LONG ABSTRACT:
Lentiviruses are efficient vectors for gene delivery to mammalian cells. Following transduction, the lentiviral genome is stably incorporated into the host chromosome and is passed on to progeny. Thus, they are ideal vectors for creation of stable cell lines, in vivo delivery of indicators, and transduction of single cell fertilized eggs to create transgenic animals. However, mouse fertilized eggs and early stage embryos are protected by the zona pellucida, a glycoprotein matrix that forms a barrier against lentiviral gene delivery. Lentiviruses are too large to penetrate the zona and are typically delivered by microinjection of viral particles into the perivitelline cavity, the space between the zona and the embryonic cells. The requirement for highly skilled technologists and specialized equipment has minimized the use of lentiviruses for gene delivery to mouse embryos. Here, we describe a protocol for permeabilizing the mouse fertilized eggs by perforating the zona with a laser. Laser-perforation does not result in any damage to embryos and allows lentiviruses to gain access to embryonic cells for gene delivery. Transduced embryos can develop into blastocyst in vitro, and if implanted in pseudopregnant mice, develop into transgenic pups. The laser used in this protocol is effective and easy to use. Genes delivered by lentiviruses stably incorporate into mouse embryonic cells and are germline transmittable. This is an alternative method for creation of transgenic mice that requires no micromanipulation and microinjection of fertilized eggs.
Keywords: Transgenesis, XYClone laser, lentivirus, mouse fertilized eggs, mouse embryos, gene delivery, transduction
SHORT ABSTRACT:
Mouse fertilized eggs and early stage embryos are protected by the zona pellucida, a glycoprotein matrix that forms a barrier against gene delivery. Here, we describe a protocol for perforating the zona with a laser to transduce embryonic cells with lentiviral vectors and to create transgenic mice.
INTRODUCTION:
Here, we describe a method for permeabilizing the zona pellucida of mouse fertilized eggs to make embryonic cells accessible for gene delivery by lentiviruses. Lentiviruses are designed by nature for efficient gene delivery to mammalian cells. They infect dividing and non-dividing cells and integrate the lentiviral genome into their host chromosomes1. The range of lentiviral host cells is readily expanded by pseudotyping the recombinant lentivirus with the vesicular stomatitis virus glycoprotein (VSV-G), due to the broad tropism of the VSV-G protein2. Following transduction, lentiviral genes are stably integrated and expressed as part of their host chromosomes creating an ideal tool for generating transgenic animals. If delivered to early stage embryonic cells, the lentiviral genome is replicated and expressed in the entire organism. Lentiviral transduction has led to the production of mice, rat, chicken, quail and pig3–7 among other species of transgenics. The typical method of lentiviral gene delivery, however, requires skilled technicians and specialized equipment to overcome the zona pellucida barrier that encapsulates the early stage embryos. The overall goal of this method is to describe how to permeabilize the zona using a laser to facilitate lentiviral gene delivery.
Mammalian eggs are surrounded by the zona pellucida which hardens following fertilization to protect the fertilized eggs against polyspermy and to limit environmental interactions8,9. The zona forms a barrier that keeps lentiviruses away from the embryonic cells until the embryos are hatched as a blastocyst. Cultured mouse fertilized eggs hatch after 4 days and must be implanted into pseudopregnant mice prior to hatching for normal development into pups. Therefore, for transduction, lentiviruses are microinjected before hatching from the zona into the perivitelline cavity, the space between the zona and the embryonic cells.
The zona pellucida is often removed for in vitro fertilization of human eggs to increase the fertilization rate10. However, chemical removal of mouse zona pellucida adversely affects mouse embryo development and is harmful to embryonic cells11,12. Other methods for gene delivery to mouse fertilized eggs overcome the zona pellucida barrier by direct microinjection of DNA into the cell nucleus13. Pronuclear microinjection is an efficient means of delivering genes to embryos. However, since each embryo is held in place individually for microinjection, the practice can be laborious and time consuming for a novice user.
Other methods such as electroporation and photoporation are useful for transient and short-term gene delivery to mouse fertilized eggs14–16. These methods are extensively used for delivering CRISPR-Cas9 components and recombinases. However, electroporation and photoporation delivery of genes cannot be used efficiently to create transgenics. Spermatozoa that are collected from punctured mouse epididymis can also be transduced by lentiviruses and used for in vitro fertilization to produce transgenic animals17–20.
Here, we facilitated the lentiviral gene delivery to mouse embryos by permeabilizing the zona using a laser. The XYClone laser was developed as an aid for in vitro fertilization21 and cultivation of embryonic stem cells22. It is a small apparatus that is simple to setup and easy to use. Once installed on a microscope, it occupies the space of an objective lens and the accompanying software allows for aiming the laser while looking through the microscope eyepieces (see PROTOCOL: section 3). Once the zona is perforated by the XYClone laser, lentiviruses can be introduced into the culture media for gene delivery23. Multiple lentiviruses could be used to simultaneously deliver several genes for chromosomal incorporation.
This protocol will describe how to isolate and culture mouse fertilized eggs, illustrates the use of laser for perforation of the zona pellucida, and demonstrates the transduction of mouse embryonic cells by lentiviruses.
PROTOCOL:
All animal procedures and treatments used in this protocol were in compliance with the NIH/NIEHS animal care guidelines and were approved by the Animal Care and Use Committee (ACUC) at the NIH/NIEHS, Animal Protocol 2010–0004.
1. Preparations
-
1.1.
Prepare/purchase recombinant non-propagating lentiviruses carrying your gene of interest. In this study, lentivirus SBI511 expressing copepod green fluorescent protein (copGFP; abbreviated to GFP) from an elongation factor 1a promoter was used for transduction. Standard protocols2 were used to produce and titer lentiviruses at titers higher than 1e8 transducing units per ml (TU/ml).
Note: Use caution and bleach all material that have been in contact with lentiviruses. Refer to your institute’s guidelines for safe use and handling of lentiviruses.
-
1.2.
Setup breeding pairs of desired mouse strain the day before harvesting embryos. In this experiment, C57BL/6J strain of mice was used. Female mice used for ovaries and oviduct collection were not treated with any hormones for superovulation.
-
1.3.
2–24 hours prior to harvesting mouse fertilized eggs, prepare several Potassium Simplex Optimized Medium (KSOM) drop plates as follows: add a 50 μl drop of KSOM medium to the middle of a 35 mm tissue culture-treated dish and cover with 2 ml of Dimethylpolysiloxane (DMPS5X) and place at 37° C, 5% CO2, 5% O2 and 90% N2 to equilibrate.
Note: Use disposable sterile plates and discard following exposure to lentiviruses.
-
1.4.
Prepare 1x solution of hyaluronidase in M2 medium from stock solution (100x, 30 mg/ml, store at −20 °C). 100x stock solution was prepared in water.
-
1.5.
24 hours post laser-assisted transduction of mouse fertilized eggs, set up breeding pairs between vasectomized male and female mice to prepare pseudopregnant female mice.
2. Isolation of Mouse Fertilized Eggs
-
2.1.
16–24 hours post mating, select female mice with vaginal plugs indicative of successful mating, humanely euthanize, and isolate ovaries and oviducts according to standard protocols24. The mice used in this study were euthanized by cervical dislocation under deep CO2 or isoflurane inhalation.
-
2.2.
Transfer the ovaries and oviducts to a 35 mm dish containing M2 media.
-
2.3.
For each ovary, tear ampulla apart to release fertilized eggs surrounded by cumulus cells.
-
2.4.
Transfer fertilized eggs and cumulus cells to a 35 mm dish containing 1x hyaluronidase in M2 medium and incubate at room temperature for 3–5 minutes.
-
2.5.
Pipet fertilized eggs up and down to release the cumulus cells.
-
2.6.
Transfer fertilized eggs to a 35 mm dish containing M2 media and pipet up and down to wash off hyaluronidase.
-
2.7.
Transfer fertilized eggs to a 35 mm dish containing KSOM and pipet up and down to wash off the remaining M2 media and hyaluronidase.
-
2.8.
Transfer fertilized eggs to the prepared KSOM drop plates from step 1.3.
Note: Mouse fertilized eggs in KSOM must be kept in a tissue culture incubator at 37 °C, 5% CO2, 5% O2 and 90% N2 to equilibrate. Removing plates from the incubator for longer than 15 minutes will result in damage to embryos.
-
2.9.
Allow fertilized eggs to recover for 2 hours in the incubator before moving to the next step.
3. Perforation of Mouse Fertilized Eggs with XYClone Laser
-
3.1.
Setup and calibrate XYClone laser according to the manufacturer’s recommendation. Briefly, attach the laser controller box wire to the laser apparatus on the microscope. Attach the laser controller box to the computer running the laser software via a USB port. Plug in the laser controller and switch it on. Looking through the eyepiece, perforate a test sample (e.g. dry-erase markings on a glass slide). Use a small screw driver (included in the laser kit) to adjust the X and Y position of the laser to match the LED light visible through the microscope eyepiece and calibrate the laser. Other lasers, typically used for in vitro fertilization, can be substituted for XYClone laser to perforate fertilized eggs.
-
3.2.
Place a KSOM drop plate containing mouse fertilized eggs on the microscope stage. Do not keep the plate outside of the incubator for longer than 15 minutes.
-
3.3.
Look through the microscope eyepiece and ensure that the embryo’s zona pellucida is in focus and the laser LED light is visible.
-
3.4.
Move the microscope stage to target the zona with the LED light/laser.
-
3.5.
Using the computer software set XYClone laser to 250 μS.
-
3.6.
Adjust the LED light size to desired dimensions (setting 5 in this experiment).
-
3.7.
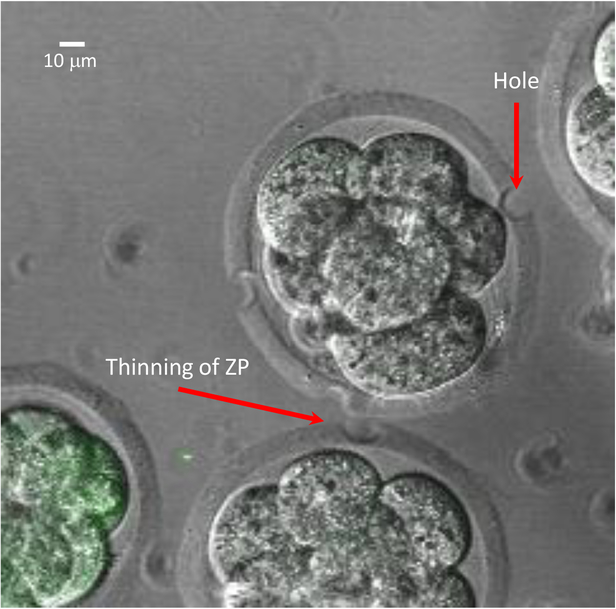
Perforate the zona of each fertilized egg thrice with the laser. The zona can be either pierced or thinned. Using the above settings, the laser will produce a hole with a diameter of 10 μm (Figure 2).
Note: Aiming close to the polar body keeps laser away from the embryonic cell.
-
3.8.
Allow fertilized eggs to recover for 2 hours in the tissue culture incubator before moving to the next step.
Figure 2. Laser-Treatment of Mouse Fertilized Eggs.
Examples of perforating the zona to produce a hole vs thinning of the zona.
4. Transduction of Mouse Fertilized Eggs following XYClone Laser Perforation
-
4.1.
Pipet 2 μl of concentrated lentivirus (greater than 1e8 TU/ml titer) into the 50 μl KSOM drop. Do not pipet up and down. Fertilized eggs readily attach to the pipet tip. Based on our experience, 1e5–5e5 transducing units of lentivirus in a volume less than 3 μl is optimal for gene delivery.
-
4.2.
Allow fertilized eggs to develop into blastocyst for 4 days in the incubator. No need to change the media.
5. Non-Surgical Transfer of Transduced Mouse Embryos to Pseudo-Pregnant Mice
-
5.1.
Use pseudopregnant mice, 3.5 day after mating, prepared in step 1.5.
-
5.2.
Use Non-Surgical Embryo Transfer (NSET) device to implant mouse embryos into pseudopregnant mice. Using a surgical or dissecting microscope, a vaginal speculum is inserted to visualize the cervix. The Non-Surgical Embryo Transfer (NSET) device is inserted approximately 5 mm into the cervix and embryos are deposited in a volume of approximately 2 μL. A sterile NSET device is used for each transfer and discarded after the procedure. All reagents used in the manipulation of the embryos must be sterile.
-
5.3.
Transfer 10–15 healthy blastocyst in 2 μl volume of KSOM to each pseudopregnant mice.
-
5.4.
Continue to monitor and measure the weight gain in pseudopregnant mice in following days to determine whether the NSET was successful.
-
5.5.
Recover pups by allowing the pregnant mice to give birth naturally or performing a caesarean section 17 days after the embryo transfer25. A C-section is often necessary if very few embryos are present and they grow too large for natural birth.
-
5.6.
Collect tissue from pups for genotyping to determine the rate of transgenesis26.
REPRESENTATIVE RESULTS:
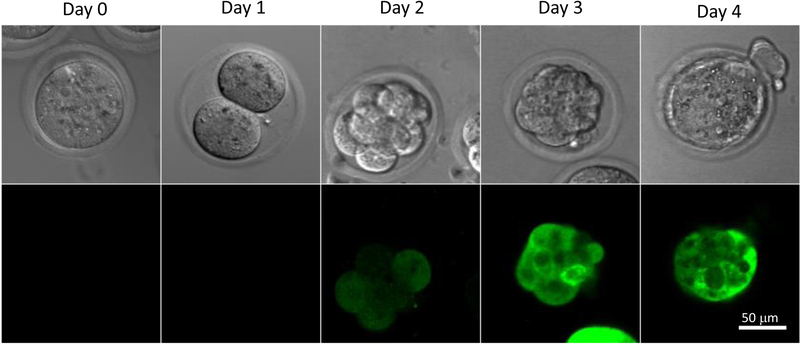
Development of isolated/transduced mouse fertilized eggs can be checked under the microscope daily (Figure 1). Healthy embryos develop into blastocyst within 3–4 days. We observed that 60–70% of untreated embryos develop into blastocyst23. Out of 114 laser-perforated transduced embryos, 54 developed into blastocyst (rate of 47%) and 46 blastocysts expressed GFP (46/54=85%)23.
Figure 1. Development of Transduced Mouse Fertilized Eggs in Culture.
The C57BL/6J mouse fertilized eggs were monitored on Day 0 (harvested fertilized egg), Day 1 (two-cell), Day 2 (8–16 cells), Day 3 (morula), and Day 4 (blastocyst). Embryos were transduced with a lentivirus carrying a GFP gene. Presence of Fluorescence in transduced embryos became evident by Day 2–3.
The mouse embryos were laser-perforated on the day of harvest. The optimal setting for the laser treatment was 3 holes per fertilized egg and 250 μs. The laser should be aimed to thin the zona instead of creating a hole (Figure 2). This allows the developing embryos to benefit from an intact encapsulating zona pellucida while becoming permissive to lentiviral transduction. In our experiments, we transduced mouse embryos with a recombinant lentivirus that expressed copepod GFP (abbreviated GFP) from an elongation factor 1a promoter. For transduction, 2 ul of lentivirus was introduced into the culture media. Increasing the amount of virus, directly affected the number of transduced embryos that expressed GFP while adversely affecting embryo development into blastocyst23. Viral volumes larger than 10% of KSOM drop volume would also adversely affect blastocyst formation (e.g. greater than 5 μl of virus in a 50 μl KSOM drop).
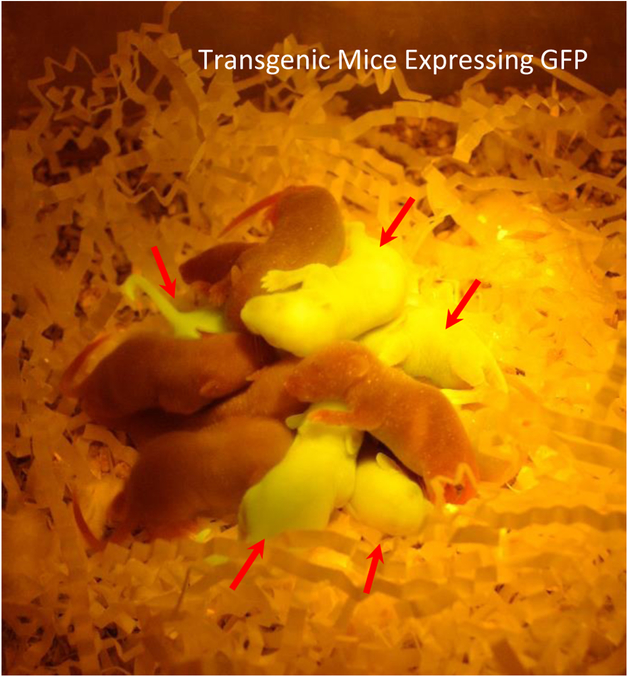
To validate the viability, transduced mouse embryos were non-surgically transferred to pseudopregnant mice. Six separate NSET events, transferring a total of 58 blastocyst, resulted in 9 GFP transgenic pups out of total of 12, yielding 75% rate of transgenesis23. The NSET protocol is reported to yield 30–35% live births27 compared to 21% yield that we observed in our experiments (12/58). We hypothesize that the lentiviral treatment may have played a role in embryo development and contributed to the low embryo transfer rate. Pups with multiple lentiviral integrations and high expression of GFP were visually green under a blue LED lamp (465–470 nm) (Figure 3). The number of incorporated GFP copies ranged from 0–6 copies and was determined by performing qualitative PCR on isolated chromosomal DNA from pup tissue{Martin, 2018 #45}.
Figure 3. Transgenic Mice Expressing GFP.
Dark Reader (DR Spot Lamp, DRSL-9S) was used to visualize GFP positive pups in a dark environment. Non-transgenic control pups were added to the group for contrast. Resulting pups (red arrows) were genotyped and they contained from 0–6 copies of the lentiviral gene/GFP.
DISCUSSION:
The ability of the lentiviruses to integrate into their host genome makes them an ideal vector for stable gene delivery. Lentiviral vectors can carry up to 8.5 kilobase pair (kbp) of genetic material that can accommodate cell-specific or inducible promoters, selection markers, or fluorescent moieties. Incorporated genomic material can replicate as part of their host genome and be regulated to express or deactivate at desired time points. These vectors allow for spatiotemporal control over gene expression at various stages of development and brand lentiviruses as powerful tools for gene delivery.
Laser-assisted lentiviral transgenesis is an effective and easy-to-use method for gene expression in vivo. This method can be used for in vivo protein production, expression of genetically encoded indicators, or functional studies. Compared to other methods, laser-assisted lentiviral gene delivery is as effective as pronuclear microinjection but requires no technical skills or costly microinjection workstations. The XYClone laser is small, portable, and can easily be shared among several laboratories.
Laser-assisted lentiviral gene delivery is stable and not transient, as in electroporation or photoporation of fertilized eggs. Transient gene delivery is more advantages for delivery of CRISPR-Cas9 components or recombinases since extended expression could lead to aberrant consequences. In this protocol, the integrase deficient lentiviruses (IDLVs) can be substituted for transient transduction of mouse fertilized eggs. IDLVs retain lentiviral infectivity but only retain a fraction (less than 8%) of integrative capability of lentiviruses25. Laser-assisted lentiviral transgenesis is an additional gene delivery option for users to evaluate based on their desired outcome.
The major disadvantage of lentiviral transduction is the random insertion of the delivered gene. Also, cells within the same embryo could host multiple lentiviral integrations that leads to mosaicism in the transgenic. Genotyping of the progeny and multiple rounds of planned breeding is necessary to establish a single locus transgenic animal. Similar breeding strategies are also employed in conventional transgenesis methods26.
A critical step in this protocol is the length of time spent on laser perforation. Mouse fertilized eggs cultured in KSOM cannot be kept outside of the incubator for longer than 15 minutes. A novice user should move the fertilized eggs to M2 medium, use Advanced KSOM Embryo Medium, or limit the number of embryos per plate. According to our result, 47% of transduced cultured mouse fertilized eggs develop into blastocysts21. Therefore, culturing 30–40 fertilized eggs per plate will ensure adequate number of transduced blastocysts in one plate for transfer into pseudopregnant mice.
Laser-assisted perforation of the mouse fertilized egg zona may also be applicable to the zona of other species and allow entry for other types of viruses or transfection reagents.
Materials.
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description | |
|---|---|---|---|---|
| CD510B-1 plasmid | System Biosciences | CD510B-1 | used to package the lentivirus expressing EF1a-copGFP | |
| Dimethylpolysiloxane | Sigma | DMPS5X | culturing embryos | |
| hyaluronidase | Sigma | H3506 | used to remove cumulus cells | |
| XYClone Laser | Hamilton Thorne Biosciences | perforating mouse fertilized eggs | ||
| Non-Surgical Embryo Transfer (NSET) Device | ParaTechs | 60010 | NSET of embryos | |
| KSOM medium | Millipore | MR-020P-5F | culturing embryos | |
| Composition of KSOM: | ||||
| Reagent Name | mg/100mL | |||
| NaCl | 555 | |||
| KCl | 18.5 | |||
| KH2PO4 | 4.75 | |||
| MgSO4 7H2O | 4.95 | |||
| CaCl2 2H2O | 25 | |||
| NaHCO3 | 210 | |||
| Glucose | 3.6 | |||
| Na-Pyruvate | 2.2 | |||
| DL-Lactic Acid, sodium salt | 0.174mL | |||
| 10mM EDTA | 100μL | |||
| Streptomycin | 5 | |||
| Penicillin | 6.3 | |||
| 0.5% phenol red | 0.1mL | |||
| L-Glutamine | 14.6 | |||
| MEM Essential Amino Acids | 1mL | |||
| MEM Non-essential AA | 0.5mL | |||
| BSA | 100 | |||
| M2 medium | Millipore | MR-015-D | culturing embryos | |
| Composition of M2: | ||||
| Reagent Name | mg/100mL | |||
| Calcium Chloride | 25.1 | |||
| Magnesium Sulfate (anhydrous) | 16.5 | |||
| Potassium Chloride | 35.6 | |||
| Potassium Phosphate, Monobasic | 16.2 | |||
| Sodium Bicarbonate | 35 | |||
| Sodium Chloride | 553.2 | |||
| Albumin, Bovine Fraction | 400 | |||
| D-Glucose | 100 | |||
| Na-HEPES | 54.3 | |||
| Phenol Red | 1.1 | |||
| Pyruvic Acid | 3.6 | |||
| DL-Lactic Acid | 295 |
ACKNOWLEDGMENTS:
This research was supported by the Intramural Research Program of the National Institute of Health (NIH), National Institute of Environmental Health Sciences (NIEHS). We are grateful to Dr. Robert Petrovich and Dr. Jason Williams for critical reading of the manuscript and helpful advice. We would also like to acknowledge and thank Dr. Bernd Gloss, the Knockout core, the Flow Cytometry Facility, the Fluorescence Microscopy and Imaging Center, and the Comparative Medicine Branch facilities of the NIEHS for their technical contributions. We would like to thank Mr. David Goulding from the Comparative Medicine Branch and Ms. Lois Wyrick of the Imaging Center at the NIEHS for providing us with photographs and illustrations.
Footnotes
DISCLOSURES:
The authors have nothing to disclose.
REFERENCES
- 1.Sakuma T, Barry MA & Ikeda Y Lentiviral vectors: basic to translational. Biochem J. 443 (3), 603–618, (2012). [DOI] [PubMed] [Google Scholar]
- 2.Salmon P & Trono D Production and titration of lentiviral vectors. Curr Protoc Hum Genet. Chapter 12 Unit 12 10, (2007). [DOI] [PubMed] [Google Scholar]
- 3.Lois C, Hong EJ, Pease S, Brown EJ & Baltimore D Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 295 (5556), 868–872, (2002). [DOI] [PubMed] [Google Scholar]
- 4.Filipiak WE & Saunders TL Advances in transgenic rat production. Transgenic Res. 15 (6), 673–686, (2006). [DOI] [PubMed] [Google Scholar]
- 5.McGrew MJ, Sherman A, Lillico SG, Taylor L & Sang H Functional conservation between rodents and chicken of regulatory sequences driving skeletal muscle gene expression in transgenic chickens. BMC Dev Biol. 10 26, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y et al. Production of transgenic pigs mediated by pseudotyped lentivirus and sperm. PLoS One. 7 (4), e35335, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z et al. Transgenic quail production by microinjection of lentiviral vector into the early embryo blood vessels. PLoS One. 7 (12), e50817, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wassarman PM Zona pellucida glycoproteins. Annu Rev Biochem. 57 415–442, (1988). [DOI] [PubMed] [Google Scholar]
- 9.Clift D & Schuh M Restarting life: fertilization and the transition from meiosis to mitosis. Nat Rev Mol Cell Biol. 14 (9), 549–562, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nijs M & Van Steirteghem AC Assessment of different isolation procedures for blastomeres from two-cell mouse embryos. Hum Reprod. 2 (5), 421–424, (1987). [DOI] [PubMed] [Google Scholar]
- 11.Gordon JW, Scangos GA, Plotkin DJ, Barbosa JA & Ruddle FH Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci U S A. 77 (12), 7380–7384, (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaneko T, Sakuma T, Yamamoto T & Mashimo T Simple knockout by electroporation of engineered endonucleases into intact rat embryos. Sci Rep. 4 6382, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosokawa Y, Ochi H, Iino T, Hiraoka A & Tanaka M Photoporation of biomolecules into single cells in living vertebrate embryos induced by a femtosecond laser amplifier. PLoS One. 6 (11), e27677, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horii T et al. Validation of microinjection methods for generating knockout mice by CRISPR/Cas-mediated genome engineering. Sci Rep. 4 4513, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamra FK et al. Production of transgenic rats by lentiviral transduction of male germline stem cells. Proc Natl Acad Sci U S A. 99 (23), 14931–14936, (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanatsu-Shinohara M et al. Production of transgenic rats via lentiviral transduction and xenogeneic transplantation of spermatogonial stem cells. Biol Reprod. 79 (6), 1121–1128, (2008). [DOI] [PubMed] [Google Scholar]
- 17.Dann CT & Garbers DL Production of knockdown rats by lentiviral transduction of embryos with short hairpin RNA transgenes. Methods Mol Biol. 450 193–209, (2008). [DOI] [PubMed] [Google Scholar]
- 18.Chandrashekran A et al. Efficient generation of transgenic mice by lentivirus-mediated modification of spermatozoa. FASEB J. 28 (2), 569–576, (2014). [DOI] [PubMed] [Google Scholar]
- 19.Woods SE et al. Laser-assisted in vitro fertilization facilitates fertilization of vitrified-warmed C57BL/6 mouse oocytes with fresh and frozen-thawed spermatozoa, producing live pups. PLoS One. 9 (3), e91892, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka N, Takeuchi T, Neri QV, Sills ES & Palermo GD Laser-assisted blastocyst dissection and subsequent cultivation of embryonic stem cells in a serum/cell free culture system: applications and preliminary results in a murine model. J Transl Med. 4 20, (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin NP et al. En masse lentiviral gene delivery to mouse fertilized eggs via laser perforation of zona pellucida. Transgenic Res. 27 (1), 39–49, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stein P & Schindler K Mouse oocyte microinjection, maturation and ploidy assessment. J Vis Exp. 10.3791/2851 (53), (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagy A, Gertsenstein M, Vintersten K & Behringer R Caesarean section and fostering. CSH Protoc. 2006 (2), (2006). [DOI] [PubMed] [Google Scholar]
- 24.Chum PY, Haimes JD, Andre CP, Kuusisto PK & Kelley ML Genotyping of plant and animal samples without prior DNA purification. J Vis Exp. 10.3791/3844 (67), (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu KC et al. Integrase-deficient lentivirus: opportunities and challenges for human gene therapy. Curr Gene Ther. 14 (5), 352–364, (2014). [DOI] [PubMed] [Google Scholar]
- 26.Sauvain MO et al. Genotypic features of lentivirus transgenic mice. J Virol. 82 (14), 7111–7119, (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]