Abstract
Genetic monogamy is rare–at least at the level of a species–and monogamy can exist in the absence of sexual fidelity. Rather than focusing on mating exclusivity, it has become common to use the term “social monogamy” to describe a cluster of social features, including the capacity for selective and lasting social bonds, central to what humans call “love.” Socially monogamous mammals often exhibit selective aggression toward strangers and form extended families. These features of social monogamy in mammals are supported by patterns of hormonal function originating in the neurobiology of maternity, including oxytocin, as well as a more primitive vasopressin pathway. Another key feature of social monogamy is reduced sexual dimorphism. Processes associated with sexual differentiation offer clues to the mysteries surrounding the evolution of monogamy. Although there is consistency in the necessary ingredients, it is likely that there is no single recipe for social monogamy. As reviewed here, genes for steroids and peptides and their receptors are variable and are subject to epigenetic regulation across the lifespan permitting individual, gender and species variations and providing substrates for evolution. Reduced sensitivity to gonadal androgens, and a concurrent increased reliance on vasopressin (for selective defense) and oxytocin (for selective affiliation) may have offered pathways to the emergence of social monogamy.
Keywords: monogamy, oxytocin, vasopressin, testosterone, estrogen, androgens, prairie vole
“A paradox is a logical puzzle that seems to contradict itself. Paradoxical statements may seem completely self-contradictory, but they can be used to reveal deeper truths.”
OVERVIEW
Social interactions are linked to the ability to mate, survive, and thrive within an always changing environment. For these reasons, explanations for species and individual variations in social behavior were initially discussed in terms of evolution, fitness, and reproduction (Kleiman, 1977; Dewsbury, 1987; Komers and Brotherton, 1997; Lukas and Clutton-Brock, 2013; Klug, 2018).
In attempts to create order in the description of socio-sexual behavior across species as well as among human cultures it became common to cluster patterns of presumed sexual behavior, and label these as mating systems including polygyny, polygamy, polyandry, or monogamy. Among these, monogamy seemed particularly difficult to understand. Having a single mate, especially in males, was not easily explained by theories based on reproductive fitness (Barash and Lipton, 2002). Adding to the confusion, genetic tests for paternity revealed that in species presumed to be “monogamous,” sexually monogamy was actually rare–at least when considered at the level of a given species (Solomon et al., 2004).
If monogamy was not primarily about sexual exclusivity, then what was it? Perhaps monogamy was better understood as a social system, and one that offered the advantages of shared parenting, protection of resources, and social support (Wilson, 1975; Gowaty, 1996). And if so, what were the features and the origins of what would come to be known as “social monogamy”? Here we offer a brief personal account of the events that led us to emphasize the importance of viewing monogamy as a social system, and a system that could be understood in terms of its’ neuroendocrinology.
Social systems are inherently variable. However, the specific neural substrates upon which hormones act differ between and among species, and can be compared in socially monogamous vs. non-monogamous rodents. This work began in the genus Microtus, but as more comparative studies have appeared variations in the effects of hormones, often due to differences in target receptors are becoming more apparent. Keeping this variation in mind, we have attempted to uncover patterns of hormone action that may help to explain the phenotype and origins of the traits of social monogamy (Table 1). From rodent data, discussed in depth throughout this review, it appears that socially monogamous traits emerge during the course of development, in part through the combined actions of steroids and peptides (Choleris et al., 2008).
TABLE 1 ∣.
Hypotheses for the roles of steroids and peptides in the emergence of social monogamy.
| Primary Hypothesis: The behavioral and morphological traits associated with social monogamy emerge during development due to the combined actions of steroids and peptides |
| • A shift from a reliance on androgens to a stronger dependence on peptides may facilitate the prosocial traits of social monogamy |
| Testosterone: Features of social monogamy emerge in part via a reduction in the functional effects of androgens |
| • Mutations in the androgen receptor gene reduce the effects of androgens, reducing morphological and behavior sex differences and masculinization |
| • Variations in the 5-α reductase gene reduce sexual dimorphism by reducing the conversion of testosterone to the more potent dihydrotestosterone |
| • High levels of glucocorticoids outcompete testosterone for the androgen receptor, reducing sexual dimorphism |
| Estrogens: Created as a metabolic by-product of testosterone, estrogens play a role in the regulation of the traits of social monogamy and sexual differentiation |
| • Testosterone is aromatized to estradiol, which in turn facilitates actions of peptides, allowing for displays of the behavioral traits of social monogamy |
| Peptides: Oxytocin has the capacity to prevent masculinization by acting as an anti-inflammatory agent that inhibits the actions of androgens |
| • Variation in the oxytocin and vasopressin systems is shaped by factors such as genetics, epigenetics, and developmental experiences; differences in these systems help to explain the variation in sociality among species and between individuals within a species |
Among the several unsolved mysteries associated with social monogamy is the apparently independent emergence of a set of shared traits in unrelated mammals ranging from rodents to canids to New World monkeys (Kleiman, 1977; Lukas and Clutton-Brock, 2013; French et al., 2018). The repeated appearance of the cluster of behavioral and anatomical features that have been termed social monogamy raises an important basic question. What are the mechanisms through which these traits have emerged on over 60 occasions in unrelated mammalian species? Has social monogamy emerged primarily through parallel or convergent evolutionary mechanisms, or some combination of the two? Did a shared physiology permit the evolution across species of social monogamy? Or are there several pathways to social monogamy?
Here we examine the hypothesis that the neurobiology of sexual differentiation and masculinization offers a template for understanding the origins of social monogamy. Research in prairie voles has repeatedly indicated that voles are insensitive to androgens. It is possible that a comparative lack of functional availability of androgens during development, for example, through changes in either the androgen receptor or based on inhibitory effects of other molecules, such as glucocorticoids and oxytocin, may help to explain some of the unique traits that have emerged in socially monogamous mammals. We further propose that alterations in mechanisms underlying the behavioral and anatomical traits of social monogamy, shifting from a reliance on androgens to a dependence on peptides would be adaptive, and also would permit the emergence of the prosocial traits of social monogamy. For example, the components of behavior prominent in males of non-monogamous species, including non-selective aggression, which rely in part on direct actions of androgens may be specifically downregulated in social monogamy. However, selective protective behaviors and selective aggression, such as that seen in socially monogamous species, are supported by estrogens and vasopressin/oxytocin and may continue to be expressed.
MONOGAMY
What Is Monogamy? “What’s in a Word?”
Confusion concerning monogamy has arisen across disciplines in part because of different uses of the word. Monogamy is often defined in dictionaries as “the habit of having only one mate.” The Greek origins of the word monogamy translate to “one wedding or marriage.” This anthropomorphic perspective implies that monogamy involves some form of ceremony and/or legal contract between two individuals. Biologists later borrowed the term monogamy, and alternatives to monogamy, such as polygamy, as a means for categorizing mating systems and social relationships, usually between males and females.
In both common usage and within the field of biology the term monogamy often infers sexual exclusivity either across the life-span or at a given point in time. Definitions of monogamy were applied to a single pair of partners or sometimes even to one individual within a pair. However, over time the word “monogamy” began to be used to classify mating systems at the level of the species (Kleiman, 1977; Dewsbury, 1987).
“Monogamy,” loosely defined, had been described by naturalists in hundreds of mammalian species and thousands of bird species (Lack, 1968). Among the estimated 4,000 or more different species of mammals for which behavioral data are available, it was estimated that between 3 and 5 percent of species, including apparently unrelated taxa, exhibited the traits of monogamy (Kleiman, 1977). A more recent survey of ~2,500 mammalian species, estimates that about 9 percent of species show features of monogamy, defined by the authors as a single breeding male and a single breeding female that share a home range and remain together for more than one breeding season, with or without offspring (Lukas and Clutton-Brock, 2013). This is in comparison to birds in which over 90 percent of species are considered to be “monogamous” (Lack, 1968). As is discussed in depth later in this review, there is also a great deal of intraspecies variation in displays of monogamy behaviors in both mammalian and avian species.
Most early behavioral studies in mammals or birds did not differentiate between mating and social systems, assuming homogeneity between these. In fact, a given species might be simply classified either as “monogamous” or “non-monogamous.” However, early studies describing a species as “monogamous” were based on the largely untested assumption that males and females living together for extended periods of time and exhibiting joint care of offspring also were showing sexual exclusivity and raising their own young (Kleiman, 1977). Sexual preferences were assumed to be the sine qua non of monogamous species. This assumption has repeatedly been shown to be incorrect.
What Is Social Monogamy?
As it became clear that in both avian and mammalian species living in long-term pairs might not always be sexually exclusive, it became increasing common in biology to narrow definitions of monogamy to describe what is now called social monogamy (Carter et al., 1995; Gowaty, 1996). A concurrent set of behavioral, anatomical, and physiological characteristics– beyond the selection of sexual partners–emerged that was found in most, but not all, apparently monogamous species (Kleiman, 1977; Carter et al., 1995; Table 2). Of particular value to identifying the biology of social monogamy were within-genera comparisons of apparently monogamous species to closely related non-monogamous relatives.
TABLE 2 ∣.
Features of social monogamy.
| Selective social interaction with an opposite-sex pair mate; may or may not include exclusive mating with the social partner |
| Selective aggression toward unfamiliar animals, perhaps to guard mating access, offspring or other resources |
| Biparental care and paternal provisioning for offspring |
| Communal living within the family group |
| Alloparental care of younger offspring in the family nest |
| Reduced sexual dimorphism |
Species categorized as socially monogamous may display some or all of these traits.
The broader use of the term social monogamy involved descriptions of animals cohabitating in male-female pairs, remaining together after mating, and jointly defending resources. Thus, the formation of selective and lasting pair bonds between two opposite sex individuals is the most consistent feature of social monogamy. Paternal or alloparental care is sometimes, but not always, observed (Kleiman, 1977; Komers and Brotherton, 1997). In addition, incest avoidance and reproductive suppression of non-breeding animals are common (Carter et al., 1995; Solomon and French, 1997).
In some cases, extended families formed, usually around the original male-female pair. Under these conditions members of a group might forego the opportunity to reproduce directly, remaining as philopatric helpers or alloparents in the natal family. The capacity to experience reproductive and juvenile growth suppression is not limited to socially monogamous species, but may be especially apparent in species that carry the traits of social monogamy. Components of this pattern also are described as cooperative breeding in which non-parents, both related and unrelated, play a major role in the care of offspring (Carter and Roberts, 1997; Solomon and French, 1997; Hrdy, 2009). In cooperative breeding groups one or more breeding females may exist with other members of the group supporting the breeders. Under some environmental conditions, social monogamy can morph into cooperative breeding and colonies, but, at least in rodents, at the core of these it is common to find one primary breeding pair (Solomon and French, 1997).
As described in more detail below, an important clue to the origins of social monogamy is a relative absence of sex differences in anatomical traits including body size and external genitalia. The same processes that have been implicated in mammalian sexual differentiation and masculinization (Arnold, 2017) are plausible substrates for differential expression of monogamous (vs. non-monogamous) traits. As detailed below, variations in the sensitivity of androgen receptors or the production of androgens are among several likely sources of the sexual dimorphism seen in monogamy. Other steroids, and especially estrogen, also regulate the behavioral effects of neuropeptides (Carter, 1998). Conversely, peptides, such as oxytocin and vasopressin, may affect behavior in part through interactions with steroids or their receptors, especially during development (Carter et al., 2009; Van Anders et al., 2011; Perkeybile et al., 2018).
A PERSONAL HISTORY OF THE STUDY OF MONOGAMY AND LOVE IN PRAIRIE VOLES
It was the desire to understand proximate mechanisms supporting lasting social attachments and parenting, that motivated one of us (CSC) to study monogamy from a neuroendocrine perspective (Carter et al., 1995; Carter, 1998). The history of that ongoing journey is summarized below. This journey weaves together several threads including documentation from Lowell Getz, accumulating in the 1970s, that prairie voles were living in life-long pairs, my personal experiences with birth, lactation and oxytocin, and finally being accused by the media of studying “love.” Extrapolations from pair bonding in prairie voles to human love had not been my original intent. However, the notion that love was “hormonal” was not novel (Klopfer, 1971), especially for someone who came of age in the 1960s. Thus, this challenge also pushed me to organize two international conferences asking “Is there a neurobiology of love” (Carter et al., 1997; Carter, 1998).
Monogamous Voles
Over the last four decades the prairie vole has become a favored model for studying the neurobiology of social monogamy. However, before we began our work with this species, both voles and lemmings, were widely studied as ecological models for understanding population dynamics (Getz, 1985). In prairie voles (Microtus ochrogaster), there was evidence of extreme and rapid variation in population density. This led Lowell Getz, a mammologist and my colleague at the University of Illinois, to conduct field and semi-natural studies, some of which continued over a period of more than 25 years (Carter and Getz, 1993; Getz and Carter, 1996). Booms and crashes in populations were not easily explained by environmental factors such as food, water, climate or predation. Emerging from those studies was evidence that prairie voles were sharing nests in long term pairs, generally remaining together for life. Furthermore, both sexually-naïve and experienced males were reliably parental when exposed to an infant. Families built around these pairs sometimes grew into communal groups, or cooperative breeders where young prairie voles help to care for new siblings, usually (but not always) with only one breeding female. In fact, about 70 percent of young prairie voles of both sexes that remained in the nest did not reproduce (Getz and Carter, 1980). In parallel studies, meadow voles (Microtus pennsylvanicus), often studied in the same fields, did not show these traits (Carter and Getz, 1993).
Monogamy: What Does Sex Have to Do With It?
In the 1970s when I began to collaborate with Getz, monogamy was generally used to refer to a mating system, and discussed in terms of reproductive fitness. At that time almost no one believed that a small rodent, such as the prairie vole, was capable of any kind of monogamy. We brought prairie voles, as well as the apparently non-monogamous meadow voles, into our laboratory. Assuming that sexual preferences would be of particular relevance to pair bonds, we repeatedly attempted to study mating preference as an index of social bonds; those attempts were unsuccessful. Both female and male prairie voles failed to show sexual preferences for familiar partners (vs. strangers).
Methods for DNA fingerprinting were first described in the mid-1980s (Jeffreys et al., 1985). The addition of DNA fingerprints increased the body of evidence indicating that social and sexual monogamy are not always coherent. Using DNA fingerprints from the offspring, we observed in prairie voles that a female given a choice voluntarily mated with and could produce mixed litters sired by both a familiar and an unfamiliar male (Carter et al., 1990). These early laboratory data, and later field studies in voles done by others (Solomon and Jacquot, 2002; Solomon et al., 2004; Ophir et al., 2007, 2008), have supported the notion that within a given individual or within a species, social and sexual preferences were not necessarily synonymous. However, despite the absence of a reliable sexual preference, careful observations of the behavior of established pairs of prairie voles revealed that even when mating preferences were not shown, prosocial contact behaviors were reliably more likely to be directed toward a familiar partner (Carter and Getz, 1993). Although sexually promiscuous, female and male prairie voles showed a high level of partner specificity for non-sexual contact, and after mating showed aggression toward intruders of both sexes presumably to guard the mate or other resources (Gavish et al., 1983).
At this same time, controversies were arising from many sources around the concept of monogamy. Other species, including birds, were beginning to be described as socially monogamous, but again evidence for genetic monogamy was rare (Wickler and Seibt, 1983; Gowaty, 1996). Sexual monogamy as a unitary concept, especially at the species level, was becoming increasingly less useful.
Oxytocin, Monogamy, and Love
In 1980, I gave birth to my first son. I was infused with oxytocin during the birth. This transforming experience left me obsessed by the possible behavioral effects of the hormones of motherhood, and especially oxytocin, for both parents and babies. I became convinced that the social bonds between adults, as well as parents and infants, and other experiences that humans call “love,” must depend on a shared underlying neural substrate (Carter, 1992,1998). Although with the exception of one study of maternal behavior in rats which had just appeared (Pedersen and Prange, 1979), there was at that time virtually no experimental data available to support this notion. This fixation led me to try to understand the effects of oxytocin on both sexual behavior and pair bonding in voles. In research first conducted in our laboratory by Diane Witt, we observed that following oxytocin treatment, female prairie voles were more likely to engage in social contact, although exogenous oxytocin did not facilitate sexual behavior (Witt et al., 1990). In this context, we then began to examine the hypothesis that oxytocin played a role in pair bond development.
Working with Jessie Williams and Kenneth Catania, we developed a paradigm for measuring partner preferences in prairie voles that continues to be used (Williams et al., 1992; Young et al., 2011). When prairie voles were given ample time to make a choice, a clear social preference could be detected (Williams et al., 1992). Under similar test conditions meadow voles did not show social preferences for a familiar partner (Carter and Getz, 1993). These findings were replicated several times. Pair bonding, as measured by a selective partner preference, could be assessed even in the absence of a sexual preference. Reluctantly, we and others abandoned the notion that sexual preference could serve as an index of monogamy.
However, using this simple choice paradigm we discovered that prior sexual interactions facilitated subsequent pair bonding, although in females mating was not essential for partner preference formation. It was already known that sexual behavior could release oxytocin a variety of species (Carter, 1992). The experiments that followed allowed us to describe a role for both oxytocin (Williams et al., 1994) as well as vasopressin (Winslow et al., 1993) in pair bond formation in both sexes. If access to either the oxytocin or vasopressin receptor was blocked within the brain, then selective preferences did not form, although animals remained indiscriminately and highly social. However, if both the oxytocin and vasopressin receptors were blocked then social preferences disappeared and both males and females showed a significant reduction in social contact (Cho et al., 1999). These experiments supported the broader notion that oxytocin was not simply a female reproductive hormone, acting on the uterus or breast or facilitating maternal behavior (Pedersen and Prange, 1979; Keverne and Kendrick, 1992). Oxytocin also was capable of increasing sociality in both sexes. If there was a hormone of “love,” then oxytocin was a prime candidate, and the study of peptide pathways in pair bonding in prairie voles was an opportunity to test this hypothesis (Carter, 1998).
Selective Aggression and Vasopressin
Although prairie voles were initially social even to unfamiliar animals, we also observed that after mating, both females (Bowler et al., 2002) and males (Gavish et al., 1983) engaged in selective aggression toward unfamiliar intruders. This aggression probably served as defense of the home range and as well as mate guarding. However, oxytocin, which in our studies usually increased measures of positive sociality, did not seem to be a likely candidate as a substrate for lethal aggression.
Contemporaneous research by Ferris et al. (1984) in golden hamsters had shown that vasopressin treatment induced territoriality and defensive aggression. Thus, vasopressin also was a likely candidate for a role in the defensive components of social monogamy. Based on behavioral studies, first conducted in our laboratory by Gavish et al. (1983) and later repeated by Nicholas Hastings, we were able to block male postmating aggression with antagonists to the vasopressin receptor (Winslow et al., 1993). During this time Geert De Vries and his colleagues also found that central vasopressin changed following mating in male prairie voles (Bamshad et al., 1994). Taken together these studies laid the foundation for the emerging hypothesis that at least two of the major traits of social monogamy (partner preferences and mate guarding) depended on interactions between oxytocin and vasopressin (Carter, 1998, 2017). Subsequent studies implicated these same peptides in male parental and alloparental behavior in prairie voles (Bales et al., 2004a,b).
THE NEUROENDOCRINOLOGY OF MONOGAMY
“What’s Love Got to Do With It?” The Peptides of Maternity as the Neuroendocrine Foundation for Mammalian Sociality
The hormones of maternity, including birth and lactation, are foundational for the emergence of mammalian social behaviors. Mammals are differentiated from non-mammals by the presence of mammary glands and lactation. Care of offspring is not unique to mammals (Maclean, 1990), but dependence of young on a mother, or an allomother, for nutrition and maternal-infant interactions (Hrdy, 2009) are universal features of mammalian life. Even the most primitive mammals, including the egg-laying platypus, show some degree of maternal engagement with their offspring and some form of lactation. Modern mammals are believed to have evolved from non-monogamous ancestors (Lukas and Clutton-Brock, 2013), but the proximate or genetic mechanisms underlying this evolution are only now becoming apparent.
The evolution of maternity depends in part on a specific cocktail of hormones including oxytocin-like molecules; these molecules and their receptors were apparently co-opted as substrates for social monogamy (Carter, 2014; Carter and Keverne, 2017). In maternal behavior (Fleming et al., 1999), as in social monogamy, hormonal effects have been traced to effects on a network of brain regions that influence approach to others and reductions in social fear (Albers, 2015; Caldwell, 2017).
Oxytocin allows immobility without fear in the presence of offspring or partners (Porges, 1998). Oxytocin also has a central role in the formation of selective social bonds between mothers and offspring (Keverne and Kendrick, 1992), between adults (Carter, 1998) and also in paternal behavior (Kenkel et al., 2012; Rilling, 2013). The more ancient mammalian neuropeptide, vasopressin, in dynamic interplay with oxytocin, also regulates birth (Arrowsmith and Wray, 2014). In conjunction with oxytocin, vasopressin was critical to pair bond formation and selective sociality (Cho et al., 1999). Vasopressin also plays a major role in defensive behaviors such as mate guarding. Both males and females are affected by oxytocin and vasopressin (Carter, 2017). However, the physiological effects of vasopressin support physical mobilization and defensive aggression, which may be especially critical in male mammals. Although both males and females synthesize oxytocin and vasopressin, there are often differences in the roles these molecules play in behavioral regulation in males vs. females (Bales et al., 2007a; Albers, 2015; Caldwell, 2017).
Oxytocin and its sibling peptide, vasopressin primarily originate in the nervous system, but both have receptors throughout the body (Grinevich et al., 2016; Chini et al., 2017). Because of the structural similarity between oxytocin and vasopressin, and their receptors, these peptides have many levels of interactions. Oxytocin evolved in mammals, at least 100 million years ago, associated with the evolution of lactation, as well as the positive sociality that much later allowed the emergence of modern humans (Carter, 2014). Over the last two decades a virtual “tsunami” of evidence has revealed that oxytocin promotes social engagement, attention, and synchrony in diverse mammalian species (Feldman, 2017; Hurlemann and Grinevich, 2018; Jurek and Neumann, 2018).
Arginine vasopressin and the vasopressin receptor (AVP V1a) are closely related to the ancestral molecule, vasotocin, and are considered more primitive than oxytocin. Vasotocin plays a role in egg production and the vasotocin receptor became the primary receptor for vasopressin (Goodson and Kingsbury, 2013). The oxytocin receptor presumably evolved more recently. Vasopressin can acutely override the actions of oxytocin. However, many of the functions of oxytocin are actually mediated via stimulation of the vasopressin receptors, possibly inhibiting at least some of the defensive and protective functions of vasopressin, and permitting the reduction of fear and emergence of prosocial behaviors (Albers, 2015; Caldwell, 2017; Carter, 2017).
The interactions of these peptides are not always antagonistic. Instead, oxytocin and vasopressin work together in a kind of dynamic dance that allows rapid changes in behavioral processes and emotional states. Of particular relevance to social monogamy is the fact that both peptides are necessary for the selective sociality that characterizes pair bonds and certain forms of parental behavior. Other molecules and neural pathways, including those that involve dopamine and opioids, are necessary for selective forms of sociality including pair bonds. These are described elsewhere in the context of social monogamy and maternal behavior (Aragona and Wang, 2009; Burkett and Young, 2012). In general, it appears that networks regulated by oxytocin and vasopressin function together with other molecules to facilitate pair bonding.
Steroids, Peptides, and Monogamy
When the field of behavioral neuroendocrinology emerged in the twentieth century most research in this area focused on steroid hormones, usually of gonadal or adrenal origins. It had become common to attempt to explain major features of social and sexual behavior, especially in males, based on variations in androgens. In addition, adrenal steroids, in the context of “challenges” across the life-cycle were described as central to the interpretations of within or between species variations in positive social behavior and aggression (Wingfield et al., 1990). However, early research in prairie voles had shown that the basic features of social monogamy, continued to be present following removal of the gonads and in females formation of social bonds was facilitated by removal of the adrenal glands (DeVries et al., 1996).
Attempts by our group and others to modify the traits of social monogamy with injections of gonadal hormones generally were not successful (Carter and Roberts, 1997). Gonadal hormones were not essential for the adult expression of either pair bonding or aggression (Williams et al., 1992). Even in early life exogenous testosterone had very little effect on either behavior or anatomy in prairie voles. For example, in adult prairie voles castration did not prevent pair bonding or male-male aggression. However, neonatal castration did disrupt pair bonding, a change which could be reversed with injections of vasopressin (Cushing et al.,2003). Findings such as these led us to focus on neuropeptides and social behavior, a journey that has continued for over three decades. Table 3 details the behavioral traits associated with social monogamy and the influence of selected steroids and neuropeptides in expression of these traits.
TABLE 3 ∣.
Features of social monogamy and functional effects of associated peptide and steroid hormones (based on the prairie vole model).
| Traits of social monogamy Functional effects of: |
Selective sociality |
Selective aggression |
Alloparental care |
Paternal behavior |
Reduced sexual dimorphism |
|---|---|---|---|---|---|
| Oxytocin | ↑ | ↓/↑? | ↑ | ↑ | ↑? |
| Vasopressin | ↑ | ↑ | ↑ | ↑ | |
| Testosterone | ↑ | ↓ or NC | ↓ | ↑? | |
| Estradiol | ↑ | ↑ | ↑ |
The peptides oxytocin and vasopressin and the steroids testosterone and estrogen are implicated in several of the common traits of social monogamy acting in some cases to increase the expression of a trait and in others to decrease it.
Variations in the Features of Social Monogamy Are Experience and Hormone Dependent
Research categorizing species according to their patterns of social behavior focused initially on attempts to identify prototypical patterns of behavior that reliably differed between species. The notion that monogamy, either considered as a mating system or a social system, was based on a set of fixed traits has been challenged by laboratory and field data. However, even within species that have been described as socially monogamous there are substantial individual differences in the expression of the traits of monogamy. As detailed below, there is now increasing evidence that genetic and endocrine changes, including variations in peptide and steroid sensitivity, are likely to be important in the emergence of both socially monogamous species and are permissive for within species variations in the traits of social monogamy.
Much of the early research on the neurobiology of social monogamy was conducted in voles of the genus Microtus, comparing closely-related species that are either non-monogamous or socially monogamous. Recent work with other rodent genera, including Peromyscus, Ctenomys, and Phodopus, continues to implicate these peptides in social monogamy (Beery, 2015). Neural and behavioral substrates affected by oxytocin, vasopressin, androgens and estrogens, and interactions among these, regulate social behaviors in each of these species. However, striking species and individual variations exist.
Understanding both the consistency and flexibility underlying the traits of behaviors may be useful to dissecting the mechanisms responsible for the evolution of social monogamy. Traits that are more variable, may be especially sensitive to evolutionary pressures.
Here we use comparative examples from closely related rodents that include both monogamous and non-monogamous species within the same genera. These studies implicate oxytocin and vasopressin in social monogamy, but also reveal between and within species variation. The sources of this variation remain to be fully identified, but as shown in the examples below, can be regulated by genetics, epigenetics, and experience across the life span (Carter et al., 2009; Perkeybile and Bales, 2017; Perkeybile et al., 2018).
OXYTOCIN AND VASOPRESSIN
Oxytocin plays a central role in social behaviors used to define social monogamy. Evidence for this comes from experiments especially in prairie voles. Oxytocin injected into the central nervous system facilitated partner preferences in female (Williams et al., 1994) and male (Cho et al., 1999) prairie voles. Mating facilitates pair bond formation (Williams et al., 1992), and also releases oxytocin (Carter, 1992; Ross et al., 2009b). More recently intranasal infusions have been successfully used in voles to examine the role of oxytocin in pair bonding (Bales et al., 2013). Endogenous variation in the oxytocin system also has been associated with social behavior across a number of species (Beery, 2015).
Specific Brain Regions as Targets for Oxytocin
Among many brain regions relevant to reproductive and social behaviors is the nucleus accumbens, a region implicated in reinforcement and reward. The nucleus accumbens is capable of being influenced by both social and hormonal experiences. In prairie voles the density of oxytocin receptors (Insel and Shapiro, 1992) has been positively related to both the female’s capacity to pair bond with a male partner and to show maternal behavior. In the case of alloparenting, females with lower levels of oxytocin receptor density in the nucleus accumbens were less likely to exhibit alloparenting and more likely to be infanticidal (Olazabal and Young, 2006a,b). Upregulating expression of the oxytocin receptor in this region using a viral vector gene transfer facilitated alloparenting behavior (Ross et al., 2009b; Keebaugh and Young, 2011), while decreasing expression of the receptor by RNA interference was associated with a decrease in alloparental behavior (Keebaugh et al., 2015).
Oxytocin activity in the nucleus accumbens also is vital in females for partner preference formation. Mating induced an increase in extracellular oxytocin concentrations within the nucleus accumbens (Ross et al., 2009a). Activating oxytocin receptors in the nucleus accumbens by administering oxytocin directly to this region induced partner preferences in virgin females, while blocking the receptors prevented partner preference formation (Young et al., 2001; Liu and Wang, 2003). Overexpression of the oxytocin receptor in the nucleus accumbens, using a viral vector gene transfer, accelerated pair bonding in adult females after a short cohabitation with a male partner (Ross et al., 2009b), providing further evidence that receptors in this region are involved in selective sociality. A similar facilitation of pair bonding was seen if the overexpression occurs developmentally (Keebaugh and Young, 2011), while a developmental knockdown (but not elimination) of the oxytocin receptor disrupted pair bonding in adulthood (Keebaugh et al., 2015).
Under natural living conditions only a subset of animals leave the natal group to form pair bonds (Getz and Carter, 1996). Individual differences in the density of oxytocin receptors in the nucleus accumbens may help to explain the variation seen in sociality across individuals in this species. Male prairie voles typically exhibit one of two mating strategies—that of a resident male, maintaining a selective social bond with a female partner, or that of a wandering male, that does not live with a single partner, but instead engages in several acute mating interactions with females, similar to the pattern observed in non-monogamous vole species (Getz et al., 1993). This flexibility in sociality in males is reflected in variation in oxytocin receptor density in the nucleus accumbens. Resident males that are selectively social with a long-term partner have higher oxytocin receptor density in this region compared to males adopting a non-monogamous social strategy (Ophir et al., 2012). Whether this is true for females remains to be studied.
Epigenetics and the Oxytocin System
Both social behavior and oxytocin receptors in the nucleus accumbens can be regulated epigenetically in early life by different amounts of parental care. For example, exposure to higher levels of parental care was associated with an increase in oxytocin receptor gene (Oxtr) expression and oxytocin receptor density and reduced levels of Oxtr DNA methylation (Perkeybile et al., 2018). Animals that experienced higher levels of parental care in early life exhibited higher levels of alloparenting and a facilitation of pair bonding later in life (Bales et al., 2007a; Perkeybile et al., 2013; Del Razo and Bales, 2016). This variability in later behavior in response to varied early experience appears to be regulated by epigenetic mechanisms, including Oxtr DNA methylation, controlling expression of Oxtr in the nucleus accumbens. Findings such as these suggest possible pathways to individual differences in sociality within prairie voles, as well as flexibility in social behavior between species.
The individual differences seen in sociality in this species have been associated with genetic variation in the Oxtr, which influences variation in receptor protein expression (King et al., 2016). Oxtr expression is sensitive to both adult and developmental epigenetic regulation via histone acetylation, and can be affected by mating. Administration of a histone deacetylase inhibitor, which serves to increase gene expression by increasing histone acetylation, facilitated preference formation without mating in both sexes and also was associated with an upregulation of Oxtr and the oxytocin receptor in the nucleus accumbens (Wang et al., 2013; Duclot et al., 2016). This provides additional support for the hypothesis that mating with a partner upregulates Oxtr gene expression in the nucleus accumbens through an epigenetic mechanism, helping to facilitate the formation of selective social bonds.
There is an increase in oxytocin receptor binding in the nucleus accumbens of female monogamous prairie voles, compared to non-monogamous meadow voles (Insel and Shapiro, 1992). This suggests that, similar to intraspecies variation in behavior, the differences in sociality between prairie voles and their non-monogamous counterparts, including both meadow and montane voles, arise due to differences in the density of oxytocin receptors in the nucleus accumbens, among other regions. In this case, high levels of oxytocin receptor density are associated with lasting selective sociality while low receptor density may be associated with acute sociality, but not a selective partner preference. While overexpression of these receptors in virgin female prairie voles induced a partner preference even without mating, the same was not true for meadow voles; upregulating oxytocin receptors in the nucleus accumbens using a viral vector transfer of prairie vole genes to the meadow vole did not alter the meadow vole’s social preference behavior (Ross et al., 2009b). Variations in sociality appear to arise from additional factors beyond oxytocin receptor density in the nucleus accumbens.
Species-Typical Variations in the Oxytocin System Associated With Sociality
Although much of the research characterizing a role for oxytocin in sociality and monogamy was originally conducted in prairie voles, additional comparative studies using closely related species have provided a broader perspective on variations in receptor system.
There are several striking differences in the patterns of peptide receptors between socially monogamous and non-monogamous species. For example, oxytocin receptor density was lower in a number of regions in the socially monogamous, territorial California mouse (Peromyscus californicus), when compared to the closely related, but non-monogamous deer mouse (Peromyscus maniculatus), especially in regions that typically control social behaviors, including the anterior olfactory nucleus, the central amygdala, and the bed nucleus of the stria terminalus (BNST), (Insel et al., 1991). This pattern of oxytocin receptor density is nearly opposite that observed in socially monogamous vs. non-monogamous voles. These findings indicate that multiple neural pathways may regulate variations in sociality. There also are few sex differences in distribution and density of oxytocin receptors in P. californicus, while sex differences are seen in most oxytocin receptor-rich regions in P. maniculatus (Insel et al., 1991). This may be related to species differences in sociality and territoriality that extend beyond pair bonding. The behavioral phenotype of male and female P. californicus tend to be similar, with both sexes engaging in high levels of parental care and both sexes working to maintain the home range and defend against intruders. However, males of this species are larger than females (Klein and Nelson, 1997) possibly implicating the demands of territoriality in sexual dimorphism in this species.
The colonial tuco-tuco (Ctenomys sociabilis) is a highly social South American rodent that forms selective and long-lasting female-based social groups, in which several females share a burrow with a single male (Lacey et al., 1997). In contrast, closely related Patagonian tuco-tucos (Ctenomys haigi), which occupy a similar habitat, are strictly solitary, with both males and females each occupying their own burrow (Lacey et al., 1998). Female colonial tuco-tucos do not show a preference for a familiar male partner over an unfamiliar male. They do, however, form longterm, selective social relationships with a small number of closely related females. This difference in sociality both between tuco-tuco species and between social tuco-tucos and monogamous prairie voles is associated with oxytocin receptor organization. C. sociabilis have higher levels of oxytocin receptor in the central amygdala compared to C. haigi. Interestingly, these two species do not have differences in oxytocin receptor density in the nucleus accumbens, such as that seen in prairie vs. meadow voles (Beery et al., 2008). Beery and colleagues argue that the upregulation of oxytocin receptors in the central amygdala of the social tuco-tuco may be the mechanism that allows this species to form long-term extended social living groups through a decrease in aggression and social anxiety. The lack of a difference in the nucleus accumbens also may suggest a unique role for this region on behaviors relevant to male-female pair bonds, as opposed to same-sex selective sociality.
In another example, in female meadow voles, a species that typically does not form social bonds, same-sex social bonds can be induced to form under short day length conditions (Parker and Lee, 2003). This is accompanied by a rise in central amygdala oxytocin receptors, to levels similar to those seen in C. sociabilis. Upregulation of oxytocin receptors in the nucleus accumbens of meadow vole females, however, has no detectable impact on social bonding (Ross et al., 2009b). Variations in selective sociality between closely related species then, appear to be regulated by oxytocin receptors acting in different regions of the social brain in a species-dependent and sociality-dependent manner.
Vasopressin and Selective Sociality
Arginine vasopressin receptor V1a activation in the lateral septum and ventral pallidum is critical for long-term bond formation in male prairie voles. Both of these regions demonstrate divergent patterns of AVP V1a receptor density between socially monogamous and non-monogamous vole species (Insel et al., 1994; Pitkow et al., 2001; Lim et al.,2004), and this divergence in receptors results in predictable variations in expressions of behavior (Lim and Young, 2004; Ophir et al., 2009). Mating also facilitates the formation of selective relationships. Blocking the activity of AVP V1a receptors in either the lateral septum or ventral pallidum inhibits bond formation even after mating (Liu et al., 2001; Lim and Young, 2004), while direct activation of receptors in the lateral septum with an injection of vasopressin facilitates a selective preference even in the absence of mating (Liu et al., 2001). Altering the expression of AVP V1a receptors in the ventral pallidum also results in variations in behavior in male prairie voles; overexpression of the receptors using a viral vector gene transfer facilitates selective sociality (Pitkow et al., 2001), while receptor knockdown eliminates this behavior (Barrett et al., 2013).
Related processes may promote variability in sociality in non-monogamous species. When the AVP V1a receptor was overexpressed in the lateral septum in non-monogamous rats, using viral vector gene transfer of the prairie vole AVP V1a receptor, social recognition, and social interactions were increased beyond what is typically seen in this species; thus, increasing vasopressin receptor in specific brain regions in rats induced more “prairie vole-like” behavior (Landgraf et al., 2003). Likewise, overexpressing AVP V1a receptors in the ventral pallidum of the male meadow vole by using an AVP V1a receptor viral vector gene transfer from the prairie vole led to development of a selective preference for a familiar opposite sex partner, a behavior not usually seen in this species (Lim et al., 2004).
As described above, associated with the formation of a selective social preference are increases in selective aggression in both male (Winslow et al., 1993) and female prairie voles (Bowler et al., 2002). Animals will show aggression toward novel animals of the same sex, likely as a form of mate guarding behavior (Kleiman, 1977; Getz et al., 1981; Winslow et al., 1993; Carter et al., 1995). Postcopulatory aggression does not occur in the non-monogamous male montane vole after mating (Shapiro and Dewsbury, 1990; Insel et al., 1995). The regulation of aggression results in part from activity of vasopressin and the AVP V1a receptor in the anterior hypothalamus. Selective bond formation is associated with increased receptor density in this region, and coincides with the onset of selective aggression in male prairie voles (Winslow et al., 1993). In socially bonded males behavioral changes in response to vasopressin in this region also occur in tests with a novel male (Gobrogge et al., 2009).
A similar pattern of vasopressin regulation of selective aggression is reported in P. californicus and P. maniculatus. Blocking activation of central AVP V1a receptors in P. californicus delayed aggression toward an intruder, but had no impact on aggression in non-territorial P. maniculatus (Bester-Meredith et al., 2005).
Taken together these data from socially monogamous rodents suggest an overlap in the role of vasopressin in the promotion of both selective social bonding and selective aggression. Both behaviors rely on an increase in vasopressin release and possibly increased sensitivity in the AVP V1a receptor, likely facilitated by mating. As vasopressin increases following mating, the male not only forms a selective preference for the female but also begins to guard access to his mate. Mated males eventually also reject unfamiliar females, even when the females are sexually receptive, helping to preserve the pair bond. These behaviors highlight the dual role of vasopressin in the formation and maintenance of social bonds. Males simultaneously engage in selective and enduring affiliative behavior with their female partner, while also engaging in selective and enduring aggressive behavior toward non-partners. The synchrony and balance between these two sets of selective behaviors may be vital for maintaining a socially monogamous system.
Comparisons in the regulation of aggression by vasopressin in socially monogamous prairie voles and Siberian hamsters (Phodopus sungorus), a non-monogamous and territorial species, highlight the sometimes contrasting role a single factor can play in generating variation in patterns of sociality across species. As discussed above, vasopressin activity in the anterior hypothalamus plays a role in the display of selective aggression in male prairie voles (Gobrogge et al., 2007, 2009). The anterior hypothalamus also plays a role in aggression in non-monogamous Syrian hamsters (Ferris and Potegal, 1988; Potegal and Ferris, 1989; Ferris et al., 1997). These displays of aggression, however, serve very different purposes for the two species. Male prairie voles display aggression toward other males only after mating, as a form of mate guarding behavior. Syrian hamsters, however, use this aggression in territorial disputes and in gaining access to a mate prior to mating. The fact that similar expressions of behavior, regulated by similar vasopressinergic activity can serve different purposes between species, highlights the flexibility within these social behavior systems; the same behavior regulated by the same endocrine mechanisms can often be used in two distinct ways by species depending on their social system. Vasopressin may play a role in aggression under both circumstances. However, as hypothesized below, it is possible that the regulation of aggression and vasopressin in non-monogamous species is more likely to be regulated by testosterone, while socially monogamous species may be less reliant on androgens and more dependent on peptides, possibly linked to estrogens.
In addition to regulating variations in selective long-term sociality and aggression both within and between species, vasopressin is also involved in variations in paternal behavior. For example, new prairie vole fathers experience an increase in vasopressin mRNA in the paraventricular nucleus and supraoptic nucleus after the birth of a litter compared to virgin males. This rise in vasopressin mRNA is not found in new fathers in the non-monogamous, non-paternal montane vole (Wang et al., 2000). An increase in vasopressin gene expression associated with birth of their own litter is particularly interesting given that male prairie voles are highly alloparental as virgins. This also suggests the possibility that different factors regulate alloparental compared to paternal behavior in this species.
Within P. californicus fathers, variation in vasopressin immunoreactivity in the BNST corresponds to variation seen in displays of paternal care; males with increased vasopressin activity spent more time in the nest with offspring, grooming, and huddling over them (Bester-Meredith and Marler, 2003). A related pattern of changes in vasopressin correlates with paternal behavior within this species. California mouse fathers have higher levels of vasopressin immunoreactivity in the BNST compared to non-monogamous and less paternal white-footed mice fathers (Bester-Meredith et al., 1999). These and other data suggest that vasopressin has a central role in paternal investment and care within socially monogamous species, but also in closely related species with divergent behavioral patterns, including social behaviors that have been termed non-monogamous.
Steroids, Peptides, and Aggression
In male prairie voles aggression is triggered within 24 h or less by mating acting via vasopressin pathways (Winslow et al., 1993). By relying on social stimuli and vasopressin to induce or support aggression (rather than acute changes in testosterone), male prairie voles also may be able to be transformed quickly—within hours—from acting as comparatively non-aggressive, non-reproductive animals to creatures capable of showing lethal aggressive toward intruders.
Female prairie voles also are capable of intruder-directed aggression, especially toward other females (Firestone et al., 1991). Although the origins of female aggression have not been well studied, preliminary studies did not implicate vasopressin (Bowler et al., 2002). The role of vasopressin in female behavior deserves additional investigation, but sex differences in the effects of vasopressin are common (Albers, 2015; Caldwell, 2017; Carter, 2017).
Circulating testosterone levels are typically found to be inversely related to expression of paternal behavior in non-monogamous males (Wingfield et al., 1990; Ketterson et al., 1992; Nunes et al., 2001). Human fathers often experience a drop in testosterone immediately after the birth of their child (Storey et al., 2000). In addition, human males who have lower levels of testosterone, whether fathers or non-fathers, respond more to the sound of infant cries than do males with higher testosterone levels (Fleming et al., 2002). In rodents declines in testosterone often coincide with a decrease in infanticidal aggression, presumably preparing the male to support, or at least not attack, his offspring (Elwood, 1977; Brown, 1986; Perrigo et al., 1991). In Mongolian gerbils (Meriones unguiculatus) castration increased time caring for offspring, including increases in grooming and huddling over pups (Clark and Galef, 1999). In typically infanticidal rats castration during adolescence and young adulthood decreases rates of infanticide, a behavior that is reinstated with testosterone replacement (Rosenberg et al., 1971; Rosenberg and Sherman, 1975). The same effect on infanticide is seen in male mice after adult castration (Svare and Mann, 1981). In both mice and rats, high levels of testosterone in these non-monogamous, non-paternal species may increase male infanticide.
In contrast, data from several species of socially monogamous rodents revealed high levels of testosterone around the time of birth. For example, male Djungarian hamsters (Phodopus campbelli), a highly social and highly paternal species, maintain elevated testosterone after the birth of a litter while still engaging in paternal care. The high testosterone levels immediately following birth might facilitate mating or mate guarding during the postpartum estrus, while the higher levels maintained several days after birth could support paternal aggression toward intruders. In contrast, the closely related but less social Siberian hamster (P. sungorus) typically engages in little paternal care, and experiences a drop in testosterone soon after birth (Reburn and Wynne-Edwards, 1999).
Comparatively high levels of testosterone are observed in other socially monogamous species, include California mice and prairie voles. As discussed more in depth below, P. californicus experience a surge in testosterone just prior to the birth of their litter that coincides with the onset of paternal behavior (Gubernick et al., 1994). In prairie voles increased testosterone appears necessary for alloparental care in males, as castration in adulthood decreased paternal care of novel pups (Wang and De Vries, 1993).
Comparisons among socially monogamous, vs. non-monogamous, rodent species suggest that testosterone does play a role in paternal behavior. However, rather than acting through androgen receptors, these effects may require conversion to estradiol and action through estrogen receptors (Trainor and Marler, 2002). There is considerable evidence for variation in estrogen receptor distribution, particularly the estrogen receptor alpha (ER-α), between socially monogamous and non-monogamous species. This is discussed in depth below. The aromatization of testosterone to estradiol, then, which facilitates the effects of both oxytocin and vasopressin, might enable males to show high levels of paternal care toward young that are in at least some cases their own, while still engaging in selective aggression toward intruders.
Estrogen and Estrogen Receptors Are Correlated With Sociality
Based on data from non-monogamous rodents, it has been proposed that ER-α activation masculinizes behavior (Scordalakes et al., 2002; Scordalakes and Rissman, 2004; Nugent et al., 2015). Data supporting a role for the ER-α as a factor inhibiting sociality comes from patterns of selective sociality in both vole and hamster species. Pine voles (Microtus pinetorum) are socially monogamous, with behavioral features similar to those seen in prairie voles. In comparison, the montane vole (M. montanus) and the meadow vole (M. pennsylvanicus) are both less social, non-monogamous species with typically lower levels of paternal or allopaternal behavior. Pine voles have decreased levels of ER-α in the medial amygdala compared to both non-monogamous vole species and in the BNST compared to montane voles (Cushing and Wynne-Edwards, 2006). Similarly, the highly social Djungarian hamster (P. campbelli) also has decreased ER-α in both the medial amygdala and BNST compared to the less social Siberian hamster (P. sungorus; Cushing and Wynne-Edwards, 2006). When ER-α levels are low then, as is seen in both vole and hamster species, prosocial contact is more commonly directed toward a specific partner and toward infants. Activity in both the medial amygdala and the BNST are has been related to displays of aggression and parental behavior in the prairie vole (Wang and De Vries, 1993; Wang et al., 1997), possibly mediated by stimulation of the ER-α.
Within prairie voles, there is population-level variability in social behavior that correlates with variation in ER-α expression. The home range of this species is large, stretching from New Mexico north into Alberta, Canada and as far east as the Great Lakes, West Virginia, and Tennessee (Stalling, 1990). This broad geographic home range is associated with variety in habitat resource availability and also in varying degrees of monogamous behavior. Voles from Illinois display relatively high rates of social monogamy (Getz et al., 1993; Roberts et al., 1998; Solomon and Jacquot, 2002), a low degree of sexual dimorphism, and high rates of paternal care (Roberts et al., 1998; Ophir et al., 2007). Voles from Kansas appear to be more promiscuous (Danielson and Gaines, 1987; Swihart and Slade, 1989). In males, the levels of ER-α expression in both the BNST and medial amygdala are reduced in the highly social Illinois prairie vole compared to the less social Kansas prairie vole. In socially monogamous species, stimulation of the ER-α, may inhibit the features of social monogamy, while stimulation of the ER-α could facilitate aggression. Taken together these findings suggest that neural mechanisms that allow a shift toward selective sociality may be inhibited by stimulation of the ER-α, possibly as a component of the association between masculine behaviors, androgens, and non-selective aggression which is more common in non-monogamous mammals.
In the Djungarian hamster, adult males have circulating estradiol levels as high as those found in females (Schum and Wynne-Edwards, 2005). Castration during the partner’s pregnancy lowers both plasma testosterone and estradiol levels in males. This decrease in steroid hormones results in decreased aggression toward an intruder compared to intact males, but has no impact on paternal behavior toward offspring. This supports a role for these steroid hormones in facilitating aggression, but does not support the hypothesis that the high plasma levels of estradiol seen in reproductive males serves to increase paternal behavior (Hume and Wynne-Edwards, 2005). This contrast between effects of differences in ER-α expression and plasma estradiol levels provides further support for the idea that flexibility in behavior is not always due to variation in the hormone, but rather due to variation in its receptor density. In this case, P. campbelli rely on low levels of ER-α in order to engage in high degrees of social behavior. Thus, following a pattern that is seen for testosterone, changes in the estrogen receptor, rather than available estradiol, may be more specifically related to observable changes in behavior.
This differs from the pattern reported in male California mice. Males show a high degree of paternal care after the birth of their first litter of offspring. This change in behavior is accompanied by a surge in plasma testosterone just prior to birth (Gubernick et al., 1994). Eliminating this rise in testosterone by gonadectomy acts to drastically reduce paternal huddling and grooming of offspring (Trainor and Marler, 2002; Marler et al., 2003), while gonadectomy coupled with testosterone replacement restores behavior to typical high levels (Trainor and Marler, 2001, 2002). In P. californicus, paternal behavior appears to be controlled by estradiol via its aromatization from testosterone. Gonadectomy with an estradiol replacement has the same impact of restoring paternal huddling and grooming as does testosterone replacement. Co-administration of testosterone plus fadrozole, an aromatase inhibitor, reduces paternal behaviors, an effect not seen with co-administration of testosterone and saline or estradiol and fadrozole (Trainor and Marler, 2002). This work provides evidence that testosterone is not directly impacting huddling and grooming, but rather is exerting its influence via conversion to estradiol. This differs from factors regulating paternal behavior in P. campbelli, in which paternal care does not appear to rely on estradiol, and provides additional evidence for species differences in how variation in sociality arises.
Taken together these findings support the hypothesis that estrogens, created as a metabolic by-product of testosterone, may play a major role in the regulation of the traits of social monogamy. In many, but perhaps not all species, estrogens act via the ER-α to increase aggression, possibly in part through downstream effects on vasopressin. However, other receptors exist for estrogen, although these are at present less well understood. It is possibly that estrogen could have effects on different receptors or actions on other brain regions, for example playing a role in the regulation of the oxytocin receptor. Thus, a given hormone, such as estrogen, might promote selective socially in some cases and aggression in others.
Coordinating Social Behaviors With Demands of the Physical and Social Environment
Steroid hormones, including androgens, estrogens, and adrenal hormones coordinate the demands of reproduction with changes in the physical environment, including seasonality and photoperiod, as well as availability of resources such as food and water (Wingfield et al., 1990). The functional properties of steroid hormones allow these molecules to play a major role in both reproductive behavior and patterns of social behavior that support reproductive success. In contrast, reproduction in socially monogamous species often includes a comparative reliance on quickly changing social cues, including those transmitted by olfaction (Dluzen et al., 1981) and behavioral experiences such as mating (Winslow et al., 1993). These effects could be more directly dependent on the rapid actions of neurosteroid hormones (Balthazart and Ball, 2016), which are being studied in avian social behavior. In addition, the actions of steroids might rely on steroid-peptide interactions with steroid effects being critical for receptor organization or physiology (Witt et al., 1991). In other cases, such as the regulation by mating of male aggression, steroids might be replaced by direct effects of peptide hormones such as vasopressin (Winslow et al., 1993).
Peptides, including oxytocin and vasopressin, have many actions, including acting as neuromodulators, neurotransmitters, and hormones (Jurek and Neumann, 2018). Neuropeptides have the capacity to respond quickly to social cues, and are positioned to influence behavior by their abundant synthesis in the brain (rather than peripheral organs), with receptors in critical tissues within the nervous system, as well as the immune system and throughout the body. The production of peptide receptors also is capable of being epigenetically tuned by experience creating longer-lasting changes in the capacity to respond to social cues (Perkeybile et al., 2018).
THE DEVELOPMENTAL ORIGINS OF SOCIAL MONOGAMY
Sexual Differentiation: An Overview
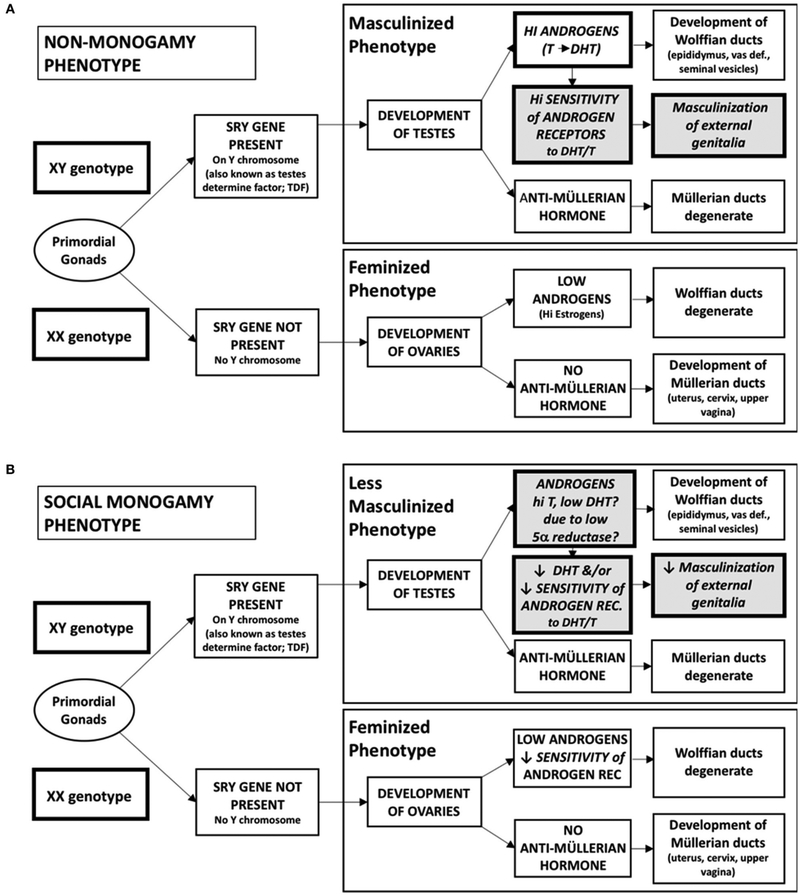
Sexual differentiation is the consequence of genes including those that regulate the synthesis or action of testosterone, estrogen and their receptors (Arnold, 2017). During early development the SRY gene (testes determining gene), located on the Y chromosome, enables the undifferentiated gonad to become masculinized, producing primarily testosterone and sperm (Figure 1). In the absence of the SRY gene the gonad is feminized, primarily producing higher levels of estrogen and allowing ovulation (Arnold, 2017).
FIGURE 1 ∣.
Sexual differentiation into masculinized and feminized physical phenotypes. (A) This classical model characterizes the differential development of the primordial gonad into testes or ovaries, depending on the presence of the SRY gene found on the Y chromosome. As testes develop, high levels of androgens, including testosterone and dihydrotestosterone (DHT), act on highly sensitive androgen receptors to developmasculinized internal (Wolffian duct) and external genitalia. Anti-Müllerian hormone is also produced, causing the Müllerian ducts to degenerate. In the absence of the SRY gene (in an XX genotype), the ovaries produce very low amounts of androgens, causing the Wolffian ducts to degenerate and allowing for feminization of external genitalia. In typical females anti-Müllerian hormone is also not produced, allowing the Müllerian ducts to develop into feminized internal genitalia. (B) In a social monogamy behavioral phenotype, we hypothesize that changes in the response to androgens produced by the testes during prenatal development may lead to changes in the masculinization of the external genitalia in XY genotype individuals. While testosterone production remains high to allow for the masculinization of the internal genitalia, decreases either in the production of DHT or in the sensitivity of the androgen receptor to either testosterone or DHT decrease the extent of masculinization of the external genitalia, a common physical feature of males of socially monogamous species.
In the classical model of sexual differentiation, derived primarily from non-monogamous rodents, it has been assumed that testicular hormones are essential for masculinization (Figure 1). Steroids implicated in genital masculinization include testosterone and dihydrotestosterone (DHT). Testosterone serves as a prohormone and can be converted locally to DHT. In addition, testosterone, in the presence of the aromatase enzyme, can be converted to an estrogen. Acting on the ER-α, it has been proposed that estrogen regulates behavioral masculinization, even in the absence of androgens (Scordalakes et al., 2002). Estrogens, possibly in conjunction with DHT, facilitate patterns of male sexual behavior including mounting and ejaculatory reflexes. Thus, at the level of the brain, testosterone per se may not be essential for masculine sex behavior, although a combination of DHT plus estrogen seems to be important to optimize adult male sexuality (Pfaff and Baum, 2018).
In mammals two X chromosomes lead to a feminine phenotype, vs. the XY genotype found in male mammals. However, the haploid nature of sex chromosomes has complex consequences for the vulnerability to modification of genes on the Y or X chromosome. For example, in females some (but not all) genes on one of the two X chromosomes are inactivated. This is accomplished by DNA methylation and in some cases parental genomic imprinting (Keverne, 2013). Genes on the X chromosome that escape X-inactivation or genes that are not imprinted may affect sexual differentiation (Arnold, 2017).
Sexual Differentiation as a Template for the Biology of Social Monogamy
The key behaviors that appear to differentiate monogamous from non-monogamous species are presented in Table 2. These are exhibited on a continuum across and within species and are sensitive to physiological changes in early life. The neuroendocrinology that regulates these behaviors also occurs on a continuum, with interspecies and intraspecies variation in these endocrine markers. Based on data primarily from rodents, it appears that the traits of social monogamy appear during the course of development as a merger of actions of steroids and peptides (Choleris et al., 2008). In this context the actions of steroids are comparatively slow, but can have long-lasting consequences, while those of neuropeptides (as well as neurotransmitters) may be more rapid, but also somewhat more transient.
In contrast to sexual behavior, social behaviors are inherently variable and must respond to a constantly changing social and physical environment. Thus, the regulation of the features of social monogamy represents a compromise between quickly adapting behavioral changes, such as those necessary for parenting and pair bonding, and more conserved traits, such as sex differences in body size or genital morphology or the distribution of receptors for both steroids and peptides.
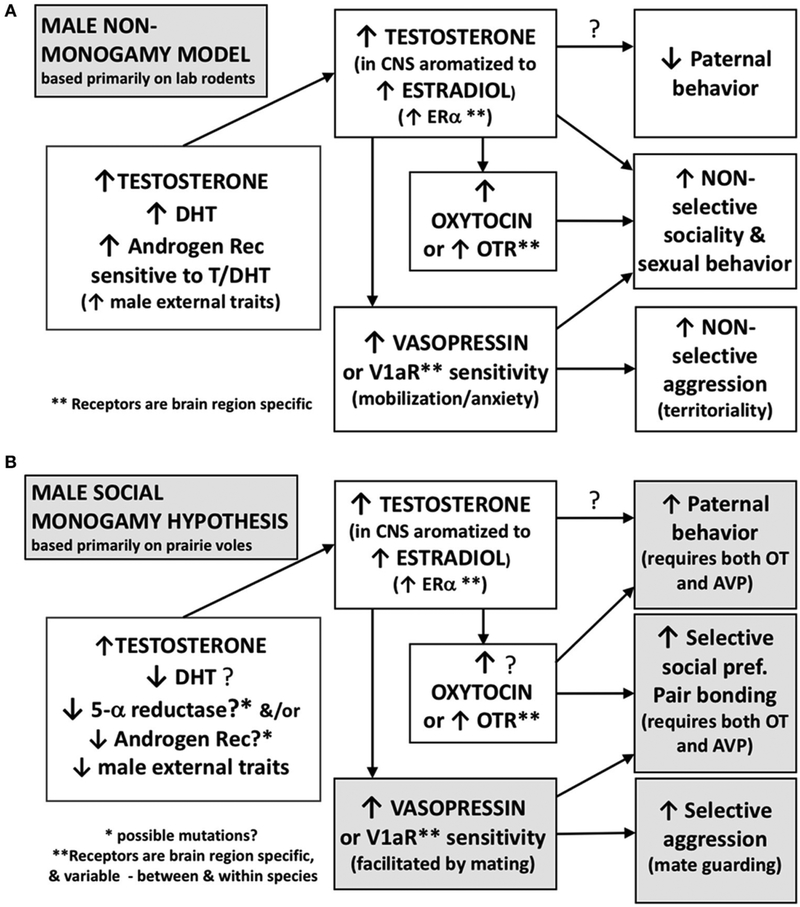
A cardinal feature of socially monogamous species includes a relative reduction in physical sexual dimorphism, presumably as an effect of sexual selection (Emlen and Oring, 1977; Kleiman, 1977). However, with a few exceptions (Kleiman, 1977; Klein and Nelson, 1997; Roberts et al., 1998), we caution that evidence for this aspect of social monogamy seems to be primarily anecdotal, possibly based on visual observations by field biologists, rather than systematic measurements of body size or genital anatomy. Based on the hypothesis that social monogamy is associated with a shift in the male phenotype toward a less masculine pattern (Figure 2), we postulate here that candidate neuroendocrine processes and genes that downregulate sexual dimorphism might also have allowed the emergence of the traits of social monogamy (Table 3).
FIGURE 2 ∣.
Functional differences in the effects of steroids and peptides may contribute to non-monogamous vs. socially monogamous behavioral phenotypes in males. This hypothesis is based primarily on data from laboratory rodents including prairie voles. (A) Males of non-monogamous species often display a suite of behaviors that include low levels of paternal investment, non-selective social and sexual behavior, and non-selective aggression that typically occurs when competing for a mate or territory. These behaviors are facilitated by high levels of androgens and a high sensitivity of the androgen receptor. High levels of testosterone, some of which is aromatized locally to an estrogen, may contribute to low levels of paternal behavior and lead to non-selective social behavior and mating. In addition, vasopressin, facilitated by androgens, is involved in mating- and territory-related aggression in these males. (B) In socially monogamous males, we hypothesize that decreased sensitivity of the androgen receptor, or possibly lower levels of DHT, with a concurrent increase in vasopressin, could contribute to high levels of paternal investment, selective social preferences, and selective mate guarding aggression. Testosterone can be aromatized locally to estrogen and also facilitate the release of oxytocin and vasopressin.
Androgens and Masculinity
Several hormones have the capacity to influence testosterone’s production or action. Among these are factors in the hypothalamic-pituitary-gonadal axis, including releasing hormones such as gonadotropin releasing hormone (GnRH). However, GnRH and testosterone are probably not the main source of the reductions in sexual dimorphism, at least in prairie voles. In fact, high levels of testosterone have been measured in prairie voles including during the perinatal period (Lansing et al., 2013).
In prairie voles, both during development and in adulthood, hormones originating in the gonads do not seem to be essential for pair bonding or male parental behavior (Carter et al., 1995; Carter and Roberts, 1997; Lansing et al., 2013). In addition, giving testosterone to female prairie voles has remarkably little behavioral effect and testosterone treatment in early life does not masculinize the genitalia in either sex. Taken together these findings suggest that prairie voles may have a deficit in the capacity to respond to testosterone.
One option through which features of social monogamy might arise more generally is via a reduction in the functional effects of androgens. This could occur through several known pathways (Table 4). The effects of testosterone differ according to whether they are mediated by androgen (AR) or estrogen receptors (including ER-α). In the central nervous system testosterone can be is aromatized locally to estradiol (E2). As mentioned above, estrogens acting on ER-α, are considered a major factor in brain masculinization (Bodo and Rissman, 2006; Cushing, 2016).
TABLE 4 ∣.
Putative pathways to reductions in physical sexual dimorphism and reduced “Masculine” traits.
| Hypothesized pathways | Evidence in the prairie vole |
|---|---|
| 1. Reduced levels of testosterone or DHT | High levels of T(Lansing et al., 2013) DHT levels have not been reported |
| 2. Failure to convert testosterone to DHT, possibly due to variation in the 5a-reductase enzyme | ?? |
| 3. Genetic or epigenetic variation in the androgen receptor (AR) | ?? |
| 4. Genes on sex chromosomes vulnerable to epigenetic modification? | ?? |
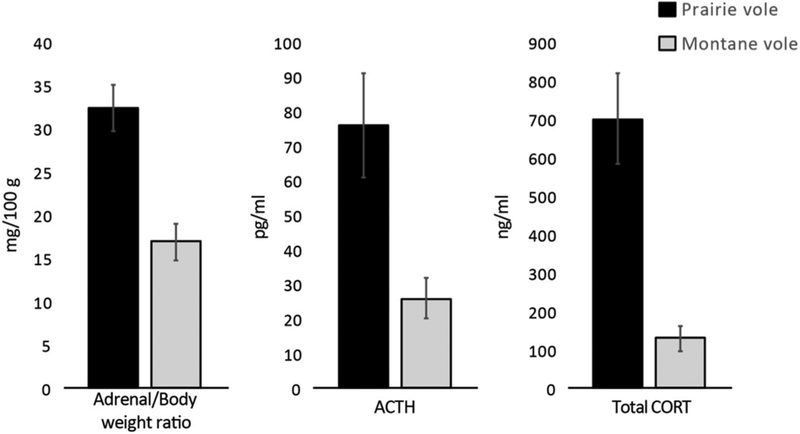
| 5. Presence of inhibitory factors such as stress or high levels of glucocorticoids | Very high corticosterone, likely across the life cycle (DeVries et al., 1995, 1996) Glucocorticoid receptor insensitivity (Taymans et al., 1997) |
| 6. Exposure to oxytocin in the perinatal period | Reduced sex difference in the brain (Yee et al., in preparation) Decreased AVP V1a receptors (both sexes; Bales et al., 2007b) May act to demasculinize via anti-inflammatory pathways (Nugent et al., 2015) |
Evidence is at present incomplete; however, studies to date suggest that although prairie voles may be comparatively insensitive to testosterone they continue to be capable of responding to estrogen. In fact estrogen, possibly by facilitating the synthesis of vasopressin (Lonstein et al., 2005), appears to play a central role in the regulation of sociality and aggression in male prairie voles (Cushing and Kramer, 2005).
Acting via the AR, testosterone and DHT also play a major role in the development of masculine physical traits including body size, anogenital distance, and phallic and scrotal development. AR is highly expressed in genitals, but also in muscle, bone, and other tissues (at least in non-monogamous mammals; Figure 1). This pathway was originally identified in humans by a genetic failure of the AR to bind androgens, known as androgen-insensitivity syndrome (AIS). In AIS, individuals with an XY chromosome pattern have an external body type that appears female, while internal organs are masculinized. Over 1,000 polymorphisms in the gene for the AR have been reported. Although the functional roles of most of these are not yet well understood (Gottlieb et al., 2012), this is a receptor already known for variation.
As mentioned above, the gene for the androgen receptor resides on the X chromosome. The X chromosome is haploid in males and may be inactivated in females. Thus, mutations in the gene for the androgen receptor or other processes that indirectly reduce the effects of androgens could be a route to reducing sex differences and masculinization. The AR gene may be subject to particularly intense evolutionary pressure (Mokkonen and Crespi, 2015). Thus, if mutations or epigenetic silencing occurs in the X chromosome in areas relevant to the expression of the AR gene, this could reduce AR production. AR expression, especially in genital tissue is a major candidate for the regulation of masculine physical traits and thus the reduced sexual dimorphism seen in some socially monogamous mammals (Figure 1).
The metabolism of testosterone to DHT is another process that could influence peripheral anatomy. The 5-α reductase enzyme, type 2 (SRD5A2) is critical for the conversion of testosterone to DHT. In the absence of DHT, masculinization of the genitalia is disrupted (Okeigwe and Kuohung, 2014). The gene for this enzyme is also variable and subject to mutation. Variation in SRD5A2 is another putative candidate for reducing sexual dimorphism.
Do High Levels of Glucocorticoids Contribute to the Social Monogamy Phenotype?
Based on research in laboratory rodents, it has been assumed that the creation of a typical male phenotype (especially external masculinization) requires the relative absence of high levels of stress and associated glucocorticoids, which can inhibit masculinization (Ward and Ward, 1985). Thus, stressful experiences during early development are another possible mechanism for suppressing masculine traits, including genital masculinization (Ward and Ward, 1985). Support for this hypothesis comes from research in rats; early life stress or increases in glucocorticoids also increase male sociality and parental behavior in late life (Kinsley and Bridges, 1988).
Prairie voles have exceptionally high levels of glucocorticoids (Figure 3), 10 times those seen in non-monogamous montane voles (Taymans et al., 1997), as well as mice and rats. Prairie voles also are insensitive to drugs that mimic the effects of glucocorticoids, indicative of glucocorticoid receptor resistance (Taymans et al., 1997). A remarkably similar pattern, including high endogenous levels of glucocorticoids and glucocorticoid resistance, has been reported in New World primates, including marmosets (Chrousos et al., 1982), which show several parallel features of social monogamy (French et al., 2018). Thus, reductions in sensitivity of the glucocorticoid receptor, with a concurrent elevation in glucocorticoids, could create a hormonal environment in which glucocorticoids compete with testosterone for the AR. This is another plausible pathway through which reductions in masculine traits might occur.
FIGURE 3 ∣.
In comparison to non-monogamous montane voles, the socially monogamous prairie vole shows higher unstimulated levels of hormones of the hypothalamic-pituitary-adrenal axis. Adapted from Taymans et al. (1997).
Could Oxytocin Play a Developmental Role in Physical Demasculinization, While Increasing the Behavioral Traits of Social Monogamy?
We hypothesize here that oxytocin may have the capacity to prevent masculinization. For example, Nugent et al. (2015) have added compelling evidence for a role for changes in inflammation as a necessary mechanism through which testosterone creates a masculine behavioral phenotype. Oxytocin is anti-inflammatory (Yuan et al., 2016), and thus might indirectly inhibit the actions of androgens on the masculine phenotype.
Socially monogamous species, including prairie voles, often have high levels of oxytocin (Kramer et al., 2004), and might be less responsive to both the masculinizing and inflammatory effects of testosterone. In addition, a brain imaging study from our group indicates, at least in prairie voles, that a brief perinatal exposure to oxytocin can demasculinize the nervous system (Yee, Ferris et al., ms in preparation). Other studies have revealed that early life manipulations of the oxytocin system alter several traits of monogamy, facilitating pair bonding and alloparental behavior in males, with little effect in females (Carter et al., 2009).
At least one source of variation in oxytocin in early life is exposure to differential parenting. The neural systems that regulate social experiences in adulthood, also are epigenetically tuned by social experience and peptides in early life (Perkeybile et al., 2018). As one other example, in prairie voles a single exposure to oxytocin on the first day of life altered the expression of the vasopressin receptor in adulthood (Bales et al., 2007b). In contrast blocking the oxytocin receptor interferes with the behavioral traits of social monogamy, again with effects that thus far have been most apparent in males (Bales et al., 2004a; Carter et al., 2009).
CONCLUSIONS
Individual differences in social behavior are regulated by genetics, epigenetics, and patterns of short-term change in peptides and steroids. While many factors contribute to the expression of social behaviors across species, at present the best studied are variations in steroids and more recently in oxytocin and vasopressin pathways. Comparative analyses, especially in closely related yet behavioral distinct species, have been useful in identifying mechanisms through which evolutionary and proximate pressures could shape the features of social monogamy. Each of the social behaviors reviewed here, as well as several others related to social monogamy, such as the social regulation of reproduction, are adaptive and exist along a continuum. Where an individual or a species falls on that behavioral continuum is controlled at least in part by peptide and steroid systems and the interactions among these. Further adding to these variations, experiences across the life cycle serve to change and refine these systems. The same hormones necessary for the expression of the features of social monogamy, also are implicated in the development of a nervous system capable of being epigenetically tuned to high levels of sociality.
The full array of unique features seen in social monogamy is observed in only a small fraction of mammalian species. However, understanding these prosocial traits, such as social attachment and parenting, has translational implications for human behavior. A topic as apparently esoteric as social monogamy in voles has uncovered neural systems relevant to the biology of love (Carter, 1998, 2017), and the role of relationships in coping with stress or trauma (Smith and Wang, 2014; Sun et al., 2014; Perkeybile and Carter, 2018). Understanding natural variations, within and across species, provides knowledge that has energized multidisciplinary sciences ranging from ethology and evolution (Ophir et al., 2008, 2012) to molecular biology (Bendesky et al., 2017), to psychology (Feldman, 2017) and emerging fields such as “precision” medicine. Findings arising from the study of socially monogamous mammals have even helped to generate interest in the broad therapeutic usefulness of peptide hormones, such as oxytocin agonists or vasopressin antagonists in the treatment of developmental disorders or other illnesses, such as autism, schizophrenia, and substance abuse (Carter, 2007; Buisman-Pijlman et al., 2014; Pedersen, 2017).
It has been suggested by French et al. (2018) that social monogamy should be treated as a “menu.” However, items on a menu can occur independently. We suggest here that social monogamy is more analogous to a biological “syndrome,” built around a recurrent set of ingredients. The features used to define social monogamy include both behavioral and anatomical traits typically, but not always, occur together. As with other syndromes, the consistent appearance of a pattern of traits suggests common underlying causes which are now being identified.
ACKNOWLEDGMENTS
This review is respectfully dedicated to Lowell Getz, whose persistence in the study of prairie voles and monogamy, has been the gold standard upon which our research is founded. We are grateful to the National Science Foundation and National Institutes of Health, who for over 40 years have been the primary source of funding for this work, including most recently P01 HD075750 (CC) and F32 HD092051 (AP).
Footnotes
Conflict of Interest Statement: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- Albers HE (2015). Species, sex and individual differences in the vasotocin/vasopressin system: relationship to neurochemical signaling in the social behavior neural network. Front. Neuroendocrinol. 36, 49–71. doi: 10.1016/j.yfrne.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, and Wang ZX (2009). Dopamine regulation of social choice in a monogamous rodent species. Front. Behav. Neurosci 3:2009. doi: 10.3389/neuro.08.015.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP (2017). A general theory of sexual differentiation. J. Neurosci. Res 95, 291–300. doi: 10.1002/jnr.23884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrowsmith S, and Wray S (2014). Oxytocin: its mechanism of action and receptor signalling in the myometrium. J. Neuroendocrinol 26, 356–369. doi: 10.1111/jne.12154 [DOI] [PubMed] [Google Scholar]
- Bales KL, Kim AJ, Lewis-Reese AD, and Carter CS (2004a). Both oxytocin and vasopressin may influence alloparental behavior in male prairie voles. Horm. Behav 45, 354–361. doi: 10.1016/j.yhbeh.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Bales KL, Lewis-Reese AD, Pfeifer LA, Kramer KM, and Carter CS (2007a). Early experience affects the traits of monogamy in a sexually dimorphic manner. Dev. Psychobiol 49, 335–342. doi: 10.1002/dev.20216 [DOI] [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM, Conley OG, Lee MH, Guoynes CD, Downing GM, et al. (2013). Chronic intranasal oxytocin causes long-term impairments in partner preference formation in male prairie voles. Biol. Psychiatry 74, 180–188. doi: 10.1016/j.biopsych.2012.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Pfeifer LA, and Carter CS (2004b). Sex differences and developmental effects of manipulations of oxytocin on alloparenting and anxiety in prairie voles. Dev. Psychobiol 44, 123–131. doi: 10.1002/dev.10165 [DOI] [PubMed] [Google Scholar]
- Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E, et al. (2007b). Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors. Neuroscience 144, 38–45. doi: 10.1016/j.neuroscience.2006.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, and Ball GF (2016). Endocrine and social regulation of adult neurogenesis in songbirds. Front. Neuroendocrinol 41, 3–22. doi: 10.1016/j.yfrne.2016.03.003 [DOI] [PubMed] [Google Scholar]
- Bamshad M, Novak MA, and Devries GJ (1994). Cohabitation alters vasopressin innervation and paternal behavior in prairie voles (Microtus ochrogaster). Physiol. Behav 56, 751–758. doi: 10.1016/0031-9384(94)90238-0 [DOI] [PubMed] [Google Scholar]
- Barash DP, and Lipton JE (2002). The Myth of Monogamy: Fidelity and Infidelity in Animals and People. New York City, NY: Macmillan Publishers. [Google Scholar]
- Barrett CE, Keebaugh AC, Ahern TH, Bass CE, Terwilliger EF, and Young LJ (2013). Variation in vasopressin receptor (Avpr1 a) expression creates diversity in behaviors related to monogamy in prairie voles. Horm. Behav 63, 518–526. doi: 10.1016/j.yhbeh.2013.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK (2015). Antisocial oxytocin: complex effects on social behavior. Curr. Opin. Behav. Sci 6, 174–182. doi: 10.1016/j.cobeha.2015.11.006 [DOI] [Google Scholar]
- Beery AK, Lacey EA, and Francis DD (2008). Oxytocin and vasopressin receptor distributions in a solitary and a social species of tuco-tuco (Ctenomys haigi and Ctenomys sociabilis). J. Compar. Neurol 507, 1847–1859. doi: 10.1002/cne.21638 [DOI] [PubMed] [Google Scholar]
- Bendesky A, Kwon YM, Lassance JM, Lewarch CL, Yao SQ, Peterson BK, et al. (2017). The genetic basis of parental care evolution in monogamous mice. Nature 544: 434. doi: 10.1038/nature22074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bester-Meredith JK, and Marler CA (2003). Vasopressin and the transmission of paternal behavior across generations in mated, cross-fostered Peromyscus mice. Behav. Neurosci 117,455–463. doi: 10.1037/0735-7044.117.3.455 [DOI] [PubMed] [Google Scholar]
- Bester-Meredith JK, Martin PA, and Marler CA (2005). Manipulations of vasopressin alter aggression differently across testing conditions in monogamous and nonmonogamous Peromyscus mice. Aggress. Behav 31, 189–199. doi: 10.1002/ab.20075 [DOI] [Google Scholar]
- Bester-Meredith JK, Young LJ, and Marler CA (1999). Species differences in paternal behavior and aggression in Peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm. Behav 36, 25–38. doi: 10.1006/hbeh.1999.1522 [DOI] [PubMed] [Google Scholar]
- Bodo C, and Rissman EF (2006). New roles for estrogen receptor beta in behavior and neuroendocrinology. Front. Neuroendocrinol 27, 217–232. doi: 10.1016/j.yfrne.2006.02.004 [DOI] [PubMed] [Google Scholar]
- Bowler CM, Cushing BS, and Carter CS (2002). Social factors regulate female-female aggression and affiliation in prairie voles. Physiol. Behav 76, 559–566. doi: 10.1016/S0031-9384(02)00755-2 [DOI] [PubMed] [Google Scholar]
- Brown RE (1986). Social and hormonal factors influencing infanticide and its suppression in adult male long evans rats (rattus rattus). J. Comp. Psychol 100, 155–161. doi: 10.1037/0735-7036.100.2.155 [DOI] [PubMed] [Google Scholar]
- Buisman-Pijlman FTA, Sumracki NM, Gordon JJ, Hull PR, Carter CS, and Tops M (2014). Individual differences underlying susceptibility to addiction: role for the endogenous oxytocin system. Pharmacol. Biochem. Behav 119,22–38. doi: 10.1016/j.pbb.2013.09.005 [DOI] [PubMed] [Google Scholar]
- Burkett JP, and Young LJ (2012). The behavioral, anatomical and pharmacological parallels between social attachment, love and addiction. Psychopharmacology 224, 1–26. doi: 10.1007/s00213-012-2794-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK (2017). Oxytocin and vasopressin: powerful regulators of social behavior. Neuroscientist 23, 517–528. doi: 10.1177/1073858417708284 [DOI] [PubMed] [Google Scholar]
- Carter CS (1992). Oxytocin and sexual behavior. Neurosci. Biobehav. Rev 16, 131–144. doi: 10.1016/S0149-7634(05)80176-9 [DOI] [PubMed] [Google Scholar]
- Carter CS (1998). Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology 23, 779–818. doi: 10.1016/S0306-4530(98)00055-9 [DOI] [PubMed] [Google Scholar]
- Carter CS (2007). Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav. Brain Res 176, 170–186. doi: 10.1016/j.bbr.2006.08.025 [DOI] [PubMed] [Google Scholar]
- Carter CS (2014). Oxytocin pathways and the evolution of human behavior. Annu. Rev. Psychol 65, 17–39. doi: 10.1146/annurev-psych-010213-115110 [DOI] [PubMed] [Google Scholar]
- Carter CS (2017). The role of oxytocin and vasopressin in attachment. Psychodyn. Psychiatry 45, 499–517. doi: 10.1521/pdps.2017.45.4.499 [DOI] [PubMed] [Google Scholar]
- Carter CS, Boone EM, Pournajafi-Nazarloo H, and Bales KL (2009). Consequences of early experiences and exposure to oxytocin and vasopressin are sexually dimorphic. Dev. Neurosci 31, 332–341. doi: 10.1159/000216544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Courtney Devries A, and Getz LL (1995). Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci. Biobehav. Rev 19, 303–314. doi: 10.1016/0149-7634(94)00070-H [DOI] [PubMed] [Google Scholar]
- Carter CS, Devries AC, Taymans SE, Roberts RL, Williams JR, and Getz LL (1997). “Peptides, steroids, and pair bonding,” in Integrative Neurobiology of Affiliation, eds. Carter CS, Lederhendler I, and Kirkpatrick B (Cambridge, MA: MIT Press; ), 260–272. [DOI] [PubMed] [Google Scholar]
- Carter CS, and Getz LL (1993). Monogamy and the prairie vole. Sci. Am 268, 100–106. doi: 10.1038/scientificamerican0693-100 [DOI] [PubMed] [Google Scholar]
- Carter CS, and Keverne EB (2017). “The neurobiology of social affiliation and pair bonding” in Hormones, Brain, and Behavior 3rd ed, eds. Pfaff DW and Joels M (Oxford: Academic Press; ), 117–143. [Google Scholar]
- Carter CS, and Roberts RL (1997). “The psychobiological basis of cooperative breeding in rodents” in Cooperative Breeding in Mammals, eds Solomon NG and French JA. (New York: Cambridge University Press; ), 231–266. [Google Scholar]
- Carter CS, Williams JR, and Witt DM (1990). “The biology of social bonding in a monogamous mammal” in Hormones, Brain, and Behaviour in Vertebrates: Behavioural Activation in Males and Females, Social Interactions and Reproductive Endocrinology, ed Balthazart J (New York, NY: Karger; ), 154–164. [Google Scholar]
- Chini B, Verhage M, and Grinevich V (2017). The action radius of oxytocin release in the mammalian cns: from single vesicles to behavior. Trends Pharmacol. Sci 38, 982–991. doi: 10.1016/j.tips.2017.08.005 [DOI] [PubMed] [Google Scholar]
- Cho MM, Devries AC, Williams JR, and Carter CS (1999). The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behav. Neurosci 113, 1071–1079. doi: 10.1037/0735-7044.113.5.1071 [DOI] [PubMed] [Google Scholar]
- Choleris E, Devidze N, Kavaliers M, and Pfaff DW (2008). “Steroidal/neuropeptide interactions in hypothalamus and amygdala related to social anxiety,” in Advances in Vasopressin and Oxytocin: From Genes to Behaviour to Disease, eds. Neumann ID and Landgraf R (Amsterdam: Elsevier Science; ), 291–303. [Google Scholar]
- Chrousos GP, Renquist D, Brandon D, Eil C, Pugeat M, Vigersky R, et al. (1982). Glucocorticoid hormone resistance during primate evolution - receptor-mediated mechanisms. Proc. Natl. Acad. Sci. U.S.A. Biol. Sci 79, 2036–2040. doi: 10.1073/pnas.79.6.2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MM, and Galef BG (1999). A testosterone-mediated trade-off between parental and sexual effort in male mongolian gerbils (Meriones unguiculatus). J. Comp. Psychol 113, 388–395. doi: 10.1037/0735-7036.113.4.388 [DOI] [PubMed] [Google Scholar]
- Cushing BS (2016). Estrogen receptor alpha distribution and expression in the social neural network of monogamous and polygynous peromyscus. PLoS ONE 11:e0150373. doi: 10.1371/journal.pone.0150373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing BS, and Kramer KM (2005). Mechanisms underlying epigenetic effects of early social experience: the role of neuropeptides and steroids. Neurosci. Biobehav. Rev 29, 1089–1105. doi: 10.1016/j.neubiorev.2005.04.001 [DOI] [PubMed] [Google Scholar]
- Cushing BS, Okorie U, and Young LJ (2003). The effects of neonatal castration on the subsequent behavioural response to centrally administered arginine vasopressin and the expression of V-1a receptors in adult male prairie voles. J. Neuroendocrinol 15, 1021–1026. doi: 10.1046/j.1365-2826.2003.01097.x [DOI] [PubMed] [Google Scholar]
- Cushing BS, and Wynne-Edwards KE (2006). Estrogen receptor-alpha distribution in male rodents is associated with social organization. J. Compar. Neurol 494, 595–605. doi: 10.1002/cne.20826 [DOI] [PubMed] [Google Scholar]
- Danielson BJ, and Gaines MS (1987). Spatial patterns in 2 syntopic species of microtines - microtus ochrogaster and Synaptomys cooperi. J. Mammal 68, 313–322. doi: 10.2307/1381470 [DOI] [Google Scholar]
- Del Razo RA, and Bales KL (2016). Exploration in a dispersal task: effects of early experience and correlation with other behaviors in prairie voles (Microtus ochrogaster). Behav. Processes 132, 66–75. doi: 10.1016/j.beproc.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, DeVries MB, Taymans S, Carter CS (1995). Modulation of pair bonding in female prairie voles (Microtus ochrogaster) by corticosterone. Proc. Natl. Acad. Sci. U.S.A 92, 7744–7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Devries MB, Taymans SE, and Carter CS (1996). The effects of stress on social preferences are sexually dimorphic in prairie voles. Proc. Natl. Acad. Sci. U.S.A 93, 11980–11984. doi: 10.1073/pnas.93.21.11980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewsbury DA (1987). The comparative psychology of monogamy. Nebraska Symposium Motiv. 35, 1–50. [PubMed] [Google Scholar]
- Dluzen DE, Ramirez VD, Carter CS, and Getz LL (1981). Male vole urine changes luteinizing hormone releasing hormone and norepinephrine in female olfactory bulb. Science 212, 573–575. doi: 10.1126/science.7010608 [DOI] [PubMed] [Google Scholar]
- Duclot F, Wang H, Youssef C, Liu Y, Wang Z, and Kabbaj M (2016). Trichostatin A (TSA) facilitates formation of partner preference in male prairie voles (Microtus ochrogaster). Horm. Behav 81, 68–73. doi: 10.1016/j.yhbeh.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwood RW (1977). Changes in responses of male and female gerbils (meriones unguiculatus) towards test pups during pregnancy of female. Anim. Behav 25, 46–51. doi: 10.1016/0003-3472(77)90066-5 [DOI] [Google Scholar]
- Emlen ST, and Oring LW (1977). Ecology, sexual selection, and evolution of mating systems. Science 197, 215–223. doi: 10.1126/science.327542 [DOI] [PubMed] [Google Scholar]
- Feldman R (2017). The neurobiology of human attachments. Trends Cogn. Sci 21,80–99. doi: 10.1016/j.tics.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Ferris CF, Albers HE, Wesolowski SM, Goldman BD, and Luman SE (1984). Vasopressin injected into the hypothalamus triggers a stereotypic behavior in golden-hamsters. Science 224, 521–523. doi: 10.1126/science.6538700 [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Koppel G, Perry KW, Fuller RW, and Delville Y (1997). Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J. Neurosci 17, 4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, and Potegal M (1988). Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiol. Behav 44, 235–239. doi: 10.1016/0031-9384(88)90144-8 [DOI] [PubMed] [Google Scholar]
- Firestone KB, Thompson KV, and Carter CS (1991). Female-female interactions and social stress in prairie voles. Behav. Neural Biol 55, 31–41. doi: 10.1016/0163-1047(91)80125-X [DOI] [PubMed] [Google Scholar]
- Fleming AS, Corter C, Stallings J, and Steiner M (2002). Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Horm. Behav 42, 399–413. doi: 10.1006/hbeh.2002.1840 [DOI] [PubMed] [Google Scholar]
- Fleming AS, O’day DH, and Kraemer GW (1999). Neurobiology of mother-infant interactions: experience and central nervous system plasticity across development and generations. Neurosci. Biobehav. Rev 23, 673–685. doi: 10.1016/S0149-7634(99)00011-1 [DOI] [PubMed] [Google Scholar]
- French JA, Cavanaugh J, Mustoe AC, Carp SB, and Womack SL (2018). Social monogamy in nonhuman primates: phylogeny, phenotype, and physiology. J. Sex Res 55,410–434. doi: 10.1080/00224499.2017.1339774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavish L, Carter CS, and Getz LL (1983). Male-female interactions in prairie voles. Anim. Behav 31, 511–517. doi: 10.1016/S0003-3472(83)80073-6 [DOI] [Google Scholar]
- Getz LL (1985). “Microtus habitats,” in Biology of Microtus, ed Tamarin RH (Stillwater, OK: Americal Society of Mammologists; ), 286–309. [Google Scholar]
- Getz LL, and Carter CS (1980). Social organization in microtus ochrogaster populations. Biologist 62, 56–69. [Google Scholar]
- Getz LL, and Carter CS (1996). Prairie-vole partnerships. Am. Sci 84, 56–62. [Google Scholar]
- Getz LL, Carter CS, and Gavish L (1981). The mating system of the prairie vole, Microtus ochrogaster - field and laboratory evidence for pair bonding. Behav. Ecol. Sociobiol 8, 189–194. doi: 10.1007/BF00299829 [DOI] [Google Scholar]
- Getz LL, Mcguire B, Pizzuto T, Hofmann JE, and Frase B (1993). Social organization of the prairie vole (Microtus ochrogaster). J. Mammal 74, 44–58. doi: 10.2307/1381904 [DOI] [Google Scholar]
- Gobrogge KL, Liu Y, Jia S, and Wang Z (2007). Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J. Comp. Neurol 502, 1109–1122. doi: 10.1002/cne.21364 [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Young LJ, and Wang ZX (2009). Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc. Natl. Acad. Sci. U.S.A 106, 19144–19149. doi: 10.1073/pnas.0908620106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, and Kingsbury MA (2013). What’s in a name? Considerations of homologies and nomenclature for vertebrate social behavior networks. Hormones Behav. 64, 103–112. doi: 10.1016/j.yhbeh.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb B, Beitel LK, Nadarajah A, Paliouras M, and Trifiro M (2012). The androgen receptor gene mutations database: 2012 update. Hum. Mutat 33, 887–894. doi: 10.1002/humu.22046 [DOI] [PubMed] [Google Scholar]
- Gowaty PA (1996). “Battles of the sexes and origins of monogamy” in Partnerships in Birds, ed. Black JM (Oxford: Oxford University Press; ), 21–52. [Google Scholar]
- Grinevich V, Knobloch-Bollmann HS, Eliava M, Busnelli M, and Chini B (2016). Assembling the puzzle: pathways of oxytocin signaling in the brain. Biol. Psychiatry 79, 155–164. doi: 10.1016/j.biopsych.2015.04.013 [DOI] [PubMed] [Google Scholar]
- Gubernick DJ, Schneider KA, and Jeannotte LA (1994). Individual differences in the mechanisms underlying the onset and maintenance of paternal behavior and the inhibition of infanticide in the monogamous biparental California mouse, Peromyscus californicus. Behav. Ecol. Sociobiol 34, 225–231. doi: 10.1007/BF00167748 [DOI] [Google Scholar]
- Hrdy SB (2009). Mothers and Others: The Evolutionary Origins of Mutual Understanding. Cambridge, MA: Harvard University Press. [Google Scholar]
- Hume JM, and Wynne-Edwards KE (2005). Castration reduces male testosterone, estradiol, and territorial aggression, but not paternal behavior in biparental dwarf hamsters (Phodopus campbelli). Horm. Behav 48, 303–310. doi: 10.1016/j.yhbeh.2005.04.001 [DOI] [PubMed] [Google Scholar]
- Hurlemann R, and Grinevich V (2018). Behavioral Pharmacology of Neuropeptides: Oxytocin. New York City, NY: Springer International Publishing. doi: 10.1007/978-3-319-63739-6 [DOI] [Google Scholar]
- Insel TR, Gelhard R, and Shapiro LE (1991). The comparative distribution of forebrain receptors for neurohypophyseal peptides in monogamous and polygamous mice. Neuroscience 43, 623–630. doi: 10.1016/0306-4522(91)90321-E [DOI] [PubMed] [Google Scholar]
- Insel TR, Preston S, and Winslow JT (1995). Mating in the monogamous male - behavioral consequences. Physiol. Behav 57, 615–627. doi: 10.1016/0031-9384(94)00362-9 [DOI] [PubMed] [Google Scholar]
- Insel TR, and Shapiro LE (1992). Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc. Natl. Acad. Sci. U.S.A 89, 5981–5985. doi: 10.1073/pnas.89.13.5981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Wang ZX, and Ferris CF (1994). Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J. Neurosci 14, 5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys AJ, Wilson V, and Thein SL (1985). Individual-specific fingerprints of human DNA. Nat. 316, 76–79. doi: 10.1038/316076a0 [DOI] [PubMed] [Google Scholar]
- Jurek B, and Neumann ID (2018). The oxytocin receptor: from intracellular signaling to behavior. Physiol. Rev 98, 1805–1908. doi: 10.1152/physrev.00031.2017 [DOI] [PubMed] [Google Scholar]
- Keebaugh AC, Barrett CE, Laprairie JL, Jenkins JJ, and Young LJ (2015). RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Soc. Neurosci 10, 561–570. doi: 10.1080/17470919.2015.1040893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh AC, and Young LJ (2011). Increasing oxytocin receptor expression in the nucleus accumbens of pre-pubertal female prairie voles enhances alloparental responsiveness and partner preference formation as adults. Horm. Behav 60,498–504. doi: 10.1016/j.yhbeh.2011.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel WM, Paredes J, Yee JR, Pournajafi-Nazarloo H, Bales KL, and Carter CS (2012). Neuroendocrine and behavioural responses to exposure to an infant in male prairie voles. J. Neuroendocrinol 24, 874–886. doi: 10.1111/j.1365-2826.2012.02301.x [DOI] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V, Wolf L, and Ziegenfus C (1992). Testosterone and avian life histories - effects of experimentally elevated testosterone on behavior and correlates of fitness in the dark-eyed junco (Junco hyemalis). Am. Nat 140, 980–999. doi: 10.1086/285451 [DOI] [Google Scholar]
- Keverne EB (2013). Importance of the matriline for genomic imprinting, brain development and behaviour. Philos. Trans. R. Soc. Lond. B Biol. Sci 368:20110327. doi: 10.1098/rstb.2011.0327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keverne EB, and Kendrick KM (1992). Oxytocin facilitation of maternal behavior in sheep. Ann. N. Y. Acad. Sci 652, 83–101. doi: 10.1111/j.1749-6632.1992.tb34348.x [DOI] [PubMed] [Google Scholar]
- King LB, Walum H, Inoue K, Eyrich NW, and Young LJ (2016). Variation in the oxytocin receptor gene predicts brain region-specific expression and social attachment. Biol. Psychiatry 80, 160–169. doi: 10.1016/j.biopsych.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsley CH, and Bridges RS (1988). Prenatal stress and maternal behavior in intact virgin rats: response latencies are decreased in males and increased in females. Horm. Behav 22, 76–89. doi: 10.1016/0018-506X(88)90032-3 [DOI] [PubMed] [Google Scholar]
- Kleiman DG (1977). Monogamy in mammals. Quart. Rev. Biol 52, 39–69. doi: 10.1086/409721 [DOI] [PubMed] [Google Scholar]
- Klein SL, and Nelson RJ (1997). Sex differences in immunocompetence differ between two Peromyscus species. Am. J. Physiol. Regul. Integr. Compar. Physiol 273, R655–R660. doi: 10.1152/ajpregu.1997.273.2.R655 [DOI] [PubMed] [Google Scholar]
- Klopfer PH(1971). Mother love - what turns it on. Am. Sci 59:404. [PubMed] [Google Scholar]
- Klug H (2018). Why monogamy? A review of potential ultimate drivers. Front. Ecol. Evol 6:30. doi: 10.3389/fevo.2018.00030 [DOI] [Google Scholar]
- Komers PE, and Brotherton PNM (1997). Female space use is the best predictor of monogamy in mammals. Proc. R. Soc. B Biol. Sci 264, 1261–1270. doi: 10.1098/rspb.1997.0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer KM, Cushing BS, Carter CS, Wu J, and Ottinger MA (2004). Sex and species differences in plasma oxytocin using an enzyme immunoassay. Can. J. Zool 82, 1194–1200. doi: 10.1139/z04-098 [DOI] [Google Scholar]
- Lacey EA, Braude SH, and Wieczorek JR (1997). Burrow sharing by colonial tuco-tucos (Ctenomys sociabilis). J. Mammal 78, 556–562. doi: 10.2307/1382907 [DOI] [Google Scholar]
- Lacey EA, Braude SH, and Wieczorek JR (1998). Solitary burrow use by adult Patagonian tuco-tucos (Ctenomys haigi). J. Mammal 79, 986–991. doi: 10.2307/1383106 [DOI] [Google Scholar]
- Lack D (1968). Ecological Adaptations for Breeding in Birds. Methuen, MA: Chapman and Hall. [Google Scholar]
- Landgraf R, Frank E, Aldag JM, Neumann ID, Sharer CA, Ren XR, et al. (2003).Viral vector-mediated gene transfer of the vole V1a vasopressin receptor in the rat septum: improved social discrimination and active social behaviour. Eur. J. Neurosci 18,403–411. doi: 10.1046/j.1460-9568.2003.02750.x [DOI] [PubMed] [Google Scholar]
- Lansing SW, French JA, and Lonstein JS (2013). Circulating plasma testosterone during early neonatal life in the socially monogamous and biparental prairie vole (Microtus ochrogaster). Psychoneuroendocrinology 38, 309. doi: 10.1016/j.psyneuen.2012.06.003 [DOI] [PubMed] [Google Scholar]
- Lim MM, Wang ZX, Olazabal DE, Ren XH, Terwilliger EF, and Young LJ (2004). Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 429, 754–757. doi: 10.1038/nature02539 [DOI] [PubMed] [Google Scholar]
- Lim MM, and Young LJ (2004). Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience 125, 35–45. doi: 10.1016/j.neuroscience.2003.12.008 [DOI] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, and Wang ZX (2001). Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster). Behav. Neurosci 115, 910–919. doi: 10.1037/0735-7044.115.4.910 [DOI] [PubMed] [Google Scholar]
- Liu Y, and Wang ZX (2003). Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121, 537–544. doi: 10.1016/S0306-4522(03)00555-4 [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Rood BD, and De Vries GJ (2005). Unexpected effects of perinatal gonadal hormone manipulations on sexual differentiation of the extrahypothalamic arginine-vasopressin system in prairie voles. Endocrinology 146, 1559–1567. doi: 10.1210/en.2004-1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas D, and Clutton-Brock TH (2013). The evolution of social monogamy in mammals. Science 341, 526–530. doi: 10.1126/science.1238677 [DOI] [PubMed] [Google Scholar]
- Maclean PD (1990). The Triune Brain in Evolution: Role in Paleocerebral Function. New York City, NY: Springer. [Google Scholar]
- Marler CA, Bester-Meredith JK, and Trainor BC (2003). “Paternal Behavior and aggression: Endocrine mechanisms and nongenomic transmission of behavior,” in Advances in the Study of Behavior, Vol 32, eds Slater PJB, Rosenblatt JS, Snowdon CT, and Roper TJ (Cambridge, MA: Academic Press; ), 263–323. [Google Scholar]
- Mokkonen M, and Crespi BJ (2015). Genomic conflicts and sexual antagonism in human health: insights from oxytocin and testosterone. Evol. Appl 8, 325. doi: 10.1111/eva.12244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, et al. (2015). Brain feminization requires active repression of masculinization via DNA methylation. Nat. Neurosci 18:690. doi: 10.1038/nn.3988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes S, Fite JE, Patera KJ, and French JA (2001). Interactions among paternal behavior, steroid hormones, and parental experience in male marmosets (Callithrix kuhlii). Horm. Behav 39,70–82. doi: 10.1006/hbeh.2000.1631 [DOI] [PubMed] [Google Scholar]
- Okeigwe I, and Kuohung W (2014). 5-Alpha reductase deficiency: a 40-year retrospective review. Curr. Opin. Endocrinol. Diabetes Obesity 21, 483–487. doi: 10.1097/MED.0000000000000116 [DOI] [PubMed] [Google Scholar]
- Olazabal DE, and Young LJ (2006a). Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience 141, 559–568. doi: 10.1016/j.neuroscience.2006.04.017 [DOI] [PubMed] [Google Scholar]
- Olazabal DE, and Young LJ (2006b). Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Horm. Behav 49, 681–687. doi: 10.1016/j.yhbeh.2005.12.010 [DOI] [PubMed] [Google Scholar]
- Ophir AG, Gessel A, Zheng DJ, and Phelps SM (2012). Oxytocin receptor density is associated with male mating tactics and social monogamy. Horm. Behav 61,445–453. doi: 10.1016/j.yhbeh.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir AG, Phelps SM, Sorin AB, and Wolff JO (2007). Morphological, genetic and behavioral comparisons of two prairie vole populations in the field and laboratory. J. Mammal 88, 989–999. doi: 10.1644/06-MAMM-A-250R.1 [DOI] [Google Scholar]
- Ophir AG, Wolff JO, and Phelps SM (2008). Variation in neural V1aR predicts sexual fidelity and space use among male prairie voles in semi-natural settings. Proc. Natl. Acad. Sci. U.S.A 105, 1249–1254. doi: 10.1073/pnas.0709116105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir AG, Zheng DJ, Eans S, and Phelps SM (2009). Social investigation in a memory task relates to natural variation in septal expression of oxytocin receptor and vasopressin receptor 1a in prairie voles (Microtus ochrogaster). Behav. Neurosci 123, 979–991. doi: 10.1037/a0016663 [DOI] [PubMed] [Google Scholar]
- Parker KJ, and Lee TM (2003). Female meadow voles (Microtus pennsylvanicus) demonstrate same-sex partner preferences. J. Comp. Psychol 117,283–289. doi: 10.1037/0735-7036.117.3.283 [DOI] [PubMed] [Google Scholar]
- Pedersen CA (2017). “Oxytocin, tolerance, and the dark side of addiction,” in Role of Neuropeptides in Addiction and Disorders of Excessive Consumption, ed Thiele TE (Cambridge, MA: Academic Press; ), 239–274. doi: 10.1016/bs.irn.2017.08.003 [DOI] [PubMed] [Google Scholar]
- Pedersen CA, and Prange AJ (1979). Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc. Natl. Acad. Sci. U.S.A 76, 6661–6665. doi: 10.1073/pnas.76.12.6661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile AM, and Bales KL (2017). Intergenerational transmission of sociality: the role of parents in shaping social behavior in monogamous and non-monogamous species. J. Exp. Biol 220, 114–123. doi: 10.1242/jeb.142182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile AM, and Carter CS (2018). “Oxytocin and attachment: clinical implications for mood disorders,” in International Handbook of Social Neuroendocrinology, eds Schultheiss OC and Mehta PH (Abingdon: Routledge; ), 674–693. [Google Scholar]
- Perkeybile AM, Carter CS, Wroblewski KL, Puglia MH, Kenkel WM, Lillard TS, et al. (2018). Early nurture epigenetically tunes the oxytocin receptor. Psychoneuroendocrinology 99, 128–136. doi: 10.1016/j.psyneuen.2018.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile AM, Griffin LL, and Bales KL (2013). Natural variation in early parental care correlates with social behaviors in adolescent prairie voles (Microtus ochrogaster). Front. Behav. Neurosci 7:21. doi: 10.3389/fnbeh.2013.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrigo G, Belvin L, and Saal FSV (1991). Individual variation in the neural timing of infanticide and parental behavior in male house mice. Physiol. Behav 50, 287–296. doi: 10.1016/0031-9384(91)90068-Y [DOI] [PubMed] [Google Scholar]
- Pfaff DW, and Baum MJ (2018). Hormone-dependent medial preoptic/lumbar spinal cord/autonomic coordination supporting male sexual behaviors. Mol. Cell. Endocrinol 15:2130. doi: 10.1016/j.mce.2017.10.018 [DOI] [PubMed] [Google Scholar]
- Pitkow LJ, Sharer CA, Ren XL, Insel TR, Terwilliger EF, and Young LJ (2001). Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J. Neurosci 21,7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW (1998). Love: an emergent property of the mammalian autonomic nervous system. Psychoneuroendocrinology 23, 837–861. doi: 10.1016/S0306-4530(98)00057-2 [DOI] [PubMed] [Google Scholar]
- Potegal M, and Ferris CF (1989). Intraspecific aggression in male hamsters is inhibited by intrahypothalamic vasopressin receptor antagonist. Aggress. Behav 15, 311–320. doi: 10.1002/ab.2480150406 [DOI] [Google Scholar]
- Reburn CJ, and Wynne-Edwards KE (1999). Hormonal changes in males of a naturally biparental and a uniparental mammal. Horm. Behav 35, 163–176. doi: 10.1006/hbeh.1998.1509 [DOI] [PubMed] [Google Scholar]
- Rilling JK (2013). The neural and hormonal bases of human parental care. Neuropsychologia 51,731–747. doi: 10.1016/j.neuropsychologia.2012.12.017 [DOI] [PubMed] [Google Scholar]
- Roberts RL, Williams JR, Wang AK, and Carter CS (1998). Cooperative breeding and monogamy in prairie voles: influence of the sire and geographical variation. Anim. Behav 55, 1131–1140. doi: 10.1006/anbe.1997.0659 [DOI] [PubMed] [Google Scholar]
- Rosenberg KM, Denenberg VH, Zarrow MX, and Frank BL (1971). Effects of neonatal castration and testosterone on rats pup-killing behavior and activity. Physiol. Behav 7:363. doi: 10.1016/0031-9384(71)90315-5 [DOI] [PubMed] [Google Scholar]
- Rosenberg KM, and Sherman GF (1975). Influence of testosterone on pup killing in rat is modified by prior experience. Physiol. Behav 15, 669–672. doi: 10.1016/0031-9384(75)90117-1 [DOI] [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, et al. (2009a). Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience 162, 892–903. doi: 10.1016/j.neuroscience.2009.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren XH, Terwilliger EF, and Young LJ (2009b). Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J. Neurosci 29, 1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schum JE, and Wynne-Edwards KE (2005). Estradiol and progesterone in paternal and non-paternal hamsters (Phodopus) becoming fathers: conflict with hypothesized roles. Horm. Behav 47, 410–418. doi: 10.1016/j.yhbeh.2004.11.009 [DOI] [PubMed] [Google Scholar]
- Scordalakes EM, Imwalle DB, and Rissman EF (2002). Oestrogen’s masculine side: mediation of mating in male mice. Reproduction 124, 331–338. doi: 10.1530/rep.0.1240331 [DOI] [PubMed] [Google Scholar]
- Scordalakes EM, and Rissman EF (2004). Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor alpha. Genes Brain Behav. 3, 20–26. doi: 10.1111/j.1601-183X.2004.00036.x [DOI] [PubMed] [Google Scholar]
- Shapiro LE, and Dewsbury DA (1990). Differences in affiliative behavior, pair bonding, and vaginal cytology in 2 species of vole (Microtus ochrogaster and Microtus montanus). J. Comp. Psychol 104, 268–274. doi: 10.1037/0735-7036.104.3.268 [DOI] [PubMed] [Google Scholar]
- Smith AS, and Wang Z (2014). Hypothalamic oxytocin mediates social buffering of the stress response. Biol. Psychiatry 76, 281–288. doi: 10.1016/j.biopsych.2013.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon NG, and French JA (1997). Cooperative Breeding in Mammals. New York, NY: Cambridge University Press. [Google Scholar]
- Solomon NG, and Jacquot JJ (2002). Characteristics of resident and wandering prairie voles, Microtus ochrogaster. Canad. J. Zool 80, 951–955. doi: 10.1139/z02-053 [DOI] [Google Scholar]
- Solomon NG, Keane B, Knoch LR, and Hogan PJ (2004). Multiple paternity in socially monogamous prairie voles (Microtus ochrogaster). Canad. J. Zool. Rev. Canad. Zool 82, 1667–1671. doi: 10.1139/z04-142 [DOI] [Google Scholar]
- Stalling DT (1990). Microtus ochrogaster. Mammal. Species 355, 1–9. doi: 10.2307/3504103 [DOI] [Google Scholar]
- Storey AE, Walsh CJ, Quinton RL, and Wynne-Edwards KE (2000). Hormonal correlates of paternal responsiveness in new and expectant fathers. Evol. Hum. Behav 21,79–95. doi: 10.1016/S1090-5138(99)00042-2 [DOI] [PubMed] [Google Scholar]
- Sun P, Smith AS, Lei K, Liu Y, and Wang Z (2014). Breaking bonds in male prairie voles: long-term effects on emotional and social behavior, physiology, and neurochemistry. Behav. Brain Res 265, 22–31. doi: 10.1016/j.bbr.2014.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svare B, and Mann M (1981). Infanticide - genetic, developmental and hormonal influences in mice. Physiol. Behav 27,921–927. doi: 10.1016/0031-9384(81)90062-7 [DOI] [PubMed] [Google Scholar]
- Swihart RK, and Slade NA (1989). Differences in home range size between sexes of Microtus ochrogaster. J. Mammal 70, 816–820. doi: 10.2307/1381718 [DOI] [Google Scholar]
- Taymans SE, Devries AC, Devries MB, Nelson RJ, Friedman TC, Castro M, et al. (1997). The hypothalamic-pituitary-adrenal axis of prairie voles (Microtus ochrogaster): evidence for target tissue glucocorticoid resistance. Gen. Comp. Endocrinol 106, 48–61. doi: 10.1006/gcen.1996.6849 [DOI] [PubMed] [Google Scholar]
- Trainor BC, and Marler CA (2001). Testosterone, paternal behavior, and aggression in the monogamous California mouse (Peromyscus californicus). Horm. Behav 40, 32–42. doi: 10.1006/hbeh.2001.1652 [DOI] [PubMed] [Google Scholar]
- Trainor BC, and Marler CA (2002). Testosterone promotes paternal behaviour in a monogamous mammal via conversion to oestrogen. Proc. R. Soc. Biol. Sci 269, 823–829. doi: 10.1098/rspb.2001.1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Anders SM, Goldey KL, and Kuo PX (2011). The steroid/peptide theory of social bonds: integrating testosterone and peptide responses for classifying social behavioral contexts. Psychoneuroendocrinology 36, 1265–1275. doi: 10.1016/j.psyneuen.2011.06.001 [DOI] [PubMed] [Google Scholar]
- Wang H, Duclot F, Liu Y, Wang ZX, and Kabbaj M (2013). Histone deacetylase inhibitors facilitate partner preference formation in female prairie voles. Nat. Neurosci 16, 919–U184. doi: 10.1038/nn.3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZX, and De Vries GJ (1993). Testosterone effects on paternal behavior and vasopressin immunoreactive projections in prairie voles (microtus ochrogaster). Brain Res. 631, 156–160. doi: 10.1016/0006-8993(93)91203-5 [DOI] [PubMed] [Google Scholar]
- Wang ZX, Hulihan TJ, and Insel TR (1997). Sexual and social experience is associated with different patterns of behavior and neural activation in male prairie voles. Brain Res. 767, 321–332. doi: 10.1016/S0006-8993(97)00617-3 [DOI] [PubMed] [Google Scholar]
- Wang ZX, Liu Y, Young LJ, and Insel TR (2000). Hypothalamic vasopressin gene expression increases in both males and females postpartum in a biparental rodent. J. Neuroendocrinol 12, 111–120. doi: 10.1046/j.1365-2826.2000.00435.x [DOI] [PubMed] [Google Scholar]
- Ward IL, and Ward OB (1985). “Sexual behavior differentiation: effects of prenatal manipulations in rats,” in Handbook of Behavioral Neurobiology, Reproduction, eds Adler N, Pfaff D, and Goy RW (New York, NY: Plenum Press; ), 77–98. [Google Scholar]
- Wickler W, and Seibt U (1983). “Monogamy: An Ambiguous Concept,” in Mate Choice, ed Bateson P (Cambridge: Cambridge University Press; ), 33–50. [Google Scholar]
- Williams JR, Catania KC, and Carter CS (1992).Development of partner preferences in female prairie voles (Microtus ochrogaster) - the role of social and sexual experience. Horm. Behav 26, 339–349. doi: 10.1016/0018-506X(92)90004-F [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, and Carter CS (1994). Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster). J. Neuroendocrinol 6, 247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x [DOI] [PubMed] [Google Scholar]
- Wilson EO (1975). Sociobiology: The New Synthesis. Cambridge, MA: Harvard University Press. [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, and Ball GF (1990). The challenge hypothesis - theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat 136, 829–846. doi: 10.1086/285134 [DOI] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, and Insel TR (1993). A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365, 545–548. doi: 10.1038/365545a0 [DOI] [PubMed] [Google Scholar]
- Witt DM, Carter CS, and Lnsel TR (1991). Oxytocin receptor binding in female prairie voles: endogenous and exogenous oestradiol stimulation. J. Neuroendocrinol 3, 155–161. doi: 10.1111/j.1365-2826.1991.tb00258.x [DOI] [PubMed] [Google Scholar]
- Witt DM, Carter CS, and Walton DM (1990). Central and peripheral effects of oxytocin administration in prairie voles (Microtus ochrogaster). Pharmacol. Biochem. Behav. 37, 63–69. doi: 10.1016/0091-3057(90)90042-G [DOI] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, and Wang ZX (2011). The neurobiology of pair bonding: insights from a socially monogamous rodent. Front. Neuroendocrinol 32, 53–69. doi: 10.1016/j.yfrne.2010.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, and Insel TR (2001). Cellular mechanisms of social attachment. Horm. Behav 40, 133–138. doi: 10.1006/hbeh.2001.1691 [DOI] [PubMed] [Google Scholar]
- Yuan L, Liu S, Bai XM, Gao Y, Liu GH, Wang XE, et al. (2016). Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. J. Neuroinflamm 13:7. doi: 10.1186/s12974-016-0541-7 [DOI] [PMC free article] [PubMed] [Google Scholar]