Abstract
18F-alfatide II has been proven to have excellent clinical translational potential. In this study, we investigated 18F-alfatide II for identifying breast cancer and compared the performances between 18F-alfatide II and 18F-FDG. Methods: Forty-four female patients with suspected primary breast cancer were recruited. PET/CT images using 18F-alfatide II and 18F-FDG were acquired within 7 d. Tracer uptake in breast lesions was evaluated by visual analysis, and semiquantitative analysis with SUVmax and SUVmean. Results: Forty-two breast cancer lesions and 11 benign breast lesions were confirmed by histopathology in 44 patients. Both 18F-alfatide II and 18F-FDG had higher uptake in breast cancer lesions than in benign breast lesions (P < 0.05 for 18F-alfatide II, P < 0.05 for 18F-FDG). The area under the curve of 18F-alfatide II was slightly less than that of 18F-FDG. Both 18F-alfatide II and 18F-FDG had high sensitivity (88.1% vs. 90.5%), high positive predictive value (88.1% vs. 88.4%), moderate specificity (54.5% vs. 54.5%), and moderate negative predictive value (54.5% vs. 60.0%) for differentiating breast cancer from benign breast lesions. By combining 18F-alfatide II and 18F-FDG, the sensitivity and negative predictive value significantly increased to 97.6% and 85.7%, respectively, with positive predictive value slightly increased to 89.1% and no change to the specificity (54.5%). The uptake of 18F-alfatide II (SUVmax: 3.77 ± 1.78) was significantly lower than that of 18F-FDG (SUVmax: 7.37 ± 4.48) in breast cancer lesions (P < 0.05). 18F-alfatide II uptake in triple-negative subtype was significantly lower than that in luminal A and luminal B subtypes. By contrast, human epidermal growth factor receptor-2 (HER-2)–overexpressing subtype had higher 18F-FDG uptake than the other 3 subtypes. There were 8 breast cancer lesions with higher 18F-alfatide II uptake than 18F-FDG uptake, which all had a common characteristic that HER-2 expression was negative and estrogen receptor expression was strongly positive. Conclusion: 18F-alfatide II is suitable for clinical use in breast cancer patients. 18F-alfatide II is of good performance, but not superior to 18F-FDG in identifying breast cancer. 18F-alfatide II may have superiority to 18F-FDG in detecting breast cancer with strongly positive estrogen receptor expression and negative HER-2 expression.
Keywords: 18F-alfatide II, integrin αvβ3, positron emission tomography, breast cancer
Angiogenesis exerts a prominent role in promoting tumor growth, progression, and metastasis. Integrin αvβ3 is highly expressed on activated endothelial cells of tumor neovasculature and thus is key to tumor angiogenesis (1–3). Arginine-glycine-aspartate (RGD) peptides have a high binding affinity with integrin αvβ3. As a result, a variety of RGD-based molecular probes have been developed to visualize integrin αvβ3 expression (4–6). Because of the superiority of PET molecular imaging technique, RGD tracers labeled with positron-emitting radionuclides such as 18F, 64Cu, 68Ga, and 89Zr have attracted much attention (7–10). As a PET tracer based on dimeric RGD peptide, 18F-alfatide II has been recently proven to possess excellent clinical translational potential in several studies (11–16). Consequently, it warrants further promotion in clinical applications.
Breast cancer is a heterogeneous disease, and angiogenesis is one of the important characteristics (17). Great strides have been made in breast cancer treatment, such as endocrine therapy, targeted human epidermal growth factor receptor-2 (HER-2) therapy, and antiangiogenic therapy. Bevacizumab, as an antiangiogenic drug, was approved by the U.S. Food and Drug Administration to treat patients with advanced breast cancer in 2008. Three years later, however, this approval was withdrawn due to a lack of evidence in improving overall survival. The unsuccessful predicament of bevacizumab could be attributed to the absence of prescreening to select for patients with specific angiogenic biomarkers (18). Accordingly, imaging angiogenesis is crucial for breast cancer patients before antiangiogenic therapy. Moreover, the present molecular classification of breast cancer is based on the status of estrogen receptor (ER), progesterone receptor (PR), and HER-2, which are all expressed on the membrane of breast cancer cells themselves. The biomarkers located in breast cancer stroma are not included in the present classification. With continuous development and improvement of the molecular classification system, some stromal biomarkers such as integrin αvβ3 might be incorporated into new classifications in the future. Consequently, the molecular imaging visualization of integrin αvβ3 expression should be valuable in exploring the molecular classification based on breast cancer stroma.
There have been a few studies using RGD-based PET probes such as 18F-galacto-RGD, 18F-AH111585, 18F-FPPRGD2, and 68Ga-PRGD2 in the clinic for breast cancer patients (19–24). Generally, these PET tracers have shown satisfactory performance in terms of safety, feasibility, and usefulness. However, the numbers of breast cancer cases included in these studies are often very small. Some diagnostic parameters such as sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) are unknown for RGD-based PET tracers due to the absence of benign breast lesions in these studies. Moreover, ductal carcinoma in situ (DCIS) cases were not included in these investigations. For this reason, the information concerning RGD uptake in early breast cancer patients remains unclear. Furthermore, there have been inadequate studies regarding RGD uptake by different subtypes based on the present molecular classification of breast cancer.
In this work, 18F-alfatide II was investigated in clinically suspected breast cancer patients for the first time, and it was meanwhile compared with 18F-FDG. We obtained diagnostic parameters of 18F-alfatide II facilitating the differentiation between breast cancer and benign breast lesions and further assessed its diagnostic performance in comparison with 18F-FDG. We also investigated 18F-alfatide II uptake in different molecular subtypes of breast cancer and evaluated the differences as compared with 18F-FDG.
MATERIALS AND METHODS
Patients
This study was approved by the ethics committee of Jinling Hospital (approval no. 2015NZYW-007) and registered at ClinicalTrials.gov (NCT02582801). Inclusion criteria consisted of clinically suspected primary breast cancer according to conventional imaging (e.g., mammography, ultrasound, MRI), no prior treatment for breast lesions, 18 y < age < 70 y. Exclusion criteria consisted of pregnancy, lactation period, and some accompanied serious diseases (e.g., impaired liver or renal function, active tuberculosis, other malignant tumor). Forty-four female patients (age range, 28–66 y; mean age ± SD, 50.73 ± 8.01 y) were included in this study and each patient signed a written informed consent form. This was a preliminary clinical study about the diagnosis of breast cancer, and a study about treatment response monitoring is ongoing in a larger group of patients.
PET/CT Acquisition and Image Analysis
18F-alfatide II was prepared according to the previously reported method (25). The injected activity was 306 ± 80 MBq (range, 155–503 MBq). No specific patient preparation such as fasting was requested for 18F-alfatide II PET/CT scanning, which was performed at 60 min after the injection using a Biography 16 PET/CT scanner (Siemens Healthcare). CT acquisition was initially performed with 120 kV, 140 mA, and a slice thickness of 5 mm. Immediately after CT acquisition, PET emission scanning was performed with an acquisition time of 3 min for each bed. PET data were corrected for attenuation using the coregistered CT data and PET images and reconstructed using an iterative algorithm. PET, CT, and fused images were displayed on a Siemens/Syngo user interface.
18F-FDG PET/CT was performed within 1–7 d before or after 18F-alfatide II PET/CT. All patients fasted for at least 6 h before receiving an intravenous injection of 18F-FDG (∼3.7 MBq/kg of body weight). Blood glucose was measured before injection to ensure that the level was below 140 mg/dL. The same procedure was used for 18F-FDG PET/CT data acquisition as was used for 18F-alfatide II PET/CT using the same scanner. The uptake time between 18F-FDG injection and PET/CT acquisition was also 60 min.
The images from 2 PET/CT scans were visually interpreted by a consensus of 2 experienced nuclear medicine physicians, who were masked to the histologic diagnosis and other imaging results. The SUVmax and the SUVmean were semiquantitatively measured by drawing regions of interest over the breast lesions.
Statistical Analysis
The data were presented as the mean ± SD. The difference between 2 groups was analyzed by Student t test, and differences among 3 or more groups were determined by ANOVA. Receiver-operating-characteristic (ROC) curve analysis was performed to evaluate the diagnostic performance. The area under the curve (AUC) and the cutoff value were further determined at the point with the highest Youden index. The sensitivity, specificity, PPV, and NPV were calculated to compare the differences in diagnostic accuracy. A Pearson correlation coefficient test was performed to determine the correlation between 18F-alfatide II and 18F-FDG groups. Statistical analysis was performed using SPSS software (version 17.0; SPSS, Inc.), and a P value of less than 0.05 was considered statistically significant.
RESULTS
Performance of 18F-Alfatide II in Diagnosis of Breast Cancer
All patients received biopsy or surgery after undergoing 2 PET/CT scans. Fifty-three breast lesions were confirmed by histopathology in 44 patients. Among them, 42 lesions were malignant and the others were benign. The malignant lesions included DCIS (4 cases) and invasive carcinoma (38 cases). The latter was divided into luminal A subtype (6 cases), luminal B subtype (15 cases), HER-2–overexpressing subtype (5 cases), and triple-negative subtype (12 cases). In luminal B subtype, 6 cases were HER-2–positive and 9 cases were HER-2 –negative. Luminal B subtype with positive HER-2 expression is completely different from HER-2–overexpressing subtype (non-luminal subtype). The former is defined as ER- and (or) PR-positive, HER-2–positive, and Ki-67 at any level. The latter is defined as both ER- and PR-negative and HER-2–positive. The benign lesions were composed of breast fibroadenoma (4 cases), breast adenosis (4 cases), breast intraduct papilloma (2 cases), and mastitis (1 case).
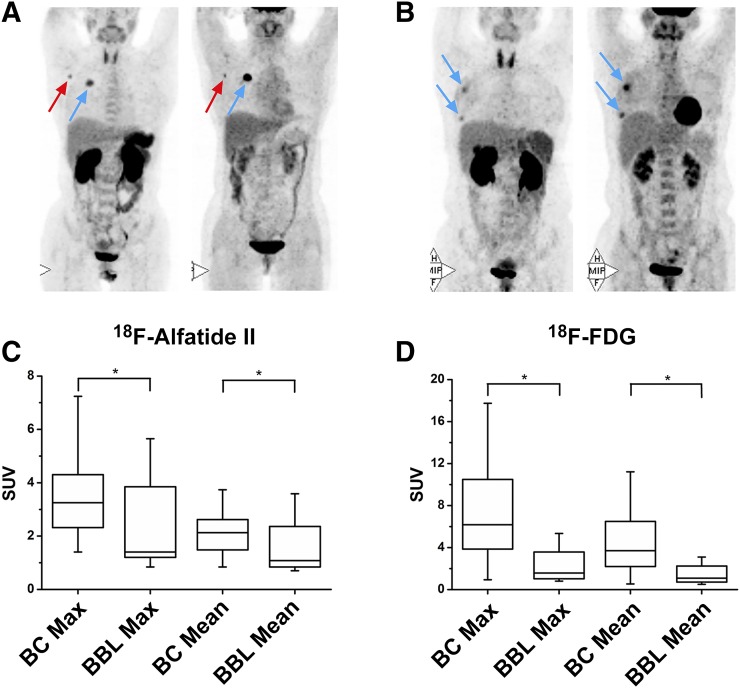
Most breast cancer lesions had high uptake (Fig. 1A). Several benign breast lesions also showed increased uptake (Fig. 1B). The SUVmax and SUVmean of breast lesions are shown in Table 1. In general, breast cancer lesions had higher 18F-alfatide II uptake than benign breast lesions (P < 0.05). Also, 18F-FDG accumulation in breast cancer lesions was more intense than that in benign breast lesions (P < 0.05). However, the difference of 18F-alfatide II SUV between breast cancer and benign lesions was less significant as compared with that of 18F-FDG SUV (Figs. 1C and 1D).
FIGURE 1.
(A) A 50-y-old patient with HER-2–overexpressing breast cancer (blue arrow), and axillary lymph node metastasis (red arrow) showing high 18F-alfatide II and 18F-FDG uptake. (B) A 47-y-old patient with 2 lesions of breast intraduct papilloma (blue arrows) showing high 18F-alfatide II and 18F-FDG uptake. Difference of SUVs between breast cancer and benign breast lesion for 18F-alfatide II (C) and 18F-FDG (D). *P < 0.05.
TABLE 1.
Comparisons of 18F-Alfatide II and 18F-FDG Uptake Between Breast Cancer and Benign Breast Lesion
|
18F-alfatide II |
18F-FDG |
||||
| Disease | SUVmax | SUVmean | SUVmax | SUVmean | |
| BC | 3.77 ± 1.78 | 2.25 ± 0.98 | 7.37 ± 4.48 | 4.54 ± 2.82 | |
| P value | P < 0.05 | P < 0.05 | P < 0.05 | P < 0.05 | |
| BBL | 2.37 ± 1.62 | 1.50 ± 0.92 | 2.88 ± 2.77 | 1.75 ± 1.50 | |
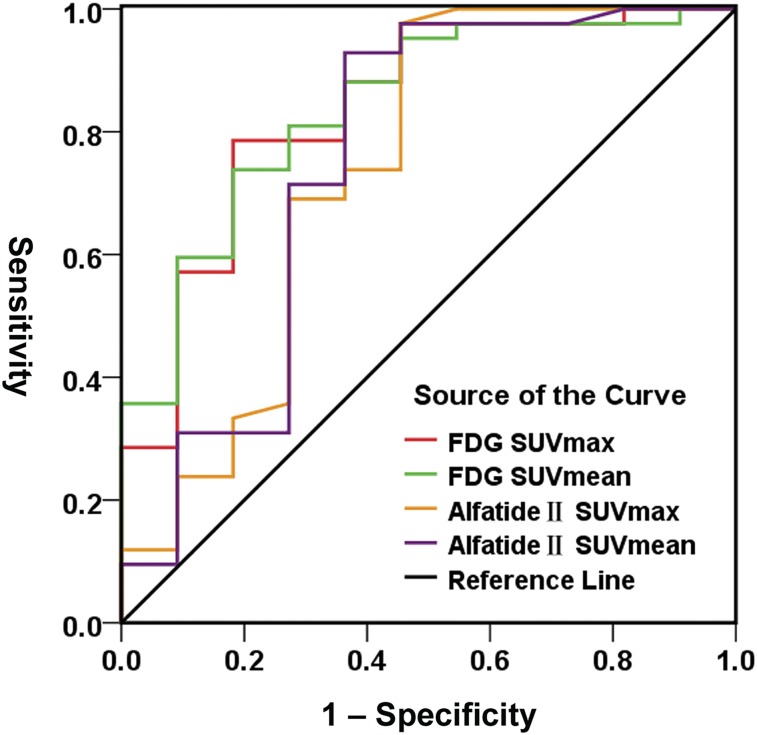
As shown in Figure 2, ROC curves of 18F-alfatide II are located at the upper left of the chance line, just as those of 18F-FDG, indicating their strong potential for identifying breast cancer. However, the AUC of 18F-alfatide II is slightly less than that of 18F-FDG. The corresponding statistics of the ROC curves are shown in Table 2. The maximum Youden index of 18F-alfatide II SUVmax (52.1%) is lower than that of 18F-FDG SUVmax (60.4%), but the gap is small. Moreover, the indices are very close between 18F-alfatide II SUVmean (56.5%) and 18F-FDG SUVmean (55.6%). The cutoff value with a maximum Youden index is 1.6 for 18F-alfatide II SUVmax, 1.28 for 18F-alfatide II SUVmean, 3.68 for 18F-FDG SUVmax, and 2.26 for 18F-FDG SUVmean. When the SUVmax or SUVmean of the breast lesion is higher than its cutoff value, breast cancer may be considered. However, 18F-FDG SUVmean has the largest AUC (0.84) and 18F-alfatide II SUVmax has the smallest AUC (0.738).
FIGURE 2.
ROC curves of SUVs of 18F-alfatide II and 18F-FDG in differentiating breast cancer from benign lesion.
TABLE 2.
ROC Quantitative Analysis of 18F-Alfatide II and 18F-FDG in Differentiating Breast Cancer from Benign Breast Lesion
| Tracer | Cutoff value | AUC | Sensitivity | Specificity | Youden index |
| 18F-alfatide II | SUVmax 1.6 | 0.738 | 97.6% | 54.5% | 52.1% |
| SUVmean 1.28 | 0.752 | 92.9% | 63.6% | 56.5% | |
| 18F-FDG | SUVmax 3.68 | 0.838 | 78.6% | 81.8% | 60.4% |
| SUVmean 2.26 | 0.840 | 73.8% | 81.8% | 55.6% |
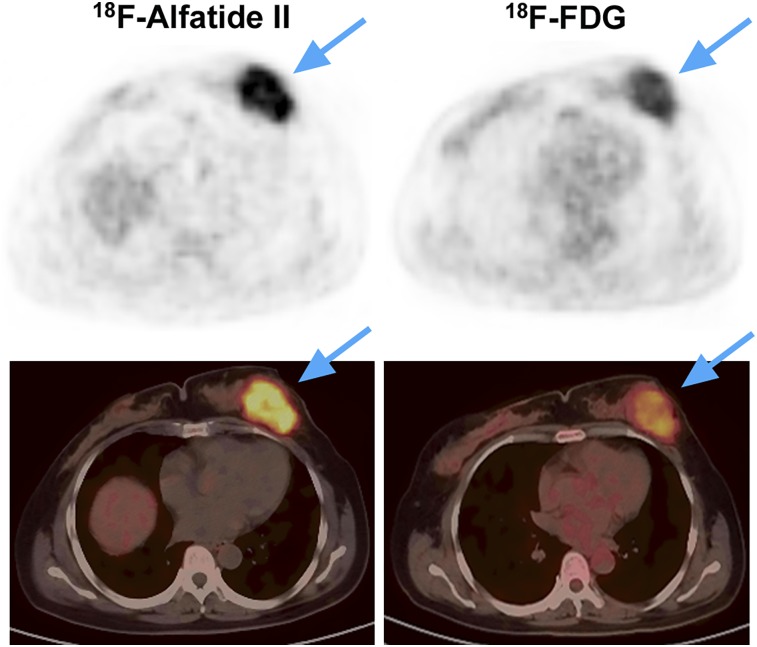
The visual analysis showed that both 18F-alfatide II and 18F-FDG for differentiating breast cancer from benign breast lesions had high sensitivity (88.1% vs. 90.5%) and PPV (88.1% vs. 88.4%). However, they had the same specificity (54.5%) and similar NPV (54.5% vs. 60%), which were moderate. The false-positive lesions were the same for 18F-alfatide II and 18F-FDG, including 2 cases of breast fibroadenoma (Fig. 3), 1 case of breast adenosis, 1 case of breast intraduct papilloma, and 1 case of mastitis. One case of DCIS was false-negative for both 18F-alfatide II and 18F-FDG. Other false-negative lesions of 18F-alfatide II included 4 cases of breast cancer with triple-negative subtype (Fig. 4). Two cases of breast cancer with luminal B subtype (HER-2–negative) and 1 case of invasive lobular carcinoma was also false-negative for 18F-FDG. As a result, the sensitivity and NPV significantly increased to 97.6% and 85.7%, respectively, by combining 18F-alfatide II and 18F-FDG. However, there was no change on the specificity and only a slight increase on PPV (Table 3).
FIGURE 3.
A 50-y-old patient with breast fibroadenoma (blue arrows) showing false-positive uptake of both 18F-alfatide II (SUVmax: 5.65) and 18F-FDG (SUVmax: 3.57).
FIGURE 4.
A 46-y-old patient with triple-negative breast cancer (blue arrows) and axillary lymph node metastasis (red arrows) showing no increased 18F-alfatide II uptake but intense 18F-FDG uptake.
TABLE 3.
Visual Analysis of 18F-Alfatide II and 18F-FDG in Differentiating Breast Cancer from Benign Breast Lesion
| Tracer | Sensitivity | Specificity | PPV | NPV |
| 18F-alfatide II | 88.1% | 54.5% | 88.1% | 54.5% |
| 18F-FDG | 90.5% | 54.5% | 88.4% | 60.0% |
| 18F-alfatide II + 18F-FDG | 97.6% | 54.5% | 89.1% | 85.7% |
Comparisons Between 18F-Alfatide II and 18F-FDG Uptake in Breast Cancer and Benign Breast Lesions
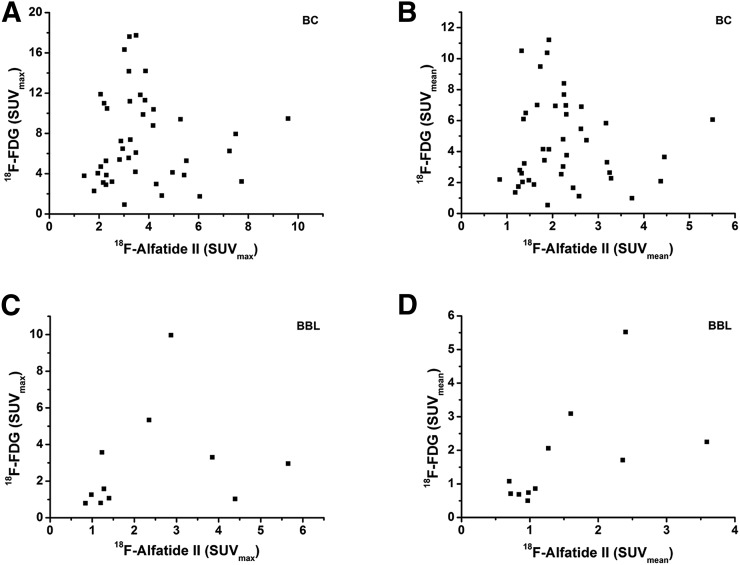
The 18F-alfatide II and 18F-FDG uptake values in breast cancer and benign breast lesions are listed in Table 4. The uptake of 18F-alfatide II was significantly lower than that of 18F-FDG in breast cancer lesions (P < 0.05), for both SUVmax and SUVmean. 18F-alfatide II uptake in benign breast lesions was also lower than 18F-FDG, but the difference was not statistically significant (P > 0.05). The correlation between 18F-alfatide II and 18F-FDG uptake in breast cancer lesions was not significant (r = 0.03, P > 0.05 for SUVmax and r = 0.003, P > 0.05 for SUVmean). There was a positive correlation between 18F-alfatide II and 18F-FDG uptake in benign breast lesions, which was present for SUVmax (r = 0.69, P < 0.05) but absent for SUVmean (r = 0.59, P > 0.05) (Fig. 5).
TABLE 4.
Comparisons of 18F-Alfatide II and 18F-FDG Uptake in Breast Cancer and Benign Breast Lesion
| BC |
BBL |
||||
| Tracer | SUVmax | SUVmean | SUVmax | SUVmean | |
| 18F-alfatide II | 3.77 ± 1.78 | 2.25 ± 0.98 | 2.37 ± 1.62 | 1.50 ± 0.92 | |
| P value | P < 0.05 | P < 0.05 | P > 0.05 | P > 0.05 | |
| 18F-FDG | 7.37 ± 4.48 | 4.54 ± 2.82 | 2.88 ± 2.77 | 1.75 ± 1.50 | |
FIGURE 5.
Correlation between 18F-alfatide II uptake and 18F-FDG uptake, respectively, based on SUVmax and SUVmean in breast cancer (A and B) and benign breast lesions (C and D).
18F-Alfatide II Uptake in Different Molecular Subtypes of Breast Cancer
The 18F-alfatide II and 18F-FDG uptake values in different molecular subtypes of breast cancer are shown in Table 5. Luminal A, luminal B, and HER-2–overexpressing subtypes have similar 18F-alfatide II uptake values, with all being higher than those of triple-negative subtype. According to statistical analysis, there was significant difference in 18F-alfatide II uptake between triple-negative subtype and luminal A subtype, as well as between triple-negative subtype and luminal B subtype (P < 0.05). No other 2 subtypes of breast cancer had significant difference in 18F-alfatide II uptake (P > 0.05) (Table 6). In comparison, 18F-FDG uptake of HER-2–overexpressing subtypes was significantly higher than that of the other 3 subtypes (P < 0.05). Moreover, 18F-FDG uptake in triple-negative subtype was significantly higher than that in luminal B subtype (P < 0.05). There was no significant difference in 18F-FDG uptake between luminal A subtype and luminal B subtype, or between luminal A subtype and triple-negative subtype (P > 0.05) (Table 7).
TABLE 5.
18F-Alfatide II and 18F-FDG Uptake in Different Molecular Subtypes of Breast Cancer
|
18F-alfatide II |
18F-FDG |
|||
| Molecular subtype | SUVmax | SUVmean | SUVmax | SUVmean |
| Luminal A | 4.72 ± 2.56 | 2.87 ± 1.42 | 6.08 ± 3.75 | 3.77 ± 2.30 |
| Luminal B | 4.35 ± 1.72 | 2.62 ± 1.10 | 5.36 ± 2.64 | 3.22 ± 1.63 |
| HER-2 overexpressing | 4.41 ± 1.92 | 2.61 ± 1.03 | 13.88 ± 3.89 | 8.61 ± 2.23 |
| Triple negative | 2.83 ± 0.82 | 1.77 ± 0.53 | 9.30 ± 4.11 | 5.80 ± 2.69 |
TABLE 6.
P Values of Multiple Comparisons Using 18F-Alfatide II SUVmax Among Different Molecular Subtypes of Breast Cancer
| Molecular subtype | Luminal A | Luminal B | HER-2 positive | Triple negative |
| Luminal A | — | 0.658 | 0.763 | 0.032 |
| Luminal B | 0.658 | — | 0.951 | 0.026 |
| HER-2 overexpressing | 0.763 | 0.951 | — | 0.088 |
| Triple negative | 0.032 | 0.026 | 0.088 | — |
TABLE 7.
P Values of Multiple Comparisons Using 18F-FDG SUVmax Among Different Molecular Subtypes of Breast Cancer
| Molecular subtype | Luminal A | Luminal B | HER-2 positive | Triple negative |
| Luminal A | — | 0.67 | 0.001 | 0.074 |
| Luminal B | 0.670 | — | 0.000 | 0.006 |
| HER-2 overexpressing | 0.001 | 0.000 | — | 0.019 |
| Triple negative | 0.074 | 0.006 | 0.019 | — |
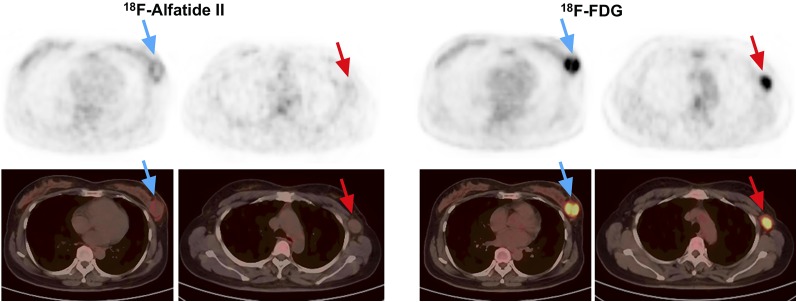
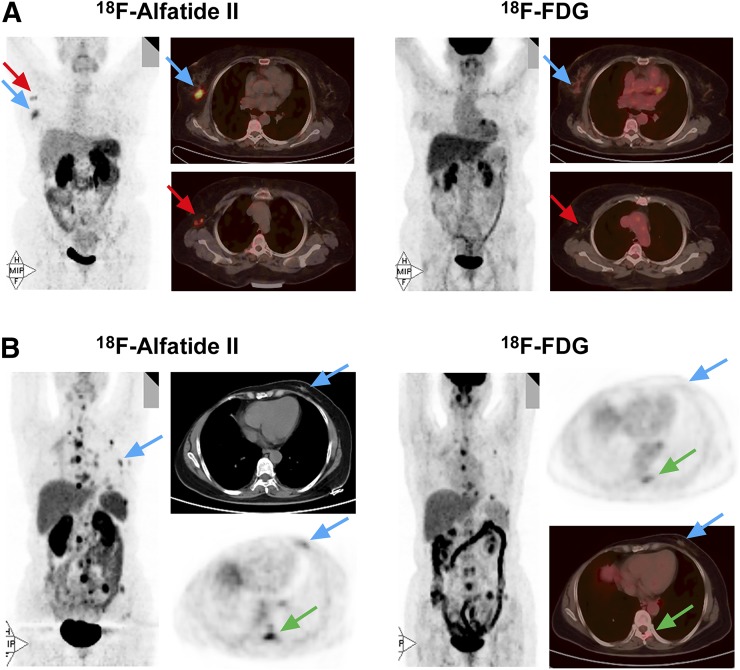
Eight breast cancer lesions had higher 18F-alfatide II uptake than 18F-FDG. Among them, 6 lesions were luminal B subtype (HER-2–negative), which accounted for 66.7% (6/9) in all cases of this subtype (Fig. 6A). The remaining 2 lesions were luminal A subtype, with a proportion of 33.3% (2/6) of this subtype (Fig. 6B). Moreover, negative HER-2 expression and strongly positive (3+) ER expression were together shown in the 8 breast cancer lesions. The PR expression was diverse in these lesions.
FIGURE 6.
(A) A 60-y-old patient with luminal B (HER-2–negative) breast cancer (blue arrows) and axillary lymph node metastasis (red arrows) showing intense 18F-alfatide II uptake but no 18F-FDG uptake. (B) A 54-y-old patient with luminal A (HER-2–negative) breast cancer (blue arrows) showing increased 18F-alfatide II uptake, but no 18F-FDG uptake, and distant metastases with larger numbers and more intense uptake (green arrows) by 18F-alfatide II than by 18F-FDG.
DISCUSSION
RGD-based PET tracers have undergone rapid development in recent years. 18F-galacto-RGD was the first noninvasive probe to target integrin αvβ3 for PET angiogenesis imaging. Like 18F-Galacto-RGD, 18F-AH111585 is another monomeric RGD PET tracer. There have been several preclinical and clinical studies suggesting the usefulness of these 2 PET tracers (20,22,26–30). However, they have relatively low tumor uptake, because of limited RGD binding of monomeric peptides and rapid clearance of the peptide tracers. Accordingly, radionuclide-labeled RGD dimers, including 18F-FPPRGD2 and 68Ga-PRGD2, have been further developed to achieve better performance (31–33). The use of a fluoride-aluminum complex, 18F-Al-NOTA-PRGD2 (denoted as 18F-alfatide), significantly simplifies the labeling procedure (34–37).
For the first time, 18F-alfatide II was clinically used in differentiating breast cancer from benign breast lesions in our study. As compared with previous studies using other RGD-based PET tracers in breast cancer patients (19,20,22–24), our study included more diverse cases, such as breast fibroadenoma, breast adenosis, DCIS, invasive carcinoma, and lobular carcinoma. Even though there were 5 cases of early cancer in our study, breast cancer overall still showed relatively high 18F-alfatide II uptake with a SUVmax of 3.77 ± 1.78 and SUVmean of 2.25 ± 0.98, higher than that in benign breast lesions, which demonstrated that 18F-alfatide II is suitable for identification of breast cancer in clinical practice.
In comparison with 18F-FDG, 18F-alfatide II had less difference in uptake between breast cancer and benign lesions. AUC of 18F-alfatide II was also lower than that of 18F-FDG in diagnosis of breast cancer. Some diagnostic parameters such as Youden index (SUVmax), sensitivity, PPV, and NPV for 18F-alfatide II were slightly lower than those for 18F-FDG, whereas the specificity was same. These results indicated that 18F-alfatide II has a diagnostic value comparable to that of 18F-FDG but is not superior in identification of breast cancer.
In our study, 5 cases of benign breast lesions displayed false-positive uptake for both 18F-alfatide II and 18F-FDG, suggesting that 18F-alfatide II does not help improve the detection specificity. As for lobular carcinoma, it has been known that the false-negative rate of 18F-FDG is as high as 65.2% (38). One case of lobular carcinoma included in our investigation also showed no 18F-FDG uptake but had intense 18F-alfatide II uptake (Fig. 4B). This phenomenon was consistent with that reported in previous studies using 18F-FPPRGD2 (19), indicating that RGD-based PET tracers such as 18F-alfatide II can be complementary to 18F-FDG in diagnosis of lobular carcinoma of the breast. Other false-negative cases of 18F-FDG included 1 case of DCIS and 2 cases of breast cancer with luminal B subtype (negative HER-2 expression). The former case was also deficient in 18F-alfatide II uptake because of the small lesion size. The latter 2 cases, however, showed increased 18F-alfatide II uptake, suggesting the additive role of 18F-alfatide II to 18F-FDG in detecting this subtype of breast cancer. On the other hand, 4 cases of triple-negative subtype without 18F-alfatide II foci showed significantly increased 18F-FDG uptake, demonstrating that 18F-FDG can compensate for the deficiency of 18F-alfatide II in this subtype. Accordingly, combining 18F-alfatide II and 18F-FDG together can significantly improve the sensitivity and NPV but may not add much in terms of the specificity and PPV.
Comparisons between 18F-alfatide II and 18F-FDG uptake were also made in our study. The result showed that tumor uptake of 18F-alfatide II was significantly lower than that of 18F-FDG in breast cancer. This finding was similar to those presented in other RGD tracer studies (19,21). It has been reported in several studies that there was no significant correlation between RGD-based PET tracers and 18F-FDG in cancer lesion uptake (21,24). Likewise, our result revealed a lack of significant correlation between 18F-alfatide II uptake and 18F-FDG uptake in breast cancer.
18F-alfatide II uptake in different molecular subtypes of breast cancer was also assessed in our study. The triple-negative subtype showed no or low 18F-alfatide II uptake, which was significantly lower than that of luminal A and luminal B subtypes. By contrast, the triple-negative subtype showed high 18F-FDG uptake, whereas luminal A and luminal B subtypes had relatively low 18F-FDG uptake. This result was similar to that from the previous study using 68Ga-PRGD2 in breast cancer patients (23).
Furthermore, our study evaluated the breast cancer lesions with higher uptake of RGD peptide than that of 18F-FDG for the first time. A total of 8 such lesions were found in our study. Interestingly, they shared a common feature of being HER-2–negative and strongly ER-positive (3+). This finding may help guide the therapeutic direction of breast cancer patients with multiple metastases, which are not classified by conventional molecular subtypes. If these metastases have higher uptake of 18F-alfatide II than that of 18F-FDG, they are very likely to be strongly ER-positive and HER-2–negative. Accordingly, they could benefit from endocrine therapy such as tamoxifen, but not from anti–HER-2 therapy such as trastuzumab.
There exist some limitations in our study. First, the number of participants is not large enough. Second, lymph node and other distant metastases were not evaluated due to the limited number of metastatic lesions in these patients. Third, immunohistochemistry tests were not performed to assess the correlation between integrin αvβ3 expression and alfatide II uptake, which has been demonstrated in several animal and clinical studies (12,16,39). Further investigations are still required in the future to elucidate the role of 18F-alfatide II in breast cancer management.
CONCLUSION
18F-alfatide II is clinically amenable for the identification of breast cancer without special patient preparation. 18F-alfatide II has good diagnostic value in distinguishing between breast cancer and benign breast lesions, but is not superior to 18F-FDG. 18F-alfatide II can exert a complementary role to 18F-FDG in breast lobular carcinoma and other breast cancers with strongly positive ER expression and negative HER-2 expression.
DISCLOSURE
This work was supported by the National Key Basic Research Program of China (973 program, 2014CB744504), the National Natural Science Foundation of China (81501537), the Natural Science Foundation of Jiangsu Province (BK20160610), Jiangsu Province Social Development Program (BE2017772), Jiangsu Planned Projects for Postdoctoral Research Funds (1601090C), and the Intramural Research Program, National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health. Jihong Tian, Chuanjin Sun, and Xingang Wang also contributed to this work. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. [DOI] [PubMed] [Google Scholar]
- 2.Felding-Habermann B, O’Toole TE, Smith JW, et al. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci USA. 2001;98:1853–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. [DOI] [PubMed] [Google Scholar]
- 4.Danhier F, Le Breton A, Preat V. RGD-based strategies to target αvβ3 integrin in cancer therapy and diagnosis. Mol Pharm. 2012;9:2961–2973. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Niu G, Wu H, et al. Clinical application of radiolabeled RGD peptides for PET imaging of integrin alphavbeta3. Theranostics. 2016;6:78–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakravarty R, Chakraborty S, Dash A. Molecular imaging of breast cancer: role of RGD peptides. Mini Rev Med Chem. 2015;15:1073–1094. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Park R, Hou Y, et al. MicroPET imaging of brain tumor angiogenesis with 18F-labeled PEGylated RGD peptide. Eur J Nucl Med Mol Imaging. 2004;31:1081–1089. [DOI] [PubMed] [Google Scholar]
- 8.Jackson AB, Nanda PK, Rold TL, et al. 64Cu-NO2A-RGD-Glu-6-Ahx-BBN(7-14)NH2: a heterodimeric targeting vector for positron emission tomography imaging of prostate cancer. Nucl Med Biol. 2012;39:377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eo JS, Jeong JM. Angiogenesis imaging using 68Ga-RGD PET/CT: therapeutic implications. Semin Nucl Med. 2016;46:419–427. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson O, Zhu L, Niu G, et al. MicroPET imaging of integrin αvβ3 expressing tumors using 89Zr-RGD peptides. Mol Imaging Biol. 2011;13:1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo J, Guo N, Lang L, et al. 18F-alfatide II and 18F-FDG dual-tracer dynamic PET for parametric, early prediction of tumor response to therapy. J Nucl Med. 2014;55:154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C, Yue X, Lang L, et al. Longitudinal PET imaging of muscular inflammation using 18F-DPA-714 and 18F-alfatide II and differentiation with tumors. Theranostics. 2014;4:546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang SY, Bao X, Wang MW, et al. Radiation dosimetry estimates of 18F-alfatide II based on whole-body PET imaging of mice. Appl Radiat Isot. 2015;105:1–5. [DOI] [PubMed] [Google Scholar]
- 14.Yu C, Pan D, Mi B, et al. 18F-alfatide II PET/CT in healthy human volunteers and patients with brain metastases. Eur J Nucl Med Mol Imaging. 2015;42:2021–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mi B, Yu C, Pan D, et al. Pilot prospective evaluation of 18F-alfatide II for detection of skeletal metastases. Theranostics. 2015;5:1115–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bao X, Wang MW, Luo JM, et al. Optimization of early response monitoring and prediction of cancer antiangiogenesis therapy via noninvasive PET molecular imaging strategies of multifactorial bioparameters. Theranostics. 2016;6:2084–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackey JR, Kerbel RS, Gelmon KA, et al. Controlling angiogenesis in breast cancer: a systematic review of anti-angiogenic trials. Cancer Treat Rev. 2012;38:673–688. [DOI] [PubMed] [Google Scholar]
- 18.Lopes G, Dent R. Weighed, measured, and still searching: bevacizumab in the treatment of unselected patients with advanced breast cancer. Oncologist. 2011;16:1669–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iagaru A, Mosci C, Shen B, et al. 18F-FPPRGD2 PET/CT: pilot phase evaluation of breast cancer patients. Radiology. 2014;273:549–559. [DOI] [PubMed] [Google Scholar]
- 20.Beer AJ, Niemeyer M, Carlsen J, et al. Patterns of αvβ3 expression in primary and metastatic human breast cancer as shown by 18F-galacto-RGD PET. J Nucl Med. 2008;49:255–259. [DOI] [PubMed] [Google Scholar]
- 21.Beer AJ, Lorenzen S, Metz S, et al. Comparison of integrin αvβ3 expression and glucose metabolism in primary and metastatic lesions in cancer patients: a PET study using 18F-galacto-RGD and 18F-FDG. J Nucl Med. 2008;49:22–29. [DOI] [PubMed] [Google Scholar]
- 22.Kenny LM, Coombes RC, Oulie I, et al. Phase I trial of the positron-emitting Arg-Gly-Asp (RGD) peptide radioligand 18F-AH111585 in breast cancer patients. J Nucl Med. 2008;49:879–886. [DOI] [PubMed] [Google Scholar]
- 23.Yoon HJ, Kang KW, Chun IK, et al. Correlation of breast cancer subtypes, based on estrogen receptor, progesterone receptor, and HER2, with functional imaging parameters from 68Ga-RGD PET/CT and 18F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2014;41:1534–1543. [DOI] [PubMed] [Google Scholar]
- 24.Minamimoto R, Jamali M, Barkhodari A, et al. Biodistribution of the 18F-FPPRGD2 PET radiopharmaceutical in cancer patients: an atlas of SUV measurements. Eur J Nucl Med Mol Imaging. 2015;42:1850–1858. [DOI] [PubMed] [Google Scholar]
- 25.Lang L, Ma Y, Kiesewetter DO, et al. Stability analysis of glutamic acid linked peptides coupled to NOTA through different chemical linkages. Mol Pharm. 2014;11:3867–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beer AJ, Haubner R, Goebel M, et al. Biodistribution and pharmacokinetics of the αvβ3-selective tracer 18F-galacto-RGD in cancer patients. J Nucl Med. 2005;46:1333–1341. [PubMed] [Google Scholar]
- 27.Beer AJ, Haubner R, Wolf I, et al. PET-based human dosimetry of 18F-galacto-RGD, a new radiotracer for imaging αvβ3 expression. J Nucl Med. 2006;47:763–769. [PubMed] [Google Scholar]
- 28.Beer AJ, Grosu AL, Carlsen J, et al. [18F]galacto-RGD positron emission tomography for imaging of alphavbeta3 expression on the neovasculature in patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6610–6616. [DOI] [PubMed] [Google Scholar]
- 29.Beer AJ, Haubner R, Sarbia M, et al. Positron emission tomography using [18F]galacto-RGD identifies the level of integrin αvβ3 expression in man. Clin Cancer Res. 2006;12:3942–3949. [DOI] [PubMed] [Google Scholar]
- 30.McParland BJ, Miller MP, Spinks TJ, et al. The biodistribution and radiation dosimetry of the Arg-Gly-Asp peptide 18F-AH111585 in healthy volunteers. J Nucl Med. 2008;49:1664–1667. [DOI] [PubMed] [Google Scholar]
- 31.Li ZB, Chen K, Chen X. 68Ga-labeled multimeric RGD peptides for microPET imaging of integrin αvβ3 expression. Eur J Nucl Med Mol Imaging. 2008;35:1100–1108. [DOI] [PubMed] [Google Scholar]
- 32.Liu S, Liu Z, Chen K, et al. 18F-labeled galacto and PEGylated RGD dimers for PET imaging of αvβ3 integrin expression. Mol Imaging Biol. 2010;12:530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mittra ES, Goris ML, Iagaru AH, et al. Pilot pharmacokinetic and dosimetric studies of 18F-FPPRGD2: a PET radiopharmaceutical agent for imaging αvβ3 integrin levels. Radiology. 2011;260:182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu S, Liu H, Jiang H, et al. One-step radiosynthesis of 18F-AlF-NOTA-RGD2 for tumor angiogenesis PET imaging. Eur J Nucl Med Mol Imaging. 2011;38:1732–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan W, Guo N, Pan D, et al. First experience of 18F-alfatide in lung cancer patients using a new lyophilized kit for rapid radiofluorination. J Nucl Med. 2013;54:691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y, Gao S, Huang Y, et al. A pilot study of 18F-Alfatide PET/CT imaging for detecting lymph node metastases in patients with non-small cell lung cancer. Sci Rep. 2017;7:2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo J, Lang L, Hu S, et al. Comparison of three dimeric 18F-AlF-NOTA-RGD tracers. Mol Imaging Biol. 2014;16:274–283. [DOI] [PubMed] [Google Scholar]
- 38.Bombardieri E, Aktolun C, Baum RP, et al. FDG-PET: procedure guidelines for tumour imaging. Eur J Nucl Med Mol Imaging. 2003;30:BP115–BP124. [DOI] [PubMed] [Google Scholar]
- 39.Kang F, Wang S, Tian F, et al. Comparing the diagnostic potential of 68Ga-alfatide II and 18F-FDG in differentiating between non-small cell lung cancer and tuberculosis. J Nucl Med. 2016;57:672–677. [DOI] [PubMed] [Google Scholar]