Abstract
The onset of motherhood is accompanied by alterations in emotional and affective behaviors. Many new mothers experience transient and mild depressive symptoms that typically resolve spontaneously (i.e. postpartum blues) but increase the risk for postpartum depression (PPD). There is little data regarding the neural adaptations occurring in response to parturition and shortly after birth that may be associated with these affective changes. Although the dopamine (DA) system is involved in affect, maternal motivation and PPD, little is known about postpartum DA function. We compared affective behavior in virgin and postpartum adult female rats at early and late time points. In vivo extracellular recordings of VTA DA neurons were performed to evaluate 3 parameters: number of active DA neurons (i.e. population activity), firing rate, and firing pattern. Compared with virgins, postpartum rats exhibited increased anxiety-like behavior in the elevated plus maze at 1-day postpartum; reduced social motivation at 1- and 3-days postpartum, reduced anxiety-like behavior in the novelty suppressed feeding test throughout the first week postpartum and increased forced swim test immobility at 1-day postpartum. 1- and 3-day postpartum females exhibited attenuated VTA population activity without changes in firing rate or pattern. None of these effects were observed in late postpartum females when compared with virgins. These data suggest that parturition induces time-dependent changes in a subset of affect-related behaviors and DA function during the postpartum period in rodents, with early postpartum females exhibiting depression-related phenotypes (i.e. low social motivation, higher immobility, blunted DA activity).
Keywords: postpartum, maternal, social behavior, dopamine, electrophysiology
1. Introduction
The period after childbirth (i.e. postpartum period) is a time of elevated risk for the development of mood and affective disorders1-3. The highest rates of anxiety and depression in women occur following childbirth4: as many as up to 75% of new mothers experience a transient mood disturbance (i.e. postpartum blues) 1-2 weeks postpartum2, 5,6, and 10-15% go on to develop postpartum depression (PPD)3, 7. Although postpartum blues is a common phenomenon and considered a normal physiologic response to the hormonal events from childbirth2, 6, 7, it is a risk factor for PPD8, 9, thereby underscoring the need to understand the normative neural changes involved in this transient state. Yet, there is little data regarding the neural adaptations in response to parturition (i.e. giving birth) and during the early postpartum, so the neural substrates that may be associated with this increased affective dysregulation remain unknown.
Alterations in maternal mood and affect have been partially attributed to rapid and robust changes in ovarian and stress hormones associated with the onset of motherhood in both humans and rodents10-13, which can influence the activity of the mesolimbic dopamine (DA) system14-16. The mesolimbic DA system originates in the ventral tegmental area (VTA) and plays a pivotal role in affective processes, maternal motivation as well as the pathophysiology of depression and PPD14, 16-19. Importantly, mesolimbic DA dysregulation has been observed in women with PPD20, 21 and animal models of PPD14, 22; and, preclinical studies in rodents have highlighted a causal link between DA dysregulation (i.e. decreased VTA DA neuron activity) and stress-induced depression-related behaviors (i.e. anhedonia, despair)23-25. Moreover, early/mid postpartum females exhibit increased depressive-like behavior26, and changes in reward-related-behaviors27, 28 compared with late postpartum rats, which may reflect time-dependent alterations in dopaminergic activity across the postpartum period. However, few studies have directly examined normative changes in behavior and VTA activity during the early postpartum, and whether a negative affect state occurs in early postpartum rodents is unknown. Thus, the primary purpose of this paper is to determine what changes in affect-related behaviors and VTA activity occur in the postpartum rat, with a focus on the early postpartum period.
We assessed parity-driven changes in affect-related behavior and DA activity by comparing virgin and postpartum female rats across distinct timepoints (early: 1-day, 3-days, 1-week; late: 21-23days). Animals underwent a behavioral test battery consisting of: elevated plus maze (EPM), three-chambered social approach test (SAT), novelty suppressed feeding (NSF), and the forced swim test (FST). In vivo single-unit extracellular recordings were conducted within the VTA of a subset of animals tested for behavior (EPM, SAT) to measure 3 parameters of DA neuron activity: number of spontaneously active DA neurons per electrode track (population activity), basal firing rate and firing pattern (percentage of spikes firing in bursts). We hypothesized that, like humans, early postpartum female rats would exhibit increased negative affect, although these effects may be limited to depression-related phenotypes and likely vary throughout the postpartum period. Moreover, given that negative affect behaviors are often associated with DA dysregulation (i.e. downregulation) in rodent models of depression25, 29, we hypothesized that early postpartum rodents would exhibit alterations in VTA DA activity.
2. Methods and Materials
2.1. Animals
Virgin or timed-pregnant (gestational day 13) adult (and age-matched) female Sprague Dawley rats were shipped overnight (Envigo, Indianapolis, IN) and arrived in our facility (~6:00-7:00AM) the next day. Rats were housed in a temperature-controlled room on a 12-h light/dark cycle (lights on 7AM-7PM) with food (Lab Diet, Rodent Diet #5001) and water available ad libitum. The day each litter was born was designated as postpartum day 0. Dams that gave birth to small litters (< 6 pups) were not included in the study and sacrificed; dams used in the study had a minimum of 8 pups and a maximum of 12 pups. Virgin rats were co-housed in pairs and dams were co-housed with their litter. Animals were kept in their home cage and undisturbed except for weekly routine animal care done by the experimenter and when undergoing behavioral testing. All postpartum females, including late postpartum females, were kept with their litter to ensure all dams had exposure to offspring during the experiment and to avoid possible disruption due to the removal of offspring just before testing30. The estrous cycle was not monitored in virgin rats because animals would have to be swabbed daily for a prolonged period of time and this would introduce a confound, as virgin females would be exposed to differential handling procedures and additional stress compared with postpartum dams, in which estrous cycle is not monitored since this reproductive stage (i.e. postpartum) is characterized by persistent diestrus and cessation of ovarian cycling 31, 32. Although we are aware of literature indicating a decrease in anxiety-like behavior during proestrus and estrus in rats33, 34, social approach/preference for same-sex conspecifics in female rats tested in the three-chamber social approach apparatus has been shown to be independent of the estrous cycle 35. With regards to VTA DA neuron activity, a previous in vivo electrophysiological study conducted in female rats indicated no change in VTA population activity (i.e. the same measure used here) across the estrous cycle36. All experiments were performed in accordance with the guidelines outlined in the National Institutes of Health Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. For timeline and experimental design, please see Figure 1.
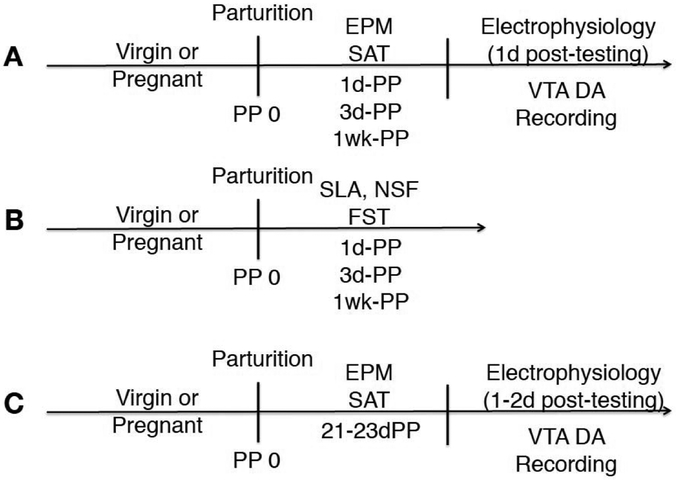
Figure 1. Timeline and experimental design.
A) Virgin or early postpartum female rats at different timepoints (1d-PP, 3d-PP, 1wk-PP) were tested for anxiety-like and social behavior in the elevated plus maze (EPM) and the three-chambered social approach test (SAT), respectively (n=9-16 per group). In vivo extracellular recordings of VTA DA neurons were conducted 1-day post-behavioral testing using an acute anesthetized preparation in the same cohort of rats used for behavior to evaluate: number of spontaneously active DA cells per track (i.e. population activity), firing rate and burst firing (n=8-12 per group). B) A separate cohort of virgin and early postpartum female rats was tested for spontaneous locomotor activity (SLA), novelty suppressed feeding (NSF) and in the forced swim test (FST) (n=6-9 per group). C)To determine whether any observed effects were specific to the early postpartum period, a separate cohort of animals consisting of virgins and late postpartum females (21-23d-PP), which were still kept with their litter, were tested in the EPM and SAT, followed by VTA recordings 1-2 days post-behavioral testing (n=8-12 per group).
2.2. Behavioral Testing
Behaviors were recorded and scored by an experimenter blind to experimental condition. All behaviors, except the social approach test, were conducted during the light cycle, similar to previously published work in early postpartum female rats37, 38 and social behavior studies conducted by our group39, 40.
2.2.1. Elevated Plus Maze
The EPM was positioned 50cm above the floor and consisted of a plus-shaped apparatus composed of 2 opposite open arms (each 50cm long × 10cm wide) crossed at a right angle by 2 arms enclosed by 40cm high opaque walls41. Animals were habituated to the testing room within the home cage for approximately 1-hour prior to testing. Each rat was placed on the central platform and its movement was recorded for 5-minutes with a camera positioned overhead39. The percentage of open arm entries (open arm entries/total × 100) and open arm time (time in open arms/total × 100), defined as front two paws and head in the arm, were used as indices of anxiety-like behavior. The total number of entries was used as an index of locomotor activity.
2.2.3. Social Approach Test
A three-chambered apparatus (total: 92cm × 45cm × 44.5 cm; side chambers: 36cm × 45cm ×44.5cm; center chamber: 20cm × 45cm × 44.5cm) was used to assess social approach (motivation), similar to previously described39. Animals were habituated to the testing room within the home cage for approximately 1-hour prior to testing. Rats were placed in a smaller center chamber adjacent to two other chambers, each containing a wire cage that allows the test rat to see and smell its content but prevents physical interactions42. After a 5-min habituation period, an unfamiliar, younger (<200g), same-sex rat (to prevent aggression and/or mating behaviors)39, 43 that had previously been habituated to the wire cage was enclosed inside it and placed in a side chamber. Social stimulus animals were used a maximum of 3 times. An inanimate object (i.e. stuffed toy rat) was placed inside the other wire cage as a novel object control. The experimental rat was then allowed to explore the entire apparatus and time spent sniffing the receptacle containing the social stimulus was recorded for 10 minutes.
2.2.4. Spontaneous Locomotor Activity
Animals were placed into an open field arena (approx. 42cm × 42cm × 39cm; Coulbourn Instruments), and spontaneous locomotor activity was monitored (under red light) for 10-minutes by beam breaks with TruScan software and indexed as total distance traveled (cm) and total time spent moving (seconds)29.
2.2.5. Novelty Suppressed Feeding
In this test, food-deprived animals are placed in a situation (i.e. brightly lit open field) that provokes conflict between the drive to eat and the fear of novel and open spaces44. Following an 18-hour home cage food deprivation, rats were placed in a rectangular open field (70 × 40 × 30 cm) baited with a chow pellet during a 10-minute trial. Latency to begin eating was recorded. This measure is thought to reflect how the animal copes with a behavioral conflict (i.e. drive to eat vs fear of novel open spaces) and is used as an index of anxiety-like behavior44, 45.
2.2.6. Forced Swim Test
The FST took place in a clear Plexiglas cylinder (50cm high, 20cm diameter) filled with water (25±1 °C) up to 38-40cm high to prevent escape or the animal’s tail touching the bottom, similar to that described previously29. Animals received a 15-minute pre-exposure (day 1; habituation) followed by a 5-minute swim session the next day (day 2; test) in which immobility behavior, defined as passive floating in which the animal is making only minor necessary movements to maintain head above water46, was measured29. Water was changed between animals on each day. Rats were dried off after each session before being placed back in the home cage. While the interpretation of FST immobility has been subject to controversy over the last couple of years47, 48, we included this test because it is still used as a standard for depression-related behavior and particularly for assaying antidepressant activity, its use is actually increasing in preclinical research, and the vast majority of researchers qualify the rodent’s floating response as depressive-like behavior, although there is an increasing trend for the interpretation of immobility as a passive coping strategy49.
2.3. In vivo Electrophysiological Recordings
2.3.1. Surgery.
Single-unit extracellular recordings were performed using an acute preparation in a subset of rats the day after behavioral testing (EPM, SAT). Prior work from our group suggests no impact of these 2 tests on VTA DA neuron activity 40, 50. Recordings were not conducted in animals that underwent the FST because this introduces a confound in VTA activity and is sufficient to induce stress-induced downregulation of VTA DA neuron activity29. Rats were anesthetized with 8% chloral hydrate (400mg/kg, intraperitoneally), placed in a stereotaxic frame (Kopf, Tujunga, CA), and maintained at 37°C using a temperature-controlled heating pad (Fine Science Tools, Foster City, CA). Anesthesia level was monitored periodically by assessing the footpinch reflex and adjusted by intraperitoneal administration of additional chloral hydrate (0.3ml supplements) as needed. After clearing the skull of skin and fascia, a burr hole was drilled in the region overlying the VTA [from bregma, anteroposterior (AP): −5.4mm, mediolateral (ML): +0.6mm] on the right side of the brain.
2.3.2. VTA sampling.
Single barrel electrodes were constructed from 2mm diameter borosilicate capillary tubes (World Precision Instruments, Sarasota, Florida) using a vertical electrode puller (Narishige, Tokyo, Japan) and broken back under microscopic control. Glass electrodes were filled with 2% Chicago Sky Blue (Sigma-Aldrich, St. Louis, MO) dissolved in 2M saline and lowered into the VTA using a hydraulic microdrive (Kopf). DA neurons were sampled by making 6-9 vertical electrode passes (tracks), each separated by 0.2mm, in a predetermined pattern spanning the antero-posterior and medio-lateral extent of the VTA [AP: 5.4-5.7mm; ML: 0.6-1.0mm from bregma, and dorsoventral (DV): 6.5-9.0mm from dura]. This procedure has been used by our group to sample DA neurons with a variety of different projection targets51 in multiple studies52, 53. Signal was acquired using a preamplifier (Dagan, Minneapolis, MN) and displayed on an oscilloscope (B&K Precision, Yorba Linda, CA) with a signal fed to a computer running Lab Chart 7 (AD Instruments, San Diego, CA).
2.3.3. Dopamine neuron identification.
DA neurons were identified with open filter settings (50Hz low cutoff, 16kHZ cutoff) using well-established electrophysiological criteria: location, slow, irregular firing pattern, long duration, variable shape biphasic action potential waveform (>2.2ms), half width (>1.1ms), temporary cessation of firing during tail/foot pinch54-56. Once identified, DA neurons were recorded for 3 minutes (1-minute minimum) when signal to noise ratio exceeded 3:1. Three parameters of DA neuron firing were measured: number of spontaneously active DA cells per electrode track (population activity, all active DA neurons in each rat divided by the number of tracks)57, basal firing rate, and proportion of spikes occurring in bursts, with burst initiation defined as the occurrence of two spikes with an interspike interval of ≤ 80ms and burst termination defined as the occurrence of an interspike interval of > 160 ms58. This procedure allows us to measure both the tonic (i.e. population activity) and phasic (i.e. burst firing) state of encountered VTA DA neurons.
2.3.4. Placement verification.
Electrode placement was marked by electrophoretic ejection of Chicago Sky Blue dye at the final recording site. Rats were then overdosed with additional chloral hydrate, decapitated, their brains removed and fixed in 8% paraformaldehyde for at least 48 hours. Brains were then transferred to 25% sucrose solution for cryoprotection, sectioned using a cryostat (Cryostar NX50, ThermoScientific, Waltham, MA) into 60μm coronal slides, mounted on to gelatin-chromalum-coated glass slides, and stained with cresyl violet and neutral red to check recording electrode placements. Animals were required to have a minimum of 6 tracks within 0.4mm of target coordinates to be included in the study.
2.4. Statistical Analyses
For behavioral data, comparisons between 3 or more groups were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison post hoc test when appropriate. Pairwise comparisons were analyzed using unpaired two-tailed t-tests. Electrophysiological data was collected with Powerlab Lab Chart (AD Instruments) to identify spike time courses and exported to Neuroexplorer (NEX Technologies, NexTech Systems) software to calculate firing rate and burst firing for each DA neuron. Track location data were analyzed by repeated measures (RM) 2-way ANOVA. Normally distributed electrophysiological data were analyzed using one-way ANOVA when comparing 3 or more groups or two-tailed unpaired t-tests for pairwise comparisons. Data sets deviating from the normal distribution were analyzed with one-way ANOVA on ranks (Kruskal-Wallis H-test) followed by Dunn’s post hoc test as appropriate or Mann-Whitney U-test, respectively. All statistics were calculated using GraphPad Prism 7.0; differences were considered significant when p < 0.05.
3. Results
3.1. Changes in affect-related behaviors during the early postpartum period
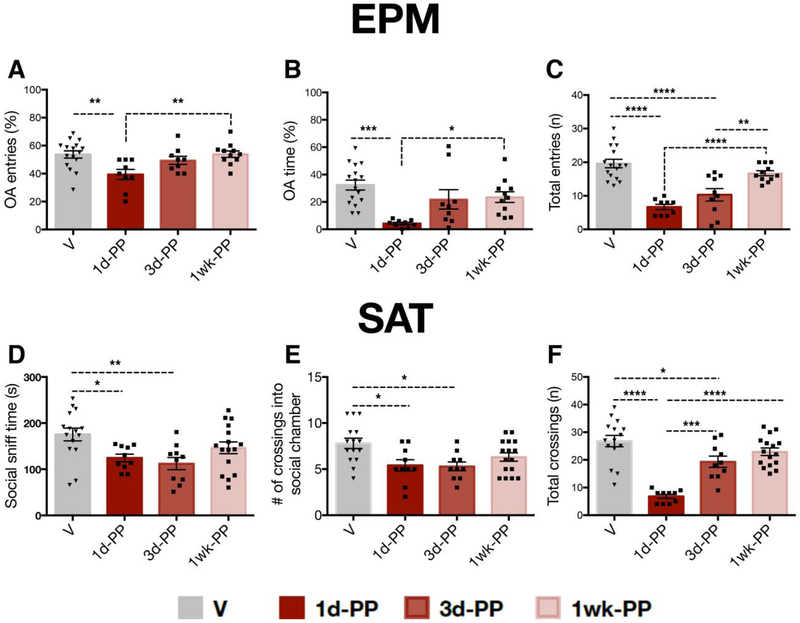
Changes in anxiety-like behavior and social motivation during the early postpartum period were assessed by testing virgin (n=16) and 1-day (n=9-10), 3-day (n=9-10) or 1-week (n=11) postpartum rats in the EPM and SAT (Figure 1A). At 1-day postpartum, females made fewer open arm entries (one-way ANOVA: F3,41=5.114, p<0.01) and spent less time in the open arm (one-way ANOVA: F3,41=7.346, p<0.01) compared with virgin or 1-week postpartum rats (Figure 2A,B). 1d-PP and 3d-PP females made fewer total entries (one-way ANOVA: F3,41=21.05, p<0.0001) compared with virgins (Tukey’s, p<0.0001) and 1-week PP females (Tukey’s, p<0.01) (Figure 2C). 1d-PP and 3d-PP females exhibited reduced social motivation, as indexed by reduced social sniff time (one-way ANOVA: F3,47=4.462, p<0.01) and fewer crossings into the social chamber (one-way ANOVA: F3,47=4.801, p<0.01) compared with virgins (Tukey’s, p<0.05) (Figure 2D,E). Changes in the total number of chamber crossings were also found (one-way ANOVA; F3,47=23.24, p<0.0001) (Figure 2F). 1d-PP and 3d-PP females made fewer total crossings compared with virgins (Tukey’s; p<0.05). Differences were also found between 1d-PP and 3d-PP females (Tukey’s; p<0.001) and between 1d-PP and 1week-PP females (Tukey’s; p<0.0001), suggesting changes in locomotor activity throughout the first week postpartum.
Figure 2. Time-dependent changes in anxiety-like and social behavior during the first week postpartum.
A,B) Females tested at 1-day postpartum exhibited increased anxiety-like behavior in the EPM with (A) fewer open arm entries (p < 0.01) and (B) less time spent in the open arm (p < 0.05) compared with virgins or 1-week postpartum females. C) 1-day and 3-day postpartum females made fewer total entries compared with virgin and 1-week postpartum females (p < 0.05). D,E) Compared with virgins, 1- and 3-day postpartum females showed reduced social motivation, as indexed by D) lower levels of social cage sniff time (p < 0.05) and E) decreased number of crossings into the social chamber (p < 0.05). F) . F) 1-day postpartum females made fewer total number of crossings compared to all other groups (p < 0.001); 3-day postpartum females made fewer total chamber crossings than virgins (p<0.05) but more total chamber crossings than 1-day postpartum females (p <0.001). *p < 0.05, ** p < 0.01, *** p < 0.001, ****p <0.0001. Error bars represent mean ± SEM. Gray bars represent virgins (n=16) and red bars represent postpartum females (n=9-11 per group).
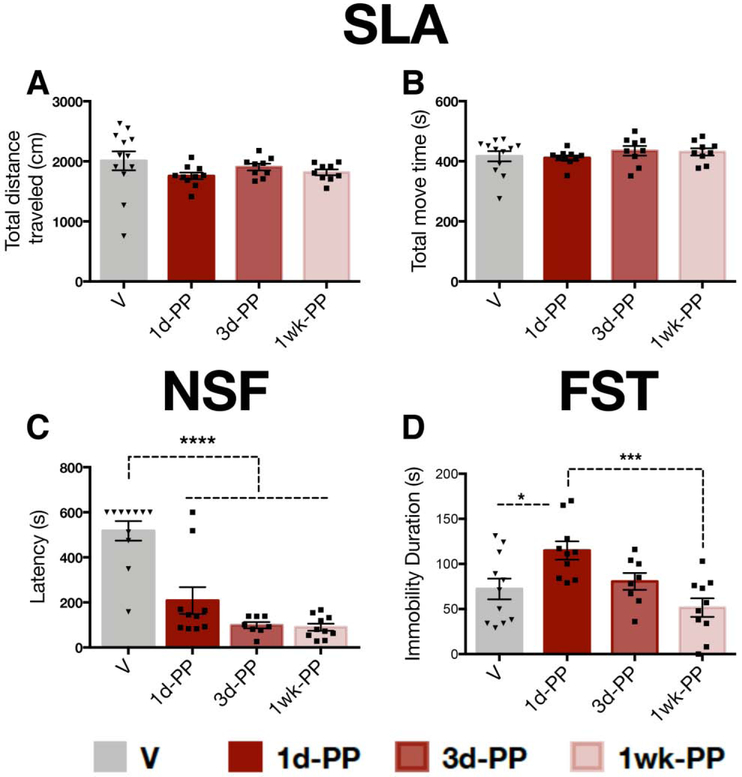
1d-PP and 3d-PP females exhibited reduced number of total entries in the EPM and total number of crossings in the SAT, suggesting altered locomotor activity. For this reason, we tested spontaneous locomotor activity as well as additional behavioral measures relevant to anxiety and depression-related phenotypes (i.e. NSF, FST) in a separate cohort of animals (n=9-16 per group; Figure 1B). No differences were found for total distance traveled (one-way ANOVA: F3,36=1.184, p=0.329) or time spent moving (one-way ANOVA: F3,36=0.610, p=0.613) between early postpartum (1d-PP, 3d-PP, 1week-PP) and virgin rats (Figure 3A,B) suggesting comparable baseline locomotor activity. Early postpartum females exhibited reduced latency to feed in a novel environment compared with virgins (one-way ANOVA: F3,35=25.78, p<0.0001; Tukey’s; p<0.00001) (Figure 3C). An effect on FST immobility was detected in 1d-PP females (one-way ANOVA: F3,35=6.364, p<0.05). Post-hoc analysis revealed increased immobility in 1d-PP females compared with virgins and 1-week PP females (Tukey’s; p<0.05) (Figure 3D). This cohort was used exclusively for behavior and did not undergo VTA recordings given our results showing that FST exposure downregulates VTA activity in female rats 29.
Figure 3. Reduced latency to feed and enhanced FST immobility in early postpartum females.
A,B) Early postpartum rats had comparable spontaneous locomotor activity (SLA) to virgins, as they exhibited A) similar distance traveled (p=0.32) and B) time spent moving (p=0.61) compared with virgins throughout the first week postpartum. C) Early postpartum females exhibited reduced latency to consume food in a novel environment across the first week postpartum compared with virgins (p<0.0001). D) 1-day postpartum females showed increased immobility in the FST compared with virgins and 1wk-PP females. Error bars represent mean ± SEM. Gray bars represent virgins, red bars represent postpartum females (n=6-9 per group). *p < 0.05, ** p < 0.01, *** p < 0.001.
3.2. Attenuated VTA population activity during the first 3 days postpartum
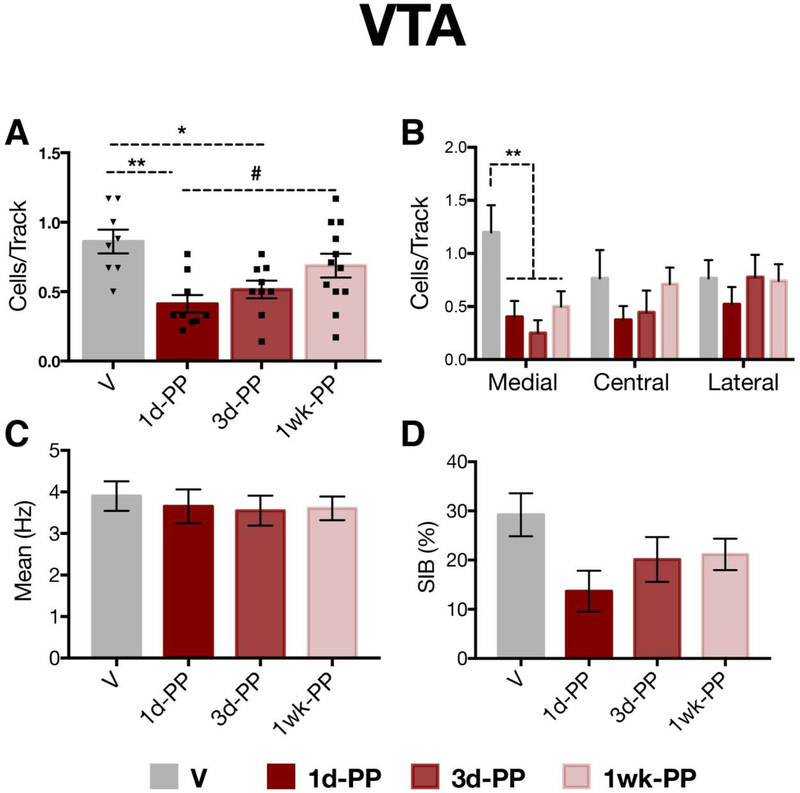
Extracellular recordings of VTA DA neurons were conducted in the same animals subjected to behavioral testing (EPM, SAT) 1-day post-test (virgins: n=8, 43 neurons, 3-8 neurons per rat; 1d-PP : n=9, 28 neurons, 2-7 neurons per rat; 3d-PP: n=9, 33 neurons, 2-7 neurons per rat; 1-week PP: n=12; 64 neurons, 1-9 neurons per rat; Figure 1A). Compared with virgin rats (0.86 ± 0.24 CPT), postpartum females exhibited lower numbers of spontaneously active DA cells per track (i.e. population activity) (one-way ANOVA: F3,34=5.86; p<0.01) at 1-day (0.41 ± 0.19 CPT; Tukey’s; p<0.01) and 3-days postpartum (0.52 ± 0.19 CPT; Tukey’s; p<0.05), but not at 1-week postpartum (0.69 ± 0.29 CPT) (Figure 3A). We also observed a trend towards a significant difference between 1d-PP and 1-week PP females (0.69 ± 0.29 CPT; Tukey’s; p=0.06). Given the evidence that the VTA is functionally segregated 51, 59, population activity data for all groups were analyzed according to location in the medial, central, or lateral VTA. For a rat to be included in this analysis, data must have been available from all three VTA locations (i.e. medial, central, lateral). Population activity was selectively reduced in the reward-related medial VTA of postpartum females (n=6-11 rats per group) when compared to virgins (n=5) (RM 2-way ANOVA; F3,26=4.73; p<0.01) (Figure 3C). No differences in firing rate (one-way ANOVA on ranks: H=0.33; p=0.95) were observed between virgin (3.90 ± 2.33 Hz) and postpartum females (1d-PP: 3.65 ± 2.17 Hz; 3d-PP: 3.55 ± 2.08 Hz; 1week-PP: 3.61 ± 2.28 Hz) (Figure 3D). No differences in the percentage of spikes fired in burst (one way ANOVA on ranks: H=6.61; p=0.08) were observed between virgin (29.22 ± 28.64 %SIB) and postpartum females (1d-PP: 13.69 ± 22.04 %SIB; 1d-PP: 20.13 ± 26.09 %SIB; 1week-PP: 21.16 ± 25.45 %SIB) (Figures 3E).
3.3. Comparable behavior and VTA DA neuron activity between virgins and late postpartum female rats
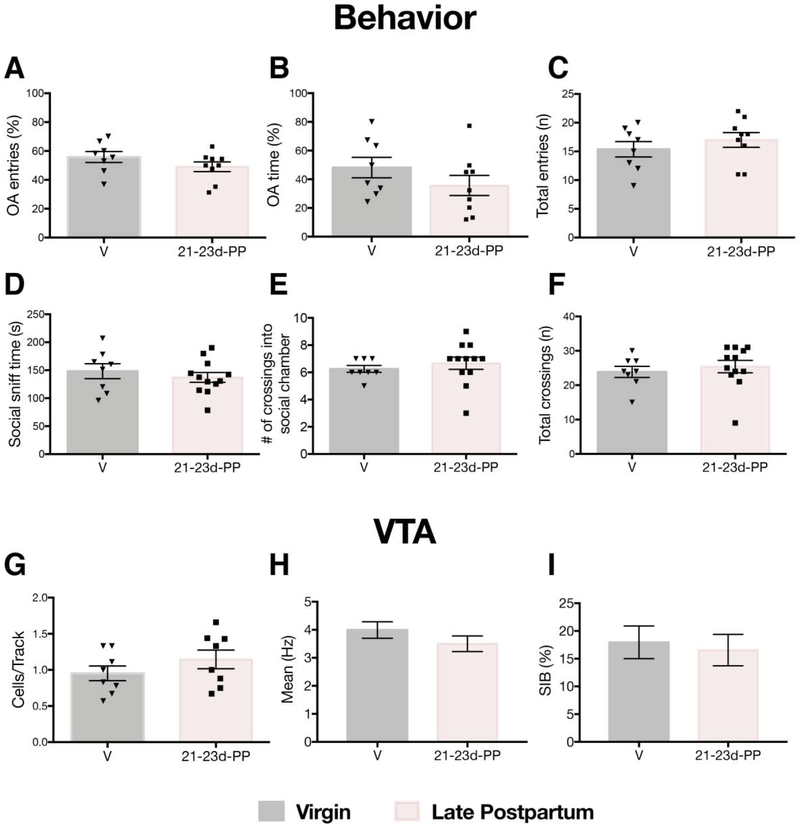
To determine whether similar behavioral and electrophysiological effects were observed in the late postpartum period, a separate cohort of rats were tested during postpartum days 21-23 (virgin: n=8; 55 neurons, 4-10 neurons per rat; late PP: n=8; 57 neurons, 4-10 neurons per rat; Figure 1C). No differences in anxiety-like behavior, as indexed by comparable percentages of open arm entries (t15=1.33; p=0.20) and open arm time (t15=1.25; p=0.23), were found (Figure 5A-C). No between group differences were found in the total number of arm entries (t15=0.87; p=0.40). Similarly, no between group differences were found for social sniff time (t18=0.73; p=0.48), crossings into the social chamber (t18=0.70; p=0.49), or total number of chamber crossings (t18=0.60; p=0.56) (Figure 5D-F). Virgin and late postpartum female rats exhibited comparable numbers of spontaneously active DA cells per electrode track (virgins: 0.95 ± 0.29 CPT, late postpartum: 1.14 ± 0.37 CPT) and were not significantly different from each other (t14=1.17; p=0.26) (Figure 5G). No between group differences in firing rate (virgins: 3.99 ± 2.18 Hz, late postpartum: 3.50 ± 2.12 Hz; t111=1.21; p=0.23) or burst firing (virgins: 17.96 ± 21.83 %SIB, late postpartum: 16.54 ± 21.59 %SIB; U=1565; p=0.23) were found (Figure 5H-I).
Figure 5. Comparable anxiety-like behavior, social behavior and VTA activity in virgin and late postpartum female rats.
A-C) No differences were found between virgin and late postpartum females in A) open arm entries (p=0.20), B) open arm time (p=0.23), or C) total number of entries in the EPM (p=0.40). D-F) No differences between virgin and late postpartum females in D) social sniff time (p=0.48), E) number of crossings into the social chamber (p=0.49) or F) total number of chamber crossings (p=0.56). G-I) Virgins and late postpartum females exhibit comparable VTA activity. No differences were found in G) population activity (p=0.26), H) firing rate (p=0.23) or I) burst firing (p=0.23). Error bars represent mean ± SEM. Gray bars represent virgins (behavior: n=8, recordings: n=8), pink bars represent late (i.e. 22-24 days) postpartum females (behavior: n=9-12 per group, recordings: n=8 per group).
4. Discussion
This is the first study to assess affect-related behaviors and the electrophysiological activity of VTA DA neurons during the early and late postpartum period in rodents. We identified time-dependent changes in a subset of affective behaviors that coexisted with an attenuation of VTA population activity, with effects being limited to early postpartum females. Late postpartum females and virgin rats exhibited comparable levels of anxiety-like behavior, social motivation and VTA activity. Collectively, these data suggest that normative changes in behavior and DA activity are limited to the first 3 days postpartum.
Behavioral changes during the early postpartum period in rodents
1-day postpartum female rats exhibited increased anxiety-like behavior (i.e. reduced percentages of open arm entries and open arm time) in the EPM compared to virgins and 1-week postpartum females, suggesting a temporary increase in anxiety-like behavior shortly after parturition. 1-day postpartum females also exhibited a reduction in the total number of arm entries, suggesting decreased locomotion in this group. These findings are in contrast with a prior study conducted in Long Evans rats indicating reduced anxiety-like behavior and increased total arm entries at 1-day postpartum37. Methodological differences including testing room habituation periods, illumination conditions, estrous cycle stage, composition of control groups, and test duration could have contributed to this discrepancy60. For example, our virgin control group was not stratified by estrous cycle and this may have contributed to our result, since anxiety-like behavior in the EPM varies throughout the estrous cycle33, 34.
A separate cohort of rats was tested for spontaneous locomotor activity (SLA) at baseline and in a different assay of anxiety-like behavior also based on approach-avoidance conflict (i.e. NSF). Early (i.e. 1d, 3d and 1-week) postpartum females and virgins did not differ in terms of total distance traveled and total time spent moving, indicating comparable baseline locomotor activity. This may suggest that the reduction in total arm entries in the EPM and SAT reflects a test and/or apparatus-dependent change in behavior. Early postpartum females tested in the NSF exhibited decreased latency to feed in a novel environment compared with virgin animals. These data are consistent with a prior study indicating that 1-day postpartum females consume more food in a novel environment than virgins61 and that early lactating females typically show a large increase in food consumption62, 63. Thus, we acknowledge that there are significant differences between females and early postpartum rats in food intake (i.e. increased food intake in early postpartum) and energy requirements (i.e. decreased energy expenditure in early postpartum)62, 64, which are associated with lactation and maternal behavior, that may contribute to the observed effects in the NSF. However, we also acknowledge that this is a confound that will be present in any study comparing virgin and postpartum rodents given that these differences are characteristic of the postpartum period. The finding of reduced latency to feed in the NSF is likely influenced by between group differences in food intake and energy expenditure and may reflect increased hunger due to metabolic needs rather than reduced anxiety-like behavior. Collectively, these data suggest alterations in anxiety-like behavior during the first week postpartum, although this effect may depend on the assay used and be influenced by task-dependent changes in locomotor activity.
Most studies assessing social behavior in postpartum female rodents have focused on aggression65, 66, sexual behavior67, 68, and maternal behaviors directed at pups69. However, there are many distinct aspects of social behavior and little is known about social approach/motivation to a novel conspecific in postpartum rats. We identified changes in social motivation to a novel conspecific during the first 3 days postpartum: 1d-PP and 3d-PP dams exhibited reduced social cage sniff time and fewer crossings into the chamber containing the younger female rat (i.e. social stimulus animal) compared with virgins, suggesting attenuated social motivation. Notably, reductions in social behavior are also observed following acute or chronic stress exposure and in rodent models relevant to depressive-like phenotypes, including females70, in which changes in glucocorticoid levels trigger social dysregulation42, 71. In the current study, our finding of reduced social motivation to a novel conspecific in 1d-PP and 3d-PP females is consistent with studies indicating elevated levels of total basal corticosterone (CORT) on postpartum day 1 and 3 in Sprague Dawley rats72-74, which may suggest that increased basal CORT levels contribute to the social behavior dysregulation observed in early postpartum females. Alternatively, reduced social approach in 1d-PP and 3d-PP dams may reflect an adaptive shift in social salience in favor of pup-related stimuli during the early stages of motherhood, as pup interaction is one of the most highly motivating behaviors in maternal mammals and can induce conditioned place preference (CPP) in postpartum rats28, 75. Since this is the first study to compare social approach in virgin and postpartum female rats, this remains to be more thoroughly explored. Nonetheless, 1d-PP and 3d-PP rats exhibiting decreased social motivation to a novel conspecific also had lower numbers of active VTA DA cells. This is significant given the role of VTA DA neurons in modulating social behavior: chemogenetic inhibition of VTA DA neurons attenuates social exploration of a nonfamiliar conspecific (i.e. social stimulus animal) in mice76 and stimulation of VTA DA neurons increases social approach/motivation in female mice77. Thus, it seems likely that attenuated social motivation in 1d-PP and 3d-PP reflects reduced dopaminergic tone within the VTA.
Finally, 1d-PP females exhibited increased FST immobility compared with virgins and 1week-PP rats. This is consistent with a prior report indicating no difference between virgins and 1-week postpartum females78, but extends it by identifying increased FST immobility at 1-day postpartum. Although this measure has been recently proposed to reflect a switch from active to passive coping in the face of an acute stressor47, 48, increased immobility is also a common outcome in rodent models relevant to depression24, 71, including females 29. Optogenetic activation of VTA DA neurons reverses increased immobility duration, increased kick frequency and promoted escape-related behaviors in stressed rats23, indicating a causal relationship between VTA DA neuron activity and FST immobility behavior regardless of whether this behavior reflects a switch in coping strategy or behavioral despair.
In sum, early postpartum females exhibit a transient behavioral dysregulation limited to a subset of tests (i.e. SAT, FST) during the first 3 days postpartum that overlaps with outcomes in rodent models of depression associated with alterations in DA function (i.e. attenuated VTA population activity). This is consistent with human studies indicating increased depressive-like symptomatology and poor social adjustment during late pregnancy and the early postpartum compared with non-childbearing women79.
Changes in VTA activity during the early postpartum period in rodents
1-day and 3-day postpartum females exhibited DA downregulation, as demonstrated by reduced numbers of spontaneously active VTA DA neurons (i.e. attenuated population activity) compared with virgin females, which was more pronounced in reward-related medial aspects of the VTA. These findings overlap with our prior reports of stress effects on the DA system in animal models of depression (i.e. learned helplessness, chronic mild stress)24, 53, in which DA downregulation (i.e. attenuation of VTA population activity) is observed25, with females showing greater effects29. The maternal HPA-axis undergoes dramatic changes during pregnancy and the postpartum12, 13, 80, 81. In rats, CORT levels peak during parturition and slowly fall over the postpartum period so that the early postpartum period is characterized by sustained high flattened levels of glucocorticoids11, 72, 82, which is a similar hormonal profile observed in depressed patients and chronically stressed female rats80, 83. Thus, in the context of our current study, elevated basal levels of CORT at 1 and 3 days postpartum72-74 may contribute to the attenuation in VTA population activity observed in 1-day and 3-day postpartum females.
Importantly, DA neurons must be spontaneously active to respond to behaviorally relevant, phasic inputs84, 85. Therefore, a change in population activity, or the number of spontaneously active DA cells, is thought to the reflect the responsivity of the DA system, which is altered in several disease states86. Our finding of reduced DA population activity specifically within the medial tracks of the VTA is consistent with prior studies by our group showing a preferential reduction of population activity within the medial and central VTA regions in rats exposed to chronic mild stress or learned helplessness52, 53. Because the majority of the DA neurons in medial VTA project to the ventromedial accumbens, reducing the active number would be expected to decrease the response of ventromedial accumbens projecting DA neurons to reward-related stimuli86, as only spontaneously active DA neurons are capable of responding to signals with burst firing87. Thus, a decrease in DA neuron population activity would attenuate stimulus-driven DA neuron responses, leading to diminished activation of the system. Prior reports have shown reduced basal DA release in the accumbens of 1d-PP rats compared with virgin rats88, which is consistent with reduced tonic activity of DA neurons within the medial VTA. Moreover, accumbens DA release is diminished in response to food in 1d-PP dams, but robustly stimulated by pup exposure88, suggesting a shift in sensitivity of phasic DA system responses towards offspring-related stimuli and away from stimuli not related to the offspring. This suggests that, in addition to changes in tonic VTA activity, we would likely find time-dependent changes in VTA activity in response to pup exposure, although this is beyond the scope of the current study. Since this is our group’s initial step in accounting for sex and reproductive status by incorporating postpartum females into our work, at this point we cannot determine whether the changes in VTA activity are driving the behavioral changes (i.e. SAT, FST) observed- only that the DA changes observed here (i.e. attenuation of population activity within the medial track of the VTA) are consistent with those we have previously seen in animal models of depression25, 29, 86. In addition, although both virgin and timed-pregnant rats were transported to our facility under the same conditions, it is possible that pregnant females may have experienced this stressor differently than virgin animals, which could be an indicator of increased stressed susceptibility.
Lastly, our results suggest that alterations in a subset of negative affect behaviors and DA activity in postpartum females may be limited to the first 3 days postpartum. Late postpartum females exhibited levels of anxiety-like and social behavior comparable to virgins, and no changes were observed in any parameter of VTA function assessed (population activity, firing rate, burst firing).
5. Conclusion
Taken together, these findings suggest normative changes in a subset of affect-related behavior and VTA activity that are confined to the first 3 days postpartum. Our findings of increased depression-related phenotypes and attenuated VTA DA activity in early postpartum female rats suggest that parity and the onset of motherhood comes with an unexpected cost involving negative (albeit transient) effects on DA system function.
Figure 4. Reduced VTA DA population activity during the first 3 days postpartum.
A) 1- and 3-day postpartum females exhibited a reduction in the number of spontaneously active DA cells in the VTA (i.e. population activity) compared with virgins (p < 0.05). B) Early postpartum females exhibited a selective attenuation of DA neuron activity in the medial aspect of the VTA (p <0.01). C,D) No changes between virgins and early postpartum females were observed in C) firing rate (p=0.95) or D) percentage of spikes firing in bursts (p=0.08). *p < 0.05, ** p < 0.01, #p=0.06, Error bars represent mean ± SEM. Gray bars represent virgins (n=8) and red bars represent postpartum females (n=9-12 per group).
Highlights:
1- and 3-day postpartum females exhibit reduced social approach compared to virgins
1-day postpartum females exhibit task-dependent changes in anxiety-like behavior
1-day postpartum females exhibit increased FST immobility compared to virgins
1- and 3-day postpartum females exhibit reduced VTA DA activity compared to virgins
Acknowledgments
We would like to thank Nicole MacMurdo and Christy Smolak for technical assistance with histology.
Funding Source
Research reported in this publication was supported by the National Institute of Mental Health (NIMH) under award numbers F32-MH110128 to M.R-C. and R01-MH101180 to A.A.G.
Footnotes
Role of Funding Source The National Institute of Mental Health (NIMH) had no role in study design, data collection, analyses, interpretation of data, writing the report or the decision to submit the article for publication.
Conflict of Interest Dr. Rincón-Cortés reports no potential conflicts of interest. Dr. Grace received consultant fees from Lundbeck, Pfizer, Otsuka, Asubio, Autofony, and Janssen, and is on the advisory board for Alkermes, Newron and Takeda.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All animal experiments comply with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978) and the manuscript clearly indicates that such guidelines have been followed under the Animals section in Materials and Methods. The sex of the animals has also been indicated, as well as the association of sex on the results of the study.
REFERENCES
- 1.O'Hara MW, Wisner KL. Perinatal mental illness: definition, description and aetiology. Best Pract Res Clin Obstet Gynaecol 2014; 28(1): 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henshaw C. Mood disturbance in the early puerperium: a review. Arch Womens Ment Health 2003; 6 Suppl 2: S33–42. [DOI] [PubMed] [Google Scholar]
- 3.Seyfried LS, Marcus SM. Postpartum mood disorders. Int Rev Psychiatry 2003; 15(3): 231–242. [DOI] [PubMed] [Google Scholar]
- 4.Pawluski JL, Lonstein JS, Fleming AS. The Neurobiology of Postpartum Anxiety and Depression. Trends Neurosci 2017; 40(2): 106–120. [DOI] [PubMed] [Google Scholar]
- 5.Pitt B. 'Maternity blues'. Br J Psychiatry 1973; 122(569): 431–433. [DOI] [PubMed] [Google Scholar]
- 6.O'Hara MW, Schlechte JA, Lewis DA, Wright EJ. Prospective study of postpartum blues. Biologic and psychosocial factors. Arch Gen Psychiatry 1991; 48(9): 801–806. [DOI] [PubMed] [Google Scholar]
- 7.Thurgood S, Avery DM, and Williamson Lloyda. Postpartum Depression (PPD). American Journal of Clinical Medicine 2009; 6(2): 17–22. [Google Scholar]
- 8.Henshaw C, Foreman D, Cox J. Postnatal blues: a risk factor for postnatal depression. J Psychosom Obstet Gynaecol 2004; 25(3-4): 267–272. [DOI] [PubMed] [Google Scholar]
- 9.Reck C, Stehle E, Reinig K, Mundt C. Maternity blues as a predictor of DSM-IV depression and anxiety disorders in the first three months postpartum. J Affect Disord 2009; 113(1-2): 77–87. [DOI] [PubMed] [Google Scholar]
- 10.Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry 2003; 44(3): 234–246. [DOI] [PubMed] [Google Scholar]
- 11.Brummelte S, Galea LA. Depression during pregnancy and postpartum: contribution of stress and ovarian hormones. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34(5): 766–776. [DOI] [PubMed] [Google Scholar]
- 12.Mastorakos G, Ilias I. Maternal hypothalamic-pituitary-adrenal axis in pregnancy and the postpartum period. Postpartum-related disorders. Ann N Y Acad Sci 2000; 900: 95–106. [DOI] [PubMed] [Google Scholar]
- 13.Rincon-Cortes M, Herman JP, S. L, Maguire J, Shansky RM. Stress: Influence of sex, reproductive status and gender. Neurobiology of Stress 2019; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Post C, Leuner B. The maternal reward system in postpartum depression. Arch Womens Ment Health 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunton PJ, Russell JA. The expectant brain: adapting for motherhood. Nat Rev Neurosci 2008; 9(1): 11–25. [DOI] [PubMed] [Google Scholar]
- 16.Champagne FA, Curley JP. Plasticity of the Maternal Brain Across the Lifespan. New Dir Child Adolesc Dev 2016; 2016(153): 9–21. [DOI] [PubMed] [Google Scholar]
- 17.Belujon P, Grace AA. Dopamine System Dysregulation in Major Depressive Disorders. Int J Neuropsychopharmacol 2017; 20(12): 1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nestler EJ, Carlezon WA Jr. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 2006; 59(12): 1151–1159. [DOI] [PubMed] [Google Scholar]
- 19.Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol 2009; 30(1): 46–64. [DOI] [PubMed] [Google Scholar]
- 20.Moses-Kolko EL, Price JC, Wisner KL, Hanusa BH, Meltzer CC, Berga SL, et al. Postpartum and depression status are associated with lower [[(1)(1)C]raclopride BP(ND) in reproductive-age women. Neuropsychopharmacology 2012; 37(6): 1422–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moses-Kolko EL, Fraser D, Wisner KL, James JA, Saul AT, Fiez JA, et al. Rapid habituation of ventral striatal response to reward receipt in postpartum depression. Biol Psychiatry 2011; 70(4): 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nephew BC, Murgatroyd C, Pittet F, Febo M. Brain Reward Pathway Dysfunction in Maternal Depression and Addiction: A Present and Future Transgenerational Risk. J Reward Defic Syndr 2015; 1(3): 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 2013; 493(7433): 537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang CH, Grace AA. Amygdala-ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol Psychiatry 2014; 76(3): 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufling J. Alterations and adaptation of ventral tegmental area dopaminergic neurons in animal models of depression. Cell Tissue Res 2019. [DOI] [PubMed] [Google Scholar]
- 26.Haim A, Sherer M, Leuner B. Gestational stress induces persistent depressive-like behavior and structural modifications within the postpartum nucleus accumbens. Eur J Neurosci 2014; 40(12): 3766–3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci 2001; 115(3): 683–694. [DOI] [PubMed] [Google Scholar]
- 28.Trezza V, Campolongo P, Vanderschuren LJ. Evaluating the rewarding nature of social interactions in laboratory animals. Dev Cogn Neurosci 2011; 1(4): 444–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rincon-Cortes M, Grace AA. Sex-Dependent Effects of Stress on Immobility Behavior and VTA Dopamine Neuron Activity: Modulation by Ketamine. Int J Neuropsychopharmacol 2017; 20(10): 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Guzman RM, Saulsbery AI, Workman JL. High nursing demand reduces depression-like behavior despite increasing glucocorticoid concentrations and reducing hippocampal neurogenesis in late postpartum rats. Behavioural brain research 2018; 353: 143–153. [DOI] [PubMed] [Google Scholar]
- 31.van der Schoot P, Lankhorst RR, de Roo JA, de Greef WJ. Suckling stimulus, lactation, and suppression of ovulation in the rat. Endocrinology 1978; 103(3): 949–956. [DOI] [PubMed] [Google Scholar]
- 32.Leuner B, Shors TJ. Learning during motherhood: A resistance to stress. Horm Behav 2006; 50(1): 38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav 2001; 74(4-5): 435–440. [DOI] [PubMed] [Google Scholar]
- 34.Diaz-Veliz G, Alarcon T, Espinoza C, Dussaubat N, Mora S. Ketanserin and anxiety levels: influence of gender, estrous cycle, ovariectomy and ovarian hormones in female rats. Pharmacol Biochem Behav 1997; 58(3): 637–642. [DOI] [PubMed] [Google Scholar]
- 35.Lukas M, Neumann ID. Social preference and maternal defeat-induced social avoidance in virgin female rats: sex differences in involvement of brain oxytocin and vasopressin. J Neurosci Methods 2014; 234: 101–107. [DOI] [PubMed] [Google Scholar]
- 36.Perez SM, Chen L, Lodge DJ. Alterations in dopamine system function across the estrous cycle of the MAM rodent model of schizophrenia. Psychoneuroendocrinology 2014; 47: 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lonstein JS. Reduced anxiety in postpartum rats requires recent physical interactions with pups, but is independent of suckling and peripheral sources of hormones. Horm Behav 2005; 47(3): 241–255. [DOI] [PubMed] [Google Scholar]
- 38.Craft RM, Kostick ML, Rogers JA, White CL, Tsutsui KT. Forced swim test behavior in postpartum rats. Pharmacol Biochem Behav 2010; 96(4): 402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rincon-Cortes M, Gagnon KG, Dollish HK, Grace AA. Diazepam reverses increased anxiety-like behavior, social behavior deficit, and dopamine dysregulation following withdrawal from acute amphetamine. Neuropsychopharmacology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klinger KK, Gomes FV, Rincon-Cortes M, Grace AA. Female rats are resistant to the long-lasting neurobehavioral changes induced by adolescent stress exposure. . European Neuropsychopharmacology 2019; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 1985; 14(3): 149–167. [DOI] [PubMed] [Google Scholar]
- 42.Sandi C, Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci 2015; 16(5): 290–304. [DOI] [PubMed] [Google Scholar]
- 43.Yan CG, Rincon-Cortes M, Raineki C, Sarro E, Colcombe S, Guilfoyle DN, et al. Aberrant development of intrinsic brain activity in a rat model of caregiver maltreatment of offspring. Transl Psychiatry 2017; 7(1): e1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bodnoff SR, Suranyi-Cadotte BE, Quirion R, Meaney MJ. Role of the central benzodiazepine receptor system in behavioral habituation to novelty. Behav Neurosci 1989; 103(1): 209–212. [DOI] [PubMed] [Google Scholar]
- 45.Britton DR, Britton KT. A sensitive open field measure of anxiolytic drug activity. Pharmacol Biochem Behav 1981; 15(4): 577–582. [DOI] [PubMed] [Google Scholar]
- 46.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature 1977; 266(5604): 730–732. [DOI] [PubMed] [Google Scholar]
- 47.Molendijk ML, de Kloet ER. Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology 2015; 62: 389–391. [DOI] [PubMed] [Google Scholar]
- 48.Commons KG, Cholanians AB, Babb JA, Ehlinger DG. The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS Chem Neurosci 2017; 8(5): 955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Molendijk ML, de Kloet ER. Coping with the forced swim stressor: Current state-of-the-art. Behavioural brain research 2019; 364: 1–10. [DOI] [PubMed] [Google Scholar]
- 50.Rincon-Cortes M, Gagnon KG, Dollish HK, Grace AA. Diazepam reverses increased anxiety-like behavior, social behavior deficit, and dopamine dysregulation following withdrawal from acute amphetamine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2018; 43(12): 2418–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev 2007; 56(1): 27–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moreines JL, Owrutsky ZL, Grace AA. Involvement of Infralimbic Prefrontal Cortex but not Lateral Habenula in Dopamine Attenuation After Chronic Mild Stress. Neuropsychopharmacology 2017; 42(4): 904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Belujon P, Grace AA. Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry 2014; 76(12): 927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience 1983; 10(2): 301–315. [DOI] [PubMed] [Google Scholar]
- 55.Ungless MA, Grace AA. Are you or aren't you? Challenges associated with physiologically identifying dopamine neurons. Trends in neurosciences 2012; 35(7): 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci 1984; 4(11): 2866–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valenti O, Cifelli P, Gill KM, Grace AA. Antipsychotic drugs rapidly induce dopamine neuron depolarization block in a developmental rat model of schizophrenia. J Neurosci 2011; 31(34): 12330–12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci 1984; 4(11): 2877–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walsh JJ, Han MH. The heterogeneity of ventral tegmental area neurons: Projection functions in a mood-related context. Neuroscience 2014; 282: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lonstein JS. Regulation of anxiety during the postpartum period. Front Neuroendocrinol 2007; 28(2-3): 115–141. [DOI] [PubMed] [Google Scholar]
- 61.Ferreira A, Hansen S, Nielsen M, Archer T, Minor BG. Behavior of mother rats in conflict tests sensitive to antianxiety agents. Behav Neurosci 1989; 103(1): 193–201. [DOI] [PubMed] [Google Scholar]
- 62.Woodside B, Budin R, Wellman MK, Abizaid A. Many mouths to feed: the control of food intake during lactation. Front Neuroendocrinol 2012; 33(3): 301–314. [DOI] [PubMed] [Google Scholar]
- 63.Widdowson EM. Changes in the body and its organs during lactation: nutritional implications. Ciba Found Symp 1976;(45): 103–118. [DOI] [PubMed] [Google Scholar]
- 64.Roberts SB, Coward WA. Lactation increases the efficiency of energy utilization in rats. J Nutr 1984; 114(12): 2193–2200. [DOI] [PubMed] [Google Scholar]
- 65.Mayer AD, Reisbick S, Siegel HI, Rosenblatt JS Maternal Aggression in Rats: Changes Over Pregnancy and Lactation in a Sprague-Dawley Strain. Aggressive Behavior 1987; 13: 29–43. [Google Scholar]
- 66.de Almeida RM, Ferreira A, Agrati D. Sensory, hormonal, and neural basis of maternal aggression in rodents. Curr Top Behav Neurosci 2014; 17: 111–130. [DOI] [PubMed] [Google Scholar]
- 67.Sodersten P, Hansen S, Eneroth P. Inhibition of sexual behaviour in lactating rats. J Endocrinol 1983; 99(2): 189–197. [DOI] [PubMed] [Google Scholar]
- 68.Eliasson M, Meyerson BJ. Sexual preference in female rats during estrous cycle, pregnancy and lactation. Physiol Behav 1975; 14(6): 705–710. [DOI] [PubMed] [Google Scholar]
- 69.Olazabal DE, Pereira M, Agrati D, Ferreira A, Fleming AS, Gonzalez-Mariscal G, et al. Flexibility and adaptation of the neural substrate that supports maternal behavior in mammals. Neurosci Biobehav Rev 2013; 37(8): 1875–1892. [DOI] [PubMed] [Google Scholar]
- 70.Iniguez SD, Flores-Ramirez FJ, Riggs LM, Alipio JB, Garcia-Carachure I, Hernandez MA, et al. Vicarious Social Defeat Stress Induces Depression-Related Outcomes in Female Mice. Biol Psychiatry 2018; 83(1): 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scheggi S, De Montis MG, Gambarana C. Making Sense of Rodent Models of Anhedonia. Int J Neuropsychopharmacol 2018; 21(11): 1049–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pawluski JL, Charlier TD, Lieblich SE, Hammond GL, Galea LA. Reproductive experience alters corticosterone and CBG levels in the rat dam. Physiol Behav 2009; 96(1): 108–114. [DOI] [PubMed] [Google Scholar]
- 73.Leuner B, Mirescu C, Noiman L, Gould E. Maternal experience inhibits the production of immature neurons in the hippocampus during the postpartum period through elevations in adrenal steroids. Hippocampus 2007; 17(6): 434–442. [DOI] [PubMed] [Google Scholar]
- 74.Fischer D, Patchev VK, Hellbach S, Hassan AH, Almeida OF. Lactation as a model for naturally reversible hypercorticalism plasticity in the mechanisms governing hypothalamo-pituitary- adrenocortical activity in rats. J Clin Invest 1995; 96(3): 1208–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fleming AS, Korsmit M, Deller M . Rat pups are potent reinforcers ot the maternal animal: Effects of experience, parity, hormones and dopamine function. Psychobiology 1994; 22(1): 44–53. [Google Scholar]
- 76.Bariselli S, Hornberg H, Prevost-Solie C, Musardo S, Hatstatt-Burkle L, Scheiffele P, et al. Role of VTA dopamine neurons and neuroligin 3 in sociability traits related to nonfamiliar conspecific interaction. Nat Commun 2018; 9(1): 3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, et al. Natural neural projection dynamics underlying social behavior. Cell 2014; 157(7): 1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walker CD, Trottier G, Rochford J, Lavallee D. Dissociation between behavioral and hormonal responses to the forced swim stress in lactating rats. J Neuroendocrinol 1995; 7(8): 615–622. [DOI] [PubMed] [Google Scholar]
- 79.O'Hara MW, Zekoski EM, Philipps LH, Wright EJ. Controlled prospective study of postpartum mood disorders: comparison of childbearing and nonchildbearing women. J Abnorm Psychol 1990; 99(1): 3–15. [DOI] [PubMed] [Google Scholar]
- 80.Gobinath AR, Mahmoud R, Galea LA. Influence of sex and stress exposure across the lifespan on endophenotypes of depression: focus on behavior, glucocorticoids, and hippocampus. Front Neurosci 2014; 8: 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lightman SL, Windle RJ, Wood SA, Kershaw YM, Shanks N, Ingram CD. Peripartum plasticity within the hypothalamo-pituitary-adrenal axis. Prog Brain Res 2001; 133: 111–129. [DOI] [PubMed] [Google Scholar]
- 82.Voogt JL, Sar M, Meites J. Influence of cycling, pregnancy, labor, and suckling on corticosterone-ACTH levels. Am J Physiol 1969; 216(3): 655–658. [DOI] [PubMed] [Google Scholar]
- 83.Brummelte S, Galea LA. Postpartum depression: Etiology, treatment and consequences for maternal care. Horm Behav 2016; 77: 153–166. [DOI] [PubMed] [Google Scholar]
- 84.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci 2007; 30(5): 220–227. [DOI] [PubMed] [Google Scholar]
- 85.Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci 2003; 6(9): 968–973. [DOI] [PubMed] [Google Scholar]
- 86.Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci 2016; 17(8): 524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology 2006; 31(7): 1356–1361. [DOI] [PubMed] [Google Scholar]
- 88.Afonso VM, King S, Chatterjee D, Fleming AS. Hormones that increase maternal responsiveness affect accumbal dopaminergic responses to pup- and food-stimuli in the female rat. Horm Behav 2009; 56(1): 11–23. [DOI] [PubMed] [Google Scholar]