Abstract
Developmental prosopagnosia (DP) is a neurodevelopmental condition characterised by difficulties recognising and discriminating faces. It is currently unclear whether the perceptual impairments seen in DP are restricted to identity information, or also affect the perception of other facial characteristics. To address this question, we compared the performance of 17 DPs and matched controls on two sensitive sex categorisation tasks. First, in a morph categorisation task, participants made binary decisions about faces drawn from a morph continuum that blended incrementally an average male face and an average female face. We found that judgement precision was significantly lower in the DPs than in the typical controls. Second, we used a sex discrimination task, where female or male facial identities were blended with an androgynous average face. We manipulated the relative weighting of each facial identity and the androgynous average to create four levels of signal strength. We found that DPs were significantly less sensitive than controls at each level of difficulty. Together, these results suggest that the visual processing difficulties in DP extend beyond the extraction of facial identity and affects the extraction of other facial characteristics. Deficits of facial sex categorisation accord with an apperceptive characterisation of DP.
Subject terms: Human behaviour, Sensory processing
Introduction
Developmental prosopagnosia (DP) is a neurodevelopmental condition characterised by difficulties recognising and discriminating faces1–3. Individuals with DP may learn to distinguish others based on non-facial features, such as hair, clothing, voice, or gait4. DP is evident in the absence of manifest brain injury and other psychiatric disorders (e.g. autism, schizophrenia), although co-occurring deficits in object processing are not uncommon5–7. The face processing impairments seen in DP appear to affect the perception of both same- and other-ethnicity faces8. Current estimates suggest that ~2% of the general population may experience lifelong face recognition difficulties severe enough to disrupt their daily lives9,10. Consistent with other neurodevelopmental disorders, DP often runs in families, indicating that the condition may have a genetic component11–13.
The nature of the impairment in DP remains unclear. Mnemonic accounts propose that DPs form accurate structural descriptions of faces, but cannot maintain these representations over time14–19. Proponents of this view point to the fact that some DPs score worse on standardised tests of face perception with a mnemonic component, than on standardised tests with little or no memory demands15,18,20. Other researchers favour an apperceptive account – that DP is an impairment of early structural face-encoding1,3,21,22. Consistent with this view, the relative size of face matching deficits seen in DP do not vary as a function of retention interval23,24 and many DPs struggle to sort unfamiliar faces presented simultaneously4,23.
A prediction of the apperceptive account is that the perceptual impairments found in DP ought to extend beyond identity processing. Leading models of face processing propose that a common structural description, formed early in the face processing stream, informs subsequent processing of identity, expression, age, and sex, that occurs in largely divergent pathways25,26. If the problems seen in DP arise from noisy structural descriptions, impairments should be seen, not only when judging identity, but also when judging the expression, age, and sex of faces. Nevertheless, whether or not the perceptual deficits seen in DP extend beyond identity processing remains controversial.
The question that has attracted most research attention is whether DPs have deficits of facial expression processing. Although case-studies have described individual DPs with expression recognition deficits27,28, early group studies failed to detect differences between DPs and controls29–31. However, many of the tasks used in the early group studies lacked sensitivity; for example, when asked to categorise prototypical ‘basic emotions’ the performance of typical observers approaches ceiling. Having used image morphing to render facial expression stimuli more ambiguous, Biotti and Cook32 found that a sample of DPs (N = 15) showed significantly poorer facial emotion categorisation precision than matched controls.
The present study sought to determine whether the perceptual problems seen in DP extend to another non-identity attribute - judgements about facial sex. Here again, the existing experimental findings on sex recognition in DP paint an inconsistent picture. Studies of individual cases suggest that discrimination of facial sex may be impaired in some cases of DP2,33–36, but not in others29,37–39. Group studies have also yielded equivocal results; some authors have described similar levels of performance in DPs and typical observers29,40–42, while others have described weak differences at the group level43.
The present study investigated facial sex discrimination in a group of DPs using sensitive psychophysical tasks. In both tasks, external cues to sex were eliminated; participants were forced to use the internal structure of a briefly presented face to make binary ‘male’/’female’ categorisation decisions. In a morph categorisation task, participants made binary decisions about faces drawn from a morph continuum that blended incrementally an average male face and an average female face. In a sex discrimination task, participants made binary decisions about female or male facial identities that had been blended with an androgynous average face. To vary task difficulty, we manipulated the relative weighting of each facial identity and the androgynous average to create four levels of signal strength. Relative to typical controls, we found that DPs were i) less precise in their categorisations of facial sex (morph categorisation task), and ii) less sensitive to the sex signal presented in faces at all levels of task difficulty (sex discrimination task).
Methods
Diagnostic testing
Seventeen adults with DP (7 males; Mage = 45.9 years, SDage = 11.8 years) were recruited through www.troublewithfaces.org. All reported life-long face recognition difficulties in the absence of brain injury or psychiatric disorder (e.g. autism, schizophrenia). Diagnostic decisions were based on participants’ scores on the Twenty-Item Prosopagnosia Index (PI20)44,45 and the Cambridge Face Memory Test (CFMT)22. We also report the DPs’ performance on the Cambridge Face Perception Test (CFPT)11 to index their face encoding ability, and the Cambridge Car Memory Test (CCMT)46, a measure of non-face object recognition ability. Diagnostic information for each DP is provided in Table 1. Ethical approval was granted by the University of Reading’s ethics committee. The study was conducted in line with the ethical guidelines provided by the 6th (2008) Declaration of Helsinki. All participants provided informed consent and were fully debriefed after the experimental procedure.
Table 1.
Scores for each developmental prosopagnosic (DP) on the Twenty-Item Prosopagnosia Index (PI20), the Cambridge Face Memory Test (CFMT), the Cambridge Face Perception Test (CFPT), and the Cambridge Car Memory Test (CCMT), with z-scores in parentheses.
| Participant | Age | PI20 | CFMT [%] | CFPT [Errors] | CCMT [%] |
|---|---|---|---|---|---|
| F1 | 56 | 88 (−5.50) | 47.22 (−4.23) | 64 (−3.70) | 76.39 (−0.23) |
| F2 | 31 | 72 (−3.74) | 51.39 (−3.77) | 60 (−3.27) | 50 (−1.87) |
| F3 | 35 | 86 (−5.28) | 45.83 (−4.39) | 58 (−3.06) | 69.44 (−0.32) |
| F4 | 36 | 78 (−4.40) | 56.94 (−3.14) | 50 (−2.20) | 65.28 (−0.66) |
| F5 | 22 | 70 (−3.52) | 59.72 (−2.83) | — | — |
| F6 | 40 | 90 (−5.72) | 50.00 (−3.92) | 66 (−3.91) | 55.56 (−1.43) |
| F7 | 38 | 78 (−4.41) | 63.89 (−2.36) | 40 (−1.13) | 56.94 (−1.32) |
| F8 | 68 | 79 (−4.51) | 61.11 (−2.67) | 40 (−1.13) | 66.67 (−0.54) |
| F9 | 44 | 76 (−4.18) | 59.72 (−2.83) | 52 (−2.42) | 56.94 (−1.32) |
| F10 | 60 | 82 (−4.84) | 62.28 (−2.54) | 42 (−1.35) | 79.17 (0.45) |
| M1 | 48 | 77 (−4.29) | 54.17 (−3.45) | 60 (−3.27) | 66.67 (−0.54) |
| M2 | 58 | 86 (−5.28) | 58.33 (−2.99) | 42 (−1.35) | 87.5 (1.11) |
| M3 | 49 | 76 (−4.18) | 54.17 (−3.45) | 76 (−4.98) | 83.33 (0.78) |
| M4 | 57 | 86 (−5.28) | 54.17 (−3.45) | 38 (−0.92) | 81.94 (0.67) |
| M5 | 41 | 79 (−4.51) | 45.83 (−4.39) | 34 (−0.49) | 55.56 (−1.43) |
| M6 | 50 | 79 (−4.51) | 56.94 (−3.14) | 42 (−1.35) | 65.28 (−0.66) |
| M7 | 47 | 83 (−4.95) | 52.78 (−3.61) | 60 (−3.27) | 52.78 (−1.65) |
| DP mean | 45.9 | 80.29 | 54.97 | 51.5 | 66.84 |
| DP SD | 11.8 | 5.58 | 5.3 | 11.51 | 11.49 |
| Comparison Mean | 29.23 | 37.96 | 84.98 | 29.41 | 73.52 |
| Comparison SD | 11.91 | 9.09 | 8.92 | 9.35 | 12.57 |
Note: To calculate z-scores, DPs’ scores on each diagnostic test were compared to a sample of typically developed controls who completed the tests under lab conditions: PI20, CFMT, and CFPT comparison sample of 54 (23 males) controls23 (Experiment 2); CCMT, comparison sample of 61 (27 males) controls6.
Morph categorisation task
In this task, a central fixation cross (250 ms) was followed by a single face presented centrally (250 ms), followed by a high-contrast mask (250 ms). Each stimulus image was drawn from a morph-continuum that blended an average male face with an average female. Facial-sex cues ranged in intensity from 20% female to 80% female in 10% increments (see Fig. 1a). Participants were asked to categorise the stimulus image presented as either male or female. We used participants’ responses to model psychometric functions that plotted how their response likelihoods varied as a function of the sex signal contained in the face.
Figure 1.
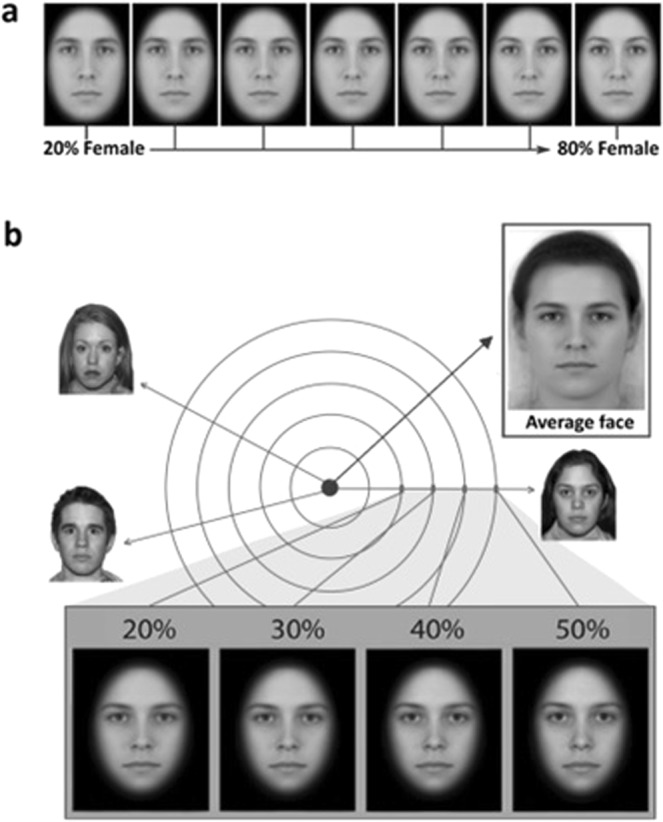
(a) Morphed stimuli used in the morph categorisation task. One male and female model were morphed along a continuum, ranging from 20% (80% male) to 80% female (20% male) in 10% increments. (b) Stimuli used in the sex discrimination task. Forty models were morphed together to create an androgynous average face. Each model was then morphed with the androgynous face to create four weighted morphs (20%, 30%, 40% and 50% identity/sex signal) along a continuum for each model.
Stimulus images were presented in greyscale and subtended 7.9° × 5.9° of visual angle when viewed at a distance of 60 cm. Hair and jaw lines were occluded by an elliptical window with a Gaussian blur at the edges. Each of the seven morphs were presented 20 times, in a random order, resulting in 140 trials.
The average male face and average female face used to create the morph continuum were themselves created by morphing together 16 males and 16 females, respectively. The original images were downloaded from the Chicago Face Database (CFD)47, and morphed using the method described by Adams, Gray, Garner and Graf48.
The performance of the DPs was compared with twenty typically developed (TD) controls recruited from the local subject pool (5 males; Mage = 41.2 years, SDage = 8.3 years). As expected, the TDs (M = 41.1, SD = 10.4) had significantly lower PI20 scores than DPs [t(35) = 13.97, p < 0.001]. Neither participant age [t(35) = 1.43, p = 0.162] nor proportion of males [X2 (1) = 1.10, p = 0.295] differed significantly between the two groups.
Sex discrimination task
A central fixation cross (250 ms) was followed by a single face presented centrally (250 ms) drawn from a set of 40 to-be-judged identities (20 male, 20 female). A high-contrast mask was presented for 250 ms immediately after stimulus offset. Participants were asked to categorise each face as male or female. We measured participants’ ability to discriminate male and female faces using signal detection theory49.
To make the task more challenging we diluted the sex signal in each stimulus image by morphing the facial identity with an androgynous average face48. The androgynous face was constructed by morphing together all 40 of the to-be-judged facial identities. To produce four levels of task difficulty, we varied the relative weighting of each facial identity and the androgynous average (Fig. 1b). In the most difficult condition, stimulus images contained only 20% of the original facial identity; in the least difficult condition, stimulus images contained 50% of the original facial identity.
The original facial images were sourced from the NimStim face set50 and the CFD47. Images were presented in greyscale and subtended 7.3° × 5.4° of visual angle when viewed at 60 cm. Each facial identity was presented four times at each of the morph levels in a random order resulting in 640 trials (40 facial identities × 4 morph levels × 4 repetitions). Both experiments were programmed in MATLAB, using the Psychophysics Toolbox extensions51,52.
The performance of the DPs was compared with twenty TD controls recruited from the local subject pool (5 males; Mage = 43.5 years, SDage = 7.5 years). Twelve out of twenty TDs from this sample also completed the sex categorisation task. Neither participant age [t(35) = .76, p = .452] nor proportion of males [X2 (1) = 1.10, p = 0.295] differed significantly between the two groups. Once again, the TDs (M = 41.1, SD = 11.0) had significantly lower PI20 scores than DPs [t(35) = 13.34, p < 0.001].
Results
Morph categorisation task
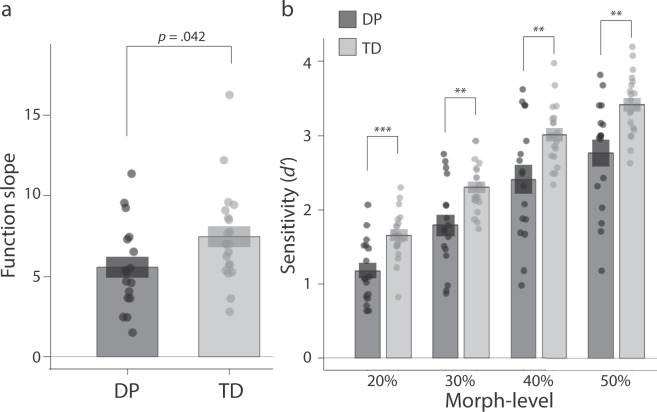
We used each participants’ binary choice responses to estimate their psychometric function by fitting Cumulative Gaussian functions using the Palamedes toolbox53. We were primarily interested in the slope of participants’ psychometric function, quantified using the reciprocal of the standard deviation of the symmetric Gaussian distribution underlying each cumulative Gaussian function. Higher slope values (steeper slopes) indicate that observers can perceive subtle differences between the stimuli and vary their responses accordingly. Slope values and the point of subjective equivalence (PSE) for DPs and TD controls were compared using independent samples t-tests (two-tailed) (α = 0.05).
There was a significant difference between slope estimates for DPs (M = 5.86, SD = 2.86) and TDs (M = 7.97, SD = 3.14) [t(35) = 2.116, p = 0.042 (two-tailed)], indicating that at the group level, the DPs were less sensitive to subtle differences in facial sex than TD controls (see Fig. 2a). There was no difference in PSE between the DPs (M = 0.50, SD = 0.08) and TD controls (M = 0.53, SD = 0.05) [t(35) = 1.15, p = 0.258 (two-tailed)], suggesting that the facial sex categorisation boundary was similar for both groups.
Figure 2.
Results from (a) the morph categorisation task and (b) the sex discrimination task. Note: bar height gives the mean, bands give the mean ± 1 SEM; *** denotes p < 0.001, ** denotes p < 0.01.
While the groups did not differ significantly, the DP group contained a slightly higher proportion of male observers than the group of typical controls. To confirm that observer gender had no influence on our results, we re-ran the foregoing analyses in an ANOVA with observer gender as an additional between-subjects factor. None of the main effects or interactions with observer gender approached significance for either the slope (all F’s < 0.1, all p’s > 0.75) or PSE analyses (all F’s < 2.0, all p’s > 0.17).
Sex discrimination task
For the purpose of the signal detection analysis, a female response to a female stimulus was regarded as a hit, whereas a female response to a male stimulus was regarded as a false alarm. We derived measures of discrimination sensitivity (d′ = z[hit rate] – z[false alarm rate]) and bias (c = −0.5(z[hit rate] + z[false alarm rate])) at each level of task difficulty. When hit or false alarm rates were 1 or 0 (respectively), total hits or false alarms were adjusted by 0.5 before calculating d′.
To assess sensitivity, d’ values were submitted to ANOVA (α = 0.05) with Difficulty (20%, 30%, 40%, 50%) as a within-participants factor and Group (TD, DP) as a between-participants factor (Fig. 2b). Sphericity violations were corrected using the Greenhouse-Geisser adjustment. The analysis revealed a main effect of Difficulty [F(3, 105) = 219.06, p < 0.001, ηp2 = 0.86], indicating that as the strength of the sex signal in the stimuli increased, participants’ sensitivity also increased. Importantly, there was also a main effect of Group [F(1, 35) = 14.16, p = 0.001, ηp2 = 0.29], whereby DPs were less sensitive than TDs. DPs were less sensitive than TDs at each Difficulty level (all ps < 0.01). The effect of Group did not vary as a function of Difficulty [F(3, 105) = 0.65, p = 0.585, ηp2 = 0.02].
To assess bias, C values were submitted to ANOVA with Difficulty (20%, 30%, 40%, 50%) as a within-participants factor and Group (TD, DP) as a between-participants factor. There was a significant main effect of Difficulty [F(1.76, 61.69) = 42.84, p < 0.001, ηp2 = 0.55]. At low morph-levels, participants were slightly biased to respond ‘female’, whereas at higher morph-levels, participants were slightly biased to respond ‘male’ (see Supplementary Materials for more details). There was no main effect of Group [F(1, 35) = 2.18, p = 0.149, ηp2 = 0.06], and no Group × Difficulty interaction [F(1.76, 61.69) = 0.24, p = 0.760, ηp2 = 0.01].
Once again, we re-ran the foregoing analyses with observer gender as an additional between-subjects factor. None of the main effects or interactions with observer gender approached significance for either the sensitivity (all F’s < 1.60, all p’s > 0.19) or bias analyses (all F’s < 1.50, all p’s > 0.20). Z-scores for each DP on both tasks are presented on Table S1 in the Supplementary Materials.
Discussion
The present study aimed to determine whether facial sex discrimination is impaired in DP. In our morph categorisation task, we found that judgment precision, inferred from the slope of individual psychometric functions, was significantly lower for DPs relative to controls. We replicated and extended this finding using a sex discrimination task. We found that DPs were significantly less sensitive to facial sex cues compared to controls at all levels of task difficulty. Despite their impairments, DPs did not differ significantly from controls in terms of categorisation threshold (PSE) or response bias (C).
Previous studies of facial sex categorisation in DP have provided equivocal results. Several studies have found no difference in task performance between DPs and typical controls29,41,42. To date, only one study has reported evidence of atypical perception of facial sex in DP at the group level43. In their study, Esins et al.43 compared 16 DPs and 21 controls, and whilst both groups were approaching ceiling (controls: 91.5% correct; DPs: 84.4% correct), DPs were significantly impaired when making binary classifications of facial sex.
We speculate that methodological issues may have prevented earlier studies from detecting the clear, widespread differences seen here. In previous studies, participants were asked to categorise the sex of unmodified facial images29,41,42. This may have let DPs use trivial pictorial cues, such as the presence of make-up or facial hair – and in some cases, actors’ hair line29 – to achieve broadly typical levels of categorisation accuracy. In several studies, stimuli were also held on screen until response execution39,42, thereby permitting DPs to detect pictorial cues and develop other heuristics. Our use of image-morphing to vary the strength of sexually dimorphic signals, together with brief presentation durations, ensured that our psychophysical tasks taxed the structural encoding processes thought to be aberrant in DP.
The widespread sex discrimination deficits seen here provide compelling support for the apperceptive account of DP. Our tasks had little or no memory component – participants had to make a simple binary-choice decision about a single stimulus. Unlike sequential matching or delayed match-to-sample tasks, there was no need to hold the target face in memory; rather, participants were free to determine their response at any point after stimulus onset. The impairments seen here are therefore hard to explain in terms of a mnemonic deficit. Instead, the fact that DPs were impaired on our sex categorisation tasks strongly suggests a problem forming structural descriptions of faces. Leading models of face processing propose that a common structural description, formed early in the face processing stream, informs subsequent processing of identity, expression, age, and sex, mediated by largely independent, divergent pathways25,26. Subtle deficits of facial sex, and facial emotion perception33, together with problems sorting simultaneously presented faces by similarity24, suggest that this early structural description may be impoverished in DP.
The nature of the apperceptive deficit seen in DP remains currently unclear. According to one influential account, a failure to process faces holistically – whereby facial features are integrated into a non-decomposable whole54–56 – may underlie the face recognition deficits seen in DP31,57–60. However, recent evidence suggests that most individuals with DP show typical susceptibility to the composite face illusion18,42,43,61,62, thought to be a key marker of holistic face processing63,64. Instead, we speculate that DPs have an apperceptive problem that affects local feature descriptions. Consistent with this possibility, many DPs are unable to accurately discriminate local face regions2,32,60. Similarly, variability in local feature encoding ability contributes substantially to the individual differences in face recognition seen in the typical population65.
It has recently been suggested that individual differences in face recognition ability may reflect different patterns of gaze fixations employed by observers. For example, some DPs appear to spend less time examining the internal feature of the face, in particular the eyes66,67. Conversely, so-called ‘super-recognizers’ – people with exceptional face recognition ability68 – spend more time inspecting the nose region than typical observers66. While these interesting differences warrant research attention in future, we believe it is unlikely that atypical gaze behaviour is driving the facial sex categorisation impairment seen here. In both tasks we employed a central fixation cross and brief stimulus presentation (250 ms) in order to limit variability in fixation behaviour as far as possible.
The principle line of evidence in favour of the mnemonic account of DP is the fact that some DPs perform worse on tests of face perception that have a memory component, than on tests that have little or no memory demand, notably the CFMT and CFPT, respectively15,16,20. It has recently been argued, however, that these apparent dissociations should be treated with caution23. In particular, these tasks are used differently in the diagnosis of DP. In many labs, a poor score on the CFMT is deemed necessary for a diagnosis of DP; i.e., people who describe face recognition problems outside the lab are excluded from DP research if their CFMT score does not fall at least two SDs below the typical mean. In contrast, the CFPT is treated as a descriptive measure, administered to assess differences in perceptual encoding ability. Thus, whereas DPs are preselected based on their low CFMT scores, their CFPT scores are free to vary. For example, only 9 out of 17 DPs in this sample performed more than two SDs below the normative mean on the CFPT. Given this treatment – akin to ‘double-dipping’ – it is not at all surprising that some DPs appear to perform worse on the CFMT than the CFPT3. It should also be noted that the psychometric properties of the CFPT are poorer than those of the CFMT, and that the simultaneous presentation of the faces in the CFPT may make it easier to employ compensatory strategies (e.g., detection of trivial pictorial cues).
We have argued that subtle deficits of facial sex categorisation may be widespread in DP, consistent with an apperceptive impairment. If this is the case, why do DPs rarely report problems making sex judgements in their daily lives37,69 ? We believe this apparent discrepancy reflects the different demands posed by sex categorisation outside of the lab and in contrived lab tasks. Outside of the lab, we rarely encounter people who seek to obscure their sex; on the contrary, individuals frequently elect to accentuate sexually dimorphic face signals through the use of make-up, personal grooming, and cosmetic surgery. Moreover, a wealth of non-face cues (e.g., facial hair, hair styles, clothes, use of make-up, body shape, voice) can be used to judge the sex of the faces we encounter outside the lab. Only when these cues are systematically removed in contrived lab-based studies, are individuals forced to base their sex categorisation judgements on facial structure. It is only under these conditions that DPs are disadvantaged relative to TD controls.
In summary, this study aimed to determine whether DPs exhibit deficits of facial sex discrimination. Our two complementary psychophysical procedures produced converging results: when judging closely controlled stimuli in which the strength of the sex signals were manipulated systematically, DPs were less able to categorise facial sex than matched TD controls. While these subtle deficits may have little impact on the day-to-day interactions of individuals with DP, they have considerable theoretical significance. Crucially, they imply that the condition has a locus of impairment early in the face processing stream, that hinders the ability of individuals to form structural descriptions of faces.
Supplementary information
Acknowledgements
J.M. is supported by a doctoral studentship awarded by University of Reading. RC is supported by a Starting Grant awarded by the European Research Council (ERC-2016-StG-715824). Development of the MacBrain Face Stimulus Set was overseen by Nim Tottenham and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development. Please contact Nim Tottenham at tott0006@tc.umn.edu for more information concerning the stimulus set.
Author contributions
J.M., R.C. and K.G. designed both experiments. J.M. and F.B. collected the data. J.M. and K.G. analysed the data. J.M., R.C. and K.G. wrote the manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-55569-x.
References
- 1.Behrmann M, Avidan G. Congenital prosopagnosia: face-blind from birth. Trends Cogn. Sci. 2005;9:180–187. doi: 10.1016/j.tics.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Duchaine BC, Nakayama K. Developmental prosopagnosia: a window to content-specific face processing. Curr. Opin. Neurobiol. 2006;16:166–173. doi: 10.1016/j.conb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Susilo T, Duchaine B. Advances in developmental prosopagnosia research. Curr. Opin. Neurobiol. 2013;23:423–429. doi: 10.1016/j.conb.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Cook R, Biotti F. Developmental prosopagnosia. R312 Curr. Biol. 2016;26:307–318. doi: 10.1016/j.cub.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Geskin J, Behrmann M. Congenital prosopagnosia without object agnosia? A literature review. Cogn. Neuropsychol. 2018;35:4–54. doi: 10.1080/02643294.2017.1392295. [DOI] [PubMed] [Google Scholar]
- 6.Gray KLH, Biotti F, Cook R. Evaluating object recognition ability in developmental prosopagnosia using the cambridge car memory test. Cogn. Neuropsychol. 2019;36:89–96. doi: 10.1080/02643294.2019.1604503. [DOI] [PubMed] [Google Scholar]
- 7.Gray KLH, Cook R. Should developmental prosopagnosia, developmental body agnosia, and developmental object agnosia be considered independent neurodevelopmental conditions? Cogn. Neuropsychol. 2018;35:59–62. doi: 10.1080/02643294.2018.1433153. [DOI] [PubMed] [Google Scholar]
- 8.Cenac Z, Biotti F, Gray KLH, Cook R. Does developmental prosopagnosia impair identification of other-ethnicity faces? Cortex. 2019;119:12–19. doi: 10.1016/j.cortex.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Kennerknecht I, et al. First report of prevalence of non-syndromic hereditary prosopagnosia (HPA) Am. J. Med. Genet. Part A. 2006;140A:1617–1622. doi: 10.1002/ajmg.a.31343. [DOI] [PubMed] [Google Scholar]
- 10.Kennerknecht I, Ho NY, Wong VCN. Prevalence of hereditary prosopagnosia (HPA) in Hong Kong Chinese population. Am. J. Med. Genet. Part A. 2008;146A:2863–2870. doi: 10.1002/ajmg.a.32552. [DOI] [PubMed] [Google Scholar]
- 11.Duchaine B, Germine L, Nakayama K. Family resemblance: Ten family members with prosopagnosia and within-class object agnosia. Cogn. Neuropsychol. 2007;24:419–430. doi: 10.1080/02643290701380491. [DOI] [PubMed] [Google Scholar]
- 12.Johnen A, et al. A family at risk: Congenital prosopagnosia, poor face recognition and visuoperceptual deficits within one family. Neuropsychologia. 2014;58:52–63. doi: 10.1016/j.neuropsychologia.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Schmalzl L, Palermo R, Green M, Brunsdon R, Coltheart M. Training of familiar face recognition and visual scan paths for faces in a child with congenital prosopagnosia. Cogn. Neuropsychol. 2008;25:704–729. doi: 10.1080/02643290802299350. [DOI] [PubMed] [Google Scholar]
- 14.Dalrymple KA, Garrido L, Duchaine B. Dissociation between face perception and face memory in adults, but not children, with developmental prosopagnosia. Dev. Cogn. Neurosci. 2014;10:10–20. doi: 10.1016/j.dcn.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalrymple KA, Palermo R. Guidelines for studying developmental prosopagnosia in adults and children. Wiley Interdiscip. Rev. Cogn. Sci. 2016;7:73–87. doi: 10.1002/wcs.1374. [DOI] [PubMed] [Google Scholar]
- 16.Jackson MC, Counter P, Tree JJ. Face working memory deficits in developmental prosopagnosia: Tests of encoding limits and updating processes. Neuropsychologia. 2017;106:60–70. doi: 10.1016/j.neuropsychologia.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Stollhoff R, Jost J, Elze T, Kennerknecht I. Deficits in long-term recognition memory reveal dissociated subtypes in congenital prosopagnosia. PLoS One. 2011;6:e15702. doi: 10.1371/journal.pone.0015702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulrich PIN, et al. Perceptual and memorial contributions to developmental prosopagnosia. Q. J. Exp. Psychol. 2017;70:298–315. doi: 10.1080/17470218.2016.1177101. [DOI] [PubMed] [Google Scholar]
- 19.Weigelt S, Koldewyn K, Kanwisher N. Face identity recognition in autism spectrum disorders: A review of behavioral studies. Neurosci. Biobehav. Rev. 2012;36:1060–1084. doi: 10.1016/j.neubiorev.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Bate S, et al. Objective patterns of face recognition deficits in 165 adults with self-reported developmental prosopagnosia. Brain Sci. 2019;9:133. doi: 10.3390/brainsci9060133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Renzi E, Faglioni P, Grossi D, Nichelli P. Apperceptive and associative forms of prosopagnosia. Cortex. 1991;27:213–21. doi: 10.1016/S0010-9452(13)80125-6. [DOI] [PubMed] [Google Scholar]
- 22.Duchaine B, Nakayama K. The cambridge face memory test: Results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic participants. Neuropsychologia. 2006;44:576–585. doi: 10.1016/j.neuropsychologia.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Biotti F, Gray KLH, Cook R. Is developmental prosopagnosia best characterised as an apperceptive or mnemonic condition? Neuropsychologia. 2019;124:285–298. doi: 10.1016/j.neuropsychologia.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Shah P, Gaule A, Gaigg SB, Bird G, Cook R. Probing short-term face memory in developmental prosopagnosia. Cortex. 2015;64:115–122. doi: 10.1016/j.cortex.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Bruce, V. & Young, A. Understanding face recognition. British Journal of Psychology77 (1986). [DOI] [PubMed]
- 26.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn. Sci. 2000;4:223–233. doi: 10.1016/S1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 27.Duchaine BC, Yovel G, Butterworth EJ, Nakayama K. Prosopagnosia as an impairment to face-specific mechanisms: Elimination of the alternative hypotheses in a developmental case. Cogn. Neuropsychol. 2006;23:714–747. doi: 10.1080/02643290500441296. [DOI] [PubMed] [Google Scholar]
- 28.Duchaine B, Murray H, Turner M, White S, Garrido L. Normal social cognition in developmental prosopagnosia. Cogn. Neuropsychol. 2009;26:620–634. doi: 10.1080/02643291003616145. [DOI] [PubMed] [Google Scholar]
- 29.Dobel C, Bölte J, Aicher M, Schweinberger SR. Prosopagnosia without apparent cause: overview and diagnosis of six cases. Cortex. 2007;43:718–33. doi: 10.1016/S0010-9452(08)70501-X. [DOI] [PubMed] [Google Scholar]
- 30.Humphreys K, Avidan G, Behrmann M. A detailed investigation of facial expression processing in congenital prosopagnosia as compared to acquired prosopagnosia. Exp. Brain Res. 2007;176:356–373. doi: 10.1007/s00221-006-0621-5. [DOI] [PubMed] [Google Scholar]
- 31.Palermo R, et al. Impaired holistic coding of facial expression and facial identity in congenital prosopagnosia. Neuropsychologia. 2011;49:1226–1235. doi: 10.1016/j.neuropsychologia.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biotti F, Cook R. Impaired perception of facial emotion in developmental prosopagnosia. Cortex. 2016;81:126–136. doi: 10.1016/j.cortex.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Ariel R, Sadeh M. Congenital visual agnosia and prosopagnosia in a child: A case report. Cortex. 1996;32:221–240. doi: 10.1016/S0010-9452(96)80048-7. [DOI] [PubMed] [Google Scholar]
- 34.De Haan EHF, Campbell R. A fifteen year follow-up of a case of developmental prosopagnosia. Cortex. 1991;27:489–509. doi: 10.1016/S0010-9452(13)80001-9. [DOI] [PubMed] [Google Scholar]
- 35.Jones RD, Tranel D. Severe developmental prosopagnosia in a child with superior intellect. J. Clin. Exp. Neuropsychol. 2001;23:265–273. doi: 10.1076/jcen.23.3.265.1183. [DOI] [PubMed] [Google Scholar]
- 36.Laeng B, Caviness VS. Prosopagnosia as a deficit in encoding curved surface. J. Cogn. Neurosci. 2001;13:556–576. doi: 10.1162/089892901750363163. [DOI] [PubMed] [Google Scholar]
- 37.Bentin S, Deouell LY, Soroker N. Selective visual streaming in face recognition: evidence from developmental prosopagnosia. Neuroreport. 1999;10:823–827. doi: 10.1097/00001756-199903170-00029. [DOI] [PubMed] [Google Scholar]
- 38.Kress T, Daum I. Developmental prosopagnosia: A review. Behav. Neurol. 2003;14:109–121. doi: 10.1155/2003/520476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nunn JA, Postma P, Pearson R. Developmental prosopagnosia: should it be taken at face value? Neurocase. 2001;7:15–27. doi: 10.1093/neucas/7.1.15. [DOI] [PubMed] [Google Scholar]
- 40.Chatterjee G, Nakayama K. Normal facial age and gender perception in developmental prosopagnosia. Cogn. Neuropsychol. 2012;29:482–502. doi: 10.1080/02643294.2012.756809. [DOI] [PubMed] [Google Scholar]
- 41.DeGutis J, Chatterjee G, Mercado RJ, Nakayama K. Face gender recognition in developmental prosopagnosia: Evidence for holistic processing and use of configural information. Vis. cogn. 2012;20:1242–1253. doi: 10.1080/13506285.2012.744788. [DOI] [Google Scholar]
- 42.Le Grand R, et al. What aspects of face processing are impaired in developmental prosopagnosia? Brain Cogn. 2006;61:139–158. doi: 10.1016/j.bandc.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Esins J, Schultz J, Stemper C, Kennerknecht I, Bülthoff I. Face perception and test reliabilities in congenital prosopagnosia in seven tests. Iperception. 2016;7:204166951562579. doi: 10.1177/2041669515625797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gray KLH, Bird G, Cook R. Robust associations between the 20-item prosopagnosia index and the cambridge face memory test in the general population. R. Soc. Open Sci. 2017;4:160923. doi: 10.1098/rsos.160923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah P, Gaule A, Sowden S, Bird G, Cook R. The 20-item prosopagnosia index (PI20): a self-report instrument for identifying developmental prosopagnosia. R. Soc. Open Sci. 2015;2:140343. doi: 10.1098/rsos.140343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dennett HW, et al. The cambridge car memory test: A task matched in format to the cambridge face memory test, with norms, reliability, sex differences, dissociations from face memory, and expertise effects. Behav. Res. Methods. 2012;44:587–605. doi: 10.3758/s13428-011-0160-2. [DOI] [PubMed] [Google Scholar]
- 47.Ma DS, Correll J, Wittenbrink B. The chicago face database: A free stimulus set of faces and norming data. Behav. Res. Methods. 2015;47:1122–1135. doi: 10.3758/s13428-014-0532-5. [DOI] [PubMed] [Google Scholar]
- 48.Adams WJ, Gray KLH, Garner M, Graf EW. High-level face adaptation without awareness. Psychol. Sci. 2010;21:205–210. doi: 10.1177/0956797609359508. [DOI] [PubMed] [Google Scholar]
- 49.Macmillan, N. A. & Creelman, C. D. Detection Theory. (Psychology Press, 2004).
- 50.Tottenham N, et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brainard DH. The Psychophysics Toolbox. Spat. Vis. 1997;10:433–436. doi: 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
- 52.Pelli DG. The videotoolbox software for visual psychophysics: transforming numbers into movies. Spat. Vis. 1997;10:437–442. doi: 10.1163/156856897X00366. [DOI] [PubMed] [Google Scholar]
- 53.Prins N, Kingdom FAA. Applying the model-comparison approach to test specific research hypotheses in psychophysical research using the palamedes toolbox. Front. Psychol. 2018;9:1250. doi: 10.3389/fpsyg.2018.01250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farah MJ, Wilson KD, Drain M, Tanaka JN. What is ‘Special’ about Face Perception? Psychol. Rev. 1998;105:482–498. doi: 10.1037/0033-295X.105.3.482. [DOI] [PubMed] [Google Scholar]
- 55.Mckone E, Yovel G. Why does picture-plane inversion sometimes dissociate perception of features and spacing in faces, and sometimes not? toward a new theory of holistic processing. Psychon. Bull. Rev. 2009;16:778–797. doi: 10.3758/PBR.16.5.778. [DOI] [PubMed] [Google Scholar]
- 56.Piepers, D. W. & Robbins, R. A. A review and clarification of the terms ‘holistic,’ ‘configural,’ and ‘relational’ in the face perception literature. Frontiers in Psychology3 (2012). [DOI] [PMC free article] [PubMed]
- 57.Avidan G, Tanzer M, Behrmann M. Impaired holistic processing in congenital prosopagnosia. Neuropsychologia. 2011;49:2541–2552. doi: 10.1016/j.neuropsychologia.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeGutis J, Cohan S, Mercado RJ, Wilmer J, Nakayama K. Holistic processing of the mouth but not the eyes in developmental prosopagnosia. Cogn. Neuropsychol. 2012;29:419–46. doi: 10.1080/02643294.2012.754745. [DOI] [PubMed] [Google Scholar]
- 59.DeGutis J, Cohan S, Nakayama K. Holistic face training enhances face processing in developmental prosopagnosia. Brain. 2014;137:1781–98. doi: 10.1093/brain/awu062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu TT, Behrmann M. Impaired holistic processing of left-right composite faces in congenital prosopagnosia. Front. Hum. Neurosci. 2014;8:750. doi: 10.3389/fnhum.2014.00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Biotti F, et al. Normal composite face effects in developmental prosopagnosia. Cortex. 2017;95:63–76. doi: 10.1016/j.cortex.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 62.Susilo T, et al. Face recognition impairments despite normal holistic processing and face space coding: Evidence from a case of developmental prosopagnosia. Cogn. Neuropsychol. 2010;27:636–664. doi: 10.1080/02643294.2011.613372. [DOI] [PubMed] [Google Scholar]
- 63.Murphy J, Cook R. Revealing the mechanisms of human face perception using dynamic apertures. Cognition. 2017;169:25–35. doi: 10.1016/j.cognition.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 64.Rossion B. The composite face illusion: A whole window into our understanding of holistic face perception. Visual Cognition. 2013;21:139–253. doi: 10.1080/13506285.2013.772929. [DOI] [Google Scholar]
- 65.DeGutis J, Wilmer J, Mercado RJ, Cohan S. Using regression to measure holistic face processing reveals a strong link with face recognition ability. Cognition. 2013;126:87–100. doi: 10.1016/j.cognition.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 66.Bobak AK, Parris BA, Gregory NJ, Bennetts RJ, Bate S. Eye-movement strategies in developmental prosopagnosia and “super” face recognition. Q. J. Exp. Psychol. 2017;70:201–217. doi: 10.1080/17470218.2016.1161059. [DOI] [PubMed] [Google Scholar]
- 67.Peterson MF, et al. Eye movements and retinotopic tuning in developmental prosopagnosia. J. Vis. 2019;19:7. doi: 10.1167/19.9.7. [DOI] [PubMed] [Google Scholar]
- 68.Russell R, Duchaine B, Nakayama K. Super-recognizers: People with extraordinary face recognition ability. Psychon. Bull. Rev. 2009;16:252–257. doi: 10.3758/PBR.16.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grüter T, Grüter M, Carbon C-C. Neural and genetic foundations of face recognition and prosopagnosia. J. Neuropsychol. 2008;2:79–97. doi: 10.1348/174866407X231001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.