tumor growth and metastasis. Unknown are the receptors that mediate NTN1 survival signaling when UNC5C is disabled. UNC5B has been reported to interact with another NTN1-dependence receptor, DCC, to regulate growth cone orientation. Specifically, DCC attracted and UNC5B repelled growth cones. However, the DCC-mediated attraction of growth cones may be converted to repulsion when UNC5B is overexpressed, and such a conversion may be initiated only by NTN1 (11). Thus, the NTN1 ligand appears to serve as an integrator that ties the two receptors with contrasting functions through a process whereby the amount of each receptor determines the final effects of NTN1, such as either to attract or repel growth cones. These findings prompted the present study to explore whether UNC5C interacts with other NTN1 receptors that are not NTN1-dependence receptors but instead mediate survival signals to regulate tumor growth and metastasis. Integrin α6/β4, whose cognate ligand is laminin-5, is one such NTN1 receptor, although its NTN1-receptor identity was first recognized in the process of pancreas development (33). In the present study, the co-immunoprecipitation assay confirmed that UNC5C interacts with integrin α6 via a mutual extracellular domain in breast cancer cells (Fig. 6). Although integrin α6 can bind to β1 or β4 subunits, integrin α6/β4 is normally expressed in the breast epithelium and upregulated in invasive breast cancer (34). Accordingly, it was presumed that UNC5C predominantly interacts with integrin α6/β4 in breast cancer. Survival signals that are transduced by integrin α6/β4 for tumor progression have been reported in various cancer types. For example, knockdown of integrin α6/β4 expression leads to enhanced apoptosis in breast cancer cells (40) and negates parathyroid hormone-related protein-promoted survival in prostate cancer cells (41). MMP9 is crucially important in tumor invasion and metastasis (42). Although, to the best of our knowledge, no studies have associated integrin α6/β4 with MMP9 expression to date, the regulation of MMP9 expression by other integrins has been frequently reported (43–45). The present study found that UNC5C-knockdown promoted the growth and metastasis of breast cancer cells, mechanistically at least partially by upregulating MMP9 expression (Fig. 4A and B). Additionally, NTN1 has been shown to promote the proliferation (46) and migration (47) of umbilical cord blood-derived mesenchymal stem cells and notably protects cells from hypoxia-induced apoptosis through the integrin α6/β4 signaling pathway (35), indicating the potential significance of the NTN1/integrin α6/β4 signaling pathway in regulating tumor growth and metastasis. A reasonable postulation is that loss of the UNC5C-receptor NTN1/integrin α6/β4 signaling pathway in SK-BR-3 and ZR-75-30 cells provokes the enhancement of tumor growth and metastasis. This is not necessarily ascribed to the upregulation of integrin α6 expression, because UNC5C-knockdown exerted no significant effects on integrin α6 expression. Notably, UNC5C-overexpression upregulated integrin α6 expression in MDA-MB-231 cells, demonstrating the complexity of cell type-dependent interactions between UNC5C and integrin α6 (Fig. 6D).
The cytoplasmic tails of both UNC5C and integrins are devoid of enzymatic features. Thus, UNC5C and integrins transmit signals by associating with adapter and signaling proteins (48,49). FAK and SRC are key kinases in the integrin (50) and NTN1/Unc5C (51) signaling cascades. Previous studies have found that NTN1 activates both FAK and SRC phosphorylation (52) and that FAK and SRC directly phosphorylates UNC5C (51). For integrin signaling, upon integrin clustering and interactions with the integrin β subunit, FAK auto-phosphorylates itself, creating a binding site for SRC whereby SRC kinase subsequently phosphorylates numerous downstream components (50). The present results demonstrated that UNC5C-knockdown enhanced FAK and SRC phosphorylation (Fig. 6D). It can therefore be proposed that the interaction between UNC5C and integrin α6 may interfere with either integrin clustering or binding between FAK and the integrin β4 subunit, thereby affecting FAK and SRC activation. UNC5C-knockdown prevented such interference, and led to enhancement of the activation of FAK and SRC. Notably, crosstalk among FAK and SRC in the p38 MAPK (53), ERK (54) and PI3K/AKT signaling pathways (55) has been well established. Specifically, FAK can directly interact with PI3K (55). Furthermore, the p38 MAPK, ERK and PI3K/AKT signaling pathways have been frequently reported to regulate MMP9 (27–29). These finding support our hypothesis that UNC5C-knockdown enhances MMP9 through the integrin α6/β4 signaling pathway by activating the FAK/SRC, p38 MAPK, ERK and PI3K/AKT signaling pathways.
Although controversial, ILK has been proposed to be both a scaffold protein and serine/threonine kinase in the integrin signaling cascade. ILK activation is PI3K-dependent (36). Although numerous studies have focused on the phosphorylation of ILK substrates, (AKT at Ser-473 and GSK at Ser-9) (36), to the best of our knowledge, the regulation of ILK activity by direct phosphorylation remains to be fully demonstrated. However, phosphorylation at Thr-173, Ser-246 (56) and Ser-343 (57) has been reported to be important for ILK function. Unexpectedly, the present study revealed that UNC5C-knockdown did not affect ILK expression, but inhibited ILK phosphorylation at Thr-173 (Fig. 6D). Based on the crucial role of ILK in stimulating MMP9 expression (58), it can be hypothesized that, at least in SK-BR-3 cells, ILK phosphorylation at Thr-173 may not exert an activating effect but rather an inhibitory effect on ILK function. However, the precise mechanism requires further research.
In summary, the present study found that UNC5C exerts an inhibitory effect on the growth and metastasis of breast cancer cells and that binding with NTN1 limits the effects of UNC5C. Moreover, UNC5C interference potentiated the survival signals that were delivered by the NTN1/integrin α6/β4 signaling pathway and subsequently enhanced tumor growth and metastasis. The NTN1/UNC5C pathway may be a therapeutic target for metastasized cancers. Strategies may be developed to exploit the potential tumor-suppressor role of UNC5C by enhancing its expression. However, the present study was preliminary research on the function of UNC5C in breast cancer, and the current study could not conduct analyses of patient survival between a high-UNC5C group and low-UNC5C group on a large scale. Given the heterogeneity of UNC5C expression in both normal and tumor tissues and the critical roles of integrin α6/β4 in cell-ECM interactions, further studies are needed on the side effects of enhancing UNC5C expression in normal tissues.
Supplementary Data
Acknowledgments
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China (grant no. 31871375) and Program of Fuzhou General Hospital for Distinguished Young Scientists Development (grant no. 2016Q03).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request
Authors' contributions
JY and LY designed the experiments and wrote the paper. MY and FX performed the RT-qPCR, western blotting, immunoprecipitation and immunohistochemistry assays. XX and KZ performed RNAi plasmid construction and functional assays, and analyzed the data. LL and SZ performed the animal experiments. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The animal protocols were approved by the Animal Ethics Committee of Xiamen University (approval no. XMULAC20120030).
Patient consent for publication
Competing interests
The authors declare that they have no competing interests.
References
- 1.Klagsbrun M, Eichmann A. A role for axon guidance receptors and ligands in blood vessel development and tumor angiogenesis. Cytokine Growth Factor Rev. 2005;16:535–548. doi: 10.1016/j.cytogfr.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Dillon AK, Jevince AR, Hinck L, Ackerman SL, Lu X, Tessier-Lavigne M, Kaprielian Z. UNC5C is required for spinal accessory motor neuron development. Mol Cell Neurosci. 2007;35:482–489. doi: 10.1016/j.mcn.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Kim D, Ackerman SL. The UNC5C netrin receptor regulates dorsal guidance of mouse hindbrain axons. J Neurosci. 2011;31:2167–2179. doi: 10.1523/JNEUROSCI.5254-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chédotal A, Kerjan G, Moreau-Fauvarque C. The brain within the tumor: New roles for axon guidance molecules in cancers. Cell Death Differ. 2005;12:1044–1056. doi: 10.1038/sj.cdd.4401707. [DOI] [PubMed] [Google Scholar]
- 5.Thiebault K, Mazelin L, Pays L, Llambi F, Joly MO, Scoazec JY, Saurin JC, Romeo G, Mehlen P. The netrin-1 receptors UNC5H are putative tumor suppressors controlling cell death commitment. Proc Natl Acad Sci USA. 2003;100:4173–4178. doi: 10.1073/pnas.0738063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehlen P, Tauszig-Delamasure S. Dependence receptors and colorectal cancer. Gut. 2014;63:1821–1829. doi: 10.1136/gutjnl-2013-306704. [DOI] [PubMed] [Google Scholar]
- 7.Coissieux MM, Tomsic J, Castets M, Hampel H, Tuupanen S, Andrieu N, Comeras I, Drouet Y, Lasset C, Liyanarachchi S, et al. Variants in the netrin-1 receptor UNC5C prevent apoptosis and increase risk of familial colorectal cancer. Gastroenterology. 2011;141:2039–2046. doi: 10.1053/j.gastro.2011.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hibi K, Mizukami H, Shirahata A, Goto T, Sakata M, Sanada Y. Aberrant methylation of the netrin-1 receptor genes UNC5C and DCC detected in advanced colorectal cancer. World J Surg. 2009;33:1053–1057. doi: 10.1007/s00268-008-9909-x. [DOI] [PubMed] [Google Scholar]
- 9.Lejmi E, Leconte L, Pédron-Mazoyer S, Ropert S, Raoul W, Lavalette S, Bouras I, Feron JG, Maitre-Boube M, Assayag F, et al. Netrin-4 inhibits angiogenesis via binding to neogenin and recruitment of Unc5B. Proc Natl Acad Sci USA. 2008;105:12491–12496. doi: 10.1073/pnas.0804008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koch AW, Mathivet T, Larrivée B, Tong RK, Kowalski J, Pibouin-Fragner L, Bouvrée K, Stawicki S, Nicholes K, Rathore N, et al. Robo4 maintains vessel integrity and inhibits angiogenesis by interacting with UNC5B. Dev Cell. 2011;20:33–46. doi: 10.1016/j.devcel.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Hong K, Hinck L, Nishiyama M, Poo MM, Tessier-Lavigne M, Stein E. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–941. doi: 10.1016/S0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- 12.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 13.Redig AJ, McAllister SS. Breast cancer as a systemic disease: A view of metastasis. J Intern Med. 2013;274:113–126. doi: 10.1111/joim.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernet A, Mazelin L, Coissieux MM, Gadot N, Ackerman SL, Scoazec JY, Mehlen P. Inactivation of the UNC5C Netrin-1 receptor is associated with tumor progression in colorectal malignancies. Gastroenterology. 2007;133:1840–1848. doi: 10.1053/j.gastro.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitamant J, Guenebeaud C, Coissieux MM, Guix C, Treilleux I, Scoazec JY, Bachelot T, Bernet A, Mehlen P. Netrin-1 expression confers a selective advantage for tumor cell survival in metastatic breast cancer. Proc Natl Acad Sci USA. 2008;105:4850–4855. doi: 10.1073/pnas.0709810105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan M, Guo H, Li J, Sui C, Qin Y, Wang J, Khan YH, Ye L, Xie F, Wang H, et al. Slit2 and Robo1 induce opposing effects on metastasis of hepatocellular carcinoma Sk-hep-1 cells. Int J Oncol. 2016;49:305–315. doi: 10.3892/ijo.2016.3506. [DOI] [PubMed] [Google Scholar]
- 17.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 18.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson AL, Wang ZC, De Nicolo A, Lu X, Brown M, Miron A, Liao X, Iglehart JD, Livingston DM, Ganesan S. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9:121–132. doi: 10.1016/j.ccr.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Diagnosis and Treatment Guidelines For Colorectal Cancer Working Group C Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines for breast cancer. 2017 doi: 10.21147/j.issn.1000-9604.2019.01.07. In Chinese. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song S, Peng P, Tang Z, Zhao J, Wu W, Li H, Shao M, Li L, Yang C, Duan F, et al. Decreased expression of STING predicts poor prognosis in patients with gastric cancer. Sci Rep. 2017;7:39858. doi: 10.1038/srep39858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C, Guo H, Li B, Sui C, Zhang Y, Xia X, Qin Y, Ye L, Xie F, Wang H, et al. Effects of Slit3 silencing on the invasive ability of lung carcinoma A549 cells. Oncol Rep. 2015;34:952–960. doi: 10.3892/or.2015.4031. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Quarto N, Senarath-Yapa K, Grey N, Bai X, Longaker MT. Enhanced activation of canonical Wnt signaling confers mesoderm-derived parietal bone with similar osteogenic and skeletal healing capacity to neural crest-derived frontal bone. PLoS One. 2015;10:e0138059. doi: 10.1371/journal.pone.0138059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao M, Alavi MV, Labelle-Dumais C, Gould DB, Type IV. Type IV collagens and basement membrane diseases: Cell biology and pathogenic mechanisms. Curr Top Membr. 2015;76:61–116. doi: 10.1016/bs.ctm.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Tryggvason K, Höyhtyä M, Pyke C. Type IV collagenases in invasive tumors. Breast Cancer Res Treat. 1993;24:209–218. doi: 10.1007/BF01833261. [DOI] [PubMed] [Google Scholar]
- 27.Zhou R, Xu L, Ye M, Liao M, Du H, Chen H. Formononetin inhibits migration and invasion of MDA-MB-231 and 4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through PI3K/AKT signaling pathways. Horm Metab Res. 2014;46:753–760. doi: 10.1055/s-0034-1376977. [DOI] [PubMed] [Google Scholar]
- 28.Kim S, Choi JH, Lim HI, Lee SK, Kim WW, Cho S, Kim JS, Kim JH, Choe JH, Nam SJ, et al. EGF-induced MMP-9 expression is mediated by the JAK3/ERK pathway, but not by the JAK3/STAT-3 pathway in a SKBR3 breast cancer cell line. Cell Signal. 2009;21:892–898. doi: 10.1016/j.cellsig.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 29.Kim ES, Kim MS, Moon A. TGF-beta-induced upregulation of MMP-2 and MMP-9 depends on p38 MAPK, but not ERK signaling in MCF10A human breast epithelial cells. Int J Oncol. 2004;25:1375–1382. [PubMed] [Google Scholar]
- 30.Li W, Liu Z, Zhao C, Zhai L. Binding of MMP-9-degraded fibronectin to β6 integrin promotes invasion via the FAK-Src-related Erk1/2 and PI3K/Akt/Smad-1/5/8 pathways in breast cancer. Oncol Rep. 2015;34:1345–1352. doi: 10.3892/or.2015.4103. [DOI] [PubMed] [Google Scholar]
- 31.Rolli M, Fransvea E, Pilch J, Saven A, Felding-Habermann B. Activated integrin alphavbeta3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:9482–9487. doi: 10.1073/pnas.1633689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung J, Bachelder RE, Lipscomb EA, Shaw LM, Mercurio AM. Integrin (alpha 6 beta 4) regulation of eIF-4E activity and VEGF translation: A survival mechanism for carcinoma cells. J Cell Biol. 2002;158:165–174. doi: 10.1083/jcb.200112015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yebra M, Montgomery AM, Diaferia GR, Kaido T, Silletti S, Perez B, Just ML, Hildbrand S, Hurford R, Florkiewicz E, et al. Recognition of the neural chemoattractant Netrin-1 by integrins alpha6beta4 and alpha3beta1 regulates epithelial cell adhesion and migration. Dev Cell. 2003;5:695–707. doi: 10.1016/S1534-5807(03)00330-7. [DOI] [PubMed] [Google Scholar]
- 34.Gilcrease MZ, Zhou X, Lu X, Woodward WA, Hall BE, Morrissey PJ. Alpha6beta4 integrin crosslinking induces EGFR clustering and promotes EGF-mediated Rho activation in breast cancer. J Exp Clin Cancer Res. 2009;28:67. doi: 10.1186/1756-9966-28-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SS, Lee SJ, Lee SH, Ryu JM, Lim HS, Kim JS, Song EJ, Jung YH, Lee HJ, Kim CH, et al. Netrin-1-induced stem cell bioactivity contributes to the regeneration of injured tissues via the lipid raft-dependent integrin α6β4 signaling pathway. Sci Rep. 2016;6:37526. doi: 10.1038/srep37526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernet A, Mehlen P. Dependence receptors: When apoptosis controls tumor progression. Bull Cancer. 2007;94:E12–E17. [PubMed] [Google Scholar]
- 38.Paradisi A, Creveaux M, Gibert B, Devailly G, Redoulez E, Neves D, Cleyssac E, Treilleux I, Klein C, Niederfellner G, et al. Combining chemotherapeutic agents and netrin-1 interference potentiates cancer cell death. EMBO Mol Med. 2013;5:1821–1834. doi: 10.1002/emmm.201302654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grandin M, Mathot P, Devailly G, Bidet Y, Ghantous A, Favrot C, Gibert B, Gadot N, Puisieux I, Herceg Z, et al. Inhibition of DNA methylation promotes breast tumor sensitivity to netrin-1 interference. EMBO Mol Med. 2016;8:863–877. doi: 10.15252/emmm.201505945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lipscomb EA, Simpson KJ, Lyle SR, Ring JE, Dugan AS, Mercurio AM. The alpha6beta4 integrin maintains the survival of human breast carcinoma cells in vivo. Cancer Res. 2005;65:10970–10976. doi: 10.1158/0008-5472.CAN-05-2327. [DOI] [PubMed] [Google Scholar]
- 41.Bhatia V, Mula RV, Weigel NL, Falzon M. Parathyroid hormone-related protein regulates cell survival pathways via integrin alpha6beta4-mediated activation of phosphatidylinositol 3-kinase/Akt signaling. Mol Cancer Res. 2009;7:1119–1131. doi: 10.1158/1541-7786.MCR-08-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Kempen LCL, Coussens LM. MMP9 potentiates pulmonary metastasis formation. Cancer Cell. 2002;2:251–252. doi: 10.1016/S1535-6108(02)00157-5. [DOI] [PubMed] [Google Scholar]
- 43.Missan DS, Mitchell K, Subbaram S, DiPersio CM. Integrin α3β1 signaling through MEK/ERK determines alternative polyadenylation of the MMP-9 mRNA transcript in immortalized mouse keratinocytes. PLoS One. 2015;10:e0119539. doi: 10.1371/journal.pone.0119539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sil H, Sen T, Chatterjee A. Fibronectin-integrin (alpha5beta1) modulates migration and invasion of murine melanoma cell line B16F10 by involving MMP-9. Oncol Res. 2011;19:335–348. doi: 10.3727/096504011X13079697132925. [DOI] [PubMed] [Google Scholar]
- 45.Lamar JM, Iyer V, DiPersio CM. Integrin alpha3beta1 potentiates TGFbeta-mediated induction of MMP-9 in immortalized keratinocytes. J Invest Dermatol. 2008;128:575–586. doi: 10.1038/sj.jid.5701042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Son TW, Yun SP, Yong MS, Seo BN, Ryu JM, Youn HY, Oh YM, Han HJ. Netrin-1 protects hypoxia-induced mitochondrial apoptosis through HSP27 expression via DCC- and integrin α6β4-dependent Akt, GSK-3β, and HSF-1 in mesenchymal stem cells. Cell Death Dis. 2013;4:e563. doi: 10.1038/cddis.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee SJ, Jung YH, Oh SY, Yong MS, Ryu JM, Han HJ. Netrin-1 induces MMP-12-dependent E-cadherin degradation via the distinct activation of PKCα and FAK/Fyn in promoting mesenchymal stem cell motility. Stem Cells Dev. 2014;23:1870–1882. doi: 10.1089/scd.2013.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taherian A, Li X, Liu Y, Haas TA. Differences in integrin expression and signaling within human breast cancer cells. BMC Cancer. 2011;11:293. doi: 10.1186/1471-2407-11-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chisholm A, Tessier-Lavigne M. Conservation and divergence of axon guidance mechanisms. Curr Opin Neurobiol. 1999;9:603–615. doi: 10.1016/S0959-4388(99)00021-5. [DOI] [PubMed] [Google Scholar]
- 50.Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009;122:159–163. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li W, Aurandt J, Jürgensen C, Rao Y, Guan KL. FAK and Src kinases are required for netrin-induced tyrosine phosphorylation of UNC5. J Cell Sci. 2006;119:47–55. doi: 10.1242/jcs.02697. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Li W, Lee J, Vikis HG, Lee SH, Liu G, Aurandt J, Shen TL, Fearon ER, Guan JL, Han M, et al. Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nat Neurosci. 2004;7:1213–1221. doi: 10.1038/nn1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aikawa R, Nagai T, Kudoh S, Zou Y, Tanaka M, Tamura M, Akazawa H, Takano H, Nagai R, Komuro I. Integrins play a critical role in mechanical stress-induced p38 MAPK activation. Hypertension. 2002;39:233–238. doi: 10.1161/hy0202.102699. [DOI] [PubMed] [Google Scholar]
- 54.Huang D, Khoe M, Befekadu M, Chung S, Takata Y, Ilic D, Bryer-Ash M. Focal adhesion kinase mediates cell survival via NF-kappaB and ERK signaling pathways. Am J Physiol Cell Physiol. 2007;292:C1339–C1352. doi: 10.1152/ajpcell.00144.2006. [DOI] [PubMed] [Google Scholar]
- 55.Chen HC, Appeddu PA, Isoda H, Guan JL. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:26329–26334. doi: 10.1074/jbc.271.42.26329. [DOI] [PubMed] [Google Scholar]
- 56.Acconcia F, Barnes CJ, Singh RR, Talukder AH, Kumar R. Phosphorylation-dependent regulation of nuclear localization and functions of integrin-linked kinase. Proc Natl Acad Sci USA. 2007;104:6782–6787. doi: 10.1073/pnas.0701999104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Persad S, Attwell S, Gray V, Mawji N, Deng JT, Leung D, Yan J, Sanghera J, Walsh MP, Dedhar S. Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: Critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem. 2001;276:27462–27469. doi: 10.1074/jbc.M102940200. [DOI] [PubMed] [Google Scholar]
- 58.Troussard AA, Costello P, Yoganathan TN, Kumagai S, Roskelley CD, Dedhar S. The integrin linked kinase (ILK) induces an invasive phenotype via AP-1 transcription factor-dependent upregulation of matrix metalloproteinase-9 (MMP-9) Oncogene. 2000;19:5444–5452. doi: 10.1038/sj.onc.1203928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request