Abstract
C–S cross-couplings are an important class of reactions applied across organic synthesis, materials science, and pharma-ceuticals. Several different methodologies have been developed to achieve this significant transformation. However, currently available synthetic procedures significantly rely on transition metals. This article describes historical developments in the field of transition-metal-catalyzed C–S cross-coupling reactions, the development of a visible-light-driven and catalyst-free approach to C–S bond formation, and future outlooks.
Keywords: C–S cross-coupling, transition-metal-free, photochemistry, charge transfer, late-stage functionalization, electron donor–acceptor complex
Graphical Abstract

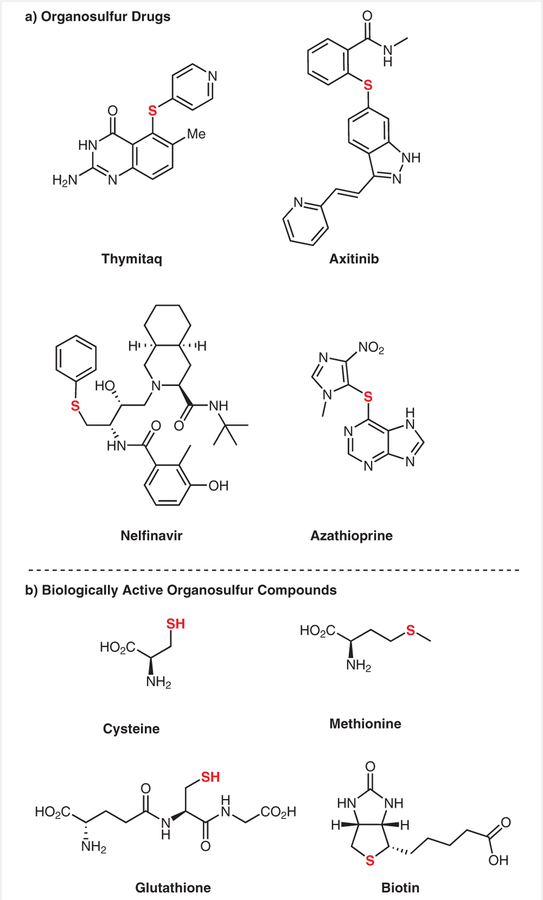
Owing to the ubiquity of aromatic thioethers in pharmaceuticals, natural products, and materials (Figure 1),1 the development of sustainable and efficient procedures for C–S bond formation is a key research goal in synthetic chemistry.2 As such, significant efforts have contributed to the development of a plethora of methodologies that expand the ability of chemists to construct C–S bonds.
Figure 1.
Selected examples of sulfur-containing drugs and biologically active compounds
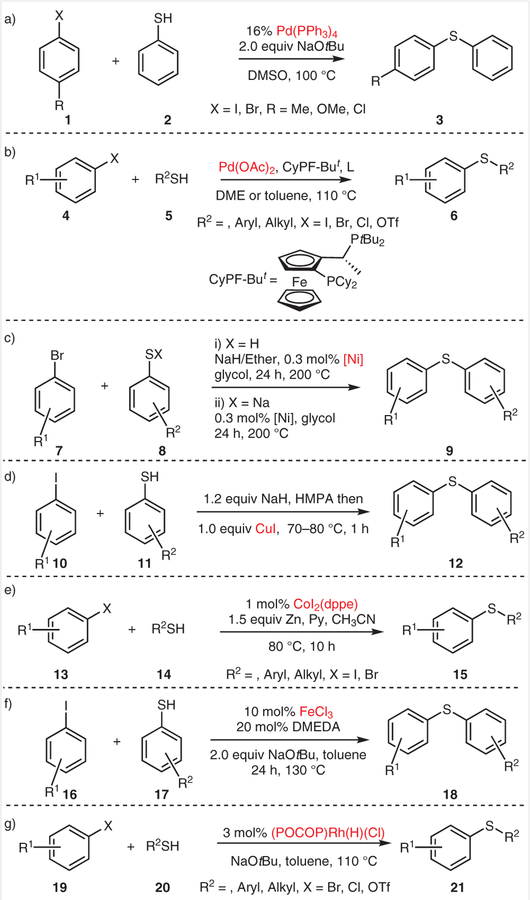
Palladium-catalyzed cross-coupling reactions between aryl iodides and thiophenols, first reported by Migita and co-workers in 1978 (Scheme 1a),3 represents an efficient tool for constructing diaryl thioethers. This method is among the most powerful catalytic C–S bond-forming reactions known and it has enabled tremendous synthetic developments in the pharmaceutical industries.4 In 2004, Murata and Buchwald developed Pd(OAc)2/1,10-bis(diisopropylphosphino) ferrocene (DiPPF) as a catalyst system for the cross-coupling of thiols and aryl halides.5 Both electronrich and sterically hindered aryl halides, as well as primary, secondary, tertiary, and aromatic thiols, were tolerated under the optimized reaction conditions, and gave the desired aromatic thioethers in good yields. Thereafter, Hartwig and co-workers reported an efficient bisphosphine ligand, CyPF-But (Scheme 1b), which made possible the cross-coupling of aryl chlorides or triflates with thiols catalyzed by Pd(OAc)2.6 This system was also expanded for synthesizing unsymmetrical diaryl thioethers with triisopropylsilanethiol (TIP-SH) as the thiol surrogate.7
Scheme 1.
Transition-metal-catalyzed C–S cross-coupling reactions
The nickel-catalyzed synthesis of diaryl sulfides was first demonstrated by Cristau and co-workers using NiBr2 as the precatalyst and o-(Ph2P)2C6H4 as the ligand (Scheme 1c).8 It is worth noting that vinyl aryl sulfides can also be obtained with this catalytic system.9 Cross-coupling reactions between nonactivated aryl iodides and aryl thiols catalyzed by copper were first discovered by Suzuki and coworkers in 1980 (Scheme 1d).10 CuI was used as a catalyst whereas hexamethylphosphoramide (HMPA) was used as the solvent. Copper (I) arenethiolates were proposed as intermediates formed in situ from CuI and arenethiolate ions. In 2002, Kwong and Buchwald reported an ethylene glycol/CuI catalytic system for the cross-coupling reactions of aryl iodides with alkyl or aryl thiols.11 A variety of functional groups, such as free anilino NH2, carboxylic acid, nitro, and free alkylamino groups, were all tolerated under the optimized reaction conditions.
The CoI2(dppe)/Zn catalytic system was first used by the Cheng group for synthesizing aromatic thioethers (Scheme 1e).12 This cross-coupling reaction proceeded readily at 80 °C when CH3CN was used as the solvent. Various (het)aryl iodides or bromides with electron-donating or -withdrawing groups were successfully coupled to diverse aryl or alkyl thiols under the optimized conditions and gave the desired aromatic thioethers in good yields. This method highlights cobalt as a user-friendly, inexpensive, and efficient catalyst for C–S bond formation. However, it suffers from a limited substrate scope (aryl bromides or iodides).
In 2008, Bolm and co-workers developed an approach for the synthesis of aromatic thioethers catalyzed by a combination of FeCl3 and N,N′-Dimethylethylenediamine (DMEDA) (Scheme 1f).13 This new cross-coupling reaction is of great interest because it avoids the use of expensive and/or air-sensitive ligands. However, only aryl iodides were successfully coupled under these reaction conditions.
A well-defined (POCOP)Rh [POCOP = 1,3-C6H4(OPPh2)2] catalyst for the catalytic coupling of aryl bromides or chlorides with aryl or alkyl thiols was demonstrated by Ozerov group (Scheme 1g).14 Both aryl bromides and chlorides were efficiently transformed into aryl thioethers. Primary and secondary alkyl thiols also worked well and reacted faster than aromatic thiols. Each elementary step of this process has been studied in detail and some intermediates involved in the reaction have been isolated; these supported the proposed mechanism of this reaction.
In summary, a variety of transition-metal-catalyzed C–S bond-formation reactions have been developed, however, all the above transition-metal-catalyzed methods require a strong base, specific or air-sensitive ligands, and high temperatures, which might limit their application scope.
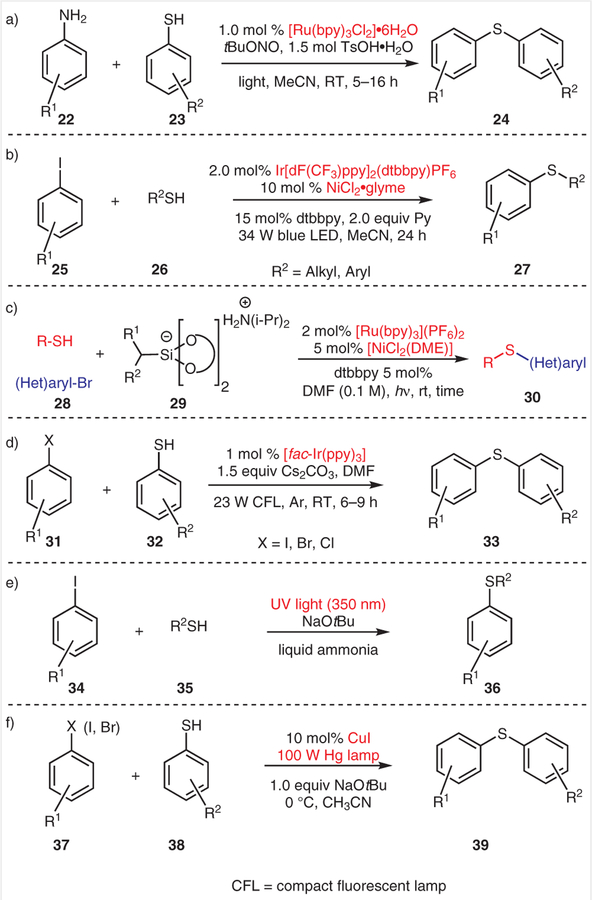
Over the last ten years, photoredox catalysis has emerged as a strategy for the construction of C–C or C– heteroatom bonds under mild conditions, without the need for highly reactive radical initiators.15 Noël and co-workers demonstrated a one-pot Stadler–Ziegler method for producing aromatic thioethers by employing Ru(bpy)3Cl2·6H2O as a photoredox catalyst (Scheme 2a).16 Arylamines containing a phenolic OH, chloro, bromo, or nitrile group were all efficiently converted into the corresponding products. Later, photoinduced Ni-catalyzed cross-coupling of thiols with (het)aryl halides was independently demonstrated by the Johannes group and the Molander group. Johannes and co-workers revealed that a thiyl radical can be generated through light-driven oxidation of a thiol followed by deprotonation of the highly acidic thiol radical cation; the thiyl radical was proposed to be the key intermediate for thioether formation (Scheme 2b).17 A variety of aryl iodides bearing organoboronate, bromide, aldehyde, or nitrile groups were all coupled efficiently with (4-methoxyphenyl) methanethiol. Moreover, thiophenols and alkyl thiols were all tolerated under these mild conditions.
Scheme 2.
Light-driven C–S cross-coupling reactions
Molander and co-workers showed that a hypervalent silicon compound can be oxidized and fragmented to give an alkyl radical that can effectively abstract a hydrogen atom from a thiol, thereby forming a thiyl radical (Scheme 2c).18 The thiyl radical generated in this manner can engage in nickel-catalyzed cross-coupling and yield the thioether product. Notably, a variety of substrates were tolerated under these reaction conditions. For example, halide-containing pyridines, isoquinolines, caffeine, and (R)-Boc-cysteine reacted efficiently with a variety of alkyl thiols.
More recently, Jiang and Fu and their co-workers developed a [fac-Ir(ppy)3]-catalyzed cross-coupling reaction between aryl halides and thiols without the need for a nickel catalyst (Scheme 2d).19 A variety of aryl halides, including aryl iodides, bromides, chlorides, and fluorides, were all efficiently coupled with aryl thiols to afford the thioether products in good to high yields.
Photoredox-catalyst-free, photoinduced C–S cross-coupling reactions have also been investigated. In 1974, a catalyst-free UV-photoinduced coupling of thiophenoxides with aryl iodides in liquid ammonia was reported by Bunnett and Creary (Scheme 2e).20 In 2013, a photoinduced, copper-catalyzed, cross-coupling of aryl thiols with aryl halides was demonstrated by the Fu group (Scheme 2f).21 Aryl thiols bearing methoxy, methyl, or fluoro groups were coupled efficiently with phenyl iodide. Notably, the light-driven C–S cross-coupling reactions highlighted in Schemes 2e and 2f were carried out in the absence of an added photo-redox catalyst to effect electron or energy transfer.
Despite the advances that have been made in aromatic thioether production, these approaches either require UV irradiation or involve the use of transition metals such as Ru, Ni, Ir, or Cu (Scheme 2). Therefore, the development of UV-free and transition-metal-free methods for constructing C–S bonds is desirable.
With the goal of establishing a transition-metal-free C–S bond-formation method using visible-light irradiation, we initially applied strongly reducing N,N-diaryldihydro-phenazines or N-arylphenoxazines as organic photoredox catalysts22 with irradiation by a white light-emitting diode (LED) of a solution containing 4′-bromoacetophenone, 4-methylbenzenethiol, and Cs2CO3 in dimethyl sulfoxide (DMSO). Encouragingly, C–S cross-coupled products were obtained in high yields; however, control experiments re-vealed that the desired product was also isolated in high yield (97%) in the absence of the organic photoredox catalyst after one hour of white LED irradiation at room tem-perature (Table 1, entry 1). This result was apparently due to a novel reaction pathway leading to C–S bond formation (see below).23
Table 1.
Effect of Reaction Parametersa
 | ||||
|---|---|---|---|---|
| Entry | Base (equiv) | Light | Solvent | Yieldb (%) |
| 1 | Cs2CO3 (1.5) | yes | DMSO | 97c |
| 2 | Cs2CO3 (1.5) | no | DMSO | trace |
| 3 | Cs2CO3 (1.0)S | yes | DMSO | 87 |
| 4 | Cs2CO3 (0.5) | yes | DMSO | 12 |
| 5 | Cs2CO3 (0.0) | yes | DMSO | 0 |
| 6 | Cs2CO3 (1.5) | yesd | DMSO | 0 |
| 7 | K2CO3 (1.5) | yes | DMSO | 91 |
| 8 | Na2CO3 (1.5) | yes | DMSO | 24 |
| 9 | Cs2CO3 (1.5) | yes | DMF | 35 |
| 10 | Cs2CO3 (1.5) | yes | CH3CN | 0 |
| 11 | Cs2CO3 (1.5) | yes | DMA | 76 |
The beaker irradiated with the LEDs was cooled to r.t. with compressed air.
NMR yield with trimethoxybenzene 1,3,5-trimethoxybenzene as an inter-nal standard.
Isolated yield.
In air.
Control experiments revealed that visible light was essential for this transformation in the absence of the organic photoredox catalyst. No reactivity was observed in the presence of oxygen (Table 1, entry 6), which is consis-tent with a radical mechanism. Furthermore, highly pure Cs2CO3 (99.995%) and K2CO3 (99.997%) were also investigat-ed, and no difference was observed between these base sources. It is noteworthy that K2CO3, which is less expensive than Cs2CO3, was also an excellent base for this transformation.
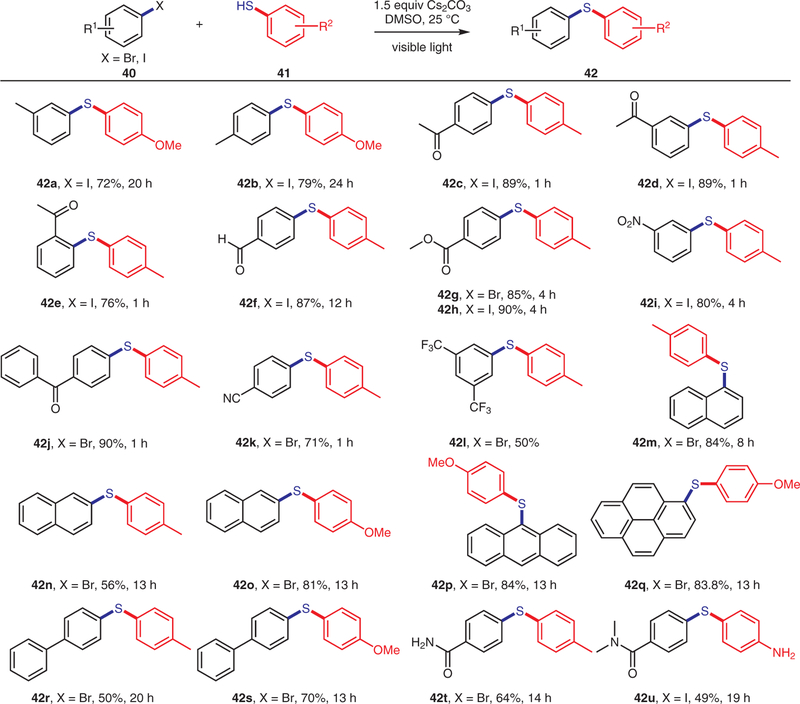
Subsequent investigation of the substrate scope revealed that a variety of aryl iodides and bromides afforded the targeted cross-coupling product in good yields (Scheme 3). Aryl bromides or iodides bearing either electron-donating (42a,b) or electron-withdrawing groups were tolerated to yield the desired biaryl thioether products. Notably, elec-tronic effects on the aryl halides resulted in an obvious difference in reactivity. For example, both longer irradiation times and the use of electron-rich thiophenols were required to obtain appreciable yields for electron-neutral or electron-rich aryl iodides.
Scheme 3.
Scope of aryl bromides and iodides
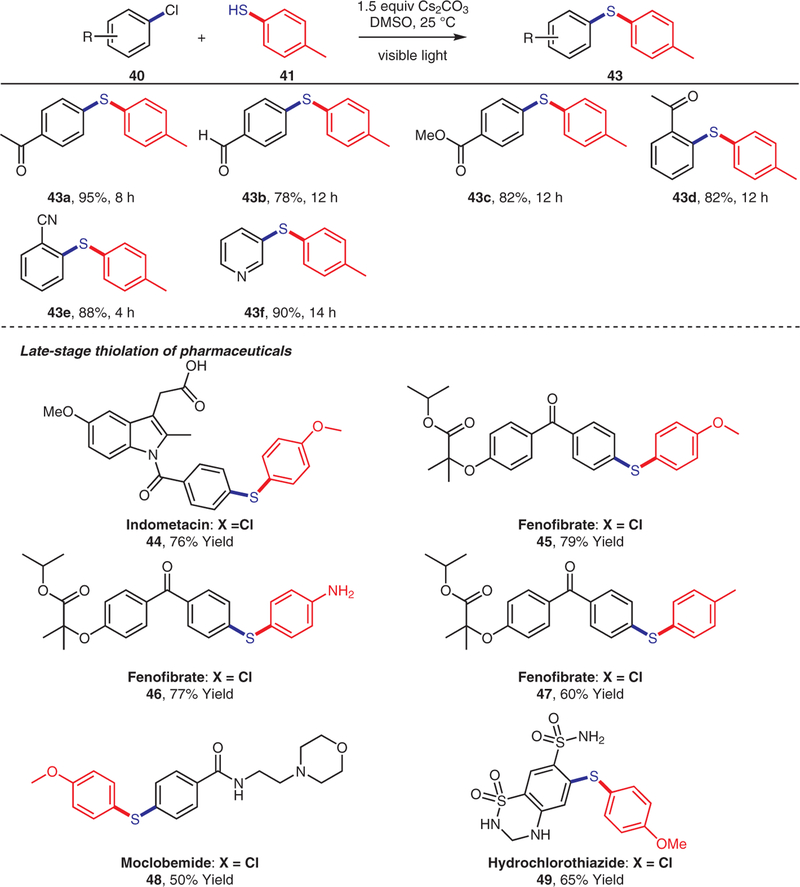
A number of aryl chlorides containing electron-with-drawing groups (43a–e) were tested with 4-methylbenzen-ethiol as the coupling partner, and the corresponding desired products were isolated in good yields (Scheme 4). 3-Chloropyridine (43f) was also a good substrate and afforded the corresponding products in high yields. More-over, when we treated indomethacin (44), fenofibrate (45– 47), moclobemide (48), or hydrochlorothiazide (49) pharmaceutical ingredients (containing an aryl chloride) with thio-phenols under our reaction conditions, we obtained the thiolated compounds in yields of 50–79%.
Scheme 4.
Scope of aryl chlorides and late-stage thiolation of pharmaceuticals
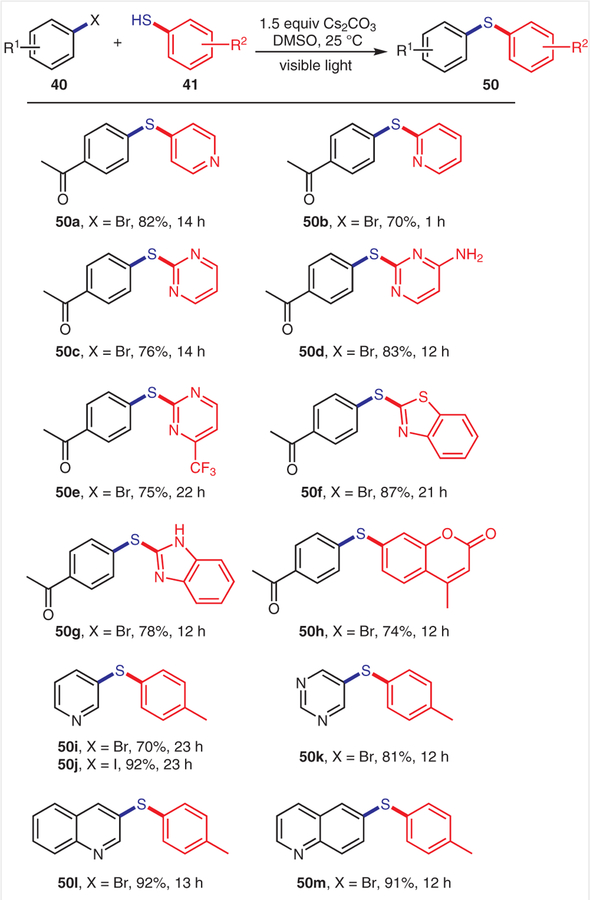
Importantly, a large variety of heterocycles were also tolerated under our reaction conditions (Scheme 5). These include (het)aryl halides (50i–l), mercaptopyridines (50a,b), mercaptopyrimidines (50c–e), 2-mercaptobenzim-idazole (50g), 2-mercaptobenzothiazole (50f), and 7-mercapto-4-methylcoumarin (50h).
Scheme 5.
Scope of (het)arenes
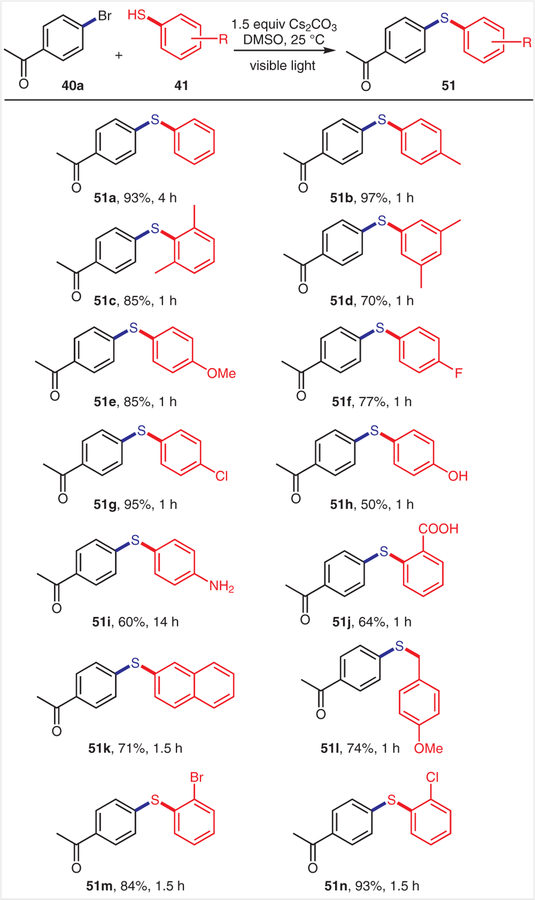
Our additional investigations of the substrate scope focused on aryl thiols (Scheme 6). Aryl thiols bearing free hydroxy, amine, or carboxyl groups were all tolerated under our C–S coupling conditions (51h–j), thus eliminating the need for protecting groups. Thiols bearing fluoro (51f), chloro (51g,n), or bromo groups (51m) were also compatible under the optimized reaction conditions, which might be synthetically useful for further modifications.
Scheme 6.
Scope of thiols
Density functional theory (DFT) calculations, in combination with UV–vis spectroscopic measurements, suggested that the catalyst-free C–S cross-coupling described above is driven by the photochemical activity of electron donor–acceptor (EDA) complexes,24 formed by the aggregation of thiolates and aryl halides. Upon light absorption, an electron is transferred from the electron-rich thiolate anion to the electron-deficient aryl halide to form thiyl and aryl radicals, which subsequently quench to form the aryl thioether product.
Despite a broad substrate scope of the C–S cross-coupling reactions with aromatic thiols, the optimized conditions do not provide C–S formation products and dehalo-genation products with alkyl thiols. We suggest that this problem might be attributed to the hydrogenatom-transfer ability of alkyl thiols. Consequently, the reaction with alkyl thiols requires further work. The evolution of reactions employing alkyl thiols might permit modifications of cysteine-residue-containing proteins or peptides to modulate their physicochemical properties and biological activities. Access to native thioarylated small biomolecules might also become possible with altered selectivity and efficiency.
We envision that this synthetic procedure will find applications in facilitating access to new thioether-containing compounds in pharmaceutical, agrochemical, and materials sciences. Future research efforts are aimed at investigating the scope of alkyl thiols, further understanding of the reac-tion mechanism of this C–S cross-coupling reaction, and extending the methodology for the syntheses of polymers.
Acknowledgments
Funding Information
This work was supported by Colorado State University and the Advanced Research Projects Agency-Energy (DE-AR0000683). Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R35GM119702. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. C.-H.L is grateful for an NIH F32 Postdoctoral Fellowship (F32GM122392).
Biography

Bin Liu (left) is a postdoctoral fellow working in the group of Garret Miyake in the Chemistry Department at the Colorado State University (USA). He received his Ph.D. with Bing-Feng Shi at Zhejiang University (P. R. of China) in 2014.
Chern-Hooi Lim (right) is a NIH Ruth L. Kirschstein postdoctoral fellow, working in the group of Garret Miyake in the Chemistry Department at the Colorado State University (USA). He earned his Ph.D. with Charles Musgrave in the Chemical and Biological Engineering Department at the University of Colorado, Boulder (USA) in 2015.
Garret M. Miyake (center) completed his Ph.D. studies with Eugene Chen at Colorado State University (USA) before conducting postdoctoral research with Robert Grubbs at the California Institute of Technology (USA). The Miyake group currently has research interests focusing on catalysis, organocatalyzed atom-transfer radical polymerization, and the synthesis of block copolymers that self-assemble to photonic crystals. He has been awarded a Sloan Research Fellowship, a Cottrell Scholar Award, and the 2017 American Chemical Society’s Division of Polymer Chemistry Mark Young Scholar Award.
References
- (1).(a) Feng M; Tang B; Liang SH; Jiang X Curr. Top. Med. Chem. (Sharjah, United Arab Emirates) 2016, 16, 1200. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ilardi EA; Vitaku E; Njardarson JT J. Med. Chem 2014, 57, 2832. [DOI] [PubMed] [Google Scholar]
- (2).(a) Song S; Zhang Y; Yeerlan A; Zhu B; Liu J; Jiao N Angew. Chem. Int. Ed 2017, 56, 2487. [DOI] [PubMed] [Google Scholar]; (b) Chauhan P; Mahajan S; Enders D Chem. Rev 2014, 114, 8807. [DOI] [PubMed] [Google Scholar]; (c) Beletskaya IP; Ananikov VP Chem. Rev 2011, 111, 1596. [DOI] [PubMed] [Google Scholar]; (d) Hartwig JF Acc. Chem. Res 2008, 41, 1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Kosugi M; Shimizu T; Migita T Chem. Lett 1978, 7, 13. [Google Scholar]
- (4).Norris T; Leeman K Org. Process Res. Dev 2008, 12, 869. [Google Scholar]
- (5).Murata M; Buchwald SL Tetrahedron 2004, 60, 7397. [Google Scholar]
- (6).Fernández-Rodríguez MA; Shen Q; Hartwig JF J. Am. Chem. Soc 2006, 128, 2180. [DOI] [PubMed] [Google Scholar]
- (7).Fernández-Rodríguez MA; Shen Q; Hartwig JF Chem. Eur. J 2006, 12, 7782. [DOI] [PubMed] [Google Scholar]
- (8).Cristau HJ; Chabaud B; Chêne A; Christol H Synthesis 1981, 892.
- (9).Cristau HJ; Chabaud B; Labaudiniere R; Christol H J. Org. Chem 1986, 51, 875. [Google Scholar]
- (10).Suzuki H; Abe H; Osuka A Chem. Lett 1980, 9, 1363. [Google Scholar]
- (11).Kwong FY; Buchwald SL Org. Lett 2002, 4, 3517. [DOI] [PubMed] [Google Scholar]
- (12).Wong Y-C; Jayanth TT; Cheng C-H Org. Lett 2006, 8, 5613. [DOI] [PubMed] [Google Scholar]
- (13).Correa A; Carril M; Bolm C Angew. Chem. Int. Ed 2008, 47, 2880. [DOI] [PubMed] [Google Scholar]
- (14).Timpa SD; Pell CJ; Ozerov OV J. Am. Chem. Soc 2014, 136, 14772. [DOI] [PubMed] [Google Scholar]
- (15).(a) Nicewicz DA; MacMillan DWC Science 2008, 322, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ischay MA; Anzovino ME; Du J; Yoon TP J. Am. Chem. Soc 2008, 130, 12886. [DOI] [PubMed] [Google Scholar]; (c) Narayanam JMR; Tucker JW; Stephenson CRJ J. Am. Chem. Soc 2009, 131, 8756. [DOI] [PubMed] [Google Scholar]
- (16).Wang X; Cuny GD; Noël T Angew. Chem. Int. Ed 2013, 52, 7860. [DOI] [PubMed] [Google Scholar]
- (17).Oderinde MS; Frenette M; Robbins DW; Aquila B; Johannes JW J. Am. Chem. Soc 2016, 138, 1760. [DOI] [PubMed] [Google Scholar]
- (18).Jouffroy M; Kelly CB; Molander GA Org. Lett 2016, 18, 876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Jiang M; Li H; Yang H; Fu H Angew. Chem. Int. Ed 2017, 56, 874. [DOI] [PubMed] [Google Scholar]
- (20).Bunnett JF; Creary X J. Org. Chem 1974, 39, 3173. [Google Scholar]
- (21).(a) Uyeda C; Tan Y; Fu GC; Peters JC J. Am. Chem. Soc 2013, 135, 9548. [DOI] [PubMed] [Google Scholar]; (b) Johnson MW; Hannoun KI; Tan Y; Fu GC; Peters JC Chem. Sci 2016, 7, 4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).(a) Theriot JC; Lim C-H; Yang H; Ryan MD; Musgrave CB; Miyake GM Science 2016, 352, 1082. [DOI] [PubMed] [Google Scholar]; (b) Pearson RM; Lim C−H; McCarthy BG; Musgrave CB; Miyake GM J. Am. Chem. Soc 2016, 138, 11399. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Du Y; Pearson RM; Lim C-H; Sartor SM; Ryan MD; Yang H; Damrauer NH; Miyake GM Chem. Eur. J 2017, 23, 10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Liu B; Lim C-H; Miyake GM J. Am. Chem. Soc 2017, 139, 13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).For reviews, see: (a) Rosokha SV; Kochi JK Acc. Chem. Res 2008, 41, 641. [DOI] [PubMed] [Google Scholar]; (b) Lima C. G. de S.; Lima T. de. M.; Duarte M; Jurberg ID; Paixão MW ACS Catal 2016, 6, 1389 For exam-ples of visible-light-induced EDA chemistry, see: [Google Scholar]; (c) Arceo E; Jurberg ID; Álvarez-Fernández A; Melchiorre P Nat. Chem 2013, 5, 750. [DOI] [PubMed] [Google Scholar]; (d) Beatty JW; Douglas JJ; Miller R; McAtee RC; Cole KP; Stephenson CRJ Chem 2016, 1, 456. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Deng Y; Wei X-J; Wang H; Sun Y; Nöel T; Wang X Angew. Chem. Int. Ed 2017, 56, 832. [DOI] [PubMed] [Google Scholar]