Abstract
Megakaryocytes have been implicated in the micro-environmental abnormalities associated with fibrosis and hematopoietic failure in the bone marrow (BM) of primary myelofibrosis (PMF) patients, the Philadelphia-negative myeloproliferative neoplasm (MPN) associated with the poorest prognosis. To identify possible therapeutic targets for restoring the BM functions in PMF, we compared the expression profiling of PMF BM with that of BM from essential thrombocytopenia (ET), a fibrosis-free MPN also associated with BM megakaryocyte hyperplasia. The signature of PMF BM was also compared to published signatures associated with liver and lung fibrosis. Gene enrichment analysis identified distinctive differences between the expression profile of PMF and ET. Notch, K-Ras, IL-8 and apoptosis pathways were altered the most in PMF as compared to controls. By contrast, cholesterol homeostasis, unfolded protein response and hypoxia were the pathways found altered to the greatest degree in ET compared to control specimens. BM from PMF expressed a non-canonical TGF-β signature which included activation of ID1, JUN, GADD45b and of genes with binding motifs for the JUN transcriptional complex AP1. By contrast, the expression of ID1 and GADD45b was not altered and there was a modest signal for JUN activation in ET. The similarities among PMF, liver and lung fibrosis were modest and included activation of integrin-α9 and tropomyosin-α1 between PMF and liver fibrosis, and ectoderm-neural cortex protein1 and FRAS1-related extracellular matrix protein1 between PMF and lung fibrosis, but not TGF-β. These data identify TGF-β as a potential target for micro-environmental therapy in PMF.
Keywords: Bone Marrow, Myeloproliferative Neoplasms, Primary Mielofibrosis, TGF-β, Fibrosis
Graphical Abstract

The expression signature of PMF BM suggests that in this organ TGF-β activates a non-canonical MAPK dependent signaling that induces stromal cells to produce fibrosis. The diagram illustrates target genes reported to be activated (red arrows) or repressed (blue arrows) by the non-canonical and canonical TGF-β signaling, respectively, in stromal and hematopoietic cells of the bone marrow, and of their respective predicted biological effects. In red and blue fonts, instead, are summarized the activated and repressed state of these same target genes in the arrays of BM from PMF patients described here. Black fonts, instead, indicate genes found to be expressed at normal levels in PMF. There is a good correlation between predicted and detected expression state of the genes of the non-canonical TGF-β signaling (4 out of 5 target genes), suggesting that in PMF bone marrow stromal cells are activated by TGF-β. By contrast there is no agreement between the predicted and the detected expression state of targets of the canonical pathway (0 out of 8 targets), supporting the hypothesis that the malignant hematopoietic cells do not respond to TGF-β.
INTRODUCTION
Mice over-expressing the transcription factor JUN, a gene activated by several inflammatory cytokines including the non-canonical MAPK-dependent TGF-β signaling1–3, develop bone marrow (BM), skin, and lung fibrosis and are predisposed to developing fibrosis in response to stresses to the liver and kidney4. These observations suggest that in mice shared mechanisms mediate development of fibrosis across organs. This hypothesis is of great clinical relevance since identification of factors that stimulate fibrosis across organs would greatly advance the development of anti-fibrotic therapies. However, whether shared mechanisms for fibrosis among organs exist also in men has not yet been established.
Bone marrow (BM) fibrosis is the hallmark of the micro-environmental abnormalities responsible for hematopoietic failure in primary myelofibrosis (PMF), the most severe of the Philadelphia-negative myeloproliferative neoplasms (MPNs)5,6. It has been hypothesized that in PMF fibrosis is induced by TGF-β, and possibly other inflammatory cytokines, produced by increased numbers of dysplastic megakaryocytes7,8. Fibrosis, however, is not observed in the BM of Essential Thrombocytemia (ET) patients, a form of MPN which presents with hyper-proliferation of polylobulated megakaryocytes rather than that of hypolobulated megakaryocytes that characterize PMF.
In order to identify the fibrosis signature in PMF and to assess whether this signature is similar to that of fibrosis in other organs, we compared the expression profiling of BM from PMF with that of BM from either ET or non-diseased volunteers, as negative controls. The PMF signature was also compared to published signatures of idiopathic pulmonary fibrosis and hepatic fibrosis9–11. The data presented indicate the presence of shared, but also distinctive signatures between PMF and ET, and between the fibrotic signature of PMF and that of other organs.
Materials and Methods
Human Subjects
Cryopreserved mononuclear cells from BM of 15 PMF and three ET patients was provided as de-identified material by IRCCS Policlinico San Matteo, Pavia. Bone marrow from eight healthy individuals was purchased from AllCells Technology (Oakland Califormia, USA). mRNA in amount sufficient for analyses was obtained from 6 PMF and all ET patients and healthy controls. The clinical data of informative patients at the time of the BM harvest are summarized in Table 1. The study was approved by the Institutional Review Board of Policlinico San Matteo, Pavia [authorization n. 20110004143, Sept 26th, 2011] and is compliant to the Declaration of Helsinki for Studies Involving Human Subjects.
Table 1:
Clinical features of the MPN patients included in the study.
| ID | Sex | Age at diagnosis | Number of Evaluations | Disease duration | IPSS | Fibrosis grade | Mutation (allele burden) | Therapy |
|---|---|---|---|---|---|---|---|---|
| PMF1 | F | 57 | 2 | 5 y | Int-2 | MF3 | JAK2 (86%) | Hydrox |
| PMF2 | F | 72 | 2 | 2 y | Int-1 | MF3 | JAK2 (Het) | Hydrox |
| PMF3 | M | 81 | 2 | 1 y | Int-1 | MF1 | CALR [del] | No |
| PMF4 | M | 38 | 2 | 16 y | Int-1 | 0/1 | JAK2 (Het) | Ruxol |
| PMF5 | M | 68 | 1 | 5 y | Int-1 | MF2 | CALR [ins] | Ruxol |
| PMF6 | M | 54 | 1 | 4 y | Int-2 | MF1 | JAK2 (76%) | Ruxol |
| ET7a | M | 51 | 2 | 4 y | High | 0 | MPL | Hydrox |
| ET7b | M | 51 | 1 | 3 y | Low | 0 | MPL | Hydrox |
| ET8 | M | 47 | 1 | 4 y | Low | 0 | JAK2 (Het) | Aspirin |
| ET9 | F | 67 | 1 | Diagnosis | High | 0 | JAK2 (5%) | Aspirin |
ET7 was analyzed twice. The first time 3 year post-diagnosis (b) and the second time one year later (a).
RNA extraction and microarray analyses
Total RNA was prepared with Trizol (Gibco-BRL, Grand Island, NY, USA) and purified with the Rneasy Mini Kit (Qiagen, Germantown, MD, USA). Hybridization to the Illumina microarray Human HT-12_v4 Bead Chip gene expression array (San Diego, CA) was performed by the Microarray Resource Facility, Icahn School of Medicine at Mount Sinai. Two sequential microarray analyses were performed. Microarray 1 included three PMF patients, two replicate measurements each (PMF1–3, Table 1), and five non-diseased control, one measurement each. Microarray 2 included three PMF patients (PMF4, and a pool of PMF5/6), three ET patients (ET7–9, Table1) and three healthy controls. PMF4 was analyzed in duplicate, the PMF5/6 pool, ET7, ET9 and the healthy controls were measured once. BM from ET7 was analyzed at 3-years (two replicates) and 4-years post diagnosis. The entire microarray data set is available in the Gene Expression Omnibus database which will be made available once the manuscript is accepted for publication.
Data Analyses
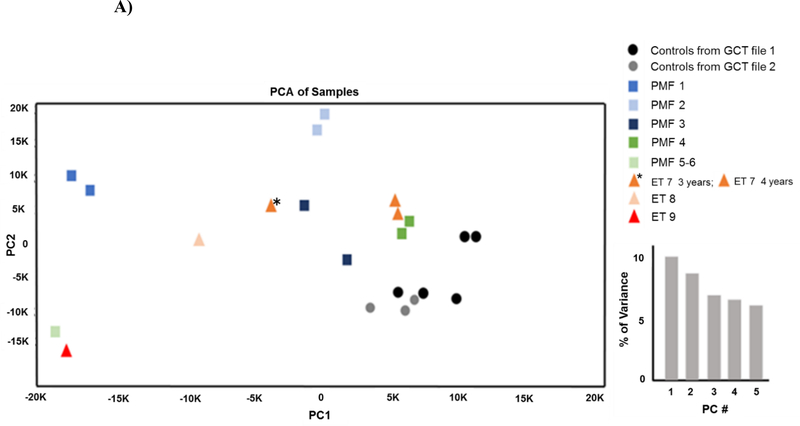
By using the GenomeStudio software (Illumina), the two microarrays were imported into gene cluster text (GCT) files (GCT file 1 and GCT file 2). In total, 47,323 RNA microarray probes were identified with a handful of missing values (out of 47,323 probes, 7 were missing from GCT file 1 and 22 from GCT file 2), which was probably due to failure during the import of the microarray data into the GCT files. By using SAS 9.4, the two separate files were combined into one GCT file and the data normalized by the IlluminaNormalizer (Illumina) module in GenePattern (Broad Institute, University of California, San Diego, CA). The data were then analyzed with the sampleFilterPercentP_R2 module of GenePattern and found of good quality (correlation to median array > 0.95 for all the samples). The collapsed data by gene (took median) was created by CollapseDataset module in GenePattern and identified 31,426 genes. Primary Component Analysis (PCA) by PCA module in GenePattern verified that the controls included in GCT file 1 and GCT file 2 were similar (Figure 1A). The data reflected the strong separation and clustering of samples, demonstrating the quality of the data. The first principal component contains the difference between controls and patients with MF, about 10% of the variance in the data (Figure 1A). Finally, we collapsed the dataset by subject (took average) and excluded genes with coefficient of variation (=SD/mean) below 0.1, to create the final dataset for analysis. Collectively, we analyzed 8,528 genes (after excluding 22,898 noise genes). Gene set enrichment analysis (GSEA) was done by GSEA module in GenePattern, utilizing the human gene sets, h.all.v6.1.symbols.gmx, comparing PMF vs. control, ET vs. control, and PMF vs. ET.
Figure 1. Comparison of the expression profiling of BM from PMF, ET and healthy controls.
A) Principal component analysis (PCA). To assess the relationship between samples, we performed PCA on the normalized data obtained in duplicate on the same sample in the two data sets. The strong separation and clustering of samples demonstrates the high reproducibility of data from the same sample. The first PCA shows differences between controls and patients with MF, about 10% of the variance in the data. B) Comparison of the 100 genes (50 most upregulated and 50 most downregulated) differentially expressed by BM from PMF with respect to health controls, from ET with respect to healthy control and from PMF with respect to ET, as indicated.
Statistical analyses
The false discovery rate (FDR) were used for multiple comparisons of gene expressions. Genes were considered up and down-regulated when fold changes were >1.4 and <0.7, respectively. In multiple comparison of genes, the level of significance was set at p<0.05 (significant) and p<0.1 (trend).
Results and Discussions
The expression profiles of the PMF and ET patients were heterogeneous while those of controls were tightly clustered (Figure 1A). Since the duplicate measurements of the same patient were also tightly clustered (Figure 1A), the variability among patients likely reflects differences in driver mutations and/or fibrosis levels (International Prognostic Score System int-1–2 and fibrosis grade 1–3) (Table 1). The expression profile of ET7 clustered with that of PMF3 at the 3 year time point and to that of PMF4 at the 4 year time point (Figure 1A). The fact that the top 50 genes differentially expressed in ET7 at the two time points remain the same and do not include any gene differentially expressed between patients vs controls (Figure 1B and S1) suggests that differences observed in ET7 at the 3 and 4 year time point underlie variability unrelated to MPN.
In spite of the heterogeneity in expression profiles among patients, common abnormalities were observed between patients and healthy controls (Figure 1B and data not shown). In PMF, there were 426–516 transcripts up- and down-regulated in the patients, as compared to controls. As expected due to great frequency of megakaryocytes in PMF, expression of the megakaryocyte specific transcription factor GATA1 was significantly upregulated in PMF (1.5 fold higher than in healthy controls, p=0.04). The transcription factor JUN (3.0-fold higher than normal controls, p=0.01) and its related genes JUNB (1.2-fold, p=0.767) and FOSB (2.8-fold, p=0.06) were also among the most enriched genes relative to controls (Figure 1B).
Since the transcription factor complex JUN/FOS binds to consensus sequences defined AP-112–14, we confirmed that the high levels of JUN/FOS expressed by BM from PMF has physiological consequences by determining that the expression of several genes with putative AP-1 binding sites was also significantly altered in the profiling of the BM from the three PMF patients analyzed using microarray 1 (Table 2).
Table 2:
Molecular pathways differentially induced between MF and controls (Gene Set Enrichment Analysis)
| Gene set database | Gene sets induced in MF | NES | p | FDR |
|---|---|---|---|---|
| Genes with AP1-related binding motif in their promoter* | AP1_01 | 1,71 | 0,000 | 0,000 |
| AP1_Q6 | 1,68 | 0,000 | 0,003 | |
| AP1_Q6_01 | 1,57 | 0,000 | 0,007 | |
| AP1_C | 1,56 | 0,002 | 0,005 | |
| AP1_Q4 | 1,54 | 0,000 | 0,005 | |
| AP1_Q4_01 | 1,44 | 0,000 | 0,010 | |
| AP1_Q2_01 | 1,43 | 0,002 | 0,009 | |
| AP1FJ_Q2 | 1,32 | 0,009 | 0,025 | |
| AP1_Q2 | 1,23 | 0,033 | 0,060 |
NES: normalized enrichment score, FDR: false discovery rate. Gene sets with FDR<0.25 or top 20 are shown. Gene sets with FDR >0.05 are gray shaded.
In the regions spanning up to 4 kb around transcription start site in TRANSFAC database ver.7.4 (http://gene-regulation.com/pub/databases.html).
The strong JUN signature of PMF BM determined in the current study confirms the high levels of expression of this transcription factor previously described by us by analyzing BM from three additional PMF patients with TGF-β specific microarrays15. It is also consistent with the high JUN and FOS content detected by immunohistochemistry in stromal cells from BM biopsies of 57 PMF patients4.
In ET, there were 332–833 transcripts up and down regulated, with respect to healthy controls (FDR, p<0.10). Of those, 81 and 77 were consistently up- and down-regulated in all the patients (Figure 1B). In the cases of ET, the gene upregulated the most was PTGS2 (prostaglandin-endoperoxide synthase 2, also known as COX), an enzyme involved in the prostaglandin biosynthetic pathway expressed at high level in BM and which is a potent mediator of inflammation16. In ET, JUN and FOS were expressed at levels lower than in PMF and there was only a trend toward activation (JUN, fold increase 3.0, p=0.097; FOSB fold increase 4.3, p=0.097) relative to controls.
In our study, mRNA was prepared from BM mono-nucleated cells thawed after cryopreservation. BM mononuclear cells are a heterogeneous population enriched for hematopoietic (mainly stem/progenitor cells, megakaryocytes and monocytes) and stromal (mesenchymal stem cells, fibroblasts and endothelial cells) cells and deprived of erythroid cells and granulocytes by the thawing procedure. Based on histological data, we infer that BM mononuclear cells from PMF and ET contain ~3-fold more megakaryocyte than normal samples while PMF is slightly enriched for CD34+ cells (2–3%) than both ET and normal controls (~ 2% in both cases). The overall content of monocytes and stromal cells is instead comparable among samples.
To assess the contribution of stem/progenitor cells to the expression signature of BM mononuclear cells, we compared the expression profiles of PMF BM with that of PMF CD34+ cells published by Guglielmelli et al.17 (Table S1). Three of the 12 genes found differentially expressed in CD34+ cells were excluded as noise before statistical evaluation. Three genes (CD9, DLK1 and NFE-2) upregulated in Guglielmelli et al.17 were also found upregulated above threshold (fold change>1.7) with respect to controls in our study. Therefore, in spite of their low numbers, we believe that CD34+ cells have contributed to the readouts of our arrays.
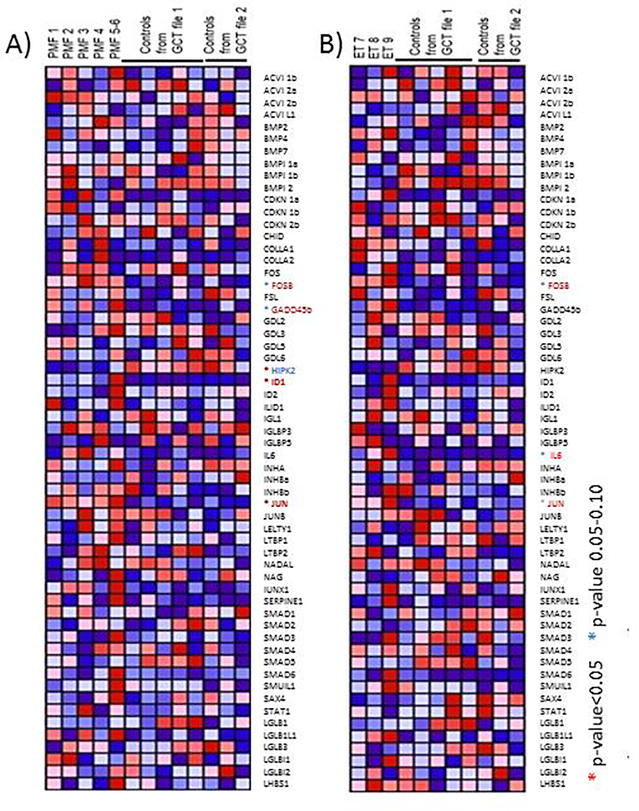
It has been suggested that the stromal and hematopoietic cells of BM respond to TGF-β by activating its non-canonical MAPK-dependent and canonical SMAD-dependent signaling pathway, respectively (Graphical Abstract). Since JUN is an important target of the non-canonical pathway, the strong JUN signature detected in PMF suggests that in these patients the TGF-β signaling is mostly active in stromal cells. To confirm that the canonical TGF-β signaling is not active in PMF and to exclude that the observed JUN over-expression is mediated by some other inflammatory cytokine18, the TGF-β gene expression profiling of PMF and ET with respect to controls was compared by gene set enrichment analysis (GSEA) (Figure 2).
Figure 2. The comparison of the expression profiling of BM from PMF (A), and ET (B) versus healthy controls indicates the presence of an activated non-canonical TGF-β signature only in PMF.
Red and blue indicate genes the expression of which is significantly or have a trend to be over- and under-expressed, respectively, with respect to controls.
The differences observed in the TGF-β data set between samples were in general modest and not consistent. In agreement with the notion that it is not the expression of TGF-β per se but rather its bioavailability in the microenvironment that is increased in PMF19, TGF-β was not detected in the PMF or ET signature. However, when compared to controls, PMF bone marrow had more (5 genes, three upregulated and two downregulated), TGF-β related genes expressed at altered levels than ET (three genes, all upregulated). In PMF, there was a significant abnormal expression of HIPK2 (downregulated) and of ID1 and JUN (upregulated) and a trend toward greater expression of FOSB and GADD45b.
In addition, comparison of the TGF-β signature of PMF patients with fibrosis grade 3 (PMF1 and 2) compared to those with fibrosis grade 0–2 (PMF 3, 4, 5 and 6) (Table S2), indicates that the upregulation of the expression of the non-canonical TGF-β target genes FOSb, GADD45b and ID1/2 increases with disease progression. Interestingly, BM from PMF grade 3 patients also express greater levels of HIPK2, homeodomain-interacting protein kinase 2, a gene encoding a serine/threonine protein kinase that interacts with homeodomain transcription factors20,21 that is activated, by over-expression or loss of heterozygosity, in animal models of kidney fibrosis22–24 and in patients with idiopathic pulmonary fibrosis25. By contrast, in ET there was a modest TGF-β signature that included a trend toward FOSB, JUN and IL6 upregulation with respect to controls.
The activation of the non-canonical p38/ERK dependent TGF-β signature JUN, ID1 and GADD45 26,27 was already reported both in PMF patients and in the Gata1low animal model of the disease28. In addition, expression of these genes was normalized in the mouse model by treatment with a TGF-β receptor 1 kinase inhibitor28, which also rescued their myelofibrosis phenotype. We believe that over-expression of these genes observed in PMF BM is an indication that the stromal cells are being activated by TGF-β to produce fibrosis (Graphical Abstract).
By contrast, the target genes expected to be activated by the canonical TGF-β signaling were either expressed at normal levels of downregulated in BM of PMF patients. These genes included CDKN1b and HIPK2. CDKN1b, cyclin-dependent kinase inhibitor 1b, encodes p27Kip1 which induces normal hematopoietic stem/progenitor cells into quiescence in response to TGF-β 29,30 and was also found downregulated in BM from PMF patients by 15 (Graphical Abstract). We believe that downregulation of CDKN1b in BM of PMF reflects the paucity or insensitivity31 of hematopoietic cells that respond to TGF-β in PMF BM.
Support for the hypothesis that the expression signatures we have identified reflect the disease status of the patient comes from the observation that IL-8 was upregulated in the BM signature of PMF but not in that of ET. The over-expression of IL-8 in PMF BM is in agreement with recent observations indicating that the plasma of PMF patients, but not that from ET, contains greater levels of IL-8 than normal, which correlates with disease prognosis32.
The differences in expression profiling between PMF and ET was further characterized by pathway analyses with the gene set enrichment analysis (GSEA) (Table 3). The expression pathways altered in PMF and ET were quite distinct. Notch signaling was the most enriched pathway in PMF (NES=1.62, p=0.046), followed by K-RAS (NES=1.51, p=0.036) and apoptosis (NES=1.47, p=0.121) as compared to healthy controls. In contrast, cholesterol homeostasis was the most enriched pathway in ET (NES=1.62, p=0.00), followed by unfolded protein response (NES=1.18, p=0.203). Notch and K-RAS were enriched in PMF even when the data were compared with ET. The individual genes abnormally expressed in each pathway are shown in Table 4.
Table 3.
GSEA enrichment terms from Gene Ontology using GSEA for PMF vs. control, ET vs. control and PMF vs. ET
| PMF vs. Control | ||||||
| Name of enrichment term | Size | ES | NES | NOM p-val | FDR p-val | FWER p-val |
| NOTCH_SIGNALING | 17 | 0.57 | 1.62 | 0.046 | 0.795 | 0.292 |
| KRAS_SIGNALING_DN | 38 | 0.48 | 1.51 | 0.036 | 0.846 | 0.465 |
| APOPTOSIS | 116 | 0.43 | 1.47 | 0.121 | 0.742 | 0.545 |
| ESTROGEN_RESPONSE_EARLY | 80 | 0.33 | 1.46 | 0.041 | 0.610 | 0.561 |
| HEME_METABOLISM | 158 | 0.58 | 1.43 | 0.192 | 0.558 | 0.596 |
| P53_PATHWAY | 120 | 0.36 | 1.40 | 0.079 | 0.536 | 0.631 |
| TNFA_SIGNALING_VIA_NFKB | 151 | 0.53 | 1.33 | 0.236 | 0.647 | 0.745 |
| CHOLESTEROL_HOMEOSTASIS | 49 | 0.41 | 1.32 | 0.190 | 0.605 | 0.757 |
| HYPOXIA | 116 | 0.33 | 1.31 | 0.102 | 0.559 | 0.771 |
| ET vs. Control | ||||||
| Name of enrichment terms | Size | ES | NES | NOM p-val | FDR p-val | FWER p-val |
| CHOLESTEROL_HOMEOSTASIS | 49 | 0.48 | 1.62 | 0.000 | 0.461 | 0.204 |
| UNFOLDED_PROTEIN_RESPONSE | 85 | 0.30 | 1.18 | 0.203 | 1.000 | 0.887 |
| HYPOXIA | 116 | 0.28 | 1.16 | 0.210 | 1.000 | 0.915 |
| ANGIOGENESIS | 17 | 0.44 | 1.16 | 0.272 | 1.000 | 0.915 |
| MYOGENESIS | 64 | 0.28 | 1.07 | 0.352 | 1.000 | 0.952 |
| ANDROGEN_RESPONSE | 59 | 0.30 | 1.06 | 0.390 | 1.000 | 0.964 |
| MTORC1_SIGNALING | 149 | 0.26 | 1.06 | 0.343 | 1.000 | 0.964 |
| COAGULATION | 59 | 0.30 | 1.03 | 0.363 | 1.000 | 0.964 |
| EPITHELIAL_MESENCHYMAL_TRANSITION | 81 | 0.32 | 1.02 | 0.599 | 1.000 | 0.967 |
| PMF vs. ET | ||||||
| Name of enrichment terms | Size | ES | NES | NOM p-val | FDR q-val | FWER p-val |
| KRAS_SIGNALING_DN | 38 | 0.53 | 1.67 | 0.000 | 0.272 | 0.113 |
| WNT_BETA_CATENIN_SIGNALING | 19 | 0.57 | 1.64 | 0.035 | 0.187 | 0.196 |
| PEROXISOME | 54 | 0.41 | 1.61 | 0.000 | 0.198 | 0.257 |
| INTERFERON_ALPHA_RESPONSE | 81 | 0.69 | 1.44 | 0.034 | 0.627 | 0.615 |
| APICAL_JUNCTION | 73 | 0.46 | 1.35 | 0.000 | 0.939 | 0.781 |
| NOTCH_SIGNALING | 17 | 0.42 | 1.32 | 0.107 | 0.985 | 0.886 |
| INTERFERON_GAMMA_RESPONSE | 152 | 0.53 | 1.26 | 0.218 | 1.000 | 0.886 |
| REACTIVE_OXIGEN_SPECIES_PATHWAY | 36 | 0.35 | 1.17 | 0.197 | 1.000 | 0.916 |
| BILE_ACID_METABOLISM | 44 | 0.30 | 1.14 | 0.320 | 1.000 | 0.931 |
ES: enrichment score; NES: normalized enrichment score
Table 4. GSEA enrichment terms from Gene Ontology using GSEA for PMF vs. control, ET vs. control and PMF vs. ET.
Gene upregulated are in red fonts, genes down regulared are in blue fonts. Genes with statistically significant fold changes are in bold.
| Pathways (number of genes) | Genes abnormally expressed |
|---|---|
| Differences between PMF and Control | |
| NOTCH_SIGNALING (17) | SKP1, HES1, FZD5, |
| KRAS_SIGNALING_DN (38) | BTG2, ADRA2C, FGFR3, IFNG, MTHFR, BARD1 |
| APOPTOSIS (116) | GPX1, JUN, LMNA, CD69, ETF1, BTG3, PMAIP1, PDGFRB, EGR3, BTG2, IL1B, SMAD7, TFN, DAP, SAT1, GADD45B, SQSTM1, CD44, DPYD, RNASEL, CASP2, CFLAR, DFFA |
| ESTROGEN_RESPONSE_EARLY (80) | ENDOD1, FHL2, PMAIP1, EGR3, NXT1, HES1, MUC1, SLC7A5, MAST4, FASN, SLC37A1, PPIF, CD44, ISG20L2, RAB31, ADD3, XBP1, |
| HEME_METABOLISM (158) | SMOX, ENDOD1, GATA1, ELL2, TAL1, CIR1, SLC10A3, OPTN, SLC11A2, PQLC1, ACP5, BTG2, ARHGEF12, MPP1, PIGQ, OSBP2, CTSB, ALDH6A1, |
| Differences between ET and Control | |
| CHOLESTEROL_HOMEOSTASIS (49) | TRIB3, SCD, DHCR7, LGMN, FAM129A, FBXO6, ATXN2, |
| UNFOLDED_PROTEIN_RESPONSE (85) | TUBB2A, HSPA9, SLC7A5, ZBTB17, ATP6V0D1, SDAD1, SPCS1, TTC37, ATF6, SEC31A, IMP3, PARN |
| HYPOXIA (116) | PRDX5, JMJD6, PLIN2, ZFP36, PPP1R15A, GRHPR, JUN, DDIT3, GAPDH, SDC4, MYH9, MAP3K1, CCNG2, BCL2, PLAC8, FOXO3, IRS2, PGM1, PRKCA, GLRX, PFKP, NAGK, ENO1, |
| ANGIOGENESIS (17) | VCAN |
| MYOGENESIS (64) | SCD, SPHK1, MYH9, OCEL1, VIPR1, MEF2C, PLXNB2, |
| Differences between PMF and ET | |
| KRAS_SIGNALING_DN (38) | No hit |
| WNT_BETA_CATENIN_SIGNALING (19) | No hit |
| PEROXISOME (54) | No hit |
| INTERFERON_ALPHA_RESPONSE (81) | No hit |
| APICAL_JUNCTION (73) | ACTB, TGF-β1 |
Red, ed (FDR); blue, underexpressed (FDR); bold, statistically significant expression (FDR, p<0.05);
To confirm the hypothesis that the high levels of GATA1 expression detected in PMF are due to increased frequency of immature megakaryocytes, the expression signature of PMF was compared with that of normal controls and of ET using the list of 26 megakaryocyte-specific genes described by 33,34(Table S3). Twelve genes were over-expressed, while 3 genes were under-expressed in PMF with respect to normal. In addition to GATA1, examples of genes over-expressed in PMF are MPL, the receptor for thrombopoietin, CD36, platelet glycoprotein IV or thrombospondin receptor, and GP9, glycoprotein IX, which are expressed at higher levels in immature megakaryocytes35. In contrast, genes expressed at greater levels by mature megakaryocytes, such as PF4, were expressed at levels similar to controls (fold change 1.14, p=0.37). The differences in gene expression (4 over-expressed and 3 under-expressed) between ET and normal controls were modest and largely overlapping with those observed in PMF. The only uniquely abnormal gene in ET was DHRS3, which encodes short-chain dehydrogenase/reductase 3. The reduced number of abnormalities observed in ET confirms that in this disease the maturation of megakaryocytes is overall normal. Of interest, CD47, the “don’t eat me” signal36, was slightly down-regulated above threshold with respect to normal both in ET (fold change 0.72, p=0.0025, Table S3) and PMF (fold change 0.82, p=0.068), a further indication that the immune control is impaired in myeloproliferative neoplasms37. Lastly, comparison between the megakaryocyte gene signature of PMF and ET largely identifies the same gene expression differences (11 upregulated) observed between PMF versus controls (Table S3).
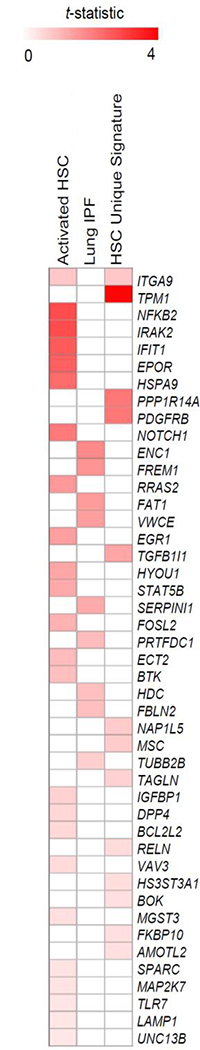
The expression profiling of the BM from PMF patients were also compared to the gene expression signatures from fibrotic tissues or cells including: activated hepatic stellate cells [HSC, 100 upregulated and 100 downregulated8; 100 upregulated9] and tissues from patients with idiopathic pulmonary fibrosis (100 upregulated and 100 downregulated, GSE4746010). The comparison with these published signatures of liver fibrosis and lung fibrosis, indicated modest similarity. Some common genes included activation of integrin-α9 (ITGA9) and tropomyosin α−1 (9TPM1) when compared with liver fibrosis signatures, and ectoderm-neural cortex protein 1 (ENC1) and FRAS1-related extracellular matrix protein 1 (FREM1) when compared to the lung fibrosis signature (Figure 3).
Figure 3. Comparison of the expression signature of the BM from PMF patients (this manuscript) with published signatures of hepatic stellate cells (HSC, activated HSC10 and HSC unique signature8,9, that are responsible for liver fibrosis, and of idiopathic pulmonary fibrosis10.
The color indicates the statistical strength of the similarity between the abnormalities.
A limitation of our study is the limited number of patients included in the analyses. In this regard, it is common experience that, due to the underlying fibrosis, the cell content of most of the BM biopsies from PMF patients is low and does not provide mRNA in amount sufficient for analyses. From a total of 15 PMF BM samples processed, we obtained mRNA for microarray analyses from only 6 patients (Table 1). This limitation prevented us from validating the expression patterns identified by quantitative RT-PCR analyses. However, because of their rarity, the availability of these data in a public database will facilitate studies on marrow fibrosis using more advanced techniques such as single cell profiling.
In summary, this study confirms the presence of a non-canonical TGF-β signature in BM from PMF supporting the hypothesis that inhibitors of TGF-β represent attractive candidates for therapies targeting the microenvironment in PMF.
Supplementary Material
Highlights.
Unlike liver and lung fibrosis and ET BM, BM from PMF express a signature of noncanonical TGF-β signaling activation
This signature identifies TGF-β as potential target for microenvironment therapy in PMF
Acknowledgements
This study was supported by grants from the National Cancer (P01-CA108671, RH and ARM), National Heart, Lung and Blood Institute (1R01-HL116329, ARM; P30 CA196521-SF), National Institute of Diabetes Digestive and Kidney Disease (R01-DK56621, SF), Department of Defense (CA150272P9, SF ) and Associazione Italiana Ricerca Cancro (AIRC 17608).
Footnotes
Disclosures
The authors have no conflicts of interest to declare. Preliminary data were presented at the 2017 annual meeting of the American Society of Hematology (Blood 2017;130:4196).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yoshida K, Kuwano K, Hagimoto N, et al. MAP kinase activation and apoptosis in lung tissues from patients with idiopathic pulmonary fibrosis. J Pathol. 2002;198(3):388–396. doi: 10.1002/path.1208 [DOI] [PubMed] [Google Scholar]
- 2.Chung WH, Bennett BM, Racz WJ, Brien JF, Massey TE. Induction of c-jun and TGF-??1 in Fischer 344 rats during amiodarone-induced pulmonary fibrosis. Am J Physiol - Lung Cell Mol Physiol. 2001. [DOI] [PubMed] [Google Scholar]
- 3.Gervasi M, Bianchi-Smiraglia A, Cummings M, et al. JunB contributes to Id2 repression and the epithelial-mesenchymal transition in response to transforming growth factor-β. J Cell Biol. 2012. doi: 10.1083/jcb.201109045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wernig G, Chen S-Y, Cui L, et al. Unifying mechanism for different fibrotic diseases. Proc Natl Acad Sci. 2017;114(18):4757–4762. doi: 10.1073/pnas.1621375114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544 [DOI] [PubMed] [Google Scholar]
- 6.Barosi G, Mesa RA, Thiele J, et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the international working group for myelofibrosis research and treatment. Leukemia. 2008;22(2):437–438. doi: 10.1038/sj.leu.2404914 [DOI] [PubMed] [Google Scholar]
- 7.Vainchenker W, Constantinescu SN, Plo I. Recent advances in understanding myelofibrosis and essential thrombocythemia. F1000Research. 2016;5:700. doi: 10.12688/f1000research.8081.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhan H, Kaushansky K. Functional interdependence of hematopoietic stem cells and their niche in oncogene promotion of myeloproliferative neoplasms: the 159th biomedical version of “it takes two to tango.” Exp Hematol. 2019. doi: 10.1016/j.exphem.2018.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drews F, Knöbel S, Moser M, et al. Disruption of the latent transforming growth factor-β binding protein-1 gene causes alteration in facial structure and influences TGF-β bioavailability. Biochim Biophys Acta - Mol Cell Res. 2008. doi: 10.1016/j.bbamcr.2007.08.004 [DOI] [PubMed] [Google Scholar]
- 10.Zhang DY, Goossens N, Guo J, et al. A hepatic stellate cell gene expression signature associated with outcomes in hepatitis C cirrhosis and hepatocellular carcinoma after curative resection. Gut. 2016. doi: 10.1136/gutjnl-2015-309655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng X, Moore M, Mathur A, et al. Plexin C1 deficiency permits synaptotagmin 7–mediated macrophage migration and enhances mammalian lung fibrosis. FASEB J. 2016;30(12):4056–4070. doi: 10.1096/fj.201600373R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prusty BK, Das BC. Constitutive activation of transcription factor AP-1 in cervical cancer and suppression of human papillomavirus (HPV) transcription and AP-1 activity in HeLa cells by curcumin. Int J Cancer. 2005;113(6):951–960. doi: 10.1002/ijc.20668 [DOI] [PubMed] [Google Scholar]
- 13.Rösl F, Das BC, Lengert M, Geletneky K, Zur Hausen H. Antioxidant-induced changes of the AP-1 transcription complex are paralleled by a selective suppression of human papillomavirus transcription. J Virol. 1997;71(1):362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antinore MJ, Birrer MJ, Patel D, Nader L, McCance DJ. The human papillomavirus type 16 E7 gene product interacts with and trans-activates the AP1 family of transcription factors. EMBO J. 1996;15(8):1950–1960. doi: 10.1002/j.1460-2075.1996.tb00546.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciaffoni F, Cassella E, Varricchio L, Massa M, Barosi G, Migliaccio AR. Activation of non-canonical TGF-beta1 signaling indicates an autoimmune mechanism for bone marrow fibrosis in primary myelofibrosis. Blood Cells Mol Dis. 2015;54(3):234–241. doi: 10.1016/j.bcmd.2014.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricciotti E, Fitzgerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011. doi: 10.1161/ATVBAHA.110.207449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guglielmelli P, Zini R, Bogani C, et al. Molecular Profiling of CD34 + Cells in Idiopathic Myelofibrosis Identifies a Set of Disease-Associated Genes and Reveals the Clinical Significance of Wilms’ Tumor Gene 1 ( WT1 ). Stem Cells. 2007;25(1):165–173. doi: 10.1634/stemcells.2006-0351 [DOI] [PubMed] [Google Scholar]
- 18.Kaminska B Molecular Characterization of Inflammation-Induced JNK/c-Jun Signaling Pathway in Connection with Tumorigenesis. In: Humana Press; 2009:249–264. doi: 10.1007/978-1-60327-530-9_13 [DOI] [PubMed] [Google Scholar]
- 19.Zingariello M, Ruggeri A, Martelli F, et al. A novel interaction between megakaryocytes and activated fibrocytes increases TGF-β bioavailability in the Gata1(low) mouse model of myelofibrosis. Am J Blood Res. 2015;5(2):34–61. [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Hofmann TG, Runkel L, et al. Isolation and characterization of cDNAs for the protein kinase HIPK2. Biochim Biophys Acta - Gene Struct Expr. 2001. doi: 10.1016/S0167-4781(00)00308-0 [DOI] [PubMed] [Google Scholar]
- 21.Ki SS, Yoon YG, Ahn JH, Young HK, Kim Y, Cheol YC. Differential interactions of the homeodomain-interacting protein kinase 2 (HIPK2) by phosphorylation-dependent sumoylation. FEBS Lett. 2005. doi: 10.1016/j.febslet.2005.04.053 [DOI] [PubMed] [Google Scholar]
- 22.Jin Y, Ratnam K, Chuang PY, et al. A systems approach identifies HIPK2 as a key regulator of kidney fibrosis. Nat Med. 2012;18(4):580–588. doi: 10.1038/nm.2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saul V V, Schmitz ML. Posttranslational modifications regulate HIPK2, a driver of proliferative diseases. J Mol Med. 2013;91(9):1051–1058. doi: 10.1007/s00109-013-1042-0 [DOI] [PubMed] [Google Scholar]
- 24.Ricci A, Cherubini E, Ulivieri A, et al. Homeodomain-interacting protein kinase2 in human idiopathic pulmonary fibrosis. J Cell Physiol. 2013. doi: 10.1002/jcp.24129 [DOI] [PubMed] [Google Scholar]
- 25.Saul V V, de la Vega L, Milanovic M, et al. HIPK2 kinase activity depends on cis-autophosphorylation of its activation loop. J Mol Cell Biol. 2013;5(1):27–38. doi: 10.1093/jmcb/mjs053 [DOI] [PubMed] [Google Scholar]
- 26.Ghosh AK, Quaggin SE, Vaughan DE. Molecular basis of organ fibrosis: Potential therapeutic approaches. Exp Biol Med. 2013. doi: 10.1177/1535370213489441 [DOI] [PubMed] [Google Scholar]
- 27.Massagué J, Blain SW, Lo RS. TGFβ Signaling in Growth Control, Cancer, and Heritable Disorders. Cell. 2000;103(2):295–309. doi: 10.1016/S0092-8674(00)00121-5 [DOI] [PubMed] [Google Scholar]
- 28.Zingariello M, Martelli F, Ciaffoni F, et al. Characterization of the TGF-b1 signaling abnormalities in the Gata1low mouse model of myelofibrosis. Blood. 2013. doi: 10.1182/blood-2012-06-439661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polyak K, Lee MH, Erdjument-Bromage H, et al. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994. doi: 10.1016/0092-8674(94)90572-X [DOI] [PubMed] [Google Scholar]
- 30.Scandura JM, Boccuni P, Massague J, Nimer SD. Transforming growth factor -induced cell cycle arrest of human hematopoietic cells requires p57KIP2 up-regulation. Proc Natl Acad Sci. 2004;101(42):15231–15236. doi: 10.1073/pnas.0406771101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ceglia I, Dueck AC, Masiello F, et al. Preclinical rationale for TGF-β inhibition as a therapeutic target for the treatment of myelofibrosis. Exp Hematol. 2016;44(12):1138–1155.e4. doi: 10.1016/j.exphem.2016.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A. Circulating Interleukin (IL)-8, IL-2R, IL-12, and IL-15 Levels Are Independently Prognostic in Primary Myelofibrosis: A Comprehensive Cytokine Profiling Study. J Clin Oncol. 2011;29(10):1356–1363. doi: 10.1200/JCO.2010.32.9490 [DOI] [PubMed] [Google Scholar]
- 33.Chen Z, Hu M, Shivdasani RA. Expression analysis of primary mouse megakaryocyte differentiation and its application in identifying stage-specific molecular markers and a novel transcriptional target of NF-E2. Blood. 2007. doi: 10.1182/blood-2006-08-038901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Psaila B, Barkas N, Iskander D, et al. Single-cell profiling of human megakaryocyte-erythroid progenitors identifies distinct megakaryocyte and erythroid differentiation pathways. Genome Biol. 2016. doi: 10.1186/s13059-016-0939-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bianchi E, Norfo R, Pennucci V, Zini R, Manfredini R. Genomic landscape of megakaryopoiesis and platelet function defects. Blood. 2016. doi: 10.1182/blood-2015-07-607952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng M, Jiang W, Kim BYS, Zhang CC, Fu Y-X, Weissman IL. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer. 2019. doi: 10.1038/s41568-019-0183-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nonino A, Nascimento JM, Mascarenhas CC, Mazzeu JF, Pereira RW, Jacomo RH. CD47 expression is decreased in hematopoietic progenitor cells in patients with myelofibrosis. Brazilian J Med Biol Res. 2019;52(1):1–7. doi: 10.1590/1414-431X20187784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brenet F, Kermani P, Spektor R, Rafii S, Scandura JM. TGFβ restores hematopoietic homeostasis after myelosuppressive chemotherapy. J Exp Med. 2013;210(3):623–639. doi: 10.1084/jem.20121610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruno E, Horrigan SK, Van Den Berg D, et al. The Smad5 gene is involved in the intracellular signaling pathways that mediate the inhibitory effects of transforming growth factor-beta on human hematopoiesis. Blood. 1998;91(6):1917–1923. [PubMed] [Google Scholar]
- 40.Zermati Y, Fichelson S, Valensi F, et al. Transforming growth factor inhibits erythropoiesis by blocking proliferation and accelerating differentiation of erythroid progenitors. Exp Hematol. 2000;28(8):885–894. doi: 10.1016/S0301-472X(00)00488-4 [DOI] [PubMed] [Google Scholar]
- 41.Geng Y, Weinberg RA. Transforming growth factor β effects on expression of G1 cyclins and cyclin-dependent protein kinases. Proc Natl Acad Sci U S A. 1993. doi: 10.1073/pnas.90.21.10315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacobsen FW, Stokke T, Jacobsen SEW. Transforming growth factor-β potently inhibits the viability-promoting activity of stem cell factor and other cytokines and induces apoptosis of primitive murine hematopoietic progenitor cells. Blood. 1995. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.