Abstract
Oocytes differentiate in diverse species by receiving organelles and cytoplasm from sister germ cells while joined in germline cysts or syncytia. Mouse primordial germ cells form germline cysts but the role of cysts in oogenesis is unknown. We find that mouse germ cells receive organelles from neighboring cyst cells and build a Balbiani body to become oocytes, whereas nurse-like germ cells die. Organelle movement, Balbiani body formation, and oocyte fate determination are selectively blocked by low levels of microtubule-dependent transport inhibitors. Membrane breakdown within the cyst and an apoptosis-like process are associated with organelle transfer into the oocyte, events reminiscent of nurse cell dumping in Drosophila. We propose that cytoplasmic and organelle transport plays an evolutionarily conserved and functionally important role in mammalian oocyte differentiation.
Keywords: mouse, oocyte, primordial follicle
Summary:
Mouse oocytes form using intercellular transport
Mammalian oocytes are the only cells that can develop into a mammalian embryo, but during the normal lifecycle, only a small number of mature oocytes are produced. Efforts to generate functional oocytes from pluripotent stem cells or from somatic cells have met with limited success (1). As a result, oocytes represent the limiting component of many genetic, biotechnological, and assisted reproductive procedures. Gaining a deeper understanding of normal oocyte development might reveal processes needed to support early embryogenesis and provide a rational framework for increasing the supply of functional, high-quality oocytes.
Oocytes differentiate in diverse species by receiving organelles and cytoplasm while joined together with sister germ cells. In many insects and lower vertebrates, synchronous mitotic divisions of precursor germ cells generate germline cysts connected by intercellular bridges known as ring canals (2, 3). In Drosophila, organelles and RNAs move into one of the 16 cyst cells along stable microtubules that span the ring canals. The single downstream cell receiving these organelles accumulates an aggregate known as a Balbiani body and becomes an oocyte (4). After further growth, the other 15 cells, known as nurse cells, actively dump most of their remaining cytoplasm into the oocyte before undergoing atypical programmed cell death (5). In nematodes and several other insect groups, early female germ cells form a syncytium through which organelles and cytoplasm are transferred into a subset of cells that become oocytes (2). In hydra, early oocytes acquire extra cytoplasm by fusing with neighboring nurse cells (2, 6). Genetic studies in Drosophila show that intercellular cytoplasmic transport is microtubule-dependent and essential for oocyte production (7).
Whether mammalian oocytes acquire organelles from sister cells and how this might be brought about has not been decisively established (Fig.1A). Following migration to the gonad at embryonic day 10.5 (E10.5), all mouse primordial germ cells form interconnected cysts of up to 30 germ cells (8, 9). Newly formed cysts sustain ongoing apoptosis and fragment into smaller cysts (9). By postnatal-day-4 (P4), 6.4 cells on average survive from the initial 30 (20%) as primary oocytes containing a Balbiani body within a primordial follicle (Fig.1A, 10). However, the importance of cysts for mouse oogenesis has been questioned. Female mice mutant for a ring canal protein, Tex14, are still fertile, suggesting that cysts are an evolutionary relic (11).
Figure 1. Germline cysts and mouse oocyte differentiation.
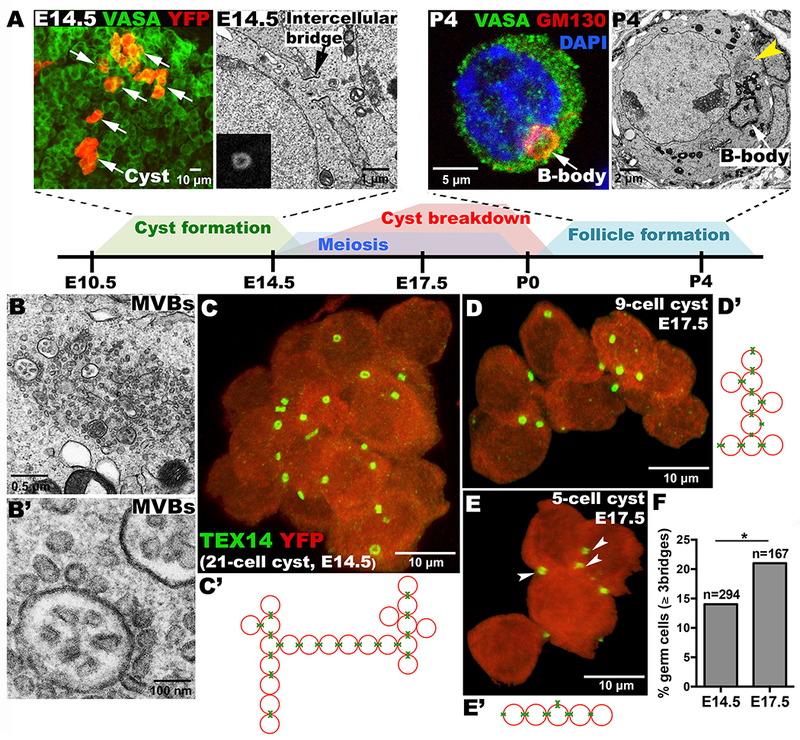
(A) Timeline of cyst formation and breakdown to form primordial follicles. Above, YFP-labeled single-germ-cell clone showing 6 cysts (arrows); EM showing an E14.5 ring canal (arrow), inset (Tex14-labeled ring canal); GM130-labeled Balbiani body (B-body); EM of a P4 oocyte showing B-body and multi-vesicular bodies (MVBs, yellow arrowhead). (B, B’) EM of oocyte MVBs at higher magnifications. Lineage-labeled E14.5 (C) and E17.5 (D, E) cysts showing ring canals (Tex14:green); and connection structure (C’-E’), arrowheads: 3-ring-canal cell. (F) “Branched” germ cells increase between E14.5-E17.5 (p<0.05;chi-squared).
Analyzing mouse cysts has been hampered by the tight packing of germ cells within the fetal ovary. Recently, single-germ-cell lineage tracing methods overcome this problem by marking just one founder germ cell’s progeny (9). We deduced the structure of individual lineage-labeled cysts stained for Tex14 using confocal microscopy and 3D-analysis software. Our studies revealed a variety of interconnection patterns within mouse cysts (Fig.1C–E). As in many other species, ring canals differ in size (Fig.1C) and sometimes cluster in one region of the cell membrane (Fig.1C–E) (2). About 14% of germ cells in E14.5 cysts contain 3 or 4 ring canals (TableS1) and these highly branched cells are selectively preserved (Fig. 1F; p<0.05,chi-square test). Tex14 antibody did not highlight ring canals in cysts younger than E14.5 (Fig.S1), consistent with the continued formation of cysts in Tex14 mutants (11).
We looked for evidence of intercellular organelle movement between sister mouse germ cells marked by lineage labeling. To examine centrosome movement, a hallmark of Drosophila cytoplasmic transfer (12), we stained fetal ovaries for Pericentrin or for γ-tubulin (Fig.2A, B). At E14.5, germ cells contained a single centrosome, as revealed by a single dot-like focus of Pericentrin staining. However, by E17.5, 20% of cells had acquired an additional centrosome, and by P0, these cells had 3-4 centrosomes (Fig.2A, B, arrows). Moreover, smaller putative meiotic centrosomes appeared that are distinct from the accumulating centrosomes (Fig. S2). These observations suggest that centrosomes are transferred through cellular interconnections into a subset of germ cells but remain distinct from meiotic centrosomes.
Figure 2. Organelle enrichment and Balbiani body formation during mouse oocyte differentiation.
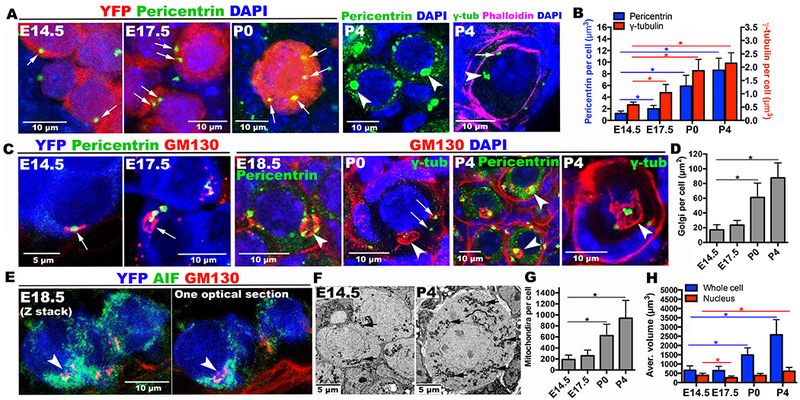
(A) Pericentrin or γ-tubulin-labeled centrosomes (green, arrows) increase in certain cyst cells (red) during E14.5-P4. Each P4 oocyte acquires a large Pericentrin-rich mass (arrowheads). γ-tubulin staining shows 5-6 elements (arrowhead, arrow) within a P4 germ cell delimited by phalloidin staining. (B) Histogram summarizing centrosome accumulation. (C) Golgi elements (red, arrows) often associated with centrosomes (green) also increase and generate the B-body (arrowheads). Pericentrin or γ-tubulin-labeled centrosomes (green) mostly cluster inside by P4. (D) Histogram of Golgi element content per cell. (E) Mitochondria are more abundant in the cyst cell containing a nascent B-body (arrow). (F) EM shows increases in mitochondria (arrows) between E14.5 and P4, as quantitated (G). (H) Germ cell volume increases with time. YFP=lineage marker, AIF (apoptosis-inducing factor)=mitochondria. *p<0.05. Note: GM130 antibody non-specifically stains basement membrane at E18.5-P4.
Further evidence connecting intercellular transport to oocyte differentiation was obtained using GM130 staining to mark Golgi (Fig.2C). At E14.5, a small cluster of Golgi material (arrow) was found in all germ cells, often near the centrosome. However, between E17.5 and P0 some cells accumulated extra Golgi material (arrow) that later coalesced into a sphere indicative of Balbiani body formation (arrowhead). Multiple Pericentrin-positive centrosomes accumulated at the Balbiani body, showing that the same cells accumulate extra Golgi and centrosomes. By P4, the transferred centrosomes fused together forming a large mass in the center of the Balbiani body (Fig.2A,C, arrowhead). γ-tubulin staining could often resolve 3-5 individual subfoci in the Balbiani body (Fig.2A,C, P4, arrowhead), and some that remain outside (Fig. 2A, P4, arrow).
Quantitation of GM130 and Pericentrin staining showed that P4 oocytes contain 5.1-times as much Golgi material, 7.3-times as much Pericentrin, and 3.7-times as much γ-tubulin as E14.5 germ cells (Fig.2B,D). In electron micrographs (Fig. 1A, Fig.1B,B’) we observed multi-vesicular bodies (MVBs) (yellow arrowhead) near P4 Balbiani bodies (white arrow). Mitochondria also increased in cells with nascent Balbiani bodies, compared to other cells in the same cyst (Fig.2E, arrowhead). Electron micrographs (Fig.2F) indicated that by P4 the number of mitochondria (arrow) in Balbiani body-containing cells had increased 4.9-fold (Fig.2G). Finally, Balbiani body-containing cells become progressively larger after E17.5 and quadrupled in size by P4 (Fig.2H). Thus, P4 oocytes acquire about five times as many centrosomes, Golgi, and mitochondria and four times the cytoplasmic volume of E14.5 germ cells.
Additional studies verified that cells acquiring cellular components form Balbiani bodies and become oocytes. Balbiani body containing cells preferentially lack expression of the early apoptosis marker AnnexinV (Fig. S3A, B; p<0.001;Fisher’s exact test) at P0 and are selectively preserved at P4 (Fig. S3C). Organelles might move between germ cells through ring canals. However, Tex14-stained structures decrease in size after E14.5 (Fig.1C–E; Fig.3A); dissociate from junctions at E17.5 (Fig. 3D, E), and disappear entirely after E19.5. The plasma membrane separating most germ cells by E17.5 develops large gaps (Fig.3B, C, E, E’; arrows). Organelle movement after E17.5 appears to utilize these direct interconnections. Some germ cells contract greatly (Fig.3G), and appear to transfer most of their cytoplasm, including centrosomes (Fig.3H, H’), Golgi (Fig.3G, H) and mitochondria (Fig.3I), leaving little more than a nucleus behind (Fig.3I, asterisk; Fig.S3D). Because cysts gradually decrease in cell number throughout E17.5 to P4 (9; TableS1), whereas oocytes increase gradually in size (Fig.2H, Fig.3J), cytoplasmic transfer and programmed cell death events likely occur sequentially within cysts over several days.
Figure 3. Balbiani body formation and oocyte differentiation are facilitated by membrane fragmentation.
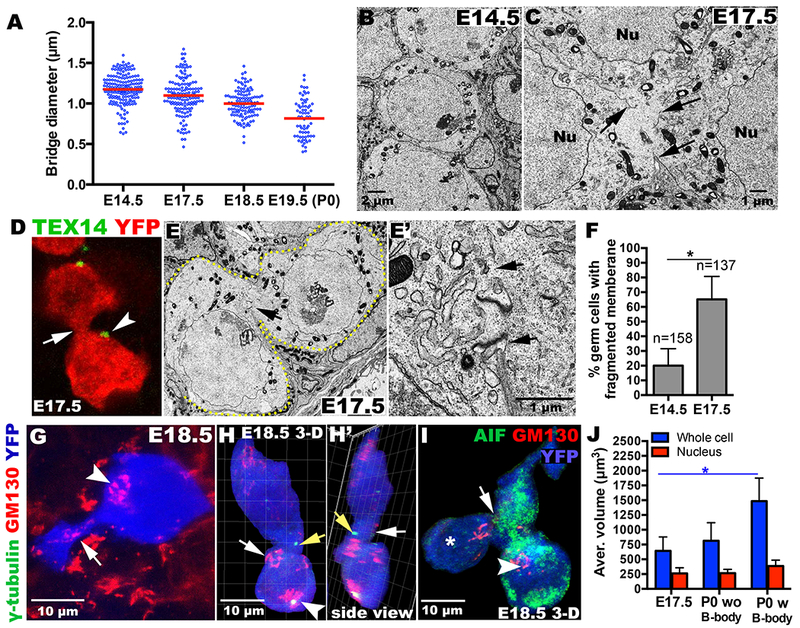
(A) Ring canal (bridge) diameter decreases in size. Cyst germ cells are distinct at E14.5 (B), but frequently contain membrane gaps by E17.5 (C, arrows; F). (D, E, E’) Ring canals (D, arrowhead; E, arrows) detach from intercellular junctions (D, E’ arrow). Golgi material from a contracting cell (G, arrow) and centrosomes (H, H’, yellow arrows) move through membrane gaps into B-body-containing cells (arrowhead). (I) Cell (*) lacking most cytoplasm is connected (arrow) to mitochondria-enriched cells (green), including one with a B-body (arrowhead). (J) P0 cells containing a B-body are larger than E17.5 cells. *p<0.05.
Developing oocytes reorganize their nuclear heterochromatin between P0 to P4 (Fig.4A). HP1-staining is juxtaposed to the nuclear lamina between E14.5 and P0, but P4 oocytes contain several nucleoplasmic aggregates (Fig.4A). To test the role of microtubule-dependent organelle transport in oocyte differentiation, we cultured fetal ovaries in vitro, under conditions where development proceeds normally for a week or more (Fig.4B). When wild type E17.5 ovarian tissue was incubated alone, cysts break down, transfer centrosomes, form Balbiani bodies, undergo apoptosis, and form primordial follicles containing single primary oocytes with similar kinetics as during development in vivo (Fig. 4C–I, Fig S4). When incubated in the presence of the anti-microtubule drug colchicine (10 nM) for the first two days of culture, corresponding to the critical E17.5-E19.5 period when most transport occurs, these processes are perturbed and the production of Balbiani bodies is significantly reduced, from 85% to 32% (Fig. 4C,H). Treatment with the dynein-specific inhibitor Ciliobrevin-D (25μM) also reduced oocyte production. Normal apoptosis (Fig.4D, I), heterochromatin reorganization (Fig. 4E), cell volume increase (Fig. 4F), and centrosome transport (Fig. 4G), and were all reduced by both inhibitors. These effects were specific, since somatic cell proliferation was little affected at these same inhibitor doses (Fig.S5A).
Figure 4. Microtubule-dependent transport is important for Balbiani body formation and oocyte differentiation.
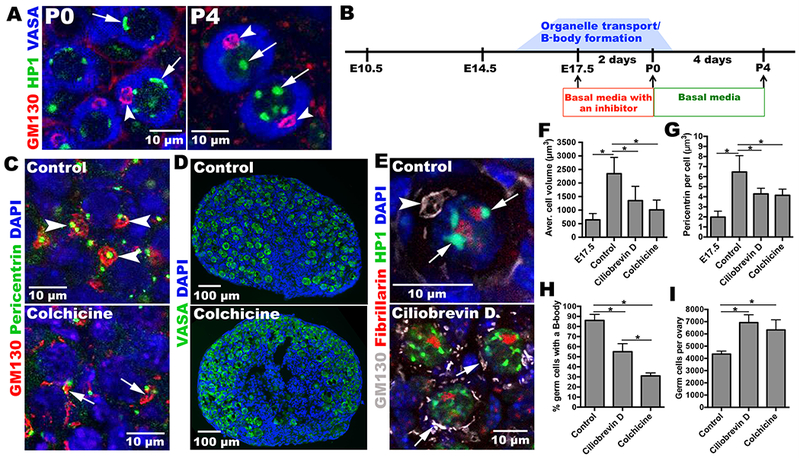
(A) Heterochromatin (arrows) re-organizes between P0 and P4. B-bodies (arrowheads). (B) Experimental design for oocyte differentiation in vitro. E17.5 ovaries are cultured with or without inhibitor for 2 d, and then cultured 4 d without inhibitor. Control ovaries efficiently develop primordial follicles (C), containing full-sized oocytes with normal B-bodies (arrowheads); ovaries treated with inhibitors form smaller cells (F) with fewer B-bodies (H) and reduced Pericentrin content (G). (D) Inhibitor-treated cultures contain more germ cells (I). (E) Heterochromatin re-organizes in controls (arrows) but remains perinuclear in inhibitor-treated cultures. *p<0.05.
Our experiments show that mouse oocytes acquire many additional centrioles, Golgi, mitochondria, and other cytoplasmic components prior to primordial follicle formation (Fig. S6). These materials derive by transfer from interconnected sister germ cells rather than by de novo synthesis since organelles were observed in transit and centrosomes and Golgi were acquired sequentially in individual units prior to fusion, rather than by the growth of a pre-existing element. The rate of organelle and cytoplasmic increase was also too large to explain by biosynthesis in a meiotic arrested cell, and the transferred centrosomes remained distinct from a meiotic centrosome (Fig. S2). Microtubule-based-transport is critical for organelle transport and oocyte formation, but other pathways and the pre-follicular somatic cells that surround each germ cell cluster likely also contribute. Our observation that membrane breakdown rather than ring canals facilitates most cytoplasmic transfer may explain why Tex14 mutant females remain fertile (11). These events lead to Balbiani body formation and strongly resemble cytoplasmic transfers into differentiating oocytes observed in a wide range of other species (2). Thus, organelle movement between the cytoplasm of connected meiotic cells, like pairing and recombination events in the nucleus, represents a conserved aspect of oocyte differentiation. Our experiments show that mammalian oogenesis is more efficient than previous believed. Intercellular transfer ensures that most germ cell cytoplasm and organelles generated during fetal development are preserved in primary oocytes despite an 80% decline in germ cell number.
Why has cytoplasmic transfer been retained throughout evolution? The acquisition of additional organelles undoubtedly helps oocytes begin to grow into the largest cells in the mammalian body, despite possessing only a 4c DNA content. Damaged organelles might simultaneously be off-loaded into cells destined to die, purifying the oocyte cytoplasm (10). Moreover, construction of a Balbiani body by transport might serve a physiological function, such as secretion.
However, we propose that intercellular RNA transport underlies the conserved differentiation of oocytes in cysts or syncytia. Germline genomes undergo epigenetic reprogramming in preparation for a new life cycle (13), a process that might put the oocyte at risk by activating transposons or by fostering non-allelic recombination. In both Drosophila and Xenopus, many RNAs associate with the Balbiani body (3, 14). Cytoplasmic RNA transfer may increase the concentration of reprogramming factors and protective factors such as piRNAs that repress transposition in the oocyte. Consistent with this model, nuage has been reported to accumulate during oocyte differentiation (15), and mutants in genes encoding nuage proteins such as MVH, Piwil2/Mili, and Malestrom cause transposon de-repression in early mouse follicles (15–17).
Finally, our findings imply that high quality mammalian oocytes are unlikely to differentiate directly from a single precursor cell in vitro (18). Consequently, our findings hold out the potential of guiding more efficient in vitro differentiation by defining the critical biological processes that must take place for normal oocyte production.
Supplementary Material
Acknowledgements
This work was supported by the Howard Hughes Medical Institute. The authors thank E, Dikovskaia and the Carnegie mouse facility staff for invaluable advice and assistance. C.-M. Fan and C. Lepper gave helpful advice on mouse husbandry. Members of the Spradling lab provided useful comments on the manuscript. We are grateful to Y. Zheng for providing anti-pericentrin antibody.
REFERENCES AND NOTES
- 1.Sun Y-C. et al. J. Genet. Genomics 41, 87–95 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Büning J, The Insect Ovary. (New York: Chapman & Hall; ) 400pp. (1994). [Google Scholar]
- 3.Matova N, Cooley L, Dev. Biol 231, 291–320 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Cox R, Spradling AC, Development 130, 1579–1590 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Jenkins VK, Timmons AK, McCall K, Trends Cell Biol. 23, 567–574 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexandrova O et al. Dev. Biol 281, 91–101 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Huynh JR, St Johnston D, Curr. Biol 14, R438–R449 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Pepling ME, Spradling AC, Development 125, 3323–28 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Lei L, Spradling AC, Development 140, 2075–81 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pepling M et al. Proc. Natl. Acad. Sci., USA 104, 187–92 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenbaum M, et al. , Biol. Reprod 80, 449–457 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahowald A, Strassheim JM, J. Cell Biol 45, 306–20 ( 1970). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rose CM et al. Biochem. Soc. Trans 41, 809–814 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Kloc M, Etkin LD, Development 121, 287–0 (1995). [DOI] [PubMed] [Google Scholar]
- 15.Lim AK et al. Development 140, 3819–25 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aravin AA et al. , PLoS Genet. 5, e1000764 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malki S et al. Dev. Cell 29, 521- (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi K et al. , Science 338, 971–75 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


