Abstract
Background.
Approximately 90% of genital warts are caused by human papillomavirus (HPV) types 6 and 11. In the United States, HPV vaccination has been recommended for girls and women aged ≤26 years, and since 2011, for boys and men aged ≤21 years and for gay, bisexual, and other men who have sex with men (MSM) aged ≤26 years.
Methods.
Data were obtained from 27 clinics participating in the STD Surveillance Network. Trends in the annual prevalence of anogenital warts (AGW) from 2010–2016 were described by sex and by the sex of sex partners.
Results.
During 2010–2016, significant declines in the prevalence of AGW were observed in women aged <40 years, men who have sex with women only (MSW) aged <40 years, and MSM of all age categories. An inflection in trend in 2012 was noted for MSW aged 20–24 or 25–29 years and for MSM aged 20–24 years.
Conclusions.
The observed declines in the prevalence of AGW suggest that HPV morbidity is declining among populations attending STD clinics, including MSW, MSM, and women. Declines in younger age groups are consistent with what would be expected following the implementation of HPV vaccination. However, declines were also observed in older age groups and are not likely to be the result of vaccination.
Keywords: Anogenital warts, genital warts, human papillomavirus (HPV), sexually transmitted infection (STI), sexually transmitted disease (STD)
Human papillomavirus (HPV) has been identified as the most common agent of sexually transmitted infections in the United States [1]. Many HPV infections appear to be transient and may not result in clinically significant outcomes [2–5]; however, persistent infections can cause adverse sequelae [4, 6]. HPV type 6 (HPV-6) and HPV-11 are associated with approximately 90% of genital warts (also referred to as anogenital warts [AGW]); HPV-16 and −18 are associated with approximately 70% of cervical and other genital cancers [2, 7, 8]. The quadrivalent HPV vaccine targets HPV-6, −11, −16, and −18 [2] and was licensed in the United States in mid-2006 for female individuals [9] and in late 2009 for male individuals [10]. Although a bivalent vaccine was also licensed for female individuals [11], almost all HPV vaccine administered in the United States through 2014 was quadrivalent [12]. A 9-valent vaccine, which protects against the quadrivalent types and 5 additional oncogenic HPV types (HPV-31, −33, −45, −52, and −58), was licensed in late 2014 for male and female individuals [13]. All HPV vaccines have been recommended by the Advisory Committee on Immunization Practices (ACIP) for routine use in US girls aged 11–12 years, with catch-up vaccination through age 26 years [9, 13]. Since October 2011, the ACIP has recommended that boys aged 11–12 years be vaccinated with either quadrivalent or 9-valent vaccine, with catch-up vaccination through age 21 years. The ACIP recommends catch-up vaccination through age 26 years for gay, bisexual, and other men who have sex with men (MSM) [14]. In the United States, HPV vaccine uptake (ie, coverage) has increased over time: in a 2016 national survey of adolescents aged 13–17 years, 65% of girls and 56% of boys had received ≥1 dose of HPV vaccine, compared with 49% and 1%, respectively, in 2010 [15, 16]. In 2014, 17% of MSM included in a National HIV Behavioral Surveillance survey self-reported receiving ≥1 dose of HPV vaccine [17], although these data have not since been updated.
Monitoring the impact of HPV vaccination in the United States remains difficult as HPV infections can result in multiple clinical sequelae with variable and often extended times to manifestation [3, 18, 19]. Furthermore, few US data sources include person-level information on both HPV vaccination and HPV infection or clinical sequelae of infection. Because AGW can manifest within weeks to months of infection with HPV-6 and/or −11 [3], the impact of vaccination on sequelae of HPV infection can most immediately be monitored through AGW surveillance [20]. Studies in the United States, Australia, and Europe have used AGW prevalence to document HPV morbidity trends [21–26]. In lieu of notifiable disease reports of AGW cases, US AGW morbidity studies have relied on healthcare claims data to identify trends in diagnoses [21–23]. However, the largest of these studies did not include populations that seek health care outside of commercial or employer-covered plans [21, 22], which may be at increased risk of STIs [23], and none assessed AGW morbidity by sex of sex partner. No US study has reported trends in AGW diagnoses in MSM since the ACIP updated its vaccination recommendations in late 2011 to include male individuals. A 2010–2011 sexually transmitted disease (STD) clinic–based study reported the prevalence of AGW to be 7.5% among men who have sex with women only (MSW), 7.5% among MSM, and 2.4% among women [20]. Our analysis uses the same STD clinic–based infrastructure to provide insight into the burden of AGW in key demographic groups. Specifically, our objectives were to describe age-specific trends in the annual prevalence of AGW among patients attending select US STD clinics from 2010–2016 overall and by sex of male patients’ sex partners.
METHODS
Data were obtained from STD clinics participating in the Centers for Disease Control and Prevention’s STD Surveillance Network (SSuN) from January 2010 to December 2016 [27]. Data from 27 STD clinics in the following jurisdictions were included: Baltimore (2 clinics), Los Angeles (12), New York City (9, 2010–2014; 8, 2015–2016), Philadelphia (2), San Francisco (1), and Seattle (1). All jurisdictions submitted data through the entire study period. Analyses were restricted to visit types in which a patient was seen by a healthcare provider (ie, clinician visits); express/follow-up visits were excluded. Within each calendar year, patient visits were deduplicated so that unique patients per year were the unit of analysis. Demographic, behavioral, and clinical information was abstracted from electronic medical records and collated across patient visits per year. Human immunodeficiency virus (HIV) status was assessed through laboratory testing or, if known to be living with diagnosed HIV, self-reporting. Sexual behavior was determined by patient self-report of male and/or female sex partners or by self-reported sexual orientation (eg, “gay” or “straight”). Men who identified as gay or bisexual or who reported having sex with a man at any of their visits for the year were classified as MSM. Men who identified as heterosexual only or reported only female sex partners at any time during the year were classified as MSW. Sex partnerships among women were not considered, as only 2% of women reported having sex with women only. Six percent of the male population for whom the sex of sex partners was unknown were excluded from the trend analyses, as were patients who reported being transgender (<1%).
Case Definition
An AGW case was defined as a patient with an AGW diagnosis, identified from electronic medical records, at ≥1 visit within a calendar year. Diagnoses were based on clinical evaluation and physical examination findings.
Statistical Analysis
Analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, NC). The annual prevalence of AGW was estimated using a random-effects meta-analysis model to account for the substantial diversity in patient demographic characteristics and AGW prevalence across the SSuN jurisdictions. The random-effects model incorporates heterogeneity across the jurisdictions by allowing each jurisdiction to have a different true prevalence, which is assumed to have been sampled from a population of true prevalences. To calculate the overall annual AGW prevalence, we first calculated the total number of unique persons with an AGW diagnosis in a year in a jurisdiction, divided by the total number of unique persons with ≥1 clinician visit in that jurisdiction that year. Then, using the year and jurisdiction-specific estimates, we estimated the overall annual AGW prevalence, using an inverse-variance weighted random effects model (the SAS macro containing programming code for model is available on request). To examine temporal trends, the percentage change in prevalence (adjusted for jurisdiction) over the study period was calculated as the difference between the prevalence of AGW in 2010 as compared to that in 2016, divided by the prevalence in 2010. Joinpoint software, version 4.4.0 (National Cancer Institute, Bethesda, MD), which fits trend data to identify the log-linear model with the fewest number of inflection points, was used to identify significant trends in adjusted annual AGW prevalences [28]. The annual percentage change (APC) in adjusted AGW prevalence was estimated by Joinpoint, using the log-linear slope of trend segments between inflection points [28]. All analyses were also conducted by age group, sex, and sex of the male patient’s sex partner; patients missing these stratifying variables were excluded from trend analyses.
RESULTS
Approximately 650 000 patients had at least one clinician visit in a participating SSuN STD clinic from 1 January 2010 through 31 December 2016. The number of patients attending the clinics declined over the study period, from 108 524 patients in 2010 to 74 690 patients in 2016 (Table 1). The median age was 26 years for women (mean age [±SD], 28.9 ± 10.4 years), 29 years for MSW (mean age [±SD], 32.5 ± 11.3 years), and 31 years for MSM (mean age [±SD], 33.6 ± 10.7 years). The mean age (±SD) of all patients increased slightly over time, from 30.5 ± 11.0 years in 2010 to 33.0 ± 11.5 years in 2016. Approximately half of the population was non-Hispanic black; 21% of patients was Hispanic, and 20% was non-Hispanic white. Sixty-three percent of the population was male, and 29% of male patients were identified as MSM. The proportion of men who were MSM increased from 12.9% in 2010 to 23.4% in 2016. Approximately 4% of the population was known to be living with diagnosed HIV infection, although the proportion increased from 3.2% in 2010 to 5.2% in 2016; among MSM only, the proportion ranged from 18.0% to 19.2% but did not significantly increase over the study period. The proportion of patients known to be living with diagnosed HIV infection was higher among MSM (18.7%) than among MSW (0.9%) or women (0.7%). During 2015–2016, compared with the preceding years, there were increases in the proportion of patients for whom the following characteristics were unknown: age (from 0% to approximately 5%), sex (from <1% to approximately 5%), race/ethnicity (from approximately 3% to approximately 8%), and sex of sex partners (from approximately 2% to approximately 8%).
Table 1.
Demographic and Clinical Characteristics of Patients Attending Sexually Transmitted Disease (STD) Clinics Participating in the US STD Surveillance Network, 2010–2016
| Characteristic | 2010 (n = 108 524) | 2011 (n = 105 984) | 2012 (n = 100 123) | 2013 (n = 95 548) | 2014 (n = 87 654) | 2015 (n = 81 324) | 2016 (n = 74 690) | Overall (n = 653 847) |
|---|---|---|---|---|---|---|---|---|
| Jurisdiction | ||||||||
| Baltimore | 13 280 (12.2) | 12 769 (12.0) | 11 554 (11.5) | 11 088 (11.6) | 9241 (10.5) | 6058 (7.4) | 5319 (7.1) | 69 309 (10.6) |
| Los Angeles | 23 826 (22.0) | 22 008 (20.8) | 19 797 (19.8) | 18 408 (19.3) | 16 771 (19.1) | 16 711 (20.5) | 15 211 (20.4) | 132 732 (20.3) |
| New York City | 43 538 (40.1) | 43 504 (41.0) | 41 972 (41.9) | 39 330 (41.2) | 35 701 (40.7) | 31 449 (38.7) | 28 565 (38.2) | 264 059 (40.4) |
| Philadelphia | 10 037 (9.2) | 9895 (9.3) | 9524 (9.5) | 9916 (10.4) | 10 061 (11.5) | 9963 (12.3) | 9677 (13.0) | 69 073 (10.6) |
| San Francisco | 10 386 (9.6) | 10 781 (10.2) | 10 117 (10.1) | 9923 (10.4) | 9730 (11.1) | 10 574 (13) | 9783 (13.1) | 71 294 (10.9) |
| Washington | 7457 (6.9) | 7027 (6.6) | 7159 (7.2) | 6883 (7.2) | 6150 (7.0) | 6569 (8.1) | 6135 (8.2) | 47 380 (7.2) |
| Age, y | ||||||||
| ≤19 | 11 931 (11.0) | 10 443 (9.9) | 8619 (8.6) | 7059 (7.4) | 5765 (6.6) | 4220 (5.2) | 3828 (5.1) | 51 869 (7.9) |
| 20–24 | 27 999 (25.8) | 26 851 (25.3) | 24 385 (24.4) | 22 296 (23.3) | 19 692 (22.5) | 15 906 (19.6) | 13 392 (17.9) | 150 521 (23.0) |
| 25–29 | 22 641 (20.9) | 23 115 (21.8) | 22,186 (22.2) | 21 755 (22.8) | 20 821 (23.8) | 18 834 (23.2) | 17 758 (23.8) | 147 110 (22.5) |
| 30–39 | 23 852 (22.0) | 23 806 (22.5) | 23,361 (23.3) | 23 264 (24.3) | 21 803 (24.9) | 20 070 (24.7) | 19 400 (26.0) | 155 556 (23.8) |
| ≥40 | 22 100 (20.4) | 21 769 (20.5) | 21,571 (21.5) | 21 174 (22.2) | 19 571 (22.3) | 17 850 (21.9) | 17 422 (23.3) | 141 457 (21.6) |
| Unknown | 1 (0.0) | 0 (0.0) | 1 (0.0) | 0 (0.0) | 2 (0.0) | 4444 (5.5) | 2890 (3.9) | 7334 (1.1) |
| Sex | ||||||||
| Female | 42 289 (39.0) | 40 925 (38.6) | 37 142 (371) | 33 835 (35.4) | 30 156 (34.4) | 25 932 (31.9) | 21 483 (28.8) | 231,762 (35.4) |
| Male | 66 076 (60.9) | 64 901 (61.2) | 62 765 (62.7) | 61 491 (64.4) | 57 252 (65.3) | 53 925 (66.3) | 46 910 (62.8) | 413,320 (63.2) |
| Transgender | 146 (0.1) | 147 (0.1) | 168 (0.2) | 184 (0.2) | 187 (0.2) | 212 (0.3) | 275 (0.4) | 1319 (0.2) |
| Unknown | 13 (0.0) | 11 (0.0) | 48 (0.0) | 38 (0.0) | 59 (0.1) | 1255 (1.5) | 6022 (8.1) | 7446 (1.1) |
| Race/ethnicity | ||||||||
| Non-Hispanic black | 56 554 (52.1) | 53 840 (50.8) | 50 128 (50.1) | 47 350 (49.6) | 42 473 (48.5) | 36 162 (44.5) | 32 862 (44.0) | 319 369 (48.8) |
| Hispanic | 22 870 (21.1) | 22 178 (20.9) | 21 011 (21.0) | 19 788 (20.7) | 18 099 (20.6) | 15 600 (19.2) | 15 469 (20.7) | 135 015 (20.6) |
| Non-Hispanic white | 20 024 (18.5) | 19 711 (18.6) | 19 420 (19.4) | 19 325 (20.2) | 18 044 (20.6) | 16 816 (20.7) | 15 422 (20.6) | 128 762 (19.7) |
| Other | 6459 (6.0) | 6543 (6.2) | 6509 (6.5) | 6308 (6.6) | 6075 (6.9) | 5758 (7.1) | 5600 (7.5) | 43 252 (6.6) |
| Unknown | 2617 (2.4) | 3712 (3.5) | 3055 (3.1) | 2777 (2.9) | 2963 (3.4) | 6988 (8.6) | 5337 (7.1) | 27 449 (4.2) |
| Patient typea | ||||||||
| Women | 42 289 (39.0) | 40 925 (38.6) | 37 144 (37.1) | 33 835 (35.4) | 30 156 (34.4) | 25 933 (31.9) | 21 484 (28.8) | 231 766 (35.4) |
| MSW | 49 097 (45.2) | 47 146 (44.5) | 44 934 (44.9) | 41 990 (43.9) | 38 604 (44.0) | 33 333 (41.0) | 26 983 (36.1) | 282 087 (43.1) |
| MSM | 14 049 (12.9) | 15 257 (14.4) | 15 928 (15.9) | 17 258 (18.1) | 16 762 (19.1) | 17 666 (21.7) | 17 489 (23.4) | 114 409 (175) |
| Transgender | 158 (0.1) | 156 (0.1) | 180 (0.2) | 196 (0.2) | 203 (0.2) | 260 (0.3) | 300 (0.4) | 1453 (0.2) |
| Unknown sex of sex partners | 2931 (2.7) | 2500 (2.4) | 1937 (1.9) | 2269 (2.4) | 1929 (2.2) | 4132 (5.1) | 8434 (11.3) | 24 132 (3.7) |
| HIV infection status | ||||||||
| Known to be living with diagnosed HIV infection | 3466 (3.2) | 3552 (3.4) | 3800 (3.8) | 4019 (4.2) | 3975 (4.5) | 3975 (4.9) | 3848 (5.2) | 26 611 (4.1) |
| Not known to be living with diagnosed HIV infection | 105 058 (96.8) | 102 432 (96.6) | 96 323 (96.2) | 91 529 (95.8) | 83 679 (95.5) | 77 373 (95.1) | 70 842 (94.8) | 627 236 (95.9) |
Data are no. (%) of patients
Abbreviations: HIV, human immunodeficiency virus; MSM, men who have sex with men; MSW, men who have sex with women only.
Takes into account the sex of male patients’ sex partners.
Substantial diversity in patient demographic characteristics and AGW prevalence existed across jurisdictions (data not shown). For example, across the study period, 31% of the patient population in Los Angeles was Hispanic, compared with 4% in San Francisco. Thirty-nine percent of the San Francisco patient population was MSM, compared with 5% in Baltimore. Eight percent of the San Francisco patient population was known to be living with diagnosed HIV infection, compared with 2% in Los Angeles. The jurisdiction with the highest prevalence of AGW during the study period was Seattle, at 5.5%, and the lowest was Baltimore, at 2.8%.
The overall prevalence of AGW, adjusted for jurisdiction, was highest in MSW (6.3%; 95% CI, 5.3%–7.4%), followed by MSM (4.6%; 95% CI, 4.2%–5.1%), and women (1.8%; 95% CI, 1.5%–2.1%). The overall unadjusted prevalence of AGW was 5.9% in MSW, 4.6% in MSM, and 1.6% in women.
Trends in the Prevalence of AGW
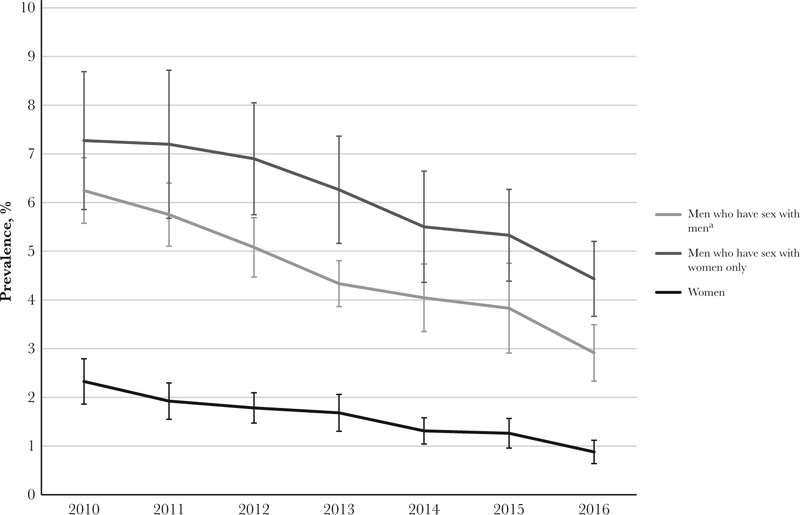
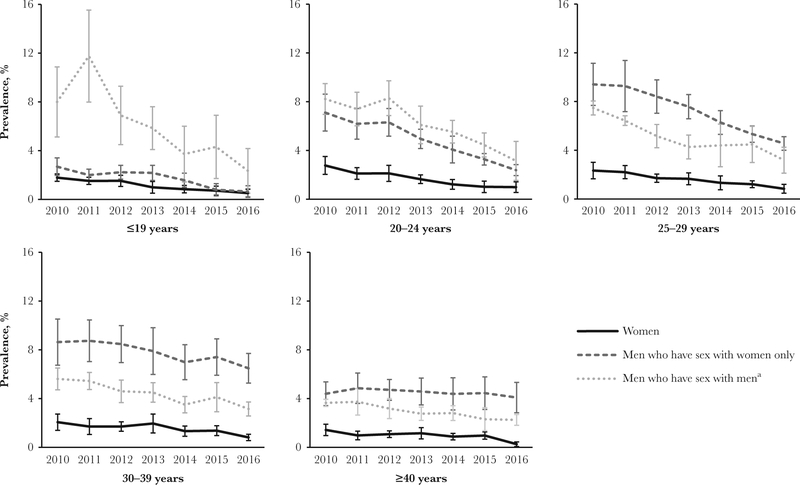
The overall prevalence of AGW among women, adjusted for jurisdiction, decreased significantly, from 2.3% in 2010 to 0.9% in 2016 (APC, −13.1%; 95% CI, −16.4% to −9.7%) (Figure 1). Among women aged ≤39 years, the annual prevalence decreased significantly during 2010–2016 (Table 2). The AGW prevalence among women aged <20 years decreased from 1.8% in 2010 to 0.5% in 2016 (APC, −17.2%; 95% CI, −21.3% to −12.8%; Figure 2). Among 20–24 year olds, the prevalence decreased from 2.8% in 2010 to 1.0% in 2016 (APC, −16.4%; 95% CI, −20.1% to −12.5%). Among 25–29 year olds, the prevalence was 2.3% in 2010, declining to 0.8% in 2016 (APC,–13.8%; 95% CI, −17.2% to −10.3%), while among 30–39 year olds, the prevalence decreased from 2.1% to 0.8% (APC, −11.7%; 95% CI, −18.7% to −4.2%). Women aged >39 years experienced declines in AGW prevalences across the study period, although the decreases were not statistically significant.
Figure 1.
Annual anogenital wart prevalence among patients attending US Sexually Transmitted Disease Surveillance Network clinics, by patient type (which takes into account the sex of male patients’ sex partners), 2010–2016. Error bars denote 95% confidence intervals. aIncludes men who have sex with men and women.
Table 2.
Trends in Prevalence of Anogenital Warts, by Patient Type and Age Category and Adjusted for Jurisdiction—US Sexually Transmitted Disease Surveillance Network, 2010–2016
| Variable | Percentage Change in Prevalencea,b | Annual Percentage Change in Prevalencea,c (95% CI) |
|---|---|---|
| Overall | −47.1 | −8.8 (−11.3 to −6.1) |
| Patient typed | ||
| Women | −62.2 | −13.1 (−16.4 to −9.7) |
| MSW | −39.0 | −8.1 (−10.4 to −5.6) |
| MSM | −53.4 | −11.3 (−13.1 to −9.4) |
| Age category, y | ||
| Among women | ||
| ≤19 | −71.1 | −17.2 (−21.3 to −12.8) |
| 20–24 | −64.3 | −16.4 (−20.1 to −12.5) |
| 25–29 | −64.0 | −13.8 (−17.2 to −10.3) |
| 30–39 | −60.4 | −11.7 (−18.7 to −4.2) |
| ≥40 | −81.9 | −9.9 (−12.3 to 3.8) |
| Among MSW | ||
| ≤19 | −76.2 | −14.5 (−25.4 to −1.9) |
| 20–24e | −66.4 | … |
| 2010–2012 | … | −4.6 (−32.4–34.5) |
| 2012–2016 | … | −20.4 (−27.3 to −12.9) |
| 25–29e | −51.5 | … |
| 2010–2012 | … | −4.3 (−14.0 to −6.5) |
| 2012–2016 | … | −14.8 (−16.6 to −13.0) |
| 30–39 | −24.9 | −5.0 (−7.1 to −2.8) |
| ≥40 | −7.1 | −1.4 (−3.8–1.2) |
| Among MSM | ||
| ≤19 | −70.7 | −18.8 (−30.0 to −5.9) |
| 20–24e | −61.8 | … |
| 2010–2012 | … | −0.2 (−27.2–36.9) |
| 2012–2016 | … | −18.1 (−32.0 to −1.3) |
| 25–29 | −57.3 | −13.2 (−16.7 to −9.6) |
| 30–39 | −43.9 | −9.5 (−12.6 to −6.3) |
| ≥40 | −38.3 | −7.7 (−9.3 to −6.1) |
Abbreviations: CI, confidence interval; MSM, men who have sex with men; MSW, men who have sex with women only.
Calculated for trend segments, defined as the period between inflection points.
Trend segments lasted from 2010 to 2016.
Trend segments lasted from 2010 to 2016, unless otherwise indicated.
Takes into account the sex of male patients’ sex partners.
Trend segments were split into 2010–2012 and 2012–2016 because 2012 was an inflection year.
Figure 2.
Annual anogenital wart prevalence among patients attending US Sexually Transmitted Disease Surveillance Network clinics, by age category and patient type (which takes into account the sex of male patients’ sex partners), 2010–2016. Error bars denote 95% confidence intervals. aIncludes men who have sex with men and women.
The overall jurisdiction-adjusted prevalence of AGW among all MSW decreased significantly during 2010–2016, from 7.3% to 4.4% (APC, −8.1%; 95% CI, −10.4% to −5.6%). MSW aged ≤39 years experienced statistically significant declines in AGW prevalence during the study period. Among MSW aged <20 years, the prevalence decreased significantly, from 2.7% in 2010 to 0.6% in 2016 (APC, −14.5%; 95% CI, −25.4% to −1.9%). Between 2010 and 2012, the prevalence among MSW aged 20–24 years was stable but decreased significantly during 2012–2016, from 6.3% to 2.4% (APC, −20.4; 95% CI, −27.3% to −12.9%) Similarly, between 2010 and 2012, the prevalence was stable among MSW aged 25–29 years but decreased significantly from 2012 to 2016, from 8.4% to 4.6% (APC, −14.8%, 95% CI, −16.6% to −13.0%). The prevalence of AGW significantly decreased among MSW aged 30–39 years (APC, −5.0%, 95% CI, −7.1% to −2.8%) but did not significantly decrease among MSW aged >39 years (APC, −1.4%, 95% CI, −3.8%–1.2%).
The prevalence of AGW among all MSM decreased significantly during 2010–2016, from 6.2% to 2.9% (APC, −11.3%;95% CI, −13.1% to −9.4%). Decreases were significant in all age categories of MSM. Among MSM aged <20 years, the prevalence decreased from 8.0% in 2010 to 2.3% in 2016 (APC, −18.8%; 95% CI, −30.0% to −5.9%). Between 2010 and 2012, the prevalence among MSM aged 20–24 years did not significantly change but decreased significantly from 2012 to 2016, from 8.3% to 3.1% (APC, −18.1; 95% CI, −32.0% to −1.3%). Additionally, from 2010 to 2016, the prevalence of AGW decreased significantly among MSM aged 25–29 years, from 7.5% to 3.2% (APC, −13.2%; 95% CI, −16.7% to −9.6%); among MSM aged 30–39 years, from 5.6% to 3.1% (APC, −9.5%; 95% CI, −12.6% to −6.3%); and among MSM aged >39 years, from 3.6% to 2.2% (APC, −7.7%; 95% CI, −9.3% to −6.1%). Similar trends in the prevalence of AGW among women, MSW, and MSM were observed in individual jurisdictions.
DISCUSSION
Using STD clinic data, we report the first temporal trends in the prevalence of AGW in the United States, by patient sex and the sex of male patients’ sex partners. We document declines in the AGW prevalence across all populations, with statistically significant declines among women aged ≤39 years, among MSW aged ≤39 years, and among MSM of all ages. These declines suggest that HPV morbidity is decreasing among patients attending SSuN STD clinics. The drivers of these decreases are unclear. It is likely that some of the observed decreases may be related to expanded HPV vaccination coverage, but it is also possible that observed declines may have been influenced by changes in the patient population accessing care in STD clinics and/or by variations in AGW testing and diagnoses by STD clinic providers.
HPV vaccination may explain some of the observed declines, particularly among populations for which HPV vaccination is recommended. For example, the observed steady declines in women aged <30 years are consistent with what would be expected following the initiation of routine HPV vaccination; all women in this group would have been age eligible for HPV vaccination before or during the study period. Additionally, there were significant declines in the AGW prevalence in both MSM and MSW aged <40 years. The ACIP has recommended catch-up HPV vaccination for men through age 21 years since 2011; all male individuals aged <20 years and most aged 20–24 years would have been age eligible for vaccination during part of the study period. The prevalence among MSW aged 20–29 years remained stable from 2010 to 2012 but decreased significantly from 2012 to 2016. This inflection in trend in 2012 coincides with the ACIP’s October 2011 recommendation to vaccinate men. Declines in the AGW prevalence among MSW may also be partially attributed to herd protection from female vaccination, as female cervicovaginal HPV prevalence and female AGW morbidity in younger age groups has continuously decreased since the ACIP recommended female vaccination in 2006 [21, 22, 29]. Since 2011, the ACIP has recommended catch-up HPV vaccination for MSM through age 26 years. Similar to MSW, an inflection in trend was observed in 2012 for MSM aged 20–24 years. However, unlike MSW or women, statistically significant declines in AGW diagnoses among MSM >39 years were observed. Although older MSM are less likely to be HPV naive and may not directly benefit from vaccination, observed decreases may be partially due to herd protection via sex partnerships with younger men, which may be more common among MSM as compared to MSW or women [30].
The observed declines in populations that were not age eligible argue against vaccination being the primary catalyst for our observed findings and may reflect changes in the patient population attending these clinics. During the study period, the overall number of patients attending SSuN STD clinics decreased by approximately 30%, which would affect the absolute number of AGW diagnoses over time. We accounted for the decreasing population size by estimating AGW morbidity as a proportion of all patients; however, we were unable to account for possible changes in the attributes of patients attending STD clinics. If, over time, more persons with AGW chose to seek care in non-STD clinics, including private facilities and community health centers, we would observe a decline in clinic prevalence independent of changes in population prevalence. Changes in where people seek care for AGW may reflect changes in the healthcare system (eg, increasing insurance coverage may lead to more use of private facilities). Additionally, fiscal cuts to STD clinics may have resulted in changes in clinic operating hours and staff shortages, leading to a prioritization of patients with acute STIs, such as syphilis or symptomatic urethritis. Finally, because we estimated AGW morbidity as a proportion to account for changing clinic population size, the observed prevalence is affected by the incidence of other STIs in the general population. For example, if the syphilis incidence increases, more patients with syphilis would access care, and the observed clinic prevalence of AGW would decrease independent of the population prevalence.
Another possible explanation for the observed declines may be related to changes in clinical practice. The clinical criteria for identifying or diagnosing AGW did not change throughout the study period. However, there is no laboratory test for AGW, and HPV testing is not routine in STD clinics, so AGW are generally diagnosed by physical examination. It is possible that the proportion of AGW cases that were present but not detected or that were misdiagnosed increased over time owing to a decrease in physical examinations. If clinics increased self-collection of specimens for STI testing (eg, self-collected vaginal swabs for chlamydia testing), fewer patients may have had direct visual inspection of their genitals, resulting in fewer diagnoses of AGW independent of changes in population prevalence. This may be especially relevant for observed trends in female patients, as male genitalia may be easier to inspect visually as compared to female genitalia, which requires a pelvic examination. Because documentation of whether a comprehensive physical examination was performed was not captured consistently during the study period, we restricted our analysis to visits where a patient was seen by a provider, to minimize this possible bias.
Our results complement the few studies that have examined US trends in HPV and AGW prevalence. A 2006–2014 study assessed AGW trends among privately insured male and female individuals and found significant declines among girls and women aged 15–19 years (2008–2014), 20–24 years (2009–2014), and 25–29 years (2009–2014) and among men aged 20–24 years (2009–2014) [22]. We found additional significant declines in older groups, including women aged 30–39 years, MSW aged 25–39 years, and MSM aged >25 years. However, our study was conducted through 2016 and likely included publicly insured and uninsured patients. To better understand the burden of disease among larger segments of the population, future studies should include both uninsured and insured (publicly or privately) individuals in a variety of healthcare settings. Additionally, our observed declines in AGW prevalence among women compliment studies that used National Health and Nutritional Examination Survey data to document declines in the prevalence of quadrivalent HPV vaccine types from cervicovaginal specimens; one study documented declines in girls and women aged 14–24 years between 2003 and 2006 (the prevaccine era) and 2009–2012 (the postvaccine era) [31]. Our study found additional significant declines in women aged 25–39 years; however, comparison between these studies is difficult because our study captured only AGW, not HPV, prevalence and was conducted through 2016.
This study has several limitations. This study used data from STD clinics located in urban centers, limiting geographical representativeness. However, substantial diversity in patient demographic characteristics and the prevalence of AGW existed across jurisdictions. Interjurisdictional variations were adjusted for and therefore should not affect the reported trends in AGW prevalence. Second, linking patients across the full study period was not possible, so our study design would not preclude the inclusion of AGW cases in the same patients across multiple years. Also, since demographic and behavioral data were abstracted from medical records in each calendar year and because matching visits by unique patients was possible within years, cases with multiple visits in a year had more opportunities for more complete ascertainment of behavioral and/or demographic patient information (eg, the ability to be identified as MSM or MSW) when compared to patients with only 1 visit. However, the overall proportion of patients with missing information was low (ie, <5%) throughout the study period; therefore, this should have a minimal effect on trends. Last, our study lacked data on patients’ HPV vaccination status. This would be especially useful for elucidating the link between HPV vaccination status and AGW prevalence among groups that were age eligible for vaccination but have had historically low coverage rates. Vaccination information would also aid in understanding whether STD clinic patients are more or less likely to be vaccinated for HPV than the general population. Many STD clinics do not routinely vaccinate for HPV [32], although individuals may be vaccinated at other venues.
Despite these limitations, these data represent the first US evaluation of AGW trends among patients attending STD clinics. While patients attending STD clinics are not likely to represent all persons with AGW, they remain an important population with a higher burden of STIs [33]. The impact of STD prevention interventions may be more easily detected among such groups with higher STI morbidity. In conclusion, given the prophylactic efficacy and high population effectiveness of the HPV vaccine [34, 35] and that the age groups that were age eligible for the HPV vaccine experienced the sharpest decreases in AGW prevalence, it is likely that the observed decline in the proportion of patients attending STD clinics with AGW is partially the result of US implementation of HPV vaccination. However, our study is ecologic by design, and declines were observed in age groups that are not targeted for vaccination and are thus less likely to be affected, suggesting that other factors may be impacting observed trends. Continued surveillance for AGW can help monitor the impact of vaccination and other prevention and control activities.
Footnotes
Publisher's Disclaimer: Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflicts of interest.
All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis 2013; 40:187–93. [DOI] [PubMed] [Google Scholar]
- 2.Garland SM, Steben M, Sings HL, et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis 2009; 199:805–14. [DOI] [PubMed] [Google Scholar]
- 3.Winer RL, Kiviat NB, Hughes JP, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis 2005; 191:731–8. [DOI] [PubMed] [Google Scholar]
- 4.Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J Clin Virol 2005; 32(Suppl 1):S16–24. [DOI] [PubMed] [Google Scholar]
- 5.Anic GM, Lee JH, Stockwell H, et al. Incidence and human papillomavirus (HPV) type distribution of genital warts in a multinational cohort of men: the HPV in men study. J Infect Dis 2011; 204:1886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Insinga RP, Perez G, Wheeler CM, et al. ; FUTURE I Investigators. Incident cervical HPV infections in young women: transition probabilities for CIN and infection clearance. Cancer Epidemiol Biomarkers Prev 2011; 20:287–96. [DOI] [PubMed] [Google Scholar]
- 7.Gissmann L, Wolnik L, Ikenberg H, Koldovsky U, Schnürch HG, zur Hausen H. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A 1983; 80:560–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saraiya M, Unger ER, Thompson TD, et al. US Assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst 2015;107:djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER; Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56:1–24. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the advisory committee on immunization practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59:630–2. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the advisory committee on immunization practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59:626–9. [PubMed] [Google Scholar]
- 12.Stokley S, Jeyarajah J, Yankey D, et al. Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014 – United States. MMWR Morb Mortal Wkly Rep 2014; 63:620–4. [PMC free article] [PubMed] [Google Scholar]
- 13.Petrosky E, Bocchini JA Jr, Hariri S, et al. ; Centers for Disease Control and Prevention (CDC). Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 2015; 64:300–4. [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males--Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011;60:1705. [PubMed] [Google Scholar]
- 15.Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2016. MMWR Morb Mortal Wkly Rep 2017; 66:874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117–23.21866084 [Google Scholar]
- 17.Oliver SE, Hoots BE, Paz-Bailey G, Markowitz LE, Meites E. Increasing human papillomavirus vaccine coverage among men who have sex with men—National HIV Behavioral Surveillance, United States, 2014. J Acq Immun Def Synd 2017; 75:S370–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong CA, Saraiya M, Hariri S, et al. Approaches to monitoring biological outcomes for HPV vaccination: challenges of early adopter countries. Vaccine 2011; 29:878–85. [DOI] [PubMed] [Google Scholar]
- 19.Egelkrout EM, Galloway DA. The biology of genital human papillomaviruses In: Holmes KK, Sparling PF, Stamm WE, et al. , eds. Sexually transmitted diseases. 4th ed. New York: McGraw Hill Medical, 2008:463–87. [Google Scholar]
- 20.Llata E, Stenger M, Bernstein K, et al. Prevalence of genital warts among sexually transmitted disease clinic patients—Sexually Transmitted Disease Surveillance Network, United States, January 2010 to December 2011. Sex Transm Dis 2014; 41:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flagg EW, Schwartz R, Weinstock H. Prevalence of anogenital warts among participants in private health plans in the United States, 2003–2010: potential impact of human papillomavirus vaccination. Am J Public Health 2013; 103:1428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flagg EW, Torrone EA. Declines in anogenital warts among age groups most likely to be impacted by human papillomavirus vaccination, United States, 2006–2014. Am J Public Health 2018; 108:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauer HM, Wright G, Chow J. Evidence of human papillomavirus vaccine effectiveness in reducing genital warts: an analysis of California public family planning administrative claims data, 2007–2010. Am J Public Health 2012; 102:833–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donovan B, Franklin N, Guy R, et al. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: analysis of national sentinel surveillance data. Lancet Infect Dis 2011; 11:39–44. [DOI] [PubMed] [Google Scholar]
- 25.Baandrup L, Blomberg M, Dehlendorff C, Sand C, Andersen KK, Kjaer SK. Significant decrease in the incidence of genital warts in young Danish women after implementation of a national human papillomavirus vaccination program. Sex Transm Dis 2013; 40:130–5. [DOI] [PubMed] [Google Scholar]
- 26.Leval A, Herweijer E, Arnheim-Dahlström L, et al. Incidence of genital warts in Sweden before and after quadrivalent human papillomavirus vaccine availability. J Infect Dis 2012; 206:860–6. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. STD Surveillance Network (SSuN). https://www.cdc.gov/std/ssun/default.htm. Accessed 1 March 2017. [Google Scholar]
- 28.National Cancer Institute. Division of Cancer Control and Population Sciences. Surveillance Research Program. Joinpoint. https://surveillance.cancer.gov/help/joinpoint. Accessed 28 March 2017. [Google Scholar]
- 29.Flagg EW, Torrone EA. Declines in anogenital warts among age groups most likely to be impacted by human papillomavirus vaccination, United States, 2006–2014. Am J Public Health. 2018; 108:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glick SN, Morris M, Foxman B, et al. A comparison of sexual behavior patterns among men who have sex with men and heterosexual men and women. J Acquir Immune Defic Syndr 2012; 60:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markowitz LE, Liu G, Hariri S, Steinau M, Dunne E, Unger E. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics 2016; 137:1–9. [DOI] [PubMed] [Google Scholar]
- 32.Meites E, Llata E, Hariri S, et al. HPV vaccine implementation in STD clinics–STD Surveillance Network. Sex Transm Dis 2012; 39:32–4. [DOI] [PubMed] [Google Scholar]
- 33.Celum CL, Bolan G, Krone M, et al. Patients attending STD clinics in an evolving health care environment. Demographics, insurance coverage, preferences for STD services, and STD morbidity. Sex Transm Dis 1997; 24:599–605. [DOI] [PubMed] [Google Scholar]
- 34.Garland SM, Hernandez-Avila M, Wheeler CM, et al. ; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356:1928–43. [DOI] [PubMed] [Google Scholar]
- 35.Drolet M, Bénard É, Boily MC, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2015; 15:565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]