Abstract
Δ9-THC suppresses cisplatin-induced vomiting through activation of cannabinoid CB1 receptors. Cisplatin-evoked emesis is predominantly due to release of serotonin and substance P (SP) in the gut and the brainstem which subsequently stimulate their corresponding 5-HT3- and neurokinin NK1-receptors to induce vomiting. Δ9-THC can inhibit vomiting caused either by the serotonin precursor 5-HTP, or the 5-HT3 receptor selective agonist, 2-methyserotonin. In the current study, we explored whether Δ9-THC and related CB1/CB2 receptor agonists (WIN55,212-2 and CP55,940) inhibit vomiting evoked by SP (50 mg/kg, i.p.) or the NK1 receptor selective agonist GR73632 (5 mg/kg, i.p.). Behavioral methods were employed to determine the antiemetic efficacy of cannabinoids in least shrews. Our results showed that administration of varying doses of Δ9-THC (i.p. or s.c.), WIN55,212-2 (i.p.), or CP55,940 (i.p.) caused significant suppression of SP-evoked vomiting in a dose-dependent manner. When tested against GR73632, Δ9-THC also dose-dependently reduced the evoked emesis. The antiemetic effect of Δ9-THC against SP-induced vomiting was prevented by low non-emetic doses of the CB1 receptor inverse-agonist/antagonist SR141716A (< 10 mg/kg). We also found that the NK1 receptor antagonist netupitant can significantly suppress vomiting caused by a large emetic dose of SR141716A (20 mg/kg). In sum, Δ9-THC and related cannabinoids suppress vomiting evoked by the nonselective (SP) and selective (GR73632) neurokinin NK1 receptor agonists via stimulation of cannabinoid CB1 receptors.
Keywords: Vomiting, Δ9-THC, SR141716A, cannabinoid CB1 receptor, Substance P, GR73632
1. Introduction
Vomiting is a protective reflex (Carpenter, 1990) which helps to remove ingested toxins from the gastrointestinal tract (GIT) (Horn, 2008). It is also a side-effect of drugs including cancer chemotherapeutics such as cisplatin (Andrews et al., 1998). Cisplatin-evoked acute and delayed emesis are mainly due to co-release of serotonin (5-hydroxytryptamine, 5-HT), dopamine, and substance P (SP) from enterochromaffin cells of the upper GIT, the vagus and/or other GIT nerves, as well as the brainstem (Andrews et al., 1998). These neurotransmitters (5-HT, dopamine, and SP), or corresponding receptor emetic drugs induce vomiting either by: i) activating their respective local serotonin 5-HT3 (5-HT3)- and SP neurokinin 1- (NK1) receptors present primarily on vagal afferents in the GIT, which eventually stimulate brainstem emetic loci, or ii) getting into the brainstem via the bloodstream to directly activate the brainstem emetic loci present in the area postrema (AP), the nucleus of the solitary tract (NTS), and the dorsal motor nucleus of the vagus (DMNX), collectively called the dorsal vagal complex (DVC) (Darmani and Ray, 2009).
The observation that NK1 receptor antagonists could suppress both the acute- and the delayed-phases of cisplatin-induced nausea and vomiting (CINV), encouraged clinical trials of several drugs including: ezlopitant, vofopitant, aprepitant and netupitant in patients undergoing chemotherapy (Andrews and Rudd 2004; Karthaus et al., 2019). Interestingly, various reports have demonstrated that NK1 receptor antagonists have a distinctive antiemetic profile from 5-HT3 receptor antagonists in their ability to inhibit both acute- and delayed phases of cisplatin-induced vomiting, as well as vomiting evoked by peripheral (e.g., abdominal vagal afferent electrical stimulation) and centrally-acting emetogens (e.g., apomorphine). Among 5-HT3 receptor antagonists, palonosetron in combination with NK1 receptor antagonists is recommended for the prevention of severe CINV caused by high-dose cisplatin therapy (Herrstedt et al., 2017). The mixture of palonosetron with an NK1 receptor antagonist such as netupitant seemingly has synergistic antiemetic efficacy against both acute and delayed emesis (Rojas et al., 2014; Darmani et al., 2011). In fact, when these two drugs were given together with dexamethasone, over 90% control of cisplatin-induced vomiting has been described (Aapro et al., 2014; Keating, 2015). Unfortunately, although the combined use of NK1 receptor- as well as 5-HT3 receptor-antagonists, has substantially lowered rates of cisplatin-mediated acute and delayed emesis, a marked number of patients continue to suffer from CINV (Karthaus et al., 2019). Furthermore, there is no available treatment exclusively for nausea or a collective anti-nausea/ antiemetic drug which would suppress both nausea and vomiting irrespective of the source (Andrews and Sanger, 2014). Subsequently, nausea is still negatively impacting patients' quality of life.
The key psychoactive component of the marijuana plant, delta 9-tetrahydrocannabinol (Δ9-THC) (Janoyan et al., 2002) and a number of its analogues/ formulations (Δ8-THC, nabilone, and dronabinol) have been used against acute- and delayed-phases of chemotherapy-evoked vomiting in patients (Voth and Schwartz, 1997). Overwhelming clinical evidence indicate that Δ9-THC pretreatment reduces emesis in some patients receiving cancer chemotherapy (Voth and Schwartz, 1997). Two oral synthetic formulations, dronabinol and nabilone, have been approved by the US Food and Drug Administration for antiemetic use against CINV. Evidence also supports cannabinoids effectiveness against nausea during the delayed phase of chemotherapy-evoked emesis which is poorly controlled by 5-HT3 receptor- and NK receptor-antagonists (Slatkin, 2007). Unlike the relatively large body of findings regarding the antiemetic potential of 5-HT3 receptor- and NK1 receptor- antagonists, only limited studies on the antiemetic effects of cannabinoids against diverse emetogens are available in vomit-competent animals (Darmani, 2002).
The mechanisms by which Δ9-THC and its structural analogs produce their antiemetic effects were revealed following the cloning of cannabinoid CB1 and CB2 G-protein coupled receptors (CB1 receptor and CB2 receptor) (Di Marzo, 2008). CB1 receptors are distributed throughout the central and the peripheral nervous system (Pertwee et al., 2010). CB2 receptors are often localized in immune tissues in the periphery (Darmani, 2010). Δ9-THC and associated cannabinoids behave as broad-spectrum agonist antiemetics in a CB1 receptor antagonist-sensitive manner (Darmani, 2010). We and others have previously tested the antiemetic efficacy of Δ9THC against diverse emetogens such as: i) cisplatin (Ray et al., 2009b), ii) the 5-HT precursor 5-hydroxytryptophan (5-HTP), iii) 5-HT3 receptor agonists, iv) Δ2 receptor agonists (Darmani and Crim, 2005), and v) the CB1 receptor antagonist/ inverse-agonist SR141716A. Since cisplatin evokes vomiting via the release of 5-HT, dopamine and substance P (SP) (etc), in the current study we investigated the antiemetic potential of Δ9-THC against vomiting evoked by the neurokinin NK1 receptor agonists SP and GR73632. Thus, we used the least shrew to evaluate: 1) the potential of Δ9-THC and its analogs (WIN55,212-2 and CP55,940) to suppress vomiting produced by SP or the NK1 receptor selective agonist GR73632, 2) the ability of low doses of the cannabinoid CB1 receptor antagonist/inverse-agonist SR141716A, to reverse the antiemetic potential of Δ9THC against SP-induced vomiting, and 3) the ability of NK1 receptor selective antagonist netupitant to suppress emesis evoked by a large dose of SR141716A since the latter agent potentiates the neuronal release of SP (Lever and Malcangio, 2002).
2. Materials and methods
2.1. Animals
Male and female (4 – 6 g, 35 – 60 days old) adult least shrews from our animal facility were used. Shrews were housed in groups of 5 – 10 on a 14:10 light: dark cycle, at a humidity-controlled room temperature of 21 ± 1°C, with an ad libitum supply of food and water (Darmani et al., 2003b) (Darmani, 2001d). All animals received care according to the Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services Publication, revised, 2011). All experimental procedures were conducted between 8:00 am and 17:00 pm. All of the procedures used in this study were approved by the Western University Institutional Animal Care and Use Committee of Western University of health Sciences (Application number R17IACUC036).
2.2. Drugs
Δ9-YHC, R(+)-WIN55,212-2, and substance P (SP) were purchased from Sigma/RBI (St. Louis, MO). CP55,940 was provided by Pfizer (Groton, CT) and SR141716A by Safoni-Synthelabo Recherche (Montpellier, France). Netupitant was a gift from the Helsinn Health Care (Lugano, Switzerland). GR73632 was purchased from Tocris Cookson Inc. (Ellisville, MO). All other reagents were obtained from Sigma (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA).
Δ9-THC, WIN55,212-2, CP55,940, SR141716A, and netupitant were dissolved to twice the stated drug dose in a 1:1:18 solution of ethanol:Emulphor™:0.9% saline. The drug doses were then diluted further with an equal volume of saline. This step was mandatory because the 1:1:18 vehicle mixture can stimulate vomiting by itself in up to 20% of animals (Darmani, 2001c). SP and GR73632 were dissolved in sterile distilled deionized water. All drugs were administered at a volume of 0.1 ml/10 g of body weight.
2.3. Emesis studies
The present protocols were based upon our previous emesis studies (Darmani, 2001b, c; Darmani et al., 1999). All experiments were performed between 8:00 am and 17:00 pm. On the day of the experiment, shrews were brought in experimental room from the animal facility, weighed, transferred to a 20 × 18 × 21 cm clean clear plastic individual cages, and allowed to acclimate for 1 h during which daily food was withdrawn. Drug-naive male and female shrews were randomly allocated to the control and the experimental groups regardless of their cage of origin. The shrews were given four meal worms (Tenebrio sp.) each 30 min before the administration of emetogens, to help identify wet vomits as described previously (Darmani et al., 1999). No animal was dropped from the experiment. For behavioral experiments, we present both the mean vomit frequency and the percentage of shrews vomiting. We have utilized the % vomit data to calculate “ID50 values.”
Both SP and its selective NK1 receptor agonist GR73632, can induce robust vomiting in least shrews at 5 and 50 mg/kg (i.p.) doses, respectively (Darmani et al., 2008). At time zero, different groups of shrews were injected with either: 1) Δ9-THC (0, 0.5, 2.5, or 5 mg/kg; n = 8 shrews/group, respectively via i.p. except for 1.25 mg/kg where n = 10) or (0, 5, 10, or 20 mg/kg; n = 8 shrews/group, respectively via s.c. except for 2.5 mg/kg dose where n = 6); 2) WIN55,212-2 (0, 1, or 2.5 mg/kg; n = 8 shrews/group, respectively via i.p. except for 5 mg/kg dose where n = 10), or 3) CP55,940 (0.025, 0.05, or 0.1 mg/kg; n = 8 shrews/group, respectively via i.p. except for 0 mg/kg dose where n = 9). Following treatment, each shrew was offered four mealworms and was placed in an observation cage for 30 min prior to the administration of SP (50 mg/kg, i.p.). Additional shrews were injected with Δ9-THC (0, 10, or 20 mg/kg; n = 8 shrews/group, respectively via i.p. except for 5 mg/kg dose where n = 6) for 30 min prior to the administration of GR73632 (5 mg/kg, i.p.) and were observed for the next 30 min. In another set of experiments, various doses of the selective NK1 receptor-antagonist netupitant (0, 1, 2.5, 5, or 10 mg/kg; n = 6 shrews/group, i.p.), were injected in different groups of shrews 30 min prior to a single emetic dose of the CB1 receptor antagonist/inverse-agonist SR141716A (20 mg/kg, i.p.). Throughout the study respective vehicles were included for each experimental condition. After the administration of each emetogen, the vomit frequency (oral ejections of food or liquid rejected for 30 min; mean ± S.E.M.) was recorded for each shrew.
To demonstrate whether the antiemetic effects of Δ9-THC is a CB1 receptor-mediated event, non-emetic (s.c.) doses of SR141716A (0, 5, or 10 mg/kg; n = 8 shrews/ group, s.c.) were used to prevent the antiemetic effect of a fully effective antiemetic dose of Δ9-THC (20 mg/kg, s.c.) against SP (50 mg/kg, i.p.)-induced vomiting. Thus, at time 0, different groups of shrews were injected with Δ9-THC and one of the discussed non-emetic doses of SR141716A, then were offered four mealworms. After 30 min, each shrew received SP (50 mg/kg, i.p.), and the frequency of evoked vomiting was recorded for the next 30 min as described earlier.
2.4. Statistical analyses
Assuming that type 1 error rate was set at 0.05, sample size estimates for behavioral studies was based on a power of 80% to detect 30% change between control and treated (assuming an expected standard deviation of 20% of mean values). This analysis results in a requirement for 8 animals in each group. The frequency of emesis data was analyzed by Kruskal–Wallis H (KW) nonparametric one-way analysis of variance (ANOVA) and post-hoc analysis by Dunn's multiple comparisons test. The incidence of emesis (percentage of animals vomiting) was analyzed by the Chi-square test to determine whether there were differences between groups. When appropriate, pairwise comparisons were also made by this method. The ID50 values (the inhibitory dose that prevented emesis in 50% of shrews) were calculated using a nonlinear regression test using GraphPad (InPlot, San Diego, CA). A P-value of < 0.05 was necessary to achieve statistical significance.
3. Results
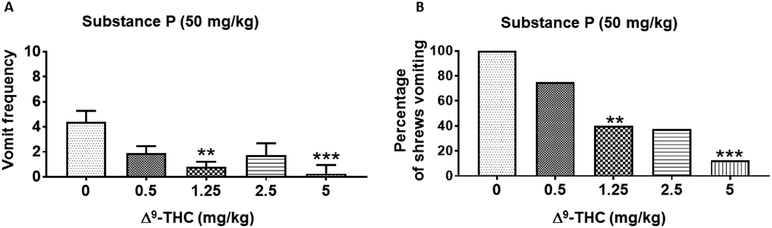
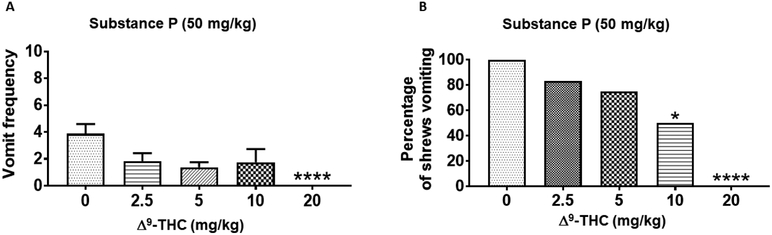
Δ9-THC reduced substance P-evoked emesis in a dose- and route-dependent manner (Figs. 1 and 2). Kruskal–Wallis nonparametric ANOVA test showed that relative to vehicle-pretreated control group, intraperitoneally (i.p.)-administered Δ9-THC (0 – 5 mg/kg) significantly reduced the mean frequency of SP-induced vomiting during the 30-min observation period (KW (4, 37) = 16.31; P < 0.01) (Fig. 1A). Significant reductions in the frequency of SP-induced vomits occurred at its 1.25 (P < 0.01) and 5 mg/kg (P < 0.001) doses (Fig. 1A). In addition, the Chi-square test indicated that Δ9-THC (i.p.) significantly protected shrews from SP-evoked vomiting (χ2 (4, 37) = 14.5; P = 0.0001) (Fig. 1B). Significant reductions in the percentage of shrews vomiting occurred at its 1.25 (60%; P < 0.01), 2.5 (62.5%; P < 0.01, and 5 (87.5%; P < 0.001) mg/kg doses (Fig. 1B). Δ9-THC potently protected shrews from SP-induced vomiting with a percentage ID50 inhibition value of 1.10 (0.55 - 2.10) mg/kg. Likewise, relative to control animals, subcutaneous (s.c.) administration of Δ9-THC (0–20 mg/kg) significantly reduced the mean frequency of SP-induced vomiting (KW (4, 33) = 19.1; P < 0.001) (Fig. 2A). Moreover, compared to the vehicle-treated animals where all the animals vomited, the frequency of SP-induced vomits was totally abrogated at a relatively much higher dose of Δ9-THC (20 mg/kg, s.c.; P < 0.0001) (Fig. 2A). The s.c.-administered Δ9-THC protected shrews from SP-evoked vomiting (χ2 (4, 33) = 19.25; P < 0.0001) (Fig. 2B) with significant reductions in the percentage of shrews vomiting at its 10 (50%; P < 0.05) and 20 mg/kg doses (100%; P < 0.0001) (Fig. 2B). Δ9-THC (s.c.) protected shrews from SP-induced emesis with a percentage ID50 inhibition value of 7.2 (4.10 – 12.50) mg/kg.
Figure 1. Antiemetic effects of different doses of intraperitoneally-administered Δ9-THC against substance P (SP)-induced emesis in the least shrew.
Different groups of shrews received either vehicle (0 mg/kg) or varying doses of Δ9-THC, 30 min prior to injection of a fully effective emetic dose of SP (50 mg/kg, i.p.). Emetic parameters were recorded for the next 30 min post emetic injection. The mean frequency of vomits (± S.E.M.) (graph A) and the percentage of animals vomiting (graph B) are presented. ** P < 0.01 and *** P < 0.001 versus 0 mg/kg control. N = 8- 10 shrews/ group.
Figure 2. Antiemetic effects of different doses of subcutaneously-administered Δ9-THC against substance P (SP)-induced emesis in the least shrew.
Different groups of shrews received either vehicle (0 mg/kg) or varying doses of Δ9-THC, 30 min prior to a fully effective emetic dose of SP (50 mg/kg, i.p.). Emetic parameters were recorded for the next 30 min post emetic injection. The mean frequency of vomits (± S.E.M.) (graph A) and the percentage of animals vomiting (graph B) are presented. * P < 0.05 and *** P < 0.001 versus 0 mg/kg control. N = 6-8 shrews/ group.
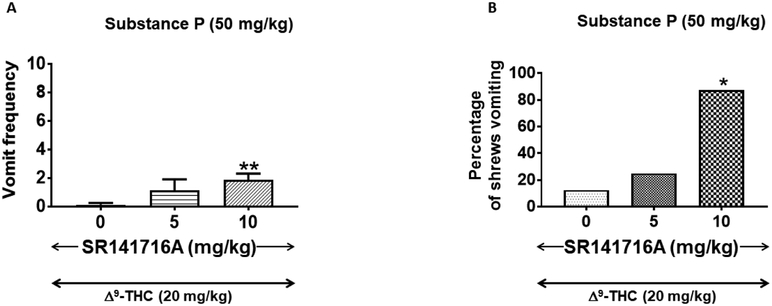
To determine whether the antiemetic effect of Δ9-THC was mediated via CB1 receptor, reversal of the antiemetic capacity of an effective dose of Δ9-THC (20 mg/kg, s.c.) against SP-induced (50 mg/kg, i.p.) emesis was investigated via co-treatment with various doses of the CB1 receptor antagonist/inverse-agonist, SR141716A (0, 5, or 10 mg/kg, s.c.). Our results demonstrate that a 10 mg/kg (s.c.) dose of SR14171A significantly prevented the antiemetic action of Δ9-THC. Thus, relative to the control group (i.e. 0 mg/kg SR14171A + 20 mg/kg Δ9-THC) where there was no vomit, SR14171A significantly prevented the ability of Δ9-THC-to block SP-evoked emesis (FW (2, 21) = 8.65; P = 0.01) (Fig. 3A) with a significant reduction at its 10 mg/kg dose (P < 0.01) (Fig. 3A). SR14171A also significantly reversed the protective efficacy of Δ9-THC against SP-evoked vomiting (χ2 (2, 21) = 9.25; P < 0.01) with a significant reversal at 10 mg/kg (87.5%; P < 0.01) (Fig. 3B). SR14171A prevented the antiemetic efficacy of Δ9-THC against SP-induced with a percentage ID50 inhibition value of 7.04 (4.03 – 11.87) mg/kg.
Figure 3. Ability of varying subcutaneously (s.c.)-administered doses of SR141716A to reverse the effects of a fully efficacious antiemetic dose Δ9-THC (20 mg/kg, s.c.) against substance P (SP)-induced emesis in the least shrew.
Different groups of shrews received either 0 mg/kg SR14171A + 20 mg/kg Δ9-THC, or varying doses of SR14171A + 20 mg/kg Δ9-THC; 30 min prior to an emetic dose of SP (50 mg/kg, i.p.). SR14171A significantly reversed the Δ9-THC-induced decrease in emesis frequency (graph A). SR141716A also reversed the ability of Δ9-THC to protect shrews from vomiting (graph B). * P < 0.05 and ** P < 0.01 versus 0 mg/kg SR141716A + 20 mg/kg Δ9-THC control group. N = 8 shrews per group.
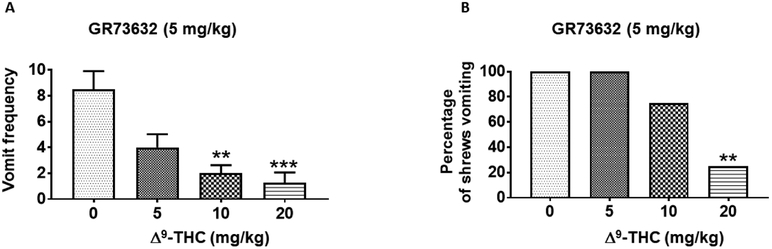
As with SP, intraperitoneal injection of various doses of Δ9-THC (i.p.) significantly suppressed the mean frequency of GR73632 (5 mg/kg, i.p.)-induced vomiting (KW (3, 26) = 16.71; P < 0.001) with significant reductions at its 10 (P < 0.01) and 20 mg/kg (P < 0.001) doses (Fig. 4A). Δ9-THC (i.p.) also significantly protected shrews from the evoked vomiting (χ2 (3, 26) = 12.3; P < 0.001) (Fig. 4B) with a significant effect at 20 mg/kg (75%; P < 0.01) (Fig. 4B). In addition, Δ9-THC protected shrews from GR73632-induced vomiting with a percentage ID50 inhibition value of 11.10 (6.50 – 18.60) mg/kg.
Figure 4. Antiemetic effects of intraperitoneally-administered Δ9-THC against GR73632-induced emesis in the least shrew.
Different groups of shrews received either vehicle (0 mg/kg) or varying doses of Δ9-THC, 30 min prior to an injection of an emetic dose of the neurokinin NK1 receptor selective agonist, GR73632 (5 mg/kg, i.p.). Emetic parameters were recorded for the next 30 min post emetic injection. The mean frequency of vomits (± S.E.M.) (graph A) and the percentage of animals vomiting (graph B) are presented. ** P < 0.01 and *** P < 0.001 versus 0 mg/kg control. N = 6-8 shrews/ group.
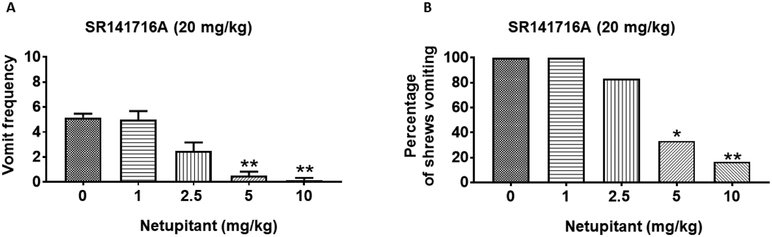
Large doses (20 - 40 mg/kg) of SR141716A can evoke vomiting in the least shrew (Darmani et al., 2003a). Thus, we used a single fully effective emetic dose of SR141716A (20 mg/kg, i.p.) to evaluate the antiemetic potential of the neurokinin NK1 receptor selective antagonist, netupitant. Netupitant (0, 1, 2.5, 5, or 10 mg/kg, i.p.) caused a dose-dependent decrease in the frequency of SR141716A-induced vomiting (KW (4, 25) = 22.5; P < 0.001) with significant reductions at its 5 (P < 0.01) and 10 mg/kg (P < 0.01) doses (Fig. 5A). Netupitant also significantly protected shrews from SR141716A-evoked vomiting (χ2 (4, 25) = 14.70; P = 0.0001) (Fig. 5B) with significant protection at its 5 (66.70%; P = 0.01) (Fig. 5B) and 10 mg/kg (83.33%; P < 0.01) doses. Moreover, netupitant potently reduced GR73632-induced vomiting with a percent ID50 inhibition value of 4.60 (2.50 - 8.80) mg/kg.
Figure 5. Antiemetic effects of intraperitoneally-administered NK1R selective antagonist netupitant against SR141716A-induced emesis in the least shrew.
Different groups of shrews received either vehicle (0 mg/kg) or varying doses of netupitant, 30 min prior to an emetic dose of SR141716A (20 mg/kg, i.p.). Emetic parameters were recorded for the next 30 min post emetic injection. The mean frequency of vomits (± S.E.M.) (graph A) and the percentage of animals vomiting (graph B) are presented. * P < 0.05 and ** P < 0.01 versus 0 mg/kg control. N = 6 shrews/ group.
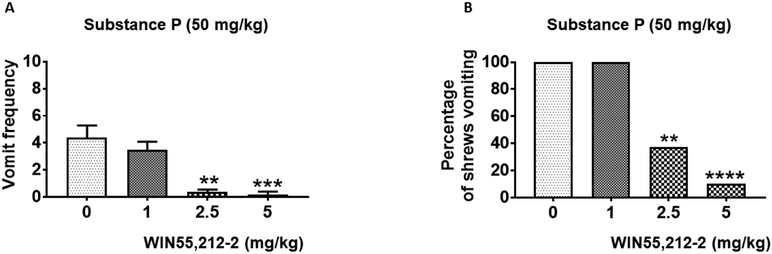
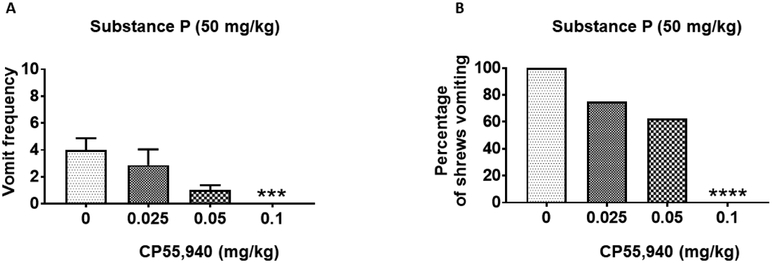
Finally, we investigated the antiemetic potential of two synthetic cannabinoids WIN55,212-2 and CP55,940 against SP (50 mg/kg, i.p.)-evoked vomiting. WIN55,212-2 (0, 1, 2.5, or 5 mg/kg, i.p.) caused dose-dependent decreases in the frequency of SP-induced emesis (KW (3, 30) = 25.20; P < 0.0001) with significant effects at 2.5 (P = 0.01) and 5 mg/kg (P = 0.001) doses (Fig. 6A). Moreover, WIN55,212-2 significantly protected shrews from SP-evoked vomiting (χ2 (3, 30) = 21.44; P < 0.0001) at its 2.5 (62.50%; P < 0.01) and 5 mg/kg (90.91%; P < 0.0001) doses (Fig. 6B). WIN55,212-2 protected shrews from SP-evoked vomiting with a percent ID50 inhibition value of 1.70 (0.73 - 3.52) mg/kg. Likewise, pretreatment with varying doses of CP55,940 (0, 0.025, 0.05, or 0.1 mg/kg, i.p.) produced substantial decreases in the mean frequency of SP-evoked vomits (KW (3, 29) = 17.10; P < 0.001) with significant reduction at 0.1 mg/kg (P < 0.001) (Fig. 7A). CP55,940 also significantly protected shrews from SP-evoked vomiting (χ2 (3, 29) = 17.02; P < 0.0001) at its 0.1 mg/kg dose (100%; P < 0.0001) (Fig. 7B). Moreover, CP55,940 potently protected shrews from SP-induced vomiting with a percent ID50 inhibition value of 0.06 (0.03 - 0.17) mg/kg.
Figure 6. Antiemetic effects of intraperitoneally-administered WIN55,212-2 against substance P (SP)-induced emesis in the least shrew.
Different groups of shrews received either vehicle (0 mg/kg) or varying doses of WIN55,212-2, 30 min following an emetic dose of SP (50 mg/kg, i.p.). Emetic parameters were recorded for the next 30 min post emetic injection. The mean frequency of vomits (± S.E.M.) (graph A) and the percentage of animals vomiting (graph B) are presented. ** P < 0.01, *** P < 0.001, and **** P < 0.0001 versus 0 mg/kg control. N = 8-10 shrews/ group.
Figure 7. Antiemetic effects of intraperitoneally-administered CP55,940 against substance P (SP)-induced emesis in the least shrew.
Different groups of shrews received either vehicle (0 mg/kg) or varying doses of CP55,940, 30 min prior to an emetic dose of SP (50 mg/kg, i.p.). Emetic parameters were recorded for the next 30 min post emetic injection. The mean frequency of vomits (± S.E.M.) (graph A) and the percentage of animals vomiting (graph B) are presented. *** P < 0.001 and **** P < 0.0001 versus 0 mg/kg control. N = 8-9 shrews/ group.
4. Discussion
This study addressed the antiemetic effects of Δ9-THC and related cannabinoids against SP-induced emesis. Our results demonstrate that Δ9-THC causes significant inhibition of SP-evoked vomiting in a dose- and route-dependent manner. Indeed, intraperitoneal administration of Δ9-THC was 6.5 times more efficacious in protecting 50% of shrews from vomiting than its s.c. injection. More importantly, the antiemetic effect of Δ9-THC against SP-induced vomiting was blocked by the CB1R inverse-agonist/antagonist, SR141716A. Furthermore, Δ9-THC (i.p.) significantly prevented vomiting caused by the NK1 receptor selective agonist, GR73632, in a dose-dependent manner. Finally, netupitant, a potent and selective NK1 receptor antagonist (Rizzi et al., 2012) caused significant and dose-dependent decreases in both the mean vomit frequency and the percentage of animals vomiting in response to administration of an emetic dose of SR141716A. Collectively, our findings strongly support the concept that Δ9-THC prevents vomiting evoked by nonselective (SP) as well as the selective (GR73632) neurokinin NK1 receptor agonists via activation of cannabinoid CB1 receptors.
Clear differences in Δ9-THC ’ antiemetic efficacy as a function of route of administration are to be expected. Relative to the s.c. route where no noticeable antiemetic effect occurred at a 5 mg/kg dose of Δ9-THC, the latter dose via the i.p. route nearly completely suppressed the mean frequency of SP-induced vomiting. Likewise, s.c. administration of Δ9-THC (0.1 - 30 mg/kg) does not seem to affect mice locomotor activity (Compton et al., 1992), whereas its i.p. injection can significantly lower both motion and rearing behaviors (Janoyan et al., 2002). One explanation for this difference relates to the highly lipophilic nature of Δ9-THC, which is expected to be absorbed from the s.c. route into the general circulation more gradually (Sharma et al., 2012). Indeed, intraperitoneally administrated drugs are exposed to a large surface of peritoneal membrane, which often allows their rapid absorption, and distribution (Lukas et al., 1971).
Another finding of this investigation is administration of relatively smaller non-emetic doses of SR141716A can prevent the antiemetic action of a totally effective dose of Δ9-THC against SP-evoked emesis. Prior studies have demonstrated that SR141716A pretreatment is able to prevent the antiemetic efficacy of Δ9-THC against LiCl- and cisplatin-induced vomiting in the house musk shrew (Kwiatkowska et al., 2004; Parker et al., 2004). Likewise, in ferrets, the ability of Δ9-THC to attenuate cisplatin- or morphine-6-glucuronide-induced emesis has been reported to be blocked by SR141716, or its analog AM251 (Van Sickle et al., 2001). Published studies from our laboratory also demonstrate that SR14171A can prevent the antiemetic effects of Δ9-THC against cisplatin- or apomorphine-evoked emesis in least shrews (Darmani, 2001c; Ray et al., 2009b; Wang et al., 2009). Furthermore, Δ9-THC suppresses cisplatin-evoked emesis in least shrews through the stimulation of CB1R both in the brainstem and the GIT (Darmani and Johnson, 2004; Ray et al., 2009b). Equally, vomiting caused by the selective NK1 receptor agonist GR73632 involves both central and peripheral mechanisms (Darmani et al., 2008; Ray et al., 2009a). Based on these facts and current findings, we postulate that stimulation of CB1 receptors by Δ9-THC leads to suppression of SP-induced vomiting in both the brainstem and the GIT, however, this entails further investigation.
As a selective NK1 receptor competitive antagonist, netupitant binds to and prevents the activity of SP on NK1 receptor (Rizzi et al., 2012; Spinelli et al., 2014), thereby inhibiting NK1 receptor binding by the endogenous SP. As with other NK1 receptor antagonists (Rojas et al., 2014), netupitant has a broad-spectrum antiemetic efficacy and can attenuate vomiting caused by diverse emetogens including the L-type calcium channel agonist FPL64176 (Zhong et al., 2018), the SERCA inhibitor thapsigargin (Zhong et al., 2016), as well as cancer chemotherapeutics such as cisplatin (Darmani et al., 2015; Rudd et al., 2016). Currently, we tested the antiemetic potential of netupitant against a large single emetic dose of the selective CB1 receptor antagonist SR141716A (20 mg/kg, i.p.) and found increasing doses of netupitant reduced the number of shrews vomiting in response to SR14171A with an ID50 inhibition value of 4.60 (2.50 - 8.80) mg/kg. Moreover, a 10 mg/kg dose of netupitant protected 83% of the shrews from the induced emesis. The latter dose of netupitant can completely protect least shrews from vomiting evoked by a 5 mg/kg (i.p.) dose of the NK1 receptor selective agonist GR73632 (Zhong et al., 2019). In line with our behavioral findings, cannabinoid agonists (e.g. HU210) seem to attenuate SP release in cultured rat dorsal ganglion cells (Oshita et al., 2005), while corresponding CB1 receptor antagonists (e.g. SR141716A) potentiate capsaicin-evoked SP release in the mouse spinal cord (Lever and Malcangio, 2002). However, exogenous administration of either SP (50 mg/kg, i.p.) or GR73632 (5 mg/kg, i.p.) can cause the release of endogenous SP in the least shrew brainstem emetic nuclei (Darmani et al., 2008; Zhong et al., 2019). In this setting, an NK1 receptor antagonist such as netupitant would probably prevent the evoked endogenous SP release in the brainstem and thus would prevent the vomiting caused by either GR73632, SP or SR141716A. We plan to investigate the interaction between CB1- and NK1-receptors in detain in future studies. Overall, these findings support the involvement of endogenous SP in SR14171A-mediated emesis.
Little is known regarding the antiemetic structure activity relationship of different cannabinoids in any species. We compared intraperitoneally injected Δ9-THC potency against SP-evoked vomiting to that of related cannabinoid CB1/2 receptor agonists WIN55,212-2 and CP55,940. We found that CP55,940 was the most potent antiemetic with the following ID50 order: CP55,940<<<Δ9-THC = WIN55,212-2. Thus, while Δ9-THC and WIN55,212-2 are essentially equipotent against SP-induced vomiting, they are 18 to 28 less efficacious than CP55,940. Moreover, while CP55,940 (0.1 mg/kg) completely protected shrews from the evoked vomiting, the other two cannabinoids could protect up to 90% of shrews from vomiting at a dose of 5 mg/kg. The present findings are consistent with our published studies where CP55,940 was 13–20 times more effective against cisplatin-induced emesis than WIN55,212-2 or Δ9-THC (Darmani, 2001a, c). Other reports seem to substantiate our findings since Δ9-THC and WIN55,212-2 have a similar affinity for the cannabinoid CB1 receptor (Pertwee, 1999) and are both less potent than CP55,940 in suppression of locomotor activity in mice.
The role of CB2 receptor in the antiemetic actions of cannabinoids is still elusive. Different CB2 receptor antagonists were unsuccessfully utilized to reverse the antiemetic efficacy of various CB1 /CB2 receptor agonists against diverse emetic stimuli (Darmani, 2001a, d). The fact that CB2 receptor is predominantly expressed in the immune tissues, and absent or very low in the emetic loci, imply that CB2 receptor may not have a role in vomiting. In support of this premise, our laboratory has shown that large doses of SR141716A and not SR144528 (a selective and potent cannabinoid CB2 receptor antagonist) can evoke vomiting in a dose-dependent manner and the induced emesis was blocked by Δ9-THC and WIN55,212-2 and CP55,940 (Darmani, 2001d). Intriguingly, Van Sickle et al., 2005 have shown that in the ferret the antiemetic actions of the endocannabinoid 2-AG (but not anandamide, another endocannabinoid) could be prevented by a CB2 receptor antagonist, which failed to prevent the antiemetic effects of Δ9-THC against cisplatin-evoked vomiting. Neither the antiemetic effects of Δ9-THC, WIN55,212-2, or CP55,940 could be reversed by SR144528 in the least shrew (Darmani, 2001a; Darmani et al., 2003b; Simoneau et al., 2001). Thus, the CB2 receptor may have no or only a modest role in emesis.
In summary, the current results extend published findings that Δ9-THC is a broad-spectrum antiemetic not only against conventional emetogens (such as cisplatin, dopamine or serotonin), but it also prevents vomiting caused by the neuropeptide SP and the corresponding neurokinin NK1 receptor selective agonist GR73632. Δ9-THC and related cannabinoids (WIN55,212-3 and CP55,940) appear to attenuate SP-evoked vomiting via activation of the cannabinoid CB1 receptor. The exact molecular mechanisms by which CB1 receptor antagonists block SP-evoked emesis remain unknown and warrants further investigation.
Acknowledgements
This work was supported in part by NIH-NCI grant RO1CA207287 and the WesternU startup fund (1395) to NAD.
Footnotes
Declarations of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aapro M, Rugo H, Rossi G, Rizzi G, Borroni ME, Bondarenko I, Sarosiek T, Oprean C, Cardona-Huerta S, Lorusso V, Karthaus M, Schwartzberg L, Grunberg S, 2014. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol 25, 1328–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PL, Naylor RJ, Joss RA, 1998. Neuropharmacology of emesis and its relevance to anti-emetic therapy. Consensus and controversies. Support Care Cancer 6, 197–203. [DOI] [PubMed] [Google Scholar]
- Andrews PL, Rudd JA, 2004. The role of tachykinins and the tachykinin NK1 receptor in nausea and emesis, in Handbook of Experimental Pharmacology, ed Holzer P, editor. (Berlin:Springer-Verlag; ), 359–440. [Google Scholar]
- Andrews PL, Sanger GJ, 2014. Nausea and the quest for the perfect anti-emetic. Eur J Pharmacol 722, 108–121. [DOI] [PubMed] [Google Scholar]
- Carpenter DO, 1990. Neural mechanisms of emesis. Can J Physiol Pharmacol 68, 230–236. [DOI] [PubMed] [Google Scholar]
- Compton DR, Gold LH, Ward SJ, Balster RL, Martin BR, 1992. Aminoalkylindole analogs: cannabimimetic activity of a class of compounds structurally distinct from delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther 263, 1118–1126. [PubMed] [Google Scholar]
- Darmani NA, 2001a. The cannabinoid CB1 receptor antagonist SR 141716A reverses the antiemetic and motor depressant actions of WIN 55, 212-2. Eur J Pharmacol 430, 49–58. [DOI] [PubMed] [Google Scholar]
- Darmani NA, 2001b. Cannabinoids of diverse structure inhibit two DOI-induced 5-HT(2A) receptor-mediated behaviors in mice. Pharmacol Biochem Behav 68, 311–317. [DOI] [PubMed] [Google Scholar]
- Darmani NA, 2001c. Delta-9-tetrahydrocannabinol differentially suppresses cisplatin-induced emesis and indices of motor function via cannabinoid CB(1) receptors in the least shrew. Pharmacol Biochem Behav 69, 239–249. [DOI] [PubMed] [Google Scholar]
- Darmani NA, 2001d. Delta(9)-tetrahydrocannabinol and synthetic cannabinoids prevent emesis produced by the cannabinoid CB(1) receptor antagonist/inverse agonist SR 141716A. Neuropsychopharmacology 24, 198–203. [DOI] [PubMed] [Google Scholar]
- Darmani NA, 2002. The potent emetogenic effects of the endocannabinoid, 2-AG (2-arachidonoylglycerol) are blocked by delta(9)-tetrahydrocannabinol and other cannnabinoids. J Pharmacol Exp Ther 300, 34–42. [DOI] [PubMed] [Google Scholar]
- Darmani NA, 2010. Mechanisms of Broad-Spectrum Antiemetic Efficacy of Cannabinoids against Chemotherapy-Induced Acute and Delayed Vomiting. Pharmaceuticals (Basel) 3, 2930–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani NA, Chebolu S, Amos B, Alkam T, 2011. Synergistic antiemetic interactions between serotonergic 5-HT3 and tachykininergic NK1-receptor antagonists in the least shrew (Cryptotis parva). Pharmacol Biochem Behav 99, 573–579. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Crim JL, 2005. Delta-9-tetrahydrocannabinol differentially suppresses emesis versus enhanced locomotor activity produced by chemically diverse dopamine D2/D3 receptor agonists in the least shrew (Cryptotis parva). Pharmacol Biochem Behav 80, 35–44. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Janoyan JJ, Kumar N, Crim JL, 2003a. Behaviorally active doses of the CB1 receptor antagonist SR 141716A increase brain serotonin and dopamine levels and turnover. Pharmacol Biochem Behav 75, 777–787. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Johnson JC, 2004. Central and peripheral mechanisms contribute to the antiemetic actions of delta-9-tetrahydrocannabinol against 5-hydroxytryptophan-induced emesis. Eur J Pharmacol 488, 201–212. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Ray AP, 2009. Evidence for a re-evaluation of the neurochemical and anatomical bases of chemotherapy-induced vomiting. Chem Rev 109, 3158–3199. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Sim-Selley LJ, Martin BR, Janoyan JJ, Crim JL, Parekh B, Breivogel CS, 2003b. Antiemetic and motor-depressive actions of CP55,940: cannabinoid CB1 receptor characterization, distribution, and G-protein activation. Eur J Pharmacol 459, 83–95. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Wang Y, Abad J, Ray AP, Thrush GR, Ramirez J, 2008. Utilization of the least shrew as a rapid and selective screening model for the antiemetic potential and brain penetration of substance P and NK1 receptor antagonists. Brain Res 1214, 58–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani NA, Zhao W, Ahmad B, 1999. The role of D2 and D3 dopamine receptors in the mediation of emesis in Cryptotis parva (the least shrew). J Neural Transm (Vienna) 106, 1045–1061. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Zhong W, Chebolu S, Mercadante F, 2015. Differential and additive suppressive effects of 5-HT3 (palonosetron)- and NK1 (netupitant)-receptor antagonists on cisplatin-induced vomiting and ERK1/2, PKA and PKC activation. Pharmacol Biochem Behav 131, 104–111. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, 2008. Endocannabinoids: synthesis and degradation. Rev Physiol Biochem Pharmacol 160, 1–24. [DOI] [PubMed] [Google Scholar]
- Herrstedt J, Roila F, Warr D, Celio L, Navari RM, Hesketh PJ, Chan A, Aapro MS, 2017. 2016 Updated MASCC/ESMO Consensus Recommendations: Prevention of Nausea and Vomiting Following High Emetic Risk Chemotherapy. Support Care Cancer 25, 277–288. [DOI] [PubMed] [Google Scholar]
- Horn CC, 2008. Why is the neurobiology of nausea and vomiting so important? Appetite 50, 430–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoyan JJ, Crim JL, Darmani NA, 2002. Reversal of SR 141716A-induced head-twitch and ear-scratch responses in mice by delta 9-THC and other cannabinoids. Pharmacol Biochem Behav 71, 155–162. [DOI] [PubMed] [Google Scholar]
- Karthaus M, Schiel X, Ruhlmann CH, Celio L, 2019. Neurokinin-1 receptor antagonists: review of their role for the prevention of chemotherapy-induced nausea and vomiting in adults. Expert Rev Clin Pharmacol 12, 661–680. [DOI] [PubMed] [Google Scholar]
- Keating GM, 2015. Netupitant/Palonosetron: A Review in the Prevention of Chemotherapy-Induced Nausea and Vomiting. Drugs 75, 2131–2141. [DOI] [PubMed] [Google Scholar]
- Kwiatkowska M, Parker LA, Burton P, Mechoulam R, 2004. A comparative analysis of the potential of cannabinoids and ondansetron to suppress cisplatin-induced emesis in the Suncus murinus (house musk shrew). Psychopharmacology (Berl) 174, 254–259. [DOI] [PubMed] [Google Scholar]
- Lever IJ, Malcangio M, 2002. CB(1) receptor antagonist SR141716A increases capsaicin-evoked release of Substance P from the adult mouse spinal cord. Br J Pharmacol 135, 21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas G, Brindle SD, Greengard P, 1971. The route of absorption of intraperitoneally administered compounds. J Pharmacol Exp Ther 178, 562–564. [PubMed] [Google Scholar]
- Oshita K, Inoue A, Tang HB, Nakata Y, Kawamoto M, Yuge O, 2005. CB(1) cannabinoid receptor stimulation modulates transient receptor potential vanilloid receptor 1 activities in calcium influx and substance P Release in cultured rat dorsal root ganglion cells. J Pharmacol Sci 97, 377–385. [DOI] [PubMed] [Google Scholar]
- Parker LA, Kwiatkowska M, Burton P, Mechoulam R, 2004. Effect of cannabinoids on lithium-induced vomiting in the Suncus murinus (house musk shrew). Psychopharmacology (Berl) 171, 156–161. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, 1999. Cannabis and cannabinoids: pharmacology and rationale for clinical use. Forsch Komplementarmed 6 Suppl 3, 12–15. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R, Ross RA, 2010. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2). Pharmacol Rev 62, 588–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray AP, Chebolu S, Ramirez J, Darmani NA, 2009a. Ablation of least shrew central neurokinin NK1 receptors reduces GR73632-induced vomiting. Behav Neurosci 123, 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray AP, Griggs L, Darmani NA, 2009b. Delta 9-tetrahydrocannabinol suppresses vomiting behavior and Fos expression in both acute and delayed phases of cisplatin-induced emesis in the least shrew. Behav Brain Res 196, 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi A, Campi B, Camarda V, Molinari S, Cantoreggi S, Regoli D, Pietra C, Calo G, 2012. In vitro and in vivo pharmacological characterization of the novel NK(1) receptor selective antagonist Netupitant. Peptides 37, 86–97. [DOI] [PubMed] [Google Scholar]
- Rojas C, Raje M, Tsukamoto T, Slusher BS, 2014. Molecular mechanisms of 5-HT(3) and NK(1) receptor antagonists in prevention of emesis. Eur J Pharmacol 722, 26–37. [DOI] [PubMed] [Google Scholar]
- Rudd JA, Ngan MP, Lu Z, Higgins GA, Giuliano C, Lovati E, Pietra C, 2016. Profile of Antiemetic Activity of Netupitant Alone or in Combination with Palonosetron and Dexamethasone in Ferrets and Suncus murinus (House Musk Shrew). Front Pharmacol 7, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Murthy P, Bharath MM, 2012. Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iran J Psychiatry 7, 149–156. [PMC free article] [PubMed] [Google Scholar]
- Simoneau II, Hamza MS, Mata HP, Siegel EM, Vanderah TW, Porreca F, Makriyannis A, Malan TP Jr., 2001. The cannabinoid agonist WIN55,212-2 suppresses opioid-induced emesis in ferrets. Anesthesiology 94, 882–887. [DOI] [PubMed] [Google Scholar]
- Slatkin NE, 2007. Cannabinoids in the treatment of chemotherapy-induced nausea and vomiting: beyond prevention of acute emesis. J Support Oncol 5, 1–9. [PubMed] [Google Scholar]
- Spinelli T, Calcagnile S, Giuliano C, Rossi G, Lanzarotti C, Mair S, Stevens L, Nisbet I, 2014. Netupitant PET imaging and ADME studies in humans. J Clin Pharmacol 54, 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA, 2005. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310, 329–332. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Oland LD, Ho W, Hillard CJ, Mackie K, Davison JS, Sharkey KA, 2001. Cannabinoids inhibit emesis through CB1 receptors in the brainstem of the ferret. Gastroenterology 121, 767–774. [DOI] [PubMed] [Google Scholar]
- Voth EA, Schwartz RH, 1997. Medicinal applications of delta-9-tetrahydrocannabinol and marijuana. Ann Intern Med 126, 791–798. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ray AP, McClanahan BA, Darmani NA, 2009. The antiemetic interaction of Delta9-tetrahydrocannabinol when combined with tropisetron or dexamethasone in the least shrew. Pharmacol Biochem Behav 91, 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Chebolu S, Darmani NA, 2016. Thapsigargin-induced activation of Ca(2+)-CaMKII-ERK in brainstem contributes to substance P release and induction of emesis in the least shrew. Neuropharmacology 103, 195–210. [DOI] [PubMed] [Google Scholar]
- Zhong W, Chebolu S, Darmani NA, 2018. Intracellular emetic signaling evoked by the L-type Ca(2+) channel agonist FPL64176 in the least shrew (Cryptotis parva). Eur J Pharmacol 834, 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Chebolu S, Darmani NA, 2019. Intracellular emetic signaling cascades by which the selective neurokinin type 1 receptor (NK1R) agonist GR73632 evokes vomiting in the least shrew (Cryptotis parva). Neurochem Int 122, 106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]