Abstract
After encouraging preclinical and human dosimetry results for the novel estrogen receptor (ER) PET radiotracer 4-fluoro-11β-methoxy-16α-18F-fluoroestradiol (18F-4FMFES), a phase II clinical trial was initiated to compare the PET imaging diagnostic potential of 18F-4FMFES with that of 16α-18F-fluoroestradiol (18F-FES) in ER-positive (ER+) breast cancer patients. Methods: Patients diagnosed with ER+ breast cancer (n = 31) were recruited for this study, including 6 who underwent mastectomy or axillary node dissection. For each patient, 18F-FES and 18F-4FMFES PET/CT scans were done sequentially (within a week) and in random order. One hour after injection of either radiotracer, a head-to-thigh static scan with a 2-min acquisition per bed position was obtained. Blood samples were taken at different times after injection to assess each tracer metabolism by reverse-phase thin-layer chromatography. The SUVmean of nonspecific tissues and the SUVmax of the tumor were evaluated for each detected lesion, and tumor-to-nonspecific organ ratios were calculated. Results: Blood metabolite analysis 60 min after injection of the tracer showed a 2.5-fold increase in metabolic stability of 18F-4FMFES over 18F-FES. Although for most foci 18F-4FMFES PET had an SUVmax similar to that of 18F-FES PET, tumor contrast improved substantially in all cases. Lower uptake was consistently observed in nonspecific tissues for 18F-4FMFES, notably a 4-fold decrease in blood-pool activity as compared with 18F-FES. Consequently, image quality was considerably improved using 18F-4FMFES, with lower overall background activity. As a result, 18F-4FMFES successfully identified 9 more lesions than 18F-FES. Conclusion: This phase II study with ER+ breast cancer patients showed that 18F-4FMFES PET achieves a lower nonspecific signal and better tumor contrast than 18F-FES PET, resulting in improved diagnostic confidence and lower false-negative diagnoses.
Keywords: receptor PET imaging, ER+, breast cancer, 18F-FES, 18F-4FMFES
Knowledge of the estrogen receptor (ER) status is of the utmost importance for the prognosis and choice of treatment modality in breast cancer. Although biopsy is the gold standard to determine ER status in the clinic, substantial effort has been made to assess this parameter noninvasively. 16α-18F-fluoroestradiol (18F-FES) was successfully used in combination with PET imaging to detect ER-positive (ER+) breast cancers in preclinical (1–3) and clinical (4–7) settings. 18F-FES uptake was shown to correlate well with ER concentrations (6) and ER expression as analyzed by the immunohistochemistry index (8,9) but not with ER gene expression (9). 18F-FES PET imaging was used in a clinical study to follow ER modulation of metastatic breast cancers under different hormone therapies (10). Furthermore, functional imaging of ER+ endometrial cancers was demonstrated using 18F-FES PET (11), and in combination with 18F-FDG PET, it was possible to assess the aggressiveness of such carcinomas (12).
Despite the demonstrated usefulness of 18F-FES for whole-body monitoring of ER status in gynecologic cancers, this tracer still has some shortcomings. First, 18F-FES is rapidly metabolized in the liver by formation of gluconate and sulfate conjugates (13), which results in an increase in low-affinity radiometabolites in the blood and nonspecific tissues. As a result, nonspecific background activity is increased, particularly in the mediastinal region, reducing the tumor contrast and the overall image quality. Second, steroid-based tracers such as 18F-FES are bound to have strong hepatic uptake and biliary excretion, resulting in high background activity in the liver and intestines, which could impair detection of lesions in those regions. Third, 18F-FES has affinity to plasma globulins such as sex hormone–binding globulin and albumin, and the percentage of bound 18F-FES depends mainly on the plasma concentration of sex hormone–binding globulin. Since globulin-bound 18F-FES is not available to target the receptors, sex hormone–binding globulin level is inversely correlated with tumoral 18F-FES uptake (14), and moreover is likely to contribute to the blood pool.
To improve the metabolic stability and overall performance of 18F-FES for ER imaging, many modifications of the parent estradiol molecule were made and the biologic effect evaluated over the past few decades (15–18). A series of 11β-methoxy– or A-ring fluorine–substituted 18F-FES derivatives were synthesized by our research group (19–21). Among these derivatives, in vivo assays showed that a combination of 4-fluoro and 11β-methoxy substitution of 18F-FES (e.g., 4-fluoro-11β-methoxy-16α-18F-FES [18F-4FMFES]) displayed the highest uterine uptake and uterus-to-blood ratios in female rats (22). This study also reported that 18F-4FMFES shows lower affinity for plasmatic globulins. Further preclinical evaluation on tumor-bearing mice revealed that 18F-4FMFES gave the best in vivo ER+ tumor uptake and image contrast (23,24). Subsequently, in a clinical phase I study, substantial uterine uptake and retention were observed in healthy women, with a faster blood clearance and lower uptake in most nonspecific organs, along with hepatobiliary excretion similar to that observed with 18F-FES (25).
After these encouraging results, a phase II study was initiated on recently diagnosed ER+ breast cancer patients to directly compare the performance of 18F-4FMFES PET with that of 18F-FES PET. A time-dependent blood metabolite analysis was performed to assess the rate of metabolism of both tracers. Static PET imaging was performed using both tracers for each patient in a 7-d interval. Finally, tumor and nonspecific organ uptake was measured using SUVmax and SUVmean, respectively, and tumor-to-nontarget ratios were calculated.
MATERIALS AND METHODS
The study was performed under the authority of Health Canada and approved by the Sherbrooke University Hospital clinical research ethics committee and institutional board, and all subjects signed a written informed consent form. Eligible patients were recruited after either biopsy or resection of the primary and axillary lymph nodes as recommended by the oncologists. Eligibility criteria included patients with newly diagnosed breast cancer at least 1 cm in size, with histologically confirmed ER+ status and suspicion of recurrence. Exclusion criteria included active infection, pregnancy, and concomitant endocrine therapy. In total, 31 patients were examined using both 18F-FES and 18F-4FMFES PET. Six patients were imaged after they underwent mastectomy or axillary dissection to assess whether unsuspected off-site lesions were present. An oncology board staged the patients according to clinical data available before ER PET imaging. Table 1 summarizes the patient characteristics in more detail.
TABLE 1.
Characteristics of ER+ Breast Cancer Patients Included in the Clinical Study
| Parameter | Data |
| Patients (n) | 31 |
| Age (y) | 54.9 ± 10.9 (median, 57; range, 26–73) |
| Premenopausal (n) | 8 |
| Detected tumors (n) | 105 |
| Histology | |
| Ductal | 22 patients, 78 foci |
| Lobular | 5 patients, 9 foci |
| Other | 4 patients, 18 foci |
| PR-positive | 28 patients |
| HER2-positive | 2 patients |
| Clinical stage | |
| I | 12 patients, 39 foci |
| II | 9 patients, 20 foci |
| III | 5 patients, 18 foci |
| IV | 4 patients, 21 foci |
| Not determined | 1 patient, 1 focus |
| Node/metastatic status | |
| N1 | 12 patients |
| N2 | 7 patients |
| N3-positive | 5 patients |
| M1 | 10 patients |
| Surgery status | |
| Presurgery | 25 patients |
| Postsurgery | 6 patients (5 18F-FES– and 18F-4FMFES–negative) |
PR = progesterone receptor; HER2 = human epidermal growth factor receptor 2.
Radiochemistry
18F was prepared by the 18O(p,n)18F reaction on 18O-enriched water as target material using the TR-19 or TR-24 cyclotrons (Advanced Cyclotron Systems, Inc.) of the Sherbrooke Molecular Imaging Center. The methods used for the synthesis of 18F-FES have been previously reported (26). The precursor for 18F-4FMFES was synthesized as previously described (20), whereas labeling at the 16α-position was accomplished via nucleophilic substitution with 18F-F− on the reactive 16β,17β-cyclic sulfate intermediate (22) using an optimized automated procedure (26). The preparation, formulation, and quality control procedures of 18F-4FMFES batches were as described previously (27). Specific activities of both tracers were similar to or higher than those reported in the literature (25–27).
Blood Metabolite Measurement
Blood samples (1 mL each) from the first 10 patients were withdrawn at 1, 2, 3, 5, 10, 20, 30, and 60 min after injection of the imaging dose of either 18F-FES or 18F-4FMFES. Blood was centrifuged at 3,000g, and plasma was collected. Five volumes of 99% methanol were added to the plasma samples, which were then centrifuged at 7,500g to precipitate proteins. Supernatants and the appropriate diluted standard tracer solution were deposited on a 20 × 20 cm preparative C18 reverse-phase thin-layer chromatography (TLC) plate (Whatman; GE Healthcare) and then eluted for 1 h in a mixture of methanol/water/glacial acetic acid (85:14.6:0.4). Radioactivity on the TLC plates was detected using an Electronic Autoradiography Instant Imager (Canberra Packard) and analyzed by region-of-interest (ROI) drawing of the individual tracer band and the complete migration lane using the Imager software (Canberra Packard). The ratios of the 18F-FES or 18F-4FMFES activities compared with total radioactivity were calculated and expressed as percentage intact radiotracer remaining for each assessed time point.
PET Imaging
Patients were injected intravenously with 189.5 ± 17.5 MBq of 18F-FES or 189.4 ± 11.8 MBq of 18F-4FMFES in a total volume of 5–8 mL of physiologic saline (0.9% NaCl), either in the arm contralateral to the primary lesion or in the foot, using a catheter; thereafter, the line was flushed with 20 mL of saline. Each patient was scanned with both tracers, with an interval ranging from 1 to 7 d between scans, selected in a random order. Patients were allowed to stand up, void, and rest in a way compatible with the blood sampling. All acquisitions were performed using a Gemini TF PET/CT scanner (Philips) from midthigh to vertex, including the upper limbs. One hour after injection, a low-dose CT acquisition was initiated, followed immediately by a PET acquisition (7–9 overlapping bed positions, 2 min each). All PET images were reconstructed using a 3-dimensional time-of-flight weighted line-of-response row-action maximum-likelihood algorithm, with attenuation correction derived from the CT attenuation map. The accuracy of the absolute count calibration of the scanner was validated against a uniform phantom containing 18F at a known concentration. The measured activity was expressed as SUV (measured tissue activity concentration/injected activity/patient weight) for each voxel (64 μL).
Data Analysis
ROIs were drawn and uptake values obtained using MIM software, version 6.0 (MIM Software Inc.). Detectable tumor foci were defined by different ROIs, and a list of organs was assessed, including the cardiac left ventricular cavity (blood), muscle and fat (thigh), and bone (vertebrae), as well as the brain, healthy breast and axillary tissues, lungs, and uterus. The maximum-intensity voxel (SUVmax) was taken for tumor and uterine ROI quantification, whereas the averaged value of the voxels included in the ROIs (SUVmean) was used for nontarget organs. Tumor contrast was evaluated by the ratio of tumor uptake to muscle, blood, and fat uptake, as well as to the background value near the lesion. Lesions with a ratio of less than 2.0 were considered equivocal.
Statistical Analysis
Data are reported as mean ± SD. Prism software, version 7.0 (GraphPad Software Inc.), was used to perform statistical analyses and to graph data. Two-way ANOVA using the Sidak method for multiple comparisons was applied to compare 18F-4FMFES and 18F-FES uptake in tumors and nonspecific tissues. The same statistical method was used to compare the tumor-to-nontarget ratios of each tracer. The threshold for significance was a priori set to a P value of less than 0.05.
RESULTS
Blood Metabolites
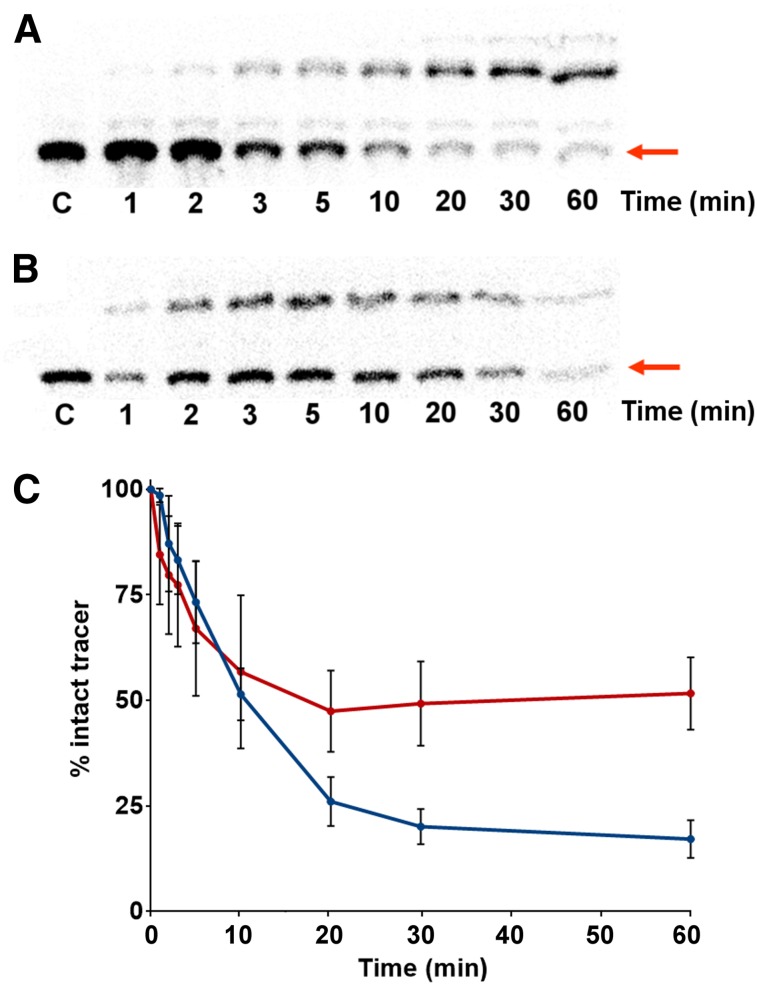
Reversed-phase TLC autoradiographs of methanol-treated plasma samples from the first 10 patients injected with either 18F-FES or 18F-4FMFES are shown in Figures 1A and 1B, respectively. In both cases, the presence of traces of radiometabolites as early as 1 min after administration is evident. Qualitative observation revealed that although 18F-FES produced up to 3 different more polar metabolites, 18F-4FMFES displayed only 1 radiometabolite of higher polarity.
FIGURE 1.
Relative metabolite quantification in plasma after injection of either 18F-FES or 18F-4FMFES. (A and B) Representative autoradiography of reverse-phase TLC of multiple blood samplings through time after injection of either 18F-FES (A) or 18F-4FMFES (B). Band corresponding to intact tracer (red arrow) is compared with total radioactivity for each lane. (C) Tracer–to–total radioactivity ratio is expressed as percentage intact tracer. 18F-4FMFES (red curve) has significantly higher percentage intact tracer than 18F-FES (blue curve) at 20 min and afterward (n = 10; P < 0.001).
Changes in the rate of metabolism, as assessed by comparing the activity of the tracer on the TLC plate to the remaining activity in the migration lane, are presented in Figure 1C. During the first 10 min after injection, both radiotracers were degraded at a similar rate. However, whereas the amount of intact 18F-FES continued to drop steadily to about 20%, the 18F-4FMFES metabolite formation plateaued after 10 min after injection. The percentage of intact 18F-4FMFES remained 2- to 2.5-fold higher (P < 0.001) than that of 18F-FES during the 20- to 60-min period.
PET Imaging of Patients
Typical examples of PET images obtained with 18F-FES and 18F-4FMFES, for the same patient, are depicted in Figures 2 and 3. The 2 tracers have similar hepatobiliary metabolism and excretion patterns, revealing comparable liver and intestinal radioactivity accumulation. Another common feature, which was noted earlier with other steroid-based radiotracers (28), is the tendency of both radiopharmaceuticals to accumulate in variable quantities in the vein proximal to the site of injection. However, overall background activity is visually lower and more uniform using 18F-4FMFES than using 18F-FES. This difference is best exemplified by the images obtained of the heart region (Figs. 3B and 3C). The whole mediastinal and thoracic region reveals a lower and more uniform activity using 18F-4FMFES than using 18F-FES, reflecting the lower concentration of the former in the blood pool and nontarget tissues.
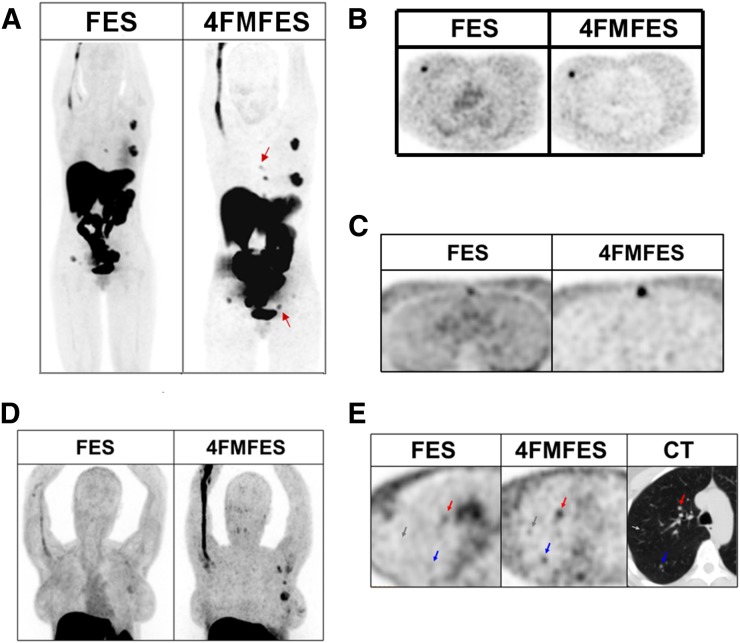
FIGURE 2.
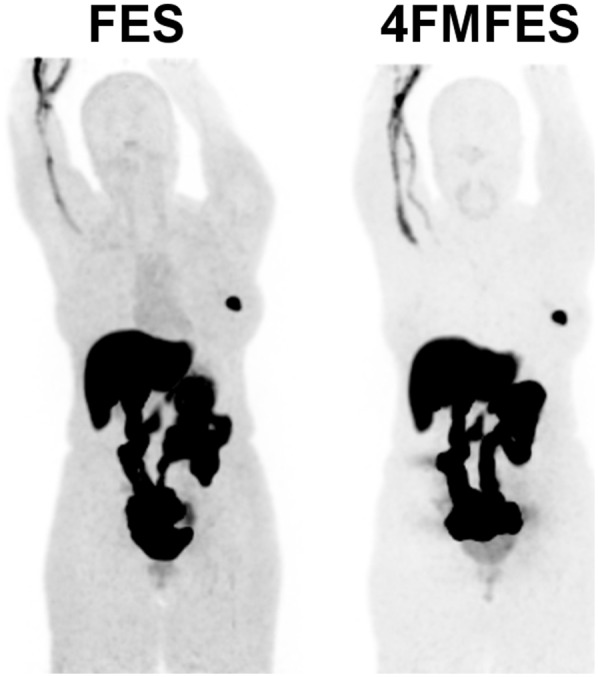
Typical maximum-intensity-projection PET images of patient with ER+ primary lesion after injection of either 18F-FES or 18F-4FMFES. Voxel saturation is set to same level for each image. Both tracers show hepatobiliary excretion that generates high background signal to liver and intestines. Less overall background is visible using 18F-4FMFES than using 18F-FES, especially in mediastinal region.
FIGURE 3.
Examples of comparative 18F-FES and 18F-4FMFES PET images. Voxel saturation is same for 18F-FES as for 18F-4FMFES in A–C; in D and E, contrast was magnified in 18F-4FMFES PET images for better clarity. (A) Whole-body maximum-intensity-projection PET images of patient initially staged T1cN2M0, in whom 18F-FES PET discovered sternal and iliac bone metastases. 18F-4FMFES PET revealed 2 more foci (arrows) not visible using 18F-FES PET. (B) Typical transaxial slice of primary lesion. Tumor contrast is improved compared with surrounding breast tissue using 18F-4FMFES instead of 18F-FES PET. Mediastinal background activity is reduced using 18F-4FMFES. (C) Transaxial slice of sternal metastasis. Although lesion was considered equivocal with 18F-FES PET, it was readily identified as ER+ foci using 18F-4FMFES PET. (D) Thoracic maximum-intensity projection of patient with suspected recurrence after resection. Although 18F-FES PET reported presence of 2 tumor-infiltrated lymph nodes with equivocal signal, 18F-4FMFES PET revealed 4 distinct ER+ foci. Axillary dissection confirmed presence of lobular carcinoma in suspected sites. (E) 18F-FES and 18F-4FMFES transaxial slices of lung metastatic burden. CT image indicated presence of 5-mm (red arrow), 4-mm (blue arrow), and 2-mm (gray arrow) tumors. 18F-FES PET failed to detect 2 smaller lesions, whereas 18F-4FMFES succeeded in distinguishing all 3 foci (although 2 were equivocal).
18F-4FMFES PET allowed detection of all ER+ lesions that were also successfully identified with 18F-FES PET. Furthermore, whereas 18F-FES PET identified a total of 96 ER+ lesions, 18F-4FMFES succeeded in detecting an additional 9 lesions, which translates into an 8.6% increase in the detection rate. Those supplemental findings were confirmed as being ER+ breast carcinomas by immunohistochemistry when biopsy was possible, and the presence of a lesion was later assessed by either 18F-FDG PET/CT or bone scintigraphy. Of the 6 postsurgery patients enrolled in the study, 5 were shown to be negative with either 18F-FDG, 18F-FES, or 18F-4FMFES PET. Overall, our data indicate that 18F-4FMFES PET provides better image contrast than 18F-FES PET for the assessment of ER+ breast cancer, with a higher detection rate of metastases and less thoracic and axillary background radioactivity.
Semiquantitative PET Analysis
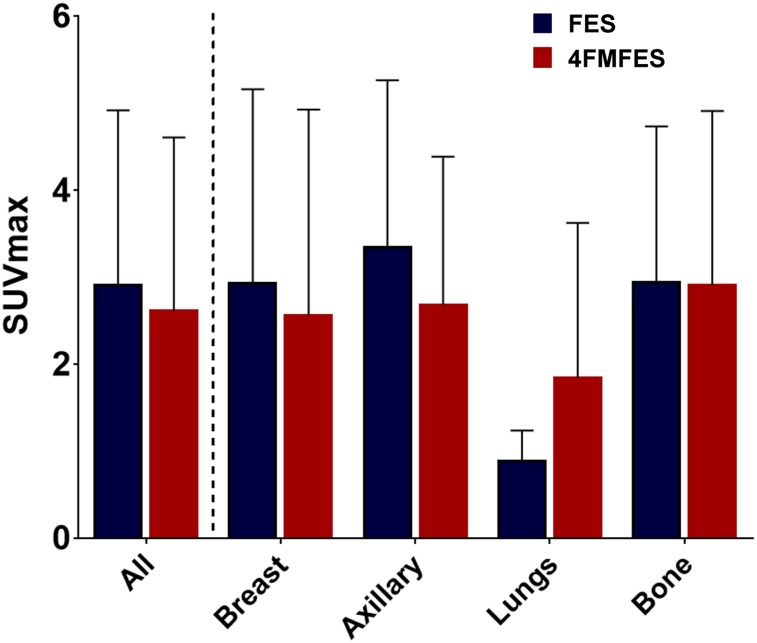
Tumor and nontarget organ uptake of 18F-FES and 18F-4FMFES was analyzed and averaged over the corresponding ROIs in all patients. The results are shown in Figure 4 for tumors and in Figure 5 for nontarget tissues. The average SUVmax of all assessed lesions did not show any significant difference between 18F-4FMFES and 18F-FES. No other uptake dissimilarities between the 2 tracers were detected when analyzing the primary, axillary, and distant metastasis subgroups, nor did we observe differences in tumor SUVs between pre- and postmenopausal patients. Uterine uptake followed the same trend, with no significant differences being observed between 18F-FES and 18F-4FMFES uptake, regardless of the menopausal status.
FIGURE 4.
18F-FES and 18F-4FMFES uptake of assessed tumors, expressed as SUVmax of representative ROIs. More tumors were identified using 18F-4FMFES PET (n = 105) than 18F-FES PET (n = 96). Although small trend toward lower uptake with 18F-4FMFES PET was observed, difference was not significant, nor did segmentation of tumor pool according to site of presentation show significantly different uptake.
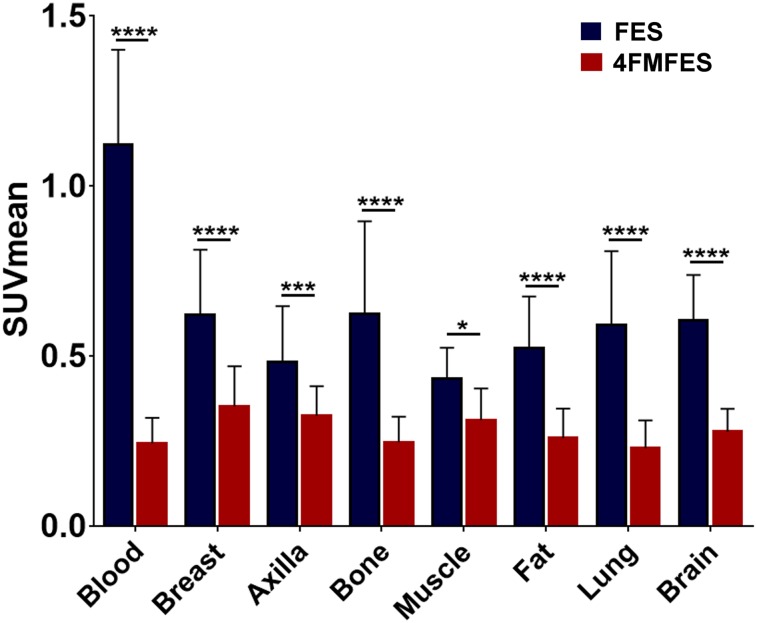
FIGURE 5.
18F-FES and 18F-4FMFES uptake of nontarget reference tissues, expressed as SUVmean of representative ROIs. Blood pool was assessed by ROI drawn into left ventricular cavity. Breast and axilla ROIs were drawn contralateral to primary lesion. Muscle and fat ROIs were derived from thigh. 18F-4FMFES consistently presented significantly lower uptake than 18F-FES in all assessed reference tissues (n = 31). *P < 0.05. ***P < 0.005. ****P < 0.001.
In contrast to the similar tumor-targeting properties of both probes, the distribution activity for 18F-4FMFES was substantially and significantly lower than that for 18F-FES in all assessed nontarget organs. In particular, blood-pool activity was 4-fold lower for 18F-4FMFES than for 18F-FES. The reduction in overall background activity was in line with the observed improved overall image contrast with 18F-4FMFES PET, especially in the mediastinal area.
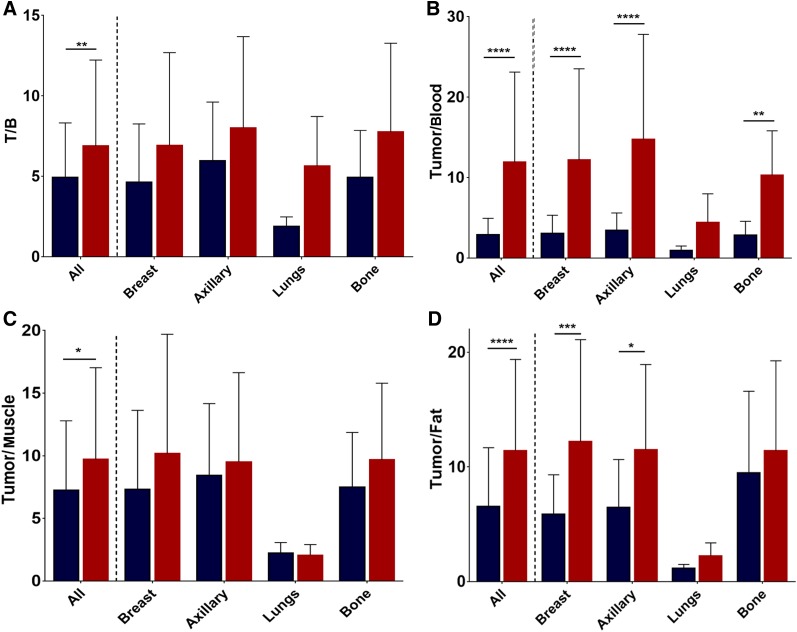
Consequently, tumor-to-blood (P < 0.0001), tumor-to-muscle (P < 0.05), tumor-to-fat (P < 0.0001), and tumor-to-background (P < 0.05) ratios were all significantly higher for 18F-4FMFES than for 18F-FES (Fig. 6), with the most noticeable difference (4-fold) being in the tumor-to-blood ratio. Primary, axillary, and metastatic subsets all saw an improvement in their tumor-to-nontarget ratios with 18F-4FMFES PET but reached significance only for tumor-to-blood ratio (all subgroups except lungs) and tumor-to-fat ratio (except lungs and bone subgroups). Moreover, 9 of the lesions detected with 18F-FES were considered equivocal, whereas only 3 of the lesions had a tumor-to-background ratio lower than 2 using 18F-4FMFES. Of those 3 tumors borderline-detectable with 18F-4FMFES, 2 were negative using 18F-FES. This quantitative contrast improvement is responsible for the easier detection of low-ER+ or smaller lesions, as well as for the higher overall rate of detection observed in our small patient sample.
FIGURE 6.
18F-FES (blue) and 18F-4FMFES (red) tumor-to-background (T/B) (A), tumor-to-blood (B), tumor-to-muscle (C), and tumor-to-fat (D) ratios. 18F-4FMFES achieved significantly better tumor contrast than 18F-FES regardless of nonspecific tissue of reference. Significantly higher tumor-to-blood and tumor-to-fat ratios were also observed for 18F-4FMFES when subcategorizing tumor pool according to tumor site. *P < 0.05. **P < 0.01. ***P < 0.005. ****P < 0.001.
DISCUSSION
ER status is one of the most important prognostic factors in breast cancer management, with ERs having to be present for endocrine therapy to be successful (29). Discrepancies between the receptor status of the primary tumor and that of distant metastases were found in up to 40% of patients in retrospective studies (30,31). However, it is impractical to take biopsy samples of every known lesion, especially at sites of distant metastasis. Also, considering that ER status can change at recurrence (32) or during therapy (33), alternative methods for whole-body ER detection are needed. The most successful and sensitive method known so far is 18F-FES PET, which was demonstrated to correlate well with ER status and immunohistochemistry-derived levels of expression (6,8,9). Recently, 18F-FES PET was shown to be successful in predicting early progression during fulvestrant therapy (34) and contributed to identifying antihormone-resistant breast cancer patients (35).
In our study, 18F-4FMFES was evaluated in a clinical phase II trial and compared with the well-known 18F-FES. The 2 tracers exhibited similar tumor uptake, and all lesions detected with 18F-FES were also revealed using 18F-4FMFES. More interestingly, 8.6% more foci were detected by 18F-4FMFES PET than by 18F-FES PET, all of which were later confirmed as true-positive by biopsy sampling or 18F-FDG PET/CT. In addition, 9 axillary, mediastinal, and bone metastases that were difficult to distinguish from the environing background with 18F-FES were more readily detected by 18F-4FMFES PET. Thus, despite comparable tumor targeting, 18F-4FMFES displayed superior imaging properties due to decreased overall background activity.
This finding was further supported by semiquantitative analyses. Although the 2 tracers exhibited a similar tumor and uterine SUVmax, uptake in all assessed nontarget organs and tissues was significantly lower with 18F-4FMFES than with 18F-FES, in accordance with the overall qualitative aspect of the produced images, and explaining the increased detection rate with 18F-4FMFES.
Many factors explain the improved imaging properties of 18F-4FMFES. This novel ER PET tracer was initially designed to improve in vivo stability by the introduction of a second (nonradioactive) fluorine at position 4 of the steroid A-ring (36). Furthermore, to decrease nonspecific binding to plasma globulins, an 11β-methoxy moiety was added to the parent 18F-FES (16,22). The improved properties are indeed reflected in the observed distribution pattern of 18F-4FMFES versus 18F-FES. First, plasma metabolite analysis at, for example, 1 h after injection—the optimal time for static PET imaging—revealed a 2.5-fold increase in intact 18F-4FMFES as compared with 18F-FES. Moreover, whereas 3 new radiometabolites were observed by radio-TLC with samples of 18F-FES, only one 18F-4FMFES–derived radiometabolite was revealed. The nature of the different metabolites was not further studied, but previous work from Mankoff et al. (13) on 18F-FES identified mainly sulfate and glucuronide addition, which is in line with our finding that all metabolites we observed were more polar than the parent tracer. Although 18F-4FMFES, because of the presence of fluoro substituents proximal to the expected metabolic sites, was deemed impervious to such processing, it may be possible that one of the steroidal hydroxyl groups is still modifiable by hepatic enzymes.
Second, binding to plasma globulins slows the metabolic and blood clearance rates of steroids (28,37). Hence, the lack of 18F-4FMFES binding affinity for sex hormone–binding globulin may facilitate its release from the blood pool, resulting in lower background activity than for 18F-FES. Moreover, 18F-4FMFES is more lipophilic than 18F-FES, potentially further accelerating blood clearance (38). Thus, notwithstanding the lower in vitro ER binding affinity of 18F-4FMFES (22) and similar tumor uptake of 18F-4FMFES and 18F-FES in patients, the 2.5-fold increase in stability of 18F-4FMFES (despite the lack of sex hormone–binding globulin binding), compared with 18F-FES, likely explains the substantial gain in tumor contrast obtained with 18F-4FMFES versus 18F-FES.
Another factor to consider is that blood estradiol levels were not monitored before imaging, nor did we consider the effect of the menstrual cycle for the premenopausal women. However, tumor, uterine, and nontarget organ SUVs did not significantly differ between pre- and postmenopausal patients, suggesting that circulating estradiol did not substantially affect tumor targeting. This possibility agrees with earlier observations defining confounding factors in 18F-FES PET imaging, where patients with an estradiol level of more than 30 pg/mL did not have significantly lower 18F-FES tumor SUVs than patients with low estrogen levels (39).
CONCLUSION
In this preliminary clinical phase II study, we showed that the novel ER PET tracer 18F-4FMFES achieved tumor uptake similar to, but nonspecific organ and blood retention lower than, that of 18F-FES. Hence, 18F-4FMFES PET allowed higher detection rates and better sensitivity than its parent, 18F-FES, toward ER+ breast carcinomas. Further investigations are in progress to evaluate the potential of 18F-4FMFES PET to assess recurrence, treatment follow-up, sensitivity, specificity, and accuracy and to complement classic 18F-FDG PET in breast cancer diagnosis and follow-up.
DISCLOSURE
This work was supported by the Canadian Breast Cancer Foundation (CBCF). Johan E. van Lier, Brigitte Guérin, Roger Lecomte, and Éric E. Turcotte are members of the Research Center of the CHUS (CRCHUS), Sherbrooke, Quebec, Canada, supported by the Fonds de la Recherche du Québec–Santé (FRQ-S). No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Aliaga A, Rousseau JA, Ouellet R, et al. Breast cancer models to study the expression of estrogen receptors with small animal PET imaging. Nucl Med Biol. 2004;31:761–770. [DOI] [PubMed] [Google Scholar]
- 2.Paquette M, Ouellet R, Archambault M, Croteau E, Lecomte R, Benard F. [18F]-fluoroestradiol quantitative PET imaging to differentiate ER+ and ERα-knockdown breast tumors in mice. Nucl Med Biol. 2012;39:57–64. [DOI] [PubMed] [Google Scholar]
- 3.He S, Wang M, Yang Z, et al. Comparison of 18F-FES, 18F-FDG, and 18F-FMISO PET imaging probes for early prediction and monitoring of response to endocrine therapy in a mouse xenograft model of ER-positive breast cancer. PLoS One. 2016;11:e0159916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mintun MA, Welch MJ, Siegel BA, et al. Breast cancer: PET imaging of estrogen receptors. Radiology. 1988;169:45–48. [DOI] [PubMed] [Google Scholar]
- 5.Dehdashti F, Mortimer JE, Siegel BA, et al. Positron tomographic assessment of estrogen receptors in breast cancer: comparison with FDG-PET and in vitro receptor assays. J Nucl Med. 1995;36:1766–1774. [PubMed] [Google Scholar]
- 6.Mortimer JE, Dehdashti F, Siegel BA, Katzenellenbogen JA, Fracasso P, Welch MJ. Positron emission tomography with 2-[18F]fluoro-2-deoxy-D-glucose and 16α-[18F]fluoro-17β-estradiol in breast cancer: correlation with estrogen receptor status and response to systemic therapy. Clin Cancer Res. 1996;2:933–939. [PubMed] [Google Scholar]
- 7.Kumar P, Mercer J, Doerkson C, Tonkin K, McEwan AJ. Clinical production, stability studies and PET imaging with 16-alpha-[18F]fluoroestradiol ([18F]FES) in ER positive breast cancer patients. J Pharm Pharm Sci. 2007;10(suppl):256s–265s. [PubMed] [Google Scholar]
- 8.Peterson LM, Mankoff DA, Lawton T, et al. Quantitative imaging of estrogen receptor expression in breast cancer with PET and 18F-fluoroestradiol. J Nucl Med. 2008;49:367–374. [DOI] [PubMed] [Google Scholar]
- 9.Gemignani ML, Patil S, Seshan VE, et al. Feasibility and predictability of perioperative PET and estrogen receptor ligand in patients with invasive breast cancer. J Nucl Med. 2013;54:1697–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linden HM, Kurland BF, Peterson LM, et al. Fluoroestradiol positron emission tomography reveals differences in pharmacodynamics of aromatase inhibitors, tamoxifen, and fulvestrant in patients with metastatic breast cancer. Clin Cancer Res. 2011;17:4799–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsujikawa T, Yoshida Y, Kiyono Y, et al. Functional oestrogen receptor α imaging in endometrial carcinoma using 16α-[18F]fluoro-17β-oestradiol PET. Eur J Nucl Med Mol Imaging. 2011;38:37–45. [DOI] [PubMed] [Google Scholar]
- 12.Tsujikawa T, Yoshida Y, Kudo T, et al. Functional images reflect aggressiveness of endometrial carcinoma: estrogen receptor expression combined with 18F-FDG PET. J Nucl Med. 2009;50:1598–1604. [DOI] [PubMed] [Google Scholar]
- 13.Mankoff DA, Tewson TJ, Eary JF. Analysis of blood clearance and labeled metabolites for the estrogen receptor tracer [F-18]-fluorestradiol (FES). Nucl Med Biol. 1997;24:341–348. [DOI] [PubMed] [Google Scholar]
- 14.Tewson TJ, Mankoff DA, Peterson LM, et al. Interactions of 16alpha-[18F]-fluoroestradiol (FES) with sex steroid binding protein (SBP). Nucl Med Biol. 1999;26:905–913. [DOI] [PubMed] [Google Scholar]
- 15.Kiesewetter DO, Kilbourn MR, Landvatter SW, Heiman DF, Katzenellenbogen JA, Welch M. Preparation of four fluorine-18-labeled estrogens and their selective uptakes in target tissues of immature rats. J Nucl Med. 1984;25:1212–1221. [PubMed] [Google Scholar]
- 16.Pomper MG, VanBrocklin H, Thieme AM, et al. 11-beta-methoxy-, 11-beta-ethyl- and 17-alpha-ethynyl-substituted 16-alpha-fluoroestradiols: receptor-based imaging agents with enhanced uptake efficiency and selectivity. J Med Chem. 1990;33:3143–3155. [DOI] [PubMed] [Google Scholar]
- 17.Ali H, Rousseau J, van Lier JE. Synthesis of A-ring fluorinated derivatives of (17α,20E/Z)-[125I]iodovinylestradiols: effect on receptor binding and receptor-mediated target tissue uptake. J Med Chem. 1993;36:3061–3072. [DOI] [PubMed] [Google Scholar]
- 18.Katzenellenbogen JA. The 2010 Philip S. Portoghese Medicinal Chemistry Lectureship: addressing the “core issue” in the design of estrogen receptor ligands. J Med Chem. 2011;54:5271–5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali H, Rousseau J, Gantchev TG, van Lier JE. 2- and 4-fluorinated, 16α-[125I]iodoestradiol derivatives: synthesis and effect on estrogen, receptor binding and receptor-mediated target tissue uptake. J Med Chem. 1993;36:4255–4263. [DOI] [PubMed] [Google Scholar]
- 20.Seimbille Y, Ali H, van Lier JE. Synthesis of 2,16α- and 4,16α-difluoroestradiols and their 11β-methoxy derivatives as potential estrogen receptor-binding radiopharmaceuticals. J Chem Soc Perkin Trans. 2002;5:657–663. [Google Scholar]
- 21.Ahmed N, Garcia G, Ali H, van Lier JE. 18F-labeling of A-ring substituted 16α-fluoro-estradiols as potential radiopharmaceuticals for PET imaging. Steroids. 2009;74:42–50. [DOI] [PubMed] [Google Scholar]
- 22.Seimbille Y, Rousseau J, Bénard F, et al. 18F-labeled difluoroestradiols: preparation and preclinical evaluation as estrogen receptor-binding radiopharmaceuticals. Steroids. 2002;67:765–775. [DOI] [PubMed] [Google Scholar]
- 23.Bénard F, Ahmed N, Beauregard JM, et al. [18F]Fluorinated estradiol derivatives for oestrogen receptor imaging: impact of substituents, formulation and specific activity on the biodistribution in breast tumour-bearing mice. Eur J Nucl Med Mol Imaging. 2008;35:1473–1479. [DOI] [PubMed] [Google Scholar]
- 24.Paquette M, Phoenix S, Ouellet R, et al. Quantitative assessment of the novel estrogen receptor PET tracer 4-fluoro-11β-methoxy-16α-[18F]fluoroestradiol (4FMFES) by PET imaging in a breast cancer murine model. Mol Imaging Biol. 2013;15:625–632. [DOI] [PubMed] [Google Scholar]
- 25.Beauregard JM, Croteau E, Ahmed N, van Lier JE, Benard F. Assessment of human biodistribution and dosimetry of 4-fluoro-11β-methoxy-16α-18F-fluoroestradiol using serial whole-body PET/CT. J Nucl Med. 2009;50:100–107. [DOI] [PubMed] [Google Scholar]
- 26.Lim JL, Zheng L, Berridge MS, Tewson TJ. The use of 3-methoxymethyl-16-beta, 17-beta-epiestriol-O-cyclic sulfone as the precursor in the synthesis of [18-F]-16α-fluoroestradiol. Nucl Med Biol. 1996;23:911–915. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed N, Langlois R, Rodrigue S, Benard F, van Lier JE. Automated synthesis of 11β-methoxy-4,16α-[16α-18F]difluoroestradiol (4F-M[18F]FES) for estrogen receptor imaging by positron emission tomography. Nucl Med Biol. 2007;34:459–464. [DOI] [PubMed] [Google Scholar]
- 28.Jonson SD, Bonasera TA, Dehdashti F, Cristel ME, Katzenellenbogen JA, Welch MJ. Comparative breast tumor imaging and comparative in vitro metabolism of 16α-[18F]fluoroestradiol-17β and 16β-[18F]fluoromoxestrol in isolated hepatocytes. Nucl Med Biol. 1999;26:123–130. [DOI] [PubMed] [Google Scholar]
- 29.Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foukakis T, Astrom G, Lindstrom L, Hatschek T, Bergh J. When to order a biopsy to characterise a metastatic relapse in breast cancer. Ann Oncol. 2012;23(suppl 10):x349–x353. [DOI] [PubMed] [Google Scholar]
- 31.Lindström LS, Karlsson E, Wilking UM, et al. Clinically used breast, cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable, throughout tumor progression. J Clin Oncol. 2012;30:2601–2608. [DOI] [PubMed] [Google Scholar]
- 32.Kuukasjärvi T, Kononen J, Helin H, et al. Loss of estrogen receptor in recurrent breast cancer is associated with poor response to endocrine therapy. J Clin Oncol. 1996;14:2584–2589. [DOI] [PubMed] [Google Scholar]
- 33.Gutierrez MC, Detre S, Johnston S, et al. Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol. 2005;23:2469–2476. [DOI] [PubMed] [Google Scholar]
- 34.van Kruchten M, de Vries EG, Glaudemans AW, et al. Measuring residual estrogen receptor availability during fulvestrant therapy in patients with metastatic breast cancer. Cancer Discov. 2015;5:72–81. [DOI] [PubMed] [Google Scholar]
- 35.van Kruchten M, Glaudemans AW, de Vries EF, Schröder CP, de Vries EG, Hospers GA. Positron emission tomography of tumour [18F]fluoroestradiol uptake in patients with acquired hormone-resistant metastatic breast cancer prior to oestradiol therapy. Eur J Nucl Med Mol Imaging. 2015;42:1674–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan P, Maggs JL, Bulman-Page PC, Hussain F, Park BK. The metabolism of 2- and 4-fluoro-17-oestradiol in the rat and its implications for oestrogen carcinogenesis. Biochem Pharmacol. 1992;43:985–993. [DOI] [PubMed] [Google Scholar]
- 37.Plymate SR, Namkung PC, Matej LA, Petra PH. Direct effect of plasma sex hormone binding globulin (SHBG) on the metabolic clearance rate of 17β-estradiol in the primate. J Steroid Biochem. 1990;36:311–317. [DOI] [PubMed] [Google Scholar]
- 38.Lukka PB, Paxton JW, Atwell GJ, Kestell P, Baguley BC. A rapid LC-MS/MS method for the quantitation of a series of benzonaphthyridine derivatives: application to in vivo pharmacokinetic and lipophilicity studies in drug development. J Pharm Biomed Anal. 2012;63:9–16. [DOI] [PubMed] [Google Scholar]
- 39.Peterson LM, Kurland BF, Linka JM, et al. Factors influencing the uptake of 18F-fluoroestradiol in patients with estrogen receptor positive breast cancer. Nucl Med Biol. 2011;38:969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]