Abstract
Background:
Glucocorticoids (GC) are considered first-line therapy for most patients with hypereosinophilic syndrome (HES). Although response rates are generally high, many patients require moderate to high doses for control of eosinophilia and symptoms, and up to 15% of patients do not respond at all. Despite this, little is known about the mechanisms of GC resistance in patients with HES.
Objective:
To explore the etiology of GC-resistance in HES
Methods:
Clinical data and samples from 26 patients with HES enrolled on a prospective study of GC-responsiveness and 23 patients with HES enrolled on a natural history study of eosinophilia for whom response to GC was known were analyzed retrospectively. Expression of GC receptor isoforms was assessed by quantitative RT-PCR in purified eosinophils. Serum cytokine levels were quantified by suspension array assay in multiplex.
Results:
Despite an impaired eosinophil response to GC after 7 days of treatment, the expected rise in absolute neutrophil count was seen in 7/7 GC-resistant patients, suggesting that GC resistance in HES is not a global phenomenon. Eosinophil mRNA expression of glucocorticoid receptor (GR) isoforms (α, β, γ and P) was similar between GC-sensitive (n=20) and GC-resistant (n=9) patients with HES. Whereas geometric mean serum levels were also comparable between GC-r (n=11) and GC-s (n=19) for all cytokines tested, serum IL-5 levels were >100 pg/mL only in GC-r patients.
Conclusions and Clinical Relevance:
These data suggest that the mechanism of GC resistance in HES is not due to a global phenomenon affecting all lineages, but may be due, at least in some patients, to impairment of eosinophil apoptosis by increased levels of IL-5.
INTRODUCTION
Hypereosinophilic syndromes (HES) are a heterogeneous group of disorders defined by blood eosinophilia ≥ 1500/mm3 on two occasions and eosinophil-related clinical manifestations. HES can be divided into a number of clinically-defined subtypes, including: 1) myeloid HES (MHES), of which FIP1L1-PDGFRA-positive myeloid neoplasms are the most common, 2) lymphoid HES (LHES), 3) clinically distinct eosinophilic disorders that meet the definition of HES, such as eosinophilic granulomatosis with polyangiitis (EGPA) and eosinophilic gastrointestinal disorders (EGID), both forms of overlap HES, and 4) idiopathic HES (IHES) [1]. A variety of conditions, such as parasitic infection, primary immunodeficiency and neoplasms, can also cause HES (Associated HES), but are approached differently [1].
Although glucocorticoids (GC) are currently considered the first line therapy for most clinical subtypes of HES, response is variable. In a multicenter retrospective study of patients with HES, 179/188 (95%) patients were treated with GC at some time during the follow-up period and 163/188 (81%) received GC as initial therapy [2]. Among the 141 patients treated with GC monotherapy, 20 (14%) failed treatment at 1 month [2], of which 10 were known to be positive for the FIP1L1-PDGFRA fusion gene. A similar rate of GC resistance (9%) was reported in a single center retrospective study of 164 patients, in which patients with PDGFRA mutations were excluded and non-responders were more strictly defined as patients with persistent AEC ≥1000/mm3 at one week despite 60 mg of prednisone daily [3]. Whereas MHES subjects were most likely to be resistant to treatment, GC resistance was not restricted to this clinical subtype [3]. Mechanisms of resistance were not explored.
Glucocorticoid receptor (GR) expression has been described on nearly all cells in the human body, including eosinophils [4]. Encoded by the Nr3c1 gene, the GR exists as several different isoforms generated by alternative splicing and translation initiation [5]. GRα is the most abundant isoform. Upon binding to GC, GRα translocates to the nucleus where it binds directly to GC-responsive elements stimulating target gene expression. GRγ is similar in function to GRα but exhibits reduced (approximately 50%) activity. In contrast to GRα and GRγ, GRβ does not bind GC and resides primarily in the nucleus where it functions as a dominant negative inhibitor, antagonizing the effects of GRα on many GC-responsive target genes. GRP does not bind GC but has been shown to modulate the transcriptional activity of GRα.
GC resistance has been studied in a wide variety of clinical disorders, including asthma, leukemia and inflammatory bowel disease. Reported mechanisms of resistance include 1) poor adherence or absorption [6, 7], 2) a non-permissive cytokine milieu [8–10], 3) abnormal steroid receptor [4, 11], 4) dysregulation of GC receptor (GR) splice variants [12, 13], 5) an impaired apoptotic response [14, 15], and (6) altered signal transduction [9]. That said, little is known about the relative importance of each of these mechanisms in patients with HES. In the present study, data and samples from patients with GC-sensitive and GC-resistant PDGFRA-negative HES were used to address this issue.
METHODS
2.1 |. Subjects
The study population was comprised of 26 GC naïve patients with PDGFRA-negative HES enrolled in a prospective study of GC response (), 23 patients with PDGFRA-negative HES enrolled on a clinical study of eosinophilia for whom samples and data were available prior to initiation of GC and for whom GC response status was known () and 19 healthy volunteers () (see Supplemental Methods and Supplemental Figure 1 for additional details). Patients in the prospective study underwent a standardized GC taper and were classified as GC-sensitive (GC-s; AEC<1000/mm3 and symptomatic improvement on ≤40 mg prednisone) or GC-resistant (GC-r; inability to taper to 40 mg prednisone due to AEC>1000/mm3 or persistent symptoms) (see Supplemental Appendix for taper details). Patients in the retrospective cohort were classified as GC-s if there was documentation of improved symptoms and AEC <1000/mm3 for a minimum of 1 week on daily oral GC. GC-r patients in the retrospective cohort had persistent AEC ≥1000/mm3 despite ≥60 mg prednisone equivalent daily). Taking both cohorts together, the geometric mean GC dose that suppressed AEC to less than <1000/mm3 in the GC-s group was a prednisone equivalent of 14 mg (range: 2.5 mg-40 mg) and all GC-r subjects had AEC≥1000/mm3 despite ≥60 mg prednisone equivalent. The National Institute of Allergy and Infectious Diseases Institutional Review Board approved all studies, and all subjects gave written informed consent.
2.2 |. Peripheral blood cell purification
Peripheral blood mononuclear cells (PBMCs) and granulocytes were isolated from whole blood by density gradient separation (Ficoll-Paque PLUS; GE Healthcare, Uppsala, Sweden). After red blood cell lysis using ice-cold ddH2O and 4X PBS, granulocytes were resuspended in eosinophil purification buffer (1X PBS, 1 mM EDTA, 0.5% endotoxin free BSA). Eosinophils were purified from the granulocyte layer by magnetic bead selection on an AutoMACS (Miltenyi Biotech, Cambridge, MA) using the Eosinophil Purification Kit (Miltenyi Biotech). Eosinophil purity was >99% for all HES samples and ≥95% for healthy control samples as determined by counting of a minimum of 300 cells on cytospin slides stained with Diff-Quik (Siemens Healthcare Diagnostics, Malvern, PA).
2.4 |. Quantitative RT-PCR analysis of gene expression
RNA was isolated using Trizol Reagent (ThermoFisher, Cat. No. 15596018), and cDNA (1 μg) was synthesized with the High Capacity cDNA Reverse Transcription Kit per manufacturer protocol (Applied Biosystems). Quantitative real-time PCR was performed in triplicate (10 μL) with TaqMan Fast Universal PCR Master Mix (Applied Biosystems) and the following custom primers and probe sets (TaqMan Gene Expression Assays; Applied Biosytems): GR-α Forward (5’ATTCTATGCATGAAGTGGTTGAAAAT3’, Reverse: 5’TTCCCCGAGATGTTA GCT GAAA3’, Probe: 5’CTATTGCTT CCAAACAT 3’), GR-β (Forward: 5’CCATTGT CAAGAGGGAAG GAAAC 3’, Reverse: 5’ GATTCTATGCATGAAAATGTTATGTG G 3’, Probe: 5’AGCCAGAACTGGCAGC 3’), GR-γ Forward: (5’TTCAAAAGAGCAGT GGAAGGTA 3’, Reverse: 5’GGTAGGGGTGAGTTGTGG TAACG 3’, Probe:5’CAC AATTACCTATGTGCTGGAAGGAATGATTGC 3’) [16] and GR-P Forward (5’ GCTG TGTTTTGCTCCTGATCTGA 3’, Reverse: 5’TGACATAAGGTGAAAAGG TGTTCTACC 3’, Probe: 5’ATGAGCAGAGAATGACTCTACCCTGCATGTACG 3’) [16]. mRNA levels are expressed in arbitrary units as 1/ΔCt normalized to 18S rRNA (mean ± SE)
2.5 |. Analysis of serum cytokine levels
Levels of IL-4, −5, −6, −8, −13, −10, −17A, and IFN-γ were quantified in serum from HES patients and normal donors using a suspension array assay in multiplex kit (Millipore, Billerica, MA) according to the manufacturer’s protocol. Samples were tested in duplicate. Lower limits of detection were 1.1 pg/mL (IL-4), 0.5 pg/mL (IL-5), 2.7 pg/mL (IL-6), 3.2 pg/mL (IL-8, IL10 and IFN-γ), 1.3 pg/mL (IL-13), and 2.4 pg/mL (IL-17A).
2.6 |. Statistical analysis
Nonparametric Mann-Whitney U test, Wilcoxon signed rank test and Fisher’s exact test were used for comparisons of group means, paired samples and proportions, respectively. p<0.05 was considered statistically significant for all analyses.
RESULTS
3.1 |. Impaired GR response is not a global phenomenon
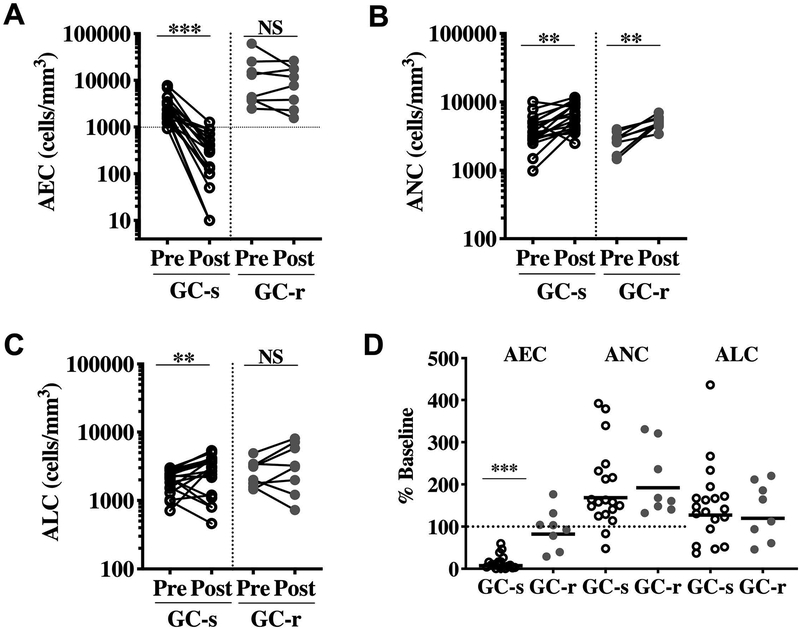
Overall, GC-r patients had significantly higher peak AEC and were more likely to have the myeloid variant of HES but were similar in age and gender to GC-s patients (Table 1). To determine whether GC resistance in HES is restricted to the eosinophil lineage, absolute eosinophil (AEC), lymphocyte (ALC) and neutrophil (ANC) counts were assessed in 27 patients with HES before and 1 week after initiation of GC monotherapy. In addition to data from the 26 subjects enrolled on the prospective study of GC-response, data from one subject treated according to the same guidelines, but prior to initiation of the prospective study, is included. Clinical and demographic information for this cohort of patients is provided in Supplemental Table 1.
Table 1.
Clinical and demographic characteristics of the study subjects
| Parameter | Healthy Volunteer (n=19) | GC-sensitive HES (n=33) | GC-resistant HES (n=16) | p-value |
|---|---|---|---|---|
| Median Age in years (range) | 39 (23–71) | 50 (21–75) | 45 (16–65) | NS* |
| Gender (M/F) | 12/7 | 15/18 | 6/10 | NS** |
| HES Clinical Subtype | ||||
| MHES | NA | 0 | 4 (25%) | 0.01** |
| LHES | NA | 6 (18%) | 4 (25%) | NS** |
| IHES | NA | 17 (52%) | 7 (44%) | NS** |
| OVERLAP | NA | 8 (24%) | 1 (6%) | NS** |
| OTHER | NA | 2 (6%) | 0 | NS** |
| Geo Mean Peak AEC in cells/mm3 (range) | 144 (50–427) | 5772 (1520–21756) | 16247 (1620–100000) | <0.01* |
GC-sensitive vs. GC-resistant; Mann-Whitney U test;
GC-sensitive vs. GC-resistant; Fisher’s exact test
Prior to the administration of oral prednisone, baseline geometric mean (GM) AEC, but not ANC or ALC, was significantly increased in GC-r as compared to GC-s patients (9120 vs. 2401, p<0.001; Supplemental Figure 2). The AEC declined in all 19 GC-s patients at day 7 following the initiation of prednisone (from GM 2401 to 178/mm3; p<0.0001; Figure 1A). Although AEC also decreased in 3/7 GC-r patients, all levels remained >1000/mm3 and GM AEC was comparable before and after 7 days of GC treatment (9120 vs. 7494/mm3; p=NS). Despite the differences in AEC response to GC in the two groups, an expected increase in ANC was observed in 17/19 GC-s and all 7 GC-r patients from GM 3610 to 6087/mm3 (p<0.001) and 2634 to 5064/mm3 (p<0.01), respectively (Figure 1B). ALC also rose above baseline levels in 14/19 GC-s and 5/8 GC-r patients (p=NS, Fisher’s exact test; Figure 1C). Whereas GM % baseline AEC was significantly lower in GC-s as compared to GC-r patients at day 7 (7% vs. 82%), GM % baseline ANC and ALC were similar between the two groups (169% vs. 192% for ANC and 127% vs. 120% for ALC; Figure 1D).
Figure 1. Cellular response to GC in HES.
Untreated subjects with HES were given a single dose of prednisone (1mg/kg) followed by prednisone (30 mg) daily for one week. (A-C) AEC, ANC, and ALC prior to and at 1 week following initiation of prednisone (D) Percent of baseline absolute cell counts at 1 week following initiation of prednisone. Symbols represent individual subject data (GC-sensitive (s; n=19; open black circles) and GC-resistant (r; n=8; closed gray circles). Solid horizontal lines indicate the GMs. The dotted horizontal line in panel D indicates 100% of baseline (no change). *p < 0.05, **p < 0.001, *** p < 0.0001
3.2 |. GR isoforms associated with resistance are not increased in GC-resistant subjects
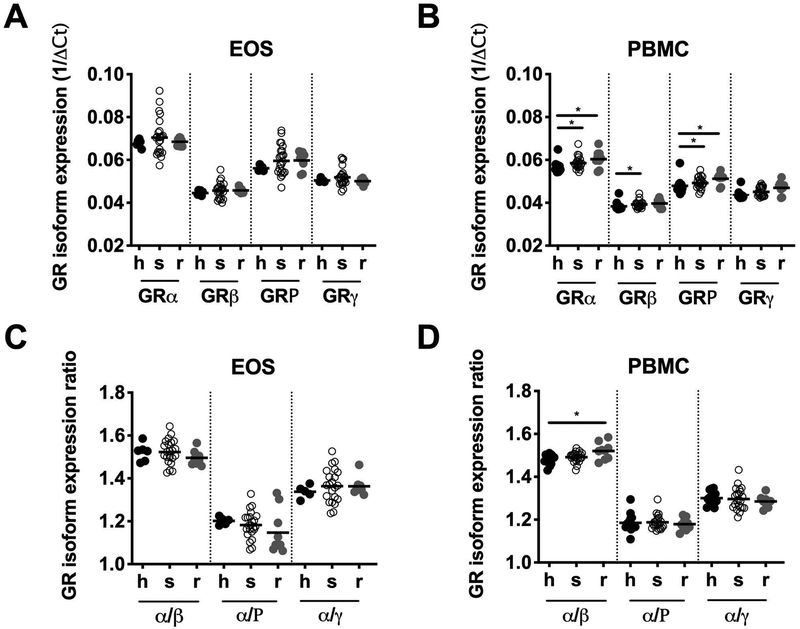
To assess the relationship between glucocorticoid receptor (GR) isoform expression and GC resistance in HES, RNA was extracted from purified eosinophils and PBMC from GC-s (n=22) and GC-r (n=9) HES patients and healthy controls (n=6 and n=14 for eosinophils and PBMCs, respectively). Eosinophils expressed more GR mRNA than PBMC (p<0.001 for all 4 splice variants tested; Supplemental Figure 3). However, there were no differences in eosinophil mRNA expression of any of the GR isoforms or in the GR α/β, α /P or α/γ isoform ratios between patients with GC-s HES, patients with GC-r HES and healthy controls (Figure 2). Although PBMC mRNA expression of GRα, GRβ, and GRP were slightly increased in the GC-r HES subjects compared to healthy controls, no significant differences were noted between the GC-s and GC-r patients. Similarly, the geometric mean GRα/β expression ratio was slightly increased in the PBMCs of GC-resistant subjects (GM 1.52 to 1.48 vs. GC-s, p < 0.01 and 1.52 to 1.49 vs. healthy controls, p < 0.06).
Figure 2. Alternations in mRNA expression of GR splice variants do not explain GC resistance in HES.
(A, B) mRNA expression of GRα, GRβ, GRP and GRγ isoforms in purified eosinophils or PBMC expressed as 1/ΔCt using 18S as a control (C, D) the ratio of GRβ, GRP and GRγ isoform mRNA expression to GRα mRNA expression in purified eosinophils or PBMC. Symbols represent the values from individual healthy controls (h; closed black circles); GC-sensitive patients with HES (s; open black circles) and GC-resistant patients with HES (r; closed gray circles). The horizontal lines denote the geometric means for each group. *p < 0.05
3.3 |. Serum IL-5 levels are markedly increased in a subset of GC-r patients with HES
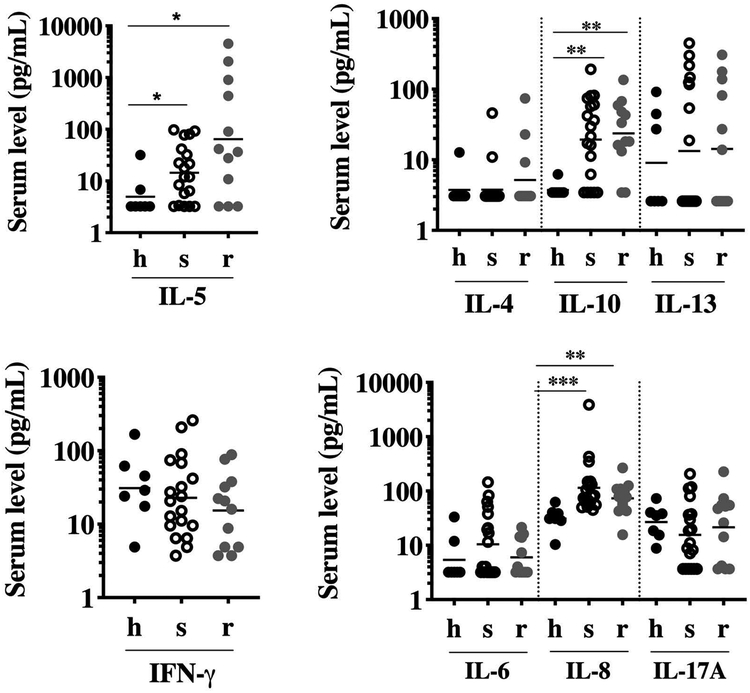
A panel of 8 cytokines previously demonstrated to be associated with GC response in vitro or in vivo were assessed in serum from 31 patients with HES prior to initiation of GC therapy and in 7 healthy controls (Figure 3). GM serum levels of IL-5, IL-8, and IL-10 were increased in patients with HES compared to healthy controls. Although GM levels of all 3 cytokines were comparable between GC-s and GC-r patients with HES, 4/12 GC-r patients had markedly elevated serum IL-5 levels (>100 pg/mL). GM serum IFN-γ, IL-4, IL-6, IL-13 and IL17A levels were comparable between the 3 groups.
Figure 3. Baseline serum cytokine levels in GC-sensitive and GC-resistant subjects.
Symbols represent the values from individual healthy controls (h; closed black circles); GC-sensitive patients with HES (s; open black circles) and GC-resistant patients with HES (r; closed gray circles). The horizontal lines denote the geometric means for each group. *p<0.05, **p<0.01, ***p<0.0001
DISCUSSION
Although GC remain the cornerstone of treatment for patients with PDGFRA-negative HES, many patients require moderate to high doses for control of eosinophilia and 9–18% of patients fail to respond even to high doses depending on the series. Despite this, little is known about the mechanism of GC resistance in HES. In fact, the only study examining this issue to date was published in 1989 and demonstrated the absence of 3H-dexamethasone binding to eosinophils in 7/16 patients with HES, 3 of whom failed to respond to prednisone at a dose of 1 mg/kg daily for 5 days [17]. Moreover, normal levels of 3H-dexamethasone binding were demonstrated in other cell lineages (neutrophils and/or lymphocytes) in 3 subjects. These findings are consistent with the results from the present study, in which normal neutrophil and lymphocyte responses to GC challenge were observed in vivo in the setting of persistent eosinophilia irrespective of clinical subtype. This suggests that GC resistance in HES is either due to abnormalities of GR expression or function in eosinophils or that it involves secondary mechanisms that uniquely affect eosinophils.
Decreased expression of GRα and/or increased expression of the other isoforms on specific cell types have been implicated in GC resistance in a wide variety of disorders, including asthma [12, 13, 18], atopic dermatitis [19] and hematologic malignancies [5, 16, 20, 21]. In the present study, mRNA expression of all 4 GC isoforms was detectable in eosinophils and PBMC from patients with HES. Although protein expression was not assessed, prior studies have demonstrated a close correlation between mRNA and protein expression of GRα [20]. As previously reported for GRα and GRβ [22], expression of all 4 isoforms was greater in eosinophils than in PBMC. More importantly, expression patterns were similar in eosinophils and PBMC from GC-r and GC-s patients with HES, suggesting that modulation of isoform expression does not play a major role in resistance to GC in HES.
Dysregulated expression of a variety of Th2 and inflammatory cytokines, including IL-4, IL-5, IL17A and IFN-γ, has been linked to steroid resistance in asthma [9, 23, 24]. Of these, IL-5 is unique in having effects that are almost completely restricted to the eosinophil lineage, including the promotion of eosinophil development, activation and survival. Moreover, IL-5 at 0.1–1 ng/mL, but not lower levels, has been shown to completely inhibit GC-mediated eosinophil apoptosis in vitro [14, 25, 26]. Although the mechanism by which cytokines inhibit GC-mediated eosinophil apoptosis is incompletely understood, recent data suggest that resistance to GC in the setting of high IL-5 levels may be due to upregulation of Nuclear Factor Interleukin-3 (NFIL3)[14]. Consistent with previously published data [27], serum IL-5 levels were increased prior to GC treatment in HES subjects in the current study compared to healthy volunteers. Although IL-5 levels were not significantly different between GC-s and GC-r patients with HES, 4/11 GC-r patients had serum IL-5 levels above the range known to inhibit GC-induced eosinophil apoptosis [25]. Serum IL-8 and IL-10 levels were also significantly elevated in HES patients compared to healthy controls. This has been reported previously[28–30] and is of unknown clinical significance, However, since neither the IL-8 nor the IL-10 receptor has been described on eosinophils, it is unlikely that the elevated serum levels of these two cytokines would have a direct effect on eosinophil survival or response to GC. Moreover, both of these cytokines are produced by eosinophils[31, 32], which may explain, at least in part, the increased serum levels in patients with HES.
Four of the patients in the GC-r group (none of whom had serum IL-5 levels >100 pg/mL) had clinical features consistent with MHES, a primary myeloid form of hypereosinophilia [1]. Although potential genetic drivers of the eosinophilia could not be identified in 3 of the 4 patients, one patient has a novel exon 13 mutation in JAK2 and 2 of the patients responded to imatinib, suggesting that they have undetected mutations in a tyrosine kinase. Patients with the most common type of MHES, FIP1L1-PDGFRA-associated myeloid neoplasm, are typically GC-r [3], but were excluded from the current study. Of note, these patients have been reported to have normal serum IL-5 levels (in contrast to patients with other non-myeloid forms of HES)[33]. Recent data suggests that activation of Lyn by FIP1L1-PDGFRA leads to increased phosphorylation of IL-5 receptor α promoting eosinophilopoiesis, activation and resistance to apoptosis [34]. This could explain GC resistance in this group of patients. Moreover, a similar mechanism could be at play in promoting eosinophilia and GC resistance in other forms of MHES.
Major limitations of the present study included the low number of GC-r patients and the retrospective study design. The relative lack of GC-r patients was due in part to the strict definition of GC-resistance selected to maximize the likelihood of identifying abnormalities and the exclusion of patients with FIP1L1-PDGFRA-associated disease, for whom GC is not first line therapy due to the efficacy of imatinib. With respect to the study design, although cell counts in the setting of GC challenge were collected prospectively as part of an ongoing clinical trial, the use of stored samples for the remaining analyses precluded analysis of GC receptor surface expression and function on eosinophils due to the inability to viably freeze eosinophils. Similarly, the role of post-translational modifications, including phosphorylation, ubiquitination, acetylation and sumoylation, all of which have been implicated in GC receptor stability and function[35], were not able to be assessed in this study.
In summary, GC resistance in HES appears to be restricted to the eosinophil lineage, irrespective of clinical subtype. Although multiple mechanisms could explain this finding, our data suggest that increased serum IL-5 may lead to GC-resistance in some patients with non-myeloid HES by impairing GC-induced eosinophil apoptosis and that the mechanism of GC-resistance in patients with MHES may be different from that in other patients with HES. Prospective studies of GC resistance in HES and further characterization of GR function and signaling pathways in eosinophils are clearly needed to better understand (and eventually circumvent) GC resistance in HES.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the clinical staff of the Laboratory of Parasitic Diseases for their assistance with patient care and sample collection and Kevin Holmes and David Stephany for assistance with flow cytometry. This study was funded by the Division of Intramural Research, NIAID, NIH. The authors declare no conflicting financial interests.
Declaration of all sources of funding: This research was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH (ZIA AI001130). The data and samples were collected under clinical protocols designed to study eosinophilic disorders (, ) or provide healthy donor samples for research (). The data that support the findings of this study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Klion AD (2015) How I treat hypereosinophilic syndromes. Blood 126:1069–1077. doi: 10.1182/blood-2014-11-551614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogbogu PU, Bochner BS, Butterfield JH, et al. (2009) Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol 124:1319–25.e3. doi: 10.1016/j.jaci.2009.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khoury P, Abiodun AO, Holland-Thomas N, et al. (2018) Hypereosinophilic syndrome subtype predicts responsiveness to glucocorticoids. J Allergy Clin Immunol Pract 6:190–195. doi: 10.1016/j.jaip.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koper JW, van Rossum EFC, van den Akker ELT (2014) Glucocorticoid receptor polymorphisms and haplotypes and their expression in health and disease. Steroids 92:62–73. doi: 10.1016/j.steroids.2014.07.015 [DOI] [PubMed] [Google Scholar]
- 5.Ramamoorthy S, Cidlowski JA (2016) Corticosteroids: mechanisms of action in health and disease. Rheum Dis Clin North Am 42:15–31, vii. doi: 10.1016/j.rdc.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milgrom H, Bender B, Ackerson L, et al. (1996) Noncompliance and treatment failure in children with asthma. J Allergy Clin Immunol 98:1051–1057. [DOI] [PubMed] [Google Scholar]
- 7.Kassi E, Nasiri-Ansari N, Papavassiliou AG (2017) Vitamin D affects glucocorticoid action in target cells. Oncotarget 8:7220–7221. doi: 10.18632/oncotarget.13997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webster JC, Oakley RH, Jewell CM, Cidlowski JA (2001) Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance. Proc Natl Acad Sci USA 98:6865–6870. doi: 10.1073/pnas.121455098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pazdrak K, Straub C, Maroto R, et al. (2016) Cytokine-Induced Glucocorticoid Resistance from Eosinophil Activation: Protein Phosphatase 5 Modulation of Glucocorticoid Receptor Phosphorylation and Signaling. J Immunol 197:3782–3791. doi: 10.4049/jimmunol.1601029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brode S, Farahi N, Cowburn AS, et al. (2010) Interleukin-5 inhibits glucocorticoid-mediated apoptosis in human eosinophils. Thorax 65:1116–1117. doi: 10.1136/thx.2009.124909 [DOI] [PubMed] [Google Scholar]
- 11.Molnár Á, Patócs A, Likó I, et al. (2018) An unexpected, mild phenotype of glucocorticoid resistance associated with glucocorticoid receptor gene mutation case report and review of the literature. BMC Med Genet 19:37. doi: 10.1186/s12881-018-0552-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung DY, Hamid Q, Vottero A, et al. (1997) Association of glucocorticoid insensitivity with increased expression of glucocorticoid receptor beta. J Exp Med 186:1567–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goleva E, Li L-B, Eves PT, et al. (2006) Increased glucocorticoid receptor beta alters steroid response in glucocorticoid-insensitive asthma. Am J Respir Crit Care Med 173:607–616. doi: 10.1164/rccm.200507-1046OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pazdrak K, Moon Y, Straub C, et al. (2016) Eosinophil resistance to glucocorticoid-induced apoptosis is mediated by the transcription factor NFIL3. Apoptosis 21:421–431. doi: 10.1007/s10495-016-1226-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gruver-Yates AL, Cidlowski JA (2013) Tissue-specific actions of glucocorticoids on apoptosis: a double-edged sword. Cells 2:202–223. doi: 10.3390/cells2020202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koga Y, Matsuzaki A, Suminoe A, et al. (2005) Differential mRNA expression of glucocorticoid receptor alpha and beta is associated with glucocorticoid sensitivity of acute lymphoblastic leukemia in children. Pediatr Blood Cancer 45:121–127. doi: 10.1002/pbc.20308 [DOI] [PubMed] [Google Scholar]
- 17.PRIN L, LEFEBVRE P, GRUART V, et al. (1989) Heterogeneity of human eosinophil glucocorticoid receptor expression in hypereosinophilic patients: absence of detectable receptor correlates with resistance to corticotherapy. Clin Exp Immunol 78:383–389. [PMC free article] [PubMed] [Google Scholar]
- 18.Sousa AR, Lane SJ, Cidlowski JA, et al. (2000) Glucocorticoid resistance in asthma is associated with elevated in vivo expression of the glucocorticoid receptor beta-isoform. J Allergy Clin Immunol 105:943–950. doi: 10.1067/mai.2000.106486 [DOI] [PubMed] [Google Scholar]
- 19.Hägg PM, Hurskainen T, Palatsi R, et al. (2010) Increased expression of glucocorticoid receptor beta in lymphocytes of patients with severe atopic dermatitis unresponsive to topical corticosteroid. Br J Dermatol 162:318–324. doi: 10.1111/j.1365-2133.2009.09518.x [DOI] [PubMed] [Google Scholar]
- 20.Haarman EG, Kaspers GJL, Pieters R, et al. (2004) Glucocorticoid receptor alpha, beta and gamma expression vs in vitro glucocorticod resistance in childhood leukemia. Leukemia 18:530–537. doi: 10.1038/sj.leu.2403225 [DOI] [PubMed] [Google Scholar]
- 21.Tissing WJE, Lauten M, Meijerink JPP, et al. (2005) Expression of the glucocorticoid receptor and its isoforms in relation to glucocorticoid resistance in childhood acute lymphocytic leukemia. Haematologica 90:1279–1281. [PubMed] [Google Scholar]
- 22.Pujols L, Mullol J, Roca-Ferrer J, et al. (2002) Expression of glucocorticoid receptor alpha- and beta-isoforms in human cells and tissues. Am J Physiol Cell Physiol 283:C1324–31. doi: 10.1152/ajpcell.00363.2001 [DOI] [PubMed] [Google Scholar]
- 23.Leung DY, Martin RJ, Szefler SJ, et al. (1995) Dysregulation of interleukin 4, interleukin 5, and interferon gamma gene expression in steroid-resistant asthma. J Exp Med 181:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morishima Y, Ano S, Ishii Y, et al. (2013) Th17-associated cytokines as a therapeutic target for steroid-insensitive asthma. Clin Dev Immunol 2013:609395. doi: 10.1155/2013/609395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallen N, Kita H, Weiler D, Gleich GJ (1991) Glucocorticoids inhibit cytokine-mediated eosinophil survival. J Immunol 147:3490–3495. [PubMed] [Google Scholar]
- 26.Bloom JW, Chacko J, Lohman IC, et al. (2004) Differential control of eosinophil survival by glucocorticoids. Apoptosis 9:97–104. doi: 10.1023/B:APPT.0000012126.06126.c4 [DOI] [PubMed] [Google Scholar]
- 27.Kuang FL, Fay MP, Ware J, et al. (2018) Long-Term Clinical Outcomes of High-Dose Mepolizumab Treatment for Hypereosinophilic Syndrome. J Allergy Clin Immunol Pract 6:1518–1527.e5. doi: 10.1016/j.jaip.2018.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khoury P, Zagallo P, Talar-Williams C, et al. (2012) Serum biomarkers are similar in Churg-Strauss syndrome and hypereosinophilic syndrome. Allergy 67:1149–1156. doi: 10.1111/j.1398-9995.2012.02873.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y-YK, Khoury P, Ware JM, et al. (2014) Marked and persistent eosinophilia in the absence of clinical manifestations. J Allergy Clin Immunol 133:1195–1202. doi: 10.1016/j.jaci.2013.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanbe N, Kurosawa M, Igarashi N, et al. (1998) Idiopathic hypereosinophilic syndrome associated with elevated plasma levels of interleukin-10 and soluble interleukin-2 receptor. Br J Dermatol 139:916–918. doi: 10.1046/j.1365-2133.1998.02525.x [DOI] [PubMed] [Google Scholar]
- 31.Nakajima H, Gleich GJ, Kita H (1996) Constitutive production of IL-4 and IL-10 and stimulated production of IL-8 by normal peripheral blood eosinophils. J Immunol 156:4859–4866. [PubMed] [Google Scholar]
- 32.Kobayashi T, Kouzaki H, Kita H (2010) Human eosinophils recognize endogenous danger signal crystalline uric acid and produce proinflammatory cytokines mediated by autocrine ATP. J Immunol 184:6350–6358. doi: 10.4049/jimmunol.0902673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helbig G, Moskwa A, Hus M, et al. (2010) Clinical characteristics of patients with chronic eosinophilic leukaemia (CEL) harbouring FIP1L1-PDGFRA fusion transcript--results of Polish multicentre study. Hematol Oncol 28:93–97. doi: 10.1002/hon.919 [DOI] [PubMed] [Google Scholar]
- 34.Li B, Zhang G, Li C, et al. (2016) Lyn mediates FIP1L1-PDGFRA signal pathway facilitating IL- 5RA intracellular signal through FIP1L1-PDGFRA/JAK2/Lyn/Akt network complex in CEL. Oncotarget 5:64984–64998. doi: 10.18632/oncotarget.11401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oakley RH, Cidlowski JA (2011) Cellular processing of the glucocorticoid receptor gene and protein: new mechanisms for generating tissue-specific actions of glucocorticoids. J Biol Chem 286:3177–3184. doi: 10.1074/jbc.R110.179325 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.