Abstract
Purpose
Postoperative vitreous hemorrhage is a vision-impacting complication of vitrectomy. This preclinical in vitro study assessed the potential ability of a nonswelling polyethylene glycol-based artificial vitreous hydrogel to maintain transparency in the vitreous cavity in the presence of vitreous hemorrhage.
Methods
Samples (1 mL) of diluted blood at concentrations of 0.1%, 0.25%, 0.5%, and 1.25% were added to 1 mL samples of polymerized hydrogel in cuvettes (gel + blood group); 2 mL samples of diluted blood at the same concentrations were prepared as controls (blood only group). Spectral transmission curves for the hydrogel (gel + blood group) and diluted blood (blood only group) were obtained before and on days 1, 2, 5, 7, 14, and 28 of the experiment. Between-group comparisons were made using the Student's t-test. The percentage of transmittance in the visible light spectrum (400–700 nm) was measured at each time point.
Results
Mean light transmittance was maintained at >90% until day 7 in the gel + blood group and was significantly greater in the gel + blood than in the blood only groups in samples containing blood diluted to 0.25%, 0.5%, and 1.25% during the 28-day study period (P < 0.05).
Conclusions
A nonswelling polyethylene glycol-based artificial vitreous hydrogel maintained high optical transparency in the presence of blood through the study period. Injection of this hydrogel into the vitreous cavity at the end of surgery might help to prevent or mitigate vitreous hemorrhage-associated postoperative visual loss.
Translational Relevance
The hydrogel may prevent visual loss due to postoperative vitreous hemorrhage.
Keywords: artificial vitreous, hydrogel, vitreous hemorrhage, transparency
Introduction
Early postoperative vitreous hemorrhage is a common and serious clinical problem that can cause a significant decrease in the patient's vision and poor visualization of the fundus. Early vitreous hemorrhage develops after vitrectomy in 13% to 45% of patients, particularly in those with proliferative diabetic retinopathy, with some patients requiring additional treatment.1–4 Intraocular tamponade with air, gas, or silicone oil at the end of surgery would afford visualization of the fundus in the early postoperative period despite postoperative vitreous hemorrhage. However, a decrease in the patient's visual function as a result of the refractive change in response to intraocular tamponade material is inevitable.5–7
Artificial vitreous bodies that resemble the natural human vitreous body, including its refractive index and transparency, have been developed recently and are expected to be available for clinical use as new-generation intraocular tamponade materials in the future.8–11 We developed an artificial vitreous body, called oligo-Tetra-PEG hydrogel, a nonswelling fast-forming hydrogel with ultralow polymeric content, and have used it successfully to treat experimental retinal detachment in an animal model over a year.12 Intraocular tamponade with an artificial vitreous body at the end of surgery could maintain transparency in the vitreous cavity as well as the patient's vision and visualization of the fundus, despite postoperative vitreous hemorrhage.
To our knowledge, this is the first study to assess the potential ability of an artificial vitreous body, that is, nonswelling oligo-Tetra-PEG hydrogel, to maintain transparency in the vitreous cavity in the presence of vitreous hemorrhage in vitro.
Methods
Preparation of PEG Solutions
In this experiment, we used oligo-Tetra-PEG hydrogels that we developed previously.12 In brief, to form these hydrogels, we used mutually reactive tetra-armed polyethylene glycol (PEG) with thiol termini (Tetra-PEG-SH, Sunbright PTE-100SH; Nichiyu Kabushikigaisha, Tokyo, Japan), maleimide termini (Tetra-PEG-MA, Sunbright PTE-100MA; Nichiyu Kabushikigaisha), and acrylamide termini (Tetra-PEG-ACR, 4arm-ACLT-10K, JenKem Technology, Plano, TX). MAex-SH PEG solution 0.6 wt % was prepared by mixing Tetra-PEG-MA and Tetra-PEG-SH in a ratio of 0.68 to 0.32 in 5 mM citrate-phosphate buffer (pH 5.0) including 149 mM NaCl. SHex-ACR PEG solution 0.6 wt % was prepared by mixing Tetra-PEG-SH and Tetra-PEG-ACR in a ratio of 0.68 to 0.32 in 2 mM phosphate buffer (pH 7.8) including 152 mM NaCl.
Preparation of Diluted Blood Samples
Whole rat blood was collected, anticoagulated with acid-citrate-dextrose, and diluted with balanced salt solution to concentrations of 1.25%, 0.5%, 0.25%, and 0.1%.
Experimental Protocol
Samples (1 mL) of PEG hydrogel were polymerized in disposable plastic cuvettes (UVC-SMAll; As One Corporation, Osaka, Japan) by injecting two oligo-Tetra-PEG solutions, that is, MAex-SH PEG solution and SHex-ACR PEG solution, using a double-syringe system. After 1 hour, which is sufficient for complete polymerization of the hydrogel, a 1-mL diluted blood sample of each concentration was added onto the polymerized hydrogel (gel + blood group). The samples were triplicated so that a total of 12 samples were prepared. The cuvettes were sealed with plastic paraffin film. The samples were stored at 4°C (to prevent contamination) on a shaker set to a speed of 100 rpm.
As controls, 2-mL diluted blood samples of each concentration without hydrogel were prepared in disposable plastic cuvettes (blood only group). The samples were triplicated so that a total of 12 samples were prepared. The samples were stored at 4°C on a shaker set to a speed of 100 rpm.
Spectral transmittance curves were measured using a spectrophotometer (UV-2700, Shimadzu, Tokyo, Japan) before and 1, 2, 5, 7, 14, 21, and 28 days after addition of the diluted blood samples onto the hydrogels in the gel + blood group, and at the time of placing the diluted blood samples on the shaker in the blood only group. Spectral transmittance curves for the hydrogel and for diluted blood without hydrogel were measured in the gel + blood and blood only groups, respectively (Fig. 1). The samples were mixed gently by inverting the cuvettes several times before each measurement. All measurements were performed at room temperature with the following settings: wavelength 220 to 800 nm, slit width 1.0 nm, slit height 12 mm, and data interval 1.0 nm. Air was used as a reference to measure transmittance. The background transmittance spectra were checked to ensure that 100% ± 0.5% transmittance was achieved. The results were expressed as the mean percentage of light transmittance in the visible spectrum (400–700 nm).
Figure 1.
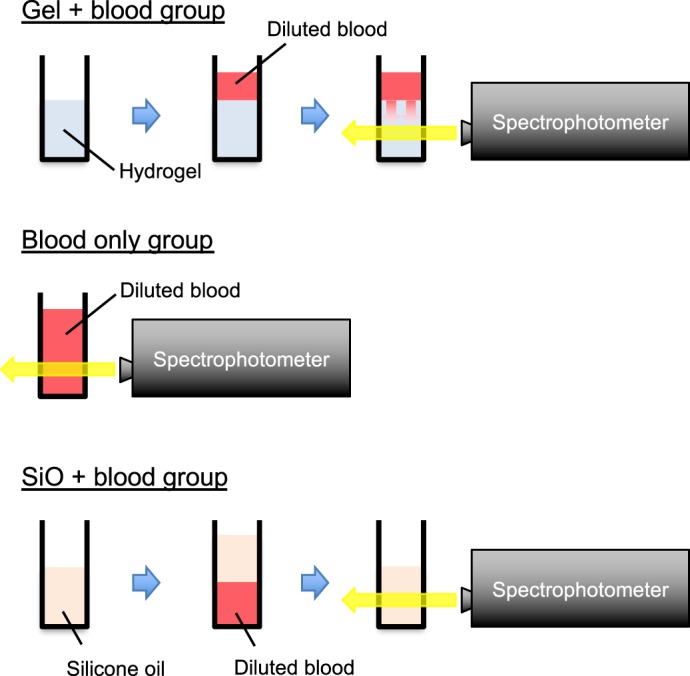
Scheme for measurement of spectral transmittance curves in the gel + blood, blood only, and SiO + blood groups. Spectral transmittance curves for hydrogel and diluted blood without hydrogel were measured in the gel + blood and blood only groups, respectively. In the SiO + blood group, silicone oil was transferred to the other cuvettes to measure the spectral transmittance curves for silicone oil.
As a referential control, 1-mL samples of silicone oil (Silicon; Alcon Laboratories, Fort Worth, TX) with a 1-mL blood sample diluted to 1.25% were prepared in disposable plastic cuvettes (SiO + blood group). Unlike hydrogel, silicone oil flows up on a diluted blood sample, so it was not possible to measure transmittance of silicone oil because the measurement light was fixed in the lower part of the cuvette. Therefore, we temporarily moved the silicone oil to the other cuvettes when measuring its transmittance (Fig. 1, SiO + blood group). After the measurements were obtained, the silicone oil was returned to the cuvettes containing the diluted blood samples. Spectral transmittance curves were measured using the spectrophotometer before and 7, 14, and 28 days after addition of the diluted blood samples to the silicone oil in the SiO + blood group. The samples were triplicated. Other conditions of measurement were the same as those mentioned above.
Statistical Analysis
The mean percentage of light transmittance in the visible spectrum was compared between the gel + blood and blood only groups at each time point using the Student's t-test. Statistical analysis was performed using Statcel (add-in software for Microsoft Excel) version 4 (Microsoft Corp., Redmond, WA).
Results
Figure 2a shows the time course of the mean percentage of light transmittance in the visible spectrum in the gel + blood and blood only groups during the 28-day experiment, along with a representative photograph of each sample taken before, and 7, 14, and 28 days after preparation.
Figure 2.
(a) Time course of mean percentage of light transmittance in the visible spectrum in the gel + blood and blood only groups during the 28-day experiment, along with a representative photograph of each sample taken before, and 7, 14, and 28 days after preparation. In the gel + blood group, >90% mean light transmittance was maintained for the first 7 days of the experiment and decreased slightly thereafter. In contrast, mean light transmittance for each concentration in the blood only group remained at <25% until day 5.
In the gel + blood group, >90% mean light transmittance was maintained for the first 7 days of the experiment and decreased slightly thereafter. In contrast, mean light transmittance for each concentration in the blood only group remained at <25% until day 5; in the samples containing blood diluted to 0.25%, 0.5%, and 1.25%, mean light transmittance remained at <10% until day 7. In the blood only group, mean light transmittance in the sample containing blood diluted to 0.1% increased dynamically from days 5 to 14, and reached a plateau after day 14. A relatively dynamic increase in mean light transmittance was observed in the blood only group from days 7 to 21, 7 to 28, and 14 to 28 in the samples diluted to 0.25%, 0.5%, and 1.25%, respectively.
Throughout the 28-day experiment, mean light transmittance in the gel + blood group was significantly greater than that in blood only group in the samples containing blood diluted to 0.25%, 0.5%, and 1.25% (P < 0.05). Mean light transmittance in the samples diluted to 0.1% was significantly greater in the gel + blood than in the blood only groups until day 7 (P < 0.01), but the difference was not statistically significant after day 14.
Figure 2b shows the time course of the mean percentage of light transmittance in the visible spectrum in the gel + blood and SiO + blood groups containing blood diluted to 1.25% during the 28-day experiment, along with a representative photograph of each sample taken before, and 7, 14, and 28 days after preparation. Throughout the experiment, mean light transmittance in the SiO + blood group was maintained (>93%) in the samples containing blood diluted to 1.25%. Mean light transmittance was significantly greater in the SiO + blood than in the gel + blood groups at day 28 (P < 0.05), but the difference was not statistically significant before or 7 and 14 days after preparation.
Figure 2.

(b) Time course of mean percentage of light transmittance in the visible spectrum in the gel + blood and SiO + blood groups (with a 1.25% diluted blood sample) during the 28-day experiment, along with a representative photograph of each sample taken before and 7, 14, and 28 days after preparation. Mean light transmittance was significantly greater in the SiO + blood than in the gel + blood groups at day 28 (P < 0.05), but the difference was not statistically significant before or 7 and 14 days after preparation.
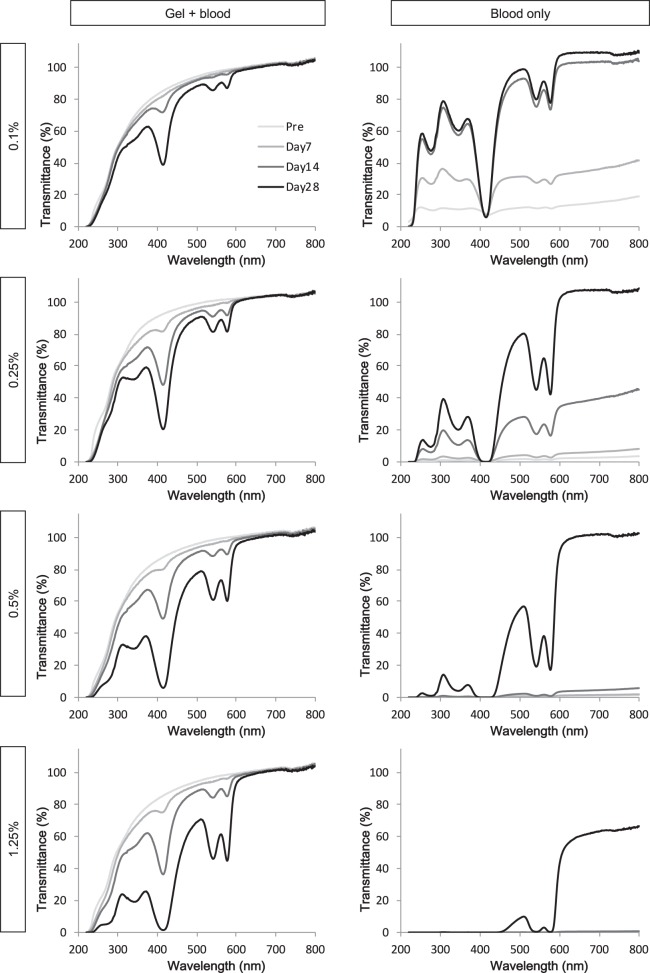
Figure 3 shows representative spectral transmittance curves for each concentration (0.1%, 0.25%, 0.5%, and 1.25%) of diluted blood samples in the two study groups on days 0, 7, 14, and 28. In the gel + blood group, there was a decrease in light transmittance around the wavelengths of 410, 530, and 575 nm after day 7, which became greater over time. In the blood only group, overall light transmittance increased over time, again with decreases at wavelengths of approximately 410, 530, and 575 nm.
Figure 3.
Representative spectral transmittance curves for each concentration (0.1%, 0.25%, 0.5%, and 1.25%) of diluted blood samples in the two study groups on days 0, 7, 14, and 28. In the gel + blood group, there was a decrease in light transmittance at wavelengths of approximately 410, 530, and 575 nm after day 7 that became greater over time.
Discussion
In this study, a nonswelling PEG-based artificial vitreous hydrogel maintained high optical transparency in the presence of blood throughout a 28-day observation period. Postoperative vitreous hemorrhage usually takes several weeks to resolve spontaneously,13 and would take even longer to resolve if it was of a thicker consistency, as represented by the 0.5% and 1.25% concentrations used in our study. Our findings suggested that good vision could be expected in the early postoperative period regardless of the presence of postoperative vitreous hemorrhage if the vitreous cavity is filled with this hydrogel at the end of surgery. Intraocular tamponade with air, gas, or silicone oil, all of which have hydrophobic behavior, also can maintain high optical transparency by preventing dispersion of the hemorrhage into the vitreous cavity. We found that the SiO + blood group maintained high light transmittance throughout the study period. However, these agents produce undesirable changes in the refraction of the eye because of their higher (1.40 in silicone oil) or lower (1.00 in air and gas) refractive index compared to the natural human vitreous (1.33).14,15 In contrast, the refractive index of oligo-Tetra-PEG hydrogel is almost the same as that of aqueous fluid, because it has a water content of 99.4%. Furthermore, the hydrophilic behavior of the hydrogel prevents disordering of refraction at the surface of the aqueous fluid, so it can maintain an optical quality similar to that of the human vitreous.
Decreases in the percentage of light transmittance at approximately 415, 540, and 580 nm were observed after day 7 in our gel + blood group. These wavelengths corresponded to the range of absorption wavelengths for oxyhemoglobin.16 This finding suggested that the oxyhemoglobin eluted during hemolysis infiltrated the hydrogel after day 7, but there was no infiltration of red blood cells (RBCs) that might decrease the optical quality of the hydrogel.
Recently, a considerable amount of research attention has been focused on development of artificial vitreous bodies with hydrophilic behavior in an effort to overcome the shortcomings of the present clinically available vitreous substitutes, such as gas and silicone oil, which are hydrophobic. One of the main advantages of using hydrophilic material as a vitreous substitute is that the mobility of bioactive agents can be preserved whereas gas and silicone oil prevent the mobility of these agents. It is reported that bioactive agents, such as fibrogenic growth factors17 and inflammatory cytokines,18 concentrate in the fluid between the silicone oil and retina, which might result in epiretinal membrane and cellular proliferation in silicone oil-filled eyes. Given that 99.4% of the hydrogel consists of water and the mobility of bioactive agents is assumed to be highly preserved, replacement of the vitreous with hydrogel would not accelerate formation of epiretinal membrane; however, further investigations will be needed to determine whether this is the case.
While a hydrophilic vitreous body has the aforementioned advantages, concerns exist about whether hydrophilic substitutes can maintain transparency in the presence of hemorrhage, which has not been a problem with hydrophobic materials, such as gas and silicone oil. To our knowledge, this is the first in vitro study to investigate the potential ability of an artificial vitreous body to maintain transparency in the presence of vitreous hemorrhage.
The reason for the decrease in light transmittance in the presence of postoperative vitreous hemorrhage is that RBCs cause scattering of light. Therefore, for a vitreous substitute to maintain transparency, infiltration of RBCs must be prevented. A hydrogel consists of a three-dimensional network of polymer chains. If the mesh size between the polymer chains is smaller than the size of RBCs (approximately 7000 nm), RBCs cannot infiltrate the hydrogel.
The mesh size is not consistent in hydrogels, so it is difficult to evaluate. The mesh size of a model PEG hydrogel that overcomes the heterogeneity problem is in the order of several nanometers,19 and some reports on hydrogels designed for medical use describe their mesh size as being in the single-digit to double-digit nanometer range.20,21 The mesh size of our nonswelling PEG hydrogel seems to be very similar to that of the above hydrogels (data not shown). Therefore, this artificial vitreous hydrogel could prevent infiltration of RBCs and maintain transparency in the vitreous cavity in the presence of postoperative vitreous hemorrhage.
On the other hand, oxyhemoglobin (size, 7 nm) is small enough to pass through the polymer network of the hydrogel. However, concerns remain that the time taken for oxyhemoglobin to elute out of the eye would be longer than that for ophthalmic irrigation solution.
This was a preliminary in vitro investigation that has little physiologic relevance to the eye and has some limitations. The samples were stored at 4°C on a shaker set to a speed of 100 rpm; however, RBCs would break down more rapidly at a physiologic temperature, and the shaker used would not necessarily reproduce the motion of the eye, which has many degrees of freedom in contrast with the shaker despite the tube being inverted. The number of RBCs in the gel + blood group was twice that in the blood only group, which should be borne in mind when interpreting the study results. Given the hydrophobic nature of silicone oil, the methodology used to measure light transmittance was not exactly the same in the other two groups, that is, the gel + blood and blood only groups. Therefore, the results for the SiO + blood group can be considered only referential. The safety of the hydrogel used in this study had been tested previously in rabbit eyes over a one-year period.12 However, further investigations are needed before it can be approved for clinical use.
The various artificial vitreous substitutes that have been developed recently using hydrogels are intended to have a tamponade effect in the treatment of retinal detachment. However, injection of an artificial vitreous hydrogel at the conclusion of vitreous surgery also may be beneficial in terms of preservation of the optical transparency of the vitreous by preventing infiltration of hemorrhage in patients with retinal detachment and a high risk of early postoperative vitreous hemorrhage, such as those with proliferative diabetic retinopathy.
Acknowledgments
The authors thank Editage (www.editage.jp) for English language editing.
Supported by the Japan Agency for Medical Research and Development, AMED (JP17lm0203022) and Nidek Co., Ltd.
Disclosure: S. Hoshi, None; F. Okamoto, None; T. Murakami, None; T. Sakai, None; Y. Shinohara, None; T. Fujii, None; M. Nakatani, None; T. Oshika, None
References
- 1.Lo W, Kim S, Aaberg T, et al. Visual outcomes and incidence of recurrent vitreous hemorrhage after vitrectomy in diabetic eyes pretreated with bevacizumab (Avastin) Retina. 2009;29:926–931. doi: 10.1097/IAE.0b013e3181a8eb88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oshima Y, Shima C, Wakabayashi T, et al. Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology. 2009;116:927–938. doi: 10.1016/j.ophtha.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Ahmadieh H, Shoeibi N, Entezari M, Monshizadeh R. Intravitreal bevacizumab for prevention of early postvitrectomy hemorrhage in diabetic patients: a randomized clinical trial. Ophthalmology. 2009;116:1943–1948. doi: 10.1016/j.ophtha.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Lee B, Yu HG. Vitreous hemorrhage after the 25-gauge transconjunctival sutureless vitrectomy for proliferative diabetic retinopathy. Retina. 2010;30:1671–1677. doi: 10.1097/IAE.0b013e3181dcfb79. [DOI] [PubMed] [Google Scholar]
- 5.Mateo-Montoya A, de Smet MD. Air as tamponade for retinal detachments. Eur J Ophthalmol. 2014;24:242–246. doi: 10.5301/ejo.5000373. [DOI] [PubMed] [Google Scholar]
- 6.Hotta K, Sugitani A. Refractive changes in silicone oil-filled pseudophakic eyes. Retina. 2005;25:167–170. doi: 10.1097/00006982-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Smith R, Smith G, Wong D. Refractive changes in silicone-filled eyes. Eye (Lond) 1990;4:230–234. doi: 10.1038/eye.1990.32. [DOI] [PubMed] [Google Scholar]
- 8.Santhanam S, Liang J, Struckhoff J, Hamilton P, Ravi N. Biomimetic hydrogel with tunable mechanical properties for vitreous substitutes. Acta Biomater. 2016;43:327–337. doi: 10.1016/j.actbio.2016.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uesugi K, Sakaguchi H, Hayashida Y, et al. A self-assembling peptide gel as a vitreous substitute: A rabbit study. Invest Ophthalmol Vis Sci. 2017;58:4068–4075. doi: 10.1167/iovs.17-21536. [DOI] [PubMed] [Google Scholar]
- 10.Schnichels S, Schneider N, Hohenadl C, et al. Efficacy of two different thiol-modified crosslinked hyaluronate formulations as vitreous replacement compared to silicone oil in a model of retinal detachment. PLoS One. 2017;12:e0172895. doi: 10.1371/journal.pone.0172895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang X, Peng Y, Yang C, Liu W, Han B. The feasibility study of an in situ marine polysaccharide-based hydrogel as the vitreous substitute. J Biomed Mater Res Part A. 2018;106:1997–2006. doi: 10.1002/jbm.a.36403. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi K, Okamoto F, Hoshi S, et al. Fast-forming hydrogel with ultralow polymeric content as an artificial vitreous body. Nat Biomed Eng. 2017;1:0044. [Google Scholar]
- 13.Schachat A, Oyakawa R, Michels R, Rice TA. Complications of vitreous surgery for diabetic retinopathy. II. Postoperative complications. Ophthalmology. 1983;90:522–530. doi: 10.1016/s0161-6420(83)34540-1. [DOI] [PubMed] [Google Scholar]
- 14.Stefansson E, Anderson M, Jr, Landers M, III, Tiedeman J, McCuen B., II Refractive changes from use of silicone oil in vitreous surgery. Retina. 1998;8:20–23. doi: 10.1097/00006982-198808010-00005. [DOI] [PubMed] [Google Scholar]
- 15.Tables of Physical & Chemical Constants 16th ed. Kaye & Laby Online; 1995. Chapter 2.5.7 Refractive index of gases. Version 1.0 (2005) Available from: www.kayelaby.npl.co.uk Accessed October 4, 2018. [Google Scholar]
- 16.Prahl S. Optical absorption of hemoglobin. (1999) Available from: http://omlc.ogi.edu/spectra/hemoglobin/index.html Accessed September 5, 2018.
- 17.Asaria R, Kon C, Bunce C, et al. Silicone oil concentrates fibrogenic growth factors in the retro-oil fluid. Br J Ophthalmol. 2004;88:1439–1442. doi: 10.1136/bjo.2003.040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaneko H, Takayama K, Asami T, et al. Cytokine profiling in the subsilicone oil fluid after vitrectomy surgeries for refractory retinal disease. Sci Rep. 2017;7:2640. doi: 10.1038/s41598-017-03124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsuji Y, Li X, Shibayama M. Evaluation of mesh size in model polymer networks consisting of tetra-arm and linear poly(ethylene glycol)s. Gels. 2018;4:50. doi: 10.3390/gels4020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao H, Munoz-Pinto D, Qu X, Hou Y, Grunlan M, Hahn MS. Influence of hydrogel mechanical properties and mesh size on vocal fold fibroblast extracellular matrix production and phenotype. Acta Biomater. 2008;4:1161–1171. doi: 10.1016/j.actbio.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uruena J, Pitenis A, Nixon R, Schulzea K, Angelini T, Sawyer WG. Mesh size control of polymer fluctuation lubrication in gemini hydrogels. Biotribology. 2015;1–2:24–29. [Google Scholar]