Abstract
E-cigarette flavored pods are increasing in use among young adults. Although marketed as a safer alternative to conventional cigarettes, the health effects of e-cigarette flavored pods are unknown. We hypothesized that e-cigarette flavored pods would cause oxidative stress, barrier dysfunction, and an inflammatory response in monocytes and lung epithelial cells. JUUL pod flavors (Fruit Medley, Virginia Tobacco, Cool Mint, Crème Brulee, Cool Cucumber, Mango, and Classic Menthol) and similar pod flavors (Just Mango-Strawberry Coconut and Caffé Latte) were tested. These pod flavors generated significant amounts of acellular ROS and induced significant mitochondrial superoxide production in bronchial epithelial cells (16-HBE). Lung epithelial cells (16-HBE, BEAS-2B) and monocytes (U937) exposed to various pod aerosols resulted in increased inflammatory mediators, such as IL-8 or PGE2. JUUL pod flavors, Crème Brulee and Cool Cucumber, caused epithelial barrier dysfunction in 16-HBE cells. Moreover, tested flavors also showed DNA damage upon exposure in monocytes. We determined the chemical constituents present in various flavors. Our data suggest that these constituents in flavored pods induce oxidative stress, inflammation, epithelial barrier dysfunction, and DNA damage in lung cells. These data provide insights into the regulation of e-cigarette flavored pods, as well as constituents in these flavors.
Subject terms: Acute inflammation, Chemokines
Introduction
Electronic cigarettes or e-cigarettes (ENDS, electronic nicotine delivery system) are battery-powered devices that can generate an aerosolized vapor from a liquid typically consisting of nicotine, propylene glycol (PG), vegetable glycerin (VG), and flavoring chemicals. Currently, there is a great diversity in flavorings, with over 8000 different flavors available in the market1. These flavors are generally regarded as safe for ingestion, but inhalation toxicity has yet to be determined2. Flavoring chemicals from e-cigarettes have previously been shown to cause inflammatory responses in lung epithelial cells and monocytes mediated by oxidative stress caused by reactive oxygen species (ROS) generation3,4.
E-cigarette use is currently on the rise in the western population, such as the United States, with an increase in the prevalence of use among high-schoolers and middle-schoolers. The use of e-cigarettes in high-schoolers and middle-schoolers was increased by 78% and 48% from 2017 to 2018, respectively5. One e-cigarette of particular interest is JUUL, since as of July 2018, JUUL devices account for 70.5% of US convenience store vapor product sales6. Using e-cigarettes is considered a harm-reduction approach for traditional tobacco smoking. Nevertheless, moderate nicotine dependence has been reported in a community-based study with correlating levels of salivary cotinine7. According to a survey-based study, 62.9% of e-cig users expressed a greater satisfaction from vaping flavored e-cigs, sweet, fruity, and minty flavors, compared to tobacco or unflavored e-cigs8.
These pod mod devices with USB charging capability and alluring flavors is a contributing factor to initiate vaping, especially among the youth8,9. JUUL pods are comprised of a heat resistant plastic enclosing the atomizer with a stainless steel vapor path, silica wick, and nichrome coil heater (Supplementary Fig. S1A). JUUL uses pre-filled pods consisting of PG, VG, nicotine, benzoic acid, and flavoring chemicals6. Benzoic acid and nicotine in these pods are likely to form nicotine benzoate during aerosolization10. Recent findings determined that average nicotine concentration was 60.9 mg/mL, levels that match the nicotine concentration of a pack of cigarettes11. The major flavoring chemicals found in JUUL pods are menthol, vanillin, and ethyl maltol11. There have been some cytotoxic effects seen from these flavoring chemicals, with menthol and ethyl maltol to cause oxidative stress, an inflammatory response, and barrier dysfunction, and vanillin to be a respiratory irritant which induces an inflammatory response2. Exposure of the airway epithelium to environmental toxicants such as JUUL pod chemical constituents, metals, and ROS can lead to disruption of the barrier junctions allowing hazardous agents to enter into subepithelial tissues inducing lung damage, repair, immunomodulation, and disease progression. The etiology of respiratory diseases such as asthma, bronchitis, bronchiolitis obliterans is the exposure to environmental insults, such as environmental tobacco smoke and airborne particulates. Hence, it is conceivable that chronic exposure to JUUL pods and similar pod aerosols potentially have similar risks, especially in developing lung.
Although JUUL pod is typically marketed as an alternative to conventional cigarette smoking with reduced harmful effects, the effects of these devices on the individuals using them are still relatively unknown. We hypothesized that flavorings used in e-liquids, JUUL pods, and other pod flavors would result in oxidative stress and inflammatory responses in lung epithelial cells and monocytes and cause airway epithelial barrier dysfunction.
Methods
Scientific rigor and reproducibility
We used a rigorous and unbiased approach throughout the experimental plans and data analyses to ensure that our data are reproducible along with full and detailed reporting of both methods and analyzed data. All the key biological and chemical resources that are used in this study were validated and authenticated and are of scientific standard from commercial sources. Our results adhere to NIH standards of reproducibility and scientific rigor.
Ethical approval: Institutional biosafety approvals
All experiments performed in this study were approved and in accordance with the University of Rochester Institutional Biosafety Committee. Cell lines (16-HBE, BEAS2B, U937) were procured from ATCC, USA. No ethical approval is required for these cell lines.
Procurement of JUUL pods and similar pod flavors
JUUL pod flavors, “Fruit Medley”, “Virginia Tobacco”, “Cool Mint”, Crème Brulee”, “Cool Cucumber”, “Mango”, and “Classic Menthol” with 5% nicotine were purchased from the JUUL online store as well as the local retail store. Other pod flavors, “Just Mango (Strawberry Coconut)” (nicotine concentration unlisted) by LCF labs and “Caffé Latte” by Eonsmoke labs with 6% nicotine, were purchased from local vape shops in Rochester, NY.
Qualitative analysis of the composition of JUUL pod constituents
JUUL pod flavors (Fruit Medley, Classic Menthol, Cool Mint, Crème Brulee, Cool Cucumber, and Virginia Tobacco) were diluted 100X into spectral grade methanol and injected into the gas chromatograph (GC) with a mass spectrometer (MS) detector (Agilent 7890 A gas chromatograph with 5975 MSD detector). The system used helium as the carrier gas, flowing 1.2 mL/min through an Agilent Technologies column (HP-5MS, 30 m × 0.250 mm, 0.25 μm, 19091S-433). The oven program initiated at 60 °C for 4 minutes, ramped to 150 °C over 2 minutes at 70 °C/min, spiked to 250 °C and then ramped again to 285 °C over 5 minutes at 25 °C/min. The total run time was almost 18 minutes, and the injection volume was 1 µL from a 10 µL syringe. The samples were analyzed by electron impact ionization in positive ion mode with a mass range of 50–550 m/z, with the source temperature at 230 °C and the quadrupole at 150 °C. Data analysis was performed using Agilent ChemStation software, with ion scans searched against the NIST database for identification. Retention times with the percentage probability of the constituents found are listed in Supplementary Table S1.
Assessment of acellular ROS
For the acellular ROS assessment, 2′7′dichlorofluoroscien diacetate (DCFH-DA) dye (Calbiochem, CA, catalog #287810) was prepared by mixing 5 mM DCFH-DA with 0.01N NaOH and followed by the addition of 25 mM phosphate buffer and HRP-streptavidin thirty minutes later. JUUL pod flavor vapors from the seven pod flavors were bubbled in the DCFH dye by a SCIREQ air pump following a puff profile of three puffs per minute lasting for three seconds with roughly 17s intervals at a flow rate of 1.6 L/min. For each pod, there were five, ten, or fifteen puffs bubbled through the DCFH dye. Using a standard curve generated from various H2O2 concentrations, produced ROS concentrations were obtained in μM H2O2 equivalents for each flavor.
Cell culture and flavor exposure/treatment
Human bronchial epithelial cells, 16-HBE cell line
16-HBE at 20,000 cells per well were cultured in 24-well transwell inserts (Corning, #3470) in complete media (DMEM media with 10% FBS, 15 mM HEPES, 1% Pen/strep, and 0.2% amphotericin B). At 80% confluency, the cells were serum-deprived at 1% FBS for 12-hours followed by three-sessions of aerosol exposure to Cool Cucumber, Classic Menthol, Just Mango (Strawberry Coconut), and Caffé Latte flavors (refer to the in vitro exposure section for more details on exposure methods). Treatment with H2O2 (600 μM) was used as a comparison positive control. Immediately after the aerosol exposure, the cells were used for the barrier dysfunction assessment and MitoStress assay. The conditioned media was stored at −80 °C for cytokine assessment with ELISA and Luminex.
Human bronchial epithelial cells, BEAS-2B cell line
BEAS-2B cells were cultured in 6-well plates with approximately 325,000 cells per well in complete media in DMEM: F12 complete media with 5% FBS, 1% pen/strep, and 15 mM HEPES. At 80% confluency, the cells were serum-deprived at 1% FBS for 12-hours followed by three sessions of aerosol exposure to Cool Cucumber, Classic Menthol, Just Mango (Strawberry Coconut), and Caffé Latte flavors (refer to the in vitro exposure section for more details on exposure methods). Tumor necrosis factor alpha (TNF-α) (10 ng/mL) treatment was used as a comparison positive control. Twenty-four hours post aerosol exposure, the conditioned media were collected and stored at −80 °C for cytokine assessment with ELISA and Luminex multiplex assay.
Monocytes, U937 cell line
U937 cells (approx. 300,000) were cultured in 12-well plates in complete RPMI 1640 media (ATCC, 30-2001) with 10% FBS complete with 1% pen/strep. Approximately 12-hours before treatments cells were serum-deprived at 1% FBS. Subsequently, designated wells were directly treated with Cool Cucumber, Classic Menthol, Just Mango (Strawberry Coconut), Caffé Latte flavor liquids at 0.5% and H2O2 (100 µM) as a positive control. Twenty-four hours post-exposure the conditioned media was used for cytokine analysis by ELISA and Luminex, and the cells were used for DNA damage assessment by Comet assay.
In-vitro aerosol exposure system
JUUL pod device was connected to one of the pumps of the Scireq Inexpose e-cig exposure system (Scireq, Monreal). The mouthpiece of the JUUL device was then connected to an Enzyscreen chamber (Enzyscreen, Netherlands). For each exposure session, a cell culture plate was placed inside the Enzyscreen chamber and the vapors were released into the chamber. (66 puffs during 22 minutes with a three second puff duration at 1.6 L/min flow rate and an interpuff interval of approximately 17s.). The aerosols were allowed to equilibrate for eight minutes post-exposure resulting in a total of 30 minutes per session before returning the cell culture plate to the incubator. Next, aerosol exposure sessions were performed at 12-hour intervals (Supplementary Fig. S1B).
Assessment of mitochondrial superoxide generation
Immediately after the last exposure with the selected flavor aerosol, the cells were trypsinized and stained with MitoSOX Red and Annexin V according to MitoStress kit protocol (Millipore, FCCH100109). For the flavors, Cool Cucumber, Classic Menthol, Just Mango (Strawberry Coconut), and Caffé Latte, six transwells were pooled together per flavor, and two pooled samples were run for each tested flavor. Sample acquisition and analysis were performed by the Guava Millipore Easycyte-8 instrument and its software, respectively.
IL-8 cytokine quantification by ELISA
Conditioned media from 16-HBE and U937 cells were collected after the specified incubation period post-treatment/exposure, and the IL-8 levels were quantified using ELISA kit (Invitrogen, CHC1303) according to the manufacturer’s instructions.
Prostaglandin E2 quantification by ELISA
Conditioned media from U937, BEAS-2B, and 16-HBE cells were collected after the specified incubation period post-treatment/exposure. A competitive ELISA assay was performed to quantify PGE2 levels according to the manufacturer’s protocol (Cayman, 514010).
Quantification of inflammatory mediators by Luminex
Conditioned media collected from BEAS-2B and U937 cells were assayed for inflammatory cytokines and growth factors by Bioplex Pro human cytokine 27-plex (BioRad, M500KCAF0Y). After the specified post-exposure/treatment incubation period, collected conditioned media was assayed for cytokines with Bioplex 3D system. Only the detectable mediators were plotted and compared with the untreated control group.
Epithelial barrier function assessment
After 16-HBE cell exposure to Crème Brulee, Classic Menthol, Just Mango (Strawberry Coconut), and Caffé Latte flavors, the barrier function was evaluated by EVOM2 (WPI instruments, FL). The transepithelial voltage and the resistance were measured three times in each transwell at different positions, and the average numbers were reported per transwell.
DNA damage assessment by JUUL pods and other pod flavors
Twenty four hours after monocytes (U937) being treated with JUUL pods and similar pod flavors, the cells were prepared for DNA damage assessment by Comet assay according to the manufacturer’s protocol (Trevigen, MD). Briefly, the alkaline procedure was followed and approximately 5000 cells per sample were used and stained with SYBR gold 1:10,000. EVOS imaging system was used to capture the fluorescence images. The images were inverted for the Opencomet analysis. The olive tail moment was obtained using Opencomet software for the representative images, and the percent change was calculated in comparison to the control group.
Statistical analysis
Statistical analysis of data was done using two-way ANOVA with a Tukey’s post-hoc test for all analyses of multiple groups with multiple variables and one-way ANOVA with Dunnett’s post-hoc test was used to analyze all comparison of multiple groups with a single variable using GraphPad Prism 8.0. Data are represented as mean ± SEM. Cells and conditioned media samples were pooled in some assays. Statistical significance was reported as *p < 0.05, **p < 0.01, and ***p < 0.001.
Disclaimer
The authors have nothing to claim or disclaim about any products used here to test their toxicological and biological effects. The authors have no personal interests or gains from the outcome of this study. The products tested are available commercially to consumers/users.
Results
JUUL pod chemical constituents
Assessment of JUUL pod constituents by GC-MS showed the presence of numerous flavoring chemicals and flavor-enhancing chemicals. Nicotine was present in all tested flavors, except Cool Mint (Supplementary Table S1).
Acellular (cell-free) ROS generated by JUUL pod flavors
To determine the ROS generation by JUUL pods, the vapors were drawn through DCFH-DA dye and the fluorescence was measured in H2O2 equivalents. JUUL pod flavors, Cool Mint, Crème Brulee, Cool Cucumber, and Fruit Medley, generated significantly higher ROS levels at each puff count compared to the respective air control group. The vapors generated from the JUUL pods followed a dose-dependent increase in ROS with an increasing number of puffs, with both Cool Mint and Crème Brulee having a significant increase between 10 and 15 puffs and 5 and 15 puffs (Fig. 1).
Figure 1.

Cell-free ROS generated by JUUL pod flavors. JUUL pods containing Fruit Medley, Virginia Tobacco, Cool Mint, Cool Cucumber, Mango, and Classic Menthol flavors and 3R4F research cigarettes were bubbled through DCFH fluorescent dye. Filtered air was bubbled as a negative control comparison group. Five, ten, and fifteen puffs were generated of these flavors each puff with three-second puff durations. ROS levels are expressed in H2O2 µM equivalents. ap < 0.001 compared to 5 puff air control, bp < 0.001 compared to 10 puff air control, and cp < 0.001 compared to 15 puff air control, 3R4F research cigarettes were not analyzed; **p < 0.01, and ***p < 0.001 vs. 5 puffs #p < 0.001 vs. 10 puffs. Two wells per group with repeated measurements in pooled samples.
Increased mitochondrial superoxide generation due to JUUL pods and other pod flavors
To determine cellular oxidative stress by JUUL pods and other pod aerosols were assessed by staining MitoStress assay. Each sample was analyzed by dividing the scatter plots into 4-quadrants, I, II, III, and IV, with each quadrant representing a cell population (Fig. 2). Quadrant I depicts the cell population MitoSOX high and Annexin V low population, quadrant II depicts MitoSOX high and Annexin V high cell population, quadrant III depicts MitoSOX low and Annexin V high cell population, and quadrant IV with MitoSOX low and Annexin V low population. With the H2O2 positive control treatment, there was a definitive shift in the population towards quadrant II, demonstrating increased mitochondrial superoxide production as well as increased cell death compared to the untreated control group. Similarly, Cool Cucumber, Classic Menthol, Just Mango (Strawberry Coconut), and Caffé Latte pod flavors showed on average a 28% shift in the cell population toward quadrant II. These data show that exposure to tested flavors increased mitochondrial superoxide production with Classic Menthol inducing the greatest mitochondrial ROS production with 12.88% and 38.04% in quadrants I and II, respectively. Cell death due to JUUL pod aerosols exposure (quadrant V) is under 8%. These data demonstrate exposure to JUUL pod aerosols induces marked mitochondrial ROS production and may result in negligible cell death.
Figure 2.
JUUL pods and other pod flavors induce the production of mitochondrial superoxide in lung epithelial cells. 16-HBE cells with 20,000 cells per transwell were grown to 80% confluency in complete media. After serum deprivation (1% FBS) the wells were exposed to three-sessions (each session 66 puffs) of JUUL pods and other pod aerosols with equal intervals between each session. Aerosolized flavors included JUUL pods (Classic Menthol and Cool Cucumber) and other pods (Just Mango -Strawberry Coconut and Caffé Latte). Air group and H2O2 (600 μM) were used as assay controls. After the last exposure, cells were collected and pooled for each treatment group (n = 3–6 pooled transwells per group) and stained with MitoSOX red and annexin V, and flow-cytometry analysis was performed. Representative scatter plots per treatment group were shown.
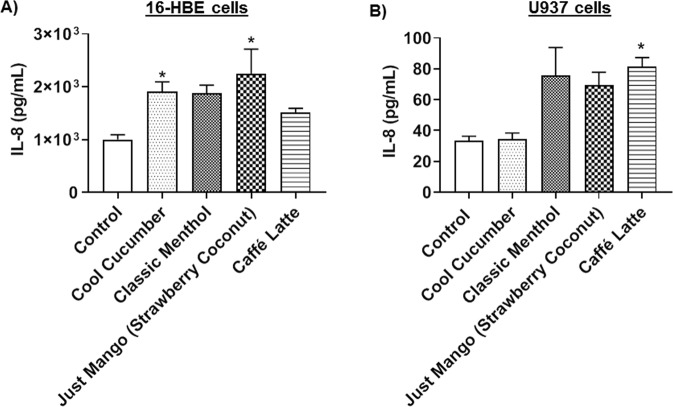
Increased IL-8 inflammatory cytokine response in 16-HBE cells
To determine the potential to elicit an inflammatory response, 16-HBE cells were exposed to various JUUL pods and other pod flavors, and the IL-8 production was measured in the conditioned media. JUUL pod flavor, Cool Cucumber significantly increased the IL-8 levels compared to the unexposed group. Similarly, Just Mango (Strawberry Coconut), also secreted significantly high IL-8 levels compared to the untreated control group (Fig. 3A).
Figure 3.
JUUL pods and other pod flavors induce the production of IL-8 in lung epithelial cells and monocytes. (A) 20,000 16-HBE cells grown in 24-well transwell plates exposed to three-sessions (each session 66 puffs) of JUUL pods and other pod aerosols with equal intervals between sessions. Aerosolized flavors were JUUL pods (Cool Cucumber and Classic Menthol) and other pod flavors (Just Mango -Strawberry Coconut and Caffé Latte). After the last exposure, IL-8 was measured in the conditioned media. *p < 0.05 vs. Control. N = 3 wells per group. (B) Approximately 300,000 U937 cells were cultured in a 12-well plate in complete medium. Cells were serum-deprived (1% FBS) for 24 hours and treated with 0.5% JUUL pods and other pod flavors. Treated flavors included JUUL pods (Cool Cucumber and Classic Menthol) and other pod flavors (Just Mango-Strawberry Coconut and Caffé Latte). Twenty-four hours later, IL-8 levels in the conditioned media were determined. *p < 0.05 vs. Control.
Increased IL-8 inflammatory cytokine response in monocytes
Monocytes directly treated with Classic Menthol flavor JUUL pod liquid showed an increase in IL-8 levels, albeit not significant. Treatment with the pod liquid, Caffé Latte, showed a significant increase in IL-8 compared to the untreated control group (Fig. 3B). Individual treatments with Classic Menthol and Caffé Latte showed significant cytotoxicity in monocytes, with a 34% and a 67% decrease in cell viability compared to the untreated, respectively, whereas all other treatments showed insignificant cytotoxicity (data not shown).
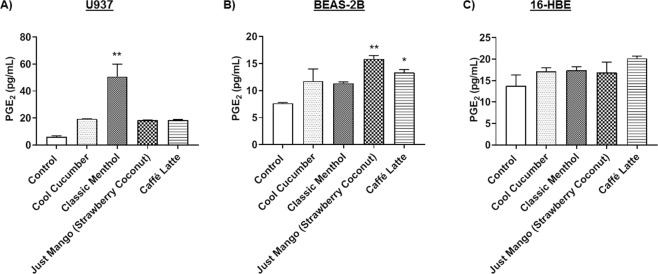
Increased prostaglandin E2α (PGE2) response in monocytes cells by JUUL pods and other pod flavors
Monocytes (U937) were directly treated with Cool Cucumber, Classic Menthol, Just Mango (Strawberry Coconut), and Caffé Latte. Among the tested pod flavors, only Classic Menthol treatment significantly increased the PGE2 levels compared to the untreated control (Fig. 4A).
Figure 4.
JUUL pods and other pod flavors induce the production of PGE2 in lung epithelial cells and monocytes. (A) U937 with 300,000 cells per well were treated with 0.5% JUUL pods and other pod flavors in serum-deprived medium. Flavors included JUULpods (Cool Cucumber and Classic Menthol) and other pod flavors (Just Mango-Strawberry Coconut and Caffé Latte). Prostaglandin E2 was measured in conditioned media. **p < 0.01 vs. control. (N = 2 treated wells per group with repeated experiments). (B) Approximately 325,000 BEAS-2B cells were cultured in 6-well plates and exposed to JUUL pods and other pod flavors in serum-deprived medium (1% FBS). Three sessions of 66-puffs were generated by Scireq Inexpose system. Aerosolized flavors included JUUL pods (Cool Cucumber and Classic Menthol) and other pods (Just Mango - Strawberry Coconut and Caffé Latte). Prostaglandin E2 was measured in conditioned media. *p < 0.05, **p < 0.01 vs. control. N = 2–6 treated wells per group. (C) 20,000 16-HBE cells cultured in 24-well transwell inserts and exposed to JUUL pods and other pod flavors in serum-deprived medium (1%). Three sessions of 66-puffs were generated by Scireq Inexpose system. Flavors included JUUL pods (Cool Cucumber and Classic Menthol) and other pods (Just Mango-Strawberry Coconut and Caffé Latte). Prostaglandin E2 was measured in conditioned media. N = 3–12 treated wells per group with repeated experiments.
Increased Prostaglandin E2α (PGE2) response in epithelial cells by JUUL pod and other- pod flavors
BEAS-2B epithelial cells exposed to JUUL pod flavors, Cool Cucumber and Classic Menthol, did not show a significant increase in PGE2 levels in conditioned media even though the levels were increased with the exposure. Just Mango (Strawberry Coconut) and Caffé Latte showed significantly elevated levels of PGE2 compared to the unexposed control group (Fig. 4B). 16-HBE cells, on the other hand, showed no significant changes in PGE2 levels compared to the control group (Fig. 4C).
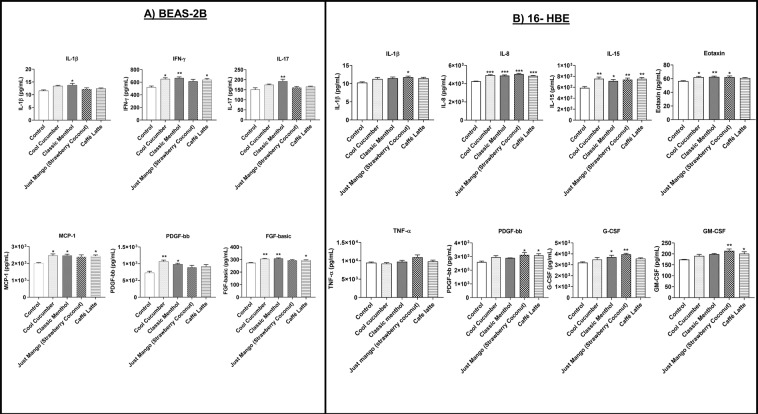
Differential inflammatory response due to JUUL pods and other pod aerosols in epithelial cells
In BEAS-2B cells, exposure to Cool Cucumber aerosol resulted in a significant increase in IFN-γ, MCP-1, PDGF-bb, and FGF-basic compared to its unexposed counterparts. Exposure to Classic Menthol resulted in a significant increase in IL-1β, IFN-γ, IL-17, MCP-1, PDGF-bb, and FGF-basic levels compared to the unexposed control group. While Just Mango (Strawberry Coconut) did not cause significant changes in inflammatory mediators, Caffé Latte significantly elevated IFN-γ, MCP-1, and FGF-basic levels compared to the unexposed group. (Fig. 5A).
Figure 5.
JUUL pods and other pod flavors produce differential effects on inflammatory mediators and growth factors in lung epithelial cells. (A) BEAS-2B cells were grown and exposed to three sessions (66 puffs per session) of JUUL pods and other pod aerosol with equal intervals between sessions. Aerosolized flavors were JUUL pods (Cool Cucumber and Classic Menthol) and other pods (Just Mango-Strawberry Coconut and Caffé Latte). The air control group was used as the negative control. After the last exposure condition media was measured using Luminex for inflammatory cytokines and growth factors. *p < 0.05, **p < 0.01 vs. control, N = 2–6 wells per group. (B) 16-HBE cells were grown and exposed to three sessions (66 puffs per session) of JUUL pods and other pod aerosols with equal intervals between sessions. Aerosolized flavors were JUUL pods (Cool Cucumber and Classic Menthol) and other pods (Just Mango-Strawberry Coconut and Caffé Latte). The air group was used as the negative control. After the last exposure condition media was measured using Luminex for inflammatory cytokines and growth factors. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. control, N = 3–12 treated wells per group.
In 16-HBE cells, exposure to Cool Cucumber resulted in a significant increase in IL-8, IL-15, and eotaxin response. Classic Menthol resulted in a significant increase in the production of inflammatory mediators IL-8, IL-15, eotaxin and G-CSF. Exposure to Just Mango (Strawberry Coconut) flavor significantly increased IL-1β, IL-8, IL-15, eotaxin, PDGF-bb, G-CSF, and GM-CSF levels. Caffé Latte exposure significantly increased IL-8, IL-15, PDGF-bb, and GM-CSF responses compared to the unexposed counterparts (Fig. 5B). Exposure to pod flavors slightly increased TNF-α levels, Just Mango (Strawberry Coconut) flavor being the highest inducer of the tested flavors (Fig. 5B).
Epithelial barrier function altered by exposure to JUUL pod aerosols
To assess the epithelial barrier dysfunction by exposure to various pod flavors, the resistance and the voltage in 16-HBE were measured subsequent to aerosol exposure. Cells exposed to Cool Cucumber resulted in a significant reduction in membrane resistance. Greater reduction in epithelial resistance was observed in cells treated with H2O2 (Fig. 6A). Exposure of 16-HBE to Crème Brulee resulted in a significant reduction in membrane voltage, similar to results seen with the exposure to H2O2 (Fig. 6B). Comparable reductions in TEER measurement was also observed when the 16-HBE cells were treated with 1.0% Crème Brulee liquid. These data in TEER and voltage measurements demonstrate that exposure to JUUL pod flavoring chemicals causes epithelial barrier dysfunction.
Figure 6.
JUUL pod flavors affects barrier function in epithelial cells. (A) 20,000 16-HBE cells grown in 24-well transwell plates exposed to three-sessions (each 66 puffs) of JUUL pod flavor cool cucumber with equal intervals of 12 hours between sessions. After the final exposure, the resistance was measured by EVOM2 device. $$$P < 0.001 vs. H2O2 and, **p < 0.01**p < 0.01, and ***p < 0.001 vs. Control. N = 3–12 wells per group. (B) 20,000 16-HBE cells were cultured in 24-well transwell plates. Designated wells were treated H2O2 (600 μM) for comparison control. JUUL pod, Crème Brulee, was aerosolized using Scireq Inexpose system. Cells were exposed to 66 puffs of Crème Brulee for 30 minutes each day for three consecutive days. Twenty-four hours post-exposure the voltage was measured by EVOM2 device. ***p < 0.001 vs. Control. N = 3–12/group.
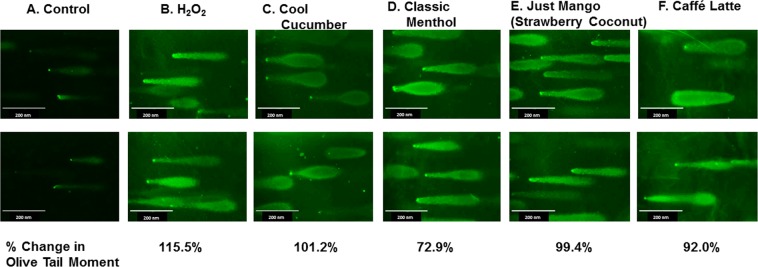
DNA damage caused by JUUL pods and other pod flavors
Based on the images from the Comet assay, treatment with H2O2 has increased the tail lengths compared to the untreated control. On average, the percent change in the olive moment was increased from 72.9% to 101.2% with Cool Cucumber, Classic Menthol, Just Mango (Strawberry Coconut), and Caffé Latte pod e-liquid treatments. As expected H2O2 caused the greatest change in olive tail moment with a 115.5% increase. These data suggest that exposure to these aerosols can cause significant DNA damage (Fig. 7).
Figure 7.
DNA damage by pod flavor liquids. Approximately 300,000 U937 cells cultured in a 12-well plate were treated with 0.5% of selected JUUL pod and other pod flavors, Cool Cucumber, Classic Menthol, Just Mango (Strawberry Coconut), and Caffé Latte. H2O2 treatment as a comparison group. After 24 hours, cells were prepared for Comet Assay. Fluorescent images were captured to visualize DNA damage. Calculated percent changes in olive moments were presented compared to the untreated control. N = 6 images per sample.
Discussion
The high prevalence of vaping among adolescents has led the Food and Drug Administration (FDA) to issue warning letters and to penalize the stores selling these products to minors5,12. However, there is still minimal regulation enforced on e-liquids and vape products. With the escalated use in e-cigs, such as pod mod devices and CBD/THC based formulations for vaping, it is pivotal to assess the toxicological and health effects of these products.
The focus of our current study was on the oxidative stress, inflammatory response, epithelial barrier function, and DNA damage by JUUL pods and other pod flavors. In this study, we tested seven JUUL pods and two other pod flavors in acellular and cellular assays to understand the effects of acute exposure. A recent study demonstrated that JUUL pods and “JUUL-a-likes pods” contain between 1.7% to 7.0% nicotine13. Another study has shown total concentrations of flavoring chemicals to be between 0.2 to 15.6 mg/ml and 0.1 to 9.1 mg/ml in JUUL pod liquid and aerosol phases respectively11. They identified high levels of menthol, ethyl maltol, and vanillin in JUUL pods11. These flavoring chemicals, which have numerous alcohols and aldehyde compounds, react and result in the perpetual formation of byproducts including acetals, such as vanillin PG acetals and vanillin VG acetals14. These products induce irritation of the lung and inflammatory responses. Moreover, our previous studies have shown that these flavoring chemicals can induce significant ROS release, oxidative stress, inflammation, and barrier dysfunction3,15. Although our findings have seen cytotoxicity in direct treatment in e-liquid of Classic Menthol, JUUL labs have conducted preliminary studies on aerosol exposures with 5% Classic Menthol had no observable cytotoxicity based on neutral red uptake, this difference could be due to the treatment method and cell lines used for assays [TSRC, Tob. Sci. Res. Conf., 2018, 72, abstr. 053].
Corroborating our data comparing ROS in JUUL pods and 3R4F cigarettes, other studies conducted on JUUL pod and other e-cigarette aerosolized vapors showed the production of harmful oxidants, such as free radicals and reactive carbonyls, although the concentration of these oxidants was lower than that of conventional cigarettes16,17. In another study, it was determined that nicotine concentration did not have an effect on ROS generation from vapors of e-cigarettes and that as the ratio of VG to PG increased there was a decrease in ROS generation18. Although the seven different JUUL pod flavors were able to generate ROS, flavors like Cool Mint, Crème Brulee, Fruit Medley, and Virginia Tobacco were not capable of reducing acellular total glutathione levels (Supplementary Fig. S2). However, studies are required to determine the redox status of the cells by determining intracellular GSH/GSSG levels.
Our data showed increased mitochondrial superoxide production in all tested flavors, including JUUL pods and other pods. The pivotal role of mitochondrial superoxide in driving pro-inflammatory cytokine production in chronic diseases has been well established19. This suggests that chronic exposure to JUUL pods and similar pod vapors may cause chronic inflammation via mitochondrial dysfunction in the lung. Consistent with our findings, in non-asthmatic human airway smooth muscle cells exposed to cigarette smoke extract led to increased mitochondrial ROS20.
We observed differential inflammatory responses by epithelial cells and monocytes depending on the flavor. Overall, there was an increased level of IL-8, a major neutrophil chemotactic factor, in lung cells exposed aerosols, JUUL pod aerosols (Cool Cucumber and Classic Menthol) and other pod aerosols (Just Mango-Strawberry Coconut and Caffé Latte). Moreover, we also observed that nicotine treatment did neither induce significant IL-8 production nor mitochondrial ROS production in epithelial cells (data not shown). JUUL pod also contains salts along with nicotine and PG/VG. It may be possible that PG/VG and salts with or without nicotine may cause oxidative stress and inflammatory responses which require investigation.
Based on these observations, flavoring chemicals present in these JUUL pods and other pod products may be responsible for the oxidative stress and inflammatory responses elicited in lung cells. Analysis of constituents in JUUL pods by GC-MS showed a wide range of flavoring chemicals, i.e. vanillin, menthol, and cyclohexanols, which are known to cause adverse health effects. Our previous work has shown that the flavors and flavoring chemicals in e-liquids can induce significant oxidative stress and inflammatory responses in lung cells3.
IL-8 stimulates neutrophil migration across endothelium and can activate its functions, including respiratory burst and free radical production. Excessive acute inflammation can result in lung injury, dysregulated repair, and impair gas exchange21. Significant increases in IL-8 have been observed in sputum and bronchoalveolar lavage fluid (BALF) of patients with pulmonary diseases such as asthma, idiopathic pulmonary fibrosis, and chronic obstructive pulmonary disease22. Based on recent case reports, PG/VG and flavorings used in JUUL pods and other pods especially the formulations with CBD/THC MCT oil with terpenes and vitamin E (alpha-tocopherol) acetate peroxyl radical formation may cause acute lung injury via foamy/lipoid (lipid-laden) macrophages leading to lipoid/hypersensitivity pneumonia and/or chemical-induced injuries, which requires investigation23. Further, endogenous phospholipids or surfactants breakdown by vaporized e-liquid/juices containing THC/CBD MCT oil along with terpenes and PG/VG can cause a similar response. Prostaglandin E2 (PGE2), an arachidonic acid-derived mediator through cyclooxygenase, is associated with pro-inflammatory cytokine response in general. PGE2 is involved in the homeostasis of immune-inflammatory response and lung remodeling process24. The increased PGE2 production observed in monocytes and epithelial cells with certain flavors may suggest that this homeostasis has been affected by the flavor constituents.
Consistent with the aforementioned IL-8 levels, we also observed elevated concentrations of other major pro-inflammatory mediators, such as IL-1β and IFN-γ by epithelial cells. Elevated IL-1β levels are known to cause pulmonary inflammation as observed in emphysema and airway remodeling in mice25. Correlating with this data, IFN-γ is a potent stimulator of CC and CXC chemokines. Cigarette smoke has been shown to induce pulmonary inflammation and emphysema via IFN-γ dependent pathways26.
GM-CSF levels were increased with Just Mango (Strawberry Coconut) and Caffé Latte pod flavor exposures. These differential responses may be attributed to the interplay between PGE2 and GM-CSF as there have been studies demonstrating regulation of fibrogenesis by GM-CSF mediated PGE2 levels27. Studies on GM-CSF have shown that BEAS-2B cells exposed to conditioned media that had e-cigarettes bubbled through it had a significant increase in GM-CSF levels28. We observed increased G-CSF levels in epithelial cells after Classic Menthol and Just Mango (Strwarberry-coconut) pod flavors exposure. In the BALF of COPD patients increased levels of G-CSF have been found29. Furthermore, deletion of G-CSF has been linked to reduced airway inflammation and tissue destruction29. These suggest that G-CSF may play a vital role in the pathogenesis of pulmonary diseases. Growth factors such as PDGF have been shown to displays chemoattractant properties at low concentrations for neutrophils, macrophages, and fibroblasts and can also display fibroblast stimulating properties. FGF has been shown to display proliferation properties but also can regulate the synthesis and deposition of extracellular components30. The observed upregulation of growth factors such as PDGF has been implicated as players in cellular proliferation and angiogenesis31. A recent study, bolstering our findings, showed that repetitive inhalation to e-cigarettes with nicotine could induce pro-fibrotic and pro-inflammatory mediators, such as angiopoietin-1/2, EGF, LIF (IL-6 member), and LIX (IL-8 homolog), in C57BL/6 and CD-1 mice32.
All tested pod flavors induced IL-15, a mediator for T-cell and NK cell-mediated inflammation, indicating the risk of chronic lung inflammation in the lungs with exposure to these flavor constituents33,34. Additionally, in accordance with the above findings, observed significant increases in eotaxin and MCP-1 also suggest eosinophilic and monocytic inflammatory processes in the lung due to flavor inhalation exposure.
Our data on epithelial barrier function show that in 16-HBE cells, exposure to JUUL pod Cool Cucumber resulted in a significant decrease in normalized resistance and exposure to JUUL pod Crème Brulee resulted in a significant decrease in membrane voltage. Other studies have shown that repeated exposure to cigarette smoke increased the epithelial monolayer permeability, and a reduction in adhesion proteins, E-cadherin and β-cadherin35. Other studies on the effects of nicotine and e-cigarette chemical flavorings on epithelial barrier function have found that both in vitro exposure to nicotine and flavoring chemicals such as diacetyl and coumarin resulted in a significant reduction in normalized resistance to the epithelial barrier function of 16-HBE15. Consistent with other studies, our results show that JUUL pod vapors resulted in decreased normalized resistance and voltage in the epithelial membrane. Vapor induced barrier dysfunction characteristics are in corroboration with elevated pro-inflammatory mediators, such as IL-1β and IFN-γ, which are known to play an intimate role in airway junctional disintegration in epithelial cells36. Epithelial barrier dysfunction can lead to acute lung injurious response by epithelial leakage. Other studies have shown cystic fibrosis transmembrane conductance regulator (CFTR) and calcium-activated chloride channels (CaCCs) dysfunction induced by acute e-cig vapor37. These adverse effects can induce thickening of the mucus and impaired mucus clearance as observed in COPD patients37.
We observed DNA damage caused by JUUL pod flavors with increasing DNA fragmentation with aerosol exposures. These data are consistent with other studies where e-cigarette exposure caused 8-hydroxy-2′-guanosine, O6-methyldeoxyguanosine, and γ-hydroxy-1,N2-propano-deoxyguanosine adduct formation in the lung tissue38,39. DNA damage can lead to increased cellular senescence and the release of inflammatory mediators by cellular secretory phenotype.
Furthermore, the risk of cardiovascular diseases (CVD) should not be ignored as the constituents of pod based devices are similar to that of other e-liquids, with high nicotine concentrations, aldehydes, oxidants, particulates, and flavor chemicals. Inhalation of these flavors potentially similar CVD risks, including atherosclerosis, hypertension, thrombogenesis, and myocardial infarction40.
In conclusion, our data show that JUUL and other pod flavors produce cellular ROS, triggering an inflammatory response, epithelial barrier dysfunction, and DNA damage in lung cells. Further studies are required to test pod based devices in inducing lung cellular stress and toxicological responses in vivo and understand the mechanisms involved by flavoring chemicals. This may provide information on flavoring chemical-induced lung injury, leading to the regulation of inhalable flavoring chemicals in pods and e-liquids/e-juices.
Supplementary information
Acknowledgements
This study was supported by NIH 1R01HL135613, WNY Center for Research on Flavored Tobacco Products (CRoFT) # U54CA228110, and toxicology training program grant T32-ES007026. We thank Ms. Monica Jackson and Ms. Samantha McDonough for technical assistance. Thanks to Dr. Alan Friedman for interpreting the GCMS data, and Petar Nastoki for GCMS technical analysis.
Author contributions
I.R.: Conceived and designed the experiments. Wrote and edited the manuscript. T.L.: Conducted acellular ROS and GSH assays/data analysis, wrote the manuscript. M.R.F.: Conducted GCMS analysis. T.M.: Conceived and designed the exposures and experiments, conducted TEER, MitoSOX, ELISA, Luminex, and Comet assays, data analysis, and wrote and revised the manuscript.
Data availability
We declare that we have provided all the data, but the primary data will be available upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Thivanka Muthumalage and Thomas Lamb.
Supplementary information
is available for this paper at 10.1038/s41598-019-51643-6.
References
- 1.Bals Robert, Boyd Jeanette, Esposito Susanna, Foronjy Robert, Hiemstra Pieter S., Jiménez-Ruiz Carlos A., Katsaounou Paraskevi, Lindberg Anne, Metz Carlos, Schober Wolfgang, Spira Avrum, Blasi Francesco. Electronic cigarettes: a task force report from the European Respiratory Society. European Respiratory Journal. 2019;53(2):1801151. doi: 10.1183/13993003.01151-2018. [DOI] [PubMed] [Google Scholar]
- 2.Kaur G, Muthumalage T, Rahman I. Mechanisms of toxicity and biomarkers of flavoring and flavor enhancing chemicals in emerging tobacco and non-tobacco products. Toxicol Lett. 2018;288:143–155. doi: 10.1016/j.toxlet.2018.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muthumalage T, et al. Inflammatory and Oxidative Responses Induced by Exposure to Commonly Used e-Cigarette Flavoring Chemicals and Flavored e-Liquids without Nicotine. Front Physiol. 2017;8:1130. doi: 10.3389/fphys.2017.01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cullen KA, et al. Notes from the Field: Use of Electronic Cigarettes and Any Tobacco Product Among Middle and High School Students - United States, 2011-2018. MMWR Morb Mortal Wkly Rep. 2018;67:1276–1277. doi: 10.15585/mmwr.mm6745a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramamurthi Divya, Chau Cindy, Jackler Robert K. JUUL and other stealth vaporisers: hiding the habit from parents and teachers. Tobacco Control. 2018;28(6):610–616. doi: 10.1136/tobaccocontrol-2018-054455. [DOI] [PubMed] [Google Scholar]
- 7.Nardone N, Helen GS, Addo N, Meighan S, Benowitz NL. JUUL electronic cigarettes: Nicotine exposure and the user experience. Drug Alcohol Depend. 2019;203:83–87. doi: 10.1016/j.drugalcdep.2019.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landry RL, et al. The role of flavors in vaping initiation and satisfaction among U.S. adults. Addict Behav. 2019;99:106077. doi: 10.1016/j.addbeh.2019.106077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leavens ELS, Stevens EM, Brett EI, Leffingwell TR, Wagener TL. JUUL in school: JUUL electronic cigarette use patterns, reasons for use, and social normative perceptions among college student ever users. Addict Behav. 2019;99:106047. doi: 10.1016/j.addbeh.2019.106047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duell AK, Pankow JF, Peyton DH. Free-Base Nicotine Determination in Electronic Cigarette Liquids by (1)H NMR Spectroscopy. Chem Res Toxicol. 2018;31:431–434. doi: 10.1021/acs.chemrestox.8b00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Omaiye Esther E., McWhirter Kevin J., Luo Wentai, Pankow James F., Talbot Prue. High-Nicotine Electronic Cigarette Products: Toxicity of JUUL Fluids and Aerosols Correlates Strongly with Nicotine and Some Flavor Chemical Concentrations. Chemical Research in Toxicology. 2019;32(6):1058–1069. doi: 10.1021/acs.chemrestox.8b00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKeganey N, Russell C. Prevalence of Awareness and Use of JUUL E-cigarettes in a National Probability Sample of Adolescents in the United States. Am J Health Behav. 2019;43:591–605. doi: 10.5993/AJHB.43.3.13. [DOI] [PubMed] [Google Scholar]
- 13.Jackler Robert K, Ramamurthi Divya. Nicotine arms race: JUUL and the high-nicotine product market. Tobacco Control. 2019;28(6):623–628. doi: 10.1136/tobaccocontrol-2018-054796. [DOI] [PubMed] [Google Scholar]
- 14.Erythropel HC, et al. Flavorant-Solvent Reaction Products and Menthol in JUUL E-Cigarettes and Aerosol. Am J Prev Med. 2019;57:425–427. doi: 10.1016/j.amepre.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerloff J, et al. Inflammatory Response and Barrier Dysfunction by Different e-Cigarette Flavoring Chemicals Identified by Gas Chromatography-Mass Spectrometry in e-Liquids and e-Vapors on Human Lung Epithelial Cells and Fibroblasts. Appl In Vitro Toxicol. 2017;3:28–40. doi: 10.1089/aivt.2016.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reilly Samantha M, Bitzer Zachary T, Goel Reema, Trushin Neil, Richie John P. Free Radical, Carbonyl, and Nicotine Levels Produced by Juul Electronic Cigarettes. Nicotine & Tobacco Research. 2018;21(9):1274–1278. doi: 10.1093/ntr/nty221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Son Y, et al. Hydroxyl Radicals in E-Cigarette Vapor and E-Vapor Oxidative Potentials under Different Vaping Patterns. Chem Res Toxicol. 2019 doi: 10.1021/acs.chemrestox.8b00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haddad C, et al. Reactive Oxygen Species Emissions from Supra- and Sub-Ohm Electronic Cigarettes. J Anal Toxicol. 2019;43:45–50. doi: 10.1093/jat/bky065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011;208:417–420. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aravamudan B, et al. Cigarette smoke-induced mitochondrial fragmentation and dysfunction in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2014;306:L840–854. doi: 10.1152/ajplung.00155.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukaida N. Pathophysiological roles of interleukin-8/CXCL8 in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol. 2003;284:L566–577. doi: 10.1152/ajplung.00233.2002. [DOI] [PubMed] [Google Scholar]
- 23.Viswam, D., Trotter, S., Burge, P. S. & Walters, G. I. Respiratory failure caused by lipoid pneumonia from vaping e-cigarettes. BMJ Case Rep2018, 10.1136/bcr-2018-224350 (2018). [DOI] [PMC free article] [PubMed]
- 24.Bozyk PD, Moore BB. Prostaglandin E2 and the pathogenesis of pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45:445–452. doi: 10.1165/rcmb.2011-0025RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol. 2005;32:311–318. doi: 10.1165/rcmb.2004-0309OC. [DOI] [PubMed] [Google Scholar]
- 26.Ma B, et al. Role of CCR5 in IFN-gamma-induced and cigarette smoke-induced emphysema. J Clin Invest. 2005;115:3460–3472. doi: 10.1172/JCI24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore BB, et al. GM-CSF regulates bleomycin-induced pulmonary fibrosis via a prostaglandin-dependent mechanism. J Immunol. 2000;165:4032–4039. doi: 10.4049/jimmunol.165.7.4032. [DOI] [PubMed] [Google Scholar]
- 28.Munakata S, et al. Oxidative stress responses in human bronchial epithelial cells exposed to cigarette smoke and vapor from tobacco- and nicotine-containing products. Regul Toxicol Pharmacol. 2018;99:122–128. doi: 10.1016/j.yrtph.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Tsantikos E, et al. Granulocyte-CSF links destructive inflammation and comorbidities in obstructive lung disease. J Clin Invest. 2018;128:2406–2418. doi: 10.1172/JCI98224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wahl SM, Wong H, McCartney-Francis N. Role of growth factors in inflammation and repair. J Cell Biochem. 1989;40:193–199. doi: 10.1002/jcb.240400208. [DOI] [PubMed] [Google Scholar]
- 31.Brock CS, Lee SM. Anti-angiogenic strategies and vascular targeting in the treatment of lung cancer. Eur Respir J. 2002;19:557–570. doi: 10.1183/09031936.02.00293002. [DOI] [PubMed] [Google Scholar]
- 32.Crotty Alexander LE, et al. Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am J Physiol Regul Integr Comp Physiol. 2018;314:R834–R847. doi: 10.1152/ajpregu.00270.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muro S, et al. Expression of IL-15 in inflammatory pulmonary diseases. J Allergy Clin Immunol. 2001;108:970–975. doi: 10.1067/mai.2001.119556. [DOI] [PubMed] [Google Scholar]
- 34.Ge N, et al. Synthesis and secretion of interleukin-15 by freshly isolated human bronchial epithelial cells. Int Arch Allergy Immunol. 2004;135:235–242. doi: 10.1159/000081309. [DOI] [PubMed] [Google Scholar]
- 35.Nishida K, et al. Cigarette smoke disrupts monolayer integrity by altering epithelial cell-cell adhesion and cortical tension. Am J Physiol Lung Cell Mol Physiol. 2017;313:L581–L591. doi: 10.1152/ajplung.00074.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aghapour M, Raee P, Moghaddam SJ, Hiemstra PS, Heijink IH. Airway Epithelial Barrier Dysfunction in Chronic Obstructive Pulmonary Disease: Role of Cigarette Smoke Exposure. Am J Respir Cell Mol Biol. 2018;58:157–169. doi: 10.1165/rcmb.2017-0200TR. [DOI] [PubMed] [Google Scholar]
- 37.Lin VY, et al. Vaporized E-Cigarette Liquids Induce Ion Transport Dysfunction in Airway Epithelia. Am J Respir Cell Mol Biol. 2019;61:162–173. doi: 10.1165/rcmb.2017-0432OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee HW, et al. E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. Proc Natl Acad Sci USA. 2018;115:E1560–E1569. doi: 10.1073/pnas.1718185115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canistro D, et al. E-cigarettes induce toxicological effects that can raise the cancer risk. Sci Rep. 2017;7:2028. doi: 10.1038/s41598-017-02317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benowitz NL, Fraiman JB. Cardiovascular effects of electronic cigarettes. Nat Rev Cardiol. 2017;14:447–456. doi: 10.1038/nrcardio.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We declare that we have provided all the data, but the primary data will be available upon request.