Abstract
Objective:
Determine the frequency of genital HIV-1 shedding in a large cohort of women on long-term suppressive antiretroviral therapy (ART) and its association with mucosal inflammation.
Design:
We measured levels of HIV-1 RNA and inflammation biomarkers in cervicovaginal lavage (CVL) from HIV-seropositive women enrolled in the Women’s Interagency HIV Study (WIHS).
Methods:
HIV-1 was quantified (Abbott RealTime HIV-1 assay) from CVL samples of 332 WIHS participants with and without clinical evidence of genital inflammation at the time of CVL collection; participants had suppressed plasma viral load (PVL) (limit of quantitation <20–4000 copies/ml depending on year of collection) for a median of 7.1 years (interquartile range=3.4–9.8, Group 1) or for a median of 1.0 years (IQR=0.5–1.0, Group 2). Twenty-two biomarkers of inflammation were measured in CVL to compare with clinical markers.
Results:
HIV-1 was detected in 47% of 38 pre-ART CVL samples (median 668 copies/ml) and detection in CVL was associated with higher pre-ART PVL. HIV-1 was detected in only 1 of 38 CVL samples from these women on suppressive antiretroviral therapy for one year. No HIV-1 RNA was detected in 294 CVL samples from a cross-sectional set of women with suppressed PVL for a median of 7 years. Clinical inflammation markers were correlated with inflammatory biomarkers in CVL specimens, although genital inflammation was not associated with measurable genital HIV-1 shedding in these WIHS participants on ART.
Conclusions:
ART that suppresses HIV-1 in the plasma of women also prevents genital tract HIV-1 shedding, even in the presence of genital tract inflammation.
Keywords: suppressed, women, WIHS, cervicovaginal lavage, genital inflammation, genital HIV-1 shedding, inflammation biomarkers
Introduction
HIV-1 shedding in the female genital tract is the source of virus for female-to-male sexual transmission [1]. Women with plasma viremia shed HIV-1 from the genital tract; studies have detected HIV-1 in the genital tract of 20–80% of women with plasma viremia [2-7]. HIV-1 is more likely to be detected in the genital tract of women with higher plasma viral loads (PVLs) than in women with low PVL [2,5,8-10]. Sexually transmitted infections and genital inflammation also appear to increase genital HIV-1 shedding [5,11]. Even when PVL is undetectable in women on antiretroviral therapy (ART), genital shedding has been detected in 2–34% of samples [4,7,11-14]. The design and methods of these studies have varied substantially with respect to duration and durability of PVL suppression, anatomic location of sampling (cervix versus vagina), and sampling technique.
ART leads to undetectable levels of HIV-1 in the blood and virtually eliminates the risk of sexual transmission [15,16]. Accordingly, public health agencies now promote the message that people whose PVL is undetectable have zero risk of sexually transmitting HIV-1 to others (Undetectable = Untransmittable, U=U) [17]. Questions persist, however, concerning the amount of virus present in the genital tract of women who receive suppressive ART. There are clinical implications for both sexual transmission and perinatal transmission of HIV-1, particularly in the setting of genital tract infection or inflammation. We evaluated the frequency and amount of HIV-1 shedding from the genital tract of women with long-term PVL suppression among participants in the Women’s Interagency HIV Study (WIHS), a well-characterized cohort of women living with or at risk for HIV infection.
Methods
Recruitment, data collection, and characteristics of WIHS participants have been previously reported [18-20]. Study visits occur every 6 months and include quantitation of PVL, clinical evaluation of genital inflammation, and collection of cervicovaginal lavage (CVL) using 10 ml of saline.
Study Groups:
We selected two different groups of WIHS participants for our present study. Women in Group 1 (n=294) were selected because they presented with suppressed plasma viremia, had longitudinal PVL and CVL samples available for five consecutive study visits post suppression, and exhibited signs of clinical inflammation in the vaginal tract during 1 or more of those 5 study visits. Clinical inflammation was defined as being positive for at least 1 of the following 7 inflammatory conditions: vaginal pH>5.5 (suggestive of bacterial vaginosis), visible cervical lesions, cervical ectopy, cervical friability, cervical exudate, trichomoniasis (diagnosed by wet mount), and inflammation noted on Pap smear (interpreted centrally for the WIHS). Upon identification of these women, one of the five longitudinal CVL samples (usually the third, or midpoint, visit) was selected for testing of genital HIV shedding. The 294 women had PVL suppressed for a median of 7.1 years (range=0.2–17.0 years, IQR=3.4–9.8 years). The Group 1 samples were collected between 2002 and 2012, during which time the lower limit of quantification (LLQ) for plasma viremia decreased from 80 cp/ml (bioMerieux NucliSens) to 20 cp/ml (Roche TaqMan) between 2002 and 2012.
Group 2 comprised 38 HIV-positive women who were selected if their CVL specimens were available at the visit prior to ART initiation and at the 1-year visit after PVL suppression (approximately 1.5 years after the first sample). These samples were collected from women between 1995 and 2013, during which the LLQ for PVL testing decreased from 4000 cp/ml (NASBA) to 20 cp/ml (Roche TaqMan). Group 2 women were further selected to be in four subgroups based on the number of clinical inflammation markers at the pre-ART and one-year-suppressed visits, having: (i) at least one clinical inflammation marker at both visits (Inflammation/Inflammation or I/I; n=10), (ii) at least one marker at only the pre-ART visit (Inflammation/No inflammation or I/N; n=10), (iii) at least one marker at only the one-year-suppressed visit (No inflammation/Inflammation or N/I; n=10), or (iv) no markers at either visit (No inflammation/No inflammation or N/N; n=8). These inflammation criteria and CVL availability constrained the number of women who could be included, so we did not expand the number of women in Group 2 beyond these 38. Unfractionated CVL samples from both time points were tested for genital shedding of HIV.
HIV-1 RNA quantitation in CVL:
The optimization of HIV-1 RNA quantitation in unfractionated CVL samples is described in the Supplemental Materials. CVL specimens were tested for HIV-1 RNA levels with the Abbott RealTime HIV-1 Assay after pre-treatment with proteinase K. Specifically, 0.06 ml Abbott Proteinase K (catalog# 03L78–060) was added to 0.74 ml CVL and manually mixed by pipetting, incubated at 53°C for 20 min, vortexed briefly, and spun at 3200g for 5 min. The mixture was run on the Abbott m2000sp with the 0.6 ml plasma program, and quantified on the Abbott m2000rt. Results were corrected for the small dilution, so the LLQ was 42 cp/ml.
Biomarker testing:
Inflammation biomarkers were quantified in CVL from Group 2 women using a Milliplex 17-plex kit (Millipore) run on Luminex MagPix (GM-CSF, IFN-γ, IL-10, IL-12p40, IL-12p70, IL-15, IL-1RA, IL-1α, IL-2, IL-4, IL-6, IL-8, IP10, MCP-1, MIP-1β, RANTES, and TNFα). Five additional biomarkers were tested by ELISA: CD163 (R&D Systems), SLPI (R&D Systems), IFN-α (PBL Assay Science), and beta-defensins 2 and 3 (Assay Biotech).
Statistical analysis:
The unadjusted association between log10 PVL and detectable viral load (VL) in CVL was analyzed using logistic regression to estimate an odds ratio (OR), and by Spearman rank correlation. Likewise, logistic regression was used to assess the association between clinical inflammation marker category (0, 1, or 2+ markers) and detectable VL in CVL; a 3-category functional form was selected using visual assessment of the model fit. Associations between the number of clinical markers (range: 0 to 5) and biomarker levels were evaluated using Kendall’s tau-b correlation coefficient at the pre-ART and post-ART time points, separately. To account for conducting 42 statistical tests (21 evaluated biomarkers x 2 time points), a Benjamini-Hochberg false discovery rate (FDR) p-value adjustment was applied. At the pre-ART visit, biomarker levels were compared by viral shedding status (detectable vs. not detectable) using a Wilcoxon rank-sum test and a Hodges-Lehmann 95% confidence interval (CI) for location shift; a Benjamini-Hochberg FDR p-value adjustment was applied. Left and right censoring of biomarker levels was handled by setting values below or above the cutoffs to the lowest or highest rank, respectively. IFNα was removed from the analysis due to a lack of variability
Results
Absence of HIV-1 shedding in CVL in women on suppressive ART for a median of 7 years.
The women in Group 1 were aged 25–74 years, with a median of 45 years (IQR=40–51 years); 52% were pre-menopausal, 12% peri-menopausal, and 36% post-menopausal (all self-reported) and had suppressed PVL for a median of 7.1 years (IQR=3.4–9.8 years). HIV-1 shedding has been associated with genital tract inflammation [5,11], and, therefore, we purposely biased our sample selection toward women on suppressive ART who showed clinical evidence of inflammation. Two-thirds of the Group 1 women (193 of 294, 66%) presented with at least 1 of the 7 clinical markers of inflammation (see Methods) at the tested visit (108 with exudate, 79 with vaginal pH<5.5, 47 with inflammation noted on Pap, 26 with cervical friability, 9 with cervical ectopy, 8 with trichomoniasis, and 6 with cervical lesions). However, HIV-1 shedding was not detected in any of the CVL specimens among the Group 1 women (95% CI: 0 to 1.2%). When we tested CVL collected at the preceding visit from 30 randomly chosen women among those with inflammation at the initially tested visit, HIV-1 was again not detectable. The absence of genital HIV shedding was confirmed by spiking with HIV-1 duplicate CVL samples from 9 randomly chosen women from Group 1 to demonstrate that HIV-1 could be detected if present (1907 cp/ml were detected in the spiked PBS control and 415–1557 cp/ml were detected in the nine spiked CVL samples).
Transition from shedding in the absence of ART to no shedding on suppressive ART.
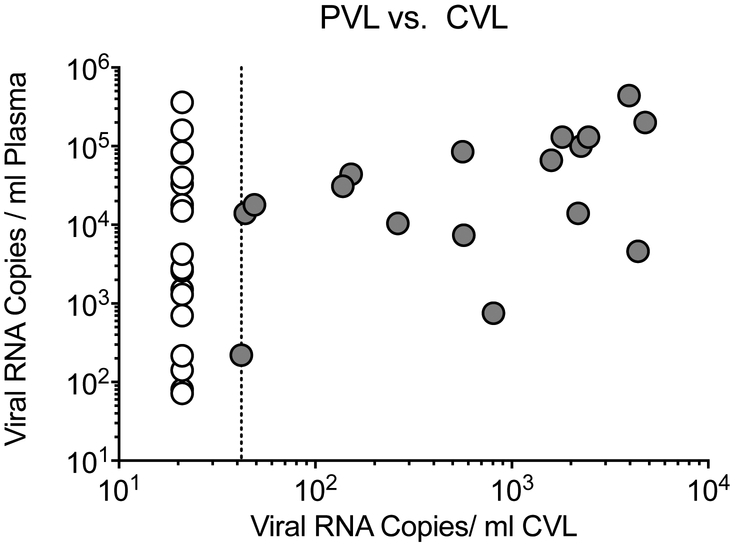
To assess the potential impact of inflammation on HIV-1 shedding in the genital tract around the time of ART initiation, we selected a second group of WIHS participants for whom two CVL specimens were available that were collected just prior to ART initiation and at approximately one year after onset of PVL suppression (Table 1). We hypothesized that one year of suppression was a time when most women should have stopped shedding, but likely close enough to ART initiation to evaluate the impact of inflammation. Close to 50% (18 of 38; 47%) of the women in Group 2 had detectable HIV-1 RNA in their pre-ART CVL samples. CVL virus load ranged from 44 to 4776 cp/ml with a median value of 668 cp/ml (IQR=235–2305 cp/ml), and was correlated with the magnitude of pre-ART plasma viremia (n=37 evaluable, Figure 1). A 1.0 log10 increase in PVL corresponded to 2.9-times the odds of detectable CVL VL (estimated OR=2.9, 95% CI 1.2 to 6.9, p=0.01; Supplemental Figure S1). This result confirmed that the failure to detect shedding virus in women with long term suppression was not due to an inability to detect virus when it is present.
Table 1.
HIV-1 quantitation in cervico-vaginal lavage (CVL) and plasma among 38 participants in the Women’s Interagency HIV Study (WIHS) before ART initiation and 1 year after achievement of viral suppression
| Pre-ART | 1 year ART suppressed | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Groupa | Age | Menopause status |
# CI markersb |
Plasma VLc |
CVL VL |
Age | Menopause status |
# CI markersb |
CVL VL |
| I/I | 54 | Post | 2 | 660003 | 1590 | 56 | Post | 4 | ND |
| I/I | 54 | Post | 1 | 15005 | NDd | 56 | Post | 2 | ND |
| I/I | 34 | Pre | 5 | 330003 | ND | 36 | Pre | 2 | ND |
| I/I | 43 | Pre | 1 | 1000003 | 2254 | 44 | Pre | 2 | ND |
| I/I | 27 | Pre | 2 | 13003 | ND | 29 | Pre | 2 | ND |
| I/I | 44 | Pre | 3 | 104545 | 263 | 46 | Pre | 3 | ND |
| I/I | 51 | Peri | 4 | 440003 | 152 | 53 | Post | 3 | ND |
| I/I | 34 | Pre | 4 | 7503 | 806 | 35 | Pre | 3 | ND |
| I/I | 48 | Pre | 1 | 308734 | 138 | 49 | Peri | 3 | ND |
| I/I | 38 | Pre | 3 | 46003 | 4391 | 39 | Pre | 2 | ND |
| N/I | 52 | Post | 0 | * | 284 | 54 | Post | 1 | ND |
| N/I | 63 | Post | 0 | 7024 | ND | 65 | Post | 1 | ND |
| N/I | 32 | Pre | 0 | 1600003 | ND | 33 | Pre | 1 | ND |
| N/I | 62 | Post | 0 | 140003 | 44 | 63 | Post | 1 | ND |
| N/I | 31 | Pre | 0 | 850001 | 562 | 33 | Pre | 1 | ND |
| N/I | 32 | Pre | 0 | 2000003 | 4776 | 34 | Pre | 1 | ND |
| N/I | 59 | Post | no data | 74002 | 569 | 61 | Post | 1 | ND |
| N/I | 48 | Pre | 0 | 160002 | ND | 49 | Pre | 1 | ND |
| N/I | 36 | Pre | 0 | 4400001 | 3958 | 38 | Pre | 1 | ND |
| N/I | 51 | Pre | 0 | 180003 | ND | 52 | Pre | 2 | ND |
| I/N | 56 | Post | 1 | 26003 | ND | 58 | Post | 0 | 770 |
| I/N | 50 | Pre | 1 | 820002 | ND | 51 | Post | 0 | ND |
| I/N | 33 | Pre | 1 | 803 | ND | 34 | Pre | 0 | ND |
| I/N | 52 | Pre | 1 | 140004 | ND | 54 | Pre | 0 | ND |
| I/N | 38 | Pre | 1 | 180003 | 2179 | 39 | Pre | 0 | ND |
| I/N | 31 | Pre | 1 | 1300003 | 49 | 33 | Pre | 0 | ND |
| I/N | 34 | Pre | 2 | 3600003 | 1806 | 36 | Pre | 0 | ND |
| I/N | 48 | Peri | 1 | 1425 | ND | 49 | Peri | 0 | ND |
| I/N | 35 | Pre | 2 | 1300003 | ND | 36 | Pre | 0 | ND |
| I/N | 52 | Post | 4 | 150003 | 2459 | 53 | Post | 0 | ND |
| N/N | 52 | Post | 0 | 27505 | ND | 53 | Post | 0 | ND |
| N/N | 32 | Pre | 0 | 401005 | ND | 34 | Pre | 0 | ND |
| N/N | 54 | Post | 0 | 836495 | ND | 56 | Post | 0 | ND |
| N/N | 35 | Pre | 0 | 2203 | ND | 37 | Pre | 0 | ND |
| N/N | 49 | Peri | 0 | 280003 | <42e | 50 | Peri | 0 | ND |
| N/N | 50 | Pre | 0 | 724 | ND | 51 | Post | 0 | ND |
| N/N | 44 | Pre | 0 | 42334 | ND | 45 | Pre | 0 | ND |
| N/N | 39 | Pre | 0 | 2164 | ND | 41 | Pre | 0 | ND |
Two letters correspond to the status of clinical inflammation (CI) at first and second visits: I indicates at least one CI marker; N indicates no CI markers.
Number of CI markers documented at visit (see Methods).
LLQ of PVL testing: 14000 cp/ml; 2400 cp/ml; 380 cp/ml; 448 cp/ml; 520 cp/ml.
ND=Not detected.
HIV-1 was detected but was below the LLQ.
Viral load not done at this visit, but ART started after this visit.
Figure 1. HIV-1 RNA levels in plasma and CVL from 37 WIHS participants before ART initiation.
CVL with detectable viral loads are shown in gray circles and CVL with undetectable viral loads are shown in white circles.
There was no clear association between detectable CVL HIV-1 RNA and clinical inflammation, although only one woman in the N/N group (no clinical inflammation markers at either visit) had detectable VL in her CVL at the pre-ART visit, and this was the sample that was detectable below the quantitation limit of the assay (listed as <42 in Table 1). Overall, 55% of CVL samples from visits with clinical inflammation markers had detectable HIV-1 RNA, compared to 39% of samples from visits without clinical inflammation markers.
After one year of suppressed PVL on ART, one CVL sample from Group 2 had detectable CVL HIV-1 RNA (770 cp/ml). This woman had no markers of clinical inflammation at the second visit, but had one inflammation marker (vaginal pH>5.5) at her pre-ART visit, when viral RNA was not detectable in her CVL specimen. The Group 2 women (at the latter visits) were aged 29–65 years, with a median of 47 years (IQR=36–53 years); 66% were pre-menopausal, 8% peri-menopausal, and 26% post-menopausal (all self-reported). In summary, only one of the 38 CVL samples collected after one year of suppressed PVL had detectable HIV-1 (2.6%, 95% CI 0.067% to 14%), while HIV-1 genital shedding was detected in 47% (18 of 38, 95% CI 31% to 64%) of the women before initiating therapy.
Role of clinical markers of inflammation in predicting HIV-1 shedding in the genital tract.
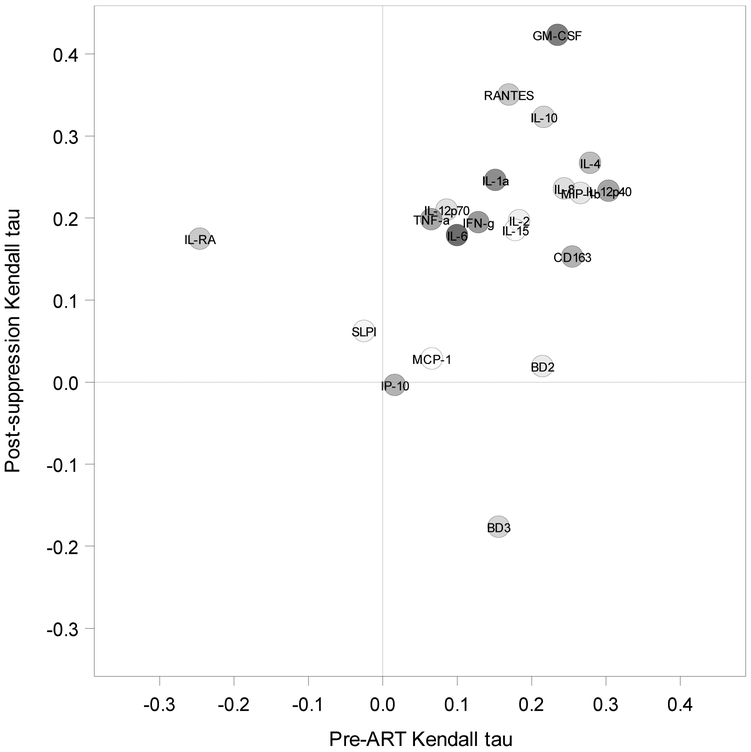
Among the women in Group 2 with clinical inflammation markers before starting ART and/or after one year of PVL suppression, the most common marker was high vaginal pH (> 5.5). The presence and distribution of the clinical inflammation markers by group and visit is summarized in Table 2. To evaluate more closely the relationship between genital tract HIV-1 shedding and the presence of specific clinical inflammation markers in women with HIV-positive CVL samples (n=18 women pre-ART and n=1 post-PVL suppression), we examined whether the type of clinical marker or a combination of markers was associated with shedding. Although the small sample size (n=38) limited the sensitivity of this analysis, we did not find an association between clinical marker category and the odds of detectable CVL viral load (OR=1.2 [95% CI 0.24 to 6.1, p=0.81] for 1 vs. 0 clinical markers, and OR=4.3 [95% CI 0.80 to 23, p=0.09] for ≥2 vs. 0 clinical markers. These results implied that genital tract HIV-1 shedding in these WIHS participants was not associated with inflammation based on clinical inflammation markers (Supplemental Figure S2). To further strengthen this conclusion, we examined whether known immune biomarkers of inflammation were correlated with clinical markers of inflammation. We measured 21 biomarkers in both the pre-ART visit and one-year-suppressed ART time point in Group 2 CVL samples (Supplemental Figure S3). Nine of the biomarkers (GM-CSF, IL-8, IL-10, IL-4, IL-1α, MIP-1β, RANTES, CD163, IL-12p40) were positively correlated with the number of clinical inflammation markers found pre-ART and/or post-suppression, with Kendall tau-b correlation coefficients between 0.22 and 0.42 and unadjusted p-values <0.10 (Figure 2). However, after false discovery rate adjustment, only the GM-CSF post-treatment correlation remained significant (p=0.04) in this small number of samples. Further, among women in Group 2 with clinical inflammation, no major differences in biomarker levels were detected at the pre-ART visit between women with detectable (n=18) and women with non-detectable (n=20) CVL HIV-1 RNA.
Table 2.
Clinical markers of genital inflammation in 38 WIHS participants before ART initiation and 1 year after achievement of viral suppressiona
| Group, Visit | # women/ visits |
High vaginal pH |
Cervical lesions |
Ectopy | Cervical friability |
Cervical exudate |
Tricho moniasis |
Inflammation in Pap smear |
|---|---|---|---|---|---|---|---|---|
| I/Nb Pre-ART | 10 | 6 | 2 | 2 | 1 | 3 | 0 | 1 |
| I/Ib Pre-ART | 10 | 6 | 1 | 2 | 4 | 7 | 2 | 4 |
| N/Ib Post-suppression | 10 | 6 | 0 | 0 | 0 | 3 | 0 | 2 |
| I/Ib Post-suppression | 10 | 8 | 0 | 1 | 5 | 5 | 2 | 5 |
Data are only shown for the 30 women in the I/I, I/N, and N/I groups; the 8 women in the N/N group had no clinical markers of inflammation.
Two letters correspond to the status of clinical inflammation (CI) at first and second visits: I indicates at least one CI marker; N indicates no CI markers.
Figure 2. Immune biomarker associations with number of clinical inflammation markers at pre-ART initiation and post-virologic suppression visits of WIHS participants.
Kendall tau-b values plotted for pre-ART (n=37) and post-suppression (n=38) for each of the 21 biomarkers (GM-CSF, IFN-γ, IL-10, IL-12p40, IL-12p70, IL-15, IL-1RA, IL-1α, IL-2, IL-4, IL-6, IL-8, IP10, MCP-1, MIP-1β, RANTES, TNFα, CD163, SLPI, IFN-α, beta-defensin 2 (BD2) and beta-defensin 3 (BD3)). Clinical inflammation markers included vaginal pH>5.5, visible cervical lesions, cervical ectopy, cervical friability, cervical exudate, trichomoniasis, and inflammation noted on Pap smear.
Discussion
The goal of this study was to assess the association between local inflammation in the female genital tract and HIV-1 shedding in women with suppressive ART. We selected two groups of women from the well characterized WIHS cohort, with women in Group 1 being virally suppressed in plasma for a median of 7.1 years, whereas women in Group 2 were suppressed for one year. Our results indicate that suppression of viremia, even in the presence of clinical inflammation, prevents genital tract HIV-1 shedding, further supporting the message that “undetectable is untransmittable.”
Based on previous studies [5,11-13,21,22], including those that reported the contribution of inflammation to genital tract HIV-1 shedding, we had expected 2–34% of the CVL samples to have detectable HIV-1 while on suppressive ART. However, within the limits of the sensitivity of our optimized HIV-1 RNA assay in CVL samples, none of the women in Group 1, who were virologically-suppressed for a median of 7.1 years, had detectable genital tract HIV-1 shedding. This result was consistent with a prior small-scale study by our group in which we also found no genital tract shedding of HIV-1 when we analyzed multiple specimen types in a group of women with suppressed plasma viremia [23].
The difference in the frequency of genital shedding during PVL suppression between our study (0% for Group 1) and other studies (2–34%) may be due to differences in the duration of PVL suppression. Some studies did not specify the length of time on suppressive ART [5,11,21], while others followed women for up to two years after starting ART [12,13], but did not evaluate risk of shedding with longer time on ART. Another study reported that risk of HIV-1 transmission persists during the first 6 months after initiation of ART, likely due in part to incomplete HIV-1 suppression in genital compartments [24].
The latter findings are indirectly supported by our results in the women in Group 2. Pre-ART, 47% of CVL samples of Group 2 women tested HIV-1 RNA positive, but only 1 woman had evidence of genital tract HIV-1 shedding after ART suppression for one year. These results confirm that ART markedly reduces genital tract HIV-1 shedding among women in the WIHS cohort, consistent with the absence of transmission in clinical studies [15,16].
In the current study, the detection of HIV-1 RNA in pre-ART CVL samples was correlated with plasma viremia. Thus, one could hypothesize that the kinetics of suppression in plasma viremia may directly influence the kinetics of suppression of genital tract shedding. Another variable that could impact the time to suppression might be the type of ART; evaluation of the relationship between different ART regimens and genital tract HIV-1 shedding was outside the scope of the current study.
The difference in frequency of genital shedding between our study and others is unlikely to be due to the use of different assays or different assay sensitivity. In fact, we identified a similar proportion of genital tract HIV-1 shedding among women who were not on ART (47%) as reported in other studies [5,11-13,21]. The odds of genital tract HIV-1 shedding were associated with PVL level at these pre-ART visits, with 2.9-times the odds of detectable shedding for each 10-fold increase in PVL (95% CI 1.2 to 6.9), which is similar to the odds ratio of 6.1 (95% CI 2.9 to 12.9) reported by Kovacs et al. [2] among WIHS participants who were younger (18–45) than those in our study. These results are also similar to those reported by Homans et al. of approximately 2.5-times the odds of detectable shedding for every 10-fold increase in initial PVL, although their study included detection of genital shedding of HIV-1 at multiple visits [3].
All of the WIHS participants studied here were selected based on the presence or absence of seven specific clinical inflammation markers documented in the WIHS cohort. Previous studies showed that genital inflammation, evidenced by either clinical inflammation markers or measurement of cytokines, was associated with higher risk of genital tract HIV-1 shedding in women both on and off ART [3,5,6,25-30]. Since most of the women in our study (213 of 332) had at least one clinical inflammation marker while on suppressive therapy and nearly all (331 of 332 women in Groups 1+2 combined) had undetectable genital tract HIV-1 shedding post-ART, our results suggest that genital inflammation does not lead to detectable shedding if sufficiently suppressive ART therapy is present. This result appears to be in contrast to other studies [3,7,11,26], including those with the WIHS cohort [3,5], that demonstrated an association between genital inflammation and the odds of genital tract HIV-1 shedding. However, as some immune biomarkers correlated with the presence of clinical inflammation, the lack of a statistically significant association between genital tract HIV-1 shedding and clinical inflammation observed in the current study was unlikely due to under-diagnosis or over-diagnosis of inflammation, but is more likely due to the small size (n=38) of the group of participants we studied.
In summary, our results are consistent with studies that have documented a decline in genital tract shedding of HIV-1 at a similar rate to that in plasma after ART initiation [4,31,32]. These data indicate that successful suppression of PVL by ART reduces female genital tract shedding to undetectable levels, in accordance with studies that show suppressive ART reduces transmission risk to near zero (untransmittable) [16,33]. The duration of HIV-1 suppression may be an important factor in the relationship between viral suppression and absence of transmission. These data should further strengthen providers’ messages as they counsel women and their sexual partners that “Undetectable is untransmittable.”
Supplementary Material
Supplemental Figure S1. Estimated probability of detectable viral load in cervicovaginal lavage before ART initiation among WIHS participants: relationship to plasma viral load. Model-based predicted probability of detectable viral load in CVL (solid line) and a 95% CI (dashed lines) are displayed. Plasma viral load (log10 cp/mL) on the x-axis was fit as linear in the logit. The observed data distribution of plasma viral load is plotted at the bottom with no associated y values.
Supplemental Figure S2. Probability of detecting HIV-1 in cervicovaginal lavage (CVL) among HIV-1 seropositive WIHS participants with 0, 1, or multiple clinical inflammation markers. Model-based predicted probability of detectable viral load in CVL (solid line) and a 95% CI (dashed lines) are displayed. Plasma viral load (log10 cp/mL) on the x-axis is shown clumped to indicate the number of observations in each group.
Supplemental Figure S3. Number of clinical inflammation markers vs. 9 biomarker levels in 38 WIHS participants at pre-ART and post-suppression visits. Clinical inflammation markers included vaginal pH>5.5, visible cervical lesions, cervical ectopy, cervical friability, cervical exudate, trichomoniasis, and inflammation noted on Pap smear.
Acknowledgments
J.A.E.N., K.D.P., C.R., A.E., R.S., J.J.E., and A.A. conceived of and designed the study. C.R., A.E., B.C.H., K.A., H.M., S.K., R.M.G., A.L.F., E.T.G., A.N.S., and C.O. collected and/or managed the data. K.C. performed CVL VL testing. J.A.E.N. performed immune biomarker testing. K.R.M, and C.P.B. analyzed the data with input from J.A.E.N and K.D.P. J.A.E.N. and K.D.P. drafted the manuscript. All authors revised the manuscript and gave final approval.
This study was funded by the National Institutes of Health through the WIHS (U01 AI103390) and the UNC Center for AIDS Research (P01 AI050410).
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): UAB-MS WIHS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos and Anjali Sharma), U01-AI-035004; Brooklyn WIHS (Deborah Gustafson and Tracey Wilson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye and Daniel Merenstein), U01-AI-034994; Miami WIHS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Bradley Aouizerat and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I – WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR000454 (Atlanta CTSA), P30-AI-050410 (UNC CFAR), and P30-AI-027767 (UAB CFAR).
This study was funded by the National Institutes of Health through the WIHS (U01 AI103390), the UNC Center for AIDS Research (P01 AI050410), and the other WIHS sites (U01-AI-103401, U01-AI-103408, U01-AI-035004, U01-AI-031834, U01-AI-034993, U01-AI-034994, U01-AI-103397, U01-AI-034989, U01-AI-042590, U01-HD-032632). WIHS data collection is also supported by NIH grants UL1-TR000004, UL1-TR000454, P30-AI-050410, and P30-AI-027767.
References
- 1.Baeten JM, Kahle E, Lingappa JR, Coombs RW, Delany-Moretlwe S, Nakku-Joloba E, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med 2011; 3:77ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovacs A, Wasserman SS, Burns D, Wright DJ, Cohn J, Landay A, et al. Determinants of HIV-1 shedding in the genital tract of women. Lancet 2001; 358:1593–1601. [DOI] [PubMed] [Google Scholar]
- 3.Homans J, Christensen S, Stiller T, Wang C-H, Mack W, Anastos K, et al. Permissive and protective factors associated with presence, level, and longitudinal pattern of cervicovaginal HIV shedding. J Acquir Immune Defic Syndr 2012; 60:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiscus SA, Cu-Uvin S, Eshete AT, Hughes MD, Bao Y, Hosseinipour M, et al. Changes in HIV-1 subtypes B and C genital tract RNA in women and men after initiation of antiretroviral therapy. Clin Infect Dis 2013; 57:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herold BC, Keller MJ, Shi Q, Hoover DR, Carpenter CA, Huber A, et al. Plasma and mucosal HIV viral loads are associated with genital tract inflammation in HIV-infected women. J Acquir Immune Defic Syndr 2013; 63:485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mauck C, Chen P-L, Morrison CS, Fichorova RN, Kwok C, Chipato T, et al. Biomarkers of Cervical Inflammation and Immunity Associated with Cervical Shedding of HIV-1. AIDS Res Hum Retroviruses 2016; 32:443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King CC, Ellington SR, Davis NL, Coombs RW, Pyra M, Hong T, et al. Prevalence, Magnitude, and Correlates of HIV-1 Genital Shedding in Women on Antiretroviral Therapy. J Infect Dis 2017; 216:1534–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iversen AK, Larsen AR, Jensen T, Fugger L, Balslev U, Wahl S, et al. Distinct determinants of human immunodeficiency virus type 1 RNA and DNA loads in vaginal and cervical secretions. J Infect Dis 1998; 177:1214–1220. [DOI] [PubMed] [Google Scholar]
- 9.Goulston C, McFarland W, Katzenstein D. Human immunodeficiency virus type 1 RNA shedding in the female genital tract. J Infect Dis 1998; 177:1100–1103. [DOI] [PubMed] [Google Scholar]
- 10.Hart CE, Lennox JL, Pratt-Palmore M, Wright TC, Schinazi RF, Evans-Strickfaden T, et al. Correlation of human immunodeficiency virus type 1 RNA levels in blood and the female genital tract. J Infect Dis 1999; 179:871–882. [DOI] [PubMed] [Google Scholar]
- 11.Low AJ, Konate I, Nagot N, Weiss HA, Mabey D, Segondy M, et al. Neisseria gonorrhoeae and Chlamydia trachomatis infection in HIV-1-infected women taking antiretroviral therapy: a prospective cohort study from Burkina Faso. Sex Transm Infect 2014; 90:100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cu-Uvin S, DeLong AK, Venkatesh KK, Hogan JW, Ingersoll J, Kurpewski J, et al. Genital tract HIV-1 RNA shedding among women with below detectable plasma viral load. AIDS 2010; 24:2489–2497. [DOI] [PubMed] [Google Scholar]
- 13.Bull ME, Mitchell C, Williams C, Soria J, Ticona E, La Rosa AM, et al. Genital HIV shedding when antiretroviral therapy (ART) suppresses plasma HIV RNA Conf Retroviruses Opportunistic Infect 2017; Seattle, WA. [Google Scholar]
- 14.Sheth AN, Evans-Strickfaden T, Haaland R, Martin A, Gatcliffe C, Adesoye A, et al. HIV-1 genital shedding is suppressed in the setting of high genital antiretroviral drug concentrations throughout the menstrual cycle. J Infect Dis 2014; 210:736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, et al. Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. JAMA 2016; 316:171–181. [DOI] [PubMed] [Google Scholar]
- 17.NIAID. Science validates undetectable = untransmittable HIV prevention message. https://www.niaid.nih.gov/news-events/undetectable-equals-untransmittable
- 18.Adimora AA, Ramirez C, Benning L, Greenblatt RM, Kempf M-C, Tien PC, et al. Cohort Profile: The Women’s Interagency HIV Study (WIHS). Int J Epidemiol 2018; 47:393–394i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology 1998; 9:117–125. [PubMed] [Google Scholar]
- 20.Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005; 12:1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fornabaio C, Carvalho ACC, Lillo F, Fiore JR, Bergamaschi V, Bigoni S, et al. Cervical human papillomavirus infection and shedding of human immunodeficiency virus in cervicovaginal fluids: a cross-sectional study. J Acquir Immune Defic Syndr 2012; 61:78–82. [DOI] [PubMed] [Google Scholar]
- 22.Buckley N, Huber A, Lo Y, Castle PE, Kemal K, Burk RD, et al. Association of High-Risk Human Papillomavirus with Genital Tract Mucosal Immune Factors in HIV-Infected Women. Am J Reprod Immunol N Y N 1989 2016; 75:146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahangdale L, Paris KD, Kashuba ADM, Nelson JAE, Cottrell M, Sykes C, et al. Immunologic, Virologic, and Pharmacologic Characterization of the Female Upper Genital Tract in HIV-Infected Women. J Acquir Immune Defic Syndr 2015; 68:420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mujugira A, Celum C, Coombs RW, Campbell JD, Ndase P, Ronald A, et al. HIV Transmission Risk Persists During the First 6 Months of Antiretroviral Therapy. J Acquir Immune Defic Syndr 2016; 72:579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell C, Hitti J, Paul K, Agnew K, Cohn SE, Luque AE, et al. Cervicovaginal shedding of HIV type 1 is related to genital tract inflammation independent of changes in vaginal microbiota. AIDS Res Hum Retroviruses 2011; 27:35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blish CA, McClelland RS, Richardson BA, Jaoko W, Mandaliya K, Baeten JM, et al. Genital Inflammation Predicts HIV-1 Shedding Independent of Plasma Viral Load and Systemic Inflammation. J Acquir Immune Defic Syndr 2012; 61:436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bull ME, Legard J, Tapia K, Sorensen B, Cohn SE, Garcia R, et al. HIV-1 shedding from the female genital tract is associated with increased Th1 cytokines/chemokines that maintain tissue homeostasis and proportions of CD8+FOXP3+ T cells. J Acquir Immune Defic Syndr 2014; 67:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keller MJ, McGinn AP, Lo Y, Huber A, Espinoza L, Minkoff H, et al. Longitudinal Assessment of Systemic and Genital Tract Inflammatory Markers and Endogenous Genital Tract E. coli Inhibitory Activity in HIV-Infected and Uninfected Women. Am J Reprod Immunol N Y N 1989 2016; 75:631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spear GT, Zariffard MR, Chen HY, Anzinger JJ, Anastos K, Rusine J, et al. Positive association between HIV RNA and IL-6 in the genital tract of Rwandan women. AIDS Res Hum Retroviruses 2008; 24:973–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zara F, Nappi RE, Brerra R, Migliavacca R, Maserati R, Spinillo A. Markers of local immunity in cervico-vaginal secretions of HIV infected women: implications for HIV shedding. Sex Transm Infect 2004; 80:108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landay A, Golub ET, Desai S, Zhang J, Winkelman V, Anastos K, et al. HIV RNA levels in plasma and cervical-vaginal lavage fluid in elite controllers and HAART recipients. AIDS 2014; 28:739–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Péré H, Rascanu A, LeGoff J, Matta M, Bois F, Lortholary O, et al. Herpes simplex virus type 2 (HSV-2) genital shedding in HSV-2-/HIV-1-co-infected women receiving effective combination antiretroviral therapy. Int J STD AIDS 2016; 27:178–185. [DOI] [PubMed] [Google Scholar]
- 33.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med 2016; 375:830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farr SL, Nelson JAE, Ng’ombe TJ, Kourtis AP, Chasela C, Johnson JA, et al. Addition of 7 days of zidovudine plus lamivudine to peripartum single-dose nevirapine effectively reduces nevirapine resistance postpartum in HIV-infected mothers in Malawi. J Acquir Immune Defic Syndr 2010; 54:515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nasioulas G, Hughes SH, Felber BK, Whitcomb JM. Production of avian leukosis virus particles in mammalian cells can be mediated by the interaction of the human immunodeficiency virus protein Rev. Proc Natl Acad Sci U S A 1995; 92:11940–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Estimated probability of detectable viral load in cervicovaginal lavage before ART initiation among WIHS participants: relationship to plasma viral load. Model-based predicted probability of detectable viral load in CVL (solid line) and a 95% CI (dashed lines) are displayed. Plasma viral load (log10 cp/mL) on the x-axis was fit as linear in the logit. The observed data distribution of plasma viral load is plotted at the bottom with no associated y values.
Supplemental Figure S2. Probability of detecting HIV-1 in cervicovaginal lavage (CVL) among HIV-1 seropositive WIHS participants with 0, 1, or multiple clinical inflammation markers. Model-based predicted probability of detectable viral load in CVL (solid line) and a 95% CI (dashed lines) are displayed. Plasma viral load (log10 cp/mL) on the x-axis is shown clumped to indicate the number of observations in each group.
Supplemental Figure S3. Number of clinical inflammation markers vs. 9 biomarker levels in 38 WIHS participants at pre-ART and post-suppression visits. Clinical inflammation markers included vaginal pH>5.5, visible cervical lesions, cervical ectopy, cervical friability, cervical exudate, trichomoniasis, and inflammation noted on Pap smear.