Abstract
The tyrosine kinase inhibitor sorafenib improves hepatopulmonary syndrome (HPS) in an experimental model. However, the efficacy and adverse effect profile in patients with HPS are unknown. We aimed to determine the effect of sorafenib on the alveolar-arterial oxygen gradient (AaPO2) at 3 months in patients with HPS. We performed a randomized, double-blind, placebo-controlled parallel trial of sorafenib in patients with HPS at 7 centers. A total of 28 patients with HPS were randomized to sorafenib 400 mg by mouth daily or a matching placebo in a 1:1 ratio. We found no statistically significant difference in the median change in AaPO2 from baseline to 12 weeks between the patients allocated to sorafenib (4.5 mm Hg; IQR, −3.8 to 7.0 mm Hg) and those allocated to placebo (−2.4 mm Hg; IQR, −4.8 to 8.2 mm Hg; P = 0.70). There was also no difference between the groups in terms of degree of intrapulmonary shunting by contrast echocardiography. Sorafenib significantly reduced circulating levels of angiogenic markers, including vascular endothelial growth factor receptors (P < 0.01) and TIE2-expressing M2 monocytes (P = 0.03), but it reduced the mental component scores of the Short Form 36 (P = 0.04), indicating a worse quality of life. In conclusion, sorafenib did not change the AaPO2 or other disease markers at 3 months in patients with HPS. Alternative antiangiogenic therapies or treatments targeting other pathways should be investigated.
Cirrhotic liver disease afflicts nearly 3 million Americans, and complications from chronic liver disease were the fourth leading cause of death in patients between the ages of 45 and 65 years in the United States in 2015.(1) Hepatopulmonary syndrome (HPS) is one such complication, which results when pulmonary microvascular dilations cause intrapulmonary shunting and abnormalities in arterial oxygenation. HPS occurs in ~20% of patients with cirrhosis and portal hypertension evaluated for liver transplantation(2)) HPS is associated with worse quality of life (specifically in cognitive domains) and doubles the already significantly elevated risk of death of patients with cirrhosis. Liver transplantation can resolve HPS but subjects a patient to the risks and adverse effects of transplantation and immunosuppression. Furthermore, many patients may not be acceptable candidates for transplantation, and transplant is limited by donor availability. Even with liver transplantation, patients with HPS may have a worse outcome compared with patients without HPS.(3–5)
The cause of lung vascular abnormalities in HPS is unknown, and there are no effective medical therapies for HPS. We have shown that variation in genetic factors that control angiogenesis (such as endostatin) was associated with HPS in candidates for liver transplantation.(6) The role of lung angiogenesis in HPS is supported by an increase in vessels in the interstitium and circulating proangiogenic hematopoietic progenitor cells/endothelial progenitor cells in our animal model with HPS.(7–9) We have also shown that overexpression of endogenous inhibitors of angiogenesis implicated in our human studies (angiostatin and endostatin) reduced lung microvessel counts and normalized alveolar-arterial oxygen gradient (AaPO2) in the experimental model, supporting the key role of lung neovascularization in HPS.(9) Finally, we have shown that the multispecific tyrosine kinase inhibitor sorafenib reversed vascular proliferation in the lungs and improved gas exchange in the rodent model when administered after the establishment of HPS.(10)
Sorafenib is a tyrosine kinase inhibitor that blocks angiogenesis predominantly via 2 receptor tyrosine kinases (vascular endothelial growth factor receptor [VEGFR] and platelet-derived growth factor receptor). Sorafenib also potently inhibits FLT-3 receptor phosphorylation and c-kit activation, and the Raf/MEK/extracellular signal-regulated kinase pathway. Sorafenib is currently approved by the US Food and Drug Administration for the treatment of renal cell carcinoma, hepatocellular carcinoma, and locally recurrent or metastatic, progressive, differentiated thyroid carcinoma.
We hypothesized that sorafenib would correct the physiologic abnormalities of HPS by reversing angiogenesis. We performed a randomized, double-blind, placebo-controlled trial to determine whether sorafenib reduced the AaPO2 at 12 weeks in patients with HPS. Secondary endpoints included the degree of intrapulmonary shunting by contrast echocardiography (CE), exercise capacity, circulating plasma biomarkers of angiogenesis, and health-related quality of life at 8–12 weeks.
Patients and Methods
STUDY DESIGN
The Sorafenib in Hepatopulmonary Syndrome (SHPS) study was a 7-center, randomized, double-blind, placebo-controlled proof-of-principle study of sorafenib in patients with HPS. The original protocol called for the recruitment of 50 patients with HPS; the National Heart, Lung, and Blood Institute requested that the target sample size be reduced to 30 patients after assessment of the variability of the primary endpoint and the enrollment rate. The first patient was randomized in October 2014, and the last was randomized in September 2017. The National Heart, Lung, and Blood Institute terminated recruitment on November 10, 2017, and sites and the Data Safety and Monitoring Board were notified. Active participants were permitted to complete the trial.
The trial protocol was approved by the institutional review boards at all participating centers and by the Data Safety and Monitoring Board. The trial was registered at clinicaltrials.gov before initiating recruitment (). Details of the methods are provided in the Supporting Materials.
STUDY PARTICIPANTS
We included adults with HPS with Child-Pugh class A or B cirrhosis (consistent with the regulatory labeling for sorafenib). HPS was defined using prior consensus criteria,(11) including the following:
AaPO2 ≥15 mm Hg (≥20 mm Hg for age >64 years).
The presence of intrapulmonary shunting (defined by the reappearance of bubbles 3 or more beats after visualization in the right atrium on CE).
Absence of significant restriction (total lung capacity <70% predicted) or obstruction (forced expiratory volume in 1 second [FEV1] <80% predicted and FEV1/forced vital capacity [FVC] <0.70).
Presence of cirrhosis/hepatic fibrosis and/or portal hypertension.
We excluded patients with recent chronic heavy alcohol consumption, active hepatic encephalopathy, portopulmonary hypertension, congenital long-QT syndrome, previous liver or other solid organ transplantation or expectation of liver transplant within 4 months of randomization, hepatocellular carcinoma that did not meet certain criteria, uncontrolled hypertension, or World Health Organization class 4 functional status. Complete inclusion and exclusion criteria are provided in Supporting Table 1. Participants were recruited from liver disease and pulmonary clinics at the University of Pennsylvania, Columbia University, University of Texas at Houston, Mayo Clinic Rochester, Mayo Clinic Arizona, Northwestern University, and the Medical University of South Carolina. All participants provided written informed consent.
STUDY PROCEDURES
Potentially eligible patients were identified by medical staff. After providing informed consent and undergoing screening, eligible patients were randomly assigned in a 1:1 ratio by a Web-based computerized system to 400 mg of sorafenib (Bayer Pharmaceuticals, Whippany, NJ) once daily or an identically encapsulated placebo. Randomization was stratified by center using randomly permuted block sizes. All patients and study personnel other than the research pharmacist, unblinded statistician, and statistical analyst preparing the closed Data Safety and Monitoring Board report were masked to sequence and treatment assignment and were not unmasked until the study was completed and the database finalized and locked.
Patients were evaluated at the study centers at baseline and at 2, 4, 8, and 12 weeks, with telephone calls at 6,10, and 14 weeks. Seated arterial blood gas (on room air, if tolerated) and CE were performed at screening and at 12 weeks (with an optional arterial blood gas at 8 weeks); the screening measures were used as the baseline values for these tests. Phlebotomy, pulse oximetry, 6-minute walk testing, World Health Organization functional class, the Medical Outcomes Study Short Form 36 (SF-36), and Mahler’s Dyspnea Index were performed at baseline and at 8 and 12 weeks. Adverse events (AEs) were monitored throughout the study period.
The primary outcome was the change in AaPO2 from baseline to 12 weeks. Secondary outcomes included changes from baseline in the degree of intrapulmonary shunting (grades 1–3; see Supporting Materials), partial pressure of arterial oxygen (PaO2), oxygen saturation by pulse oximetry (sitting and supine), 6-minute walk distance, World Health Organization functional class, SF-36, Mahler’s Dyspnea Index, hematopoietic progenitor cells (HPCs) and other plasma biomarkers at 8 and 12 weeks, and AEs.
STATISTICAL ANALYSIS
All analyses proceeded according to the intent-to-treat principle. Continuous variables are presented as median (interquartile range [IQR]) and categorical variables as n (%). We compared the changes from baseline to follow-up between the sorafenib and placebo groups using Wilcoxon rank sum tests. Fisher’s exact tests were used to compare categorical outcomes at 12 weeks between the groups. Patients who underwent transplant prior to 12 weeks were included in the primary analysis if data were available. The original statistical analysis plan had included several model-based analyses using linear mixed-effects models, time-varying covariates, and multiple imputation, which were not performed because of the small sample size at termination. No interim analyses or stopping rules were planned a priori for the trial. All reported P values are nominal without correction for multiple comparisons.
We expected 30 patients to enroll in the study and anticipated a 10% dropout rate, accounted for in our updated power calculations. Assuming that 1 standard deviation in AaPO2 was ~10 mm Hg, we anticipated sufficient power to detect a difference of 11.5 mm Hg in the changes in AaPO2 over 12 weeks between patients allocated to sorafenib and placebo.
DATA-SHARING STATEMENT
In accordance with International Committee of Medical Journal Editor standards, we plan to share individual deidentified clinical, biomarker, echocardiographic, and event data from the analyzable data set that underlie the results reported in this article. Data dictionaries, study protocol, and analytic code will be available upon request. Even with the removal of identifiers, we believe that the possibility remains of deductive disclosure of patients. Thus, we will make the data and associated documentation available to investigators based in academic centers whose proposed use of the data has been approved by a committee of SHPS investigators only under a data-sharing agreement that provides for the following:
A commitment to using the data only for research purposes and for ancillary studies approved by the parent study review committee and not to identify any individual participant.
A commitment to securing the data using appropriate computer technology.
A commitment to destroying or returning the data after the approved analyses are completed.
These data will be made available beginning in October 2019, which is 18 months from the completion of the study/database lock, and they will be available for 2 years (until October 2021).
Results
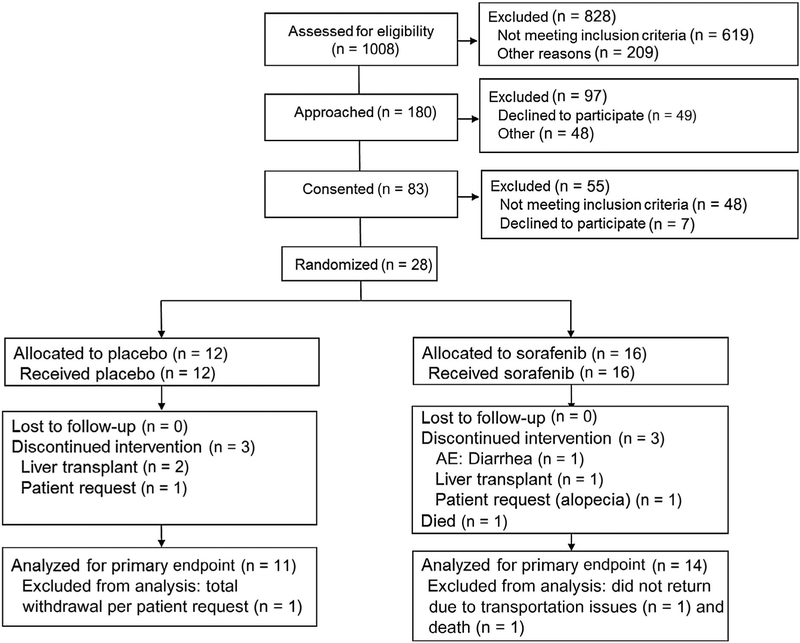
We prescreened 1008 patients during the enrollment period (Fig. 1). Of these, 180 appeared potentially eligible and were approached for possible screening and enrollment; 97 declined to participate or were excluded for other reasons. A total of 83 patients provided informed consent, of whom 48 did not meet inclusion criteria at screening and 7 declined to participate after giving consent. Finally, 28 patients were randomized: 16 to the sorafenib group and 12 to the placebo group. No patients were lost to follow-up. In the placebo arm, 3 patients discontinued the study drug because of liver transplantation (n = 2) or patient request (n = 1). One patient totally withdrew from the study, leaving 11 patients for analysis of the primary endpoint in the placebo arm. In the sorafenib arm, 3 patients discontinued the study drug because of an AE (diarrhea, n = 1), liver transplantation (n = 1), and patient request (due to alopecia, n = 1). There was 1 patient in this arm who died, and 1 patient did not return for the final visit because of lack of transportation, leaving 14 patients for analysis of the primary endpoint in the sorafenib arm. The 2 groups were similar in terms of age and sex, but the sorafenib group had more non-Hispanic whites (Table 1). The underlying liver disease diagnoses and severity of liver disease (reflected by the Model for End-Stage Liver Disease score and Child-Pugh class) were similar between the arms. Although spirometry was similar (Table 2), the PaO2, AaPO2, oxygen saturation, and intrapulmonary shunting at baseline may have been different between the groups, with the sorafenib group having 11 mm Hg lower PaO2 and 13 mm Hg higher AaPO2. Approximately 50% of both groups had World Health Organization functional class III symptoms, and the 6-minute walk distances were similar.
FIG. 1.
Consolidated Standards of Reporting Trials diagram.
TABLE 1.
Baseline Characteristics of Patients
| Characteristics | Placebo Group (n = 12) |
Sorafenib Group (n = 16) |
|---|---|---|
| Age, years | 59 (54–63) | 60 (53–64) |
| Sex, female | 6 (50) | 10 (62) |
| Race/ethnicity | ||
| White (non-Hispanic) | 6 (50) | 15 (94) |
| Hispanic or Latino | 4 (33) | 1 (6) |
| Black | 0 (0) | 0 (0) |
| Other | 2 (17) | 0 (0) |
| Body mass index, kg/m2 | 32 (29–33) | 34 (27–37) |
| Liver disease etiology* | ||
| Hepatitis C | 3 (25) | 4 (25) |
| Nonalcoholic fatty liver disease | 3 (25) | 5 (31) |
| Alcohol | 2 (17) | 5 (31) |
| Autoimmune | 2 (17) | 0 (0) |
| Cryptogenic | 1 (8) | 1 (6) |
| Alpha-1-antitrypsin deficiency | 1 (8) | 1 (6) |
| Other | 0 (0) | 3 (19) |
| Severity and complications of liver disease | ||
| Model for End-Stage Liver Disease | 13 (10–15) | 13 (11–14) |
| Child-Pugh class | ||
| A | 3 (25) | 7 (44) |
| B | 9 (75) | 9 (56) |
| Ascites | 4 (33) | 5 (31) |
| Encephalopathy | 6 (50) | 5 (31) |
| Esophageal varices | 10 (83) | 11 (69) |
| World Health Organization functional class | ||
| Class 1 | 4 (33) | 3 (19) |
| Class 2 | 2 (17) | 6 (37) |
| Class 3 | 6 (50) | 7 (44) |
NOTE: Data are given as median (IQR) or n (%).
Patients may have had more than 1 liver disease etiology.
TABLE 2.
Baseline Physiologic, Laboratory, Exercise, and Quality-of-Life Data
| Data | Placebo Group (n = 12) |
Sorafenib Group (n = 16) |
|---|---|---|
| Pulmonary function testing | ||
| Predicted FVC, % | 86 (81–105) | 87 (77–100) |
| Predicted FEV1, % | 95 (83–104) | 85 (74–96) |
| FEV1/FVC | 81 (74–83) | 74 (72–77) |
| Predicted total lung capacity, % | 98 (90–108) | 93 (85–106) |
| Arterial blood gas | ||
| PH | 7.5 (7.4–7.5) | 7.5 (7.4–7.5) |
| PaCO2, mm Hg | 30 (28–32) | 29 (28–32) |
| PaO2, mm Hg | 69 (55–87) | 58 (54–69) |
| AaPO2, mm Hg | 39 (24–56) | 52 (45–63) |
| Seated oxygen saturation from pulse oximetry, % | 95 (92–97) | 92 (88–94) |
| Supine oxygen saturation from pulse oximetry, % | 97 (92–97) | 93 (91–95) |
| Grade of intrapulmonary shunting by CE | ||
| Grade 1 | 2 (17) | 2 (13) |
| Grade 2 | 5 (42) | 1 (6) |
| Grade 3 | 5 (42) | 13 (81) |
| Laboratory test results | ||
| Sodium, mmol/L | 139 (137–142) | 141 (139–143) |
| Blood urea nitrogen, mg/dL | 15 (12–19) | 16 (10–19) |
| Creatinine, mg/dL | 0.9 (0.6–1.0) | 0.9 (0.8–1.1) |
| Hemoglobin, g/dL | 14 (12–15) | 14 (13–15) |
| Platelets, × 109 per liter | 76 (59–84) | 93 (78–106) |
| INR | 1.3 (1.2–1.4) | 1.3 (1.3–1.5) |
| Albumin, g/dL | 3.3 (3.2–3.7) | 3.6 (3.1–4.0) |
| Total bilirubin, mg/dL | 1.7 (1.1–2.4) | 1.9 (1.2–2.5) |
| 6-minute walk distance, m | 350 (281–459) | 329 (262–395) |
| SF-36 PCS | 38 (27–48) | 36 (27–42) |
| SF-36 MCS | 53 (41–58) | 53 (46–60) |
| Mahler’s Baseline Dyspnea Index | 5.5 (2.0–8.5) | 6.5 (4.5–9.0) |
NOTE: Data are given as median (IQR) or n (%).
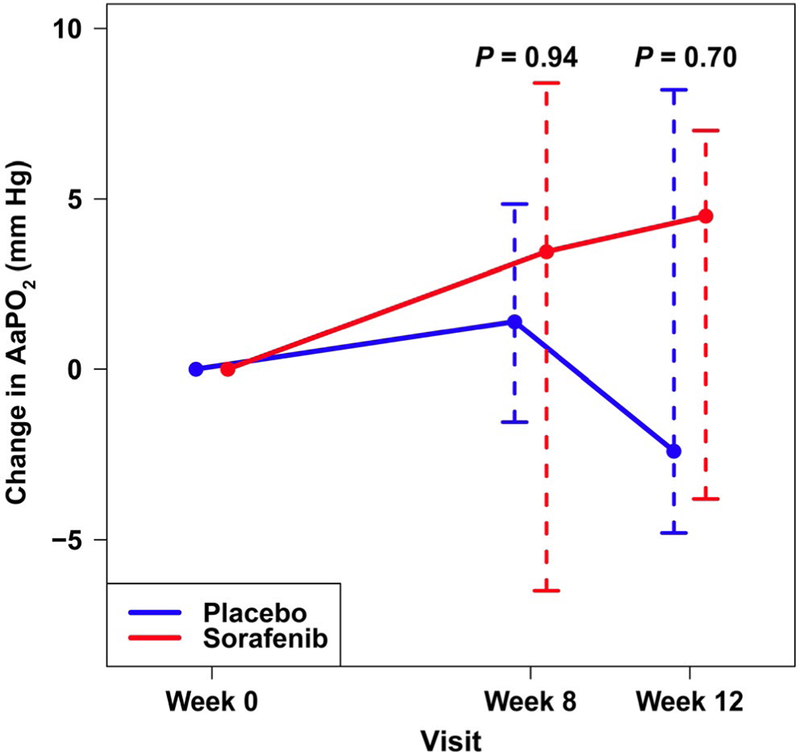
The median change in AaPO2 from baseline to 12 weeks did not differ significantly between the sorafenib group (4.5 mm Hg; IQR, −3.8 to 7.0 mm Hg) and the placebo group (−2.4 mm Hg; IQR, −4.8 to 8.2 mm Hg; P = 0.70; Fig. 2). CE was available and could be assessed in 12 patients randomized to sorafenib and 9 patients randomized to placebo. A total of 3 (25%) patients allocated to sorafenib had an improvement in intrapulmonary shunting compared with 6 (67%) patients allocated to placebo (P = 0.09). There were no differences between the groups in PaO2 or sitting or supine oxygen saturation by pulse oximetry (data not shown).
FIG. 2.
Median (whiskers, IQR) absolute change in AaPO2 (mm Hg) from baseline to 8 and 12 weeks in patients receiving sorafenib (red) or placebo (blue). P values are for comparison with baseline.
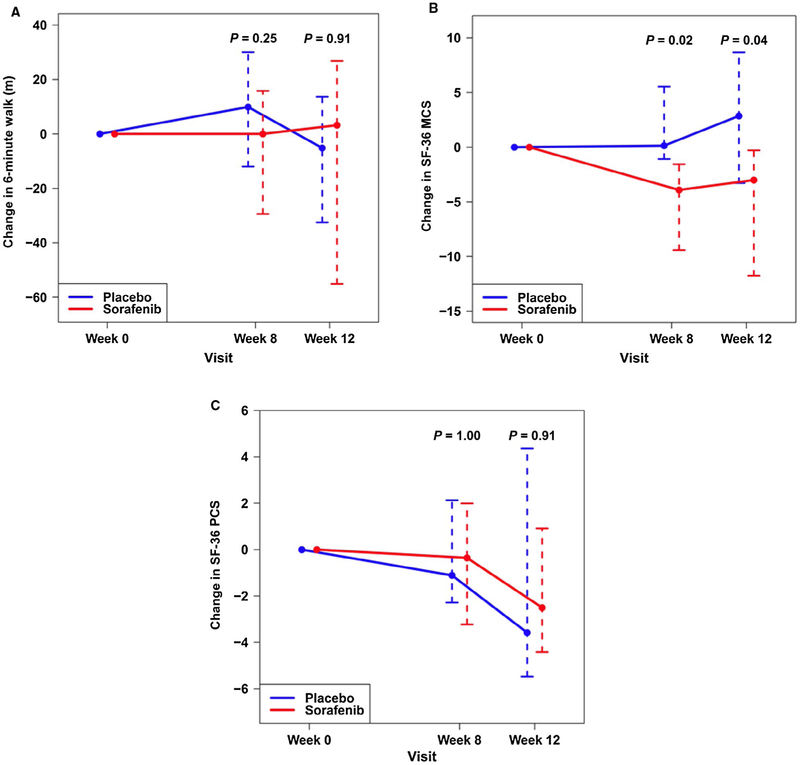
The changes in the 6-minute walk distance (Fig. 3A) and the Mahler s Dyspnea Index (Supporting Table 2) were similar between the groups as was the functional class (data not shown). Allocation to sorafenib appeared to worsen the mental component score (MCS) of the SF-36 at 12 weeks compared to placebo (Fig. 3B; Supporting Table 2), but it had no effect on the physical component score (PCS; Fig. 3C; Supporting Table 2).
FIG. 3.
Median (whiskers, IQR) absolute change in (A) 6-minute walk distance (meters), (B) SF-36 MCS, and (C) SF-36 PCS from baseline to 8 and 12 weeks in patients receiving sorafenib (red) or placebo (blue). P values are for comparison with baseline.
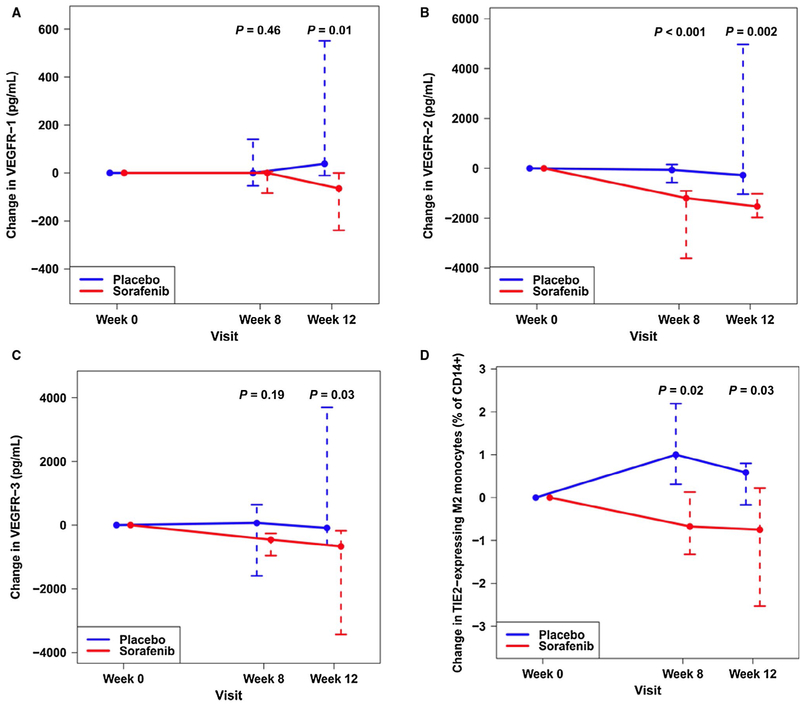
Sorafenib significantly reduced the levels of some markers of angiogenesis (including VEGFR-1, −2, and −3) and TIE2-expressing M2 monocytes (Fig. 4), but it had no effect on others, including vascular endothelial growth factor, c-kit, fractalkine, angiopoietin 2, and HPCs (Supporting Tables 3 and 4).
FIG. 4.
Median (whiskers, IQR) absolute change in circulating (A) VEGFR-1 (pg/mL), (B) VEGFR-2 (pg/mL), (C) VEGFR-3 (pg/mL), and (D) TIE2-expressing M2 monocytes (% of CD14+ cells) from baseline to 8 and 12 weeks in patients receiving sorafenib (red) or placebo (blue). P values are for comparison with baseline.
The most common AEs were diarrhea, abdominal pain, and weight loss (Table 3). There were no clear differences in AEs between sorafenib and placebo; non-liver transplant serious AEs occurred in 5 patients receiving sorafenib and 3 patients receiving placebo (Table 4). The only death in the study was due to Fournier’s gangrene and Escherichia colt sepsis in a patient who had been receiving sorafenib for 2 weeks.
TABLE 3.
Patients Experiencing AEs
| AE | Placebo Group (n = 12) |
Sorafenib Group (n = 16) |
|---|---|---|
| Diarrhea/loose stools | 4 (33) | 5 (31) |
| Abdominal pain | 5 (42) | 2 (13) |
| Weight loss | 2 (17) | 5 (31) |
| Fatigue | 3 (25) | 3 (19) |
| Nausea | 3 (25) | 3 (19) |
| Pruritus | 3 (25) | 3 (19) |
| Cough | 3 (25) | 2 (13) |
| Hypertension | 2 (17) | 3 (19) |
| Rash (maculopapular) | 1 (8) | 4 (25) |
| Headache | 0 (0) | 4 (25) |
| Myalgia | 2 (17) | 2 (13) |
| Alopecia/abnormal hair growth | 1 (8) | 2 (13) |
| Dry skin | 0 (0) | 3 (19) |
| Edema/edema in the limbs | 1 (8) | 2 (13) |
| Mucositis (oral) | 0 (0) | 3 (19) |
| Nasal congestion | 2 (17) | 1 (6) |
| Pain in extremity | 1 (8) | 2 (13) |
| Upper respiratory infection | 2 (17) | 1 (6) |
NOTE: Data are given as n (%). AEs that occurred in 3 or more individuals are shown, and all AEs were CTCAE grade 1 or 2.
TABLE 4.
Patients Experiencing Serious AEs (Non-Liver Transplant)
| Serious AE | Placebo Group (n = 12) |
Sorafenib Group (n = 16) |
|---|---|---|
| Encephalopathy | 0 | 1 (6)* |
| Neoplasm | 1 (8)* | 1 (6)† |
| Diagnostic procedure | 0 | 1 (6)* |
| Hypoxia | 0 | 1 (6)‡ |
| Mania | 1 (8)‡ | 0 |
| Low platelet count | 1 (8)‡ | 0 |
| Encephalopathy and skin infection (Fournier’s gangrene) | 0 | 1 (6)§ |
NOTE: Data are given as n (%).
CTCAE grade 1.
CTCAE grade 2.
CTCAE grade 3.
CTCAE grade 5.
We found no differences in safety laboratory results or systemic blood pressure between the groups (data not shown). Analyses excluding patients who underwent liver transplantation during the study showed similar results.
Discussion
This is the first randomized clinical trial of an anti-angiogenic therapy for HPS. We found no effect of sorafenib on AaPO2 or on the degree of intrapulmonary shunting. Sorafenib did not have an impact on exercise capacity or symptoms of dyspnea. Sorafenib reduced circulating levels of VEGFR-1, −2, and −3 and TIE2-expressing M2 monocytes but did not affect circulating hematopoietic progenitor cells or angiopoietin 2, which have been linked to angiogenesis. Sorafenib worsened the mental component of health-related quality of life. AEs occurred at similar rates in the 2 groups. The multicenter design lends to the generalizability of the findings.
Experimental and human data supporting the role of angiogenesis in HPS have been reported. Historical studies and pathologic specimens from patients demonstrate increased vascularity in the lung.(12,13) Administration of sorafenib to rats after common bile duct ligation (which recapitulates HPS) reverses abnormal oxygenation, reduces intrapulmonary vascular dilations and shunting, and decreases circulating monocytes and progenitor cells.(10)
Although we expected sorafenib to have these same effects in patients with HPS, there are several possible explanations for our results. First, it is possible that the dose used was too low. For the usual indications of cancer, the target dose of sorafenib is 800 mg each day. We chose a lower dose than this because of the following:
We thought that excess adverse effects with higher doses would lead to poor adherence in a nononcologic study.
Most patients treated with sorafenib for cancer do not tolerate the maximal dose because of adverse effects.
Our animal studies suggested that this lower dose would be sufficient.
Although inadequate dosing could be an issue, we found that several biomarkers of angiogenesis decreased in patients randomized to sorafenib, including TIE2-expressing M2 monocytes and VEGFR-2, a known biomarker of sorafenib administration,(14) and there was decreased quality of life with the active drug, both suggesting systemic and biological effects of the dose used.
Second, it is possible that the length of the study was inadequate to see an effect. We based the study duration on the usual rapid correction of gas exchange abnormalities after liver transplant(15) while trying to avoid a long study, which would be complicated by many patients undergoing liver transplantation. It would not be feasible (or ethical) to forbid liver transplant during the study duration (because this is the standard of care for HPS), so we tried to focus on patients who were unlikely to undergo transplant within 4 months from screening. Even so, 3 transplants occurred during the study, requiring withdrawal of the study drug.
Third, the early termination of the study, withdrawal of the study drug for transplant or adverse effects, and patient withdrawals could have led to a loss of the ability to detect differences between sorafenib and placebo that were actually there. However, the changes in AaPO2 were very similar between the groups, and there was, if anything, possible improvement in intrapulmonary shunting in the placebo group compared with the sorafenib group. In addition, we found significant effects of sorafenib on VEGFRs and other circulating markers of angiogenesis. Therefore, although the sample size was small, it is unlikely that lack of power explains the results. Fourth, although the patients randomized to sorafenib may have had more severe HPS, it is unlikely that this imbalance would have entirely negated the effect of the intervention. Finally, and most likely, sorafenib may not decrease lung angiogenesis and may be ineffective at treating the abnormalities in oxygenation and intrapulmonary shunting in HPS.
Our study had several limitations. Recruitment for this study was more difficult than anticipated because of challenges in routine screening for HPS in the liver transplant programs with arterial blood gas (rather than pulse oximetry, which is relatively insensitive for detecting HPS),(16) lack of patient awareness regarding the existence and implications of HPS, and hesitation from potential subjects regarding the side effect profile of sorafenib. The sample size was reduced, and the sponsor terminated the trial before reaching the target sample size. Arterial blood gases can be subject to measurement error due to the technique of arterial puncture, timing of analysis, problems with accurately quantifying the fraction of inspired oxygen in a patient using supplemental oxygen by nasal cannula, and measurement of barometric pressure. Recent studies have shown significant within-patient variability in AaPO2 in a cohort of patients with liver disease being evaluated for HPS(17); such variability may have biased to the null. Several patients underwent liver transplantation, which not only can have effects on HPS but also required stopping sorafenib because of potential effects on healing postoperatively, both of which could have affected the results. There were some missing data, which were not imputed because of the small size of the study population. Finally, we report a large number of statistical tests, so that type 1 error (ie, false positives) is possible.
In conclusion, sorafenib did not reduce the AaPO2 gradient in patients with HPS, despite reductions in VEGFR and other biomarkers of angiogenesis. The lack of clinically significant impact on the primary outcome in the treatment group and potential reduction in quality of life may decrease the priority of future studies of sorafenib for HPS. Additional studies of other possible interventions for angiogenesis or novel pathobiological pathways are required.
Supplementary Material
Acknowledgments:
We thank the following individuals for their assistance with this study: Scarlett Bellamy, M.Sc., and Michael Sprys, M.S.
This work has received funding from grants UMI HL116886 and K24 HL103844. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Grace Lin consults for Prothena and is on the speakers bureau for Boston Scientific. David E. Kaplan received research grants/contracts from Bayer. David Goldberg received research grants/contracts from Merck, Zydus Pharmaceuticals, and Gilead Sciences.
Abbreviations:
- AaPO2
alveolar-arterial oxygen gradient
- AE
adverse event
- CE
contrast echocardiography
- CTCAE
Common Terminology Criteria for Adverse Events
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- HPCs
hematopoietic progenitor cells
- HPS
hepatopulmonary syndrome
- INR
international normalized ratio
- IQR
interquartile range
- MCS
mental component score
- PaCO2
partial pressure of arterial carbon dioxide
- PaO2
partial pressure of arterial oxygen
- PCS
physical component score
- SF-36
Short Form 36
- SHPS
Sorafenib in Hepatopulmonary Syndrome
- VEGFR
vascular endothelial growth factor receptor
Footnotes
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1).CDC/NCHS. National Vital Statistics System. Mortality. 2015. [Google Scholar]
- 2).Fallon MB, Krowka MJ, Brown RS, Trotter JF, Zacks S, Roberts KE, et al. Impact of hepatopulmonary syndrome on quality of life and survival in liver transplant candidates. Gastroenterology 2008;135:1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Goldberg DS, Krok K, Batra S, Trotter JF, Kawut SM, Fallon MB. Impact of the hepatopulmonary syndrome MELD exception policy on outcomes of patients after liver transplantation: an analysis of the UNOS database. Gastroenterology 2014;146:1256–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Swanson KL, Wiesner RH, Krowka MJ. Natural history of hepatopulmonary syndrome: impact of liver transplantation. Hepatology 2005;41:1122–1129. [DOI] [PubMed] [Google Scholar]
- 5).Iyer VN, Swanson KL, Cartin-Ceba R, Dierkhising RA, Rosen CB, Heimbach JK, et al. Hepatopulmonary syndrome: favorable outcomes in the MELD exception era. Hepatology 2013;57:2427–2435. [DOI] [PubMed] [Google Scholar]
- 6).Roberts KE, Kawut SM, Krowka MJ, Brown RS Jr, Trotter JF, Shah V, et al. Genetic risk factors for hepatopulmonary syndrome in patients with advanced liver disease. Gastroenterology 2010;139:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Thenappan T, Goel A, Marsboom G, Fang YH, Toth PT, Zhang HJ, et al. A central role for CD68(+) macrophages in hepatopulmonary syndrome. Reversal by macrophage depletion. Am J Respir Crit Care Med 2011;183:1080–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Zhang J, Ling Y, Tang L, Luo B, Pollock DM, Fallon MB. Attenuation of experimental hepatopulmonary syndrome in endothelin B receptor-deficient rats. Am J Physiol Gastrointest Liver Physiol 2009;296:G704–G708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Zhang J, Luo B, Tang L, Wang Y, Stockard CR, Kadish I, et al. Pulmonary angiogenesis in a rat model of hepatopulmonary syndrome. Gastroenterology 2009;136:1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Yang W, Zhang J, Hu B, Wu W, Venter J, Alpini G, et al. The role of receptor tyrosine kinase activation in cholangiocytes and pulmonary vascular endothelium in experimental hepatopulmonary syndrome. Am J Physiol Gastrointest Liver Physiol 2014;306:G72–G80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Rodriguez-Roisin R, Krowka MJ, Herve P, Fallon MB; for ERB Task Force Pulmonary-Hepatic Vascular Disorders (PHD) Scientific Committee. Pulmonary-hepatic vascular disorders (PHD). Eur Respir J 2004;24:861–880. [DOI] [PubMed] [Google Scholar]
- 12).Berthelot P, Walker JG, Sherlock S, Reid L. Arterial changes in the lungs in cirrhosis of the liver-lung spider nevi. N Engl J Med 1966;274:291–298. [DOI] [PubMed] [Google Scholar]
- 13).Williams A, Trewby P, Williams R, Reid L. Structural alterations to the pulmonary circulation in fulminant hepatic failure. Thorax 1979;34:447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Thomeas V, Chow S, Gutierrez JO, Karovic S, Wroblewski K, Kistner-Griffin E, et al. Technical considerations in the development of circulating peptides as pharmacodynamic biomarkers for angiogenesis inhibitors. J Clin Pharmacol 2014;54:682–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Gupta S, Castel H, Rao RV, Picard M, Lilly L, Faughnan ME, et al. Improved survival after liver transplantation in patients with hepatopulmonary syndrome. Am J Transplant 2010;10: 354–363. [DOI] [PubMed] [Google Scholar]
- 16).Forde KA, Fallon MB, Krowka MJ, Sprys M, Goldberg DS, Krok KL, et al. Pulse oximetry is insensitive for detection of hepatopulmonary syndrome in patients evaluated for liver transplantation. Hepatology 2018;69:270–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Gupta S, Nayyar D, Pomier-Layrargues G. Variability of oxygenation in possible hepatopulmonary syndrome: effects of requiring two abnormal arterial blood gas results for diagnosis. Dig Dis Sci 2015;60:1848–1855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.