Abstract
Background
To understand real-world human papillomavirus (HPV) vaccine impact, continuous evaluation using population-based data is critical. We evaluated the early impact of the school-based HPV immunization program on cervical dysplasia in women in British Columbia, Canada.
Methods
Data linkage was performed using records from provincial cervical screening and immunization registries. Precancerous outcomes were compared between unvaccinated and HPV-vaccinated women born 1994–2005. Incidence rate, relative rate (RR), and vaccine effectiveness (VE), using unadjusted and adjusted Poisson regression of cytology (HSIL) and histopathology (CIN2, CIN3, and CIN2+) outcomes, were compared across vaccination status groups.
Results
Women who received a complete series of vaccine on schedule between age 9 and 14 years had an adjusted RR = 0.42 (95% confidence interval [CI], 0.31–0.57) for CIN2+ over 7 years of follow-up compared to unvaccinated women, resulting in a VE of 57.9% (95% CI, 43.2%–69.0%). Adjusted RR for HSIL was 0.53 (95% CI, .43–.64), resulting in a VE of 47.1% (95% CI, 35.6%–56.7%).
Conclusion
Women vaccinated against HPV have a lower incidence of cervical dysplasia compared to unvaccinated women. Immunization between 9 and 14 years of age should be encouraged. Continued program evaluation is important for measuring long-term population impact.
Keywords: human papillomavirus, papillomavirus vaccines, vaccine effectiveness, cervical intraepithelial neoplasia, immunization programs
A data linkage of the cervix screening program registry to the immunization registry in British Columbia showed that women vaccinated against HPV have a lower incidence of cervical dysplasia compared to unvaccinated women.
Randomized controlled trials have demonstrated that human papillomavirus (HPV) vaccines are highly efficacious for the prevention of HPV infections and precancerous lesions [1–6]. However, progression of an HPV infection to cervical cancer takes decades, thus continuous evaluation using population-based data offers early and critical insight into the real-world impact of vaccination. In countries with cervical cancer screening and HPV vaccination programs, women vaccinated in adolescence are now becoming age eligible for routine cervical cancer screening. Various countries that have implemented population-based vaccination programs have reported decreases in HPV infection prevalence, genital warts, and precancerous lesions, even in localities with lower HPV vaccine uptake [7–15]. However, very few studies have documented the population-level impact of HPV vaccination in young women on precancerous lesions as they enter cervical cancer screening programs.
The province of British Columbia (BC), Canada, implemented a voluntary school-based HPV immunization program in 2008 with the quadrivalent HPV vaccine, providing protection against HPV 6, 11, 16, and 18. The BC HPV immunization program’s evaluation framework includes annual vaccine uptake rates, an ongoing province-wide HPV prevalence study, and ecological vaccine impact studies [16–19]. Since introduction of the BC HPV immunization program, vaccine uptake has remained below the Canadian program target of 90% [20], with only 66.9% of eligible grade 6 girls having completed HPV vaccination in 2017–2018 [16]. Despite suboptimal uptake across BC, ecological analysis revealed rates of cervical intraepithelial neoplasia (CIN) have significantly declined in young women after the introduction of HPV vaccine [19].
In BC, HPV vaccination status for all girls in the province is maintained in 2 electronic immunization registries. Since 1960, cervical cancer screening in BC has been centrally coordinated by the BC Cancer Cervix Screening Program, which conducts and records information on every cervical screen done in the province. By using data from the comprehensive provincial HPV vaccine and cervical screening registries, BC is uniquely able to evaluate long-term population impact of the HPV vaccine using linkage on an individual level.
The results of a province-wide data linkage between the BC Cancer Cervix Screening Program’s registry and records from the immunization registries are presented here. As the first cohort of women receiving HPV vaccination in the school-based immunization program enter eligibility for the Cervix Screening Program, the early impact of the HPV immunization program on individual outcomes for cervical cancer prevention can be evaluated. Cytological (high-grade squamous intraepithelial lesion [HSIL]) and histological (CIN2, CIN3, and CIN2+) outcomes in a screening cohort of women born in 1994 through 2005 were compared between those who were vaccinated against HPV and those who were unvaccinated.
METHODS
BC HPV Vaccination Program and Cervix Screening Program
The BC school-based HPV immunization program was introduced in 2008 for grade 6 girls (11 years old, birth year 1997), which included a 3-year catch-up program for grade 9 girls (14 years old, birth year 1994) until June 2011 (birth year 1996), although several health authorities have since continued to offer HPV vaccine in grade 9 to unimmunized eligible students. In 2014–2015, the vaccine schedule was changed from a 3-dose to a 2-dose schedule based on results from a Canadian immunogenicity trial [21, 22].
Two electronic registries (Panorama and Primary Access Regional Information System) capture all school-based HPV immunizations in BC. The registries capture individual date of birth, number and timing of vaccine doses, immunization dates, consent, and adverse events. Both registries were used in this data linkage analysis to provide immunization coverage information for the province.
In BC, cervical cancer screening is performed with the Papanicolaou (Pap) smear (conventional cytology). All provincial cervical cytology specimens are processed and interpreted at 1 centralized laboratory and results maintained in 1 database, which contains complete cytology, colposcopic treatment, and histopathology results, including disease outcomes, for all women participating in cervix screening in BC.
Between 2011 and 2016, provincial cervical cancer screening guidelines recommended biennial cytology-based screening commencing at age 21 years or 3 years after sexual debut. In June 2016, the Cervix Screening Program updated the guidelines to recommend women 25–69 years of age receive cytology-based cervical screening every 3 years, regardless of age of sexual debut [23].
HPV Vaccination Status Cohort
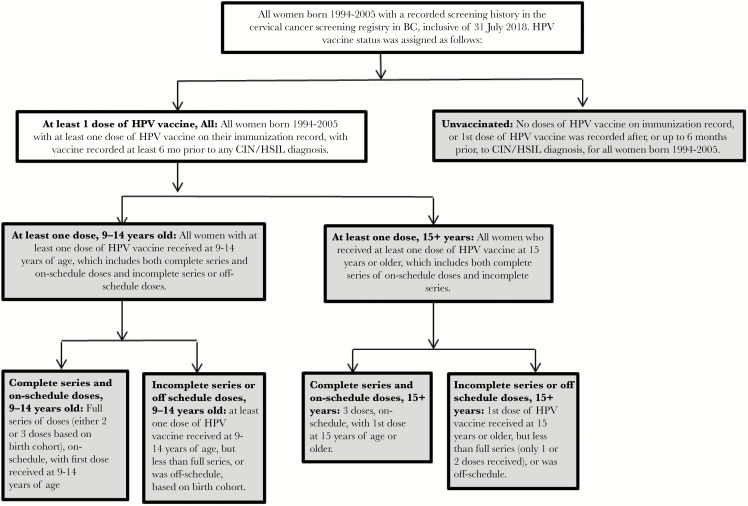
For this analysis, the vaccine cohort was restricted to women who received the HPV vaccine at the age of 9 or later. Vaccination status was defined based on age at the first dose and the number of doses received relative to the recommended HPV immunization schedule for a given birth cohort at the time of eligibility for vaccination. Specific vaccine status definitions and subgroups are described in Figure 1.
Figure 1.
Definitions of vaccination status and subgroups, based on age of first dose of human papillomavirus (HPV) vaccine and number of doses received relative to HPV immunization schedule for given birth cohort at time of eligibility for vaccination. Abbreviations: BC, British Columbia; CIN, cervical intraepithelial neoplasia; HSIL, high-grade squamous intraepithelial lesion.
For this analysis, we included the following groups: unvaccinated, vaccinated with at least 1 dose at 9–14 years old or 15+ years old, complete series and on-schedule doses at 9–14 years old or 15+ years old, and incomplete series or off-schedule doses at 9–14 years old or 15+ years old. Vaccination status criteria for women varied by birth cohort due to changes in HPV vaccine schedule over time (Supplementary Figure 1). Women with cervical dysplasia were defined as vaccinated only if any dose of the HPV vaccine was administered at least 6 months prior to diagnosis.
Cervix Screening Program Cohort
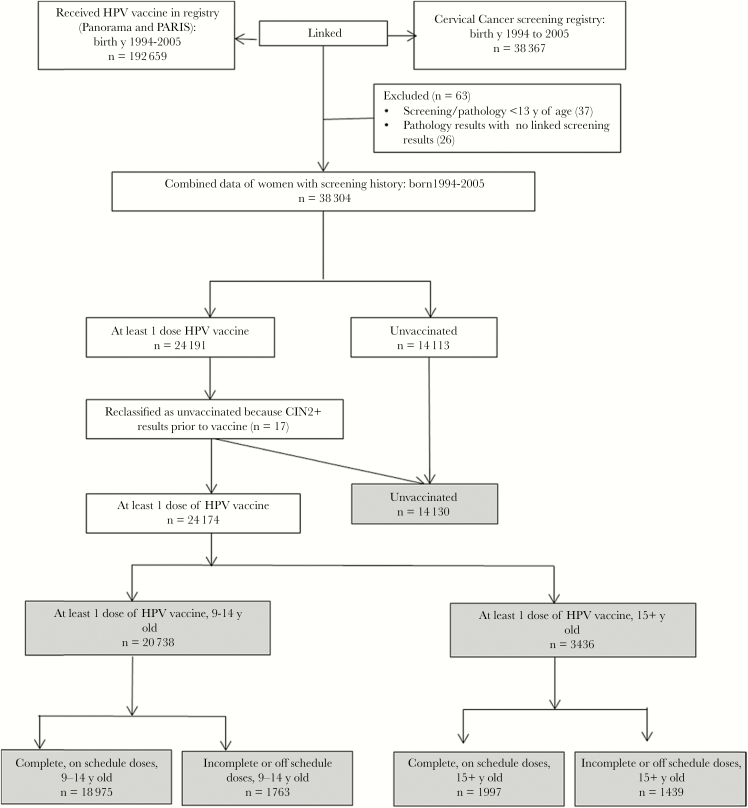
We used the screening cohort of women from the Cervix Screening Program’s registry born in 1994 through 2005 who had ≥1 Pap smear taken on or after age 13 years, up to 31 July 2018. Records were excluded from the analysis if the prior screening result was not available for a pathology diagnosis or if a pathology diagnosis was received prior to a screening result due to a data error (Figure 2). Pathology diagnoses of high-grade histological outcomes CIN2, CIN3, CIN2+ (combination of CIN2 and CIN3), and the cytology outcome HSIL from a screening result, were selected as analysis end points, as these are well-established markers for progression to invasive cancer [24].
Figure 2.
Flow chart of the data process between the British Columbia (BC) cervical cancer screening registry and the BC Panorama and Primary Access Regional Information System (PARIS) immunization registries, inclusive to 31 July 2018. Abbreviations: CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; y, years.
Data Linkage
In BC, health care is publicly funded and all citizens, permanent residents, and long-term visa holders are provided a unique Personal Health Number (PHN) used for accessing universal health care, which includes the cost of cervical cancer screening and adolescent HPV vaccination. The Cervix Screening Program and immunization registries were linked using PHNs. All women with an HPV vaccine record who were born in 1994 through 2005 were linked to the Cervix Screening Program’s registry to create the vaccinated cohort, covering people who have received at least 1 dose of the HPV vaccine (n = 24 191). The remainder of the women in the Cervix Screening Program’s registry born in 1994 through 2005 with ≥1 Pap smear taken on or after age 13 years, up to 31 July 2018, were defined as the unvaccinated cohort. Once linkage occurred, all personal identifiers were removed and the dataset was provided to the study analyst. Individual informed consent was not required for this study. Ethics approval was obtained from University of British Columbia Clinical Research Ethics Board (H16-01466). The study was conducted in adherence with the Declaration of Helsinki.
Statistical Analysis
Incidence Rates
Incidence rates (IRs) and 95% confidence intervals (CIs) for HSIL, CIN2, CIN3, and CIN2+ were calculated for unvaccinated and the vaccination status groups (at least 1 dose 9–14 years old or 15+ years, incomplete or off schedule 9–14 years old or 15+ years, complete and on schedule 9–14 years old and 15+ years old). IRs were calculated based on 1000 person-years, with the individual years at risk for HPV acquisition based on age at which first vaccination was given and the number of years between the first screening visit minus 3 years and/or the date of the most severe pathology result, or 31 July 2018, whichever came first. First screening date minus 3 years was chosen as the best approximate age of sexual debut, as program recommendations up until 2016 were to begin screening at age 21 or 3 years after sexual debut. In cases with multiple pathology results, the most severe pathology was included in the analysis. In cases with more than 1 equivalent cytology result, the date of the first result was included in the analysis.
Relative Rate
Relative incidence rate (RR) and 95% CIs for each of HSIL, CIN2, CIN3, and CIN2+ were calculated using (un)adjusted Poisson regression models. Model adjustment included birth year and age at first screening test less 3 years. Women vaccinated at 9–14 years of age were compared to unvaccinated women. Women vaccinated at 9–14 years with complete series and on-schedule doses were selected as the main comparison group, for comparison with those 15+ years, as this younger age group is the target age group of the BC HPV immunization program, and was therefore deemed the most relevant comparator for public health practice.
Vaccine Effectiveness
RR, adjusted for birth year and age at first cervical screen, was used to estimate relative vaccine effectiveness (VE), calculated as VE = (1 − RR) × 100%. VE was estimated for complete series and on schedule and incomplete series/off schedule, and then compared to unvaccinated women.
RESULTS
Vaccine Cohort
There were 192 659 women in the vaccine registry born in 1994 through 2005 who received their first dose of HPV vaccine at age 9 or later, with the mean age of first vaccine at 12.2 years (SD ± 1.8) (Supplementary Table 1). The majority (92.6%) received the HPV vaccine (at least 1 dose) through the school-based immunization targeted age range of 9–14 years of age. The 7.4% who initiated the HPV vaccine series at 15 years of age or later would have done so outside the school-based HPV immunization program.
Screening Cohort
There were 38 367 women born in 1994 through 2005 with at least 1 Pap smear recorded through 31 July 2018. The analysis excluded 63 (0.2%) women due to inconsistencies in their screening or pathology record (Figure 2). Of the 38 304 women who were screened, 24 174 (63.1%) of them received at least 1 dose of HPV vaccine at least 6 months prior to pathology results. Of the 24 174 women, 20 738 (85.8%) received at least 1 dose at 9–14 years old, of which 18 975 had complete series and on-schedule dosing at 9–14 years old and 1763 had incomplete series at 9–14 years old. Fourteen percent (3436 of 24 174 women) received at least 1 dose at 15 years or older, of which 1997 had complete series and on-schedule dosing at 15 + years and 1439 received incomplete series or off-schedule doses starting at 15+ years of age. The number of unvaccinated women was 14 130. Among the 38 304 women with a cervical screening history, 258 (1.4%) had at least 1 colposcopy and received the complete vaccination series at 9–14 years, compared to 28 (1.8%) women with at least 1 colposcopy and received an incomplete vaccination series. In addition, there were 304 (2.2%) women with at least 1 colposcopy and were unvaccinated. The mean age of first screening visit was 18.7 (SD ± 2.2) years overall, and differed significantly between vaccinated and unvaccinated women (P < .0001); furthermore, vaccinated women had on average more screening visits compared to those unvaccinated (Table 1).
Table 1.
Description of the Screening Cohort Overall and Stratified by HPV Vaccine Status
| Characteristic | Total (N = 38 304) | HPV Vaccine Status | |
|---|---|---|---|
| Unvaccinated (n = 14 130) | Vaccinatedb (n = 24 174) | ||
| Age at first screening, mean (SD) | 18.7 (± 2.2) | 19.1 (± 2.2) | 18.5 (± 2.1) |
| Number of screening visits, median (IQR) | 2.0 (1.0–3.0) | 1.0 (1.0–3.0) | 2.0 (1.0–3.0) |
| Age at first vaccine, mean (SD) | 8.7 (± 6.8) | 0.0 (± 0.0) | 13.8 (± 1.7) |
| Age at first vaccine, No. (%) | |||
| Unvaccinated | 14 130 (36.9) | 14 130 (100) | 0 (0) |
| 9–14 y | 20 738 (54.1) | 0 (0) | 20 738 (85.8) |
| 15–17 y | 2537 (6.6) | 0 (0) | 2537 (10.5) |
| 18 y or older | 899 (2.3) | 0 (0) | 899 (3.7) |
| Birth cohort,a No. (%) | |||
| 1994 | 14 127 (36.9) | 5685 (40.2) | 8442 (34.9) |
| 1995 | 10 253 (26.8) | 3872 (27.4) | 6381 (26.4) |
| 1996 | 6559 (17.1) | 2227 (15.8) | 4332 (17.9) |
| 1997 | 3887 (10.1) | 1299 (9.2) | 2588 (10.7) |
| 1998 | 2019 (5.3) | 628 (4.4) | 1391 (5.8) |
| 1999 | 905 (2.4) | 259 (1.8) | 646 (2.7) |
| 2000 | 375 (1.0) | 110 (0.8) | 265 (1.1) |
| 2001 | 135 (0.4) | 37 (0.3) | 98 (0.4) |
| Cytology,c No. (%) | |||
| No abnormal cytology | 33 898 (88.5) | 12 301 (87.1) | 21 380 (88.4) |
| ASCUS/LSIL | 3918 (10.2) | 1499 (10.6) | 2419 (10.0) |
| HSIL | 488 (1.3) | 229 (1.6) | 259 (1.1) |
| Pathology,c No. (%) | |||
| No CIN2+ | 38 087 (99.4) | 14 015 (99.2) | 24 072 (99.6) |
| CIN2 | 120 (0.3) | 55 (0.4) | 65 (0.3) |
| CIN3 | 97 (0.3) | 60 (0.4) | 37 (0.2) |
| CIN2+ d | 217 (0.6) | 115 (0.8) | 102 (0.4) |
Abbreviations: ASCUS, atypical squamous cells of undetermined significance; CIN, cervical intraepithelial neoplasia (grade 2, 3, or combined 2+); HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; IQR, interquartile range; LSIL, low-grade squamous intraepithelial lesion; y, years.
a2002–2005 inclusive, not reported due to small numbers.
bVaccinated: at least 1 dose of HPV vaccine on immunization record, at least 6 months prior to any CIN/HSIL diagnosis, for all women born in 1994–2005.
cOnly reported the highest cytology and pathology outcome for each individual.
dCIN2 and CIN3 outcomes combined.
Incidence Rates and Relative Rates for Cervical Pathology
At Least 1 HPV Dose, 9–14 Years of Age, Including Complete and Incomplete Series
Unvaccinated women had an IR for CIN2+ of 1.20 per 1000 person-years (95% CI, 1.00–1.42) and for CIN3 of 0.62 per 1000 person-years (95% CI, .48–.79). Women with at least 1 dose of HPV vaccine administered at 9–14 years of age had an IR for CIN2+ of 0.52 per 1000 person-years (95% CI, .42–.64) and the IR for CIN3 was 0.17 per 1000 person-years (95% CI, .12–.24) (Supplementary Figure 2; Table 2). Overall, a significant reduction in the RR of HSIL, CIN2, CIN3, and CIN2+ in both unadjusted and adjusted models was observed for those with at least 1 HPV vaccine dose administered at 9–14 years of age compared to those who were unvaccinated (Table 3). The adjusted RR for CIN2+ for women with at least 1 dose of HPV vaccine administered at 9–14 years of age was 0.43 (95% CI, .32–.58) compared to unvaccinated women.
Table 2.
Incidence Rates Adjusted for Person Time at Risk and Stratified by Dosage and Age at First Vaccine
| Vaccine Status (n) | HSIL, IR (95% CI) | No. of HSIL | CIN 2, IR (95% CI) | No. of CIN2 | CIN 3, IR (95% CI) | No. of CIN3 | CIN 2+, IR (95% CI) | No. of CIN 2+ |
|---|---|---|---|---|---|---|---|---|
| Unvaccinated (14 130) | 2.96 (2.60–3.36) | 229 | 0.57 (.44–.73) | 55 | 0.62 (.48–.79) | 60 | 1.20 (1.00–1.42) | 115 |
| At least 1 dose of vaccine 9–14 y (20 738) | 1.61 (1.40–1.84) | 191 | 0.35 (.27–.45) | 51 | 0.17 (.12–.24) | 25 | 0.52 (.42–.64) | 76 |
| Incomplete series or off schedule 9–14 y (1763) | 1.88 (1.22–2.79) | 20 | 0.46 (.22–.90) | 6 | 0.23 (.08–.56) | <5a | 0.69 (.37–1.22) | 9 |
| Complete series on schedule 9–14 y (18 975) | 1.58 (1.36–1.83) | 171 | 0.34 (.26–.44) | 45 | 0.16 (.11–.24) | 22 | 0.50 (.40–.63) | 67 |
| At least 1 dose of vaccine 15 y and older (3436) | 2.62 (2.04–3.33) | 68 | 0.54 (.29–.91) | 14 | 0.46 (.24–.81) | 12 | 1.00 (.66–1.47) | 26 |
| Incomplete series or off schedule 15 y and older (1439) | 3.32 (2.32–4.59) | 36 | 0.55 (.20–1.20) | 6 | 0.64 (.26–1.33) | 7 | 1.20 (.64–2.05) | 13 |
| Complete series, on schedule 15 y and older (1997) | 2.59 (1.84–3.57) | 32 | 0.53 (.27–.96) | 8 | 0.33 (.15–.68) | 5 | 0.87 (.51–1.40) | 13 |
Abbreviations: CI, confidence interval; CIN, cervical intraepithelial neoplasia; HSIL, high-grade squamous intraepithelial lesion; IR, incidence rate per 1000 person-years.
aSuppressed due to small numbers.
Table 3.
Relative Rate Estimates From Poisson Regression for 4 Outcomes Comparing at Least 1 Dose Administered 9–14 Years of Age, Complete On-schedule Dosing, and Incomplete or Off-schedule Dosing Versus Unvaccinated
| Vaccine Status (n) | HSIL | CIN 2 | CIN 3 | CIN 2+ | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |||||||||
| RR (95%CI) | P Value | RR (95%CI) | P Value | RR (95%CI) | P Value | RR (95%CI) | P Value | RR (95% CI) | P Value | RR (95%CI) | P Value | RR (95%CI) | P Value | RR (95% CI) | P Value | |
| Unvaccinated (14 130) | Ref | … | Ref | … | Ref | … | Ref | … | Ref | … | Ref | … | Ref | … | Ref | … |
| At least 1 dose of vaccine 9–14 y (20 738) | 0.54 (.45–.66) | <.0001 | 0.54 (044–.65) | <.0001 | 0.61 (.42–.89) | .01 | 0.61 (.42–.90) | .01 | 0.27 (.17–.43) | <.0001 | 0.27 (.17–.43) | <.0001 | 0.43 (.32–.58) | <.0001 | 0.43 (.32–.58) | <.0001 |
| Incomplete series or off schedule 9–14 y (1763) | 0.64 (.39–.98) | .052 | 0.61 (.37–.94) | .03 | 0.81 (.31–1.73) | .62 | 0.80 (.31–1.72) | .6 | 0.37 (.09–1.00) | .09 | 0.36 (.09–.98) | .08 | 0.58 (.27–1.08) | .12 | 0.57 (.27–1.06) | .1 |
| Complete series on schedule 9–14 y (18 975) | 0.53 (.44–.65) | <.0001 | 0.53 (.43–.64) | <.0001 | 0.59 (.40–.87) | .01 | 0.59 (.40–.88) | .01 | 0.26 (.16–.42) | <.0001 | 0.26 (.16–.42) | <.0001 | 0.42 (.31–.57) | <.0001 | 0.42 (.31–.57) | <.0001 |
All rates are calculated per 1000 person-adjusted rates are adjusted for birth year and age at first screening.
Abbreviations: CI, confidence interval; CIN, cervical intraepithelial neoplasia; HSIL, high-grade squamous intraepithelial lesion; Ref, reference; RR, relative rate.
Complete Series and On-schedule HPV Vaccine Doses, 9–14 Years of Age
The IR for CIN2+ for women with complete series and on-schedule vaccination administered at 9–14 years of age was 0.50 per 1000 person-years (95% CI, .40–.63) and for CIN3 the IR was 0.16 (95% CI, .11–.24) (Table 2). There was a significant reduction in the RR of HSIL, CIN2, CIN3, and CIN2+ in both unadjusted and adjusted models for 9–14 old girls with complete series and on-schedule vaccination compared to unvaccinated. Women who received complete series and on-schedule HPV dosing administered at 9–14 years of age had a significant adjusted RR for CIN2+ of 0.42 (95% CI, .31–.57) compared to unvaccinated women and of 0.26 (95% CI, .16–.42) for CIN3 (Table 3). The adjusted RR for CIN3 among women with incomplete series/off-schedule doses administered at 9–14 years of age was 0.36 (95% CI, .09–.98) compared to unvaccinated women (Table 3).
Complete Series and On-schedule HPV Vaccine Doses Administered at 9–14 Years Old Compared to HPV Vaccine Doses Administered at 15 Years or Older
Given the low number of women vaccinated at 15 years or older in our study, these findings should be interpreted with caution. Women vaccinated at age 15 or older with complete on-schedule doses had an IR of CIN2+ at 0.87 per 1000 person-years (95% CI, .51–1.40) and for CIN3 of 0.33 per 1000 person-years (95% CI, .27–.96). In women vaccinated at age 15 or older with at least 1 dose of vaccine the IR for CIN2+ was 1.00 per 1000 person-years (95% CI, .66–1.47) and for CIN3 the IR was 0.46 per 1000 person-years (95% CI, .24–.81) (Table 2). Overall, there was a trend for increased observed cervical lesions in women who received their first dose of HPV vaccine at 15 years or older compared to those who initiated HPV vaccination at 9–14 years of age amongst those vaccinated with complete, on-series schedules. A significant increase in adjusted RR of HSIL was estimated (RR = 1.56; 95% CI, 1.05–2.24); however, the adjusted RR for the combined outcome of CIN2+ (RR = 1.53; 95% CI, .81–2.69) did not reach significance when compared to those who were completely vaccinated at 9–14 years of age (Table 4).
Table 4.
Relative Rate Estimates From Poisson Regression for 4 Outcomes in Those With Complete On-schedule Vaccine Dosing Comparing Women Vaccinated Starting at 9–14 Years of Age to Those Vaccinated Starting at Ages 15 and Above, and Incompletely Vaccinated 15 and Above
| Vaccine Status (n) | HSIL | CIN 2 | CIN 3 | CIN 2+ | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |||||||||
| RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | RR (95% CI) | P Value | |
| Complete series on schedule 9–14 y (18 975) | Ref | … | Ref | … | Ref | … | Ref | … | Ref | … | Ref | … | Ref | … | Ref | … |
| Complete series on schedule 15 + y (1997) | 1.66 (1.12–2.39) | .008 | 1.56 (1.05–2.24) | .02 | 1.58 (.69–3.17) | .23 | 1.39 (.61–2.79) | .40 | 2.02 (.68–4.92) | .16 | 1.84 (.61–4.52) | .22 | 1.72 (.91–3.01) | .07 | 1.53 (.81–2.69) | .16 |
| Incomplete series 15+ y (1439) | 2.59 (1.78–3.66) | <.0001 | 2.41 (1.66–3.42) | .02 | 1.64 (.63–3.55) | .26 | 1.47 (.56–3.19) | .38 | 3.91 (1.55–8.71) | .002 | 3.48 (1.37–7.79) | .004 | 2.38 (1.26–4.17) | .004 | 2.13 (1.12–3.73) | .01 |
Abbreviations: CI, confidence interval; CIN, cervical intraepithelial neoplasia; HSIL, high-grade squamous intraepithelial lesion; Ref, reference; RR, relative rate.
Vaccine Effectiveness
VE against CIN2+ for women with at least 1 dose of HPV vaccine at 9–14 years old was estimated at 56.6% (95% CI, 42.1%–67.7%) compared to those who were unvaccinated (Table 5). Complete series and on-schedule vaccine doses received at 9–14 years of age resulted in an estimated VE against CIN2+ of 57.9% (95% CI, 43.2%–69.0%) compared to those who were unvaccinated. The VE for complete series and on-schedule vaccine doses at 15 years or older was 36.8% (95% CI, 0%–66.1%) for CIN2+ compared to unvaccinated.
Table 5.
Adjusted Vaccine Effectiveness for Vaccine Status Groups at Least 1 Dose of Vaccine at 9–14 and Complete Series On-schedule at 9–14 Years of Age Versus Unvaccinated
| Vaccine Status | n | HSIL | CIN2 | CIN3 | CIN 2+ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Cases | Average Follow-up y ± SD | Adjusted VE, % (95% CI) | No. of Cases | Average Follow-up y ± SD | Adjusted VE, % (95% CI) | No. of Cases | Average Follow-up y ± SD | Adjusted VE, % (95% CI) | No. of Cases | Average Follow-up y ± SD | Adjusted VE, % (95% CI) | ||
| Unvaccinated | 14 130 | 229 | 5.5 ± 2.1 | Ref | 55 | 6.8 ± 2.1 | Ref | 60 | 6.8 ± 2.1 | Ref | 115 | 6.8 ± 2.1 | Ref |
| At least 1 dose 9–14 y | 20 738 | 191 | 5.7 ± 2.1 | 46.4 (35.0–55.9) | 51 | 7.1 ± 2.1 | 38.7 (0.00–58.3) | 25 | 7.1 ± 2.1 | 72.7 (57.0–83.2) | 76 | 7.1 ± 2.1 | 56.6 (42.0–67.7) |
| Complete series on schedule 9–14 y | 18 975 | 171 | 5.7 ± 2.1 | 47.1 (35.6–56.7) | 45 | 7.0 ± 2.1 | 40.6 (0.00–60.1) | 22 | 7.0 ± 2.1 | 73.6 (57.5–84.1) | 67 | 7.0 ± 2.1 | 57.9 (43.2–69.0) |
| At least 1 dose 15+ y | 3436 | 68 | 6.2 ± 2.2 | 1.2 (0.00–25.3) | 14 | 7.5 ± 2.2 | 18.4 (0.00–56.5) | 12 | 7.5 ± 2.2 | 32.0 (0.00–65.3) | 26 | 7.5 ± 2.2 | 25.3 (0.00–52.4) |
| Complete series on schedule 15+ y | 1997 | 32 | 6.2 ± 2.2 | 20.3 (0.00–46.0) | 8 | 7.5 ± 2.2 | 20.8 (0.00–65.2) | 5 | 7.5 ± 2.2 | 52.1 (0.00–83.3) | 13 | 7.5 ± 2.2 | 36.8 (0.00–66.1) |
Abbreviations: CI, confidence interval; CIN, cervical intraepithelial neoplasia; HSIL, high-grade squamous intraepithelial lesion; VE, vaccine effectiveness.
DISCUSSION
This analysis presents findings from the first population-level impact evaluation of the BC school-based quadrivalent HPV vaccine program. Findings indicate the school-based HPV immunization program for girls has led to significant declines in cervical dysplasia and precancerous lesions in BC. Women who received at least 1 dose of the HPV vaccine between 9 and 14 years old had a 57% reduction in the incidence of CIN2+ and a 73% reduction in the incidence of CIN3, the outcome most likely to be associated with HPV 16/18 [25]. It is important to note that our VE estimates are for CIN regardless of HPV type; however, HPV 16 and 18 historically have been attributed to 70% of cervical cancer cases [26]. Although less evident than the bivalent HPV vaccine, the quadrivalent HPV vaccine might result in cross-protection to other oncogenic HPV types [8, 27, 28].
The observed decline in cytological abnormalities for those vaccinated against HPV is similar to other data linkage studies [9, 11, 13]. In Australia, Gertig et al. [10] estimated VE at 36.4% against CIN3 with at least 1 dose of HPV vaccine and 47.5% with 3 doses; however, their cohort was predominately comprised of women vaccinated as part of a catch-up program (up to age 26). The higher VE estimated in the BC cohort is likely due to the younger age at vaccination, thus girls were less likely to have been exposed to HPV prior to vaccination.
In a Swedish cohort of females 13–30 years of age, a higher VE of 75% for CIN2+ was observed among girls vaccinated before age 17. However, prior to 2012, HPV vaccination and most screening in this cohort was opportunistic and likely not representative of an organized vaccination and screening program [29].
Our analysis focused on girls vaccinated as part of the school-based HPV immunization program, with the primary offering at age 11 (grade 6), where the Australian and Swedish study’s cohorts comprised predominately of women who received vaccination in late adolescence as part of a national catch-up program.
In regard to the estimated population-level effectiveness of the bivalent HPV vaccine, a similar VE estimate of 55% against CIN3 was observed in a preliminary population analysis [30].
A decline in CIN2+ was observed in this analysis for women with incomplete HPV series at 9–14 years of age compared to those who were unvaccinated; however, it did not reach significance likely due to low power, as 90% of women who received vaccine at 9–14 years of age had complete series and on-schedule vaccine doses recorded. The observed nonsignificant decline in high-grade histological outcomes for women with incomplete vaccine series was also observed in an impact evaluation of the school HPV immunization program in Victoria, Australia [10].
While women who received complete series and on-schedule HPV vaccination at 15+ years old showed a VE of 36.8% compared to unvaccinated women, they had a trend for increased risk for CIN2+ compared to women vaccinated completely and on schedule at 9–14 years of age. These findings validate current BC recommendations to receive the vaccine before the age of 15. Because few women were vaccinated after age 15 and wide confidence intervals were observed, these results should be interpreted with caution. The lower RR reduction in women who received HPV vaccine at an older age is possibly due to the higher prevalence of prevaccination exposure to HPV and a higher likelihood for cervical cancer screening [15], with only 7% of young people in BC reporting first sexual intercourse before the age of 15 [31]. These findings validate that the current BC recommendations for HPV vaccine receipt before the age of 15, with the primary offering at grade 6 (age 11 years) [32], is prudent.
Our analysis has numerous strengths, including robust deterministic data linkage between comprehensive population registries for the HPV immunization program and cervical screening program, which represents the entire provincial population. There was a 7-year average length of follow-up in our cohort of women who predominately received HPV vaccination as part of an organized school-based immunization program. Finally, the outcome selection method used the most severe abnormal result end point, allowing for VE estimates of high-grade histopathological outcomes most likely to progress to invasive cervical cancer.
The analysis is not without limitations. There is the potential for misclassification bias, as women may have been classified as unvaccinated who had a Pap smear recorded in the cervical screening registry but may have received HPV vaccine outside the publicly funded program or out of province and were subsequently not captured in the immunization registries. Similarly, women classified as receiving incomplete doses may have received additional doses outside of the publicly funded program or out of province. The potential misclassifications may have resulted in underestimating the effect of HPV vaccine on cervical lesion development.
Another limitation is that we could not adress potential confounders in relation to socio-demographics and therefore not estimate the impact on screening participation and outcomes. A previous study indicated that girls being vaccinated for HPV were more likely to have received all childhood vaccines compared to HPV unvaccinated adolescents. In addition, parents with higher education levels were less likely to have their adolescent girl vaccinated against HPV [33]. Our model adjustment included first screening date less 3 years, which was used as a proxy for sexual debut based on the 2011 Cervix Screening Program guidelines, to control for women who were at a higher risk of developing abnormal cervical outcomes due to earlier sexual debut or behavioral differences in the cohort over time. However, further adjustment was not undertaken for socioeconomic, geographical, or immigration variables due to a lack of availability in the registry data.
The introduction of a school-based HPV immunization program has significantly reduced high-grade cervical lesions by a half in women who receive HPV vaccination compared to those who do not. BC’s centralized immunization and cervical screening registries allow for robust data linkage to evaluate the impact of HPV vaccine across the whole population. As the first cohorts of women vaccinated against HPV become age eligible for cervical cancer screening, continued evaluation of the long-term public health impact of the HPV vaccine on cervical cancer and HPV-related diseases is needed. However, our initial findings contribute to the growing body of evidence illustrating the positive population impact HPV vaccination has had on rates of cervical dysplasia.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The funding source had no involvement in the design, analysis, interpretation, or preparation and approval of the submitted manuscript. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
Financial support. This work was supported the Canadian Institutes of Health Research (Foundation Grant number FDN-143339).
Potential conflicts of interest. L. S. reports personal fees from Roche Molecular outside the submitted work. P. D. B. reports grants from Public Health Agency of Canada Immunization Partnership Fund, outside the submitted work. M. S. reports institution-funded grants from GlaxoSmithKline, VBI Vaccines, and Merck, outside the submitted work. M. K. reports grants from Roche, Hologic, and Siemens, outside the submitted work. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: International Papillomavirus Conference, 4–6 October 2018, Sydney, Australia and the Canadian Immunization Conference, 4–6 December 2018, Ottawa, Canada.
References
- 1. Apter D, Wheeler CM, Paavonen J, et al. ; HPV PATRICIA Study Group Efficacy of human papillomavirus 16 and 18 (HPV-16/18) AS04-adjuvanted vaccine against cervical infection and precancer in young women: final event-driven analysis of the randomized, double-blind PATRICIA trial. Clin Vaccine Immunol 2015; 22:361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dillner J, Kjaer SK, Wheeler CM, et al. ; FUTURE I/II Study Group Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ 2010; 341:c3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–27. [DOI] [PubMed] [Google Scholar]
- 4. Kjaer SK, Nygård M, Dillner J, et al. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 Nordic countries. Clin Infect Dis 2018; 66:339–45. [DOI] [PubMed] [Google Scholar]
- 5. Lu B, Kumar A, Castellsagué X, Giuliano AR. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review and meta-analysis. BMC Infect Dis 2011; 11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muñoz N, Kjaer SK, Sigurdsson K, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst 2010; 102:325–39. [DOI] [PubMed] [Google Scholar]
- 7. Benard VB, Castle PE, Jenison SA, et al. ; New Mexico HPV Pap Registry Steering Committee Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol 2017; 3:833–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drolet M, Bénard É, Boily MC, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2015; 15:565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garland SM, Kjaer SK, Muñoz N, et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clin Infect Dis 2016; 63:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gertig DM, Brotherton JM, Budd AC, Drennan K, Chappell G, Saville AM. Impact of a population-based HPV vaccination program on cervical abnormalities: a data linkage study. BMC Med 2013; 11:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herweijer E, Sundström K, Ploner A, Uhnoo I, Sparén P, Arnheim-Dahlström L. Quadrivalent HPV vaccine effectiveness against high-grade cervical lesions by age at vaccination: a population-based study. Int J Cancer 2016; 138:2867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kavanagh K, Pollock KG, Cuschieri K, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect Dis 2017; 17:1293–1302. [DOI] [PubMed] [Google Scholar]
- 13. Kim J, Bell C, Sun M, et al. Effect of human papillomavirus vaccination on cervical cancer screening in Alberta. CMAJ 2016; 188:E281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Markowitz LE, Hariri S, Lin C, et al. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003-2010. J Infect Dis 2013; 208:385–93. [DOI] [PubMed] [Google Scholar]
- 15. Baldur-Felskov B, Dehlendorff C, Munk C, Kjaer SK. Early impact of human papillomavirus vaccination on cervical neoplasia–nationwide follow-up of young Danish women. J Natl Cancer Inst 2014; 106:djt460. [DOI] [PubMed] [Google Scholar]
- 16. BC Centre for Disease Control. Immunization uptake in grade 6 students 2002–2018. Vancouver, BC: BC Centre For Disease Control, 2018. [Google Scholar]
- 17. Moore RA, Ogilvie G, Fornika D, et al. Prevalence and type distribution of human papillomavirus in 5000 British Columbia women–implications for vaccination. Cancer Causes Control 2009; 20:1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ogilvie GS, Cook DA, Taylor DL, et al. Population-based evaluation of type-specific HPV prevalence among women in British Columbia, Canada. Vaccine 2013; 31:1129–33. [DOI] [PubMed] [Google Scholar]
- 19. Ogilvie GS, Naus M, Money DM, et al. Reduction in cervical intraepithelial neoplasia in young women in British Columbia after introduction of the HPV vaccine: an ecological analysis. Int J Cancer 2015; 137:1931–7. [DOI] [PubMed] [Google Scholar]
- 20. Canadian Immunization Committee. Recommendations for human papillomavirus programs. Ottawa, Ontario: Public Health Agency of Canada, 2007. [Google Scholar]
- 21. Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013; 309:1793–802. [DOI] [PubMed] [Google Scholar]
- 22. Ogilvie G, Sauvageau C, Dionne M, et al. Immunogenicity of 2 vs 3 doses of the quadrivalent human papillomavirus vaccine in girls aged 9 to 13 years after 60 months. JAMA 2017; 317:1687–8. [DOI] [PubMed] [Google Scholar]
- 23. BC Cancer Agency. Cervical cancer screening policy change. Vancouver, BC: BC Cancer Agency, 2016. [Google Scholar]
- 24. World Health Organization, IARC HPV Working Group. Primary end-points for prophylactic HPV vaccine trials. France: International Agency for Research on Cancer, 2014. [PubMed] [Google Scholar]
- 25. Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115 789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012; 131:2349–59. [DOI] [PubMed] [Google Scholar]
- 26. de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer 2017; 141:664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Markowitz LE, Liu G, Hariri S, Steinau M, Dunne EF, Unger ER. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics 2016; 137:e20151968. [DOI] [PubMed] [Google Scholar]
- 28. Merck Canada Inc. Product monograph Gardasil: quadrivalent human papillomavirus (Types 6, 11, 16, 18) recombinant vaccine. Quebec, Canada: Merck Canada Inc, 2015. [Google Scholar]
- 29. Herweijer E, Feldman AL, Ploner A, et al. The participation of HPV-vaccinated women in a national cervical screening program: population-based cohort study. PLoS One 2015; 10:e0134185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pollock KG, Kavanagh K, Potts A, et al. Reduction of low- and high-grade cervical abnormalities associated with high uptake of the HPV bivalent vaccine in Scotland. Br J Cancer 2014; 111:1824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Poon C, Smith A Saewyc E, Society MC.. Sexual health of youth in BC. Vancouver, BC: McCreary Centre Society, 2015. [Google Scholar]
- 32. Vaccine Preventable Diseases Service, BC Centre for Disease Control. Communicable disease control manual. Chapter 2: immunization, Part 1: immunization schedules. Vancouver, BC: BC Centre for Disease Control, 2017. [Google Scholar]
- 33. Ogilvie G, Anderson M, Marra F, et al. A population-based evaluation of a publicly funded, school-based HPV vaccine program in British Columbia, Canada: parental factors associated with HPV vaccine receipt. PLoS Med 2010; 7:e1000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.