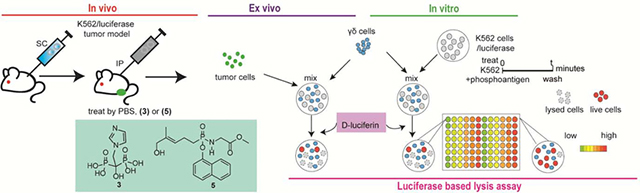
Abstract
Human Vγ9Vδ2 T cells respond to small phosphorus-containing compounds, often called phosphoantigens, which are now known to be intracellular ligands of the immune receptor butyrophilin 3A1 (BTN3A1). In order to compare the efficiency of butyrophilin ligands, we developed a luciferase-based lysis assay that measures the direct cytolysis by Vγ9Vδ2 T cells of luciferase-expressing K562 leukemia cells sensitized by phosphoantigen prodrugs. Our results show that the luciferase-based lysis assay allows in vitro and in vivo assessment of phosphoantigen activity in a way that does not require the extensive processing of flow cytometry or ELISA based approaches. In cellular assays, the structure activity relationships of phosphoantigen prodrugs correlate with ELISA-based activation assays, though phosphoantigen induced target cell lysis occurs at lower concentrations relative to T cell interferon γ production measured by ELISA. In mice dosed with phosphoantigens, a racemic aryl phosphonamidate prodrug, methyl 2-[[[(E)-5-hydroxy-4-methyl-pent-3-enyl]-(1-naphthyloxy)phosphoryl]amino]acetate (1-Nap/GlyOMe C-HMBP, 5), sensitized subcutaneous K562 tumors within minutes, and this effect was maintained at least four hours after treatment. In vivo activity of compound 5 was stronger than that of an equivalent dose of zoledronate. This luciferase lysis assay can be used for evaluation of phosphoantigens due to its time efficiency, high sensitivity, and in vivo compatibility and demonstrates rapid in vitro and in vivo sensitization of tumor cells by phosphoantigen prodrugs.
Graphical Abstract
1. Introduction
T cells are important lymphocytes involved in immune responses. The γδ T cells are a subset of T cells with a different type of T cell receptor (TCR) composed of a γ chain paired with a δ chain. The γδ T cells only account for 1–5% of circulating T cells, though this population is larger in certain tissues and disease states [1–3]. The Vγ9Vδ2 T cell is the dominant subset of γδ T cells in peripheral blood and is unique to primates [4]. Vγ9Vδ2 T cells are activated by small phosphorus-containing compounds called phosphoantigens rather than peptide antigens [5–7]. The Vγ9Vδ2 T cells exhibit a mix of innate and adaptive characteristics that make them of interest for treatment of diseases such as cancer [8].
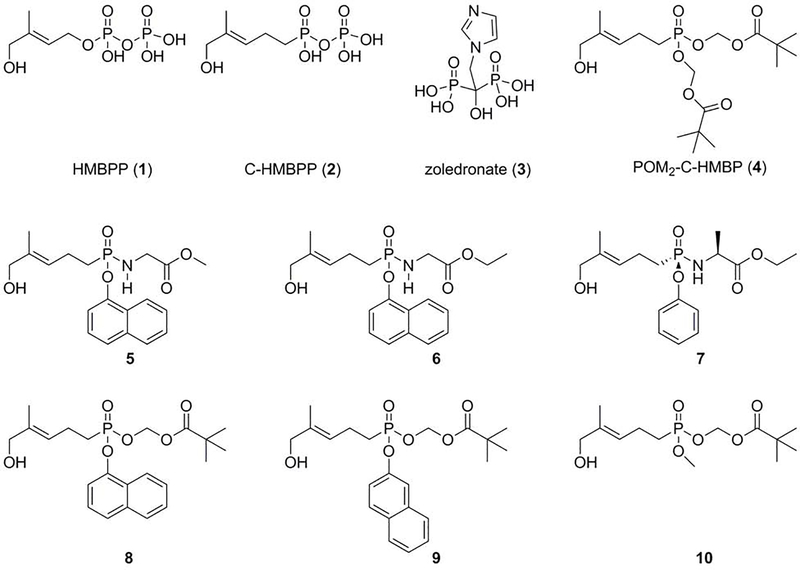
Examples of phosphoantigens include (E)-4-hydroxy-3-methyl-but-2-enyl diphosphate (HMBPP) (Figure 1) and isopentenyl diphosphate (IPP) [4, 9]. HMBPP is an isoprenoid metabolite produced by bacteria and apicomplexan parasites using the non-mevalonate pathway [10]. While HMBPP is the most potent natural phosphoantigen, its potential as a drug is limited due to its poor pharmacokinetic properties. For example, HMBPP has rapid clearance from the plasma because of phosphatase-mediated degradation. Furthermore, it is difficult for HMBPP to enter target cells by passive diffusion because it is negatively charged at physiological pH [4, 9]. The clinical bisphosphonate drug zoledronate can also indirectly stimulate the Vγ9Vδ2 T cells via elevation of endogenous IPP, and is more metabolically stable than HMBPP. However, its high charge to mass ratio also restricts its access to the intracellular target farnesyl diphosphate synthase [11, 12]. Therefore, we prepared and characterized a series of phosphoantigen prodrugs [9, 13–18], which are charge neutral at physiological pH and predicted to have better membrane permeability relative to HMBPP or zoledronate [19]. Once the prodrugs enter into the target cells, the modified groups are cleaved by cytoplasmic enzymes to release the active form.
Figure 1.
Chemical structures of the compounds evaluated in this study.
In contrast to traditional T cell antigens, the phosphoantigens and their prodrugs function in a manner that is independent of the major histocompatibility complex (MHC), through mechanisms that are still not fully understood [20–22]. Required in this process is target cell expression of the transmembrane immunoglobulin BTN3A1 [23], which also contains an intracellular domain in which phosphoantigens bind to a shallow basic binding pocket [9, 17, 24]. The ligand binding event triggers changes in conformation and clustering of the protein leading to TCR signaling in the Vγ9Vδ2 T cell [14, 25–27].
When activated, Vγ9Vδ2 T cells can perform a variety of effector functions similar to CD8 T cells or NK cells. Specifically, signaling from the Vγ9Vδ2 TCR leads them to proliferate, produce cytokines, and directly lyse target cells [7, 28–33]. Monitoring these effector functions is often time intensive with low throughput for compound screening [34] and may not be compatible with in vivo/ex vivo analysis. Here, we tested the hypothesis that phosphoantigen prodrugs could sensitize K562 tumor cells to lysis by Vγ9Vδ2 T cells. Our primary endpoint to these studies is our newly developed in vitro luciferase-based lysis assay which we used to study Vγ9Vδ2 T cell response to various phosphoantigens and their prodrugs in K562 cells and cells derived from K562 tumors in mice.
2. Materials and Methods
2.1. Reagents
The K562 cell line labeled with luciferase (K562/luciferase, #SL019) was purchased from GeneCopoeia (Rockville, MD). The cells were aliquoted upon arrival and then maintained less than 2 months in culture for use in experiments. RPMI-1640, fetal bovine serum (FBS), 2-mercaptoethanol (BME), 100x nonessential amino acids, pyruvate, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 200x penicillin/streptomycin, bovine serum albumin (BSA), and cell-permeable D-luciferin potassium salt (#88294) were purchased from Fisher Scientific (Pittsburgh, PA). Interleukin 2 (IL-2) was obtained from Miltenyi Biotec (Bergisch Gladbach, Germany).
2.2. Test compounds
HMBPP (1) and C-HMBPP (2) were purchased from Echelon Biosciences (Salt Lake City, UT). Zoledronate (3) was purchased from Fisher Scientific. Compounds (4–10) were previously synthesized [9, 13, 15, 18] by our group.
2.3. Mice
Rag2−/− γc−/− mice (strain C;129S4-Rag2tm1.1Flv Il2rgtm1.1Flv/J) were purchased from Jackson Labs (Mount Desert Island, ME). Mice were housed in the University of Connecticut Animal Care Services facility. Experiments were conducted according to a protocol approved by the Institutional Animal Care and Use Committee (IACUC).
2.4. Vγ9Vδ2 T cells
Human peripheral blood mononuclear cells (PBMCs) were purified from human buffy coat (purchased from Research Blood Components, Brighton, MA) by density centrifugation as described [9]. PBMCs were resuspended in T cell medium (10% heat-inactivated fetal bovine serum in RPMI-1640 medium containing 1.5 g/L sodium bicarbonate, supplemented with 10 mM HEPES buffer, 1 mM pyruvate, 1x non-essential amino acids, 1x Pen-Strep solution, and 50 μM 2-mercaptoethanol) and stimulated with 10 nM HMBPP for 72 hours and cultured for up to 14 days in an incubator at 37 °C containing 5% CO2. IL-2 (5 ng/mL) was provided every 3 days. After 7–14 days, Vγ9Vδ2 T cells were purified by negative selection using the Miltenyi TCR γ/δ+ T Cell Isolation Kit (#130–092-892) as directed.
2.5. Luciferase lysis assay
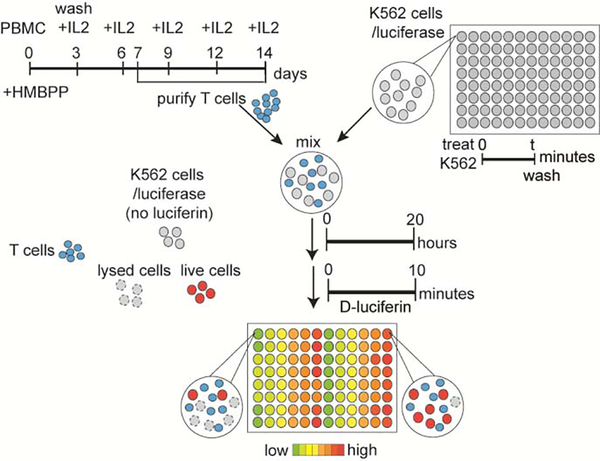
The K562 cells stably expressing firefly luciferase were exposed to cell-permeable luciferin to determine live cell number. Indicated numbers of K562/luciferase cells were treated with indicated concentrations of test compounds, as shown in Figure 1, for 15 minutes, 60 minutes or 240 minutes. Cells were then washed five times after HMBPP or C-HMBPP treatments and twice after prodrug or zoledronate treatments by T cell media, and diluted/mixed with Vγ9Vδ2 T cells at an effector: target (E:T) ratio of 3:1 in 200 μL final volume in a 96 well white opaque tissue culture plate (Fisher Scientific). Mixtures were incubated for various times at 37 °C before addition of cell-permeable D-luciferin (as indicated in the text) for 10 minutes incubation at 37 °C. The plates were read by a Perkin-Elmer VICTOR X multilabel plate reader (Waltham, MA, USA) for luminescence counts per second (relative light units, RLU). The reading time was 1 second/well. The flow chart of the experiments is shown in Figure 2.
Figure 2.
Flow chart of the luciferase-based lysis assay. K562/luciferase cells were pre-loaded with test compounds, washed and mixed with purified effector Vγ9Vδ2 T cells for defined times to allow for cellular interactions and lysis. The cell-mediated lysis was measured by adding the cell-permeable D-luciferin and detection of luminescence using a plate reader.
2.6. K562 mouse model
K562/luciferase cells were maintained at exponential phase growth. Each mouse (8 to 12 weeks old, approximately 25 g) was injected with a single injection of 1 million cells (in 100 μL matrigel) subcutaneously into the rear flank. Both male and female mice were used for the experiments. Tumors were measured using calipers. When the average size of tumor reached 50–150 mm3, mice were then randomized into different groups and dosed with different treatments, including PBS and compounds 3 (zoledronate) or 5 (1-Nap/GlyOMe C-HMBP) at 10 mg/kg by IP injection. Mice were euthanized after times described in the text and tumors were excised for further processing.
2.7. Ex vivo K562 tumor cell luciferase lysis assay
Following in vivo phosphoantigen treatment, K562 tumor cells were isolated from the mouse tumor for incubation with Vγ9Vδ2 T cells. Single cell suspensions were made from each individual tumor by physically dissociating the tumor and forcing the cells through a 40 μm sterile nylon mesh cell strainer and these cells were washed twice with media. An initial estimate of the amount of K562 cells in each tumor-derived single cell suspension was obtained using the luciferase assay on a small sample of cells from each tumor. The tumor cells were then aliquoted into wells based on the relative light unit (RLU) values of the initial sample. Similar RLU values among each treatment condition were used. The K562 tumor cells were incubated with Vγ9Vδ2 T cells at an E:T ratio of 3:1, and same amount of tumor cells incubated without Vγ9Vδ2 T cells were used as reference. The PBS treated tumor cells were also treated ex vivo with compound 4 (1 μM) for 1 hour in vitro as an additional control. All the cells were incubated for times as indicated before addition of cell-permeable D-luciferin for analysis of surviving cells using the plate reader. The RLU values of the Vγ9Vδ2 T cell exposed group were divided by the RLU values of the corresponding control cells that were not exposed to Vγ9Vδ2 T cells to determine the percentage of luciferase positive K562 cells remaining after exposure to Vγ9Vδ2 T cells.
2.8. Statistics
All the experiments were performed at least three times using cells from at least two different donors. Data were analyzed using GraphPad Prism 6. Dose response curves were analyzed using a log (inhibitor) versus response-variable slope (four parameters) model. Data is presented as the mean +/− standard deviation as indicated in the figure legends. For clarity, in lieu of graphing standard deviations in Figures 4 and 5 the error has been presented as 95% confidence intervals in Tables 1 and 2. EC50 values were calculated and the EC50 with 95% confidence intervals, the Hill slope, and R2 values were shown in Table 1. Log EC50 values for each compound and time point were plotted on the X-axis versus log time on the Y axis. Power constants (k1) and additive constants (k2) were calculated as described in our previous publication [35]. In brief, k1 = log t + α log C, where C is the concentration (μM) and t the incubation time (min). The fit (R2) was assessed for each compound. The k2 values were calculated using α = 1 for each compound, and the fit (R2) was assessed using the calculated k2 values.
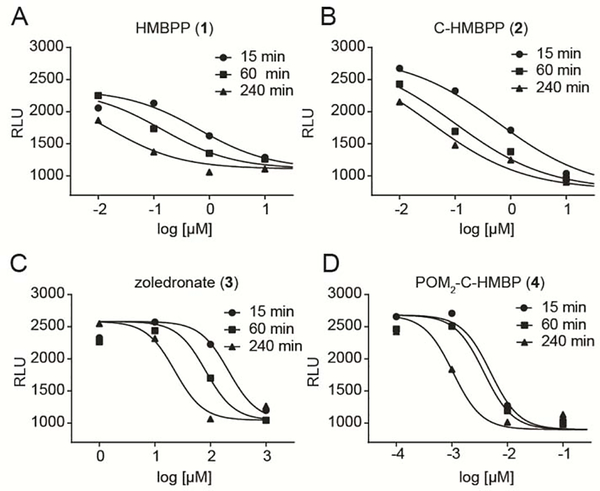
Figure 4.
Dose- and time-dependent activation of Vγ9Vδ2 T cells by indicated compounds. K562 cell lysis by Vγ9Vδ2 T cells after K562 cell exposure to A) HMBPP, B) C-HMBPP, C) zoledronate, or D) POM2-C-HMBP for varied time points. The average RLU for each panel in the absence of test compound was 2100, 2700, 2100, and 2600 in panels A-D, respectively. Data points represent the mean values of three independent experiments (n=3).
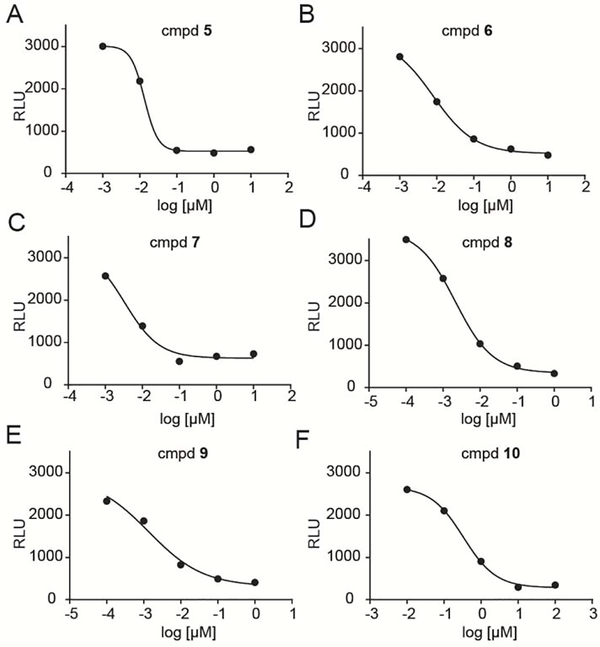
Figure 5.
Dose-dependent activation of Vγ9Vδ2 T cells by K562 cells exposed to A) compound 5, B) 6, C) 7, D) 8, E) 9, or F) 10 for 60 minutes. Data points represent the mean values of three independent experiments (n=3).
Table 1.
Compound characteristics for Vγ9Vδ2 T cells lysis assay by pre-loaded K562 cells (n=3).
| Cmpd (n=3) | 15 min IC50 [μM] (95% CI) | 60 min IC50 [μM] (95% CI) | 240 min IC50 [μM] (95% CI) | Hill slope | Hill R2 | Power constant (k1)=log t+α log C |
Power R2 | Log-additive constant (k2)=log t+ log C | Log-additive R2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| k1 | α | |||||||||
| 1 | 0.65 0.27 to 1.5 |
0.14 0.059 to 0.33 |
0.017 0.0065 to 0.042 |
−0.67 | 0.94 | 1.1 | 0.75 | 0.99 | 0.84 | 0.88 |
| 2 | 0.60 0.37 to 0.97 |
0.098 0.059 to 0.16 |
0.036 0.022 to 0.061 |
−0.52 | 0.98 | 0.93 | 0.96 | 0.97 | 0.89 | 0.97 |
| 3 | 210 86 to 520 |
82 43 to 160 |
23 9.0 to 58 |
−1.7 | 0.98 | 4.1 | 1.2 | 0.99 | 3.6 | 0.96 |
| 4 | 0.0050 0.0027 to 0.0091 |
0.0039 0.0019 to 0.0074 |
0.0011 0.00068 to 0.0016 |
−1.7 | 0.97 | −2.2 | 1.6 | 0.87 | −0.79 | 0.76 |
Table 2.
EC50 and characteristics of the compounds in lysis assay (n=3).
| Cmpd (n=3) | 60 min EC50 [μM] (95% CI) | Hill slope | Hill R2 |
|---|---|---|---|
| 5 | 0.013 (0.0015 to 0.12) |
−2.4 | 1.0 |
| 6 | 0.0079 (0.0052 to 0.012) |
−0.77 | 1.0 |
| 7 | 0.0033 (0.0010 to 0.011) |
−0.93 | 0.98 |
| 8 | 0.0021 0.00046 to 0.0099 |
−0.87 | 1.0 |
| 9 | 0.0014 (0.00023 to 0.0080) |
−0.59 | 0.99 |
| 10 | 0.33 (0.018 to 6.3) |
−0.99 | 1.0 |
3. Results
3.1. Optimization of the firefly luciferase lysis assay
Firefly luciferase is an enzyme that requires oxygen and ATP to oxidize the substrate D-luciferin and emit light. Due to this characteristic, luciferase can be applied to cytotoxicity assays [36–38]. Through treatment of these cells with a cell-permeable luciferin, it is possible to use luciferin activity to determine live cell numbers. Here, we tested whether luciferase expressing K562 leukemia cells could be useful as Vγ9Vδ2 T cell target cells. These K562 cells would be pretreated with phosphoantigen prodrugs to engage and activate their BTN3A1. Vγ9Vδ2 T cells would then sense the phosphoantigen loaded K562 cell and lyse them when the two types of cells are mixed [19]. The cell-permeable firefly luciferase substrate D-luciferin would then be oxidized by live luciferase positive K562 cells but not dead (or lysed) K562 cells, thereby allowing the quantification of target cell lysis by T cells (as outlined in Figure 2).
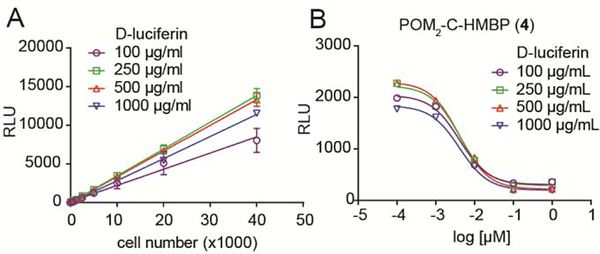
The number of target cells and the concentration of cell-permeable D-luciferin were optimized for this assay as shown in Figure 3. Different numbers of K562 cells were plated and different concentrations of the cell-permeable firefly luciferase substrate D-luciferin were added into the wells. The RLU and the cell numbers showed excellent linear regression over a wide range of cell numbers and luciferin concentrations (Figure 3A). Therefore, the luciferase assay can be used to quantify the number of live K562 cells. To minimize the use of the effector cells while still generating a good signal, the number of target cells was chosen to be 10,000 target cell/well for subsequent assays.
Figure 3.
Luciferase lysis assay optimization. A) Different numbers of K562/luciferase cells were mixed with different concentrations of cell-permeable D-luciferin. The live cell number is linearly proportionate to the luciferase signal. Data points represent the mean values and standard deviations of three independent experiments (n=3). B) 104 K562 cells were treated with indicated doses of compound 4 for 1 hour, washed, and then mixed with Vγ9Vδ2 T cells at an E:T = 3:1 for 20 hours. Different doses of cell-permeable D-luciferin were added to the cell mixtures after the incubation. Data points represent the mean values of three independent experiments (n=3). The RLU values for the 100, 250, 500, and 1000 μg/mL conditions for K562 cells alone were 3500, 3300, 3100, and 3100 and for the K562/T cell co-cultures were 1600, 1900, 2200, and 1800. Experiments were performed using cells from at least two different donors.
For the lysis assay, an E:T ratio of 3:1 and a co-culture time of 20 hours were maintained in the co-culture system, which were both consistent with the previous flow cytometry based lysis assay [19] and the ELISA based IFN-γ secretion assay [35]. We next optimized the dose of the cell-permeable luciferase substrate D-luciferin. K562 cells were pretreated with compound 4 for 1 hour and washed twice with T cell medium before they were mixed with purified Vγ9Vδ2 T cells. After co-incubation, different concentrations of cell-permeable D-luciferin (100, 250, 500, and 1000 μg/mL) were added. Results showed there is a dose dependent decrease in the RLU after compound 4 treatment (Figure 3B). Therefore, compound 4 caused a dose dependent lysis of K562 cells by Vγ9Vδ2 T cells. Different doses of cell-permeable D-Luciferin all showed R2 > 0.99 while 500 μg/mL D-luciferin, which gave the peak RLU values in untreated control cells, was used in the following experiments.
3.2. Test compounds enable Vγ9Vδ2 T cells to lyse target cells in a dose- and time- dependent manner in the luciferase assay
Four compounds were evaluated using this optimized lysis assay: HMBPP (1), C-HMBPP (2), zoledronate (3) and POM2-C-HMBP (4). Dose response and time course experiments were performed as follows. The K562/luciferase cells were treated with indicated concentrations of the test compounds for 15 minutes, 60 minutes and 240 minutes. Concentrations varied among compounds and were chosen based on their relative activity in an earlier ELISA study [35]. After removal of the compounds, the cells were mixed with purified Vγ9Vδ2 T cells. Dose and time dependent increases in the lysed cells were triggered by all four compounds in these washout experiments (Figure 4). The compound characteristics, including the EC50 values and Hill slopes are shown in Table 1. POM2-C-HMBP is more potent in this experiment compared to HMBPP, C-HMBPP or zoledronate. POM2-C-HMBP is more than 20,000-fold more potent than zoledronate at the 240 minute time point, an effect that increased at the short 15 minute exposure time. Hill slopes of both POM2-C-HMBP and zoledronate were steep compared to HMBPP and C-HMBPP. POM2-C-HMBP showed efficient cellular activity compared to the charged compounds (k1 = −2.2). HMBPP and C-HMBPP were 120–130 fold less potent than POM2-C-HMBP at 15 minutes, however, the differences were reduced at 60 minutes and 240 minutes. This may be due to the energy dependent cellular uptake of HMBPP and C-HMBPP, they are less active following short exposures but more active following longer exposure times. In addition, HMBPP and C-HMBPP showed a shallow Hill slope consistent with prior studies [14, 35].
3.3. The phosphoantigen prodrugs induce dose dependent lysis of K562 cells, consistent with cytokine release
After optimization of the conditions with the preceding controls, additional compounds were tested to both better understand the structure activity relationships and aid in selection of a candidate for in vivo evaluation. We focused on compounds from our recently described phosphonamidate series (5–7) [15] and our mixed aryl acyloxyalkyl series (8 and 9) [13, 16], all of which previously showed improved plasma stability relative to prodrug 4. Less active compound 10 was used as an additional control. The six additional prodrugs (compounds 5–10) showed maximal activity at high concentrations following 60 minutes of exposure. Dose dependent lysis of K562 cells was observed for all of them (Figure 5A–F). The IC50 values and Hill slopes are shown in Table 2. Compound 10 caused the lowest cell lysis compared to the other 5 compounds.
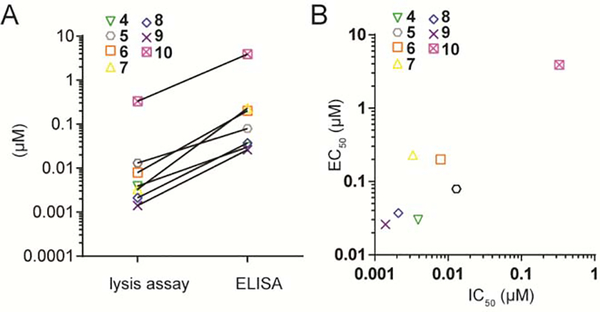
We next compared the consistency and the differences of compound potency using the newly generated IC50 values from the luciferase-based lysis assay and the previously published EC50 values from interferon-γ ELISA assays and the results are shown in Figure 6. The prior ELISA results were obtained using the same K562 incubation time (1 hour), co-culture time (20 hours) and E:T ratio (3:1), though they varied in the absolute number of cells used (10,000 K562 cells/well here versus 4,000/well in the ELISA assay [35]). The compound potency was generally lower in the lysis assay compared to the ELISA (Figure 6A), while the pattern of activity was generally correlated (Figure 6B) and consistent with our previous findings regarding the structure activity relationships [19].
Figure 6.
Comparison of the IC50 values from the luciferase-based lysis assay and EC50 values from the ELISA, both at the 1 hour time point. A) line graph, B) scatter plot.
3.4. Compound 5 distributes to the tumor and sensitizes cells in vivo more efficiently than zoledronate
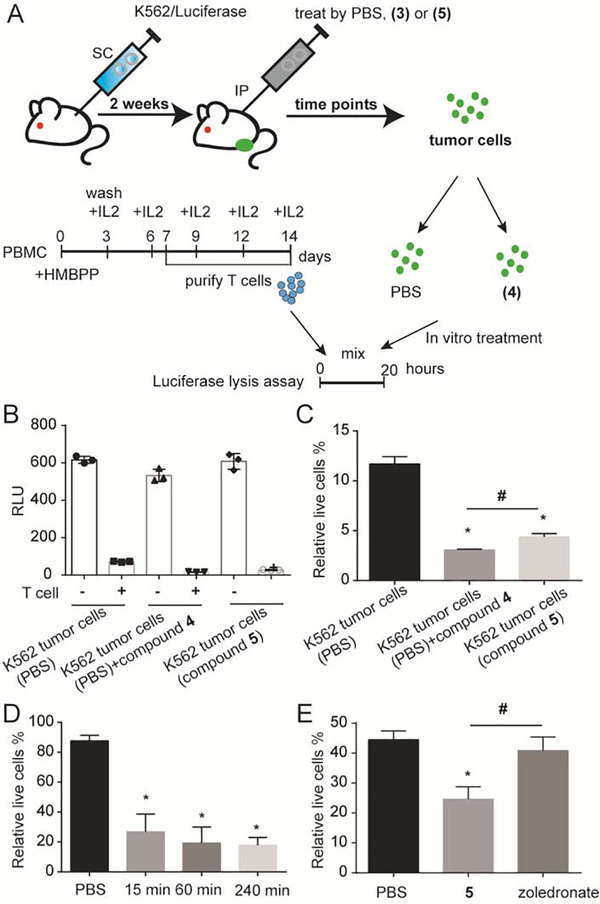
In order to evaluate the ability of phosphoantigen prodrugs to distribute to tumors and to evaluate the applicability of the luciferase assay for this purpose, we performed an in vivo test. Compound 5 was selected for these studies due to its availability and high plasma stability [15, 18]. Mice bearing subcutaneous K562 tumors were dosed by intraperitoneal injection with compound 5 at a dose of 10 mg/kg for indicated time points. The dose and route were chosen based on a prior study that used 10 mg/kg of pamidronate in a mouse model of Vγ9Vδ2 T cell activation [39]. The K562 tumor cells were harvested into a single cell suspension (Figure 7). Each tumor sample was divided into two groups- one group was exposed to the Vγ9Vδ2 T cells while the other was not. The luciferase assay was performed, and the percentage of K562 luciferase cells remaining after incubation with Vγ9Vδ2 T cells was quantified.
Figure 7.
In vivo phosphoantigen dosing and ex vivo K562 tumor lysis by Vγ9Vδ2 T cells. A) Mice bearing subcutaneous K562 tumors in the rear flank were treated with 10 mg/kg of the test compounds using IP injection. Mice were euthanized at different time points after injection to harvest and isolate tumor cells for incubation with Vγ9Vδ2 T cells. B) Lysis of the luciferase-positive tumor-derived K562 cells by Vγ9Vδ2 T cells. K562 tumor bearing mice were dosed with PBS or compound 5 for 1 hour. Tumors were extracted and dissociated into single cell suspensions. Some tumor cells from PBS-treated mice were also treated with compound 4 (1 μM) for 60 minutes in vitro as a positive control. Bars represent mean RLU values and the standard deviation from three mice (n=3). C) The data from the preceding panel is represented as a percentage of live cells under each condition in the presence of T cells. D) K562 tumor bearing mice were dosed with PBS or compound 5. After different time points, tumors were extracted then tumor lysis was analyzed using luciferase assay relative to controls incubated without any Vγ9Vδ2 T cell treatment. Bars represent mean values and the standard deviation from three mice (n=3). E) Subcutaneous K562 tumor bearing mice were treated with PBS, compound 5 or zoledronate. Mice were euthanized after 1 hour to harvest and isolate tumor cells for incubation with Vγ9Vδ2 T cells. Bars represent mean values and the standard deviation from three mice (n=3). Statistics were determined by ANOVA with Tukey’s post-hoc analysis and with p < 0.05. *indicates significant difference compared to the tumor cells from PBS-treated mice. #indicates significant difference compared to each other.
In vivo treatment with phosphoantigen sensitized the tumor cells to ex vivo lysis by Vγ9Vδ2 T cells. As shown in Figure 7B, addition of T cells to K562 tumor cells reduced the number of viable cells as judged by luciferase activity. Although a high degree of background lysis was observed in this experiment, the tumor cells of the mice treated by compound 5 for 1 hour exhibited a significantly higher lysis compared to that of the mice treated by PBS (Figure 7C). As a positive control, some tumor cells from the PBS treated mice were also exposed after extraction from the mouse to 1 μM of compound 4. These control cells showed the strongest lysis by Vγ9Vδ2 T cells and could be used to establish the maximum activity of the text compounds, since not all of the tumor cells would be expected to be luciferase positive K562 cells. The magnitude of the lysis observed following in vivo treatment with compound 5 was similar to the magnitude of lysis observed following ex vivo treatment with 4.
The experiment was further validated using Vγ9Vδ2 T cells from a different healthy donor. In this experiment, less background lysis was observed. Figure 7D shows that 15 minutes, 60 minutes and 240 minutes after the injection, there was significant phosphoantigen induced lysis relative to untreated mice. No difference was observed for different time points, indicating that the prodrug distributed to the tumor tissue by 15 minutes post injection. Compared to the PBS treated mice, tumor cells from the prodrug-treated mice demonstrated significant higher cell lysis when incubated with Vγ9Vδ2 T cells, indicating that compound 5 accumulation in the tumor reaches a concentration that is sufficient to activate Vγ9Vδ2 T cells for cell lysis.
An additional experiment was performed to compare the in vivo efficacy of compound 5 to that of compound 3 (zoledronate) (Figure 7E). Here, tumor bearing mice were dosed with equivalent masses of test compounds (10 mg/kg). Compound 5 again significantly induced lysis of the K562 tumor cells, while no impact of treatment with zoledronate was observed. Taken together, compound 5 distributes to K562 tumors to provide a sustained sensitization to lysis by Vγ9Vδ2 T cells that reaches maximal efficacy and is more efficient than zoledronate.
Discussion
In this study, the in vitro and in vivo efficacy of a series of phosphoantigen prodrugs was measured using an optimized luciferase lysis assay. The lysis assay can detect phosphoantigen activity in a manner that is more amenable to high-throughput approaches and more sensitive relative to other effector function assays. The test compounds enable Vγ9Vδ2 T cells to lyse K562 cells in a dose- and time- dependent manner. The phosphoantigen prodrugs stimulate a dose-dependent lysis of the K562 cells by Vγ9Vδ2 T cells and achieve maximal activation and similar patterns of structure activity relationships relative to other assays. We also showed for the first time the in vivo activity of a phosphoantigen prodrug. The aryl phosphonamidate prodrug (compound 5) clearly distributed to the tumor and sensitized subcutaneous K562 tumors, in a way that was more efficient than zoledronate.
This optimized luciferase-based lysis assay has several advantages over other lysis assays. Compared to the most common method, the [51Cr] chromium release assay [40] which requires the radio-labeled target cells, this luciferase-based assay avoids the use of radioactive materials. A recent publication [41] measured the Vγ9Vδ2 T cell cytotoxicity using a chelate-forming prodrug (BM-HT) combined with europium (Eu3+). In contrast, our luciferase-based lysis assay reduces the cost of the assay because luciferin can be readily obtained in high quantities. In addition, compared to the flow cytometry based lysis assay, which requires an extra Annexin V staining step after the co-incubation of the T cells and the target cells and relatively slow flow acquisition [19], the luciferase-based assay requires fewer processing procedures and faster data acquisition using a plate reader. While our studies only focused on a single cell line that is already well established as a target for Vγ9Vδ2 T cells, it is possible this approach would be more broadly applicable to other cells lines, though further studies would be needed to support this broader applicability.
Here, the results we obtained from the luciferase-based lysis assay are consistent with our previous findings using flow cytometry-based lysis assay and ELISA-based activation assays [19, 35]. The phosphoantigen prodrugs demonstrated higher potency in the cytotoxicity compared to the charged diphosphates or bisphosphonates due to the neutralization of the negative charges under physiological pH. In these assays, the potency from luciferase-based lysis assays showed higher sensitivity than the potency we observed from the ELISA, which is consistent with our previous study in which the flow based lysis assay showed higher sensitivity than ELISA [19]. This implies that the cytolytic activity of the Vγ9Vδ2 T cells might be more sensitive to the phosphoantigen.
Moreover, we successfully applied the luciferase-based lysis assay into the in vivo experiment using tumor bearing mice. We showed that the tumor cells from the prodrug treated mice can be lysed by the in vitro expanded Vγ9Vδ2 T cells. This is strong evidence that the prodrug can reach the tumor site, deliver the phosphoantigen payload, and engage the molecular target BTN3A1, though further studies would be necessary to determine if the tumors can be recognized by the Vγ9Vδ2 T cells in vivo. This is the first in vivo activity of the prodrugs, and it also demonstrates an improvement relative to zoledronate, a bisphosphonate drug that was studied in multiple clinical studies [42] and pre-clinical studies [43–45].
In the in vivo experiments, there was a high degree of variability in the rate of lysis of the untreated tumor cells (Figure 7B–7E). We attribute some of this variability to differences among human donors, as these cells are polyclonal with respect to their Vγ9Vδ2 T cell receptors and the frequency of clones may vary among donors. While some donor variability also has been observed in our prior co-culture experiments, the in vivo component of the present study adds an additional complicating factor with respect to counting the target cells. Furthermore, processing time involved in extracting the tumor cells from the mouse may also increase the variability we observed in this study. We cannot rule out a role for the mouse bystander cells in influencing the assay, however we continue to believe that the advantages of using the pre-labeled luciferase cells (speed, ease of experiment) outweigh the potential negatives (variability).
Although continuous dosing or high dose levels are required, diphosphate phosphoantigens, such as HMBPP, have been tested using in vivo models [46], These in vivo experiments usually use in vitro expanded Vγ9Vδ2 T cells and then an adoptive transfer of the T cells to the model. Luciferase expressing target cells have potential for in vivo testing of the phosphoantigens. For example, when combined with in vivo live imaging systems, luciferase is widely used to visualize the distribution and progress of the tumors in the animal models [47]. Alternatively, as our assays have shown the luciferase assay can be applied to in vivo study to show the successful delivery of the prodrug to the tumor site of the mice. We observed some donor differences in this assay, but the trend is consistent and indicates a fast distribution to the tumor and ability to engage BTN3A1 in the tumor cells. This will largely improve the convenience of the in vivo testing of the prodrugs.
In summary, the luciferase lysis assay successfully measured the activity of the phosphoantigens and their prodrugs in vitro. In addition, it can be used in mice to show the delivery of the prodrug to the tumor within minutes of the administration. It is possible to scale up the assay to enable high-throughput screening of a large number of small molecule drug candidates as immune activation agents for Vγ9Vδ2 T cells. This method potentially can be used in different cytotoxicity experiments of other immune cells, such as CD8+ T cells, NK cells, as well as other genetically modified T cells.
Acknowledgments
Additional compounds were provided by Prof. Rocky Barney, Dr. Rebekah Shippy, and Dr. Ben Foust while at the University of Iowa. Helpful insight into the animal model was provided by Prof. Dennis Wright at the University of Connecticut. Research reported in this publication was supported by the National Cancer Institute of the United States National Institutes of Health under Award Number R01CA186935 (A.J.W., P.I.) and the Herman Frasch Foundation for Chemical Research, Bank of America, N.A., Trustee (HF17) (A.J.W., PI).
Footnotes
Disclosure
D.F.W and A.J.W. are founders of Terpenoid Therapeutics. The current work did not involve the company.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Nielsen MM, Witherden DA, Havran WL, γδ T cells in homeostasis and host defence of epithelial barrier tissues, Nat. Rev. Immunol. 17 (12) (2017) 733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chien YH, Meyer C, Bonneville M, γδ T cells: first line of defense and beyond, Annu. Rev. Immunol. 32 (1) (2014) 121–55. [DOI] [PubMed] [Google Scholar]
- [3].Vermijlen D, Gatti D, Kouzeli A, Rus T, Eberl M, γδ T cell responses: How many ligands will it take till we know?, Semin. Cell Dev. Biol. 84 (2018) 75–86. [DOI] [PubMed] [Google Scholar]
- [4].Sicard H, Ingoure S, Luciani B, Serraz C, Fournie JJ, Bonneville M, et al. , In vivo immunomanipulation of Vγ9Vδ2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model, J. Immunol. 175 (8) (2005) 5471–80. [DOI] [PubMed] [Google Scholar]
- [5].Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR, Natural and synthetic non-peptide antigens recognized by human γδ T cells, Nature 375 (6527) (1995) 155–8. [DOI] [PubMed] [Google Scholar]
- [6].Morita CT, Beckman EM, Bukowski JF, Tanaka Y, Band H, Bloom BR, et al. , Direct presentation of nonpeptide prenyl pyrophosphate antigens to human γδ T cells, Immunity 3 (4) (1995) 495–507. [DOI] [PubMed] [Google Scholar]
- [7].Wrobel P, Shojaei H, Schittek B, Gieseler F, Wollenberg B, Kalthoff H, et al. , Lysis of a broad range of epithelial tumour cells by human γδ T cells: involvement of NKG2D ligands and T-cell receptor- versus NKG2D-dependent recognition, Scand. J. Immunol. 66 (2–3) (2007) 320–8. [DOI] [PubMed] [Google Scholar]
- [8].Zumwalde NA, Sharma A, Xu X, Ma S, Schneider CL, Romero-Masters JC, et al. , Adoptively transferred Vγ9Vδ2 T cells show potent antitumor effects in a preclinical B cell lymphomagenesis model, JCI insight 2 (13) (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hsiao CH, Lin X, Barney RJ, Shippy RR, Li J, Vinogradova O, et al. , Synthesis of a phosphoantigen prodrug that potently activates Vγ9Vδ2 T-lymphocytes, Chem. Biol. 21 (8) (2014) 945–54. [DOI] [PubMed] [Google Scholar]
- [10].Wiemer AJ, Hsiao CH, Wiemer DF, Isoprenoid metabolism as a therapeutic target in gram-negative pathogens, Curr. Top. Med. Chem. 10 (18) (2010) 1858–71. [DOI] [PubMed] [Google Scholar]
- [11].Thompson K, Rogers MJ, Coxon FP, Crockett JC, Cytosolic entry of bisphosphonate drugs requires acidification of vesicles after fluid-phase endocytosis, Mol. Pharmacol. 69 (5) (2006) 1624–32. [DOI] [PubMed] [Google Scholar]
- [12].Gan LM, Lagerstrom-Fermer M, Ericsson H, Nelander K, Lindstedt EL, Michaelsson E, et al. , Safety, tolerability, pharmacokinetics and effect on serum uric acid of the myeloperoxidase inhibitor AZD4831 in a randomized, placebo-controlled, phase I study in healthy volunteers, Br. J. Clin. Pharmacol. 85 (4) (2019) 762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Foust BJ, Poe MM, Lentini NA, Hsiao CC, Wiemer AJ, Wiemer DF, Mixed Aryl Phosphonate Prodrugs of a Butyrophilin Ligand, ACS Med. Chem. Lett. 8 (9) (2017) 914–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shippy RR, Lin X, Agabiti SS, Li J, Zangari BM, Foust BJ, et al. , Phosphinophosphonates and Their Tris-pivaloyloxymethyl Prodrugs Reveal a Negatively Cooperative Butyrophilin Activation Mechanism, J. Med. Chem. 60 (6) (2017) 2373–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lentini NA, Foust BJ, Hsiao CC, Wiemer AJ, Wiemer DF, Phosphonamidate Prodrugs of a Butyrophilin Ligand Display Plasma Stability and Potent Vγ9 Vδ2 T Cell Stimulation, J. Med. Chem. 61 (19) (2018) 8658–8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Foust BJ, Li J, Hsiao CC, Wiemer DF, Wiemer AJ, Stability and Efficiency of Mixed Aryl Phosphonate Prodrugs, ChemMedChem 14 (17) (2019) 1597–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Poe MM, Agabiti SS, Liu C, Li V, Teske KA, Hsiao CC, et al. , Probing the Ligand-Binding Pocket of BTN3A1, J. Med. Chem. 62 (14) (2019) 6814–6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lentini NA, Hsiao CC, Crull GB, Wiemer AJ, Wiemer DF, Synthesis and Bioactivity of the Alanyl Phosphonamidate Stereoisomers Derived from a Butyrophilin Ligand, ACS Med. Chem. Lett 10 (9) (2019) 1284–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kilcollins AM, Li J, Hsiao CH, Wiemer AJ, HMBPP Analog Prodrugs Bypass Energy-Dependent Uptake To Promote Efficient BTN3A1-Mediated Malignant Cell Lysis by Vγ9Vδ2 T Lymphocyte Effectors, J. Immunol. 197 (2) (2016) 419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Morita CT, Jin C, Sarikonda G, Wang H, Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens, Immunol. Rev. 215 (2007) 59–76. [DOI] [PubMed] [Google Scholar]
- [21].Boutin L, Scotet E, Towards Deciphering the Hidden Mechanisms That Contribute to the Antigenic Activation Process of Human Vγ9Vδ2 T Cells, Front. Immunol. 9 (828) (2018) 828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Riano F, Karunakaran MM, Starick L, Li J, Scholz CJ, Kunzmann V, et al. , Vγ9Vδ2 TCR-activation by phosphorylated antigens requires butyrophilin 3 A1 (BTN3A1) and additional genes on human chromosome 6, Eur. J. Immunol. 44 (9) (2014) 2571–6. [DOI] [PubMed] [Google Scholar]
- [23].Harly C, Guillaume Y, Nedellec S, Peigne CM, Monkkonen H, Monkkonen J, et al. , Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset, Blood 120 (11) (2012) 2269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sandstrom A, Peigné C-M, Léger A, Crooks JE, Konczak F, Gesnel M-C, et al. , The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2 T cells, Immunity 40 (4) (2014) 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gu S, Sachleben JR, Boughter CT, Nawrocka WI, Borowska MT, Tarrasch JT, et al. , Phosphoantigen-induced conformational change of butyrophilin 3A1 (BTN3A1) and its implication on Vγ9Vδ2 T cell activation, Proc. Natl. Acad. Sci. U. S. A. 114 (35) (2017) E7311–E7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nguyen K, Li J, Puthenveetil R, Lin X, Poe MM, Hsiao CC, et al. , The butyrophilin 3A1 intracellular domain undergoes a conformational change involving the juxtamembrane region, FASEB J. 31 (11) (2017) 4697–4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yang Y, Li L, Yuan L, Zhou X, Duan J, Xiao H, et al. , A Structural Change in Butyrophilin upon Phosphoantigen Binding Underlies Phosphoantigen-Mediated Vγ9Vδ2 T Cell Activation, Immunity 50 (4) (2019) 1043–1053. [DOI] [PubMed] [Google Scholar]
- [28].Ota K, Yagi T, Iwagaki H, Morimoto Y, Sadamori H, Inagaki M, et al. , Fas-mediated cytotoxicity by γδ T cells during acute rejection in xenotransplantation of spheroidal aggregate-cultured hepatocytes, Res. Commun. Mol. Pathol. Pharmacol. 105 (1–2) (1999) 43–54. [PubMed] [Google Scholar]
- [29].Boulland ML, Kanavaros P, Wechsler J, Casiraghi O, Gaulard P, Cytotoxic protein expression in natural killer cell lymphomas and in αβ and γδ peripheral T-cell lymphomas, J. Pathol. 183 (4) (1997) 432–9. [DOI] [PubMed] [Google Scholar]
- [30].Li B, Bassiri H, Rossman MD, Kramer P, Eyuboglu AF, Torres M, et al. , Involvement of the Fas/Fas ligand pathway in activation-induced cell death of mycobacteria-reactive human γδ T cells: a mechanism for the loss of γδ T cells in patients with pulmonary tuberculosis, J. Immunol. 161 (3) (1998) 1558–67. [PubMed] [Google Scholar]
- [31].Hayday AC, γδ T cells and the lymphoid stress-surveillance response, Immunity 31 (2) (2009) 184–96. [DOI] [PubMed] [Google Scholar]
- [32].Vantourout P, Hayday A, Six-of-the-best: unique contributions of γδ T cells to immunology, Nat. Rev. Immunol. 13 (2) (2013) 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Courtney AH, Lo WL, Weiss A, TCR Signaling: Mechanisms of Initiation and Propagation, Trends Biochem. Sci. 43 (2) (2018) 108–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wiemer AJ, Hegde S, Gumperz JE, Huttenlocher A, A live imaging cell motility screen identifies prostaglandin E2 as a T cell stop signal antagonist, J. Immunol. 187 (7) (2011) 3663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hsiao CC, Wiemer AJ, A power law function describes the time- and dose-dependency of Vγ9Vδ2 T cell activation by phosphoantigens, Biochem. Pharmacol. 158 (2018) 298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Matta H, Gopalakrishnan R, Choi S, Prakash R, Natarajan V, Prins R, et al. , Development and characterization of a novel luciferase based cytotoxicity assay, Sci. Rep. 8 (1) (2018) 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Karimi MA, Lee E, Bachmann MH, Salicioni AM, Behrens EM, Kambayashi T, et al. , Measuring cytotoxicity by bioluminescence imaging outperforms the standard chromium-51 release assay, PLoS One 9 (2) (2014) e89357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fu X, Tao L, Rivera A, Williamson S, Song XT, Ahmed N, et al. , A simple and sensitive method for measuring tumor-specific T cell cytotoxicity, PLoS One 5 (7) (2010) e11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tu W, Zheng J, Liu Y, Sia SF, Liu M, Qin G, et al. , The aminobisphosphonate pamidronate controls influenza pathogenesis by expanding a γδ T cell population in humanized mice, J. Exp. Med. 208 (7) (2011) 1511–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Brunner KT, Mauel J, Cerottini JC, Chapuis B, Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs, Immunology 14 (2) (1968) 181–96. [PMC free article] [PubMed] [Google Scholar]
- [41].Tagod MSO, Mizuta S, Sakai Y, Iwasaki M, Shiraishi K, Senju H, et al. , Determination of human γδ T cell-mediated cytotoxicity using a non-radioactive assay system, J. Immunol. Methods 466 (2019) 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bertaina A, Zorzoli A, Petretto A, Barbarito G, Inglese E, Merli P, et al. , Zoledronic acid boosts γδ T-cell activity in children receiving αβ(+) T and CD19(+) cell-depleted grafts from an HLA-haplo-identical donor, Oncoimmunology 6 (2) (2017) e1216291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Abe Y, Muto M, Nieda M, Nakagawa Y, Nicol A, Kaneko T, et al. , Clinical and immunological evaluation of zoledronate-activated Vγ9γδ T-cell-based immunotherapy for patients with multiple myeloma, Exp. Hematol. 37 (8) (2009) 956–68. [DOI] [PubMed] [Google Scholar]
- [44].Benzaid I, Monkkonen H, Stresing V, Bonnelye E, Green J, Monkkonen J, et al. , High phosphoantigen levels in bisphosphonate-treated human breast tumors promote Vγ9Vδ2 T-cell chemotaxis and cytotoxicity in vivo, Cancer Res. 71 (13) (2011) 4562–72. [DOI] [PubMed] [Google Scholar]
- [45].Benzaid I, Monkkonen H, Bonnelye E, Monkkonen J, Clezardin P, In vivo phosphoantigen levels in bisphosphonate-treated human breast tumors trigger Vγ9Vδ2 T-cell antitumor cytotoxicity through ICAM-1 engagement, Clin. Cancer. Res. 18 (22) (2012) 6249–59. [DOI] [PubMed] [Google Scholar]
- [46].Chen ZW, Multifunctional immune responses of HMBPP-specific Vγ2Vδ2 T cells in M. tuberculosis and other infections, Cell. Mol. Immunol. 10 (1) (2013) 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Li J, Gu B, Meng Q, Yan Z, Gao H, Chen X, et al. , The use of myristic acid as a ligand of polyethylenimine/DNA nanoparticles for targeted gene therapy of glioblastoma, Nanotechnology 22 (43) (2011) 435101. [DOI] [PubMed] [Google Scholar]