Abstract
Carcinoma-associated fibroblasts (CAFs) secrete factors that increase the expression and/or activities of proteins in breast cancer cells and induce resistance to anti-estrogens, such as fulvestrant. A major factor is interleukin-6 (IL-6). This study demonstrated that, across estrogen receptor (ER) α-positive and -negative cell lines, recombinant human IL-6 (rhIL-6) mimicked most of the CAF-conditioned medium (CM)-induced changes in protein expression patterns; however, in most cases, it failed to recapitulate CAF-CM-triggered alterations in ERK1/2 and AKT activities. The ability of rhIL-6 to induce fulvestrant resistance was dependent upon the culture conditions. In 3D, but not in 2D cultures, rhIL-6 increased the survival of fulvestrant-treated cells, although not to the same extent as observed with CAF-CM. In 2D cultures, rhIL-6 acted in a pro-apoptotic manner and decreased the expression of ATP-binding cassette transporter G2 (ABCG2). The inhibition of the PI3K/AKT pathway had similar effects on apoptosis and ABCG2 expression, linking the failure of rhIL-6 to induce fulvestrant resistance to its inability to activate the PI3K/AKT pathway. In 3D cultures, both CAF-CM and rhIL-6 acted in an anti-apoptotic manner. These activities are likely independent on the PI3K/AKT pathway and ABCG2. Experiments on ERα-negative breast cancer cells revealed a growth-inhibitory effects of both CAF-CM and rhIL-6, which coincided with a reduction in the c-Myc level. These data suggest that IL-6 plays a role in several effects of CAF-CM, including alterations in protein expression patterns, fulvestrant resistance in 3D cultures and growth inhibition. By contrast, IL-6 is unlikely to be responsible for the CAF-CM-induced activation of the PI3K/AKT pathway and fulvestrant resistance in 2D cultures.
Keywords: breast cancer, carcinoma-associated fibroblasts, anti-estrogen resistance, spheroid culture, interleukin-6
Introduction
Breast cancer is the most common type of cancer and the leading cause of cancer-associated mortality among women worldwide (1). Breast cancer is a heterogenous disease which can be divided into immunohistochemical and molecular subtypes (2,3). The most frequent subtype expresses estrogen receptor (ERα), which can be targeted by anti-estrogens or aromatase inhibitors (2). Less frequent subtypes are human epidermal growth factor receptor 2 (Her-2)-positive breast cancers, which are vulnerable to Her2 inhibitors (4) and triple-negative breast cancers (TNBCs), which are devoid of ERα, Her2 and progesterone receptor. For TNBCs, selective drugs are still not available (5). Breast cancers also exhibit intratumoral heterogeneity (6), part of which is caused by cancer stem cells (CSCs) (7). CSCs have been linked to tumor initiation, metastasis and drug resistance (8).
Drug resistance is a major challenge in the treatment of patients with breast cancer (9,10). Different mechanisms leading to drug resistance are known. Endocrine resistance often involves the activation of the phosphoinositol-3-kinase (PI3K)/AKT and/or Ras/Raf/mitogen-activated protein kinase (MEK-1)/extracellular-signal-regulated kinase (ERK) kinase-1)/ERK1/2 pathways, which are both able to activate ERα independently of estrogen (11). The activation of these pathways can occur intrinsically or extrinsically. By interacting with breast cancer cells, stromal cells, such as mesenchymal stem cells (MSCs) and carcinoma-associated fibroblasts (CAFs), can extrinsically activate these pathways and thereby promote the development of endocrine resistance (12).
Recently, the authors demonstrated that MSCs and CAFs are able to induce resistance to the selective ERα down-regulator, fulvestrant, in breast cancer cells by increasing the expression of the atypical inhibitor of nuclear factor-κB (IκB) family member B-cell leukemia/lymphoma 3 (Bcl-3) (13). Bcl-3, whose expression can also be induced by the withdrawal of estrogen (14), has been associated with higher metastatic activities in breast cancer (15). Bcl-3 is a potent activator of the nuclear factor (NF)-κB pathway (16), a pathway that has also been linked to endocrine resistance (17). In addition, the activity of Bcl-3 is positively regulated by the PI3K/AKT and Ras/Raf/MEK-1/ERK1/2 signaling pathways (18).
The stromal cell-induced increase in Bcl-3 expression has been shown to be causally linked to a prior downregulation of the insulin-like growth factor binding protein 5 (IGFBP5) level (13). A main function of IGFBP5 is to regulate the availability of insulin-like growth factor (IGF) for the interaction of this growth factor with its receptor, IGF1 receptor (IGF1R) (19). In addition, IGFBP5 can also act without targeting IGF (19). Such an IGF-independent effect may be responsible for the regulation of Bcl-3 by IGFBP5. There is evidence to indicate that IGFBP5 plays an important role in breast cancer progression and that it is of prognostic value (20,21). Notably, IGFBP5 expression is higher in ERα-positive tumors (20).
The stromal cell-induced desensitization of MCF-7 cells to fulvestrant does not require direct contact between stromal and breast cancer cells (13); however, it can be fully recapitulated by conditioned medium (CM) derived from stromal cells. CM can also mimic all effects of stromal cells on signaling pathways, namely the Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3), PI3K/AKT and the Ras/Raf/MEK-1/ERK1/2 pathways, as well as on the expression of proteins, namely Bcl-3, integrin β1, IGF1R and carbonic anhydrase IX (CAIX). This suggests that factors secreted by stromal cells are responsible for the desensitization to fulvestrant and for alterations in protein expression.
Interleukin (IL)-6 belongs to the IL-6 family of cytokines (22). By forming a complex with the IL-6 receptor (IL-6R) and glycoprotein 130 (gp130) (23), IL-6 activates JAKs (24). Downstream targets of JAKs are STATs, MAPK and the PI3K/AKT pathways, whereby the activation of STAT3 is a major cellular response to IL-6 (25). Soluble IL-6R allows trans signaling. In trans signaling, an extracellular complex of IL-6 and IL-6R activates gp130-expressing targets cells (24). Since, in this case, the target cells do not need to express IL-6R by themselves, the number of cells that can respond to IL-6 increases. IL-6 is primarily secreted by leukocytes to regulate hematopoietic cells involved in inflammation and adaptive immunity (22). In addition, IL-6 acts on non-hematopoietic cells, such as fibroblasts, adipocytes, endothelial and epithelial cells and may, when deregulated, lead to the development of certain diseases, such as fibrosis. Epithelial cells benefit from the survival-promoting activity of IL-6, helping damaged epithelia to be repaired (26). Intriguingly, IL-6 also supports the survival of premalignant epithelial cells, which links IL-6 to cancer progression. Strikingly, IL-6 has often been found to be upregulated in the bodily fluids of cancer patients (27) and activated STAT3 is a common feature of numerous cancer types (28). IL-6 has been associated with inflammation and multidrug resistance in cancer (29,30). In breast cancer, IL-6 has been found to induce resistance to the anti-estrogen tamoxifen and the Her2 antibody trastuzumab and has been shown to contribute to chemoresistance (12). Evidence for a role of IL-6 in maintaining cancer stem cell activity in breast cancer has also been provided (8). IL-6 is able to increase the cancer stem cell population and, along with it, the expression of crucial stemness factors, such as octamer-binding transcription factor 4 (Oct4) (31). IL-6 also induces epithelial-to-mesenchymal transition (32,33), which promotes cancer stem cell activity (34). The ability of IL-6 to induce drug resistance has been found to be linked to its stemness-supporting activity (35,36). IL-6 has further been shown to be involved in a cytokine network between MSCs, CSCs and non-CSC breast cancer cells (37). Based on the assumption that CSCs are the likely drivers of metastasis (38), it is noteworthy that IL-6 serum levels are higher in breast cancer patients with metastatic disease (39).
Given its multiple effects on cancer progression, IL-6 has been discussed as a promising target for drug intervention in breast cancer (40,41). IL-6- or IL-6R-directed drugs are already routinely used for treatment of diseases with excessive IL-6 expression, such as inflammatory arthritis (22) and could therefore be made available for cancer treatment. Since the major source of IL-6 are MSCs and CAFs in cancer (12), in this study, the potential of recombinant IL-6 to mimic the effects of stromal cells on fulvestrant resistance and on the expression and activities of those proteins which may be involved therein was examined.
This study demonstrates that IL-6 is the mediator of the majority of the CAF-CM-induced effects on protein expression and on STAT3 phosphorylation, although not on PI3K/AKT pathway activity. It is further demonstrated that IL-6 participates in CAF-CM-induced fulvestrant resistance in 3D spheroid cultures, but not in 2D adherent cultures. In addition, it was found that IL-6 likely contributes to the growth-inhibitory effects of CAF-CM on ERα-negative breast cancer cells.
Materials and methods
Cell lines and agents
MCF-7, BT474, T47D, SKBR3 and MDA-MB-231 cells, which were authenticated by SNP analysis (Genolytic), were propagated in RMPI-1640 supplemented with 10% fetal calf serum (PAN Biotech). The MCF-7 subline, AnD5 cells, and the generation of CAF-CM have been described previously (42). One part CAF-CM was mixed with four parts fresh medium/serum (20% CAF-CM). Recombinant human IL-6 (rhIL-6) was purchased from PeproTech and reconstituted in water as recommended by the provider. Fulvestrant (LKT Laboratories) was added to the cells at a final concentration of 1 µM.
RNA interference
The p110α (pik3ca)-specific siRNA siPIK (5′-GUACAGGACUUCCGAAGAA-3′), siBcl-3 (5′-UGGUC UUCUCUCCGCAUCA-3′) and the control siRNA and Firefly luciferase-siRNA siLuc (5′-CUUACGCUG AGUACUUCGA-3′), were purchased from Eurofins MWG. Transfections were performed by electroporation by using a Bio-Rad GenePulserX-Cell as previously described (42). Briefly, following electroporation using a Bio-Rad GenePulserX-cell (250 V, 800 µF), cells were seeded on a 10 cm culture dish and incubated for 3 days to allow the siRNA to downregulate its specific target.
Western blot analysis
Protein extractions and western blot analysis were carried out as previously described (42). Blots were incubated with primary and secondary antibodies at room temperature for 1 h. The primary antibodies used are listed below. Rabbit polyclonal antibodies: Anti-p(S473)-AKT (1:2,000, D9E, #4060, Cell Signaling Technology), anti-Bcl-3 (1:1,000, C-14, sc-185, Santa Cruz Biotechnology), anti-ERα (1:2,000, HC-20, sc-543, Santa Cruz Biotechnology), anti-p(Thr202, Tyr204)-ERK1/2 and anti-ERK1/2 (both 1:2,000, #9101 and #9102, Cell Signaling Technology), anti-IGF1Rβ (1:2,000, #3027, Cell Signaling Technology), anti-protein kinase Cα (PKCα; 1:2,000, C-20, sc-208, Santa Cruz Biotechnology), anti-p(Tyr705)-STAT3 (1:1,000, D3A7, #9145, Cell Signaling Technology) and anti-STAT3 (1:1,000, 79D7, #4904, Cell Signaling Technology); rabbit monoclonal antibodies: Anti-PI3 linase p110α (1:1,000, C73F8, #4249, Cell Signaling Technology), anti-Her2 (1:1,000, 29D8, #2165, Cell Signaling Technology), anti-NF-κB1 p105/p50 (1:1,000, D4P4D, #13586, Cell Signaling Technology), anti-p21 Waf1/Cip1 (1:1,000, 12D1, #2947, Cell Signaling Technology), anti-integrin β1 (1:2,000, EPR1040Y, ab134179, Abcam), anti-ABCG2 (1:1,000, EPR20080, ab207732, Abcam), anti-Ki67 (1:2,000, EPR3610, ab92742, Abcam), anti-c-Myc (1:500, EP121, AC-0116, Epitomics) and anti-poly(ADP-ribose) polymerase 1 (PARP-1; cleaved 25 kDa; 1:10,000, #1051-1, Epitomics); mouse monoclonal antibodies: Anti-(pan) AKT (1:2,000, 40D4, #2920, Cell Signaling Technology). Anti-CAIX antibody was kindly provided by Professor S. Pastorekova (Slovak Academy of Sciences). Secondary antibody conjugates (anti-rabbit/anti-mouse horse radish peroxidase, 1:2,000, #7074 and #7076) were purchased from Cell Signaling Technology. Protein loading was examined by either staining proteins with Coomassie Blue (Blue G, SERVA Electrophoresis) or with Fast Green (MERCK). Incubations with either staining agent were carried out at room temperature for 1 h. Antibodies against housekeeping proteins were not used for this purpose, since they are not reliable markers for protein loading (43,44).
Immunocytochemistry
Immunocytochemical analysis of formaldehyde-fixed and paraffin-embedded 3D cell aggregates was carried out as previously described (45).
Growth/survival assays
In 3D and high-density 2D cultures, the mass of living cells was determined by an ATP-based assay (Vialight Plus kit, Lonza). For spheroid formation in 3D suspension cultures, the cells were incubated in ultra-low attachment 96-well microplates (Corning) at a density of 5×103 cells/well. For incubation in high-density 2D cultures, the cells were seeded at a density of 1×104 per well (24-well plate). The cells were then exposed to fulvestrant and/or CAF-CM or left untreated for 3-7 days as indicated. Following the removal of the growth medium, the 2D-cultured cells were lysed by the addition of a mixture of 100 µl PBS and 50 µl lysis buffer to each well. After mixing 50 µl of the lysate with 50 µl luciferase stock solution, the luciferase activity was measured in a Sirius luminometer (Berthold). For measuring ATP in 3D-cultured cells, 50 µl of the lysis buffer was directly added to the 100-µl culture medium the cells were grown in. For examining cell growth in low-cell density 2D cultures, the cells were seeded at a density of 3×104 cells per ø 10 cm dish. Following treatment either the average size (MCF-7, MDA-MB-231 cells) or the average cell number (SKBR3 cells) of individual colonies was determined. Colony size was measured using an AxioCAM MRc5 camera and the AxioVision R 4.5 imaging software (Zeiss) as described previously (45). For each condition, 50 randomly selected colonies were examined. For measuring spheroid/aggregate size in 3D suspension cultures, the area occupied by the spheroid or aggregate was measured. Of note, some cell lines did not form regularly shaped spheroids. Instead they formed randomly clusters (T47D) or discs (MDA-MB-231, SKBR3) (data not shown).
Reverse transcription-quantitative PCR (RT-qPCR)
RNA isolation, cDNA synthesis and quantitative (q)PCR were carried out as described previously (46). Briefly, NucleoSpin RNA II (Macherey & Nagel) and Superscript II (Invitrogen; Thermo Fisher Scientific) were used for RNA isolation and cDNA synthesis, respectively. Following the addition of ABsolute qPCR SYBR-Green Fluorescein Mix (Thermo Fisher Scientific), qPCRs were run on a Bio-Rad iCycler and analyzed using iQ5 Optical System software version 2.1. Relative RNA levels of genes were calculated by the comparative Cq (2−∆∆Cq) method using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and hypoxanthine-guanine phos-phoribosyltransferase (HPRT) as reference genes for normalization (47). Primers for Bcl-3, fibroblast growth factor (FGF)18, GAPDH, HPRT, IGFBP5, kinesin family member 12 (KIF12), kelch like family member 4 (KLHL4), Kallikrein-11 (KLK11), RAB30, receptor activity modifying protein 3 (RAMP3), selenoprotein P (SEPP1), transforming growth factor β receptor III (TGFBR3), transmembrane protein 26 (TMEM26), UDP-glucuronosyltransferase 2B15 (UGT2B15) and yippee like 1 (YPEL1) were as previously described (13). The primers used for the detection of aldehyde dehydroge-nase 1 family, member A1 (ALDH1A1), aldehyde dehydrogenase 3 family, member A1 (ALDH3A1) and STAT3 were as follows: ALDH1A1 (forward 5′→3′, CAAAGAAGC TGCCGGGAAA and reverse 5′→3′, TCCAAGCTCCAGGGT CACC), ALDH3A1 (forward 5′→3′, GTCCCTGAGACCACG GAGC and reverse 5′→3′, CCCGTGTACAGGATATGGTCG) and STAT3 (forward 5′→3′, GGACAATATCATTGACCTTGT GAAAA and reverse 5′→3′, CTTCGTTCCAAAGGGCCAG).
Antibody array
To identify proteins that are abundant in CAF-CM, the Human Obesity Antibody Array I (RayBiotech) was used, containing 62 different antibodies. Among the proteins, which can be detected by this array are a number of ILs, stromal cell-derived factor 1 (SDF-1) and TGFβ. The incubation of this array with CAF-CM and control medium/serum was carried out according to the manufacturer's instructions.
Statistical analyses
Data obtained from colony growth assays were analyzed by Kruskal-Wallis test followed by pairwise comparison with Bonferroni-corrected test. All other statistical analyses were carried out by using one-way ANOVA. The Bonferroni correction was applied for post-hoc analysis. A P-value <0.05 was considered to indicate a statistically significant difference.
Results
IL-6 is a major mediator of the effects of CAF-CM on protein expression and phosphorylation in MCF-7 cells
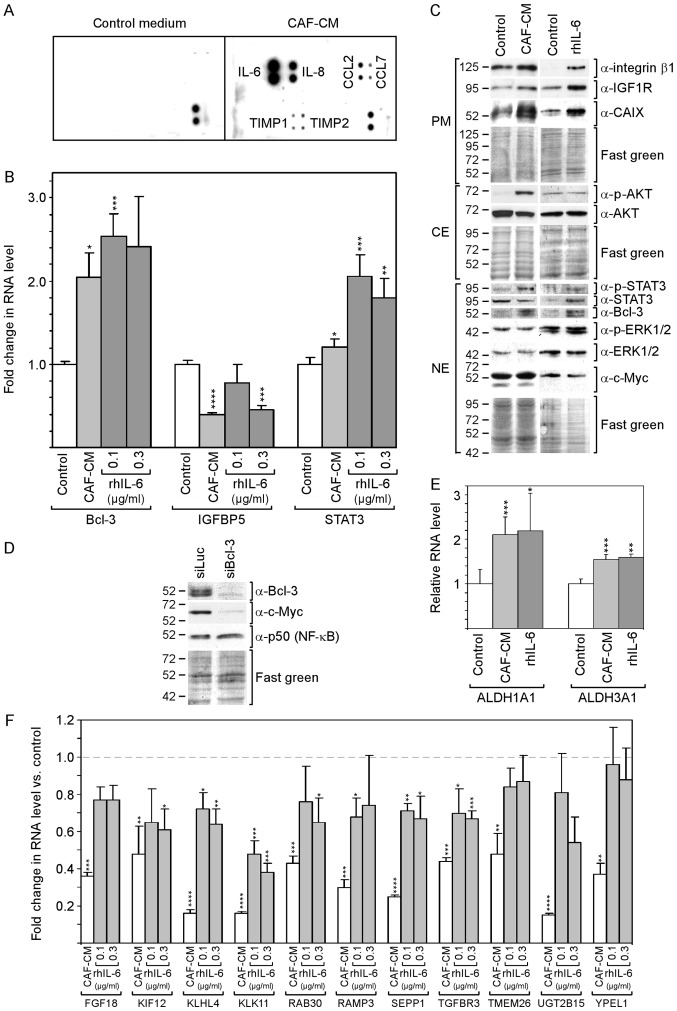
By selecting an antibody array that was able to detect the majority of factors reported to be secreted by CAFs (48), it was found that, in the CAF-CM preparations in this study, IL-6 was the most prominent component among other proteins [IL-8, CC-chemokine ligand (CCL)2 and CCL7, and tissue inhibitor of metallopro-teinases (TIMP)1 and TIMP3] (Fig. 1A).
Figure 1.
rhIL-6 recapitulates many of the effects of CAF-CM on protein expression in MCF-7 cells. (A) Antibody arrays of CAF-CM and control medium. (B) Effects of CAF-CM and rhIL-6 on relative RNA expression on Bcl-3, IGFBP5 and STAT3 as measured by RT-qPCR. (C) Western blot analyses of protein extracts derived from cells treated with either CAF-CM or rhIL-6 (0.3 µg/ml) or of untreated cells (control). Cells were incubated for 3 days before plasma membrane (PM), cytosolic (CE) and nuclear (NE) proteins were extracted. (D) Effect of a Bcl-3-specific siRNA (siBcl3) on the protein expression of Bcl-3, c-Myc and p50 (NF-κB) as determined by western blot analyses of nuclear protein extracts. (E) Effects of CAF-CM and rhIL-6 (0.3 µg/ml) on the relative RNA expression of ALDH1A1 and ALDH1A3 as measured by RT-qPCR. (F) Effect of CAF-CM and rhIL-6 (0.1 and 0.3 µg/ml) on the relative RNA expression of 11 CAF-CM-responsive genes. (B, E and F) Each bar represents the average values of 3 independent experiments. Statistical analyses were carried out by ANOVA and the Bonferroni post-hoc test. Asterisk(s) indicate statistical significance in comparison to the control. *P<0.05, **P<0.01, ***P<0.005 and ****P<0.001. rhIL-6, recombinant human interleukin 6; CAF-CM, carcinoma-associated fibroblast-conditioned medium; ALDH1A1, aldehyde dehydrogenase 1 family, member A1; ALDH1A3, aldehyde dehydrogenase 3 family, member A1; STAT3, signal transducer and activator of transcription 3.
Of note, SDF-1, vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), tumor necrosis factor (TNF) and interferon (IFN)γ could not be detected in considerable amounts. rhIL-6 was used to determine to which extent IL-6 is able to mimic the effects of CAF-CM on MCF-7 cells.
rhIL-6 was as potent as CAF-CM in increasing Bcl-3 RNA and protein expression (Fig. 1B and C). Of note, several bands of Bcl-3 were visible in the western blot analysis, which likely represent Bcl-3 isoforms with a different phosphorylation status (49). In the CAF-CM-treated MCF-7 cells, Bcl-3 expression is linked to IGFBP5 downregulation (13), and in rhIL-6-treated cells, STAT3 has been shown to drive Bcl-3 expression (49). In this study, rhIL-6 was found to be as capable as CAF-CM in activating STAT3 (Fig. 1C) and, above that, even increased STAT3 mRNA expression (Fig. 1B). However, at 0.1 µg/ml, it failed to mimic the downregulating effect of CAF-CM on IGFBP5 expression (Fig. 1B). This suggests that, in contrast to CAF-CM, rhIL-6 upregulates Bcl-3 in MCF-7 cells through STAT3 and not through IGFBP5.
The authors have previously reported that Bcl-3 modulates the expression of IGF1R and CAIX (13). The levels of both could be increased in a similar manner by CAF-CM and rhIL-6 (Fig. 1C). Another potential target of Bcl-3 is c-Myc (50), a mitogenic oncoprotein (51). After it was confirmed that c-Myc expression also depends on Bcl-3 in MCF-7 cells (Fig. 1D), the effect of CAF-CM and rhIL-6 on the c-Myc level was examined. However, neither agent interfered with c-Myc expression (Fig. 1C) suggesting that the basal level of Bcl-3 is sufficient to maintain high c-Myc levels. It was also determined whether Bcl-3 modulates the expression of p50 (NF-κB), a Bcl-3-binding transcription factor through which Bcl-3 activates transcription (52). However, p50 expression was not affected by Bcl-3 knockdown (Fig. 1D).
Integrin β1, another protein whose expression can be upregulated by CAF-CM, also exhibited a higher level in the presence of rhIL-6 (Fig. 1C). In addition, the RNA expression levels of the cancer stem cell markers, ALDH1A1 and ALDH1A3 (8) were increased in a similar manner by CAF-CM and rhIL-6 (Fig. 1E). However, rhIL-6 failed to increase AKT phosphorylation (Fig. 1C). In addition, it was not able to down-regulate a number of genes to the same extent as CAF-CM (Fig. 1F), whereby two of these genes (TMEM26 and YPEL1) exhibited no response to rhIL-6 at all. Of note, the 11 genes depicted in Fig. 1F shared the ability to react to inhibitors of the PI3K/AKT pathway by increasing their expression (data not shown). This links the weaker effect of rhIL-6 on these genes to its inability to induce AKT phosphorylation.
On the whole, these data demonstrate that rhIL-6 recapitulates a number of the effects of CAF-CM on RNA and protein expression in MCF-7 cells. However, it fails to activate the PI3K/AKT pathway and has a weaker or no effect on a number of PI3K/AKT-responsive genes.
rhIL-6 partly mimics CAF-CM-induced fulvestrant resistance in 3D, but not in 2D cultures
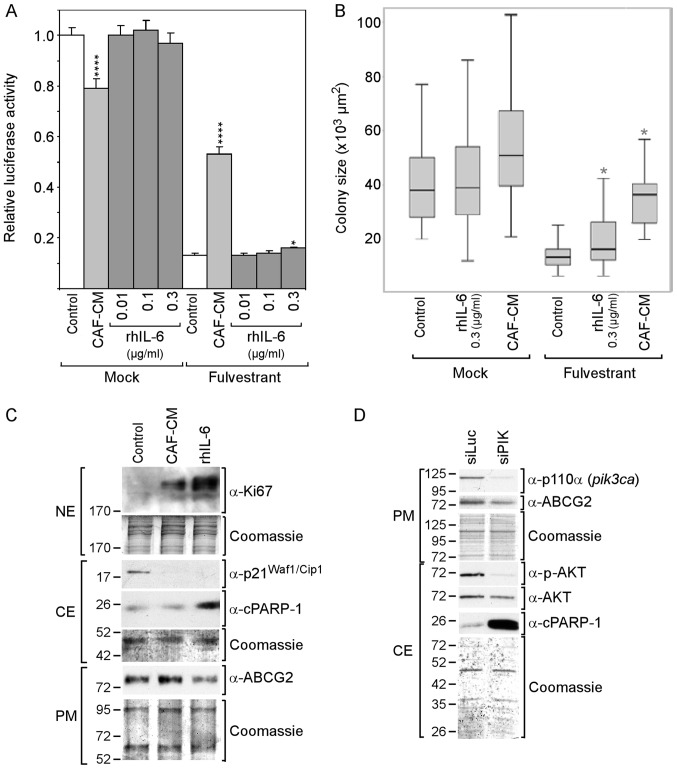
The PI3K/AKT pathway and Bcl-3 play important roles in CAF-induced anti-estrogen resistance (13,53). The PI3K/AKT pathway is able to phosphorylate ERα and restore ERα activity in the presence of anti-estrogens. In addition, it increases Bcl-3 protein stability and fosters its nuclear localization (18). As demonstrated above, CAF-CM and rhIL-6 increased the expression of Bcl-3, but only CAF-CM was able to activate the PI3K/AKT pathway. We therefore examined whether rhIL-6 is capable of inducing fulvestrant resistance to the same extent as CAF-CM. To this end, MCF-7 cells were grown in the absence and presence of fulvestrant for 5 or 7 days at high or low density, respectively, and the mass of viable cells was determined by an ATP/luciferase-based assay (high density) or average colony size (low density). In the absence of fulvestrant, CAF-CM negatively affected growth in high-density cultures and had a slight positive effect in low-density cultures, whereas rhIL-6 had no effect (Fig. 2A and B). In the presence of fulvestrant, CAF-CM potently increased cell mass both in low- and high-density cultures, whereas rhIL-6 had no effect at the concentration of 0.01 and 0.1 µg/ml, and slightly increased cell mass at 0.3 µg/ml. This indicates that, in contrast to CAF-CM, rhIL-6 is unable to induce fulvestrant resistance.
Figure 2.
rhIL-6 fails to mimic the growth-stimulatory effect of CAF-CM on MCF-7 cells in the presence of fulvestrant. (A and B) Cells were incubated for 6 days in the presence or absence of fulvestrant and exposed to either CAF-CM or rhIL-6 (at concentrations as indicated) or to none of these two agents after cells have been seeded at (A) high or (B) low density. Viability/growth was either measured by (A) an ATP/luciferase-based assay or (B) examined by determining the average size of 50 randomly selected individual colonies. Statistical analyses were either performed by (A) ANOVA or (B) by the Kruskal-Wallis test and the Bonferroni post-hoc test. Asterisk(s) indicate statistical significance in comparison to the control. *P<0.05 and ****P<0.001. (C) Cells were incubated for 7 days in the presence of fulvestrant and exposed to either CAF-CM, rhIL-6 (0.3 µg/ml) or none of these agents, before plasma membrane (PM), cytosolic (CE) and nuclear proteins (NE) were extracted and examined by western blot analysis for the proteins as indicated. (D) Effects of a PI3KCA (p110α)-specific siRNA (siPIK) on the levels of p110α, ABCG2, p-AKT, AKT and cPARP-1 as determined by western blot analysis. rhIL-6, recombinant human interleukin 6; CAF-CM, carcinoma-associated fibroblast-conditioned medium; cPARP-1, cleaved poly(ADP-ribose) polymerase-1; ABCG2, ATP-binding cassette transporter G2.
To examine whether CAF-CM and rhIL-6 differently affect proliferation and/or apoptosis in the presence of fulvestrant, the proliferation marker Ki67 and the apoptosis marker cPARP, which is the 25-kDa fragment of cleaved PARP1, were analyzed. Both CAF-CM and rhIL-6 increased the Ki67 levels in the fulvestrant-treated MCF-7 cells and, along with this, abrogated the expression of cytoplasmic p21Waf1/Cip1, a marker of both senescence and apoptosis resistance (54,55). Unlike CAF-CM, rhIL-6 increased cPARP-1 expression (Fig. 2C). This suggests that, in contrast to CAF-CM, rhIL-6 exhibited pro-apoptotic activity in MCF-7 cells, which is consistent with the findings of a previous study demonstrating that, at 5 ng/ml, rhIL-6 induced apoptosis-linked DNA laddering in these cells (56). Along with the increased expression of cPARP-1, the level of ABCG2, a multidrug resistance protein acting as drug efflux pump (57), was found to be reduced (Fig. 2C). This suggest that the pro-apoptotic activity of rhIL-6 is linked to a decline in the activity of ABCG2.
Subsequently, it was determined whether the PI3K/AKT pathway is involved in this process by using siRNA directed to the PI3K subunit p110α. This siRNA completely abolished p110α expression and markedly reduced AKT phosphorylation (Fig. 2D). It also potently increased cPARP-1 expression and downregulated ABCG2 expression. These data suggest that the PI3K/AKT pathway is important for protecting MCF-7 cells against apoptosis and that ABCG2 plays a role in this process (Fig. 2D).
These data suggest that, in the presence of fulvestrant, CAF-CM increased cell growth by stimulating proliferation. While rhIL-6 shares with CAF-CM the ability to stimulate proliferation, it also acts in a pro-apoptotic manner, which seems to lead to a steady state between proliferation and apoptosis and therefore prevents growth. The data further suggest that the pro-apoptotic activity of rhIL-6 is linked to ABCG2 downregulation and its inability to raise AKT activity.
The effects of CAF-CM and rhIL-6 on the sensitivity of MCF-7 cells to fulvestrant in 3D spheroid cultures were then analyzied for two reasons. These culture conditions more likely resemble in vivo conditions (57) and cells in 3D cultures react differently to external stimuli as compared to cells in 2D cultures (59,60). In 3D cultures, MCF-7 cells form regularly-shaped spheroids (Fig. S1A). Spheroid formation is complete after three days of incubation. Thereafter, the spheroids become larger (Fig. S1A), which coincides with cell growth (Fig. S1B) and a high expression of Ki67 (Fig. S1C). Later, growth ceases, possibly since cells die in the center of the spheroid, as indicated by cPARP-1 expression (61). In the presence of fulvestrant, cells form smaller spheroids, which later on appear disheveled (Fig. S1A). These morphological changes were accompanied by progressive cell death (Fig. S1B).
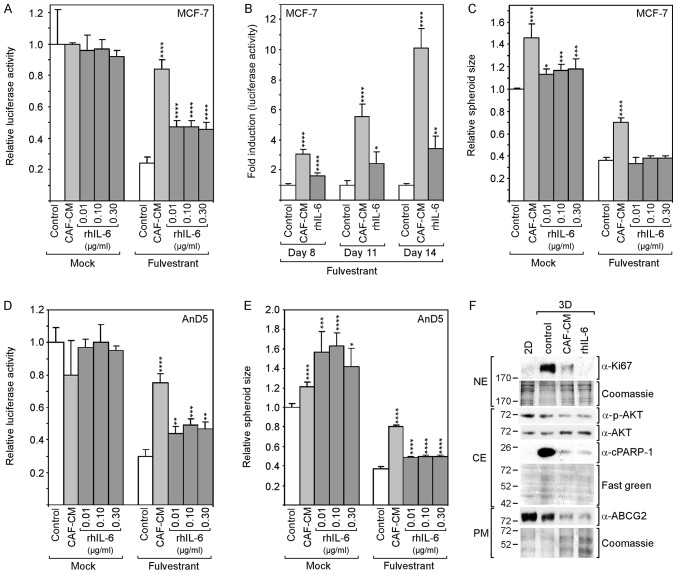
To assess the effects of CAF-CM and rhIL-6 on spheroid-assembled MCF-7 cells, cell growth and spheroid size were measured. In the absence of fulvestrant, neither CAF-CM nor rhIL-6 affected cell growth (Fig. 3A). In the presence of fulvestrant, both CAF-CM and rhIL-6 increased cell mass; however, the effect of CAF-CM was more potent (3.5- vs. 1.9-fold). The difference became even more pronounced, when the incubation time was extended to 11 days (5.5- vs. 2.4-fold) and 14 days (10- vs. 3.4-fold) (Fig. 3B). Spheroid size was increased by CAF-CM both in absence and presence of fulvestrant; however, rhIL-6 only weakly enlarged the spheroids in the absence of fulvestrant and had no effect on spheroid size in the presence of fulvestrant (Fig. 3C).
Figure 3.
rhIL-6 partly mimics the growth-stimulatory effects of CAF-CM in 3D cultures of MCF-7 and AnD5 cells. (A-C) MCF-7 cells were incubated for (A and C) 6 days or (B) for 8, 11 and 14 days in the presence or absence of fulvestrant and exposed to either CAF-CM or rhIL-6 (A and C, at concentrations as indicated; B, at 0.3 μg/ml) or to none of these two agents. (A and B) Viability was measured by an ATP/luciferase-based assay, whereby, in (B) fold induction is shown, calculated relative to the control value for each time point. (C) Spheroid size was measured as described in the Materials and methods. (D and E) AnD5 cells were incubated for 3 days in the presence or absence of fulvestrant and exposed to either CAF-CM or rhIL-6 (at concentrations as indicated) or to none of these two agents. (D) Viability was measured by an ATP/luciferase-based assay. (E) Spheroid size was measured as described in the Materials and methods. (A-E) Statistical analyses were carried out by ANOVA and the Bonferroni post-hoc test. Asterisk(s) indicate statistical significance in comparison to the control. *P<0.05, **P<0.01, ***P<0.005 and ****P<0.001. (F) Western blot analysis of plasma membrane (PM), cytosolic (CE) and nuclear protein extracts (NE) derived from 2D- or 3D-cultured MCF-7 cells after 7-day-incubation in the presence of fulvestrant and exposure to CAF-CM, rhIL-6 (0.3 μg/ml) or to none of these agents (control). rhIL-6, recombinant human interleukin 6; CAF-CM, carcinoma-associated fibroblast-conditioned medium; cPARP-1, cleaved poly(ADP-ribose) polymerase-1; ABCG2, ATP-binding cassette transporter G2.
These experiments were repeated with the AnD5 cell line (42). This MCF-7 subline forms smaller spheroids and develops them more rapidly than the parental MCF-7 cell line (data not shown). The results obtained with the AnD5 cells were similar to those obtained with the parental cell line, with the exception that, in the absence of fulvestrant, rhIL-6 affected spheroid size more potently than CAF-CM and that, in the presence of fulvestrant, rhIL-6 increased spheroid size, although still not as efficiently as CAF-CM (Fig. 3D and E).
The examination of the Ki67 and cPARP-1 levels after 7 days of fulvestrant treatment revealed that the levels of both proteins were higher in the 3D cultures compared to their levels in 2D cultures, and that CAF-CM and rhIL-6 reduced the levels of both proteins (Fig. 3F). While the effects of CAF-CM and rhIL-6 were similar on cPARP-1 expression, rhIL-6 had a more potent suppressive effect on Ki67 expression. These data suggest that, in 3D cultures, CAF-CM- and rhIL-6-induced fulvestrant resistance is based on their anti-apoptotic activities. The residual proliferative activity in the presence of CAF-CM and rhIL-6 may as well play a role. If so, the more potent suppressive effect of rhIL-6 on Ki67 expression may explain why rhIL-6 is not as effective as CAF-CM in inducing fulvestrant resistance. The examination of p-AKT and ABCG2 expression revealed that the anti-apoptotic effect of CAF-CM and rhIL-6 was not linked to these proteins. In the presence of CAF-CM and rhIL-6, the levels of both proteins were rather reduced (Fig. 3F). This suggests that, in 3D cultures, the PI3K/AKT pathway and ABCG2 are not involved in the regulation of apoptosis.
Collectively, these data suggest that CAF-CM induces fulvestrant resistance in 2D and 3D cultures through different mechanisms, one by inducing proliferation, the other by inhibiting apoptosis, respectively. To a large extent, rhIL-6 was able to mimic the protective effect of CAF-CM against fulvestrant in 3D cultures, likely due to the fact that it was as able as CAF-CM to block apoptosis. By contrast, in 2D cultures, rhIL-6 shares with CAF-CM a mitogenic effect; however, by also acting in a pro-apoptotic manner under these culture conditions, it prevents cell growth and instead induces a steady-state between cell proliferation and cell death.
rhIL-6 fails to induce fulvestrant resistance also in other ERα-positive cell lines in 2D cultures
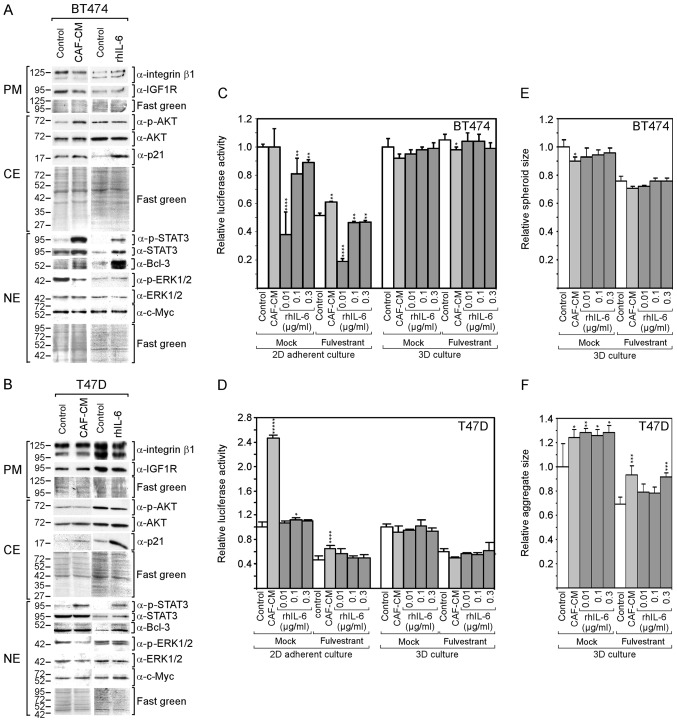
The question of whether the findings that were obtained with the MCF-7 cells are also applicable for other ERα-positive cell lines was then examined. The BT474 and T47D cell lines were selected, which have been previously found by the authors to respond to CAF-CM (13), both in terms of protein expression patterns and survival in the presence of fulvestrant. Both cell lines exhibited an increased STAT3 phosphorylation in response to CAF-CM and rhIL-6 (Fig. 4A and B). In the BT474 cells, both CAF-CM and rhIL-6 upregulated Bcl-3 expression, although the effect of rhIL-6 was more potent. In the T47D cells, only rhIL-6 increased the Bcl-3 level. In neither case was a gain in the Bcl-3 level accompanied by a substantial change in c-Myc or IGF1R expression; nor did CAIX expression increase to detectable levels (data not shown). In addition, neither CAF-CM nor rhIL-6 upregulated the level of integrin β1. Different effects of CAF-CM and rhIL-6 were observed on cytoplasmic p21, ERK1/2 and AKT phosphorylation. In both cell lines, rhIL-6, but not CAF-CM, substantially increased the level of cytoplasmic p21, while CAF-CM, but not rhIL-6, decreased ERK1/2 phosphorylation. Importantly, as was observed with the MCF-7 cells, rhIL-6 also failed to mimic the positive effect of CAF-CM on AKT phosphorylation in BT474 cells.
Figure 4.
rhIL-6 shares with CAF-CM many effects on protein expression in BT474 and T47D cells, but fails to mimic growth-stimulatory effects of CAF-CM. (A and B) Western blot analyses of protein extracts derived from (A) BT474 or (B) T47D cells treated with either CAF-CM or rhIL-6 (0.3 µg/ml) or of untreated cells (control). Cells were incubated for 3 days before plasma membrane (PM), cytosolic (CE) and nuclear (NE) proteins were extracted. (C-F) Measurement of (C and D) viability/growth or (E and F) spheroid size of (C) BT474 and (D) T47D cells after (C and E) 7 days or (D and F) 4 days of incubation with CAF-CM or rhIL-6 (at concentrations as indicated) or with none of these agents (control) in the presence or absence of fulvestrant in 2D or 3D cultures. Statistical analyses were carried out by ANOVA and the Bonferroni post-hoc test. Asterisk(s) indicate statistical significance in comparison to the control. *P<0.05, **P<0.01, ***P<0.005 and ****P<0.001. rhIL-6, recombinant human interleukin 6; CAF-CM, carcinoma-associated fibroblast-conditioned medium; STAT3, signal transducer and activator of transcription 3.
As was previously demonstrated (13), CAF-CM increased the resistance of BT474 and T47D cells to fulvestrant in 2D cultures (Fig. 4C and D), although these effects were not as potent as with the MCF-7 cells (Fig. 2A). Again, with rhIL-6, these results could not be reproduced.
In the absence of fulvestrant, CAF-CM had no effect on BT474 cell growth, while it potently increased the growth of T47D cells. By contrast, rhIL-6 failed to mimic the growth-stimulatory effect on the T47D cells and even had a growth-inhibitory effect on BT474 cells (Fig. 4C and D).
In 3D cultures, neither CAF-CM nor rhIL-6 had a substantial effect on the growth of these cells (Fig. 4C and D). The spheroid size of the BT474 cells and aggregate size of the T47D cells (which do not form uniformly shaped spheroids) were affected by CAF-CM and rhIL-6 in a similar manner. Both agents slightly reduced the average spheroid size of the BT474 cells and increased the average aggregate size of the T47D cells (Fig. 3E and F).
Collectively, these data demonstrate that rhIL-6 also fails to mimic the growth-stimulatory effects of CAF-CM on BT474 and T47D cells in 2D cultures. In the case of the BT474 cells, this failure may again be linked to the inability of rhIL-6 to increase AKT phosphorylation. Additionally, cytoplasmic p21 may play a role, which was highly upregulated in both BT474 and T47D cells in response to rhIL-6, but not in response to CAF-CM.
rhIL-6 mimics the majority of the growth-inhibitory effects of CAF-CM on ERα-negative cell lines
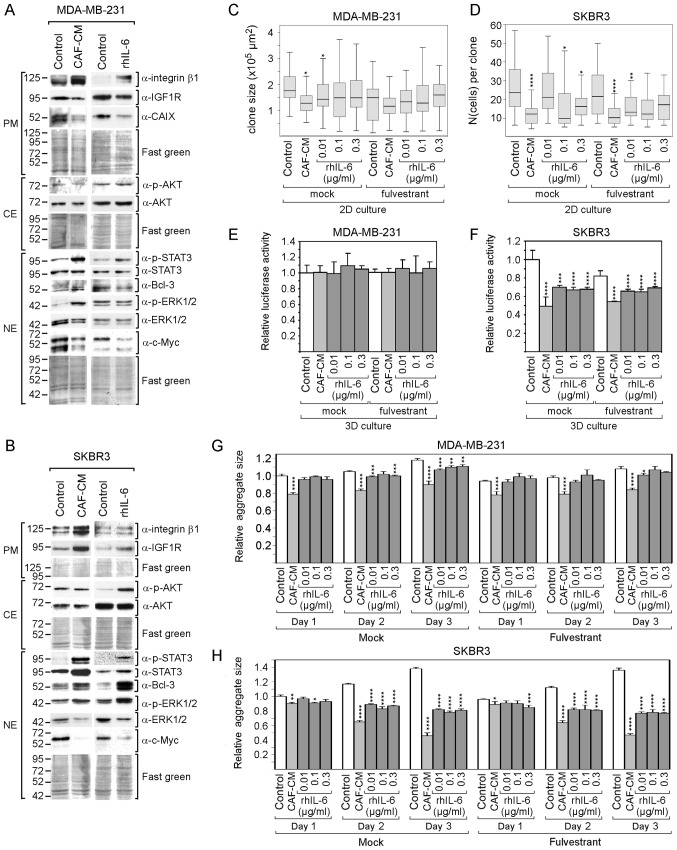
Furthermore, ERα-negative cell lines (triple-negative MDA-MB-231 and Her2-positive SKBR3 cells) were analyzed. The lack of ERα expression in MDA-MB-231 and SKBR3 cells and Her2 expression in SKBR3 cells were confirmed by western blot analysis (Fig. S2). Of note, the MDA-MB-231 cells exhibited a high basal activity of STAT3 (Fig. S2), which is the consequence of their high production of IL-6 (61) and an IL-6/IL-6 receptor autocrine loop (63). This cell line also exhibited the highest basal level of integrin β1 of all the cell lines tested (Fig. S2). However, due to their ERα deficiency, no effect of fulvestrant on MDA-MB-231 and SKBR3 cells was expected; for reasons of completeness, the analyses were also carried out in the presence of fulvestrant. In addition, since these cells grow rapidly, growth in 2D cultures was examined at a low density and either the average size of individual colonies (MDA-MB-231) or the average number per colony (SKBR3) was determined.
Despite the high basal levels of p-STAT3 and integrin β1 in the MDA-MB-231 cells, CAF-CM and rhIL-6 were able to further upregulate the levels of both proteins in these cells (Fig. 5A). By contrast, none of the two agents induced Bcl-3 expression and both even negatively affected the expression of IGF1R and CAIX. In SKBR3 cells, CAF-CM and rhIL-6 similarly upregulated STAT3 phosphorylation, Bcl-3 and IGF1R expression, while only CAF-CM enhanced integrin β1 expression (Fig. 5B). In both cell lines, CAF-CM increased ERK1/2 phosphorylation and decreased total ERK1/2 levels, while having no effect on AKT phosphorylation (Fig. 5A and B). The effects on ERK1/2 were mimicked by rhIL-6 in the SKBR3, but not in the MDA-MB-231 cells. In addition, in the SKBR3 cells, rhIL-6 increased AKT phosphorylation. Of note, in both cell lines, CAF-CM, as well as rhIL-6, potently decreased the expression of c-Myc.
Figure 5.
rhIL-6 mimics the CAF-CM growth-inhibitory effects on ERα-negative breast cancer cells. (A and B) Western blot analyses of protein extracts derived from MDA-MB-231 (A) or SKBR3 cells (B) treated with either CAF-CM or rhIL-6 (0.3 µg/ml) or of untreated cells (control). Cells were incubated for 3 days before plasma membrane (PM), cytosolic (CE) and nuclear (NE) proteins were extracted. (C and D) Colony growth assays of MDA-MB-231 and SKBR3 cells after 6 days of incubation in the presence or absence of fulvestrant and exposure to CAF-CM, rhIL-6 (at concentrations as indicated) or with none of these agents (control). Either average colony size (MDA-MB-231) was measured as described in the Materials and methods or average cell number per colony (SKBR3) was determined. (E-H) Viability (E and F) or spheroid size measurement (G and H) of MDA-MB-231 and SKBR3 cells after (E and F) 3 days or (G and H) 1-3 days of exposure to CAF-CM, rhIL-6 (at concentrations as indicated) or with none of these agents (control) in 3D cultures in the presence or absence of fulvestrant. Statistical analyses were done by the Kruskal-Wallis test (C and D) or by ANOVA (E-H) and the Bonferroni post-hoc test. Asterisk(s) indicate statistical significance in comparison to the control. *P<0.05, **P<0.01, ***P<0.005 and ****P<0.001. rhIL-6, recombinant human interleukin 6; CAF-CM, carcinoma-associated fibroblast-conditioned medium; STAT3, signal transducer and activator of transcription 3; CAIX, carbonic anhydrase IX.
Cell growth analysis revealed that both CAF-CM and rhIL-6 reduced the growth of MDA-MB-231and SKBR3 cells in 2D cultures (Fig. 5C and D). With the SKBR3 cells, but not with the MDA-MB-231 cells, this growth-inhibitory effect was also observed in 3D cultures (Fig. 5E and F). In addition, the average sizes of the 3D SKBR3 cell aggregates decreased significantly, while the aggregates of the MDA-MB-231 cells, which form discs rather than spheroids (data not shown), remained unaltered (Fig. 5G and H). In general, the negative effects of CAF-CM on the growth of MDA-MB-231 and SKBR3 cells were more potent than the corresponding effects of rhIL-6.
Collectively, these data demonstrate that rhIL-6 also mimics the majority of the effects of CAF-CM on protein expression in MDA-MB-231 and SKBR3 cells. rhIL-6 also shares the growth-inhibitory effects of CAF-CM on both cell lines, without being as potent as CAF-CM. The decline in growth activity may be linked to the potent reduction in c-Myc expression as inflicted by both agents in these cell lines. In the case of the MDA-MB-231 cells, additionally, the decline in IGF1R and CAIX may play a role here. c-Myc, IGF1R and CAIX have been reported to be key players driving MDA-MB-231 cell proliferation (64-66).
Discussion
The findings of this study suggest that IL-6 is not only a major component of CAF-CM, but also mimics the majority of the effects of CAF-CM on protein expression in both ERα-positive and -negative breast cancer cells (Fig. 6). In agreement with previous reports that IL-6 is a classical activator of STAT3 (24,25,67), this study found that an increase in the p-STAT3 level was a common response of all tested breast cancer cell lines to rhIL-6. This effect was also observed with CAF-CM, suggesting that IL-6 is the general mediator of the STAT3-activating effect of CAF-CM. In most cases, rhIL-6 also shared with CAF-CM the ability to increase the expression of Bcl-3. Bcl-3 gene expression can be upregulated by rhIL-6 through STAT3 (49), which may suggest that the increase in STAT3 activity was responsible for the increase in Bcl-3 expression in response to rhIL-6 and CAF-CM. However, in MCF-7 cells, the CAF-CM-induced decrease in IGFBP5 expression was shown to be the major mediator of Bcl-3 upregulation (13). Since rhIL-6 had no effect on IGFBP5 expression at a lower concentration, it is likely that CAF-CM and rhIL-6 induced Bcl-3 expression through two different mechanisms: CAF-CM by decreasing IGFBP5 expression, rhIL-6 by upregulating STAT3 activity. A downregulatory effect of CAF-CM on the IGFBP5 level was as also observed in BT474 and SKBR3 cells (data not shown). RNA interference confirmed that, also in these cells, the downregulation of IGFBP5 caused an increase in Bcl-3 protein expression (data not shown). Of note, in MDA-MB-231 cells, IGFBP5 RNA was barely detectable (data not shown) and basal STAT3 activity was the highest of all cell lines tested (Fig. S2). In these cells, neither CAF-CM nor rhIL-6 increased the Bcl-3 level.
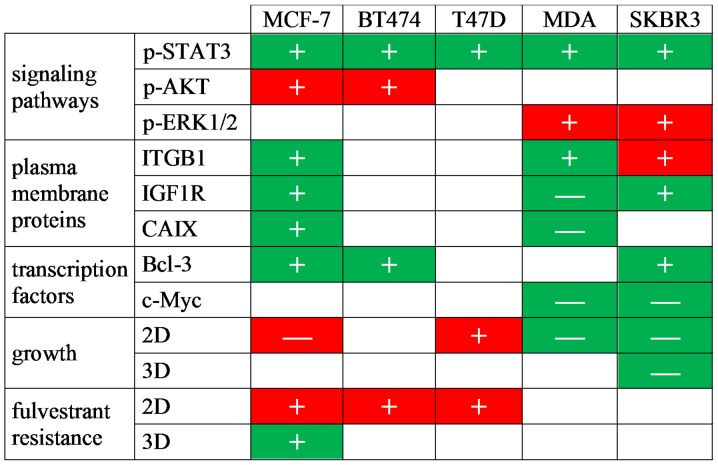
Figure 6.
Summary of the effects of CAF-CM and rhIL-6 on molecular and cellular activities of breast cancer cells. Symbols denote effects induced by 20% CAF-CM (+, upregulation; −, downregulation). Green shading indicates that the effects of CAF-CM can be mimicked by rhIL-6 and red shading indicates that the effects of CAF-CM cannot be mimicked by rhIL-6. MDA, MDA-MB-231 cells; rhIL-6, recombinant human interleukin 6; CAF-CM, carcinoma-associated fibroblast-conditioned medium; STAT3, signal transducer and activator of transcription 3; ITGB1, integrin β1; IGF1R, insulin-like growth factor receptor; CAIX, carbonic anhydrase IX.
There were a number of other effects on protein expression shared by CAF-CM and rhIL-6, which may be related to their abilities to increase the p-STAT3 and Bcl-3 levels. Among these is IGF1R, a major driver of the PI3K pathway in MCF-7 cells and important for ERα-driven proliferation (68,69). In MCF-7 cells, Bcl-3 mediates the CAF-CM-induced expression of IGF1R (13). In SKBR3 cells, the IGF1R levels were also found to increase along with Bcl-3. However, in BT474 and T47D cells, a higher Bcl-3 expression did not coincide with higher IGF1R levels and, in the MDA-MB-231 cells, the IGF1R level even decreased in response to CAF-CM and rhIL-6, while no change in Bcl-3 expression was observed. This suggests that IGF1R expression is regulated by CAF-CM and rhIL-6 in an either Bcl-3-dependent or -independent manner. In tongue squamous cell carcinoma cells, the IGF1R promoter has been reported to be regulated by c-Myc (70). Hence, in MDA-MB-231 cells, the CAF-CM- and rhIL-6-induced loss of c-Myc may be responsible for the decrease in the IGF1R level. However, in the other cell lines tested, c-Myc expression was not associated with IGF1R expression. In MCF-7 cells, c-Myc expression was closely linked to Bcl-3 expression (Fig. 1D), which is consistent with the observation that, in colon cancer cells, Bcl-3 increases the expression and stability of c-Myc (50). However, the CAF-CM- and rhIL-6-induced increase in Bcl-3 expression did not lead to a higher expression of c-Myc (Fig. 1C), arguing against the possibility that in MCF-7 cells, Bcl-3 increased IGF1R expression indirectly through c-Myc.
Another protein, whose expression was modulated by both CAF-CM and rhIL-6 was CAIX, which is important for the growth of MCF-7 and MDA-MB-231 cells (65,71). In MCF-7 cells, Bcl-3 and, to a minor extent, also STAT3 contribute to the CAF-CM-induced increase in CAIX expression (13). The classical activator of CAIX expression is hypoxia-inducible factor 1α (HIF1α) (72). CAF-CM has been shown to induce the expression of HIF1α in MCF-7 cells under normoxic conditions, although not as potently as the hypoxia mimetic, CoCl2 (13). NF-κB, through which Bcl-3 activates genes (16), and STAT3 have been shown to be able to upregulate HIF1α expression under normoxic conditions (73,74). In MDA-MB-231 cells, in which CAF-CM and rhIL-6 increased the p-STAT3 level, while leaving the Bcl-3 level unaffected, CAIX expression decreased (Fig. 5A). This suggests that additional factors are involved in CAIX regulation. In the other cell lines tested, the CAIX levels were not detectable neither in the absence of CAF-CM or rhIL-6 (Fig. S2), nor in their presence (data not shown).
Integrin β1, a protein linked to anti-estrogen resistance (42,53), also exhibits a higher expression in response to CAF-CM and rhIL-6 in some of the breast cancer cell lines tested (MCF-7, SKBR3 and MDA-MB-231). The expression of both the slower migrating, N-glycosylated (75) and the rapidly migrating, unglycosylated forms of integrin β1 were similarly affected, suggesting that CAF-CM and rhIL-6 did not modulate the glycosylation of this protein. The regulation of integrin β1 expression is not yet well understood. In MCF-7 cells, neither STAT3 nor Bcl-3 affect integrin β1 expression positively and exhibit a rather downregulatory effect (13). The transcription factor, forkhead box protein M1 (FoxM1), has recently been identified as a potential regulator of integrin β1 expression in TNBC cells (76). However, in SKBR3 cells, CAF-CM and rhIL-6 decreased the level of FoxM1 (data not shown), while CAF-CM increased the level of integrin β1, and rhIL-6 did not affect integrin β1 expression. This argues against an involvement of FoxM1 in CAF-CM- and rhIL-6-induced integrin β1 expression. Another candidate that potentially may have contributed to the increase in integrin β1 expression is TMEM26. Not only does its knockdown increase integrin β1 expression (75), but CAF-CM also downregulated its expression in MCF-7 cells (Fig. 1F). Furthermore, an inverse association between TMEM26 and integrin β1 levels was also found with BT474 and SKBR3 cells (data not shown). Hence, a downregulated TMEM26 level may have contributed to the stimulatory effect of CAF-CM on integrin β1 expression. However, it does not explain the effect of rhIL-6 on integrin β1, as rhIL-6 fails to downregulate TMEM26 (Fig. 1F).
Although rhIL-6 mimicked almost all the effects of CAF-CM on protein expression, in most cases, it failed to recapitulate the effects of CAF-CM on ERK1/2 and AKT phosphorylation. In the BT474 and T47D cells, CAF-CM decreased and, in the MDA-MB-231 and SKBR3 cells, it increased ERK1/2 phosphorylation, while, only in the SKBR3 cells, rhIL-6 was able to mimic the effect of CAF-CM. Likewise, CAF-CM, but not rhIL-6 increased the phosphorylation of AKT in the MCF-7 and BT474 cells. The failure of rhIL-6 (10 ng/ml) to modulate AKT and ERK1/2 activities in breast cancer cell lines has also been demonstrated by others (40).
The failure to activate the PI3K/AKT pathway in MCF-7 and BT474 cells coincided with the inability of rhIL-6 to mimic CAF-CM-induced fulvestrant resistance in 2D cultures. The authors demonstrate that, in MCF-7 cells, rhIL-6 induced apoptosis in 2D cultures, probably by downregulating ABCG2 expression, and that the apoptotic activity and ABCG2 expression are under the control of the PI3K/AKT pathway. This suggests a connection between the failure of rhIL-6 to activate the PI3K/AKT pathway and its pro-apoptotic activity. Apart from stimulating apoptosis, rhIL-6 is as capable as CAF-CM in stimulating proliferation in the presence of fulvestrant. Hence, a likely scenario is that CAF-CM induces fulvestrant resistance by promoting proliferation, while rhIL-6 fails to foster growth in the presence of fulvestrant by causing a steady-state between cell proliferation and apoptotic cell death. The PI3K/AKT pathway is not only important in regulating apoptosis, but is also able to phosphorylate ERα, thereby rendering the activity of ERα independent of estrogen (11). Furthermore, the PI3K/AKT pathway can activate Bcl-3 (18). These PI3K/AKT pathway-related activities may as well play a role in CAF-CM-induced fulvestrant resistance.
CAF-CM also promoted the growth of T47D cells in the presence of fulvestrant. T47D cells differ from the MCF-7 and BT474 cells, as they exhibit a higher growth activity in response to CAF-CM also in the absence of fulvestrant. The effect on cell growth in the absence of fulvestrant was more potent than that in its presence. Neither of these effects were mimicked by rhIL-6. These findings are supported by those of other studies. It was previously demonstrated that co-cultures with CAFs upregulated the proliferation of T47D cells (77), whereas, at concentrations between 20 pg/ml and 100 ng/ml, rhIL-6 downregulated growth of these cells (56,78,79). The p-AKT levels did not differ in the presence of CAF-CM and rhIL-6 (Fig. 4B), ruling out the possibility that the PI3K/AKT pathway was involved. In addition, both agents upregulated the phosphorylation of STAT3, a factor that T47D cells rely on for growth (80,81), in a similar manner. However, unlike CAF-CM, rhIL-6 potently increased the expression of cytoplasmic p21 and Bcl-3 (Fig. 4B). The activation of NF-κB, as can be induced by Bcl-3 (16), can lead to the reduced growth of T47D cells (82). In addition, IKKβ (IκB kinase β), which activates NF-κB by triggering its dissociation from IκB, was found to cause the cytoplasmic p21 level in breast cancer cells to rise (83). Since cytoplasmic p21 is associated with cellular senescence (54), rhIL-6 may have induced senescence through a Bcl-3/cytoplasmic p21 route. It should be noted that cytoplasmic p21 has also been linked to apoptosis protection (55). However, the data of this study suggest that, at least in MCF-7 cells, the cytoplasmic p21 level is an indicator of proliferative and not apoptotic activity (Fig. 2C).
By summarizing the data on the growth activity of ERα-positive breast cancer cells in 2D cultures, it becomes clear that all the growth-promoting effects of CAF-CM could not be recapitulated by rhIL-6 and must therefore be mediated by a CAF-CM component other than IL-6. By contrast, in 3D cultures, the growth-stimulatory effects of CAF-CM on MCF-7 and AnD5 cells in the presence of fulvestrant were mimicked by rhIL-6 to a large extent (Fig. 3A and B). Of note, the mechanism through which CAF-CM induces fulvestrant resistance differs between 2D and 3D cultures. In 2D cultures, CAF-CM promotes growth in the presence of fulvestrant by stimulating proliferation, whereas in 3D cultures this is achieved by the inhibition of apoptosis. rhIL-6 shares with CAF-CM the anti-apoptotic effect and is therefore also able to induce fulvestrant resistance in 3D cultures. Hence, there are opposite effects of rhIL-6 on apoptosis in 2D vs. 3D cultures, whereby the regulation of apoptosis in 2D cultures is dependent on the PI3K/AKT pathway, whereas, in 3D cultures, it is not. In contrast to 3D-cultured MCF-7 cells, neither the 3D-cultured BT474 nor 3D-cultured T47D cells responded to CAF-CM or rhIL-6 by a considerable change in growth activity. These data again emphasize that responses to external factors can be quite different between 2D and 3D cultures.
In contrast to the ERα-positive breast cancer cell lines tested, the ERα-negative cell lines MDA-MB-231 and SKBR3 responded to CAF-CM in 2D cultures by a decrease in growth activity. The CAF-CM-treated SKBR3 cells also exhibited a lower growth activity in 3D cultures. Depending on the concentration used, all these growth-inhibitory actions were more or less mimicked by rhIL-6. Notably, CAF-CM and rhIL-6 potently decreased c-Myc expression in both cell lines. Since c-Myc is essential for cell cycling in MDA-MB-231, as well as in SKBR3 cells (66,84), the loss of c-Myc is the likely reason for the reduced growth activity as inflicted by CAF-CM and rhIL-6. The loss of c-Myc does not seem to be connected to Bcl-3. In the SKBR3 cells, the c-Myc levels were decreased, while the Bcl-3 levels were increased; in the MDA-MB-231 cells, the c-Myc levels decreased, while the Bcl-3 levels remained unaltered. In the MDA-MB-231 cells, the loss of c-Myc may be related to the decrease in the levels of IGF1R and CAIX, which are both important for MDA-MB-231 cell proliferation (64,65).
Differences in the reactivity to CAF-CM and rhIL-6 in terms of growth activity in 2D as opposed to 3D culture have also been found with MDA-MB-231 cells, demonstrating that ERα-negative breast cancer cells are react differently between 2D and 3D cultures. This was also shown in a previous study, in which the B-Raf inhibitor, RAF-265, was found to be more effective in inhibiting the growth of 3D-cultured than that of 2D-cultured MDA-MB-231 cells (60). Likewise, the growth inhibition of SKBR3 cells by the Her2 inhibitor, lapatinib, has been shown to be more pronounced in 3D as compared to 2D cultures (85).
Although the stroma is the major source of IL-6 expression in primary breast cancers (86), breast cancer cells also secrete IL-6, whereby TNBC cells produce the highest amounts (56,62,87). For instance, MDA-MB-231 cells secrete a ~1,000-fold greater amount of IL-6 than MCF-7 cells (88). MDA-MB-231 cells use the endogenous production of IL-6 to stimulate a IL-6/IL-6 receptor loop for keeping STAT3 activity high (63). In fact, their p-STAT3 level was the highest of all p-STAT3 levels in the breast cancer cell lines we have tested (Fig. S2). Nevertheless, CAF-CM and rhIL-6 were able to further increase STAT3 activity (Fig. 5A) suggesting that the amount of IL-6 they secrete does not reach the maximum concentration for optimal stimulation of STAT3 activity. The inhibition of the IL-6/IL-6R loop or STAT3 activity has been shown to lead to the decreased proliferation and the increased apoptosis of MDA-MB-231 cells (63,89). On the other hand, the addition of rhIL-6 to MDA-MB-231 cells has been demonstrated to decrease proliferation, while not having any effect on apoptosis (56). In this study, rhIL-6 also acted growth-inhibitory on MDA-MB-231 cells in 2D cultures. As discussed above, the downregulation of the c-Myc, IGF1R and CAIX levels may be the reasons for the rhIL-6-induced growth inhibition.
IL-6 plays a role in tissue remodeling and can stimulate both reprogramming and senescence (90). In this study, at the concentrations of 0.1 and 0.3 µg/ml, rhIL-6 was found to recapitulate the effects of CAF-CM on the expression of CSC-related ALDH1A1 and ALDH3A1 in MCF-7 cells (Fig. 1E). This is in agreement with earlier findings indicating that 0.1 µg/ml rhIL-6 increased ALDEFLOUR activity in SUM159 cells (37).
In conclusion, the findings of this study suggest that, in all cell lines tested, CAF-CM component IL-6 mediates CAF-CM-induced STAT3 phosphorylation and the majority of the changes in protein expression pattern. However, almost all CAF-CM-induced alterations in ERK1/2 and AKT activities are not mediated by IL-6. The failure to activate AKT seems to be linked to its inability to mimic CAF-CM-induced fulvestrant resistance in common adherent 2D cultures. Yet, it likely mediates CAF-CM-induced fulvestrant resistance in 3D cultures. This suggests that the ability of IL-6 to participate in the acquisition of fulvestrant resistance depends on culture conditions. Notably, IL-6 acts in a pro-apoptotic manner in 2D and in an anti-apoptotic manner in 3D cultures. Differences in the apoptotic activities of cells in 2D and 3D cultures have also been found by others (91). Tissue architecture has a potent effect on the behavior of cells. One reason may be that cells become polarized in 3D cultures, but not in 2D cultures. Given that cells in tissues are organized in three and not in two dimensions, the data obtained with 3D cultures are more likely to resemble the in vivo situation. The Kaplan-Meier-Plotter (http://kmplot.com/analysis/index.php?p=service&default=true) allows the assessment in-silico of the importance of IL-6 on the outcome of patients based on its mRNA level. Such a survival analysis of 494 ERα+/Her2− breast cancer samples for IL-6 mRNA revealed no significant difference in relapse-free survival at high and low levels of IL-6 mRNA (data not shown). However, IL-6 mRNA levels may not necessarily reflect the levels of secreted IL-6. Therefore, future studies on primary ERα+ breast cancer samples, comparing low and high IL-6 protein expresser, are warranted.
Supplementary Data
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
AD performed most of the growth assays and RNA interference experiments and carried out Western blot analyses. TL performed most of the Western blot analyses and contributed to the growth assay studies. BL performed antibody arrays and RT-qPCR analyses. AD, TL and BL contributed to the interpretation of the results. JD designed the study, interpreted the data and wrote the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, et al. Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011;5:5–23. doi: 10.1016/j.molonc.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hudis CA. Trastuzumab - mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 5.Omarini C, Guaitoli G, Pipitone S, Moscetti L, Cortesi L, Cascinu S, Piacentini F. Neoadjuvant treatments in triple-negative breast cancer patients: Where we are now and where we are going. Cancer Manag Res. 2018;10:91–103. doi: 10.2147/CMAR.S146658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner J, Rapsomaniki MA, Chevrier S, Anzeneder T, Langwieder C, Dykgers A, Rees M, Ramaswamy A, Muenst S, Soysal SD, et al. A Single-Cell Atlas of the Tumor and Immune Ecosystem of Human Breast Cancer. Cell. 2019;177:1330–1345.e18. doi: 10.1016/j.cell.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong D, Fritz AJ, Zaidi SK, van Wijnen AJ, Nickerson JA, Imbalzano AN, Lian JB, Stein JL, Stein GS. Epithelial-to-mesenchymal transition and cancer stem cells contribute to breast cancer heterogeneity. J Cell Physiol. 2018;233:9136–9144. doi: 10.1002/jcp.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dittmer J. Breast cancer stem cells: Features, key drivers and treatment options. Semin. Cancer Biol. 2018;53:59–74. doi: 10.1016/j.semcancer.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Bailey TA, Luan H, Clubb RJ, Naramura M, Band V, Raja SM, Band H. Mechanisms of Trastuzumab resistance in ErbB2-driven breast cancer and newer opportunities to overcome therapy resistance. J Carcinog. 2011;10:28. doi: 10.4103/1477-3163.90442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–643. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 11.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–247. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dittmer J, Leyh B. The impact of tumor stroma on drug response in breast cancer. Semin Cancer Biol. 2015;31:3–15. doi: 10.1016/j.semcancer.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Leyh B, Dittmer A, Lange T, Martens JW, Dittmer J. Stromal cells promote anti-estrogen resistance of breast cancer cells through an insulin-like growth factor binding protein 5 (IGFBP5)/B-cell leukemia/lymphoma 3 (Bcl-3) axis. Oncotarget. 2015;6:39307–39328. doi: 10.18632/oncotarget.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pratt MA, Bishop TE, White D, Yasvinski G, Ménard M, Niu MY, Clarke R. Estrogen withdrawal-induced NF-kappaB activity and bcl-3 expression in breast cancer cells: Roles in growth and hormone independence. Mol Cell Biol. 2003;23:6887–6900. doi: 10.1128/MCB.23.19.6887-6900.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Cao X, Sun X, Lei R, Chen P, Zhao Y, Jiang Y, Yin J, Chen R, Ye D, et al. Bcl-3 regulates TGFβ signaling by stabi-lizing Smad3 during breast cancer pulmonary metastasis. Cell Death Dis. 2016;7:e2508. doi: 10.1038/cddis.2016.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuster M, Annemann M, Plaza-Sirvent C, Schmitz I. Atypical IκB proteins - nuclear modulators of NF-κB signaling. Cell Commun Signal. 2013;11:23. doi: 10.1186/1478-811X-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sas L, Lardon F, Vermeulen PB, Hauspy J, Van Dam P, Pauwels P, Dirix LY, Van Laere SJ. The interaction between ER and NFκB in resistance to endocrine therapy. Breast Cancer Res. 2012;14:212. doi: 10.1186/bcr3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang VY, Li Y, Kim D, Zhong X, Du Q, Ghassemian M, Ghosh G. Bcl3 Phosphorylation by Akt, Erk2, and IKK is required for its transcriptional activity. Mol Cell. 2017;67:484–497.e5. doi: 10.1016/j.molcel.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baxter RC. IGF binding proteins in cancer: Mechanistic and clinical insights. Nat Rev Cancer. 2014;14:329–341. doi: 10.1038/nrc3720. [DOI] [PubMed] [Google Scholar]
- 20.Mita K, Zhang Z, Ando Y, Toyama T, Hamaguchi M, Kobayashi S, Hayashi S, Fujii Y, Iwase H, Yamashita H. Prognostic significance of insulin-like growth factor binding protein (IGFBP)-4 and IGFBP-5 expression in breast cancer. Jpn J Clin Oncol. 2007;37:575–582. doi: 10.1093/jjco/hym066. [DOI] [PubMed] [Google Scholar]
- 21.Becker MA, Hou X, Harrington SC, Weroha SJ, Gonzalez SE, Jacob KA, Carboni JM, Gottardis MM, Haluska P. IGFBP ratio confers resistance to IGF targeting and correlates with increased invasion and poor outcome in breast tumors. Clin Cancer Res. 2012;18:1808–1817. doi: 10.1158/1078-0432.CCR-11-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West NR. Coordination of Immune-Stroma Crosstalk by IL-6 Family Cytokines. Front Immunol. 2019;10:1093. doi: 10.3389/fimmu.2019.01093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boulanger MJ, Chow DC, Brevnova EE, Garcia KC. Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science. 2003;300:2101–2104. doi: 10.1126/science.1083901. [DOI] [PubMed] [Google Scholar]
- 24.Schaper F, Rose-John S. Interleukin-6: Biology, signaling and strategies of blockade. Cytokine Growth Factor Rev. 2015;26:475–487. doi: 10.1016/j.cytogfr.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Avalle L, Pensa S, Regis G, Novelli F, Poli V. STAT1 and STAT3 in tumorigenesis: A matter of balance. JAK-STAT. 2012;1:65–72. doi: 10.4161/jkst.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacina L, Brábek J, Král V, Kodet O, Smetana K., Jr Interleukin-6: A molecule with complex biological impact in cancer. Histol Histopathol. 2019;34:125–136. doi: 10.14670/HH-18-033. [DOI] [PubMed] [Google Scholar]
- 28.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Morrow RJ, Etemadi N, Yeo B, Ernst M. Challenging a Misnomer? The Role of Inflammatory Pathways in Inflammatory Breast Cancer. Mediators Inflamm. 2017;2017:4754827. doi: 10.1155/2017/4754827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghandadi M, Sahebkar A. Interleukin-6: A Critical Cytokine in Cancer Multidrug Resistance. Curr Pharm Des. 2016;22:518–526. doi: 10.2174/1381612822666151124234417. [DOI] [PubMed] [Google Scholar]
- 31.Kim SY, Kang JW, Song X, Kim BK, Yoo YD, Kwon YT, Lee YJ. Role of the IL-6-JAK1-STAT3-Oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cells. Cell Signal. 2013;25:961–969. doi: 10.1016/j.cellsig.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, Oberyszyn TM, Hall BM. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yadav A, Kumar B, Datta J, Teknos TN, Kumar P. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol Cancer Res. 2011;9:1658–1667. doi: 10.1158/1541-7786.MCR-11-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brooks MD, Burness ML, Wicha MS. Therapeutic Implications of Cellular Heterogeneity and Plasticity in Breast Cancer. Cell Stem Cell. 2015;17:260–271. doi: 10.1016/j.stem.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korkaya H, Kim GI, Davis A, Malik F, Henry NL, Ithimakin S, Quraishi AA, Tawakkol N, D'Angelo R, Paulson AK, et al. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol Cell. 2012;47:570–584. doi: 10.1016/j.molcel.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saha S, Mukherjee S, Khan P, Kajal K, Mazumdar M, Manna A, Mukherjee S, De S, Jana D, Sarkar DK, et al. Aspirin Suppresses the Acquisition of Chemoresistance in Breast Cancer by Disrupting an NFκB-IL6 Signaling Axis Responsible for the Generation of Cancer Stem Cells. Cancer Res. 2016;76:2000–2012. doi: 10.1158/0008-5472.CAN-15-1360. [DOI] [PubMed] [Google Scholar]
- 37.Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, Korkaya H, Heath A, Dutcher J, Kleer CG, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71:614–624. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dittmer J. Mechanisms governing metastatic dormancy in breast cancer. Semin Cancer Biol. 2017;44:72–82. doi: 10.1016/j.semcancer.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Zhang GJ, Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res. 1999;19(2B):1427–1432. [PubMed] [Google Scholar]
- 40.Casneuf T, Axel AE, King P, Alvarez JD, Werbeck JL, Verhulst T, Verstraeten K, Hall BM, Sasser AK. Interleukin-6 is a potential therapeutic target in interleukin-6 dependent, estrogen receptor-a-positive breast cancer. Breast Cancer (Dove Med Press) 2016;8:13–27. doi: 10.2147/BCTT.S92414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang W, Guo J, Li S, Ma T, Xu D, Han C, Liu F, Yu W, Kong L. Discovery of monocarbonyl curcumin-BTP hybrids as STAT3 inhibitors for drug-sensitive and drug-resistant breast cancer therapy. Sci Rep. 2017;7:46352. doi: 10.1038/srep46352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dittmer A, Dittmer J. Long-term exposure to carcinoma-associated fibroblasts makes breast cancer cells addictive to integrin β1. Oncotarget. 2018;9:22079–22094. doi: 10.18632/oncotarget.25183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dittmer A, Dittmer J. Beta-actin is not a reliable loading control in Western blot analysis. Electrophoresis. 2006;27:2844–2845. doi: 10.1002/elps.200500785. [DOI] [PubMed] [Google Scholar]
- 44.Moritz CP. Tubulin or Not Tubulin: Heading Toward Total Protein Staining as Loading Control in Western Blots. Proteomics. 2017;17:17. doi: 10.1002/pmic.201600189. [DOI] [PubMed] [Google Scholar]
- 45.Dittmer A, Schunke D, Dittmer J. PTHrP promotes homotypic aggregation of breast cancer cells in three-dimensional cultures. Cancer Lett. 2008;260:56–61. doi: 10.1016/j.canlet.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 46.Oerlecke I, Bauer E, Dittmer A, Leyh B, Dittmer J. Cyclic AMP enhances TGFβ responses of breast cancer cells by upregulating TGFβ receptor I expression. PLoS One. 2013;8:e54261. doi: 10.1371/journal.pone.0054261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 48.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 49.Brocke-Heidrich K, Ge B, Cvijic H, Pfeifer G, Löffler D, Henze C, McKeithan TW, Horn F. BCL3 is induced by IL-6 via Stat3 binding to intronic enhancer HS4 and represses its own transcription. Oncogene. 2006;25:7297–7304. doi: 10.1038/sj.onc.1209711. [DOI] [PubMed] [Google Scholar]
- 50.Liu Z, Jiang Y, Hou Y, Hu Y, Cao X, Tao Y, Xu C, Liu S, Wang S, Wang L, et al. The IκB family member Bcl-3 stabilizes c-Myc in colorectal cancer. J Mol Cell Biol. 2013;5:280–282. doi: 10.1093/jmcb/mjt020. [DOI] [PubMed] [Google Scholar]
- 51.Bretones G, Delgado MD, León J. Myc and cell cycle control. Biochim Biophys Acta. 2015;1849:506–516. doi: 10.1016/j.bbagrm.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 52.Massoumi R, Chmielarska K, Hennecke K, Pfeifer A, Fässler R. Cyld inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-kappaB signaling. Cell. 2006;125:665–677. doi: 10.1016/j.cell.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 53.Pontiggia O, Sampayo R, Raffo D, Motter A, Xu R, Bissell MJ, Joffé EB, Simian M. The tumor microenvironment modulates tamoxifen resistance in breast cancer: A role for soluble stromal factors and fibronectin through β1 integrin. Breast Cancer Res Treat. 2012;133:459–471. doi: 10.1007/s10549-011-1766-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sánchez-Pérez Y, Chirino YI, Osornio-Vargas AR, Herrera LA, Morales-Bárcenas R, López-Saavedra A, González-Ramírez I, Miranda J, García-Cuellar CM. Cytoplasmic p21(CIP1/WAF1), ERK1/2 activation, and cytoskeletal remodeling are associated with the senescence-like phenotype after airborne particulate matter (PM(10)) exposure in lung cells. Toxicol Lett. 2014;225:12–19. doi: 10.1016/j.toxlet.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 55.Yousefi B, Rahmati M, Ahmadi Y. The roles of p53R2 in cancer progression based on the new function of mutant p53 and cytoplasmic p21. Life Sci. 2014;99:14–17. doi: 10.1016/j.lfs.2014.01.063. [DOI] [PubMed] [Google Scholar]
- 56.Chiu JJ, Sgagias MK, Cowan KH. Interleukin 6 acts as a paracrine growth factor in human mammary carcinoma cell lines. Clin Cancer Res. 1996;2:215–221. [PubMed] [Google Scholar]
- 57.Kathawala RJ, Gupta P, Ashby CRJ, Jr, Chen ZS. The modulation of ABC transporter-mediated multidrug resistance in cancer: A review of the past decade. Drug Resist Updat. 2015;18:1–17. doi: 10.1016/j.drup.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DuFort CC, Paszek MJ, Weaver VM. Balancing forces: Architectural control of mechanotransduction. Nat Rev Mol Cell Biol. 2011;12:308–319. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dittmer A, Fuchs A, Oerlecke I, Leyh B, Kaiser S, Martens JW, Lützkendorf J, Müller L, Dittmer J. Mesenchymal stem cells and carcinoma-associated fibroblasts sensitize breast cancer cells in 3D cultures to kinase inhibitors. Int J Oncol. 2011;39:689–696. doi: 10.3892/ijo.2011.1073. [DOI] [PubMed] [Google Scholar]
- 61.Dittmer A, Hohlfeld K, Lützkendorf J, Müller LP, Dittmer J. Human mesenchymal stem cells induce E-cadherin degradation in breast carcinoma spheroids by activating ADAM10. Cell Mol Life Sci. 2009;66:3053–3065. doi: 10.1007/s00018-009-0089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM. Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. FASEB J. 2007;21:3763–3770. doi: 10.1096/fj.07-8832com. [DOI] [PubMed] [Google Scholar]
- 63.Bharti R, Dey G, Ojha PK, Rajput S, Jaganathan SK, Sen R, Mandal M. Diacerein-mediated inhibition of IL-6/IL-6R signaling induces apoptotic effects on breast cancer. Oncogene. 2016;35:3965–3975. doi: 10.1038/onc.2015.466. [DOI] [PubMed] [Google Scholar]
- 64.Klinakis A, Szabolcs M, Chen G, Xuan S, Hibshoosh H, Efstratiadis A. Igf1r as a therapeutic target in a mouse model of basal-like breast cancer. Proc Natl Acad Sci USA. 2009;106:2359–2364. doi: 10.1073/pnas.0810221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ward C, Meehan J, Mullen P, Supuran C, Dixon JM, Thomas JS, Winum JY, Lambin P, Dubois L, Pavathaneni NK, et al. Evaluation of carbonic anhydrase IX as a therapeutic target for inhibition of breast cancer invasion and metastasis using a series of in vitro breast cancer models. Oncotarget. 2015;6:24856–24870. doi: 10.18632/oncotarget.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen CR, Kang Y, Massagué J. Defective repression of c-myc in breast cancer cells: A loss at the core of the transforming growth factor beta growth arrest program. Proc Natl Acad Sci USA. 2001;98:992–999. doi: 10.1073/pnas.98.3.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Friedrich K, Dolznig H, Han X, Moriggl R. Steering of carcinoma progression by the YIN/YANG interaction of STAT1/STAT3. Biosci Trends. 2017;11:1–8. doi: 10.5582/bst.2016.01250. [DOI] [PubMed] [Google Scholar]
- 68.Marconett CN, Singhal AK, Sundar SN, Firestone GL. Indole-3-carbinol disrupts estrogen receptor-alpha dependent expression of insulin-like growth factor-1 receptor and insulin receptor substrate-1 and proliferation of human breast cancer cells. Mol Cell Endocrinol. 2012;363:74–84. doi: 10.1016/j.mce.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gaben AM, Sabbah M, Redeuilh G, Bedin M, Mester J. Ligand-free estrogen receptor activity complements IGF1R to induce the proliferation of the MCF-7 breast cancer cells. BMC Cancer. 2012;12:291. doi: 10.1186/1471-2407-12-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun J, Lu Z, Deng Y, Wang W, He Q, Yan W, Wang A. Up-regulation of INSR/IGF1R by C-myc promotes TSCC tumorigenesis and metastasis through the NF-κB pathway. Biochim Biophys Acta Mol Basis Dis. 2018;1864(5 Pt A):1873–1882. doi: 10.1016/j.bbadis.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 71.Ansari MF, Idrees D, Hassan MI, Ahmad K, Avecilla F, Azam A. Design, synthesis and biological evaluation of novel pyridine-thiazolidinone derivatives as anticancer agents: Targeting human carbonic anhydrase IX. Eur J Med Chem. 2018;144:544–556. doi: 10.1016/j.ejmech.2017.12.049. [DOI] [PubMed] [Google Scholar]
- 72.Sedlakova O, Svastova E, Takacova M, Kopacek J, Pastorek J, Pastorekova S. Carbonic anhydrase IX, a hypoxia-induced catalytic component of the pH regulating machinery in tumors. Front Physiol. 2014;4:400. doi: 10.3389/fphys.2013.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17:2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- 74.Wang K, Zhu X, Zhang K, Yin Y, Chen Y, Zhang T. Interleukin-6 contributes to chemoresistance in MDA-MB-231 cells via targeting HIF-1a. J Biochem Mol Toxicol. 2018;32:e22039. doi: 10.1002/jbt.22039. [DOI] [PubMed] [Google Scholar]
- 75.Nass N, Dittmer A, Hellwig V, Lange T, Beyer JM, Leyh B, Ignatov A, Weiβenborn C, Kirkegaard T, Lykkesfeldt AE, et al. Expression of transmembrane protein 26 (TMEM26) in breast cancer and its association with drug response. Oncotarget. 2016;7:38408–38426. doi: 10.18632/oncotarget.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamurcu Z, Kahraman N, Ashour A, Ozpolat B. FOXM1 transcriptionally regulates expression of integrin β1 in triple-negative breast cancer. Breast Cancer Res Treat. 2017;163:485–493. doi: 10.1007/s10549-017-4207-7. [DOI] [PubMed] [Google Scholar]
- 77.Gache C, Berthois Y, Martin PM, Saez S. Positive regulation of normal and tumoral mammary epithelial cell proliferation by fibroblasts in coculture. In. Vitro Cell Dev Biol Anim. 1998;34:347–351. doi: 10.1007/s11626-998-0012-2. [DOI] [PubMed] [Google Scholar]
- 78.Chen L, Shulman LM, Revel M. IL-6 receptors and sensitivity to growth inhibition by IL-6 in clones of human breast carcinoma cells. J Biol Regul Homeost Agents. 1991;5:125–136. [PubMed] [Google Scholar]
- 79.Badache A, Hynes NE. Interleukin 6 inhibits proliferation and, in cooperation with an epidermal growth factor receptor autocrine loop, increases migration of T47D breast cancer cells. Cancer Res. 2001;61:383–391. [PubMed] [Google Scholar]
- 80.Ikeda O, Sekine Y, Mizushima A, Nakasuji M, Miyasaka Y, Yamamoto C, Muromoto R, Nanbo A, Oritani K, Yoshimura A, Matsuda T, et al. Interactions of STAP-2 with Brk and STAT3 participate in cell growth of human breast cancer cells. J Biol Chem. 2010;285:38093–38103. doi: 10.1074/jbc.M110.162388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Proietti C, Salatino M, Rosemblit C, Carnevale R, Pecci A, Kornblihtt AR, Molinolo AA, Frahm I, Charreau EH, Schillaci R, et al. Progestins induce transcriptional activation of signal transducer and activator of transcription 3 (Stat3) via a Jak- and Src-dependent mechanism in breast cancer cells. Mol Cell Biol. 2005;25:4826–4840. doi: 10.1128/MCB.25.12.4826-4840.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Segev DL, Ha TU, Tran TT, Kenneally M, Harkin P, Jung M, MacLaughlin DT, Donahoe PK, Maheswaran S. Mullerian inhibiting substance inhibits breast cancer cell growth through an NFkappa B-mediated pathway. J Biol Chem. 2000;275:28371–28379. doi: 10.1074/jbc.M004554200. [DOI] [PubMed] [Google Scholar]
- 83.Ping B, He X, Xia W, Lee DF, Wei Y, Yu D, Mills G, Shi D, Hung MC. Cytoplasmic expression of p21CIP1/WAF1 is correlated with IKKβ overexpression in human breast cancers. Int J Oncol. 2006;29:1103–1110. [PubMed] [Google Scholar]
- 84.Neve RM, Sutterlüty H, Pullen N, Lane HA, Daly JM, Krek W, Hynes NE. Effects of oncogenic ErbB2 on G1 cell cycle regulators in breast tumour cells. Oncogene. 2000;19:1647–1656. doi: 10.1038/sj.onc.1203470. [DOI] [PubMed] [Google Scholar]
- 85.Weigelt B, Lo AT, Park CC, Gray JW, Bissell MJ. HER2 signaling pathway activation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D micro-environment. Breast Cancer Res Treat. 2010;122:35–43. doi: 10.1007/s10549-009-0502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hugo HJ, Lebret S, Tomaskovic-Crook E, Ahmed N, Blick T, Newgreen DF, Thompson EW, Ackland ML. Contribution of Fibroblast and Mast Cell (Afferent) and Tumor (Efferent) IL-6 Effects within the Tumor Microenvironment. Cancer Microenviron. 2012;5:83–93. doi: 10.1007/s12307-012-0098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mao Y, Zhang Y, Qu Q, Zhao M, Lou Y, Liu J, Huang O, Chen X, Wu J, Shen K. Cancer-associated fibroblasts induce trastuzumab resistance in HER2 positive breast cancer cells. Mol Biosyst. 2015;11:1029–1040. doi: 10.1039/C4MB00710G. [DOI] [PubMed] [Google Scholar]
- 88.Gallo M, Frezzetti D, Roma C, Chicchinelli N, Barbieri A, Arra C, Scognamiglio G, Botti G, De Luca A, Normanno N. RANTES and IL-6 cooperate in inducing a more aggressive phenotype in breast cancer cells. Oncotarget. 2018;9:17543–17553. doi: 10.18632/oncotarget.24784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singh S, Murillo G, Chen D, Parihar AS, Mehta RG. Suppression of Breast Cancer Cell Proliferation by Selective Single-Domain Antibody for Intracellular STAT3. Breast Cancer (Auckl) 2018;12:1178223417750858. doi: 10.1177/1178223417750858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mosteiro L, Pantoja C, de Martino A, Serrano M. Senescence promotes in vivo reprogramming through p16INK4a and IL-6. Aging Cell. 2018;17:17. doi: 10.1111/acel.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Correia AL, Bissell MJ. The tumor microenvironment is a dominant force in multidrug resistance. Drug Resist Updat. 2012;15:39–49. doi: 10.1016/j.drup.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.