Abstract
BACKGROUND:
Skin cancer is an uncommon cause of skull invasion, dural infiltration and brain parenchyma involvement.
CASE REPORT:
We report on a series of three elderly patients who presented with squamous cell carcinoma of the scalp with skull bone and cerebral invasion and discuss the diagnostic and therapeutic challenges.
CONCLUSION:
A major factor of delayed diagnosis of this potentially life-threatening skin cancer feature is patients’ neglecting.
Keywords: Skin cancer, Skull invasion, Squamous cell carcinoma, Treatment
Introduction
Malignant skin tumours of the scalp with skull invasion, dural infiltration and brain involvement are uncommon. However, in advanced cases, skin cancer may be associated with infiltration of the skull bone and even the brain. In a 10-year retrospective analysis, Brunner et al. (2017) analysed 62 patients with malignant craniofacial skin tumours. Brain invasion, surgical margin involvement, and dural margin involvement were the factors that significantly reduce survival. They concluded that the resection of bone is a reasonable surgical option in the treatment of patients with advanced skin cancer of the face and scalp [1]. In a retrospective study of 25 patients with aggressive squamous cell carcinoma (SCC) of the scalp from Catania (Italy) a multidisciplinary approach achieved a complete response in 22 patients with a follow-up of seven years. Three patients died from other causes not related to the cutaneous malignancy [2].
Case Reports
Case 1
An 80-year-old male patient presented a fast-growing exophytic tumour parietal right, measuring 8 x 7 cm. A keratoacanthoma or keratoacanthoma-like SCC was suspected (Figure 1, A and B). He had several comorbidities including dementia, arterial hypertension, glaucoma and atrial fibrillation. Five years ago, another SCC had been removed surgically. He was treated with rivaroxaban and anti-hypertensive drugs.
Figure 1.
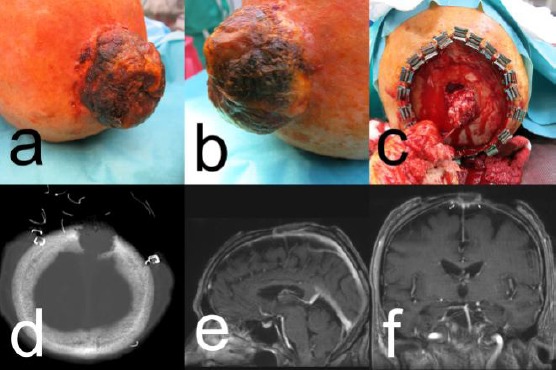
Patient #1 with SCC; A) and B) clinical presentation of the tumour; C) Surgical situs with infiltration of the skull; D) MRI scan with osteolytic skull lesion; E) and F) CT scan with skull penetrating the tumour
Lymph node sonography and chest-X-ray were unremarkable. Computerised tomography (CT) and magnetic resonance imaging (MRI) of the head demonstrated an osteolytic defect of the skull measuring 2.6 cm in diameter with the involvement of the tabula interna. The MRI suggested a tumour infiltration of the sinus sagittalis and the dura mater (Figure 1, C and D). The surgical situs demonstrated an exophytic, the skull infiltrating tumour with a pulsation at the bottom of the skull defect (Figure 1C). In association with the neurosurgeon, it was decided to perform a temporary closure with a meshed graft. Histopathology confirmed the diagnosis of an SCC, pT3, G2. Imaging techniques excluded a metastatic spread leading to the final tumour stage of pT3 cN0 cM0, G2, stage III.
Because of the R1-resection status, the patient was presented to the interdisciplinary tumour board. Adjuvant radiotherapy and complete neurosurgical excision of the tumour were discussed. Both methods bear a mortality risk. After consultation with the family, and because of dementia, palliative treatment was initiated.
Case 2
An 83-year old female patient presented with a long-standing occipital scalp tumour, that was hidden by hair and crusts. The lesion developed over several years. Due to recent bleeding, the patient had been referred to our department (Figure 2A).
Figure 2.
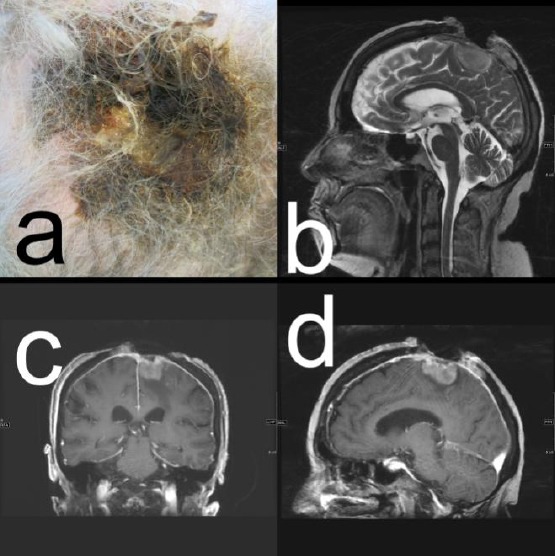
Patient # 2; A) Clinical presentation of the SCC; C) to D) CT scan with a large osteolytic skull lesion due to tumour infiltration
She was living in a nursing home. Her medical history was positive for arterial hypertension, depression and diabetes type II, which were pharmacologically treated. A diagnostic skin biopsy revealed an SCC, G2. On CT, a 42 x 53 mm large full-thickness skull defect was noted (Figure 2, B and D). By imaging techniques, there was no evidence of a metastatic spread. In association with the neurosurgeon, complete surgical removal had been discussed but was denied by the patient and her family. Palliative care was initiated. The patient was lost to follow-up.
Case 3
A 67-year-old man presented to the emergency room with very large bleeding, the ulcerated, malodorous, exophytic tumour of the scalp, infested by maggots, that was slowly growing over more than two years. His medical history was remarkable for chronic alcohol abuse. He had no medical drug therapy.
We observed cauliflower-like exophytic growth with partial necrosis and discharged measuring 23 cm x 12 cm x 2.4 cm localised in the midline of the frontal skull (Figure 3A). There were neither clinical signs of tumour spread to regional lymph nodes nor any neurological impairment. A diagnostic biopsy was taken that confirmed the diagnosis of a tricholemmal SCC (Broders grade 3) (Figure 3D). Routine laboratory demonstrated anaemia and hypoproteinemia: erythrocyte count 3.1 Tpt/L (normal range 4.6-6.2), haemoglobin 6.4 mmol/L (normal range 8.6-12.1), and serum protein 43.3 g/L (normal range 60-85).
Figure 3.
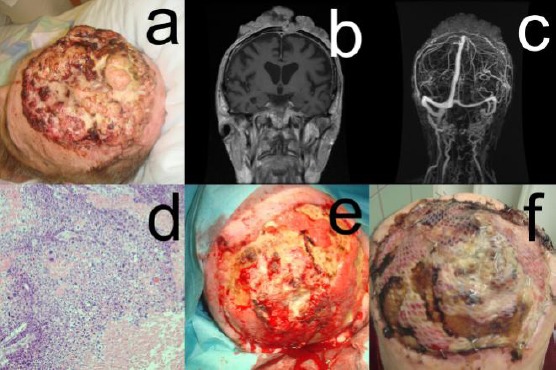
Patient #3: a, cauliflower-like exophytic growth with partial necrosis and discharged measuring 23 cm x 12 cm x 2.4 cm localised in the midline of the frontal skull; b, c, Cranial CT, MRI, and gadolinium-enhanced vascular MRI disclosed parietal parasagittal cranial tumor invasion with a continuous extension to the meninges on the left side with infiltration (3.5 cm x 3.7 cm) and partial closure of the medial part of the superior sinus sagittalis (remaining open lumen 2.4 cm); d, A diagnostic biopsy was taken that confirmed the diagnosis of a tricholemmal SCC (Broders grade 3); e, f, The defect was covered by oxygenised regenerated cellulosis (Tabotamp, Johnson & Johnson—Ethicon) and split-thickness skin meshes graft transplant
Microbial swabs from the tumour surface revealed Proteus mirabilis, Alcaligens faecalis, and beta-hemolytic streptococci that were sensitive to levofloxacin.
Staging with thoracic X-ray, lymph node sonography, and cranial CT did not show any metastases. Cranial CT, MRI, and gadolinium-enhanced vascular MRI disclosed parietal parasagittal cranial tumor invasion with a continuous extension to the meninges on the left side with infiltration (3.5 cm x 3.7 cm) and partial closure of the medial part of the superior sinus sagittalis (remaining open lumen 2.4 cm) (Figure 3, B and C).
After good ulcer care and initiation of intravenous levofloxacin therapy (2 x 250 mg/day), we performed a debulking surgery in association with the neurosurgeon with lateral safety margins of 2 cm down to the skull bone. During surgery, a large full-thickness skull bone defect in the neighbourhood of the sinus sagittalis was visible.
The defect was covered by oxygenised regenerated cellulosis (Tabotamp, Johnson & Johnson—Ethicon) and split-thickness skin meshes graft transplant (Figure 3, E and F).
There was an uneventful postoperative course, and the patient could leave the intensive care unit after 24 hours, but the transplant was lost after seven days. A second surgery with complete debridement of the defect and thinning of the outer tabula of the skull bone by a larger rose drill was performed. After that, a smaller rose drill was used to make penetrations into the spongiosa to reach diploe veins for transplant nutrition. The whole area was covered with a dermal template of porcine collagen (mediCipio, V-CARE Biomedical, Leipzig). Split skin was obtained from the abdomen, meshed 1:1.5, and transplanted onto the dermal template. A light protective helmet was provided to support mobilisation. The patient was re-staged after two months when a collateral circulation around the thrombosed sagittal sinus was evident. We planned a neuro-vascular surgery for complete removal of the remaining tumour. One week before the scheduled operation, he died in a traffic accident.
Discussion
Skull and cranial invasion are rare among cutaneous malignancies. Cancer that has been most often reported to cause this rare but potentially life-threating complication is SCC [2], [3], [4].
Other tumours with a potential of cranial infiltration are adenoid carcinoma, basal cell carcinoma, and cutaneous melanoma [5], [6], [7].
We presented three patients (one female and three males) with full-thickness skull bone penetration by SCC and intracranial extension. The main cause for the late diagnosis and treatment was the neglection of the tumours by the patients. But GP’s, nurses and family members were unable to overcome this disadvantage. Interestingly, none of these patients presented with neurological symptoms. This argues for a slow process of intracranial tumour invasion.
The differential diagnosis includes metastases. The most common site of metastases is the bony skull, while dural metastasis occurs less frequently and may mimic subdural hematoma [8].
The treatment of these advanced SSC’s should be multidisciplinary with dermatology, neurosurgery, radiotherapy, and rehabilitative medicine. In the case of dura and brain invasion, the mortality is increased [1], [2]. Newer pharmaceutical treatment options include epidermal growth factor receptor inhibitors such as cetuximab-alone or in combination with radiotherapy-and immune checkpoint inhibitors like cemiplimab (anti-programmed death-1) [9], [10], [11]. The response rate in real life, however, is less than 50% and short-lived. Surgery remains the cornerstone of treatment [1], [2], [10].
In conclusion, in addition to metastases into the brain and the meninges, nonmelanoma skin cancer can cause other types of cancer involvement by local bone infiltration and perineural invasion. Often, a multidisciplinary approach for diagnosis and treatment of these cancers will be needed.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Brunner M, Ch'ng S, Shannon K, Clifford A, Ashford B, Elliott M, Clark JR. Bone resection for facial cutaneous malignancies. J Surg Oncol. 2017;116(4):545–549. doi: 10.1002/jso.24693. https://doi.org/10.1002/jso.24693. PMid:28628727. [DOI] [PubMed] [Google Scholar]
- 2.Soma PF, Chibbaro S, Makiese O, Marsella M, Diemidio P, Fricia M, Passanisi M, Catania V, Siragò P, Ventura F. Aggressive scalp carcinoma with intracranial extension:a multidisciplinary experience of 25 patients with long-term follow-up. J Clin Neurosci. 2008;15(9):988–92. doi: 10.1016/j.jocn.2007.09.014. https://doi.org/10.1016/j.jocn.2007.09.014. PMid:18653348. [DOI] [PubMed] [Google Scholar]
- 3.Wollina U, Helm C, Schreiber A, Brandl HG. Extensive cranial infiltration by basal cell carcinoma. J Cutan Med Surg. 2006;10(5):257–8. doi: 10.2310/7750.2006.00050. https://doi.org/10.2310/7750.2006.00050. PMid:17234111. [DOI] [PubMed] [Google Scholar]
- 4.Schwarze HP, Loche F, Gorguet MC, Kuchta J, Bazex J. Invasive cutaneous squamous cell carcinoma associated with actinic keratosis:a case with orbital invasion and meningeal infiltration. Dermatol Surg. 1999;25(7):587–9. doi: 10.1046/j.1524-4725.1999.99009.x. https://doi.org/10.1046/j.1524-4725.1999.99009.x. PMid:10469120. [DOI] [PubMed] [Google Scholar]
- 5.Keck M, Ueberreiter K, Tanzella U, Doll D, Krapohl BD. Primary cutaneous adenoid carcinoma of the scalp. GMS Interdiscip Plast Reconstr Surg DGPW. 2012;1:Doc04. doi: 10.3205/iprs000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sicinska J, Rakowska A, Czuwara-Ladykowska J, Mroz A, Lipinski M, Nasierowska-Guttmejer A, Sikorska J, Sklinda K, Slowinska M, Kowalska-Oledzka E, Walecka I, Walecki J, Rudnicka L. Cylindroma transforming into basal cell carcinoma in a patient with Brooke-Spiegler syndrome. J Dermatol Case Rep. 2007;1(1):4–9. doi: 10.3315/jdcr.2007.1.1002. https://doi.org/10.3315/jdcr.2007.1.1002. PMid:21886698 PMCid:PMC3157764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wollina U, Hansel G, Schmidt N, Schönlebe J, Kittner T, Nowak A. Very rare amelanotic lentigo maligna melanoma with skull roof invasion. Open Access Maced J Med Sci. 2017;5(4):458–461. doi: 10.3889/oamjms.2017.113. https://doi.org/10.3889/oamjms.2017.113. PMid:28785332 PMCid:PMC5535657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grisold W, Grisold A. Cancer around the brain. Neurooncol Pract. 2014;1(1):13–21. doi: 10.1093/nop/npt002. https://doi.org/10.1093/nop/npt002. PMid:26034610 PMCid:PMC4369702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wollina U. Update of cetuximab for non-melanoma skin cancer. Expert Opin Biol Ther. 2014;14(2):271–6. doi: 10.1517/14712598.2013.876406. https://doi.org/10.1517/14712598.2013.∼06. PMid:24387664. [DOI] [PubMed] [Google Scholar]
- 10.Hillen U, Leiter U, Haase S, Kaufmann R, Becker J, Gutzmer R, Terheyden P, Krause-Bergmann A, Schulze HJ, Hassel J, Lahner N, Wollina U, Ziller F, Utikal J, Hafner C, Ulrich J, Machens HG, Weishaupt C, Hauschild A, Mohr P, Pföhler C, Maurer J, Wolff P, Windemuth-Kieselbach C, Schadendorf D, Livingstone E Dermatologic Cooperative Oncology Group (DeCOG) Advanced cutaneous squamous cell carcinoma:A retrospective analysis of patient profiles and treatment patterns-Results of a non-interventional study of the DeCOG. Eur J Cancer. 2018;96:34–43. doi: 10.1016/j.ejca.2018.01.075. https://doi.org/10.1016/j.ejca.2018.01.075. PMid:29665511. [DOI] [PubMed] [Google Scholar]
- 11.Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, Chung CH, Hernandez-Aya L, Lim AM, Chang ALS, Rabinowits G, Thai AA, Dunn LA, Hughes BGM, Khushalani NI, Modi B, Schadendorf D, Gao B, Seebach F, Li S, Li J, Mathias M, Booth J, Mohan K, Stankevich E, Babiker HM, Brana I, Gil-Martin M, Homsi J, Johnson ML, Moreno V, Niu J, Owonikoko TK, Papadopoulos KP, Yancopoulos GD, Lowy I, Fury MG. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N Engl J Med. 2018;379(4):341–351. doi: 10.1056/NEJMoa1805131. https://doi.org/10.1056/NEJMoa1805131. PMid:29863979. [DOI] [PubMed] [Google Scholar]


