Abstract
Bullous pemphigoid (BP) is an autoantibody-mediated blistering disease that is often associated with neurologic disease. BP antibodies target two epidermal adhesion molecules, known as BP180 and BP230. Homologues to these proteins are found in the brain, and it is hypothesized that neurologic disease leads to the production of autoantibodies that can cross react with their cutaneous forms. To better understand the link between BP and neurologic disease, we evaluated primary demographic features (age, sex, race, ethnicity and elapsed time between onset of skin symptoms and BP diagnosis), severity of BP, and IgG and IgE autoantibody levels in BP controls and BP patients with preceding Parkinson’s disease (PD), dementia (DEM), and stroke (STR). The main findings of this study are that BP patients with preceding neurologic disease have a shorter elapsed time between onset of skin disease and BP diagnosis and that subjects with preceding PD or DEM, but not STR, are significantly older than BP patients without neurologic disease. However, no significant differences in clinical presentation, BP severity scores or autoantibody (IgG and IgE) responses were observed amongst the groups. These findings suggest that, despite the age difference, the clinical phenotype of BP is not affected by preceding neurologic disease.
Keywords: bullous pemphigoid, autoantibody, Collagen XVII, neurologic disease, dementia
INTRODUCTION
Bullous pemphigoid (BP) is an autoimmune blistering disease characterized by autoantibodies targeting epidermal adhesion molecules, BP180 (collagen XVII) and BP230 (Diaz et al., 1990, Stanley et al., 1988). BP primarily affects individuals ≥ 70 years old and disease risk increases with age (Hubner et al., 2016, Langan et al., 2008). Worldwide, there is an increasing incidence of BP that varies by geographic location; reported rates range from 2.4 to 50 cases per million individuals per year (Brick et al., 2014, Hubner et al., 2016, Joly et al., 2012, Langan et al., 2008, Marazza et al., 2009). An association of BP with neurologic disease was first reported in a 1985 case series of three patients with multiple sclerosis (MS) who developed severe, generalized BP (Simjee et al., 1985). Subsequently, several large case-control and population-based studies estimate that individuals with neurologic disease, such as MS, dementia (DEM) and stroke (STR), are 1.8 – 10.7 times more likely to develop BP than the general population (Bastuji-Garin et al., 2011, Chen Y. J. et al., 2011, Cordel et al., 2007, Forsti et al., 2016, Kibsgaard et al., 2017, Langan et al., 2011, Ren et al., 2017, Taghipour et al., 2010, Yu Phuan et al., 2017). Based on these studies, neurologic disease is now recognized as one of the most common BP comorbidities, affecting 30-60% of patients.
The link between neurologic disease and BP suggests a common mechanism of disease susceptibility or pathogenesis. One possibility is that progressive neurodegeneration and inflammation leads to exposure of neuronal isoforms of the BP180 and BP230 proteins, which facilitates cross reactivity to the skin isoforms (Brown et al., 1995, Kunzli et al., 2016, Li et al., 2009, Seppänen, 2013, Seppanen et al., 2006). Evidence in support of this hypothesis is provided by studies demonstrating serum IgG reactivity to the skin isoforms of BP180 and BP230 in patients who have neurologic disease but not BP (Chen J. et al., 2011, Foureur et al., 2006, Kokkonen et al., 2017, Messingham et al., 2016). Similarly, IgG reactivity to 230-kDa protein of human brain extract was detected in serum from BP patients who did not have neurologic disease (Chen J. et al., 2011).
The significance of the BP180- and BP230-specific antibodies in the pathogenesis of neurologic disease, and their role in the eventual development of BP, remains unclear. Serum IgG antibody reactivity to skin antigens is reported only in a subset (20% or less) of patients with neurologic disease (Foureur et al., 2006, Messingham et al., 2016, Recke et al., 2016). Furthermore, IgE antibodies have not been assessed in patients with BP and neurologic disease, despite the critical role of this autoantibody subclass in the pathogenesis of BP (Fairley et al., 2009). To better understand the association between neurologic disease and BP, we examined patient demographics and circulating IgG and IgE autoantibody levels in BP patients with and without neurologic disease and further determined if particular features were associated with preceding neurologic disease.
RESULTS
Of the 112 BP patients initially identified in our database, 35 (31%) were diagnosed with a preexisting neurologic disease; two of these were excluded due to an unspecified neurologic disease that could not be classified. The remaining 110 BP patients were categorized as follows: 77 BP patients without neurologic disease (BP CONtrol) and 33 with preceding neurologic disease (ND + BP) which could be sub-divided as 11 Parkinson’s disease (PD) + BP, 11 dementia (DEM) + BP, and 11 stroke (STR) + BP. Analysis of patient demographics (Table S1) revealed a similar male to female incidence when ND + BP (43% male) and BP CON (45% male) were compared. When broken down by type of neurologic disease, the STR + BP group was also 45% male, while the PD + BP was 90% male, and the DEM + BP was only 27% male, but these differences were not statistically significant using a chi-square test. Additionally, no differences in race or ethnicity were noted among the study groups, which is typical of BP (Table S1).
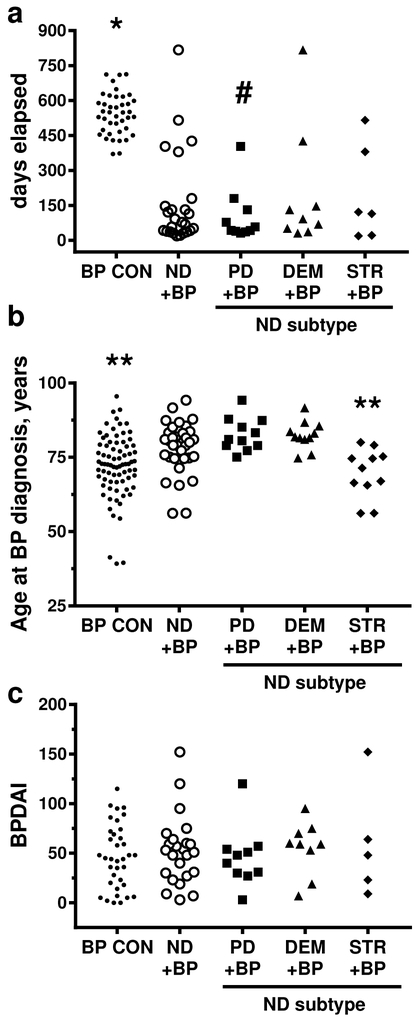
Although preceding neurologic disease increases individual risk of developing BP (Bastuji-Garin et al., 2011, Chen Y. J. et al., 2011, Ren et al., 2017), it is not established how it impacts the progression to clinical BP. Studies investigating the association of neurologic disease and BP are problematic. The main issue is that it is difficult to estimate the time between the onset of neurologic disease and development of BP since cognitive decline is gradual and, often, self-reported. In this study, this is further complicated because most of the patients were referred by outside physicians specifically for treatment of BP, but their neurologist was off-site. Based on the information available, the time between onset of skin disease and BP diagnosis (days elapsed) and the age of BP onset were evaluated (Figure 1A, B). The days elapsed was calculated based on the date of first skin symptoms, as perceived by the patient. This information was available for 41 BP CON, 10 PD + BP, 8 DEM + BP and 6 STR + BP subjects. Overall, subjects with all types neurologic disease had a significantly (p<0.0001) shorter time between symptom onset and definitive diagnosis of BP (Table 1). In addition, subjects with PD had a significantly shorter (p<0.0001) interval to diagnosis of BP than subjects with preceding DEM or STR. Interestingly, when the age of subjects at the time of BP diagnosis was compared (Table 1), subjects with preceding PD (82.7 ± 5.6 years) or DEM (82.6 ± 4.9 years) were significantly older than either BP CON (71.6 ± 10.8 years) or STR+ BP (69.6 ± 8.2 years)(p-values range from 0.0030-0.0052). Upon clinical examination, no differences in morphologic presentation of BP, such as localization of lesions, or BPDAI (Murrell et al., 2012) scores were observed with any type preceding neurologic disease (Figure 1C, Table 1).
Figure 1. Initial presentation of BP with or without preceding neurologic disease.
Subjects were BP controls (BP CON), and BP patients with preceding neurologic disease (ND); Parkinson’s disease (PD+BP), dementia (DEM+BP), stroke (STR+BP). A) Days elapsed was calculated based on the time between the onset of skin symptoms and BP diagnosis; B) Age at BP onset, years; C) Disease severity calculated using the Bullous Disease Area Index (BPDAI). Each point represents an individual patient. Using generalized linear models with a Bonferroni correction a p ≤ 0.0071 was required for significance; * = vs. all other groups, # = vs. DEM or STR, ** = vs. PD or DEM.
Table 1:
Analysis of days elapsed, age at diagnosis and BPDAI scores in study subjects.
| Days elapsed 2 | Age at diagnosis, years 3 | BPDAI 3, 4 | ||||
|---|---|---|---|---|---|---|
| Comparison 1 | Mean Ratio 5 95% CI |
p-value | Mean Ratio 95% CI |
p-value | Mean Ratio 95% CI |
p-value |
| ND+BP vs. BP CON | 0.515 0.496-0.534 |
<.0001 | 1.085 1.021-1.124 |
0.0085 | 1.059 0.707-1.587 |
0.7762 |
| PD+BP vs. BP CON | 0.333 0.312-0.355 |
<.0001 | 1.146 1.048-1.252 |
0.0030 | 0.935 0.543-1.610 |
0.8042 |
| DEM+BP vs. BP CON | 0.661 0.627-0.696 |
<.0001 | 1.143 1.046-1.249 |
0.0036 | 1.120 0.635-1.974 |
0.6911 |
| STR+ BP vs. BP CON | 0.624 0.587-0.663 |
<.0001 | 0.965 0.883-1.055 |
0.4297 | 1.200 0.582-2.476 |
0.6154 |
| PD+BP vs. STR+BP | 0.534 0.491-0.581 |
<.0001 | 1.187 1.056-1.335 |
0.0046 | 0.779 0.340-1.783 |
0.5474 |
| DEM+BP vs. STR+BP | 1.060 0.982-1.144 |
0.1337 | 1.184 1.053-1.331 |
0.0052 | 0.933 0.401-2.168 |
0.8693 |
| PD+BP vs. DEM+BP | 0.504 0.466-0.545 |
<.0001 | 1.003 0.892-1.128 |
0.9643 | 0.835 0.417-1.672 |
0.6045 |
Subjects included BP controls (BP CON), and BP patients with preceding neurologic disease (ND); Parkinson’s disease (PD+BP), dementia (DEM+BP), stroke (STR+BP)
calculated based on the interval between the onset of skin symptoms and BP diagnosis. N’s = 41 BP CON, 10 PD + BP, 8 DEM + BP, 6 STR + BP.
N’s = 77 BP CON, 11 PD + BP, 11 DEM + BP, 11 STR + BP.
Bullous Disease Area Index.
Mean ratio, 95% confidence interval (CI), and p-value are indicated for each comparison. A p-value <0.0071 was considered significant (bold font).
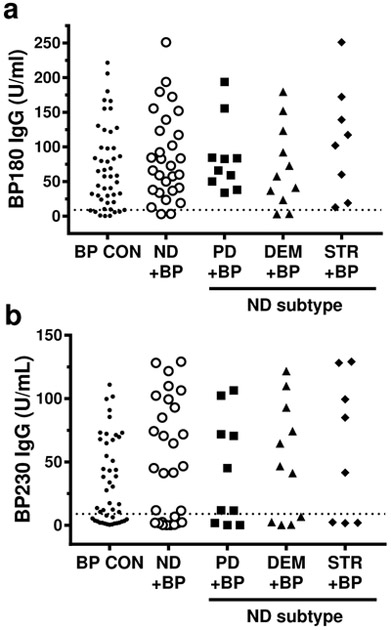
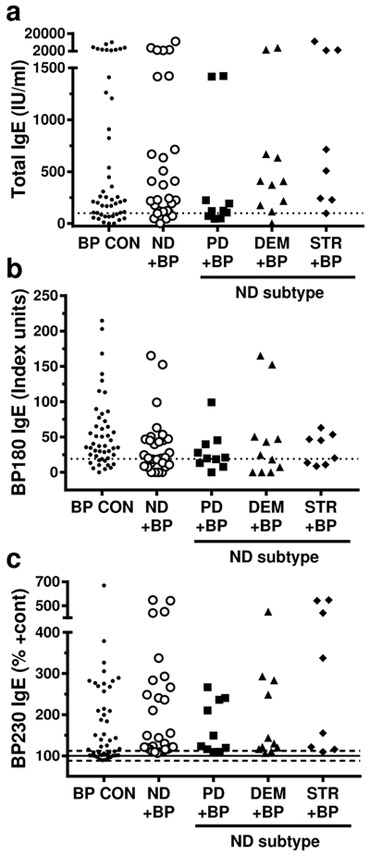
If preceding neurologic disease facilitates the development of autoantibodies reactive to cutaneous antigens, this could affect the autoantibody response observed in BP. Thus, circulating antibody levels were measured in patient sera by ELISA. Comparison of BP180 and BP230 IgG levels did not uncover any significant differences in the incidence or specificity of the IgG response in BP patients with or without neurologic disease; however, it is of interest that the highest levels of both antibodies were found in the STR + BP sera (Figure 2, Table 2, Table S2). Total IgE and BP antigen specific IgE, which have not been previously described in BP patients with neurologic disease, were also evaluated (Figure 3, Table 3, Table S2). Consistent with previous studies of BP examining IgE in BP (Iwata et al., 2008, Messingham et al., 2009), subjects with pre-existing neurologic disease exhibited robust production of both total and antigen-specific IgE. Although, no significant differences in the incidence or specificity of the IgE response were observed, the IgE antibody profiles did vary amongst the groups. Specifically, the median BP180 IgE concentration was highest in the BP CON group, while BP230 and total IgE was the highest in STR + BP (Figure 3, Table S2).
Figure 2. Serum IgG autoantibody levels in BP patients with and without neurologic disease.
Subjects were BP controls (BP CON), and BP patients with preceding neurologic disease (ND); Parkinson’s disease (PD+BP), dementia (DEM+BP), stroke (STR+BP). A) BP180- and B) BP230-specific IgG were measured by ELISA. The dashed line indicates the minimum value for a positive test, ≥ 9 Units/ml. Each point represents an individual patient. No statistically significant differences were observed.
Table 2:
Analysis of serum IgG autoantibodies.
| BP180 IgG 1 | BP230 IgG 1 | |||
|---|---|---|---|---|
| Comparison2 | Mean Ratio 3 95% CI |
p-value | Mean Ratio 95% CI |
p-value |
| ND+BP vs. BP CON | 1.234 0.788-1.933 |
0.3536 | 1.493 0.823-2.708 |
0.1840 |
| PD+BP vs. BP CON | 1.029 0.626-2.337 |
0.5675 | 1.241 0.516-2.981 |
0.6257 |
| DEM+BP vs. BP CON | 1.020 0.541-1.922 |
0.9510 | 1.500 0.646-3.487 |
0.3408 |
| STR+ BP vs. BP CON | 1.560 0.756-3.217 |
0.2249 | 1.798 0.687-4.711 |
0.2284 |
| PD+BP vs. STR+BP | 0.775 0.316-1.092 |
0.5735 | 0.690 0.209-2.277 |
0.5373 |
| DEM+BP vs. STR+BP | 0.654 0.271-1.575 |
0.3387 | 0.834 0.259-2.688 |
0.7585 |
| PD+BP vs. DEM+BP | 1.186 0.519-2.711 |
0.6827 | 0.827 0.275-2.484 |
0.7314 |
IgG antibodies specific for BP180 and BP230 were measured by ELISA.
Subjects included BP controls (BP CON), and BP patients with preceding neurologic disease (ND); Parkinson’s disease (PD+BP), dementia (DEM+BP), stroke (STR+BP)
Mean ratio, 95% confidence interval (CI), and p-value are indicated for each comparison. A p-value <0.0071 was considered significant.
Figure 3. Serum IgE antibody levels in BP patients with and without neurologic disease.
Subjects were BP controls (BP CON), and BP patients with preceding neurologic disease (ND); Parkinson’s disease (PD+BP), dementia (DEM+BP), stroke (STR+BP). A) Circulating total IgE was measured with electrochemiluminescence; normal level ≤100 U/ml (dashed line); B) BP180-specific IgE was measured by ELISA, positive test ≥ 19 units/ml; C) BP230-specific IgE was measured by ELISA; mean ± SD is indicated for N = 23 healthy controls is indicated. Each point represents an individual patient. No statistically significant differences were observed.
Table 3:
Analysis of serum IgE antibodies.
| BP180 IgE2 | BP230 IgE2 | Total IgE3 | ||||
|---|---|---|---|---|---|---|
| Comparison1 | Mean Ratiod 95% CI |
p-value | Mean Ratio 95% CI |
p-value | Mean Ratio 95% CI |
p-value |
| ND+BP vs. BP CON | 0.760 0.504-1.139 |
0.1801 | 1.195 0.934-1.530 |
0.1540 | 0.889 0.443-1.787 |
0.7383 |
| PD+BP vs. BP CON | 0.581 0.325-1.038 |
0.0661 | 0.944 0.665-1.340 |
0.7429 | 0.290 0.107-0.787 |
0.0159 |
| DEM+BP vs. BP CON | 1.135 0.617-2.089 |
0.6789 | 1.084 0.774-1.519 |
0.6331 | 0.778 0.297-2.035 |
0.6040 |
| STR+ BP vs. BP CON | 0.586 0.318-1.077 |
0.0804 | 1.663 1.132-2.443 |
0.0100 | 1.792 0.598-5.372 |
0.2933 |
| PD+BP vs. STR+BP | 0.992 0.458-2.150 |
0.9844 | 0.568 0.353-0.914 |
0.0205 | 0.162 0.041-0.631 |
0.0094 |
| DEM+BP vs. STR+BP | 1.939 0.875-4.296 |
0.1013 | 0.652 0.409-1.040 |
0.0720 | 0.434 0.114-1.648 |
0.2164 |
| PD+BP vs. DEM+BP | 0.512 0.236-1.109 |
0.0884 | 0.870 0.561-1.350 |
0.5299 | 0.372 0.106-1.306 |
0.1209 |
Subjects included BP controls (BP CON), and BP patients with preceding neurologic disease (ND); Parkinson’s disease (PD+BP), dementia (DEM+BP), stroke (STR+BP)
IgE autoantibodies specific for BP180 and BP230 were measured by ELISA.
Total IgE was measured electrochemiluminescence.
Mean ratio, 95% confidence interval (CI), and p-value are indicated for each comparison. A p-value <0.0071 was considered significant.
A Spearman’s correlation matrix was performed to evaluate the relationships between circulating antibodies, symptom duration, age of BP onset and BPDAI score. Subjects with neurologic disease were evaluated as a group and also by their individual diagnoses. The duration of skin symptoms (days elapsed) was not associated with any other measures in the BP CON group, but was correlated (p=0.036, r=0.459) with total IgE levels in the neurologic disease + BP group. Although the subjects with preceding neurologic disease were significantly older when diagnosed with BP, no correlations with age were observed in this group. However, in the BP CON group, age at BP diagnosis was inversely related (p=0.0003, r = −0.5086) to BP180 IgG levels.
Studies evaluating autoantibody profiles in BP show that disease severity often correlates with IgG and/or IgE autoantibody levels (Hashimoto et al., 2017, Iwata et al., 2008). In agreement, BPDAI scores of all study subjects strongly correlated with BP180 IgG levels (p=0.002, r = 0.5850 for BP CON; p=0.0212, r=0.468 for ND +BP). In in subjects without neurologic disease, BPDAI scores also correlated moderately with BP180 IgE (p=0.0173, r = 0.3944).
Relationships between different the different classes/specificities of antibodies were also evaluated. While several correlations were identified in the BP CON group, none were observed when subjects with neurologic disease were considered as a whole, nor were they consistent when broken down by type of neurologic disease. Briefly, BP180 IgG levels correlated moderately with BP180 IgE (p=0.0187, r=0.3491) in the BP CON group, but instead correlated strongly (p=0.0279, r=0.7857) with BP230 IgG in the STR + BP group. In contrast, BP230 IgG correlated (p=0.292, r=0.3183) only with total IgE in the BP CON group. Finally, BP180 IgE levels were inversely associated with both BP230 IgG (p = 0.0202, r=−0.7333) and BP230 IgE (p=0.034, r=−0.6848) only in subjects with PD + BP.
DISCUSSION
Preceding neurologic disease is one of the most common comorbidities of BP (Bastuji-Garin et al., 2011, Chen Y. J. et al., 2011, Ren et al., 2017). However, it has not been established whether the clinical phenotype of BP differs in the presence or absence of neurologic disease. In this report, patient demographics and circulating autoantibody levels were examined in BP patients with PD, DEM or STR and BP patients without neurologic disease. The main findings of this study are that BP patients with preceding neurologic disease have a shorter elapsed time between onset of skin disease and BP diagnosis and that subjects with preceding PD or DEM, but not STR, are significantly older than BP patients without neurologic disease. However, no significant differences in clinical presentation, BP severity scores or autoantibody (IgG and IgE) responses were observed amongst the groups.
It is hypothesized that the association between neurologic disease and subsequent BP results from antibodies initially generated in response to the inflammatory processes associated with neurologic disease (Chen J. et al., 2011, Li et al., 2009, Messingham et al., 2016, Seppänen, 2013). In this scenario, generation of cutaneous autoantibodies is dependent on transit of neuronal proteins and/or sensitized immune cells through the blood brain barrier. Thus, the progression to BP is likely dictated by the nature and timing of these events. We propose that the progression to BP is influenced by a combination of neurodegeneration and inflammation that is unique to each type of neurologic disease. This idea is consistent with a relatively short gap (5.5 years) between cerebral infarction and development of BP compared to a much longer gap (3-18 years) between the initial diagnosis of PD or DEM and the development of BP (Chen J. et al., 2011, Forsti et al., 2016, Simjee et al., 1985, Taghipour et al., 2010). We were unable to reliably ascertain the onset of neurologic disease in this study; however, based on the established age of onset of PD at 60 years (Pagano et al., 2016) and the onset of BP at mean age of 82.7 ± 5.6 years, a gap of 17-27 years is observed here.
Although serum IgG reactivity to brain and/or skin isoforms of BP180 and BP230 has been reported in patients with neurologic disease, it is not known if or how the specificity of these antibodies changes over time or what changes are associated with the development of BP (Chen J. et al., 2011, Foureur et al., 2006, Kokkonen et al., 2017, Messingham et al., 2016). Further, differences in the immunologic mechanisms leading to BP (with or without neurologic disease) could influence the manifestation of disease, autoantibody profiles, or both.
This study found a significantly shorter duration of skin symptoms prior to BP diagnosis in subjects with preceding neurologic disease. Although this observation may be biologically significant, it must be interpreted with caution. In subjects with neurologic disease, the duration of skin symptoms, such as itch, erosions or eczema, could be easily underestimated or go unnoticed by their representatives or caretakers. Indeed, the fact preceding neurologic disease was not associated with any differences in clinical presentation or severity of BP suggests that this may be the case.
In this report, preceding neurologic disease was not associated with any significant differences in the incidence or specificity of the IgG or IgE antibody response, which is in agreement with a previous study that examined only IgG (Gornowicz-Porowska et al., 2017). Additionally, BP180 IgG levels correlated with both the age of BP onset and disease severity regardless of neurologic disease. Thus, although the events leading to the development of cutaneous antibodies might differ, preceding neurologic disease may not alter the pathogenic mechanisms driving BP. In regard to the IgE autoantibody profiles, a larger number of patients are needed to tease out some potential differences associated with type of neurologic disease. Going forward, it would also be interesting to compare serum IgE reactivity to brain isoforms of BP180 and BP230 based the relative abundance of BP230 in the CNS (Brown et al., 1995) and the robust BP230-IgE response found here.
The possibility remains that preceding neurologic disease results in differences in autoantibody specificity that are not evident when the commercially available ELISAs are used. These kits only detect antibodies targeting a relatively small domain of the protein, known as NC16A, since their pathogenicity in BP is well established (Giudice et al., 1993, Zillikens et al., 1997). However, it is widely accepted that BP patients often have serum reactivity to regions of BP180 that are outside of NC16A (Fairley et al., 2013). Further, the epitope specificity may depend on the protein isoform driving the antibody response. In agreement, differences in serum reactivity to brain- or skin-derived proteins suggest that subjects with neurologic disease (and not BP) recognize different auto-epitopes than BP patients (Foureur et al., 2006, Kokkonen et al., 2017, Messingham et al., 2016). Indeed, a recent study (Tuusa et al., 2018) demonstrated, via epitope mapping of recombinant proteins, that sera from subjects with MS or Alzheimer’s disease recognize different regions of the BP180 protein than sera from BP patients. These findings explain why skin symptoms are not observed when BP180 antibodies are observed in patients with ND but not BP. To determine if BP180 and BP230-reactive antibodies play a role in the progression to BP, and if epitope spreading is required for the development of BP, longitudinal studies utilizing detailed epitope mapping of reactive sera from patients with well-characterized neurologic disease are needed.
Since only a fraction of individuals with neurologic disease go on to develop BP, the goal of this study to determine if any specific characteristics of these patients could be discerned. However, other than the timing of disease, we did not see any clinical or serologic phenotype that differentiates patients with neurologic disease compared to those who do not. Further, the medications for neurologic disease are commonly used and have not themselves been associated with BP at this point. Thus, additional studies are needed to further characterize these patients, and as personalized medicine advances, we may be able to identify predictive factors for the development of BP in the context of neurologic disease.
MATERIALS & METHODS
Study subjects
Patients with clinical and histologic characteristics of BP were recruited from the University of Iowa Hospitals and Clinics and written informed consent was obtained in compliance with the guidelines of the Institutional Review Board (IRB #201106752) and the Declaration of Helsinki Accords. A BP diagnosis was confirmed by detection of cutaneous autoantibodies via direct immunofluorescence, BP180/BP230 ELISA or immunoblot against the recombinant extracellular domain of BP180 (Fairley et al., 2013).
A self-reported history and medical records were used to identify preceding neurologic disease. Of the112 BP patients initially identified, two patients diagnosed with an unspecified autoimmune central nervous system disease were excluded. The remaining 110 BP patients were included: 77 BP CON, 11 PD + BP, 11 DEM + BP, and 11STR + BP. The type of DEM, and also the location, number of strokes and elapsed time between the stroke and the onset of BP, was heterogeneous. None of the patients in our database had MS.
Disease severity
Disease severity was scored using the bullous pemphigoid disease area index (BPDAI) (Murrell et al., 2012). Because some patients were enrolled prior to validation of the BPDAI, scores were available only on a subset of patients; 37 BP CON, 11 PD + BP, 9 DEM + BP, and 7 STR + BP.
Serum antibody detection
Serum antibodies were measured only subjects who had not received any prior corticosteroids or immunomodulatory treatment, resulting in the following N’s: 47 BP CON, 10 PD + BP, 11 DEM + BP and 8 STR + BP. IgG antibodies specific for BP180 and BP230 were evaluated using commercial ELISAs (MBL International, Woburn, MA). Of note, this study included 9 subjects (7 BP CON, 2 DEM+ BP) who were negative using the BP180 ELISA that targets the immunodominant NC16A region of BP180. BP180 ELISA-negative patients were included in the study if serum IgG reactivity to recombinant full length BP180 was verified with immunoblot (Fairley et al., 2013). Additionally, IgE autoantibodies specific for BP180 and/or BP230 were typically observed in these patients.
Total serum IgE levels were quantified using electrochemiluminescence performed by the institutional pathology laboratory. BP180 (NC16A)-specific IgE was evaluated by ELISA as described (Messingham et al., 2009). BP230-specific IgE was evaluated using the IgG kit with the substitution of an anti-IgE detection antibody that was previously verified as IgE-specific via immunoblot and ELISA.
Statistical Analysis
Statistical analysis was performed with assistance from Dr. Patrick Ten Eyck at the Institute for Clinical and Translational Science Biostatistics Core at the University of Iowa. A chi-square test was used to assess differences in sex ratios. Generalized linear models (GLMs) were used to compare the values of several outcome measures between different groups. Since all outcomes followed a right-skewed distribution, a gamma distribution and log link was specified to provide pairwise group estimates for the ratio of group means along with p-values. SAS 9.4 was used for all GLM analyses. We decided on a type I error rate, α, of 0.05 which necessitates a p-value ≤ 0.0071 for significance, based on the Bonferroni correction for 7 separate comparisons.
Supplementary Material
CREDIT STATEMENT.
Kelly Messingham played a role in Conceptualization, Formal Analysis, Funding Acquisition, Investigation, Project Administration, Supervision, Visualization, Writing-Original Draft and Writing-Review and Editing.
Adam Miller played a role in Investigation and Writing-Original Draft, Writing-Review and Editing.
Nandekumar Naranyan played a role in Conceptualization, Funding Acquisition, Investigation, Resources, Writing-Review and Editing.
Samuel Connell played a role in Investigation and Validation of data.
Janet Fairley played a role in Conceptualization, Data Curation, Funding Acquisition, Investigation, Methodology, Project Administration, Resources, Supervision, Validation, Writing-Original Draft, Writing-Review and Editing.
ACKNOWLEDGMENTS
This study was supported in part by The University of Iowa Clinical and Translational Science Award (KNM, NN, JAF) granted with funds from the NIH (U54 TR001356 and UL1 TR002537). We thank Julie McKillip, RN, for her assistance with the IRB, patient consent, and sample collection.
Abbreviations used:
- BP
bullous pemphigoid
- neurologic disease
neurologic disease
- DEM
dementia
- STR
stroke
- PD
Parkinson’s disease
- MS
multiple sclerosis
- CON
control
- BPDAI
bullous disease area index
Footnotes
CONFILICT OF INTEREST
The authors state no conflict of interest.
DATA AVAILABILITY STATEMENT
Primary research data, including de-identified patient demographics and classification, disease severity scores and raw ELISA values, are available free of charge to all researchers wherever possible and with minimal reuse restrictions.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bastuji-Garin S, Joly P, Lemordant P, Sparsa A, Bedane C, Delaporte E, et al. Risk factors for bullous pemphigoid in the elderly: a prospective case-control study. J Invest Dermatol 2011;131(3):637–43. [DOI] [PubMed] [Google Scholar]
- Brick KE, Weaver CH, Lohse CM, Pittelkow MR, Lehman JS, Camilleri MJ, et al. Incidence of bullous pemphigoid and mortality of patients with bullous pemphigoid in Olmsted County, Minnesota, 1960 through 2009. J Am Acad Dermatol 2014;71(1):92–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A, Bernier G, Mathieu M, Rossant J, Kothary R. The mouse dystonia musculorum gene is a neural isoform of bullous pemphigoid antigen 1. Nat Genet 1995;10(3):301–6. [DOI] [PubMed] [Google Scholar]
- Chen J, Li L, Chen J, Zeng Y, Xu H, Song Y, et al. Sera of Elderly Bullous Pemphigoid Patients with Associated Neurological Diseases Recognize Bullous Pemphigoid Antigens in the Human Brain. Gerontology 2011;57(3):211–6. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Wu CY, Lin MW, Chen TJ, Liao KK, Chen YC, et al. Comorbidity profiles among patients with bullous pemphigoid: a nationwide population-based study. Br J Dermatol 2011;165(3):593–9. [DOI] [PubMed] [Google Scholar]
- Cordel N, Chosidow O, Hellot MF, Delaporte E, Lok C, Vaillant L, et al. Neurological disorders in patients with bullous pemphigoid. Dermatology 2007;215(3):187–91. [DOI] [PubMed] [Google Scholar]
- Diaz LA, Ratrie H 3rd, Saunders WS, Futamura S, Squiquera HL, Anhalt GJ, et al. Isolation of a human epidermal cDNA corresponding to the 180-kD autoantigen recognized by bullous pemphigoid and herpes gestationis sera. Immunolocalization of this protein to the hemidesmosome. Journal of Clinical Investigation 1990;86(4):1088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairley JA, Baum CL, Brandt DS, Messingham KA. Pathogenicity of IgE in autoimmunity: successful treatment of bullous pemphigoid with omalizumab. J Allergy Clin Immunol 2009;123(3):704–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairley JA, Bream M, Fullenkamp C, Syrbu S, Chen M, Messingham KN. Missing the target: characterization of bullous pemphigoid patients who are negative using the BP180 enzyme-linked immunosorbant assay. J Am Acad Dermatol 2013;68(3):395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsti AK, Jokelainen J, Ansakorpi H, Seppanen A, Majamaa K, Timonen M, et al. Psychiatric and neurological disorders are associated with bullous pemphigoid - a nationwide Finnish Care Register study. Sci Rep 2016;6:37125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foureur N, Mignot S, Senet P, Verpillat P, Picard-Dahan C, Crickx B, et al. [Correlation between the presence of type-2 anti-pemphigoid antibodies and dementia in elderly subjects with no clinical signs of pemphigoid]. Ann Dermatol Venereol 2006;133(5 Pt 1):439–43. [DOI] [PubMed] [Google Scholar]
- Giudice GJ, Emery DJ, Zelickson BD, Anhalt GJ, Liu Z, Diaz LA. Bullous pemphigoid and herpes gestationis autoantibodies recognize a common non-collagenous site on the BP180 ectodomain. Journal of Immunology 1993;151(10):5742–50. [PubMed] [Google Scholar]
- Gornowicz-Porowska J, Seraszek-Jaros A, Bowszyc-Dmochowska M, Kaczmarek E, Pietkiewicz P, Bartkiewicz P, et al. Analysis of the autoimmune response against BP180 and BP230 in ethnic Poles with neurodegenerative disorders and bullous pemphigoid. Cent Eur J Immunol 2017;42(1):85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Ohzono A, Teye K, Numata S, Hiroyasu S, Tsuruta D, et al. Detection of IgE autoantibodies to BP180 and BP230 and their relationship to clinical features in bullous pemphigoid. British Journal of Dermatology 2017;177(1):141–51. [DOI] [PubMed] [Google Scholar]
- Hubner F, Recke A, Zillikens D, Linder R, Schmidt E. Prevalence and Age Distribution of Pemphigus and Pemphigoid Diseases in Germany. J Invest Dermatol 2016;136(12):2495–8. [DOI] [PubMed] [Google Scholar]
- Iwata Y, Komura K, Kodera M, Usuda T, Yokoyama Y, Hara T, et al. Correlation of IgE autoantibody to BP180 with a severe form of bullous pemphigoid. Archives of Dermatology 2008;144(1):41–8. [DOI] [PubMed] [Google Scholar]
- Joly P, Baricault S, Sparsa A, Bernard P, Bedane C, Duvert-Lehembre S, et al. Incidence and mortality of bullous pemphigoid in France. J Invest Dermatol 2012;132(8):1998–2004. [DOI] [PubMed] [Google Scholar]
- Kibsgaard L, Rasmussen M, Lamberg A, Deleuran M, Olesen AB, Vestergaard C. Increased frequency of multiple sclerosis among patients with bullous pemphigoid: a population-based cohort study on comorbidities anchored around the diagnosis of bullous pemphigoid. Br J Dermatol 2017;176(6):1486–91. [DOI] [PubMed] [Google Scholar]
- Kokkonen N, Herukka SK, Huilaja L, Kokki M, Koivisto AM, Hartikainen P, et al. Increased Levels of the Bullous Pemphigoid BP180 Autoantibody Are Associated with More Severe Dementia in Alzheimer's Disease. J Invest Dermatol 2017;137(1):71–6. [DOI] [PubMed] [Google Scholar]
- Kunzli K, Favre B, Chofflon M, Borradori L. One gene but different proteins and diseases: the complexity of dystonin and bullous pemphigoid antigen 1. Exp Dermatol 2016;25(1):10–6. [DOI] [PubMed] [Google Scholar]
- Langan SM, Groves RW, West J. The relationship between neurological disease and bullous pemphigoid: a population-based case-control study. J Invest Dermatol 2011;131(3):631–6. [DOI] [PubMed] [Google Scholar]
- Langan SM, Smeeth L, Hubbard R, Fleming KM, Smith CJ, West J. Bullous pemphigoid and pemphigus vulgaris--incidence and mortality in the UK: population based cohort study. BMJ 2008;337:a180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chen J, Wang B, Yao Y, Zuo Y. Sera from patients with bullous pemphigoid (BP) associated with neurological diseases recognized BP antigen 1 in the skin and brain. Br J Dermatol 2009;160(6):1343–5. [DOI] [PubMed] [Google Scholar]
- Marazza G, Pham HC, Scharer L, Pedrazzetti PP, Hunziker T, Trueb RM, et al. Incidence of bullous pemphigoid and pemphigus in Switzerland: a 2-year prospective study. Br J Dermatol 2009;161(4):861–8. [DOI] [PubMed] [Google Scholar]
- Messingham KA, Aust S, Helfenberger J, Parker KL, Schultz S, McKillip J, et al. Autoantibodies to Collagen XVII Are Present in Parkinson's Disease and Localize to Tyrosine-Hydroxylase Positive Neurons. J Invest Dermatol 2016;136(3):721–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messingham KA, Noe MH, Chapman MA, Giudice GJ, Fairley JA. A novel ELISA reveals high frequencies of BP180-specific IgE production in bullous pemphigoid. J Immunol Methods 2009;346(1–2):18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell DF, Daniel BS, Joly P, Borradori L, Amagai M, Hashimoto T, et al. Definitions and outcome measures for bullous pemphigoid: recommendations by an international panel of experts. J Am Acad Dermatol 2012;66(3):479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano G, Ferrara N, Brooks DJ, Pavese N. Age at onset and Parkinson disease phenotype. Neurology 2016;86(15):1400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recke A, Oei A, Hubner F, Fechner K, Graf J, Hagenah J, et al. Parkinson disease and multiple sclerosis are not associated with autoantibodies against structural proteins of the dermal-epidermal junction. Br J Dermatol 2016;175(2):407–9. [DOI] [PubMed] [Google Scholar]
- Ren Z, Hsu DY, Brieva J, Silverberg NB, Langan SM, Silverberg JI. Hospitalization, inpatient burden and comorbidities associated with bullous pemphigoid in the U.S.A. Br J Dermatol 2017;176(1):87–99. [DOI] [PubMed] [Google Scholar]
- Seppanen A Collagen XVII: A Shared Antigen in Neurodermatological Interactions? Clinical and Developmental Immunology 2013;2013:240570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppänen A, Autio-Harmainen H, Alafuzoff I, Sarkioja T, Veijola J, Hurskainen T, et al. Collagen XVII is expressed in human CNS neurons. Matrix Biol 2006;25(3):185–8. [DOI] [PubMed] [Google Scholar]
- Simjee S, Konqui A, Razzaque Ahmed A. Multiple sclerosis and bullous pemphigoid. Dermatologica 1985;170(2):86–9. [DOI] [PubMed] [Google Scholar]
- Stanley JR, Tanaka T, Mueller S, Klaus-Kovtun V, Roop D. Isolation of complementary DNA for bullous pemphigoid antigen by use of patients' autoantibodies. Journal of Clinical Investigation 1988;82(6):1864–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghipour K, Chi CC, Vincent A, Groves RW, Venning V, Wojnarowska F. The association of bullous pemphigoid with cerebrovascular disease and dementia: a case-control study. Arch Dermatol 2010;146(11):1251–4. [DOI] [PubMed] [Google Scholar]
- Tuusa J, Lindgren O, Tertsunen H-M, Nishie W, Kokkonen N, Huilaja L, et al. BP180 Autoantibodies Target Different Epitopes in Multiple Sclerosis or Alzheimer’s Disease than in Bullous Pemphigoid. Journal of Investigative Dermatology 2018. [DOI] [PubMed] [Google Scholar]
- Yu Phuan CZ, Yew YW, Tey HL. Bullous pemphigoid and antecedent neurological diseases: An association with dementia. Indian J Dermatol Venereol Leprol 2017;83(4):457–61. [DOI] [PubMed] [Google Scholar]
- Zillikens D, Rose PA, Balding SD, Liu Z, Olague-Marchan M, Diaz LA, et al. Tight clustering of extracellular BP180 epitopes recognized by bullous pemphigoid autoantibodies. Journal of Investigative Dermatology 1997;109(4):573–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.