Synopsis
With thrombectomy cerebral ischemic stroke treatment may change significantly now that clots are actually physically removed from the patient. This allows for analysis of the content of the clots as well as the correlation of the imaging findings and the clot behavior and morphology. In this paper we illustrate how the interaction of different clots varies in the clinical setting and how analysis of clot composition, as well as the search for new pharmacological targets, can lead to a better understanding of the pathophysiology and therapy resistance, in turn providing possibilities for a better approach in the treatment.
Conventional treatment of cerebral ischemic stroke was based on recanalizing arteries by use of thrombolytic agents and, if indicated, hemicraniectomy. The results of this approach, with only 10% of patients receiving the drug and only 50% thereof responding postively1,2, has led to the continued search for alternative treatments. Since 2015 the first line treatment of ischemic stroke has changed dramatically with the publication of the so-called ‘positive trials’ 3–7, which showed benefit of endovascular stroke treatment. The neurointerventional field has started to implement this new strategy in a rapid pace in conjunction with, and sometimes even replacing, traditional medical stroke therapy.
With the development of newer devices and extensive research that is being done, we are confronted with previously unknown hurdles in our endovascular procedures. One of the most important obstacles is the fact that clots tend to differ in consistency and removability. On one hand we can face a clot that comes out with simple proximal aspiration or thrombectomy but in a next case we may be faced with a clot that is too resilient to be removed by any of the devices we have at hand. The next case examples show the high variability and, so far, unpredictable nature of the clots in the stroke cases we face on a daily basis.
Case 1
A 67 y/o female presented with sudden onset right sided hemiparesis and aphasia. Thrombectomy was performed after CT and CTA showed an M1 occlusion without hyperdense vessel sign. Four passes with a Solitaire 4×40 (Medtronic, Minneapolis, Minnesota, USA) were needed in order to achieve full recanalization. (Figure 1)
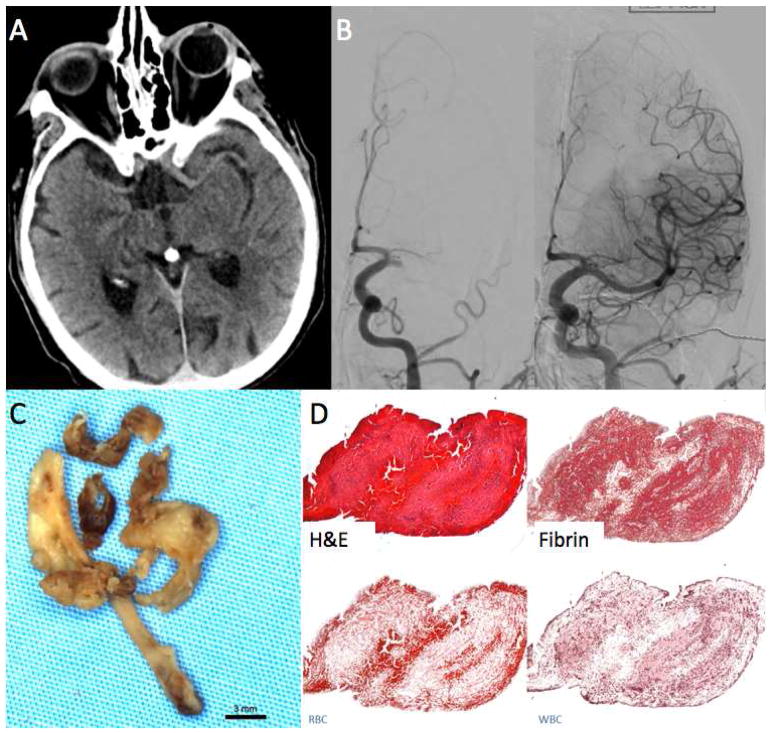
FIGURE 1.
Clot Attenuation and Composition and Revascularization: Fibrin Rich Clot. 67-year-old female with acute onset right sided hemiparesis and aphasia.
A. Non-contrast CT using thin-section 1mm reconstruction shows no hyperdense vessel.
B. Left ICA cerebral angiogram shows occlusion of the left MCA. Four passes with a Solitaire 4×40 were needed to open the vessel.
C. Gross photo of the retrieved clot shows white clot consistent with fibrin rich thrombus.
D. H&E stain shows that a majority of the clot was composed of fibrin, the overall clot composition was 90% fibrin and 7%RBCs.
Case 2
A 40 year old female, previously known for an occipital infarct 1 month earlier, came to the hospital for cardiac complications due to her anorexia nervosa. During her stay in the ward she developed new stroke like symptoms which, based on the CT angiography and CT perfusion images was caused by a thrombus in the left MCA bifurcation. Thrombectomy was attempted but the clot seemed very sticky and only after the 6th pass with several devices (Solitaire, Medtronic, Minneapolis, Minnesota, Capture Mindframe/Medtronic) the clot was removed. As a complication a small amount of blood and contrast was seen in the subarachnoid space. The image of the clot shows how the texture is totally different from other clots we normally encounter and that have a more heterogenous appearance. (Figure 2)
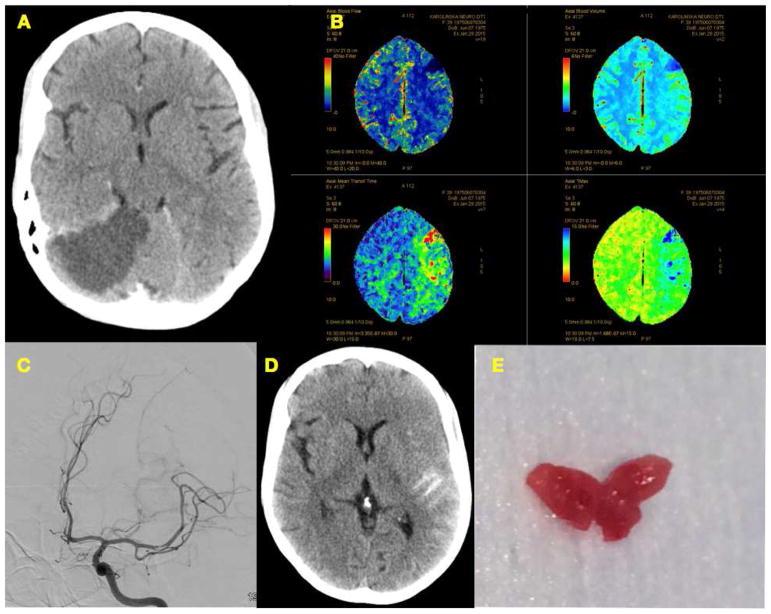
FIGURE 2.
A Older right occipital infarct, no clear infarct signs in left hemisphere
B Perfusion imaging showing tissue at risk in the left hemisphere and a frontal manifest infarction
C Resilient clot taken out after 6 attempts
D Subarachnoid hemorrhage due to thrombectomy
E The clot appears relatively homogeneous, glazy, reddish-purple and from a uncommon consistency
Case 3
A 72 y/o female with progressive dyspnea and a history of 60 pack years, and a malignant melanoma in the face 3 years earlier, was found lying on the floor showing a full hemiplegia and aphasia. Thrombectomy of a non-hyperdense MCA trifurcation clot, performed within 3 hours from onset, resulted in a 10 pass procedure using three different devices (Solitaire, Medtronic, Minneapolis, Minnesota, Capture Mindframe/Medtronic, Embotrap Neuravi/Cerenovus, Galway, Ireland). The clot could not be removed and appeared to be firmly attached to the wall of the artery. The size and shape of the clot changed due to manipulations and the rounded structure can be seen as a filling defect in the artery. After the futile thrombectomy the patient was further analyzed for increasing dyspnea. She died of, previously undiagnosed, lung cancer within the next three weeks after only moderately recovering from her stroke. (Figure 3)
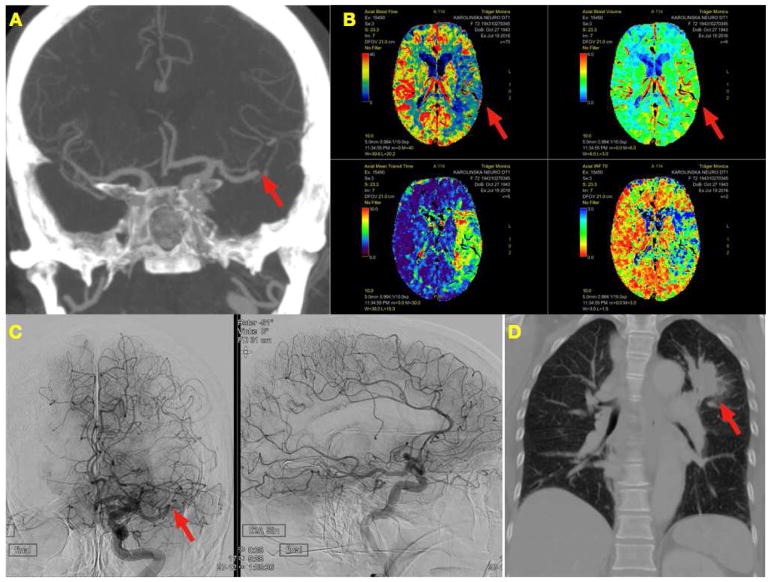
FIGURE 3.
A CTA showing the occlusion (arrow) in the MCA trifurcation and good collaterals
B Perfusion imaging showing a defect in the Blood Flow but maintained Blood Volume (arrows)
C Persistent occlusion (arrow) after 10 passes with multiple devices. Procedure stopped because of to futile attempts and moderate to good collateral circulation
D Tumor in left apical segment of the lung (arrow)
Case 4
A 24 y/o female, who underwent an aortic root replacement, did not wake up after the surgery. She was analysed using non-contrast CT which showed a long hyperdense clot on the left side reaching from the carotid termination into the left MCA trifurcation. She was immediately taken to the angiosuite and underwent successful thrombectomy with full flow restoration. The clot had a dark appearance, which is consistent with a high red blood cell content, subsequently supported by the histological imaging using H&E staining. (Figure 4)
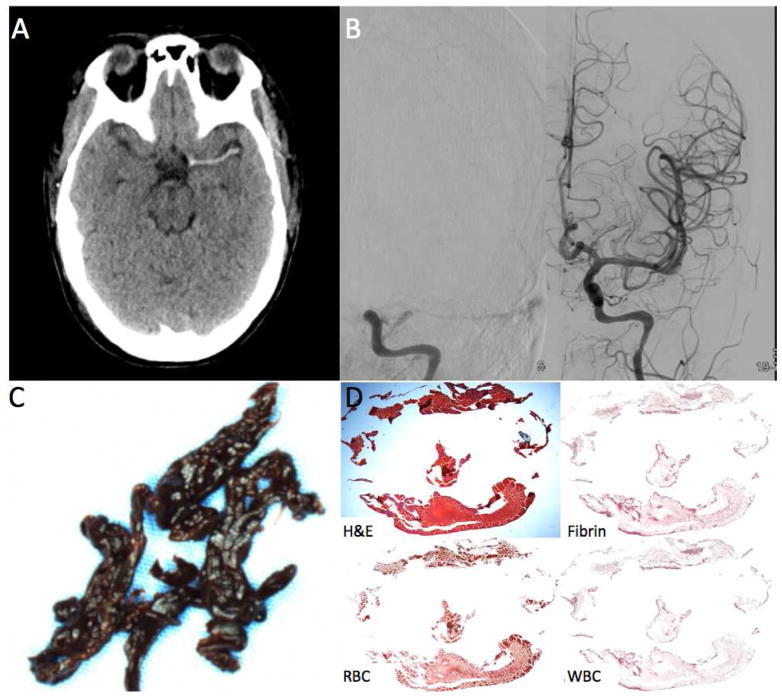
FIGURE 4.
Clot Attenuation and Composition and Revascularization: RBC Rich Clot. A 24 year old female status post aortic root replacement who was not waking up two hours after surgery.
A. Non-contrast CT shows a long hyperdense thrombus of the left MCA.
B. The patient was taken straight to angiography which confirmed the left MCA occlusion. One pass with a 6cm Solitaire was needed to revascularized the occlusion.
C. Gross photo of the clot shows dark red clot consisted with an RBC rich thrombus.
D. H&E stain shows that a majority of the clot was composed of RBCs (94% RBC density).
These four examples show that both efficacy of treatment and appearance of clot can be highly variable between patient cases. In our own experience thrombectomies are more likely to be difficult in patients with for example lung cancer or long standing clot that is dislodged from the aortic arch than the fresh red blood cell rich clots. The mechanisms behind this are still largely unclear, and one can only postulate that paraneoplastic clots have a different composition, and therefore behavior, compared to atherosclerotic clots or clots of cardiac origin. Bench top observations showed that fibrin rich clots are more sticky than pure red blood cell clots on thrombectomy. This further supports the hypothesis that, besides patient related factors, clot properties influence thrombectomy failure or success to a large extent.
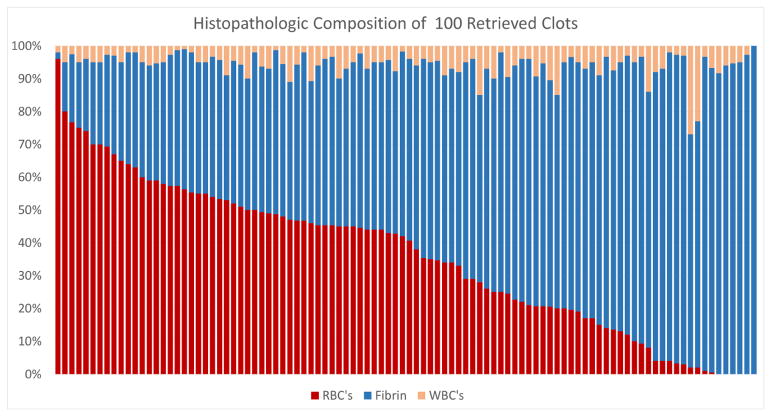
The problems due to clot variations are, however, not specific for endovascular therapies. For both pharmacological thrombolysis and mechanical thrombectomy, the culprit thrombus itself is indeed the primary target of therapy. Therefore, better understanding of clot composition, physical properties, behavior, and how these occlusive thrombi interact with their environment will contribute to future advancements in patient treatment and hopefully improved clinical outcomes. The clot analysis studies of Liebeskind et al., Boeckh-Behrens et al. and Cline et al. showed the distribution of fibrin, leucocytes and red blood cells in clots harvested after thrombectomy.8–10 The spread in content of each of the components, between the different clots, is striking and a composite of the results of these three cohorts is presented in Figure 5. Since the clot analysis could only be performed on clots that were actually retrieved, it remains a question what the exact content of the resilient, unremovable, clots has been.
FIGURE 5.
Histopathologic clot composition
Composition distribution of Red Blood Cells (RBC) Fibrin and White Blood Cells (WBC) in 100 clots retrieved from cerebral ischemic stroke patients. (Courtesy of M. Mirza MD, Neuravi/Cerenovus, Galway, Ireland)
Gunning et al. recently published a study looking into the friction coefficient in relation to red blood cell and fibrin content of the clot.11 The study showed that the fibrin rich clots had a significantly higher friction than the clots with high red blood cell content. In the same study clots were tested after compression simulating ‘repeated stent retriever manipulation’. The increase in friction coefficient after manipulation was dramatic, suggesting that there may as well be a potential increase in friction after multiple thrombectomy attempts in real life. This would explain why a resilient clot seems to be responding even less and less to subsequent thrombectomy attempts.
Previous studies indicate that thrombus composition can provide insights into stroke etiology, recanalization outcomes, and moreover guide development of newer technologies in stroke treatment.8,12–16 A number of recently published studies have even demonstrated that clot composition can impact the choice of revascularization techniques due to differences in clot-device interactions17,18. The ability to retrieve and study fresh thrombi in acute stroke patients has opened a whole new door in the study of stroke pathogenesis and treatment. In this section we will review the current state-of-the-art literature in the study of clot in stroke.
Clot Composition Analysis
As seen in other vascular beds, the typical composition of clot in stroke is a mixture of fibrin, platelets, white blood cells and red blood cells. Various histological stains have been applied in the study of clot including standard hematoxylin and eosin (H&E), Elastic van Gieson, Mallory’s Trichrome, and Martius Scarlet Blue which allow for detailed analysis of the above-listed clot components. Most of the current studies in the literature report quantitative values for each of these components when studying clot phenotype9. The most commonly used terms in describing clot composition are Red Blood Cell (RBC) rich/poor and Fibrin rich/poor as these components make up a majority of the composition of clots seen in acute ischemic stroke patients.19
More recently, there has been growing interest in molecular markers of clot composition including evaluation of von Willebrand Factor (VWF), T-cells (CD3), neutrophils macrophages and endothelial cells. There is growing interest in electron microscopy as a tool in clot characterization, however it is not yet widely applied.19 For the purposes of clarity we will, in this section, focus primarily on the ‘basics’ of clot composition: fibrin, platelets, white blood cells and red blood cells and we will cover the VWF and neutrophils in the separate section on ‘potential pharmacological thrombus targets for treatment’ in this paper.
Clot Composition and Stroke Etiology Recanalization Outcomes
There is a growing body of literature examining the association between clot composition and stroke etiology. To date, a majority of published studies have failed to identify an association between red blood cell and fibrin composition and stroke etiology. Thus, in order to better identify clot characteristics associated with stroke etiology, some investigators have turned to the study of components including white blood cell (WBC) composition and characteristics.20 T-cells have been an interesting target in some studies as these cells have been shown to be a major component of atherosclerotic lesions. In a study of 54 consecutive thrombi retrieved during mechanical thrombectomy, one group found that the number of T-cells was significantly higher in thrombi from atherosclerotic plaques than those from a cardioembolic source.21 Because the final step in plaque destabilization and rupture includes the release of elastase and metalloproteinases from activated T-cells, the idea that T-cell composition would be higher in atheroembolic clots seems logical.21 Ultimately, larger studies are needed to determine if indeed there is a consistent association between clot composition, both cellular and molecular, and stroke etiology.
Clot Composition and Recanalization
Studies examining association between clot composition and recanalization rates have more or less consistently found a strong association between RBC density and ease of recanalization with both fibrinolytic therapies and endovascular techniques. The improved recanalization rates of RBC rich clots over those which are composed of fibrin is likely due to a combination of decreased stiffness of RBC rich clots-thus allowing for improved clot-device interaction, decreased friction of RBC rich clots with the vessel wall, thus allowing for easier clot extraction and increased permeability of RBC rich clots, thus allowing improved permeability of thrombolytic agents.20
To date there have been no in vivo studies comparing recanalization rates of various techniques and devices in revascularization of fibrin rich or RBC rich clots. However, there are some in vitro data suggesting the benefit of unique stent-retriever designs for the treatment of stiffer, fibrin rich thrombi. In a study comparing the Geometric Clot Extractor (GCE), a stent-retriever with a curved spiral configuration, to the Solitaire Stent-retriever, Fennell et al. found that the GCE had a success rate of 100% for retrieving fibrin rich thrombi compared to just 8% for the Solitaire device.22 There are also data to suggest that RBC rich thrombi are more prone to fragmentation, highlighting the need for proximal flow arrest with balloon guide catheters when revascularizing these types of clots.23,24
Clot Imaging Techniques
CT and MRI are the mainstay of stroke imaging in North America and Europe. Both non-contrast CT and CT angiography are useful in characterization of clot. When evaluating clot on non-contrast CT, the use of thin-slice (<2.5mm) CT is strongly recommended. This is due to the fact that a number of studies have shown that thin slice CT is more sensitive and specific in identifying hyperdense thrombus and more accurately measures thrombus length due to the fact that thin-slice CT minimizes partial volume averaging with the adjacent brain parenchyma and CSF.25 (see Figure 6) Characteristics that can be easily discerned on thin-slice CT include thrombus density (in Hounsfield units), clot location and clot length-variables which have been shown to be strongly correlated with angiographic outcomes after mechanical thrombectomy.
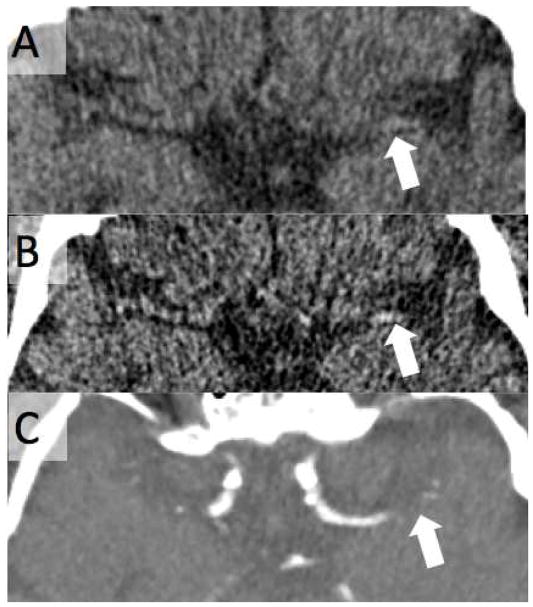
FIGURE 6.
Utility of Thin-Slice CT in Identifying Hyperdense Artery Sign. 75 year old male with acute onset right sided hemiparesis and aphasia. A. Non-contrast CT with 5mm slice thickness shows no evidence of hyperdense artery sign on the left (arrow). B. 0.5mm slice thickness reconstruction shows a hyperdense left MCA (arrow). C. The occlusion was confirmed on CT angiography (arrow).
CT angiography (also using thin-section CT) is a very useful tool in clot imaging. Identification of the ‘contrast gap’ between the proximal and distal end of the clot is useful in assessing clot length and thus aiding in device choice, especially stent-retriever type and length.26 CTA can also be useful in assessing the clot-burden score, a semiquantitative method of measuring the extent of thrombus on CTA.27 Delayed-phase CTA is particularly useful in assessing clot burden and thrombus length as it can take time for contrast to make it to the distal face of the clot. There has been growing interest in the assessment of clot perviousness/permeability to iodinated contrast as increasing density/enhancement of clot on CTA has been shown to be associated with revascularization outcomes.28
MRI has substantial theoretical advantages over CT. The use of multi-contrast MRI allows one to assess multiple different elements of thrombus composition. While there are currently some in vitro data supporting the use T1, T2 and FLAIR in clot evaluation, susceptibility weighted imaging (SW) is the mainstay of clot imaging on MRI due to the consistent presence of blooming artifact associated with most clots. SWI can also allow for evaluation of tiny distal emboli that may have showered in the initial ictus. One disadvantage of MRI in clot evaluation is the inability to assess clot length on non-contrast MRI.29 The presence of blooming artifact on SWI results in overestimation of clot length. This can be obviated by using post-contrast MR angiography. Evaluation of MIP images in post-contrast MR angiography for the presence of a contrast gap has been shown be a useful tool in assessment of clot length.29
Association Between Imaging Findings and Clot Composition
Conventional imaging techniques (i.e. non-contrast CT and MRI) are fairly accurate in characterization of clot composition. Studies examining the association between non-contrast CT findings and clot composition are very consistent in demonstrating that the proportion of RBCs is strongly correlated with clot density. In general, hyperdense thrombi on CT are RBC rich across all energies. There is some preliminary in vitro data to suggest that dual energy CT may have some added utility in distinguishing between RBC and fibrin rich clots, however this has yet to be studied in vivo.30 In terms of MRI, hypointense thrombi on T2* imaging have been shown to have higher RBC content.31 This is secondary to increased iron density in the RBC rich thrombi which results in blooming artifact.8
alization
A number of studies have reported the association between imaging findings and recanalization rates. In a systematic review and meta-analysis, one group found that patients with good angiographic outcomes had higher mean clot density than those with poor angiographic outcomes-both with tPA and mechanical thrombectomy.20 In addition, patients with a hyperdense artery sign were more likely to have a good angiographic outcome than those without. This is logical given the fact that hyperdense artery sign is associated with RBC rich thrombi, and RBC rich thrombi are easier to be removed by thrombectomy.20 Increasing clot length has also been shown to be associated with poorer angiographic outcomes.32
Studies of clot perviousness have generally demonstrated a consistent association between clot perviousness and revascularization outcomes. In a study of 221 patients receiving thin-slice multiphase CTA, Santos et al. found that increased attenuation of thrombus during the arterial phase of CTA was strongly associated with functional and angiographic outcomes, while delayed phase imaging added no value.28 A similar finding was demonstrated by the publication by the entire MR CLEAN group.33 In a study examining clot perviousness and tPA outcomes, the DUST investigators found that permeable thrombi were more responsive to tPA than those which were impermeable to contrast.34
It is likely that the knowledge about clot consistency before treatment would influence the choice of the most effective thrombectomy device. The medical management of stroke may however benefit to a similar extent. If the target is clear the therapy can be adapted accordingly by using the right pharmacological agents.
Potential pharmacological thrombus targets for treatment
Given the limitations of current fibrinolytic therapy, there is an unmet need for alternative and improved thrombolytic agents. Depending on local guidelines, t-PA can only be administered in the limited time window of 4.5–6 hours after the onset of stroke due to the unacceptable risk of cerebral bleeding when treatment is delayed. As a consequence, t-PA treatment is available to less than 10% of patients.1 Remarkably, t-PA results in recanalization only in less than half of the patients that receive it.2 Factors that contribute to this so-called ‘t-PA resistance’ are not well understood but thrombus composition is a likely candidate determining fibrinolytic success rates. Various studies demonstrated that in particular arterial platelet-rich clots are more resistant to thrombolysis with t-PA.35–38 By studying stroke thrombi, our understanding of fibrin clot composition has much improved over the last years and other new potential targets for thrombolytic therapy have been identified. Some of those will be discussed in the next section
Fibrin
Since its FDA approval in 1996, t-PA has become the standard pharmacological intervention for thrombolysis in acute ischemic stroke. As part of the plasminogen activator system, t-PA promotes proteolytic activation of plasminogen to plasmin, which in turn is responsible for the enzymatic degradation of the fibrin mesh in a thrombus. Histological studies of ischemic stroke thrombi typically revealed a heterogenous pattern of fibrin-rich areas in thrombi, with a wide range of fibrin amounts.9,40,41 Various preclinical studies demonstrated that, in particular, arterial platelet and fibrin-rich clots are more resistant to thrombolysis with t-PA compared to erythrocyte-rich thrombi.35,36,38 Interestingly, Choi et al. recently found that also patients with higher amounts of fibrin and platelets in their thrombi were less responsive to intravenous thrombolysis.41 Fibrin structure is controlled by the environment in which the thrombus has been formed. In particular, stroke thrombi of cardioembolic origin are associated with a higher content of fibrin.9,39,40,42,43 More research on human thrombus material is needed to investigate how fibrin characteristics determine success rates of t-PA treatment. In this context, it will also be interesting to investigate the relative presence of potential inhibitors of fibrinolysis, such as α2-antiplasmin, plasminogen activator inhibitor-1 and thrombin activatable fibrinolsyis inhibitor.
Von Willebrand factor
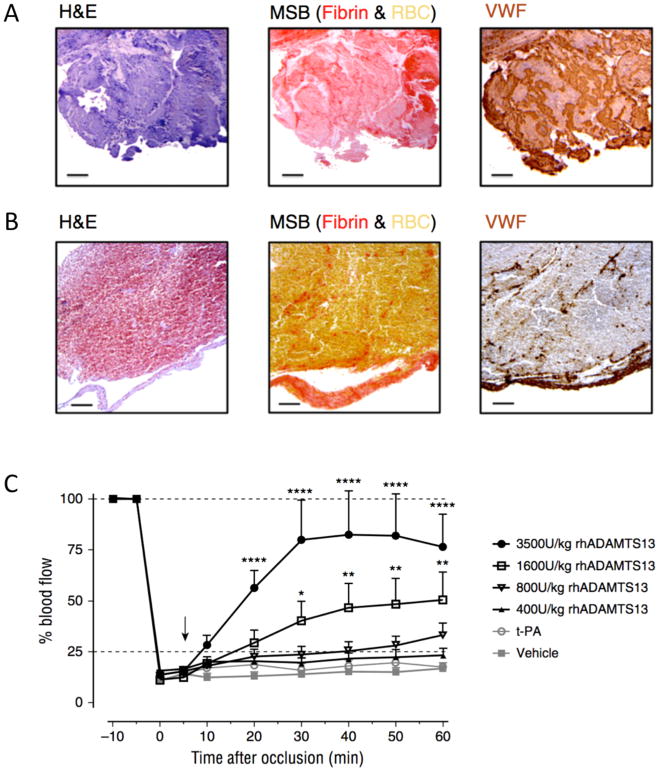
In the past decade, von Willebrand factor (VWF) is gaining increasing attention as an important factor in stroke pathology.44 VWF is a large multimeric plasma glycoprotein that mediates thrombus formation by recruiting platelets at sites of vascular injury. Together with fibrin(ogen), VWF links platelets together, further stabilizing the platelet thrombus.44,45 Besides its thromboinflammatory role in cerebral ischemia/reperfusion injury 46–49, recent findings point towards VWF as an attractive novel target to enhance thrombolysis. Via immunohistochemical staining for VWF, a recent study revealed that stroke thrombi retrieved from stroke patients contained significant amounts of VWF (Figure 7, A and B).50 VWF was present in all thrombi, with amounts ranging from 5 to 50% of the thrombus content.
FIGURE 7.
VWF in ischemic stroke thrombi.
Intracranial thrombi retrieved from stroke patients that underwent thrombectomy procedure were collected for histological analysis. Consecutive thrombi sections were stained with H&E, Martius Scarlet Blue (MSB) and anti-VWF antibodies. Classical H&E staining show overall thrombus composition and organization. On MSB staining, red areas show the presence of fibrin whereas red blood cells appear yellow. Varying amounts of VWF (brown color) were found in all the thrombi Two representative patient thrombi are shown illustrating a VWF-rich thrombus (A) and a RBC-rich, VWF-poor thrombus (B). Scale bar: 50μm. (C) An occlusive thrombus was generated in the right MCA of C57Bl/6J mice. Five minutes after occlusion, vehicle, t-PA or different doses of rhADAMTS13 were intravenously administered (arrow) and MCA blood flow was monitored for 60 minutes. Average blood flow profiles show that rhADAMTS13 restores MCA blood flow in a dose dependent way, while t-PA was unable to restore blood vessel patency. (n = 10 and 8 mice respectively for vehicle and 3500U/kg rhADAMTS13, n = 5 for the lower doses of rhADAMTS13 and t-PA; *, p < 0.05; **, p < 0.01; ****, p < 0.001; compared with vehicle).
Adapted from Denorme F, Langhauser F, Desender L, et al. ADAMTS13-mediated thrombolysis of t-PA-resistant occlusions in ischemic stroke in mice. Blood. 2016;127(19):2337–2345; with permission.
VWF can be cleaved by the metalloprotease ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13). By cleaving the Y1605–M1606 bond in the VWF A2 domain, ADAMTS13 digests VWF into smaller, less thrombogenic multimers. Following the hypothesis that ADAMTS13 can exert a thrombolytic effect in the setting of stroke, a mouse model was used in which the middle cerebral artery (MCA) was occluded by VWF-rich thrombi.50 Interestingly, infusion of t-PA did not lyse these MCA occlusions but administration of ADAMTS13 dose-dependently dissolved these t-PA-resistant thrombi without bleeding side effects (Figure 7, C). As a result, fast restoration of MCA patency was achieved, which was associated with reduced cerebral infarct sizes 24 hours after initial occlusion.50 These results indicate that VWF could become a new target for improved thrombolytic activity in stroke. Besides cleaving by ADAMTS13, VWF can also be targeted to promote thrombolysis by blockade of the VWF-platelet interaction or by reducing the VWF monomer-monomer disulfide bonds by N-acetyl-cysteine.51–53 Targeting VWF could prove particularly helpful for cases in which t-PA is ineffective. VWF content was significantly higher in thrombi retrieved after IV rt-PA use, compared to thrombi retrieved in primary thrombectomy, supporting the idea that thrombi resistant to intravenous thrombolysis may indeed be rich in VWF.54 Of note, an inverse VWF can be cleaved by the metalloprotease ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13). By cleaving the Y1605–M1606 bond in the VWF A2 domain, ADAMTS13 digests VWF into smaller, less thrombogenic multimers. Following the hypothesis that ADAMTS13 can exert a thrombolytic effect in the setting of stroke, a mouse model was used in which the middle cerebral artery (MCA) was occluded by VWF-rich thrombi.50 Interestingly, infusion of t-PA did not lyse these MCA occlusions but administration of ADAMTS13 dose-dependently dissolved these t-PA-resistant thrombi without bleeding side effects (Figure 7, C). As a result, fast restoration of MCA patency was achieved, which was associated with reduced cerebral infarct sizes 24 hours after initial occlusion.50 These results indicate that VWF could become a new target for improved thrombolytic activity in stroke. Besides cleaving by ADAMTS13, VWF can also be targeted to promote thrombolysis by blockade of the VWF-platelet interaction or by reducing the VWF monomer-monomer disulfide bonds by N-acetyl-cysteine.51–53 Targeting VWF could prove particularly helpful for cases in which t-PA is ineffective. VWF content was significantly higher in thrombi retrieved after IV rt-PA use, compared to thrombi retrieved in primary thrombectomy, supporting the idea that thrombi resistant to intravenous thrombolysis may indeed be rich in VWF.54 Of note, an inverse correlation was found between the amount of VWF and the amount of red blood cell content in stroke thrombi.50 Since radiological imaging upon patient admission is able to predict thrombus red cell content,8,55 such information could become helpful to identify those patients who particularly benefit from ADAMTS13 treatment.
Neutrophil extracellular traps
Despite the large general variability in clot composition, leukocytes have been found to be consistently present in ischemic stroke thrombi.8,9,39 Via specific immunostaining of stroke patient thrombi, Laridan et al. showed that neutrophils are a commonly found leukocyte in these thrombi.56 Furthermore, this study also revealed the presence of neutrophil extracellular traps (NETs). Initially described as a novel form of neutrophil-mediated immunity, NETs form through the release of decondensated chromatin that is lined with granular components, creating fibrous structures.57 It has become clear that, apart form their antimicrobial properties, NETs are also implicated in thrombus formation.58 NETs form a scaffold for platelets and red blood cells and influence the coagulation cascade.59
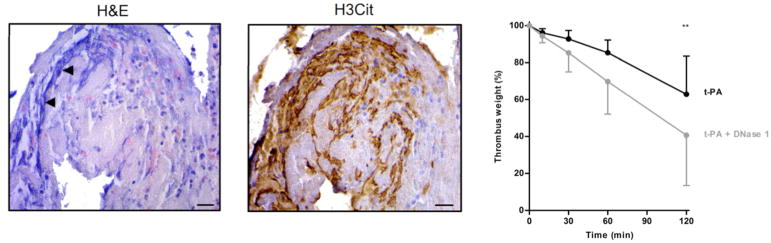
Histological H&E analysis of stroke thrombi revealed prominent extracellular nucleic acid-rich areas that were located in neutrophil-rich zones.56 Selected immunostainings showed the presence of specific NETs-markers on these extracellular DNA strands, demonstrating their neutrophilic origin (Figure 8). Such extensive DNA networks were found in all thrombi, indicating that NETs are a common component of ischemic stroke thrombi. Leukocytes, but also NETs, are reported to be more abundant in stroke thrombi of cardioembolic origin. 9,56 This observation suggests that NETs might be specifically involved in thrombi formed in conditions of stasis, which is in agreement with Savchenko et al. who found NET accumulation in human venous thrombi.60 These new insights led to the hypothesis that NETs could also become a new target for thrombolysis. Serving as a thrombotic scaffold, NETs most likely contribute to overall thrombus stability and might confer resistance to fibrinolytic therapy as recently shown by Ducroux et al.61 DNA is know to be degraded by DNases, which were recently shown to prevent vascular occlusion.62 Interestingly, addition of DNase 1 significantly improved t-PA-mediated ex vivo dissolution of thrombi freshly retrieved from stroke patients (Figure 8).57 DNase 1 was similarly shown to improve thrombolytic activity of coronary thrombi 63 and, in addition, it also reduces ischemic brain injury in mice.64
FIGURE 8.
Neutrophil extracellular traps in ischemic stroke thrombi. Intracranial thrombi retrieved from stroke patients that underwent thrombectomy procedure were collected for histological analysis. Consecutive thrombi sections were stained with H&E and antibodies against citrullinated histones (H3Cit), a marker of NET-formation. Extracellular zones of nuclear material were often observed on H&E stainings (left), which were also positive for H3Cit (right). Scale bars: 10 μm. Right: Fresh thrombi (n=8) retrieved from ischemic stroke patients, were used for ex vivo lysis experiments. The thrombus parts were incubated for 120 min at 37°C in the presence of either t-PA alone (black) or t-PA plus DNase 1 (grey). Thrombus weight (percentage of original weight) was measured at time points 0, 10, 30, 60 and 120 minutes. Data are represented as mean with SD (** p < 0.01).
Adapted from Laridan E, Denorme F, Desender L, et al. Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol. 2017; 313:1451–10; with permission.
DNase 1 already is a safe, low-cost, FDA-approved drug routinely used for cystic fibrosis to clear extracellular DNA in the lungs. Additional studies are needed to further assess the prothrombolytic potential of DNase 1 in the setting of stroke.
Conclusion
Stroke thrombi are complex targets, containing various amounts of different cellular and molecular components. Emerging insights on thrombus composition provide valuable information that can stimulate the development of alternative and better strategies to efficiently remove the occluding thrombus. Promising new approaches are under development to improve mechanical thrombectomy but also for improved pharmacological approaches, including targeting VWF and NETs by ADAMTS13 and DNase 1. Combination of these new strategies with standard t-PA could potentially allow decreasing the dose of t-PA utilized, limiting its side effects and potentially increasing the therapeutic time window. Especially in light of current limitations in stroke therapy, it will be interesting to follow future research in this area.
Acknowledgments
S. De Meyer was supported by the FWO (Fonds voor Wetenschappelijk Onderzoek Vlaanderen G.0A86.13 and 1509216N to S.F.D.M), by an ‘Onderzoekstoelage’ grant from KU Leuven (OT/14/099, to S.F.D.M) and by a research grant from the Queen Elisabeth Medical Foundation (S.F.D.M). S.F.D.M. has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 77072.
Footnotes
Disclosures:
P.A. Brouwer is consultant for Cerenovus/Neuravi, Medtronic, Stryker
W. Brinjikji is consultant for Cerenovus, Superior Medical Editing and CEO of Marblehead Medical LLC. He is funded by NIH grant R01NS105853
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Patrick A. Brouwer, Email: patrickbrouwermd@gmail.com.
Waleed Brinjikji, Email: brinjikji.waleed@gmail.com.
Simon F. De Meyer, Email: simon.demeyer@kuleuven.be.
References
- 1.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 2.Rha J-H, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38(3):967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 3.Berkhemer O, Beumer F, van den Berg L, et al. A randomized trial of intraarterial treatment for acute ischemic stroke (MR CLEAN) N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 4.Goyal M, Demchuk A, Menon B. Randomized assessment of rapid endovascular treatment of ischemic stroke (ESCAPE) N Engl J Med. 2015;372:1019–30. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 5.Campbell B, Mitchell P, Kleinig T, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection (EXTEND-IA) N Engl J Med. 2015;372:1009–18. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 6.Saver J, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke (SWIFT-PRIME) N Engl J Med. 2015;372:2285–95. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 7.Jovin T, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke (REVASCAT) N Engl J Med. 2015;372:2296–306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 8.Liebeskind DS, Sanossian N, Yong WH, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42:1237–1243. doi: 10.1161/STROKEAHA.110.605576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boeckh-Behrens T, Schubert M, Förschler A, et al. The Impact of Histological Clot Composition in Embolic Stroke. Clin Neuroradiol. 2014:1–9. doi: 10.1007/s00062-014-0347-x. [DOI] [PubMed] [Google Scholar]
- 10.Cline B, Vos J, Carpenter J, Rai A. Pathological Analysis Of Extracted Clots In embolectomy patients with acute ischemic stroke. J NeuroIntervent Surg. 2013;5:A15–16. doi: 10.1136/neurintsurg-2013-010870.27. [DOI] [Google Scholar]
- 11.Gunning GM, McArdle K, Mirza M, et al. Clot friction variation with fibrin content; implications for resistance to thrombectomy. J Neurointerv Surg. 2018 Jan;10(1):34–38. doi: 10.1136/neurintsurg-2016-012721. [DOI] [PubMed] [Google Scholar]
- 12.Ahn SH, Choo IS, Hong R, et al. Hyperdense arterial sign reflects the proportion of red blood cells in the thromboemboli of acute stroke patients. Cerebrovascular Diseases. 2012;33:236. [Google Scholar]
- 13.Chueh JY, Wakhloo AK, Hendricks GH, et al. Mechanical characterization of thromboemboli in acute ischemic stroke and laboratory embolus analogs. AJNR Am J Neuroradiol. 2011;32:1237–1244. doi: 10.3174/ajnr.A2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Froehler MT, Tateshima S, Duckwiler G, et al. The hyperdense vessel sign on ct predicts successful recanalization with the merci device in acute ischemic stroke. J Neurointerv Surg. 2013;5:289–293. doi: 10.1136/neurintsurg-2012-010313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guthrie S, Huang X, Moreton F, et al. The significance of the hyperdense vessel sign (hvs) International Journal of Stroke. 2012;7:3. [Google Scholar]
- 16.Mehta BP, Nogueira RG. Should clot composition affect choice of endovascular therapy? Neurology. 2012;79:S63–67. doi: 10.1212/WNL.0b013e3182695859. [DOI] [PubMed] [Google Scholar]
- 17.van der Marel K, Chueh JY, Brooks OW, et al. Quantitative assessment of device-clot interaction for stent retriever thrombectomy. J Neurointerv Surg. 2016 doi: 10.1136/neurintsurg-2015-012209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nogueira RG, Levy EI, Gounis M. The trevo device: Preclinical data of a novel stroke thrombectomy device in two different animal models of arterial thrombo-occlusive disease. J Neurointerv Surg. 2012;4:295–300. doi: 10.1136/neurintsurg-2011-010053. [DOI] [PubMed] [Google Scholar]
- 19.De Meyer SF, Andersson T, Baxter B, et al. Analyses of thrombi in acute ischemic stroke: A consensus statement on current knowledge and future directions. Int J Stroke. 2017;12:606–614. doi: 10.1177/1747493017709671. [DOI] [PubMed] [Google Scholar]
- 20.Brinjikji W, Duffy S, Burrows A, et al. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: A systematic review. J Neurointerv Surg. 2017;9:529–534. doi: 10.1136/neurintsurg-2016-012391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dargazanli C, Rigau V, Eker O, et al. High cd3+ cells in intracranial thrombi represent a biomarker of atherothrombotic stroke. PLoS One. 2016;11:e0154945. doi: 10.1371/journal.pone.0154945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fennell VS, Setlur Nagesh SV, et al. What to do about fibrin rich ‘tough clots’? Comparing the solitaire stent retriever with a novel geometric clot extractor in an in vitro stroke model. J Neurointerv Surg. 2018 doi: 10.1136/neurintsurg-2017-013507. [DOI] [PubMed] [Google Scholar]
- 23.Chueh JY, Kuhn AL, Puri AS, et al. Reduction in distal emboli with proximal flow control during mechanical thrombectomy: A quantitative in vitro study. Stroke. 2013;44:1396–1401. doi: 10.1161/STROKEAHA.111.670463. [DOI] [PubMed] [Google Scholar]
- 24.Brinjikji W, Starke RM, Murad MH, et al. Impact of balloon guide catheter on technical and clinical outcomes: A systematic review and meta-analysis. J Neurointerv Surg. 2017 Jul 28; doi: 10.1136/neurintsurg-2017-013179. pii: neurintsurg-2017–013179. [DOI] [PubMed] [Google Scholar]
- 25.Riedel CH, Zoubie J, Ulmer S, et al. Thin-slice reconstructions of nonenhanced ct images allow for detection of thrombus in acute stroke. Stroke. 2012;43:2319–2323. doi: 10.1161/STROKEAHA.112.649921. [DOI] [PubMed] [Google Scholar]
- 26.Heo JH, Kim K, Yoo J, et al. Computed tomography-based thrombus imaging for the prediction of recanalization after reperfusion therapy in stroke. J Stroke. 2017;19:40–49. doi: 10.5853/jos.2016.01522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaschka IN, Kloska SP, Struffert T, et al. Clot burden and collaterals in anterior circulation stroke: Differences between single-phase cta and multi-phase 4d-cta. Clin Neuroradiol. 2016;26:309–315. doi: 10.1007/s00062-014-0359-6. [DOI] [PubMed] [Google Scholar]
- 28.Santos EMM, d’Esterre CD, Treurniet KM, et al. Added value of multiphase cta imaging for thrombus perviousness assessment. Neuroradiology. 2018;60:71–79. doi: 10.1007/s00234-017-1907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganeshan R, Nave AH, Scheitz JF, et al. Assessment of thrombus length in acute ischemic stroke by post-contrast magnetic resonance angiography. J Neurointerv Surg. 2017 doi: 10.1136/neurintsurg-2017-013454. [DOI] [PubMed] [Google Scholar]
- 30.Brinjikji W, Michalak G, Kadirvel R, et al. Utility of single-energy and dual-energy computed tomography in clot characterization: An in-vitro study. Interv Neuroradiol. 2017;23:279–284. doi: 10.1177/1591019917694479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SK, Yoon W, Kim TS, et al. Histologic analysis of retrieved clots in acute ischemic stroke: Correlation with stroke etiology and gradient-echo mri. AJNR Am J Neuroradiol. 2015;36:1756–1762. doi: 10.3174/ajnr.A4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jindal G, Miller T, Shivashankar R, et al. Relationship of thrombus length to number of stent retrievals, revascularization, and outcomes in acute ischemic stroke. J Vasc Interv Radiol. 2014;25:1549–1557. doi: 10.1016/j.jvir.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Borst J, Berkhemer OA, Santos EMM, et al. Value of thrombus ct characteristics in patients with acute ischemic stroke. AJNR Am J Neuroradiol. 2017;38:1758–1764. doi: 10.3174/ajnr.A5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santos EMM, Dankbaar JW, Treurniet KM, et al. Permeable thrombi are associated with higher intravenous recombinant tissue-type plasminogen activator treatment success in patients with acute ischemic stroke. Stroke. 2016;47:2058–2065. doi: 10.1161/STROKEAHA.116.013306. [DOI] [PubMed] [Google Scholar]
- 35.Jang IK, Gold HK, Ziskind AA, et al. Differential sensitivity of erythrocyte-rich and platelet-rich arterial thrombi to lysis with recombinant tissue-type plasminogen activator. A possible explanation for resistance to coronary thrombolysis. Circulation. 1989;79(4):920–928. doi: 10.1161/01.cir.79.4.920. [DOI] [PubMed] [Google Scholar]
- 36.Booth NA, Robbie LA, Croll AM, et al. Lysis of platelet-rich thrombi: the role of PAI-1. Ann N Y Acad Sci. 1992;667:70–80. doi: 10.1111/j.1749-6632.1992.tb51599.x. [DOI] [PubMed] [Google Scholar]
- 37.Rusak T, Piszcz J, Misztal T, et al. Platelet-related fibrinolysis resistance in patients suffering from PV. Impact of clot retraction and isovolemic erythrocytapheresis. Thromb Res. 2014;134(1):192–198. doi: 10.1016/j.thromres.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 38.Tomkins AJ, Schleicher N, Murtha L, et al. Platelet rich clots are resistant to lysis by thrombolytic therapy in a rat model of embolic stroke. Exp Transl Stroke Med. 2015;7(1):1317. doi: 10.1186/s13231-014-0014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boeckh-Behrens T, Kleine JF, Zimmer C, et al. Thrombus Histology Suggests Cardioembolic Cause in Cryptogenic Stroke. Stroke. 2016 Jul;47(7):1864–71. doi: 10.1161/STROKEAHA.116.013105. [DOI] [PubMed] [Google Scholar]
- 40.Simons N, Mitchell P, Dowling R, et al. Thrombus composition in acute ischemic stroke: a histopathological study of thrombus extracted by endovascular retrieval. J Neuroradiol. 2015;42(2):86–92. doi: 10.1016/j.neurad.2014.01.124. [DOI] [PubMed] [Google Scholar]
- 41.Choi MH, Park GH, Lee JS, et al. Erythrocyte Fraction Within Retrieved Thrombi Contributes to Thrombolytic Response in Acute Ischemic Stroke. Stroke. 2018 Jan 26; doi: 10.1161/STROKEAHA.117.019138. pii STROKEAHA.117.019138. [DOI] [PubMed] [Google Scholar]
- 42.Sporns PB, Hanning U, Schwindt W, et al. Ischemic Stroke: What Does the Histological Composition Tell Us About the Origin of the Thrombus? Stroke. 2017 Aug;48(8):2206–2210. doi: 10.1161/STROKEAHA.117.016590. [DOI] [PubMed] [Google Scholar]
- 43.Niesten JM, van der Schaaf IC, van Dam L, et al. Histopathologic composition of cerebral thrombi of acute stroke patients is correlated with stroke subtype and thrombus attenuation. PLoS ONE. 2014;9(2):e88882. doi: 10.1371/journal.pone.0088882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Meyer SF, Stoll G, Wagner DD, et al. von Willebrand factor: an emerging target in stroke therapy. Stroke. 2012;43(2):599–606. doi: 10.1161/STROKEAHA.111.628867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Meyer SF, Deckmyn H, Vanhoorelbeke K. von Willebrand factor to the rescue. Blood. 2009;113(21):5049–5057. doi: 10.1182/blood-2008-10-165621. [DOI] [PubMed] [Google Scholar]
- 46.De Meyer SF, Denorme F, Langhauser F, et al. Thromboinflammation in Stroke Brain Damage. Stroke. 2016;47(4):1165–1172. doi: 10.1161/STROKEAHA.115.011238. [DOI] [PubMed] [Google Scholar]
- 47.Verhenne S, Denorme F, Libbrecht S, et al. Platelet-derived VWF is not essential for normal thrombosis and hemostasis but fosters ischemic stroke injury in mice. Blood. 2015;126(14):1715–1722. doi: 10.1182/blood-2015-03-632901. [DOI] [PubMed] [Google Scholar]
- 48.Kleinschnitz C, De Meyer SF, Schwarz T, et al. Deficiency of von Willebrand factor protects mice from ischemic stroke. Blood. 2009;113(15):3600–3603. doi: 10.1182/blood-2008-09-180695. [DOI] [PubMed] [Google Scholar]
- 49.De Meyer SF, Schwarz T, Deckmyn H, et al. Binding of von Willebrand factor to collagen and glycoprotein Ibalpha, but not to glycoprotein IIb/IIIa, contributes to ischemic stroke in mice--brief report. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(10):1949–1951. doi: 10.1161/ATVBAHA.110.208918. [DOI] [PubMed] [Google Scholar]
- 50.Denorme F, Langhauser F, Desender L, et al. ADAMTS13-mediated thrombolysis of t-PA-resistant occlusions in ischemic stroke in mice. Blood. 2016;127(19):2337–2345. doi: 10.1182/blood-2015-08-662650. [DOI] [PubMed] [Google Scholar]
- 51.Momi S, Tantucci M, Van Roy M, et al. Reperfusion of cerebral artery thrombosis by the GPIb-VWF blockade with the Nanobody ALX-0081 reduces brain infarct size in guinea pigs. Blood. 2013;121(25):5088–5097. doi: 10.1182/blood-2012-11-464545. [DOI] [PubMed] [Google Scholar]
- 52.Le Behot A, Gauberti M, de Lizarrondo SM, et al. GpIbα-VWF blockade restores vessel patency by dissolving platelet aggregates formed under very high shear rate in mice. Blood. 2014;123(21):3354–3363. doi: 10.1182/blood-2013-12-543074. [DOI] [PubMed] [Google Scholar]
- 53.Martinez de Lizarrondo S, Gakuba C, Herbig BA, et al. Potent Thrombolytic Effect of N-Acetylcysteine on Arterial Thrombi. Circulation. 2017 Aug 15;136(7):646–660. doi: 10.1161/CIRCULATIONAHA.117.027290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.López-Cancio E, Millán M, Pérez de la Ossa N, et al. Immunohistochemical study of clot composition in thrombi retrieved from MCA with mechanical thrombectomy. Cerebrovasc Dis. 2013;35(Suppl 3):255. [Google Scholar]
- 55.Niesten JM, van der Schaaf IC, van der Graaf Y, et al. Predictive value of thrombus attenuation on thin-slice non-contrast CT for persistent occlusion after intravenous thrombolysis. Cerebrovasc Dis. 2014;37(2):116–122. doi: 10.1159/000357420. [DOI] [PubMed] [Google Scholar]
- 56.Laridan E, Denorme F, Desender L, et al. Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol. 2017;313:1451–10. doi: 10.1002/ana.24993. [DOI] [PubMed] [Google Scholar]
- 57.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 58.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107(36):15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martinod K, Wagner DD. Thrombosis: tangled up in NETs. Blood. 2014;123(18):2768–2776. doi: 10.1182/blood-2013-10-463646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Savchenko AS, Martinod K, Seidman MA, et al. Neutrophil extracellular traps form predominantly during the organizing stage of human venous thromboembolism development. J Thromb Haemost. 2014 Jun;12(6):860–70. doi: 10.1111/jth.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ducroux C, Di Meglio L, Loyau S, et al. Thrombus Neutrophil Extracellular Traps Content Impair tPA-Induced Thrombolysis in Acute Ischemic Stroke. Stroke. 2018 doi: 10.1161/STROKEAHA.117.019896. STROKE AHA.117.019896. [DOI] [PubMed] [Google Scholar]
- 62.Jiménez-Alcázar M, Rangaswamy C, Panda R, et al. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science. 2017;358(6367):1202–1206. doi: 10.1126/science.aam8897. [DOI] [PubMed] [Google Scholar]
- 63.Mangold A, Alias S, Scherz T, et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res. 2015;116(7):1182–1192. doi: 10.1161/CIRCRESAHA.116.304944. [DOI] [PubMed] [Google Scholar]
- 64.De Meyer SF, Suidan GL, Fuchs TA, et al. Extracellular chromatin is an important mediator of ischemic stroke in mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(8):1884–1891. doi: 10.1161/ATVBAHA.112.250993. [DOI] [PMC free article] [PubMed] [Google Scholar]