Abstract
In this review, we cover the evolution of knowledge on the biology of prostate-specific membrane antigen (PSMA) and its translation to therapy. The usual key to discovery is a realistic model for experimentation and for testing a hypothesis. A realistic model is especially needed in the case of the human prostate, which differs significantly from the prostate of species often used as research models. We will emphasize the genetic characterization of PSMA, the nature of the PSMA protein, and its role as a carboxypeptidase, with differing important substrates and products in different tissues. We give special prominence to the importance of PSMA as a target for imaging and therapy in prostate cancer and its underdeveloped role for imaging and targeting the neovasculature of tumors other than prostate cancer. Lastly, we bring attention to its importance in other nonprostatic tissues.
Keywords: prostate cancer, angiogenesis, tumor neovasculature, endoradiotherapy, inflammatory bowel disease, folate
NOTEWORTHY
The high expression of PSMA in the human prostate is unique, as no other mammal, with the possible exception of the dog, expresses detectable protein; this expression increases with increasing grade of prostate cancer and in metastatic disease.
PSMA expression is also seen in tumor-associated angiogenesis of almost all solid tumors other than prostate but not in normal vasculature.
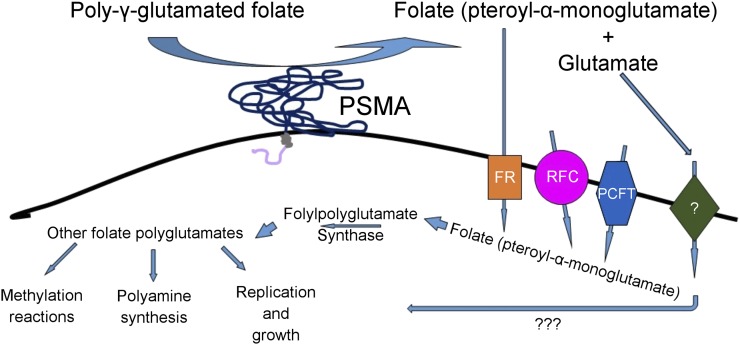
The role of PSMA in the metabolism of folates may contribute to cancer progression, as shown in Figure 1.
The theranostic evolution of radionuclide PSMA-targeted ligands has generated encouraging improvements in imaging and therapy, especially with low-molecular-weight–linked positron and α-emitters.
The story of the prostate-specific membrane antigen (PSMA) began with development of the prostate cancer cell line LNCaP by Horoszewicz et al. (1) in 1983. LNCaP was the first cell model that maintained several key biomarkers in keeping with real human prostate cancers both in vitro and in vivo. Horoszewicz’s group then developed a monoclonal mouse antibody, 7E11-C5, to the membranes of this cell line (2). This antibody eventually was used to develop the first Food and Drug Administration–approved imaging agent for prostate cancer, 111In-capromab pendetide (ProstaScint; Jazz Pharmaceuticals).
FIGURE 1.
Proposed mechanisms by which PSMA contributes to tumor growth and progression. Poly-γ-glutamated folates released from dead and dying tumor cells are hydrolyzed to folate by PSMA and can then be taken up by nearby healthy tumor cells via proton-coupled folate transporter (PCFT), folate receptor (FR), or reduced folate carrier (RFC). Once inside the cell, folate is again polyglutamated and used for polyamine synthesis, methylation reactions, and the nucleotide synthesis required for cell proliferation. Free glutamate released by this reaction may be taken up by glutamate receptor, stimulating proliferative growth pathways; however, the role of glutamate in this process, whether metabolic or signaling, is still being clarified.
CLONING AND EXPRESSION OF PSMA
We used the 7E11-C5 antibody for immunoprecipitation, peptide microsequencing, and subsequently degenerate primers to clone and sequence the complementary DNA encoding PSMA (3). A ribonuclease protection assay showed that PSMA was predominantly expressed in the prostate, with much lower levels of expression in the brain, salivary gland, and small intestine. Therefore, we used the term prostate-specific membrane antigen for the encoded protein (4). The deduced amino acid sequence established that the gene encoded for a type 2 membrane protein with a region of homology to the transferrin receptor. In vitro expression of PSMA was decreased 3- to 10-fold by treatment with androgens (4). The short intracellular region of 19 amino acids was subsequently determined to be the target recognized by the 111In-capromab pendetide antibody, likely explaining the poor performance of 111In-capromab pendetide as an imaging agent (5).
We were concerned about our initial findings suggesting that PSMA mapped to 2 nearly identical regions: one on chromosome 11p11.2 and one on 11q14.3 (6). We designated the one on 11q14.3 as PSMA-like. We were able to distinguish the PSMA gene from the PSMA-like gene because our group had fully sequenced the entire PSMA gene before completion of the human genome project and reported its full characterization (7).
Although the 2 genes are similar, the PSMA-like encoded protein lacks the transmembrane domain and is therefore a cytosolic protein and will not interfere with clinical targeting of PSMA (7). There was also reason to consider that the level of PSMA messenger RNA may underrepresent protein expression, because membrane proteins can be relatively stable and radioimmunoassay measurement of PSMA protein found the amount of PSMA in the prostate to be 1,000 times the amount found in the liver or kidney, the tissues where both PSMA and PSMA-like messenger RNA are expressed (8).
In collaboration with investigators in Australia, we were able to identify which regions of the PSMA gene are responsible for the high level of expression in the prostate and prostate cancers (9). Identification of this enhancer sequence allowed several groups to design gene therapies specifically targeted toward prostate tumors (10). The lack of PSMA expression in the prostate of most mammals, including rodents and apes, likely relates to a gene duplication event that occurred 22 million years ago, followed later—at some time after the separation of chimps from humans, 6–7 million years ago—by the acquisition of factors able to activate expression in the prostate (6,7,11).
As noted, PSMA has substantial sequence and structural homology with transferrin receptors. The crystal structure of PSMA was first solved using the existing transferrin receptor 1 crystal structure as the model for molecular replacement (12). Like the transferrin receptor, the extracellular portion of PSMA has 3 domains: apical, helical, and protease. PSMA exists as a symmetric homodimer with a large (∼4,600 Å2) dimerization interface from the association of the 2 helical domains. The apical domain of PSMA contains the binding site of the J591 antibody. The active site and substrate-binding cavity of the peptidase lies deep within the PSMA structure and is formed with a contribution from all 3 domains. There are 2 zinc atoms at the active site; the zinc atoms are coordinated by residues from the protease domain. As expected, the position of zinc atoms, the catalytic water, and their coordinating amino acid residues are nearly identical to other binuclear zinc exopeptidases (12).
TISSUE DISTRIBUTION OF THE PSMA PROTEIN
To determine the tissue distribution of PSMA protein expression, we applied immunohistochemistry using the 7E11-C5 antibody. In a subset of kidney proximal tubules in normal tissue, we observed expression of duodenal brush border cells and cells in the colonic crypts. Most other cell types lacked expression. In normal and hyperplastic prostate tissue, staining was weak or absent. In prostate cancer, 33 of 35 primary tumors were positive, 7 of 8 metastatic lymph nodes were positive, and 8 of 18 bone metastases were positive. Other cancer cell types were PSMA-negative, but in many nonprostatic tumors, the tumor-associated neovasculature was positive for PSMA expression whereas the neovasculature in prostate cancers was negative (13). Other investigators using the 7E11-C5 antibody also found benign prostatic hyperplasia to have less expression than normal prostate tissue, which in turn had less expression than prostatic intraepithelial neoplasia. PSMA expression in cancer increased with grade and was highest in metastatic deposits, with 94% of lymph nodes and 100% of bone metastases exhibiting strong apical membrane staining, although it did not correlate with tumor stage (14). In another investigation, Ross et al. reported that both pathologic stage (P = 0.0018) and PSMA (P = 0.002) were independent predictors of biochemical recurrence, with overexpression of PSMA in the primary tumor independently predicting disease outcome (15).
BIOLOGIC FUNCTION OF PSMA
The PSMA messenger RNA transcript encodes a protein of 750 amino acids and a predicted molecular weight of 84 kD; however, because of protein glycosylation, the molecular weight appears to be greater than 100 kD (16,17). PSMA glycosylation is required for enzymatic activity (16,17) and exists as both dimer and monomeric forms on the apical cell surface (18). A second-generation antibody, J591, developed in the Bander laboratory was found to increase the rate of internalization of PSMA (19). Similar to transferrin, when PSMA is internalized it follows intracellular trafficking to the recycling endosomal compartment (19). The short 19-amino-acid intracellular domain has a novel MXXXL motif that mediates internalization by interacting with the clathrin adaptor protein 2 complex (18). The intracellular domain binds to the structural protein filamin A as part of a larger macromolecular complex including integrin β1, p130 Crk-associated substrate, c-SRC, and the epidermal growth factor receptor that ultimately activates the protein kinase B (AKT) pathway and the mitogen-activated protein kinase pathway to promote proliferation and survival (20). These in vitro findings are supported by recent in vivo findings in xenograft models showing that PSMA activates the AKT pathway (21).
The enzymatic activity of PSMA is considered part of the cocatalytic zinc metallopeptidase family M28 (22). We observed strong expression of PSMA in the proximal small intestine and could identify poly-γ-glutamated folate as a substrate (23). PSMA removes the γ-linked glutamates from folate, providing deglutamated folate for absorption and nutrition and leaving a single α-linked glutamate attached. The official gene name of PSMA is folate hydrolase 1 (FOLH1), reflecting its major role in folate uptake; however, although PSMA usually acts as a glutamate carboxypeptidase (GCP), its major substrate can differ in different tissues.
Role of PSMA in Normal Prostate and Prostate Cancer
The location, lobation, and histologic features of the prostate can differ substantially among different species of mammals (24). All mammalian prostates are dependent on androgens for growth and maintenance. The different mammalian prostates, however, can differ dramatically in the nature of their secretions. Indeed, the mouse prostate does not produce PSMA to any extent, is multilobed, and rarely develops cancer, whereas the human prostate is encased as one lobe and makes large amounts of proteins, such as prostate-specific antigen (PSA), prostatic acid phosphatase, and PSMA. The development of prostate cancer is common in humans but rare in other mammalian species, with the exception of dogs (24). Thus, the mouse prostate cannot be considered a good model for aspects of prostate function that differ significantly from those in humans, such as PSMA.
We cloned the mouse homolog of human PSMA and generated a mouse model in which the FOLHI gene has been knocked out (25–27). Others have also reported that the mouse prostate is lacking in PSMA/Folh1 expression (the protein is also referred to as GCPII) or have reported on another related type, GCPIII, which has similar enzymatic features but with different tissue distributions (11,28). PSMA expression in human cancers increases with increasing cancer aggressiveness. Despite no expression of PSMA/Folh1 in the mouse prostate or in genetic tumor models such as the popular “transgenic adenocarcinoma of the mouse prostate” model, commonly known as TRAMP, expression of PSMA/Folh1 at other sites, such as brain, kidney, and salivary glands, still makes the mouse a useful in vivo model for studying off-target side effects in targeting approaches that use xenotransplanted human tumors (28). One should also remember that GCPIII would also likely exhibit uptake of PSMA-targeted low-molecular-weight ligands, and off-site targets such as ovary, uterus, testes, heart, lung, and skeletal muscle should be kept in mind as well (28). Fortunately, PSMA is located on the apical surface of cells and thus is less accessible to targeting.
To humanize the mouse prostate, Yao et al. developed a transgenic mouse model expressing human PSMA in the luminal epithelial cells of the ventral, lateral, and dorsal lobes of the prostate (29). Because the expression of human PSMA in these mice did not lead to cancer in the short term, the investigators explored the effect of human PSMA expression specifically in the tissue-recombinant prostatic recapitulation model, comparing human PSMA–expressing mice versus wild-type mice. Of the PSMA transgenic prostate recombinants, 47% demonstrated adenocarcinoma by 24 wk, whereas no cancer was observed in the wild-type tissue recombinants. The conclusion was reached that PSMA played a role in promoting prostate carcinogenesis, with moderate expression being associated with prostate cancer development and PSMA-negative cells being unable to become cancers (29). This conclusion is in keeping with the findings of Rajasekaran et al., who observed that high PSMA expression decreases cell-cycle time in G2/M from its association with the anaphase-promoting complex, setting cells up for potential aneuploidy and carcinogenesis (30).
Role of PSMA in Folate Level and Prostate Cancer
The intracellular folate (vitamin B9) concentration has been described as a 2-edged sword (31). Low levels are associated with increased susceptibility to chromosome damage, whereas high levels increase tumor growth. Indeed, the LNCaP and PC-3 prostate cancer cell lines have been reported to show increased growth and invasiveness in vitro when exposed to high folate concentrations (32). However, grains and cereals in the United States are now supplemented with folic acid to reduce the potential for neural-tube defects in newborns. Cole et al. hypothesized that folic acid supplementation could prevent colorectal adenoma in patients with a history of the disease. However, the investigators found that, in fact, mild supplementation with folic acid led to an increased risk of advanced adenomas (33). Furthermore, there was a significant overrepresentation of prostate cancer in the folate-supplemented group (age-adjusted hazard ratio, 2.63), even years after the trial ended (34). Intriguing in this regard is a case report of a patient with hormone-refractory prostate cancer who had a rising PSA level while undergoing docetaxel treatment. The PSA rise occurred as the patient began taking folate supplements, with his serum folate level reaching 304 nM. Within 2 wk after the supplementation and fortified foods had been stopped, his serum folate dropped to 9 nM and his PSA dropped to 2 ng/mL (35).
Folate levels also modulate the activity of PSMA; Yao et al. demonstrated increased invasiveness under low-folate in vitro conditions, as well as a growth advantage for PSMA-transfected PC-3 cells in physiologic folate compared with PC-3 cells lacking PSMA (32). Because PSMA may also internalize its ligands, it may be involved in folate uptake in prostate tissue. Indeed, Yao et al. found that PSMA-expressing cells also demonstrated a 2-fold increased uptake of folate, suggesting that PSMA may be associated with folate transport (32).
Jan Grimm’s research team has documented that the level of PSMA expression and the generation of glutamate from its substrate, polyglutamated vitamin B9 (which is the general term for folates), provides the signal necessary to activate the AKT pathway in vitro and that PSMA expression correlates with prostate cancer aggressiveness (21). The team used Memorial Sloan Kettering’s cohort of 168 primary prostate cancers that combined full clinical annotation with extensive gene expression and copy number profiling at the time of surgery. In this well-annotated cohort, elevated PSMA is associated with disease relapse and metastasis, and PSMA levels also correlate with the phosphorylation with eIF4E-binding protein 1, a downstream target of the mammalian target of rapamycin. In addition, PSMA expression levels positively correlate with phosphorylation of AKT and its downstream targets. Using in vitro studies with polyglutamated folates, the team demonstrated that glutamate released by the enzymatic activity of PSMA was responsible for signaling via the metabotropic glutamate receptor 1, activating the phospholipase C pathway. Recently, it was reported that combined androgen receptor and phosphoinositide 3-kinase inhibition is required for therapeutic efficacy in prostate cancer (36). Because inhibitors of the latter pathway have off-target effects, Grimm’s research group asked whether inhibition of PSMA could be an alternative approach (21). They examined a combination therapy of androgen receptor or PSMA blockade using enzalutamide or 2-phosphonomethyl pentanedioic acid (an inhibitor of PSMA enzymatic activity) in mouse xenograft models in vivo. They found that either enzalutamide or 2-phosphonomethyl pentanedioic acid could retard tumor growth, but what was most dramatic was that the combination resulted in regression of 90% of the tumors. The Grimm team then asked whether imaging could identify patients in whom this pathway is operative and combination therapy therefore appropriate. They examined patients using PET/MRI with the PSMA-specific probe 68Ga-PSMA-11-CC before prostatectomy had been performed. After prostatectomy, they stained the tissue for phospho-AKT and found a strong correlation between staining and PSMA imaging. They argue not only that this approach should be considered in prostate cancer but also that, because PSMA is strongly expressed in the neovasculature of some tumors, and several tumors express metabotropic glutamate receptors, combined therapies should be considered in those situations as well (21).
We previously reported that mice carrying LNCaP xenografts in which PSMA expression either is present or has been knocked out have superior take rates for tumors expressing PSMA (16/18 vs. 10/18 tumors grew/tumors implanted, respectively). Furthermore, the tumors that expressed PSMA grew significantly larger in response to increasing dietary folate, although the non–PSMA-expressing tumors remained small and there was no significant difference in tumor size among diets containing different levels of folate (37). Given that most prostate tumors express PSMA, patients should therefore be mindful about which vitamins and fortified foods they consume.
Role of PSMA in Tumor-Associated Neovasculature
PSMA is expressed in the neovasculature of some tumors other than prostate cancer (13,38). The question was whether this is a human-only aspect or is common in other animal models as well. In a collaboration with Shapiro’s research group on our knockout mouse model, we used an in vivo assay to evaluate activation of endothelial cell ingrowth (neovascularization) into an extracellular matrix plug (Matrigel; Corning). The PSMA-knockout animals responded with dramatically attenuated vascular growth and reduced hemoglobin content, compared with the wild-type animals (39). Similarly, the PSMA inhibitor 2-phosphonomethyl pentanedioic acid also blocked neovascularization. Examination of different extracellular matrix proteins demonstrated that vascular invasion by PSMA was mediated by laminin. Further studies showed that the carboxypeptidase activity of PSMA resulted in endothelial cell activation through integrin focal-adhesion-kinase phosphorylation and subsequent PAK activation (39) and that the laminin-derived peptides of LQE, IEE, and LNE were substrates for PSMA (39). The investigators further found that LQ, a product of PSMA’s enzymatic cleavage of LQE, efficiently activates endothelial cells in vitro, enhances angiogenesis in vivo, and is dependent on integrin β1 activation (39).
PSMA-BASED IMAGING AND TARGETED THERAPY
Antibody-Based Strategies
There has been a dramatic theranostic evolution from imagined silver-bullet antibody specific for cancer to the reality of delivering a radionuclide dose sufficient to treat cancer while avoiding toxicity to normal tissues. In that light, PSMA as a target for imaging and therapy has been evolving. The first Food and Drug Administration–approved imaging agent for PSMA was 111In-capromab pendetide, a mouse monoclonal antibody (7E11-C5) linked to 111In for SPECT imaging in patients with rising PSA levels after prostatectomy. However, there were specificity and sensitivity issues (40). Dr. Bander’s research group identified another antibody, J591, that binds to the extracellular portion of PSMA and was more sensitive than technetium scans for detection of bone metastatic disease. In a phase 2 study, treatment using J591 chelated with 177Lu (2,406–2,590 MBq [65–70 mCi]/m2) was studied in 47 patients whose disease progressed on hormonal therapy, and half of whom had received prior cytotoxic chemotherapy (41). Ten percent of patients had a PSA decline of 50% or greater. One of 12 with measurable disease experienced a radiographic response, and 44 of 47 targeted sites were sites of prostate cancer as determined by planar imaging. All patients experienced reversible hematologic toxicity. The J591 antibody, too, is evolving; it has been humanized and undergone genetic engineering to generate a minibody, 89Zr-Df-IAB2M, which is 80 kD in size, compared with the 150-kD J591 antibody (42). Monomeric and dimeric single-chain Fv fragment anti-PSMA agents are also being developed, as well as multimers using different targets, including PSMA (43). These agents have the advantage of more rapid clearance, which might reduce the toxicity found with the full-sized antibody and reduce the time required before the antibody can be readily imaged. Interestingly, the antibodies and derivatives used to date have not imaged the salivary glands, nor have the salivary glands been a site of toxicity.
Ligand-Based Strategies
The further evolution of PSMA targeting involves low-molecular-weight ligands based on the glutamate–urea cargo-carrying derivatives (MIP-1095, MIP-1404, PSMA-11/617/1007, DCFPyl, PSMA I&T [imaging and therapy]) with first-in-human studies demonstrating good imaging and therapeutic activity for the 18F- and 131I-linked radionuclides that are being evaluated in clinical trials at several U.S. sites in preparation for regulatory approval (the results should be available in early 2019 (44–47)). In studies outside the United States, especially Germany, imaging and treatment-targeting PSMA in hormone-refractory disease has been used with other low-molecular-weight compounds linked to 68Ga for PET imaging and 177Lu for endoradiotherapeutic treatment. These low-molecular-weight ligands have demonstrated excellent imaging of prostate cancer at distant metastatic sites, as well as locoregional nodes and areas of aggressive cancer within the prostate itself (48).
Recent experience with 177Lu ligands has been extensive. In one German study (49), patients are being treated on a compassionate-use basis; nevertheless, a retrospective compilation of data from 12 centers and 145 patients receiving doses of 2–8 GBq per cycle for up to 4 cycles achieved an overall biochemical response rate of 45% (≥50% decline in PSA), with 40% of patients responding after a single dose. Bone pain improved in 33%–77% of patients, and grade 3–4 hematotoxicity occurred in 18 patients, with 10%, 4%, and 3% experiencing anemia, thrombocytopenia, and leukopenia, respectively. Xerostomia occurred in 8%, a finding that is quite different from antibody treatment, in which no salivary gland toxicity is observed. An elevated alkaline phosphatase level and visceral metastases were negative predictors of response (49,50).
Even though low-molecular-weight anti-PSMA 177Lu treatment produced an impressive response for such heavily pretreated patients, 40% of the patients nevertheless did not respond. Kratochwil et al. began a trial using 225Ac in heavily pretreated castration-resistant prostate cancer patients and found that treatment with an α-emitter could overcome resistance seen to β-emitters (51). In an empiric dose-finding series, they learned that treatment with a 100 kBq/kg dose of 225Ac-PSMA-617 every 2 mo was superior for continuing tumor control. Similar results were obtained with 213Bi-PSMA-617 by Sathekge et al. (52). Indeed, many dramatic responses were seen in these heavily pretreated patients (51,52).
These are impressive results considering this is third-line therapy facing bulky metastatic disease. Drs. Czernin and Eiber have called on the Food and Drug Administration to approve these agents, as they have already undergone extensive testing in humans and shown minimal toxicity and excellent imaging and therapeutic responses, even in heavily pretreated patients (53).
Neovasculature-Based Strategies
In addition to imaging for prostate cancer, PSMA expression in the vasculature of most solid tumors has been exploited by other groups. Using the J591 antibody radiolabeled with 111In, Pandit-Taskar et al. were able to image most metastatic deposits to the skeleton, lymph nodes, and soft tissues from patients with renal, breast, and colorectal cancer (54). Because PSMA-based detection frequently identifies sites in addition to those visible by standard scanning, a recent study included a therapy-resistant patient with renal cancer who was imaged shortly before death, and on death a rapid autopsy was performed. All sites detected by PSMA imaging were histologically proven clear cell renal cancer with PSMA expression in the tumor vasculature, suggesting that PSMA imaging is more sensitive than standard imaging techniques (55). Indeed the urgent need to address the potential for imaging and therapy of PSMA expression in the tumor neovasculature of solid tumors other than prostate cancer was recently addressed by Fragomeni et al. (56).
OTHER CONSIDERATIONS
Brain PSMA
Understanding the biologic role of PSMA is aided by examining its role in other tissues. Neurochemistry researchers have been working on a protein that they call NAALADASE, which hydrolyzed the brain dipeptide N-acetylaspartylglutamate, NAAG, to yield N-acetylaspartate and glutamate (57). Carter et al. cloned rat brain NAALADASE and found that it has 85% homology with human PSMA (58). In the brain, NAALADASE is a membrane-bound enzyme found on the extracellular face of glia. Its substrate, NAAG, is found in many types of neuronal cells in the brain, spinal cord, and peripheral nerves. There are at least 2 distinct neurotransmitter functions for NAAG (57). Under basal conditions, when PSMA/GCPII activity is relatively low, NAAG dampens synaptic activity via metabotropic glutamate receptor III activation and N-methyl-d-aspartate receptor blockade. However, stimulated conditions enhance NAAG release and PSMA/GCPII activity, resulting in excess glutamate generation and pathologic receptor activation (57).
Using the Bacich PSMA knockout mouse, it was observed that mice lacking PSMA/GCPII were resistant to nerve damage induced via physical, chemical, and hypoxic conditions, confirming that PSMA under these conditions cleaves the neuroprotective NAAG released by damaged neurons to N-acetylaspartate and excitotoxic glutamate, exacerbating nerve damage (27). PSMA/GCPII is expressed in the spinal cord and peripheral nerves. Nevertheless, PSMA imaging has not demonstrated uptake in spinal cords that do not have prostate cancer metastasis, and because of the blood–brain barrier no imaging of brain PSMA/GCPII has been observed.
Intestinal and Colonic PSMA
PSMA expression in the gastrointestinal tract is responsible for hydrolysis of the γ-linked glutamates from poly-γ-glutamated folates found in foods, leaving intact the α-linked glutamate of folate. Folate, with its remaining α-linked glutamate, is then transported into enterocytes and subsequently into the liver to provide folate for nutrition (23).
We originally observed PSMA expression in the colonic crypt in what we thought were neuroendocrinelike cells (13). Slusher’s research group, citing a recent gene-profiling analysis that showed significant upregulation of PSMA expression in patients with inflammatory bowel disease, experimentally determined that there was a 4- to 41-fold increase in PSMA/GCPII enzymatic activity in patient surgical specimens of affected inflammatory colon, compared with noninflamed areas. To examine this relationship, they applied an animal model of inflammatory bowel disease either using dextran sodium sulfate or using interleukin-10–deficient mice and found an increase in PSMA/GCPII expression (59). Slusher’s group then demonstrated that this inflammation could be attenuated, and disease activity index lowered, either using the PSMA inhibitor 2-phosphonomethyl pentanedioic acid or using the PSMA/GCPII knockout animals of Bacich (26). The PSMA/GCPII knockout mice not only were resistant to dextran treatment but also had a longer colon length with a healthier mucosa, less neutrophil infiltration, and preserved crypts and goblet cells.
Kidney PSMA
Most model systems demonstrate PSMA/GCPII/FOLH1 expression in a subset of the kidney proximal tubules (13). The kidneys are a site imaged with the low-molecular-weight ligands for PSMA, which are rapidly excreted. Indeed, in an initial study of prostate cancer tumor imaging, uptake was higher in normal kidneys than in the kidneys of the PSMA knockout mice, indicating some PSMA-specific binding (60). The knockout animals demonstrated no retention of the low-molecular-weight ligands, and the animals themselves appeared to be healthy, with nothing to suggest any negative aspect in kidneys that lack PSMA expression. There is no evidence of any specific enzymatic substrate for PSMA in the kidneys, nor is it evident why just a subset of proximal tubules appears to express PSMA. Of note, the knockout mice do have a slight difference in blood pressure (27).
CONCLUSION
The PSMA gene was cloned in the Urology Research Laboratory at Memorial Sloan Kettering Cancer Center in 1993. William R. Fair was chair of urology at that time, with Willet F. Whitmore having previously stepped down as chair. Dr. Whitmore was considered the Dean of Urologic Oncology. He was not a fan of PSA and was a proponent of expectant management at the time (noted as active surveillance in today’s parlance). He died from metastatic prostate cancer in 1995. He was noted for raising the question, “Is cure necessary for those in whom it is possible, and is cure possible for those in whom it is necessary?” For he himself, a cure was necessary but not possible. Here we are 23 y later, and at the Prostate Cancer Foundation meetings a presentation was made on PSMA-targeted radiotherapy in a patient with castration-resistant prostate cancer. The patient was imaged with 68Ga-labeled low-molecular-weight PSMA ligand, which identified hundreds of metastatic sites involving bone and soft tissues. He was subsequently treated with a PSMA-targeted low-molecular-weight ligand linked with 225Ac. All those sites of metastatic disease disappeared, and his PSA decreased from the thousands to an undetectable level—an amazing result. When that patient was asked how he was doing, he replied that he felt great. When asked about the symptom of dry mouth, he said he drinks a lot of water. So today there are patients in whom the answer to Dr. Whitmore’s question will be that cure is both necessary and possible. Indeed, besides being used to image prostate cancer that is high-grade and localized, has spread locoregionally, or has reached distant metastatic sites, PSMA can be used for therapy of prostate cancer. It may even be that PSMA inhibition along with androgen deprivation therapy may further slow progression of the disease (21). In terms of toxicity, the salivary glands need to be considered. Both for imaging and for therapy, one strategy might be to alter the structure of the low-molecular-weight PSMA-targeting ligand to decrease uptake in off-target sites while retaining high uptake in the tumor (60).
Dr. Fair died from complications of metastatic colon cancer. Given the presence of PSMA expression in the neovasculature of many types of tumors other than prostate cancer, it is hoped that evolving strategies and innovative PSMA-targeted payloads will eventually prove useful against these nonprostate cancers so that, in such patients as well, cure will be possible for those in whom it is necessary.
DISCLOSURE
This work was supported in part by NIH grant R01 CA138444 to Denise O’Keefe and Dean Bacich. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Horoszewicz JS, Leong SS, Kawinski E, et al. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–1818. [PubMed] [Google Scholar]
- 2.Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 1987;7:927–935. [PubMed] [Google Scholar]
- 3.Israeli RS, Powell CT, Fair WR, Heston WD. Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer Res. 1993;53:227–230. [PubMed] [Google Scholar]
- 4.Israeli RS, Powell CT, Corr JG, Fair WR, Heston WD. Expression of the prostate-specific membrane antigen. Cancer Res. 1994;54:1807–1811. [PubMed] [Google Scholar]
- 5.Troyer JK, Beckett ML, Wright GL. Location of prostate-specific membrane antigen in the LNCaP prostate carcinoma cell line. Prostate. 1997;30:232–242. [DOI] [PubMed] [Google Scholar]
- 6.O’Keefe DS, Bacich DJ, Heston WD. Comparative analysis of prostate-specific membrane antigen (PSMA) versus a prostate-specific membrane antigen-like gene. Prostate. 2004;58:200–210. [DOI] [PubMed] [Google Scholar]
- 7.O’Keefe DS, Su SL, Bacich DJ, et al. Mapping, genomic organization and promoter analysis of the human prostate-specific membrane antigen gene. Biochim Biophys Acta. 1998;1443:113–127. [DOI] [PubMed] [Google Scholar]
- 8.Sokoloff RL, Norton KC, Gasior CL, Marker KM, Grauer LS. A dual-monoclonal sandwich assay for prostate-specific membrane antigen: levels in tissues, seminal fluid and urine. Prostate. 2000;43:150–157. [DOI] [PubMed] [Google Scholar]
- 9.Watt F, Martorana A, Brookes DE, et al. A tissue-specific enhancer of the prostate-specific membrane antigen gene, FOLH1. Genomics. 2001;73:243–254. [DOI] [PubMed] [Google Scholar]
- 10.O’Keefe DS, Uchida A, Bacich DJ, et al. Prostate-specific suicide gene therapy using the prostate-specific membrane antigen promoter and enhancer. Prostate. 2000;45:149–157. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal S, Ricklis RM, Williams SA, Denmeade SR. Comparative study of PSMA expression in the prostate of mouse, dog, monkey, and human. Prostate. 2006;66:903–910. [DOI] [PubMed] [Google Scholar]
- 12.Davis MI, Bennett MJ, Thomas LM, Bjorkman PJ. Crystal structure of prostate-specific membrane antigen, a tumor marker and peptidase. Proc Natl Acad Sci USA. 2005;102:5981–5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 14.Wright GL, Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol. 1995;1:18–28. [DOI] [PubMed] [Google Scholar]
- 15.Ross JS, Sheehan CE, Fisher HA, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res. 2003;9:6357–6362. [PubMed] [Google Scholar]
- 16.Barinka C, Sácha P, Sklenár J, et al. Identification of the N-glycosylation sites on glutamate carboxypeptidase II necessary for proteolytic activity. Protein Sci. 2004;13:1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh A, Heston WD. Effect of carbohydrate moieties on the folate hydrolysis activity of the prostate specific membrane antigen. Prostate. 2003;57:140–151. [DOI] [PubMed] [Google Scholar]
- 18.Rajasekaran AK, Anilkumar G, Christiansen JJ. Is prostate-specific membrane antigen a multifunctional protein? Am J Physiol Cell Physiol. 2005;288:C975–C981. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Rajasekaran AK, Moy P, et al. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 1998;58:4055–4060. [PubMed] [Google Scholar]
- 20.Perico ME, Grasso S, Brunelli M, et al. Prostate-specific membrane antigen (PSMA) assembles a macromolecular complex regulating growth and survival of prostate cancer cells “in vitro” and correlating with progression “in vivo.” Oncotarget. 2016;7:74189–74202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaittanis C, Andreou C, Hieronymus H, et al. Prostate-specific membrane antigen cleavage of vitamin B9 stimulates oncogenic signaling through metabotropic glutamate receptors. J Exp Med. 2018;215:159–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rawlings ND, Barrett AJ. Structure of membrane glutamate carboxypeptidase. Biochim Biophys Acta. 1997;1339:247–252. [DOI] [PubMed] [Google Scholar]
- 23.Pinto JT, Suffoletto BP, Berzin TM, et al. Prostate-specific membrane antigen: a novel folate hydrolase in human prostatic carcinoma cells. Clin Cancer Res. 1996;2:1445–1451. [PubMed] [Google Scholar]
- 24.Coffey DS. Similarities of prostate and breast cancer: evolution, diet, and estrogens. Urology. 2001;57:31–38. [DOI] [PubMed] [Google Scholar]
- 25.Bacich DJ, Pinto JT, Tong WP, Heston WD. Cloning, expression, genomic localization, and enzymatic activities of the mouse homolog of prostate-specific membrane antigen/NAALADase/folate hydrolase. Mamm Genome. 2001;12:117–123. [DOI] [PubMed] [Google Scholar]
- 26.Bacich DJ, Ramadan E, O’Keefe DS, et al. Deletion of the glutamate carboxypeptidase II gene in mice reveals a second enzyme activity that hydrolyzes N-acetylaspartylglutamate. J Neurochem. 2002;83:20–29. [DOI] [PubMed] [Google Scholar]
- 27.Bacich DJ, Wozniak KM, Lu XC, et al. Mice lacking glutamate carboxypeptidase II are protected from peripheral neuropathy and ischemic brain injury. J Neurochem. 2005;95:314–323. [DOI] [PubMed] [Google Scholar]
- 28.Knedlík T, Vorlová B, Navrátil V, et al. Mouse glutamate carboxypeptidase II (GCPII) has a similar enzyme activity and inhibition profile but a different tissue distribution to human GCPII. FEBS Open Bio. 2017;7:1362–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao V, Parwani A, Maier C, Heston WD, Bacich DJ. Moderate expression of prostate-specific membrane antigen, a tissue differentiation antigen and folate hydrolase, facilitates prostate carcinogenesis. Cancer Res. 2008;68:9070–9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajasekaran SA, Christiansen JJ, Schmid I, et al. Prostate-specific membrane antigen associates with anaphase-promoting complex and induces chromosomal instability. Mol Cancer Ther. 2008;7:2142–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rycyna KJ, Bacich DJ, O’Keefe DS. Opposing roles of folate in prostate cancer. Urology. 2013;82:1197–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao V, Berkman CE, Choi JK, O’Keefe DS, Bacich DJ. Expression of prostate-specific membrane antigen (PSMA), increases cell folate uptake and proliferation and suggests a novel role for PSMA in the uptake of the non-polyglutamated folate, folic acid. Prostate. 2010;70:305–316. [DOI] [PubMed] [Google Scholar]
- 33.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–2359. [DOI] [PubMed] [Google Scholar]
- 34.Figueiredo JC, Grau MV, Haile RW, et al. Folic acid and risk of prostate cancer: results from a randomized clinical trial. J Natl Cancer Inst. 2009;101:432–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tisman G, Garcia A. Control of prostate cancer associated with withdrawal of a supplement containing folic acid, L-methyltetrahydrofolate and vitamin B12: a case report. J Med Case Rep. 2011;5:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carver BS, Chapinski C, Wongvipat J, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bacich D, Flores S, Pennetti S, et al. MP66-19 prostate-specific membrane antigen interacts with dietary folate to facilitate prostate carcinogenesis and progression. J Urol. 2016;195(suppl):e880. [Google Scholar]
- 38.Chang SS, O’Keefe DS, Bacich DJ, Reuter VE, Heston WD, Gaudin PB. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin Cancer Res. 1999;5:2674–2681. [PubMed] [Google Scholar]
- 39.Conway RE, Rojas C, Alt J, et al. Prostate-specific membrane antigen (PSMA)-mediated laminin proteolysis generates a pro-angiogenic peptide. Angiogenesis. 2016;19:487–500. [DOI] [PubMed] [Google Scholar]
- 40.Schuster DM, Savir-Baruch B, Nieh PT, et al. Detection of recurrent prostate carcinoma with anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT and 111In-capromab pendetide SPECT/CT. Radiology. 2011;259:852–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tagawa ST, Milowsky MI, Morris M, et al. Phase II study of lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin Cancer Res. 2013;19:5182–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandit-Taskar N, O’Donoghue JA, Ruan S, et al. First-in-human imaging with 89Zr-Df-IAB2M anti-PSMA minibody in patients with metastatic prostate cancer: pharmacokinetics, biodistribution, dosimetry, and lesion uptake. J Nucl Med. 2016;57:1858–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freise AC, Wu AM. In vivo imaging with antibodies and engineered fragments. Mol Immunol. 2015;67:142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kopka K, Benešová M, Bařinka C, Haberkorn U, Babich J. Glu-ureido-based inhibitors of prostate-specific membrane antigen: lessons learned during the development of a novel class of low-molecular-weight theranostic radiotracers. J Nucl Med. 2017;58(suppl):17S–26S. [DOI] [PubMed] [Google Scholar]
- 45.Rowe SP, Gorin MA, Allaf ME, et al. PET imaging of prostate-specific membrane antigen in prostate cancer: current state of the art and future challenges. Prostate Cancer Prostatic Dis. 2016;19:223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szabo Z, Mena E, Rowe SP, et al. Initial evaluation of [18F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015;17:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zechmann CM, Afshar-Oromieh A, Armor T, et al. Radiation dosimetry and first therapy results with a 124I/131I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur J Nucl Med Mol Imaging. 2014;41:1280–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eiber M, Fendler WP, Rowe SP, et al. Prostate-specific membrane antigen ligands for imaging and therapy. J Nucl Med. 2017;58(suppl):67S–76S. [DOI] [PubMed] [Google Scholar]
- 49.Fendler WP, Rahbar K, Herrmann K, Kratochwil C, Eiber M. 177Lu-PSMA radioligand therapy for prostate cancer. J Nucl Med. 2017;58:1196–1200. [DOI] [PubMed] [Google Scholar]
- 50.Baum RP, Kulkarni HR, Schuchardt C, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med. 2016;57:1006–1013. [DOI] [PubMed] [Google Scholar]
- 51.Kratochwil C, Bruchertseifer F, Rathke H, et al. Targeted alpha therapy of mCRPC with 225actinium-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor-control. J Nucl Med. January 11, 2018 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 52.Sathekge M, Knoesen O, Meckel M, Modiselle M, Vorster M, Marx S. 213Bi-PSMA-617 targeted alpha-radionuclide therapy in metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:1099–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Czernin J, Eiber M. Acceleration of PSMA-targeted theranostics to the clinic: can common sense prevail? J Nucl Med. 2017;58:1186–1187. [DOI] [PubMed] [Google Scholar]
- 54.Pandit-Taskar N, O’Donoghue JA, Divgi CR, et al. Indium 111-labeled J591 anti-PSMA antibody for vascular targeted imaging in progressive solid tumors. EJNMMI Res. 2015;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gorin MA, Rowe SP, Hooper JE, et al. PSMA-targeted 18F-DCFPyL PET/CT imaging of clear cell renal cell carcinoma: results from a rapid autopsy. Eur Urol. 2017;71:145–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fragomeni RAS, Amir T, Sheikhbahaei S, et al. Imaging of nonprostate cancers using PSMA-targeted radiotracers: rationale, current state of the field, and a call to arms. J Nucl Med. 2018;59:871–877. [DOI] [PubMed] [Google Scholar]
- 57.Vornov JJ, Hollinger KR, Jackson PF, et al. Still NAAG’ing after all these years: the continuing pursuit of GCPII inhibitors. Adv Pharmacol. 2016;76:215–255. [DOI] [PubMed] [Google Scholar]
- 58.Carter RE, Feldman AR, Coyle JT. Prostate-specific membrane antigen is a hydrolase with substrate and pharmacologic characteristics of a neuropeptidase. Proc Natl Acad Sci U S A. 1996;93:749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rais R, Jiang W, Zhai H, et al. FOLH1/GCPII is elevated in IBD patients, and its inhibition ameliorates murine IBD abnormalities. JCI Insight. 2016;1:e88634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang SS, Wang X, Zhang Y, Doke A, DiFilippo FP, Heston WD. Improving the biodistribution of PSMA-targeting tracers with a highly negatively charged linker. Prostate. 2014;74:702–713. [DOI] [PubMed] [Google Scholar]