Abstract
PET is a functional imaging method that can exploit various aspects of tumor biology to enable greater detection of prostate cancer than can be provided by morphologic imaging alone. Anti-1-amino-3-18F-flurocyclobutane-1-carboxylic acid (18F-fluciclovine) is a nonnaturally occurring amino acid PET radiotracer that was recently approved by the U.S. Food and Drug Administration for detection of suspected recurrent prostate cancer. The tumor-imaging features of this radiotracer mirror the upregulation of transmembrane amino acid transport that occurs in prostate cancer because of increased amino acid metabolism for energy and protein synthesis. This continuing medical education article provides an overview on 18F-fluciclovine PET diagnostic capabilities for primary and metastatic disease, including reviews of published comparisons to conventional imaging and other molecular imaging agents. Additionally, the imaging procedure and image interpretation are detailed, including physiologic and pathologic uptake patterns and pitfalls.
Keywords: 18F-fluciclovine, prostate cancer, amino acid
Prostate cancer is the most common type of cancer in men and the third most common cause of cancer-related death in men (1). In the initial phase, prostate cancer spreads almost exclusively through lymphatic channels and bones, with pelvic nodes being the primary and most frequent site of nodal dissemination. However, in 10%–15% of cases, presacral, paraaortic, and paracaval nodes can be the primary site of nodal disease. Prostate-specific antigen (PSA) doubling time, time to PSA relapse, Gleason score, and pathologic stage are the principal clinical parameters used to determine the likelihood of local or distant recurrent disease.
Current guidelines recommend the use of 99mTc-methylene diphosphonate bone scans, as well as CT or MRI, to determine the extent of disease in men with high-risk primary, recurrent, or metastatic prostate cancer. These conventional imaging modalities have limited sensitivity for detecting small-volume sites of prostate cancer and may underestimate the burden of disease.
18F-FDG has been extensively studied, but its role in the detection of metastatic prostate cancer is limited because the low glycemic activity of hormone-naïve prostate cancer cells limits 18F-FDG uptake (2). Phospholipid precursors such as 11C-choline have been widely used in detecting both localized prostate cancer and metastases. 11C-choline was approved by the U.S. Food and Drug Administration (FDA) for use in men with biochemically recurrent prostate cancer (3). However, the utility of radiolabeled choline PET is limited in patients with low PSA values (<1.0 ng/mL), and increased uptake in areas of inflammation may affect specificity. Prostate-specific membrane antigen (PSMA) is a transmembrane type II glycoprotein that is expressed by prostate cancer cells, and several PSMA-specific radiotracers have been developed for detection of prostate metastasis.
Amino acids are essential to cell metabolism and growth, and the nutrient demand of cancerous cells is much higher than that of normal tissues (4). Several amino acid transporter systems are overexpressed in prostate cancer—specifically, large neutral amino acid transporters (system L: LAT1, LAT3, and LAT4) and alanine-serine-cysteine transporters (system ASC: ASCT1 and ASCT2) (5,6). Of these, LAT3, ASCT1, and ASCT2 are upregulated with androgen simulation and LAT1 and ASCT2 are associated with a more aggressive tumor phenotype (7). Anti-1-amino-3-18F-flurocyclobutane-1-carboxylic acid (18F-fluciclovine) is a nonnaturally occurring amino acid first synthesized by Goodman’s group and initially developed for the evaluation of cerebral gliomas (8). 18F-fluciclovine transport is primarily mediated by sodium-dependent amino acid transporters, specifically system ASC (ASCT2) with a contribution from sodium-independent system L (LAT1) (5). Because these amino acid transporters that are most involved in 18F-fluciclovine transport mediate influx and efflux of amino acids, washout of radiotracer occurs over time. Thus, early imaging is recommended to maximize lesion conspicuity (9). In addition, though amino acid PET imaging is less prone to inflammatory uptake than is 18F-FDG, it may still occur, potentially confounding interpretation.
Early 18F-fluciclovine biodistribution studies demonstrated relatively low urinary excretion, with urinary bladder doses ranging from 12 to 25 μGy/MBq (10), compared with 73 ± 42 μGy/MBq for 18F-FDG (11) and 60 μGy/MBq for O-(2-18F-fluoroethyl)-l-tyrosine (12). For this reason, in combination with early imaging times, attention was turned from brain glioma imaging to renal and pelvic malignancies. One of these studies involved a patient with renal cell carcinoma who incidentally was found to have intense 18F-fluciclovine uptake in retroperitoneal lymph nodes, which were biopsy-proven to be metastatic prostate cancer (9). Subsequent studies with 18F-fluciclovine demonstrated uptake in both primary and metastatic prostate carcinoma, as well as increased uptake in recurrent prostate carcinoma within the prostatectomy bed, lymph nodes, and bone (13).
A New Drug Application was accepted in December 2015 by the FDA for priority review based on data collected from 877 subjects, including 797 patients with prostate cancer in the United States and Europe. Approval was granted to 18F-fluciclovine (Axumin; Blue Earth Diagnostics, Inc.) in May 2016 for the clinical indication of suspected prostate cancer recurrence based on elevated PSA levels after prior treatment. 18F-fluciclovine was also approved for Medicare pass-through reimbursement effective January 2017. 18F-fluciclovine has also been studied for the imaging of other malignancies, such as breast (14) and glioma (15), but discussion of these applications is beyond the scope of this article.
18F-FLUCICLOVINE PET DIAGNOSTIC PERFORMANCE
Primary Disease and Staging
Several studies have evaluated 18F-fluciclovine PET in the setting of primary prostate cancer with the conclusion that 18F-fluciclovine PET should not be used by itself to completely characterize primary lesions. Additionally, primary tumor characterization is not an FDA-approved indication. In a multicenter trial, 68 patients who had primary prostate cancer and were scheduled for either radical prostatectomy or hormone therapy underwent 18F-fluciclovine PET/CT, which was found to have a high sensitivity and specificity of 92.5% and 90.1% for the focus of primary disease (16). Yet, additional studies reported lower specificity in the prostate. In a study with 10 prostate cancer patients who were scheduled for radical prostatectomy and underwent dynamic pelvic imaging (17), the highest combined sensitivity and specificity were 81.3% and 50.0%, respectively. Although sextants with prostate cancer had the highest SUVmax, there was overlap between prostate cancer and areas of benign tissue. In a more comprehensive study involving 22 patients with primary prostate cancer, 18F-fluciclovine PET was compared with multiparametric MRI. In these patients, the mean SUVmax was significantly higher in the tumor than in the normal prostate, but there was again overlap between the 18F-fluciclovine mean SUVmax in prostate cancer and that in benign prostate hyperplasia, at 4.5 ± 0.5 versus 4.3 ± 0.6, respectively. Interestingly, combining the information from 18F-fluciclovine PET and MRI in this study increased the positive predictive value (PPV) from 50% for 18F-fluciclovine PET and 76% for MRI to 82% for the combined data (18). Similar findings were reported for a recent study of 26 men with primary prostate cancer who underwent 18F-fluciclovine PET/CT followed by PET/MRI (19); high sensitivity and low specificity were found for primary tumor identification, but 18F-fluciclovine PET did not outperform MRI alone in prostate lesion detection. Elschot et al. have explored the value of delayed-time-point imaging (18–23 min and 33–38 min) for differentiating between prostate cancer and benign tissue and reported improved performance (20).
Limited data have been reported for staging in the setting of primary prostate cancer. A study involving 68 patients with primary prostate cancer compared 18F-fluciclovine PET/CT with whole-body contrast-enhanced CT and found a sensitivity, specificity, and accuracy of 64.5%, 99.6%, and 95.5%, respectively, for extraprostatic nodal disease with 18F-fluciclovine PET/CT, versus 71%, 100%, and 96.6%, respectively, for contrast-enhanced CT for nodal disease larger than 1 cm in short-axis diameter (16). Though unable to be confirmed with the reference standard, 18F-fluciclovine PET detected subcentimeter (5–9 mm) lymph nodes in 13 patients negative on CT. A recent study of 28 patients with high-risk prostate cancer underwent simultaneous 18F-fluciclovine PET/MRI before surgery and found a high specificity (100%) but low sensitivity (30%) for detection of regional lymph node metastases (21). Therefore, in the setting of primary prostate cancer, 18F-flucicloivne PET does not replace lymph node dissection and histopathologic confirmation but can be useful to guide biopsy.
Recurrent Disease
The American Urological Association defines biochemical recurrence after radical prostatectomy as a PSA level of at least 0.2 ng/mL, followed by a subsequent confirmatory PSA level of at least 0.2 ng/mL (22). After radiation therapy, the American Society for Therapeutic Radiation and Oncology defines 3 successive PSA rises above the nadir as consistent with biochemical evidence of recurrence; however, this applies only to patients with external-beam radiotherapy. A consensus committee concluded that any rise in PSA levels of 2 ng/mL or more above the nadir, regardless of the type of radiation therapy given, is biochemical evidence of prostate cancer recurrence (Phoenix Definition) (23).
The defining factor in therapy planning for recurrent prostate carcinoma is determining whether disease is confined to the prostate or prostatic bed or whether there is extraprostatic disease. Identification of pelvic metastatic disease will result in modification of the radiation field to cover pelvic lymph nodes, and the presence of extrapelvic disease will change the therapeutic approach from potential curative salvage therapy to systemic hormonal treatment. On a per-patient basis, the sensitivity of 18F-fluciclovine PET for detecting recurrent disease varies with PSA values, with reported detection rates in the postprostatectomy biochemical failure setting of 72.0%, 83.3%, and 100% at PSA levels of less than 1, of 1–2, and of 2 or more ng/mL, respectively (24). A mixed postprostatectomy and nonprostatectomy cohort demonstrated a 18F-fluciclovine detection rate of 37.5% at a PSA level of less than 1, 77.8% at 1–2, 91.7% at more than 2, and 83.3% at more than 5 ng/mL (25). Additional studies have found 18F-fluciclovine detection rates ranging from 21% to 38.7% at a PSA level of less than 1 ng/mL (26,27). It is believed that differences in detection rate at low PSA levels between studies is likely related to PSA kinetics. For example, higher original Gleason scores and a shorter PSA doubling time are correlated with positive findings on 18F-fluciclovine PET/CT; in 1 study, the average PSA doubling time was 3.25 ± 2.09 mo in patients with positive findings, versus 31.2 ± 22.02 mo in patients with negative findings (28).
In identifying recurrent disease in the treated prostate or prostatectomy bed, 18F-fluciclovine PET/CT has demonstrated high sensitivity, low specificity, and moderate PPV (Fig. 1). An analysis of 93 patients found an overall sensitivity, specificity, PPV, and accuracy of 90.2%, 40.0%, 75.3%, and 73.6%, respectively (29). That series consisted of a relatively high proportion of patients who had undergone cryotherapy and brachytherapy, potentially leaving residual viable prostate tissue, which may be more prone to confounding uptake due to inflammation or prostatic hypertrophy. Further analysis on postprostatectomy cohorts is ongoing. Similar diagnostic performance for local recurrence was found in a large, multisite study of 596 patients (sensitivity of 88.1%, specificity of 32.6%, and PPV of 71.8%) (27). Therefore, as with primary disease, histologic confirmation of findings in the prostate or prostate bed is recommended.
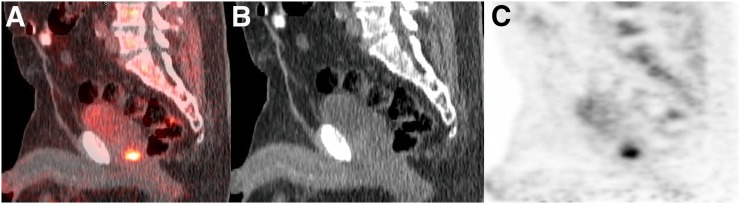
FIGURE 1.
A 66-y-old man with biochemical recurrence of prostate cancer (PSA level, 29.3 ng/mL). Sagittal 18F-fluciclovine PET/CT (A), CT (B), and PET (C) found single suggestive focus of uptake in prostate resection bed. Patient underwent external-beam radiation of lesion, with corresponding PSA nadir (0.27 ng/mL)
In the detection of recurrent extraprostatic disease, not accounting for PSA levels or doubling times, the sensitivity, specificity, PPV, and accuracy of 18F-fluciclovine have been reported to be 55.0%, 96.7%, 95.7%, and 72.9%, respectively (29). This high PPV in the detection of extraprostatic disease (95.7%) was mirrored by the large, multisite study of 596 patients (27), which had a PPV of 92.3%. In a study of 53 patients comparing 18F-fluciclovine with standard CT criteria for pathologically enlarged lymph nodes, the presence of extraprostatic true-positive lesions was noted in 29% patients with 18F-fluciclovine, whereas CT was true-positive in 7% patients. The smallest short-axis diameters reported positive on 18F-fluciclovine PET/CT and CT were 0.4 cm and 0.9 cm, respectively (25). Thus, 18F-fluciclovine PET/CT demonstrates clear superiority over CT for the detection of both local and distant disease in the clinical scenario of biochemical failure (Fig. 2). Test–retest reproducibility in target tissues and untreated malignant lesions in patients with follow-up 18F-fluciclovine PET/CT found an SUVmean difference of less than 20%. 18F-fluciclovine uptake in malignant lesions without interim therapy increased or remained stable over time (30).
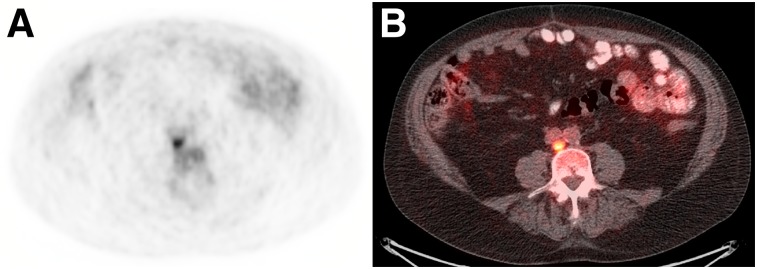
FIGURE 2.
A 73-y-old man with biochemical recurrence of prostate cancer after prostatectomy and lymphadenectomy (PSA level, 1.02 ng/mL; doubling time, 4.5 mo). Transaxial 18F-fluciclovine PET (A) and PET/CT (B) found single 0.9-cm retrocaval lymph node with SUV higher than that of bone marrow. Surgical excision was positive for metastasis and subsequent PSA nadir (0.08 ng/mL)
Osseous Disease
18F-fluciclovine has been shown to accumulate in osteolytic and osteoblastic lesions, including early-stage osteoblastic bone metastatic lesions that have abundant cellular components (31). Additionally, 18F-fluciclovine has been shown to have uptake in osseous lesions before morphologic changes can be detected by CT. 18F-fluciclovine typically demonstrates intense focal uptake in lytic prostate metastatic osseous lesions, moderate uptake in mixed sclerotic lesions, and mild to no uptake in dense sclerotic lesions (Fig. 3). Because there may be little to no 18F-fluciclovine activity in dense sclerotic lesions, skeleton-specific imaging to supplement 18F-fluciclovine PET/CT is recommended in these cases.
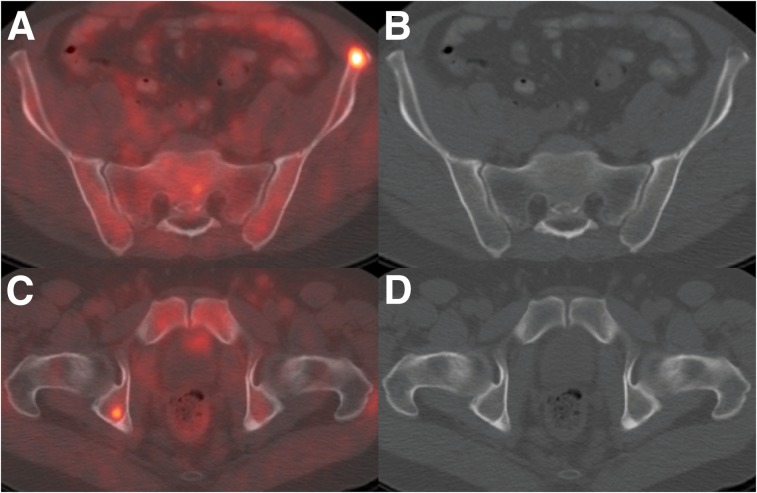
FIGURE 3.
A 50-y-old man with biochemical recurrence of prostate cancer after prostatectomy (PSA level, 3.2 ng/mL; doubling time, 1.4 mo). MRI of abdomen, initially interpreted as negative, and subsequent 18F-fluciclovine PET/CT found multiple osseous metastases. Shown are transaxial PET/CT (A) and CT (B) through subtle left iliac bone lytic lesion and transaxial PET/CT (C) and CT (D) through silent right acetabular lesion.
Compared with standard osseous scintigraphy, 18F-fluciclovine demonstrated equivalent or better results in limited studies. A phase 2A clinical trial of 10 patients with untreated prostate cancer found that 7 patients had increased 18F-fluciclovine uptake in osseous metastatic lesions—a finding that was comparable to those of conventional imaging (32). In a study involving 68 patients, 18F-fluciclovine PET/CT had moderate concordance with combined bone scintigraphy and contrast-enhanced CT for osseous metastatic disease; however, 7 patients did have positive 18F-fluciclovine findings that were negative on the combined modality (16). Although it might be concluded that a dedicated bone scan may not be necessary if a patient has definitively positive osseous disease on 18F-fluciclovine PET, most early clinical trials used a positive bone scan as an exclusion criterion. Thus, it is not recommended at this time that 18F-fluciclovine PET replace dedicated bone scintigraphy.
Comparison to Other Molecular Imaging Agents
Several studies have compared 18F-fluciclovine with other molecular imaging probes. PSMA is a transmembrane protein that is highly overexpressed in most prostate cancers, with only 5%–10% of primary prostate cancer lesions shown to be PSMA-negative (33). Currently, the only FDA-approved PSMA agent is a radiolabeled anti-PSMA antibody, capromab pendetide (ProstaScint; EUSA Pharma). However, it targets an intracellular epitope of PSMA that cannot be accessed in viable tumor cells, therefore limiting diagnostic performance. Direct comparisons between 18F-fluciclovine PET/CT and 111In-capromab pendetide demonstrate superior diagnostic performance for 18F-fluciclovine PET/CT in detecting both recurrence in the prostate or prostatectomy bed and extraprostatic recurrence (29).
Direct comparison between 18F-fluciclovine and 11C-choline PET/CT have demonstrated overall superior imaging performance for 18F-fluciclovine in biochemically recurrent prostate cancer. In a comparison study of 89 patients with biochemical relapse after radical prostatectomy, sensitivity, specificity, and accuracy for nodal disease were 37%, 67%, and 38%, respectively, for 18F-fluciclovine, versus 32%, 40%, and 32%, respectively, for 11C-choline. 18F-fluciclovine was positive for osseous uptake in 5 of 89 patients, including 5 false-negative, whereas 11C-choline was positive for osseous uptake in 6 of 89 patients, with 1 false-positive and 5 false-negative as determined by clinical and imaging follow-up (26,34,35). Overall, in a side-by-side comparison, 18F-fluciclovine detected more disease on a per-patient and per-lesion basis, with a higher PPV than 11C-choline. Although no direct comparison has been performed between 11C-choline and 18F-fluciclovine in the setting of primary prostate cancer, 11C-choline has also been reported to have relatively limited accuracy for localization of tumors within the prostate (36,37).
Several small-molecule PSMA agents have been explored that bind to the active site in the extracellular domain of PSMA. In one study, 10 patients with prostate cancer recurrence underwent sequential 68Ga PSMA-11 and 18F-fluciclovine PET/CT (38). The authors reported a better detection rate with 68Ga PSMA-11 than with 18F-fluciclovine, as is in keeping with other published literature reporting that lesions targeted by PSMA are highly conspicuous, compared with lesions targeted by other imaging agents, such as 11C-choline (39). There has also been an individual-comparison report on a man with prostate cancer recurrence who was imaged with both 18F-fluciclovine and the PSMA radiotracer 18F-DCFPyL (40). The authors similarly described superior lesion conspicuity with 18F-DCFPyL, compared with 18F-fluciclovine; however, 18F-DCFPyL demonstrated significant activity in the ureters and bladder, which the authors noted might obscure disease in the pelvis. Though both papers concluded that more data are needed to compare the merits and drawbacks of each modality, it seems likely that the newer PSMA radiotracers will have superior detection, especially at lower PSA values.
THERAPY MANAGEMENT
A wide range of factors, including PSA, PSA doubling time, Gleason score, and imaging results, are considered before a decision on whether to offer salvage radiotherapy is made (41). Poor patient selection is believed to be a contributor to the high biochemical failure rates seen after salvage radiotherapy, with accurate imaging being essential for correct planning. Current treatment planning relies mostly on conventional imaging, which may be unrevealing in the initial stages of prostate cancer recurrence. In a study involving 42 postprostatectomy patients randomized to undergo 18F-fluciclovine PET/CT after conventional radiotherapy planning, radiotherapy decisions were changed in 40.5% of patients after the 18F-fluciclovine PET results were known (24). However, the authors noted that the decision on whether to actually offer radiotherapy had no statistically significant impact, as evidence of extrapelvic disease caused radiotherapy to be cancelled in only 2 of 42 patients. In another analysis from this trial, use of 18F-fluciclovine PET changed the planning volumes for 83% of lesions without a change in toxicity (42,43). These results are comparable to studies involving other PET agents, such as 11C-choline, resulting in salvage radiotherapy adjustments in 13%–33% of patients (44,45).
PATIENT PREPARATION AND IMAGING PROTOCOL
Patient preparation includes for 4 h before the study to equalize plasma amino acid levels, except for small amounts of water taken to ingest medications. The effects of suboptimal fasting on 18F-fluciclovine biodistribution have not been evaluated in humans. Patients are encouraged to avoid heavy exercise because this has been observed to increase muscle uptake.
Gastrointestinal contrast medium may be administered to improve visualization. It is best to have the patient void the urinary bladder before receiving oral contrast medium at approximately 30 min before scanning. Because of the potential for early bladder activity, the patient should be encouraged to withhold from voiding immediately before being placed in the PET/CT scanner, since a relatively distended bladder may mitigate occasional early 18F-fluciclovine excretion.
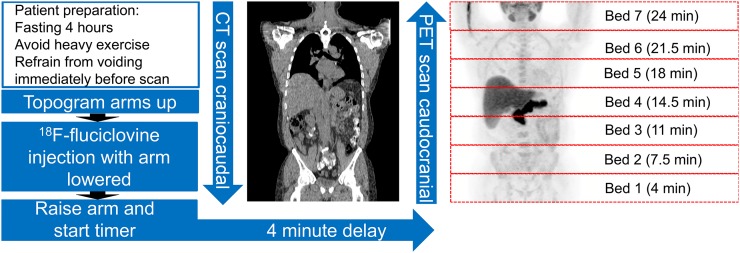
Several different protocols for imaging recurrent prostate cancer with 18F-fluciclovine PET have been reported, incorporating early and delayed sequences (29,46). Comparable results have been obtained for a more practical protocol using an early single time point (3–5 min), which is the current recommendation (26). Though scanner-dependent (47), a successful protocol used at our facility on a time-of-flight PET scanner involves first obtaining a CT scan from skull base to thigh in the craniocaudal direction with arms raised (Fig. 4). The patient is then removed from the scanner without shifting position, and an arm is lowered for injection. Unlike 18F-FDG and other radiotracers, no prolonged uptake phase is required, and the patient is injected with a 370-MBq (10 mCi) intravenous bolus while on the table. The injected arm is then raised, followed by PET imaging commencing at 3–5 min (target, 4 min) after 18F-fluciclovine injection in a caudocranial direction at 3.5 min per table position (Fig. 4), which results in adequate coverage to the skull base by 25 min after injection. When the indication is suspected recurrence of prostate cancer, starting the acquisition caudally is especially critical with 18F-fluciclovine because its relatively rapid kinetics cause the tumor-to-background activity to be highest at an average of up to 20–30 min after injection, though individual lesions may have prolonged retention. If the scan is started too early (<3 min), biodistribution may be altered with increased blood pool.
FIGURE 4.
Schema of 18F-fluciclovine PET/CT acquisition adapted from Emory clinical protocol. Maximum-intensity-projection 18F-fluciclovine images show normal biodistribution. Pancreas uptake is relatively higher than liver uptake, consistent with ideal time of image acquisition after injection. PET bed-position times are after injection (p.i.).
IMAGE INTERPRETATION
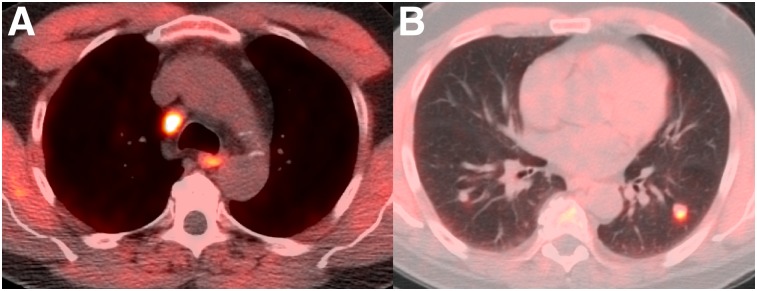
18F-fluciclovine PET/CT should be interpreted with knowledge of the physiologic biodistribution of 18F-fluciclovine and the uptake patterns typical of prostate cancer recurrence (e.g., prostatectomy bed and deep pelvic lymph nodes vs. peripheral inguinal or distal external iliac nodes), though distant disease may also be present (Fig. 5).
FIGURE 5.
A 60-y-old man with biochemical recurrence of prostate cancer after prostatectomy (PSA level, 16.4 ng/mL; doubling time, 6.4 mo). 18F-fluciclovine PET/CT found metastatic mediastinal (A) and pulmonary nodules (B) on transaxial PET/CT. Patient was started on hormonal therapy with subsequent resolution of lymphadenopathy and pulmonary nodules and undetectable PSA.
Typically, the liver and pancreas demonstrate the most intense physiologic uptake, and there may be mild to moderate uptake in the pituitary gland, salivary glands, lymphoid tissue of the Waldeyer ring, thyroid gland, breast parenchyma, esophagus, stomach, bowel, and renal parenchyma. Uptake in a renal mass should be considered suggestive of malignancy. Papillary renal cell carcinoma has been shown to have increased uptake, whereas clear cell carcinoma has been reported to have uptake equal to that of the renal parenchyma (9). The urinary bladder wall typically has physiologic diffuse mild to moderate activity. Periurethral tissue may have mild to moderate parallel activity, and therefore, sagittal images can help differentiate physiologic uptake in the urethra from disease in the prostatectomy bed (Fig. 1). Early bladder activity in a small percentage of patients may interfere with evaluation of the prostatectomy bed, prostate, and seminal vesicles. Attention should be paid to the ureters in these cases so as to avoid confusion with nodal uptake. The adrenal glands have unilateral or bilateral mild to moderate physiologic uptake. The arm or subclavian vein on the side of the injection may retain radiotracer and can be differentiated from abnormal nodal uptake by careful correlation on PET/CT.
Several benign conditions can demonstrate increased 18F-fluciclovine uptake, leading to false-positive findings (48). Benign prostatic hypertrophy, acute and chronic inflammation (including postradiation inflammation), and infection all demonstrate varying levels of 18F-fluciclovine uptake. Reactive lymph nodes with uptake have been described adjacent to vascular grafts. Cutaneous and musculoskeletal inflammation has variable activity.
The general criterion for positivity is uptake clearly greater than the level in the bone marrow (preferred L3 vertebra) for lesions larger than 1 cm. Soft-tissue lesions and lymph nodes smaller than 1 cm, subject to the partial-volume effect, may still be considered suggestive if uptake is visually equal to or approaches that in the marrow and is significantly greater than that in the blood pool, as observed in the abdominal aorta.
For patients after prostatectomy, uptake in the prostatectomy bed and seminal vesicles greater than that in the bone marrow is considered suggestive of recurrence. In patients with an intact prostate (e.g., after radiation therapy, high-intensity focal ultrasound, or cryotherapy), focal asymmetric uptake equal to or greater than that in the bone marrow should be considered suggestive of cancer recurrence. Diffuse and symmetric homogeneous uptake should be significantly greater than uptake in the marrow to be considered suggestive. Heterogeneous uptake should be treated as multifocal. It has also been anecdotally noted that median lobe uptake may have greater false positivity.
Lymph node uptake greater than bone marrow uptake, with a distribution typical of recurrent prostate cancer, is suggestive of malignancy. Uptake in lymph nodes in a location atypical of recurrence (e.g., inguinal, distal external iliac) should be considered suggestive only if the uptake is asymmetric, intense, or in the context of other clearly malignant disease. Otherwise, mild symmetric uptake in these nodal groups is considered physiologic.
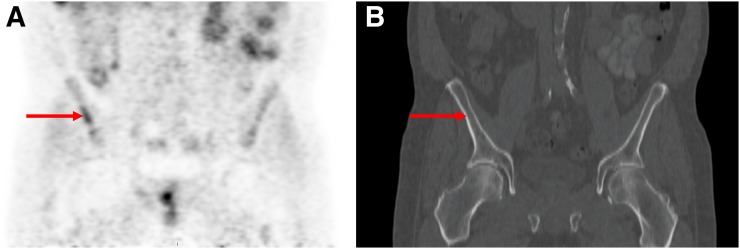
Bone marrow may have heterogeneous activity, more so than is typically seen with 18F-FDG PET. Focal uptake clearly visualized on maximum-intensity-projection images in osseous tissue is considered suggestive of cancer. 18F-fluciclovine uptake in lytic metastatic lesions is typically intense, and there is moderate intensity in mixed sclerotic lesions. Indolent sclerotic lesions may not demonstrate 18F-fluciclovine uptake. A suspected bone abnormality visualized on CT only (i.e., dense sclerosis without uptake) is considered nonspecific and does not exclude the presence of metastasis. Suggestive CT findings without 18F-fluciclovine uptake may be evaluated with alternative imaging modalities for further characterization (e.g., MRI, 18F-NaF PET/CT, 99mTc-methylene diphosphonate SPECT/CT). Degenerative disk and facet uptake is less commonly seen with 18F-fluciclovine than with 18F-FDG. Malignant uptake in what initially appeared to be a Schmorl node with irregular borders has been described. Uptake in benign and malignant bone lesions (e.g., osteoid osteoma and multiple myeloma) has been reported (48). Finally, single pelvic, nonfocal, or nonmasslike 18F-fluciclovine activity not associated with a CT abnormality with benign follow-up has been uncommonly seen in our experience and should therefore be considered equivocal, warranting further evaluation with MR or biopsy (Fig. 6).
FIGURE 6.
A 60-y-old man with biochemical recurrence of prostate cancer (PSA level, 6.2 ng/mL). 18F-fluciclovine PET/CT demonstrated uptake in right iliac bone (arrows; without CT correlate) and in prostate bed (not shown) on coronal PET (A) and CT (B). Biopsy of right iliac bone found benign sclerosis. Uptake was linear and ill-defined, atypical of prostate metastases.
CONCLUSION
18F-fluciclovine is FDA-approved for the localization of recurrent prostate cancer in patients with elevated PSA levels. Comprehensive clinical data demonstrate that 18F-fluciclovine is beneficial in the identification of the site of suspected recurrent disease. 18F-fluciclovine demonstrates improved accuracy when compared with conventional imaging modalities for whole-body staging. Knowledge of normal physiologic distribution and variants, as well as typical patterns of prostate cancer spread, is important for proper interpretation of 18F-fluciclovine PET findings. Further studies will be needed to compare 18F-fluciclovine with PSMA radiotracers and to more completely characterize patterns of bone and other malignant uptake.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Jadvar H. Is there use for FDG-PET in prostate cancer? Semin Nucl Med. 2016;46:502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giovacchini G, Giovannini E, Leoncini R, Riondato M, Ciarmiello A. PET and PET-CT with radiolabeled choline in prostate cancer: a critical reappraisal of 20 years of clinical studies. Eur J Nucl Med Mol Imaging. 2017;44:1751–1776. [DOI] [PubMed] [Google Scholar]
- 4.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell. 2012;21:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okudaira H, Shikano N, Nishii R, et al. Putative transport mechanism and intracellular fate of trans-1-amino-3-18F-fluorocyclobutanecarboxylic acid in human prostate cancer. J Nucl Med. 2011;52:822–829. [DOI] [PubMed] [Google Scholar]
- 6.Sakata T, Ferdous G, Tsuruta T, et al. L-type amino-acid transporter 1 as a novel biomarker for high-grade malignancy in prostate cancer. Pathol Int. 2009;59:7–18. [DOI] [PubMed] [Google Scholar]
- 7.Segawa A, Nagamori S, Kanai Y, Masawa N, Oyama T. L-type amino acid transporter 1 expression is highly correlated with Gleason score in prostate cancer. Mol Clin Oncol. 2013;1:274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shoup TM, Olson J, Hoffman JM, et al. Synthesis and evaluation of [18F]1-amino-3-fluorocyclobutane-1-carboxylic acid to image brain tumors. J Nucl Med. 1999;40:331–338. [PubMed] [Google Scholar]
- 9.Schuster DM, Nye JA, Nieh PT, et al. Initial experience with the radiotracer anti-1-amino-3-[18F]fluorocyclobutane-1-carboxylic acid (anti-[18F]FACBC) with PET in renal carcinoma. Mol Imaging Biol. 2009;11:434–438. [DOI] [PubMed] [Google Scholar]
- 10.McParland BJ, Wall A, Johansson S, Sorensen J. The clinical safety, biodistribution and internal radiation dosimetry of [18F]fluciclovine in healthy adult volunteers. Eur J Nucl Med Mol Imaging. 2013;40:1256–1264. [DOI] [PubMed] [Google Scholar]
- 11.Hays MT, Watson EE, Thomas SR, Stabin M. MIRD dose estimate report no. 19: radiation absorbed dose estimates from 18F-FDG. J Nucl Med. 2002;43:210–214. [PubMed] [Google Scholar]
- 12.Pauleit D, Floeth F, Herzog H, et al. Whole-body distribution and dosimetry of O-(2-[18F]fluoroethyl)-L-tyrosine. Eur J Nucl Med Mol Imaging. 2003;30:519–524. [DOI] [PubMed] [Google Scholar]
- 13.Schuster DM, Votaw JR, Nieh PT, et al. Initial experience with the radiotracer anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid with PET-CT in prostate carcinoma. J Nucl Med. 2007;48:56–63. [PubMed] [Google Scholar]
- 14.Ulaner GA, Goldman DA, Gonen M, et al. Initial results of a prospective clinical trial of 18F-fluciclovine PET-CT in newly diagnosed invasive ductal and invasive lobular breast cancers. J Nucl Med. 2016;57:1350–1356. [DOI] [PubMed] [Google Scholar]
- 15.Kondo A, Ishii H, Aoki S, et al. Phase IIa clinical study of [18F]fluciclovine: efficacy and safety of a new PET tracer for brain tumors. Ann Nucl Med. 2016;30:608–618. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki H, Inoue Y, Fujimoto H, et al. Diagnostic performance and safety of NMK36 (trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid)-PET-CT in primary prostate cancer: multicenter phase IIb clinical trial. Jpn J Clin Oncol. 2016;46:152–162. [DOI] [PubMed] [Google Scholar]
- 17.Schuster DM, Taleghani PA, Nieh PT, et al. Characterization of primary prostate carcinoma by anti-1-amino-2-[18F]-fluorocyclobutane-1-carboxylic acid (anti-3-[18F] FACBC) uptake. Am J Nucl Med Mol Imaging. 2013;3:85–96. [PMC free article] [PubMed] [Google Scholar]
- 18.Turkbey B, Mena E, Shih J, et al. Localized prostate cancer detection with 18F FACBC PET-CT: comparison with MR imaging and histopathologic analysis. Radiology. 2014;270:849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jambor I, Kuisma A, Kahkonen E, et al. Prospective evaluation of 18F-FACBC PET-CT and PET-MRI versus multiparametric MRI in intermediate- to high-risk prostate cancer patients (FLUCIPRO trial). Eur J Nucl Med Mol Imaging. 2018;45:355–364. [DOI] [PubMed] [Google Scholar]
- 20.Elschot M, Selnaes KM, Sandsmark E, et al. A PET-MRI study towards finding the optimal [18F]fluciclovine PET protocol for detection and characterisation of primary prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:695–703. [DOI] [PubMed] [Google Scholar]
- 21.Selnaes KM, Kruger-Stokke B, Elschot M, et al. 18F-fluciclovine PET-MRI for preoperative lymph node staging in high-risk prostate cancer patients. Eur Radiol. January 2, 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22.Cookson MS, Aus G, Burnett AL, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association prostate guidelines for localized prostate cancer update panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–545. [DOI] [PubMed] [Google Scholar]
- 23.Roach M, III, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix consensus conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. [DOI] [PubMed] [Google Scholar]
- 24.Akin-Akintayo OO, Jani AB, Odewole O, et al. Change in salvage radiotherapy management based on guidance with FACBC (fluciclovine) PET-CT in postprostatectomy recurrent prostate cancer. Clin Nucl Med. 2017;42:e22–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Odewole OA, Tade FI, Nieh PT, et al. Recurrent prostate cancer detection with anti-3-[18F]FACBC PET-CT: comparison with CT. Eur J Nucl Med Mol Imaging. 2016;43:1773–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nanni C, Zanoni L, Pultrone C, et al. 18F-FACBC (anti1-amino-3-18F-fluorocyclobutane-1-carboxylic acid) versus 11C-choline PET-CT in prostate cancer relapse: results of a prospective trial. Eur J Nucl Med Mol Imaging. 2016;43:1601–1610. [DOI] [PubMed] [Google Scholar]
- 27.Bach-Gansmo T, Nanni C, Nieh PT, et al. Multisite experience of the safety, detection rate and diagnostic performance of fluciclovine (18F) positron emission tomography/computerized tomography imaging in the staging of biochemically recurrent prostate cancer. J Urol. 2017;197:676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kairemo K, Rasulova N, Partanen K, Joensuu T. Preliminary clinical experience of trans-1-amino-3-18F-fluorocyclobutanecarboxylic acid (anti-18F-FACBC) PET-CT imaging in prostate cancer patients. Biomed Res Int. 2014;2014:305182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuster DM, Nieh PT, Jani AB, et al. Anti-3-[18F]FACBC positron emission tomography-computerized tomography and 111In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol. 2014;191:1446–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Odewole OA, Oyenuga OA, Tade F, et al. Reproducibility and reliability of anti-3-[18F]FACBC uptake measurements in background structures and malignant lesions on follow-up PET-CT in prostate carcinoma: an exploratory analysis. Mol Imaging Biol. 2015;17:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oka S, Kanagawa M, Doi Y, Schuster DM, Goodman MM, Yoshimura H. PET tracer 18F-fluciclovine can detect histologically proven bone metastatic lesions: a preclinical study in rat osteolytic and osteoblastic bone metastasis nodes. Theranostics. 2017;7:2048–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inoue Y, Asano Y, Satoh T, et al. Phase IIa clinical trial of trans-1-amino-3-18F-fluoro-cyclobutane carboxylic acid in metastatic prostate cancer. Asia Ocean J Nucl Med Biol. 2014;2:87–94. [PMC free article] [PubMed] [Google Scholar]
- 33.Budäus L, Leyh-Bannurah SR, Salomon G, et al. Initial experience of 68Ga-PSMA PET-CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur Urol. 2016;69:393–396. [DOI] [PubMed] [Google Scholar]
- 34.Nanni C, Schiavina R, Brunocilla E, et al. 18F-FACBC compared with 11C-choline PET-CT in patients with biochemical relapse after radical prostatectomy: a prospective study in 28 patients. Clin Genitourin Cancer. 2014;12:106–110. [DOI] [PubMed] [Google Scholar]
- 35.Nanni C, Schiavina R, Brunocilla E, et al. 18F-fluciclovine PET-CT for the detection of prostate cancer relapse: a comparison to 11C-choline PET-CT. Clin Nucl Med. 2015;40:e386–e391. [DOI] [PubMed] [Google Scholar]
- 36.Testa C, Schiavina R, Lodi R, et al. Prostate cancer: sextant localization with MR imaging, MR spectroscopy, and 11C-choline PET-CT. Radiology. 2007;244:797–806. [DOI] [PubMed] [Google Scholar]
- 37.Farsad M, Schiavina R, Castellucci P, et al. Detection and localization of prostate cancer: correlation of 11C-choline PET-CT with histopathologic step-section analysis. J Nucl Med. 2005;46:1642–1649. [PubMed] [Google Scholar]
- 38.Calais J, Fendler WP, Herrmann K, Eiber M, Ceci F. Head-to-head comparison of 68Ga-PSMA-11 PET-CT and 18F-fluciclovine PET-CT in a case series of 10 patients with prostate cancer recurrence. J Nucl Med. December 14, 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 39.Afshar-Oromieh A, Babich JW, Kratochwil C, et al. The rise of PSMA ligands for diagnosis and therapy of prostate cancer. J Nucl Med. 2016;57(suppl 3):79S–89S. [DOI] [PubMed] [Google Scholar]
- 40.Gorin MA, Pienta KJ, Pomper MG, Rowe SP. Prostate cancer local recurrence detected with both 18F-fluciclovine and PSMA-targeted 18F-DCFPyL PET-CT. Urology. 2017;107:e9–e10. [DOI] [PubMed] [Google Scholar]
- 41.Cotter SE, Chen MH, Moul JW, et al. Salvage radiation in men after prostate-specific antigen failure and the risk of death. Cancer. 2011;117:3925–3932. [DOI] [PubMed] [Google Scholar]
- 42.Jani AB, Schreibmann E, Rossi PJ, et al. Impact of 18F-fluciclovine PET on target volume definition for postprostatectomy salvage radiotherapy: initial findings from a randomized trial. J Nucl Med. 2017;58:412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schreibmann E, Schuster DM, Rossi PJ, Shelton J, Cooper S, Jani AB. Image guided planning for prostate carcinomas with incorporation of anti-3-[18F]FACBC (fluciclovine) positron emission tomography: workflow and initial findings from a randomized trial. Int J Radiat Oncol Biol Phys. 2016;96:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Souvatzoglou M, Krause BJ, Purschel A, et al. Influence of 11C-choline PET-CT on the treatment planning for salvage radiation therapy in patients with biochemical recurrence of prostate cancer. Radiother Oncol. 2011;99:193–200. [DOI] [PubMed] [Google Scholar]
- 45.Ceci F, Herrmann K, Castellucci P, et al. Impact of 11C-choline PET-CT on clinical decision making in recurrent prostate cancer: results from a retrospective two-centre trial. Eur J Nucl Med Mol Imaging. 2014;41:2222–2231. [DOI] [PubMed] [Google Scholar]
- 46.Schuster DM, Savir-Baruch B, Nieh PT, et al. Detection of recurrent prostate carcinoma with anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET-CT and 111In-capromab pendetide SPECT/CT. Radiology. 2011;259:852–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savir-Baruch B, Zanoni L, Schuster DM. Imaging of prostate cancer using fluciclovine. PET Clin. 2017;12:145–157. [DOI] [PubMed] [Google Scholar]
- 48.Schuster DM, Nanni C, Fanti S, et al. Anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid: physiologic uptake patterns, incidental findings, and variants that may simulate disease. J Nucl Med. 2014;55:1986–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]