Abstract
Hypothesis:
Local administration of the calcium-channel blocker (CCB), diltiazem, via intratympanic (IT) chitosan-glycerophosphate (CGP) hydrogel will protect against cisplatin-induced ototoxicity.
Background:
Cisplatin induces calcium-mediated apoptosis of cochlear outer hair cells (OHCs). Previous work demonstrated otoprotection and reduced auditory brainstem response (ABR) threshold shifts in a cisplatin-induced ototoxicity mouse model treated with multiple doses of IT diltiazem given in solution. Here, we evaluated the role of a single dose of IT CGP-diltiazem as a novel otoprotectant against cisplatin-induced ototoxicity.
Methods:
Baseline pure-tone and click-evoked ABRs were performed in control (IT CGP-saline, n=13) and treatment (IT CGP-diltiazem 2mg/kg, n=9) groups of female CBA/J mice. A single dose of IT CGP hydrogel was administered just prior to intraperitoneal injection of cisplatin (14mg/kg). On Day 7 post-treatment, ABRs were performed and cochleae were harvested. Hair cells were quantified using anti-myosin VIIa immunostaining and inner hair cell ribbon synapses were quantified using Ctbp2 immunostaining.
Results:
There was a statistically significant effect of treatment on click- and tone-evoked ABRs between groups. The mean threshold shifts were significantly reduced in both click- and tone-evoked ABRs on Day 7 in IT CGP-diltiazem treated mice compared to CGP-saline control mice. There were no significant differences in OHC counting between groups, but there appears to be an otoprotection against loss of synapses in the apical turn from IT CGP-diltiazem treated mice (p<0.05).
Conclusions:
This preliminary work suggests that IT CGP-diltiazem reduces ABR threshold shifts with possible mechanisms of protecting ribbon synapses in the setting of cisplatin-induced ototoxicity. More work is necessary to determine the mechanism underlying this otoprotection.
Introduction:
Cisplatin is a well-known systemic medication used to treat head and neck, ovarian, and other solid tumors. A dose-limiting side effect of cisplatin is permanent sensorineural hearing loss (SNHL) in some subjects mediated by cochlear damage, with outer hair cells (OHCs) in the basal turn most affected.1 Cochlear damage occurs via apoptosis,2,3 which is a complex and dynamic process that requires the influx of calcium as an essential co-factor.4,5 Currently, there are limited therapeutic options to prevent cisplatin-induced ototoxicity and no therapies are available to stimulate regeneration of lost OHCs in the mature cochlea. While many newer systemic agents with the potential to neutralize the free radical production induced by cisplatin have shown promise as otoprotective agents,6 there is concern that administration of systemic agents will neutralize the chemotherapeutic effects of cisplatin. One approach to efficiently evaluate the wide range of potential otoprotective agents is to consider repurposing FDA-approved drugs for newer indications.7 With this in mind, we investigated a potential role for intratympanic (IT) administration of the calcium-channel blocker (CCB), diltiazem, as an otoprotective agent against cisplatin-induced ototoxicity.8
Previous in vitro work demonstrated that the CCB, nifedipine, can prevent calcium influx in cochlear hair cells in the setting of oxidative stress.9 Similarly, in vitro use of the CCB, flunarizine, demonstrated protective effects after cisplatin treatment through reduction in lipid peroxidation and mitochondrial permeability.10 There has been limited in vivo work performed with CCBs, but IT diltiazem has been shown to reduce auditory brainstem response (ABR) threshold shifts in mice and guinea pig models.11,12 In these models, multiple IT injections were given on consecutive days to administer diltiazem in solution, however, morphological analysis of the cochlea was not performed. Thus, more research is necessary to understand the potential otoprotective effects and to offer a more practical technique for the application of diltiazem.
A shortcoming of IT administration of medications in solution is that the solution is likely to diffuse out of the Eustachian tube quickly and diffusion into the inner ear can be unpredictable.13,14 Recently, chitosan-glycerophosphate (CGP) hydrogel has been described as a vehicle for drug delivery that can provide controlled, sustained delivery of drugs into the inner ear.15,16 Compared to repeat IT administration of diltiazem in solution, CGP-hydrogel administration offers the advantage of a single injection. Previously it was used for direct administration over the round window membrane (RWM).16 While this offers a more direct approach, it requires a bullostomy, which is an invasive procedure. The ideal translational approach to inner ear drug delivery would be to deliver sustained-release therapies via an IT approach.
Here, we evaluated the otoprotective effects of IT diltiazem against cisplatin-induced ototoxicity delivered via a novel CGP-diltiazem hydrogel and given as a single injection. We hypothesized that IT CGP-diltiazem will prevent cisplatin-induced ototoxicity by protecting OHCs and synapses. The primary aim of this work was to correlate cochlear morphology with ABR thresholds after IT CGP-diltiazem in the setting of cisplatin ototoxicity, and the secondary aim was to determine the feasibility of IT administration of CGP-based hydrogel in a mouse model.
Materials and Methods:
This study was approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania (Protocol # 804352).
Hydrogel Preparation
The CGP-hydrogel was freshly prepared as previously described.17 Briefly, a 2% w/v solution of ultra-pure chitosan (DDA 91.7%; Biosyntech, Quebec, Canada) in 0.1M HCl was prepared by stirring at room temperature. The solution was kept at 4°C until use. To obtain a thermosensitive hydrogel, a 80% w/v water soluble glycero-2-phosphate (EMD Millipore, Billerica, MA) solution was added dropwise while stirring by hand until the pH of the solution reached 7.2 ± 0.1. The crosslinked hydrogel obtained was a highly viscous thermosensitive CGP-hydrogel, and it was kept on ice until use within 2 hours of preparation.
After preparation of the CGP-hydrogel, a 1:1 ratio of saline (control) or diltiazem (Sigma-Aldrich, St. Louis, MO) (20 mg/cc) (treatment) solution was added to the hydrogel and mixed vigorously. Assuming the weight of a mouse is roughly 25g at 4 weeks of age and the volume of the middle ear roughly 5 μl, this concentration of diltiazem, when mixed with CGP-hydrogel offers a final CGP-diltiazem concentration of 2mg/kg as previously described.11
Animal Subjects:
Thirty, 4-week-old female CBA/J mice from The Jackson Laboratories (Bar Harbor, ME), were used. The mice were weighed prior to any procedure. They were kept in standard housing with free access to food and water. Each day procedures took place, they were observed closely for any signs of weight loss, isolation, poor social interaction, and general distress.
Injections/Anesthesia:
On the day of IT and cisplatin injections, anesthesia was achieved by intraperitoneal (IP) injection of a ketamine/xylazine (Sigma-Aldrich, St. Louis, MO) (100mg/kg:10mg/kg) cocktail. With the mouse adequately anesthetized, the left tympanic membrane (TM) was visualized, and a 22-gauge needle connected to a microsyringe was placed into the middle ear through the TM. A minimum of 5 μl of CGP-saline or CGP-diltiazem was delivered into the middle ear until it was visualized filling the middle ear space. Previous work demonstrated that compounds injected IT can diffuse to the contralateral ear through the cochlear aqueduct.18 Therefore, only the injected ear was evaluated and separate animals were used for injection of CGP-saline and CGP-diltiazem. Immediately after the IT injection, mice were given an IP injection of cisplatin (Sigma-Aldrich, St. Louis, MO), 14 mg/kg, followed by a 1 ml bolus of saline to ensure hydration. After the injections, the mouse remained under anesthesia with the treated ear facing up for at least 15 minutes.
Auditory Testing
ABRs were recorded using a Tucker Davis System II (Tucker-Davis Technologies, Alachua, FL). The left, injected ear was evaluated in all mice. Click-and tone-evoked ABRs were recorded at Baseline and Day 7 post-cisplatin. Mice were anesthetized with ketamine/xylazine (100mg/kg:10mg/kg) cocktail, and subcutaneous electrodes were placed at the vertex (active electrode), near the left postauricular bulla (reference electrode), and in the right postauricular bulla (ground electrode). The left ear was placed 10cm from an open field speaker. The acoustic stimulus, generated by the TDT SigGen system, consisted of 10 millisecond tone pips at 4, 8, 16, 24, and 32 kHz, presented at a rate of 20/second in the tone-evoked ABRs, while click-evoked responses were measured in response to alternating polarity clicks of 100 microseconds. Responses were averaged over 500 stimuli and intensity increments were attenuated in 5dB (sound pressure level (SPL)) steps. Threshold was determined as the lowest intensity at which an observable response could be detected in wave I. Threshold shifts were calculated for each mouse comparing their Baseline and Day 7 post-cisplatin ABR.
Cochlear Histology
Following Day 7 post-cisplatin ABR, temporal bones (TBs) were collected and post-fixed for 24 hours in 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO) diluted in 10 mM PBS at room temperature and then stored in 10 mM PBS at 4⁰C. TBs were then decalcified for ~48 hours in 120 mM EDTA (Sigma-Aldrich, St. Louis, MO) diluted in 10 mM PBS, with the EDTA changed daily. Whole mount dissection and immunostaining was performed as previously described.19 Rabbit anti-myosin VIIa primary antibody (1:200; cat. #25-6790; Proteus Biosciences, Ramona, CA) was used to label hair cells and mouse anti-Ctbp2 primary antibody (1:500, cat #BDB612044, BD Biosciences) was used to label inner hair cell (IHC) ribbon synapses. Alexa-conjugated secondary antibodies (Thermo Fisher Scientific, Hampton, NH) were used at 1:1000 dilution and nuclei were counterstained with Hoechst 33342 (1:2,000; Thermo Fisher Scientific, Hampton, NH). Individual cochlear turns were mounted in Prolong™ Gold antifade reagent (cat #P36930; Thermo Fisher Scientific, Hampton, NH). Samples were imaged using a Zeiss LSM800 (Oberkochen, Germany) confocal microscope. Hair cells were quantified by a blinded researcher from two representative regions (150 μm) of each cochlear turn. IHC ribbon synapses were quantified by a blinded researcher in 10 IHCs from each cochlear turn.
Statistical Analysis
Using previous protocols and a method described by Berndtson as a guide,20 we determined that a minimum of 8 animals was needed to detect differences between study groups in our animal model. Normality of distributions for click-and tone-ABRs was tested using Shapiro-Wilk test. Statistical comparisons of ABR threshold shift between each group were performed. For click evoked ABRs, one-way ANOVA was performed. For tone-evoked ABRs, two-way ANOVA tests were performed followed by Tukey post-hoc testing. ABR statistics were performed using SPSS (Chicago, IL). Hair cell and synapse counts were compared between groups and cochlear turns with a two-way ANOVA followed by a Sidak’s post-hoc test using GraphPad Prism v6 (San Diego, CA). A p-value of less than or equal to 0.05 was considered statistically significant.
Results:
Mice were randomly assigned to either the CGP-saline or CGP-diltiazem group and Baseline ABRs were performed. Following the Baseline ABRs, mice received IT administration of either CGP-saline or CGP-diltiazem (2mg/kg), immediately followed by IP injection of cisplatin (14mg/kg). A post-treatment ABR was performed 7 days after treatment and cochleae were harvested for histology.
There were 15 mice assigned to each group. Two mice in the control, CGP-saline group died from the combined effects of cisplatin and the procedure. In the CGP-diltiazem group, four mice died from the effects of cisplatin, and one mouse was euthanized due to weight loss > 30% of its original body weight. Only one mouse in the CGP-diltiazem group was eliminated due to a technical problem in administering CGP-diltiazem into the middle ear. Thus, 9 CGP-diltiazem and 13 CGP-saline mice were included in the ABR study. During TB collection, the cochlea from one mouse in the control, CGP-saline group was damaged, therefore only 12 samples were included in this group for the hair cell and synapse quantifications. Single-dose models of the 14mg/kg dose of cisplatin have previously generated a 20% mortality rate, thus mortality of 7 out of 30 mice falls within this expected rate.21
ABRs Thresholds
ABR threshold shifts were calculated as the difference between Baseline and Day 7 post-cisplatin ABR thresholds for each individual mouse. The mean threshold shift for click-evoked ABRs was 16.5 dB SPL (± 1.00 dB, standard error of the mean (SEM)) in CGP-saline mice compared to 7.7 dB SPL (± 1.08dB) in CGP-diltiazem treated mice (p<0.001). The mean ABR threshold shift in tone-evoked ABRs was significantly reduced at all frequencies tested in CGP-diltiazem treated mice compared to CGP-saline mice. At 4, 8, and 16 kHz, the CGP-saline mice had threshold shifts of 9.6 dB SPL(± 1.84 dB), 17.3 dB SPL(± 1.93 dB), and 16.5 dB SPL(± 1.67 dB), respectively compared to CGP-diltiazem mice with shifts of 0.56 dB SPL(± 2.29 dB), 3.89 dB SPL(± 1.24 dB), and 7.22 dB SPL(± 1.31 dB), respectively (Figure 1; p=0.009, p<0.001, and p=0.001, respectively). The largest reduction in threshold shifts was at 24 and 32 kHz, where CGP-saline mice had a shift of 28.0 dB SPL(± 4.97 dB) and 31.9 dB SPL(± 4.82 dB), compared to CGP-diltiazem mice with shifts of 7.78 dB SPL(± 2.2 dB) and 10 dB SPL(± 3.8 dB) (Figure 1; p=0.006 and 0.005, respectively).
Figure 1:
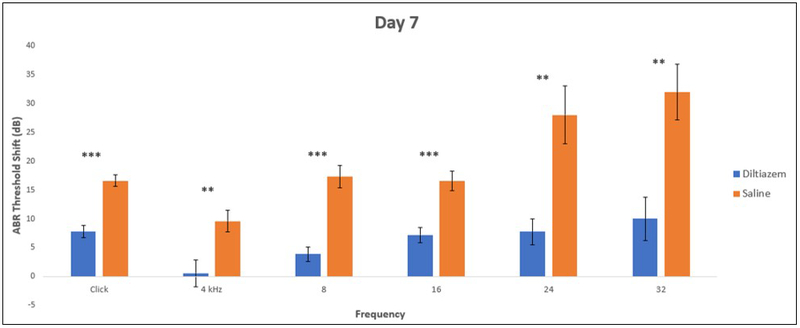
Threshold shifts between baseline and Day 7 post-cisplatin using click- and tone-evoked ABR for CGP-diltiazem (blue) and CGP-saline (orange) treated subjects. * represents p<0.05, ** p<0.01, and ***p<0.001 between treatment and control groups.
Quantification of Cochlear Hair Cells
Whole mount dissection and immunofluorescent staining with the hair cell-specific marker myosin VIIa was used to quantify IHCs and OHCs from the apical, middle, and basal turns of the cochlea. In both groups, there was no hair cell loss in the apical turn of the cochlea [CGP-saline group: 62.7 (± 0.5) OHCs and 20.0 (± 0.2) IHCs; CGP-diltiazem group: 60.1 (± 2.0) OHCs (p=0.222) and 20.5 (± 0.3) IHCs] (Figure 2A-B, G-H). There was a small loss of OHCs and no effect on IHCs in the middle turn, with no statistical differences detected between groups [CGP-saline group: 56.9 (± 2.0) OHCs and 21.3 (± 0.3) IHCs; CGP-diltiazem group: 51.8 (± 4.4) OHCs and 20.8 (± 0.3) IHCs] (Figure 2C-D, G-H). The basal turn of the cochlea suffered the most OHC damage, yet there were still no statistical differences detected between groups [CGP-saline group: 33.9 (± 6.5) OHCs; CGP-diltiazem group: 31.9 (± 6.3) OHCs] (Figure 2E-F, G). While the number of IHCs appeared similar between the two groups, there was a statistically significant difference in the basal turn [CGP-saline group: 18.7 (± 0.4) IHCs; CGP-diltiazem group: 17.2 (± 0.5) IHCs (p< 0.01)] (Figure 2E-F, H).
Figure 2:
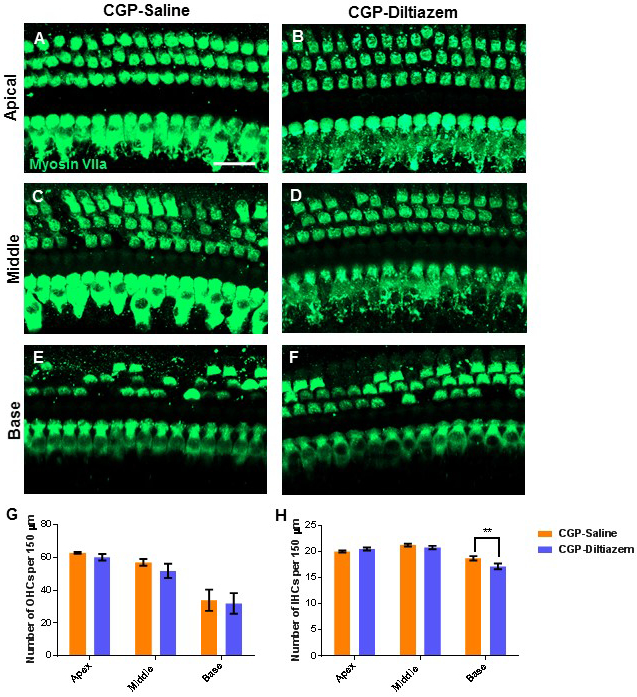
Representative confocal projection images of IHCs and OHCs (myosin VIIa, green) in the apical (A-B), middle (C-D), and basal (E-F) turns of the cochlea from CGP-saline (A, C, E) and CGP-diltiazem (B, D, F) groups. Scale bar = 20μm. Quantification of OHCs (G) and IHCs (H) from these images in CGP-Diltiazem (blue) and CGP-Saline (orange) groups. ** represents p<0.01.
Quantification of Synapses
Immunostaining with C-terminal binding protein 2 (Ctbp2) was used to quantify the number of ribbon synapses in 10 IHCs from the apical (Figure 3A-D), middle, and basal turns of the cochlea (Figure 3E). Although we did not include a control group without cisplatin treatment, several other studies have shown that each IHC in the mouse cochlea contains ~15-20 ribbon synapses.22,23 Thus there appears to be a loss of synapses in all turns of the cochlea after cisplatin treatment in the CGP-saline group [apex: 8.0 (± 1.4); middle: 9.7 (± 1.6); and base: 9.6 (± 1.7)] (Figure 3E). However, this is less clear in the CGP-diltiazem group [apex: 14. ± (0.7); middle 13.61± (1.1); base 9.39± (2.0)]. There was a significant difference between the synapse counts between groups in the apical turn of the cochlea (p<0.05) (Figure 3E), suggesting protection by CGP-diltiazem. There were no significant differences between groups in the middle or basal turns of the cochlea.
Figure 3:
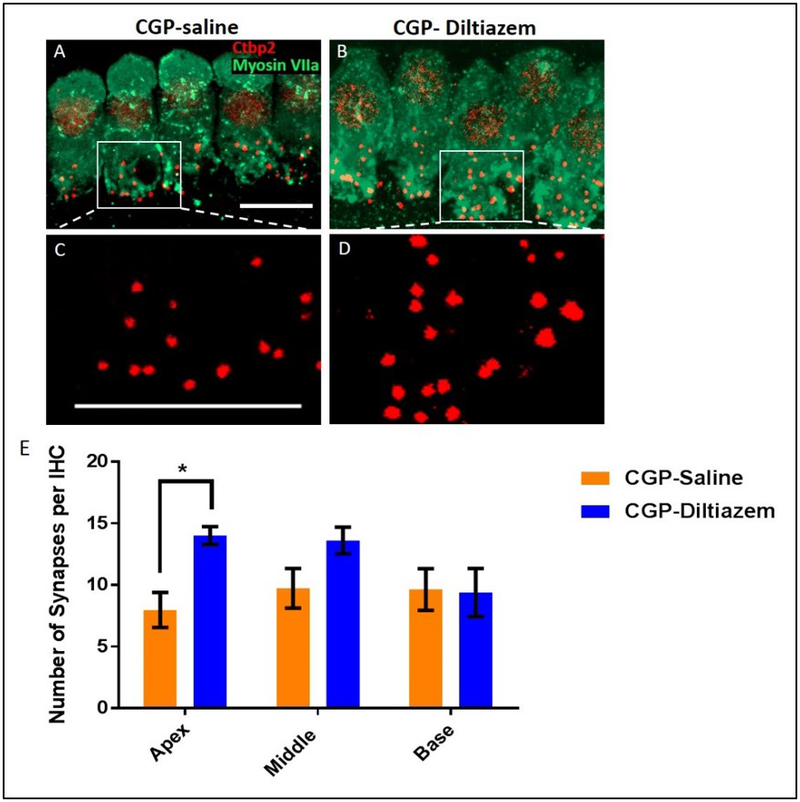
Representative confocal projection images of ribbon synpases (Ctbp2, red) located on IHCs (myosin VIIa, green) in the apical turn of the cochlea from CGP-saline (A, C) and CGP-dilitiazem (B, D), Scale bar = 10μm. E, Quantification of IHC ribbon synapses in CGP-diltiazem (blue) and CGP-Saline (orange) groups. * represents p<0.05.
Discussion:
Despite considerable research effort and improved understanding of cisplatin-induced ototoxicity, therapeutic options remain elusive in treating this permanent, acquired SNHL.4,6,24 Recent in vivo work showed evidence for the CCB, diltiazem, as an otoprotectant when delivered via an IT approach as a solution, yet only ABR threshold analysis was performed.11,12 Our current research builds upon this prior work, and demonstrates feasibility for a single IT administration of diltiazem as a CGP-hydrogel hydrogel. Additionally, this work represents the first histological analysis of the cochlea after administration of IT diltiazem in a mouse model of cisplatin-induced ototoxicity.
Clinically, cisplatin ototoxicity primarily affects the high-frequency regions.25 This concept is clear in our ABR results which demonstrate increased threshold shifts at 24 and 32 kHz and reduced OHC counts in the basal turn of the cochlea in both groups. While the CGP-diltiazem group demonstrated a statistically significant reduction in ABR threshold shifts across all frequencies, there were no significant differences in the number of OHCs from the CGP-saline group in any cochlear turn. The IHC counts were also similar between groups, and although IHC counts were statistically different in favor of the CGP-saline group in the basal turn of the cochlea, this difference was only a single cell and is likely physiologically insignificant. Previous work has shown that cisplatin decreases the number of ribbon synapses that connect IHCs to spiral ganglion neurons.26,27 Our results confirm this effect of cisplatin in the control group at all locations within the cochlea. However, in the CGP-diltiazem treated-group, we observed protection against cisplatin-induced synapse loss in the apical turn of the cochlea. A previous study showed that IT therapy can penetrate the otic capsule and cause higher drug concentrations in the cochlear apex by filling the bulla.28 This may explain the apical protection we observed.
We noted variability in the number of OHCs in the basal turn of the cochlea in both groups. This may be caused by technical challenges with the IT delivery of the CGP-hydrogel and failure of the therapy to get to the inner ear. However, this is unlikely given that only one subject had to be eliminated from analysis due to challenges with IT injection in our cohort. OHC count variability may also be caused by variability in the metabolism of cisplatin in a single dose model.29 We chose to give a single dose of cisplatin based on previous work,21 however other administration protocols with multiple doses of cisplatin may provide more consistent OHC damage.30,31 Single dose cisplatin protocols in various animal models demonstrate ABR threshold shifts across all frequencies despite hair cell loss primarily occurring in the basal turn of the cochlea.24,26,32,33 This suggests that there are other factors beyond the OHCs that contribute to ABR threshold shifts, and that CGP-diltiazem may provide its protective effects at a location outside the organ of Corti.
While ABR shifts suggest otoprotection with IT diltiazem, the site of otoprotection within the cochlea remains unclear. We evaluated the hair cells and IHC ribbon synapses because cisplatin damage has been demonstrated in these locations in other models,27 however there is evidence to suggest that cisplatin can also negatively impact the stria vascularis34 and spiral ganglion neurons.35 Recent work has suggested that cisplatin remains in the stria vascularis for a long time after administration.30 With this in mind, future work should evaluate the potential otoprotective properties against cisplatin at the level of the stria vascularis or spiral ganglion cells. There is evidence to suggest that CCBs may have otoprotective properties at the spiral ganglion neurons in the setting of noise-trauma.36
In addition to understanding the site of otoprotection within the cochlea, the mechanism of CCBs’ protection against cisplatin-induced ototoxicity needs to be established. Early work in the clinical setting evaluated the role of systemic administration of CCBs as vasodilators in the setting of otologic insult and sudden hearing loss.37 We hypothesized that diltiazem, when administered locally, works at the level of the calcium channels present within the cochlea.24,38 By blocking calcium influx, it theoretically prevents upstream influx of an essential co-factor responsible for catalytic activation of pro-apoptotic enzymes.39-41 Previous work has suggested that CCBs prevent calcium influx to OHCs in the setting of free radicals,9 and prevent lipid peroxidation and mitochondrial permeability in the setting of cisplatin.10 There is a preponderance of cardiovascular literature suggesting that CCBs have antioxidant properties as well,42-44 which may provide a secondary role in neutralizing the oxidative stress caused by cisplatin.6 All of these mechanisms are plausible for diltiazem as an otoprotectant, and more research is necessary to understand these details.
The use of the CGP-hydrogel delivery system addresses some of the shortcomings in previous protocols that require multiple, consecutive days of IT injections for administration of diltiazem in solution. Our findings also confirm previous work that the IT route of administration of chitosan-based hydrogel is feasible and less invasive compared to bullostomy.45 The properties that make CGP-hydrogel advantageous for our study is that the compound requires a single administration that releases drug for more than 4 days after initial placement.16 Clinically, this is particularly useful in the setting of cisplatin-induced effects, because, unlike other types of acquired SNHL, the time of administration and potential insult can be anticipated. Additionally, a concern about systemic administration of otoprotective therapies is their potential to neutralize the chemotherapeutic effects of the cisplatin, and with IT administration, this concern is reduced. Pharmacokinetic studies of diltiazem and other CCBs after IT administration are necessary to determine concentrations of diltiazem within the perilymph, CSF, and bloodstream. Although, the chemical properties of diltiazem46 suggest it is likely to diffuse into the cochlea without issue.14
We delivered IT CGP-diltiazem as a single dose on the same day as cisplatin. Previous animal protocols have evaluated ABR threshold shifts at various time points post-cisplatin.2,11,47 However, we chose to evaluate thresholds at Day 7 post-cisplatin because the CGP-hydrogel is eliminated from the middle ear at this point,16 and the most dramatic effects of cisplatin on hair cells and ABRs occur before Day 7.2,48 Assessments of cochlear function before Day 7 are difficult to obtain in experimental models because middle ear fluid associated with IT administration techniques result in ABR threshold shifts that would confound the effects of cisplatin.16,49,50 Beyond Day 7, ABR changes can be small,51 and electrophysiological and morphological recovery has been described in some species after cisplatin.52,53 More work will be necessary to evaluate the effects of IT CGP-diltiazem at different time points after cisplatin administration.
This work introduces a novel approach to otoprotection, and there are various directions for this research to continue. Exploration of other CCBs as otoprotective agents may be useful as both L- and T-type calcium channels are present in the inner ear,24 and other CCBs demonstrate antioxidant properties which may also play a role in otoprotection.10,42,43 We chose to evaluate CGP-hydrogel as the delivery vehicle because of the properties it demonstrates as a slow-eluting compound and our lab’s previous expertise with this compound. Other vehicles for drug delivery warrant exploration such as compounds that offer slow-release therapy to the inner ear. Finally, targeted delivery to the specific site of injury within the inner ear will be necessary as precision medicine evolves and improved delivery of drugs to the inner ear becomes standardized within clinical practice.
Through this research, we have introduced a novel concept that incorporates a CGP-hydrogel system to deliver diltiazem. Our work suggests that CGP-diltiazem reduces the effects of cisplatin-induced ototoxicity by reducing ABR threshold shifts and prevents synapse loss in the apical turn of the cochlea. While more work is necessary to understand the location and mechanism of otoprotection, we see clinical potential with this concept that may avoid the traditionally long timeframe that accompanies new drug development.
Acknowledgments
Funding Sources: This work was supported by an institutional Pilot Grant from the University of Pennsylvania
This work was supported by NIH grant R01DC014441 (BCC) and Office of the Assistant Secretary of Defense for Health Affairs Grant W81XWH-15-1-0475 (BCC).
This work was supported by the National Institute for Deafness and Communication Disorders (NIDCD R01 DC014464, 2015). NIDCD had no involvement in study design, the collection, analysis and interpretation of data, writing of this report or the decision to submit this article for publication.
Footnotes
Conflicts of Interest: Brandon Cox is a consultant for Otonomy, Inc and Turner Scientific, LLC. No other authors have potential conflicts of interest to report.
References
- 1.Callejo A, Sedo-Cabezon L, Juan ID, Llorens J. Cisplatin-Induced Ototoxicity: Effects, Mechanisms and Protection Strategies. Toxics 2015; 3:268–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Berrocal JR, Nevado J, Ramirez-Camacho R et al. The anticancer drug cisplatin induces an intrinsic apoptotic pathway inside the inner ear. British journal of pharmacology 2007; 152:1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casares C, Ramirez-Camacho R, Trinidad A, Roldan A, Jorge E, Garcia-Berrocal JR. Reactive oxygen species in apoptosis induced by cisplatin: review of physiopathological mechanisms in animal models. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies 2012; 269:2455–2459. [DOI] [PubMed] [Google Scholar]
- 4.Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hearing research 2007; 226:157–167. [DOI] [PubMed] [Google Scholar]
- 5.Clerici WJ, DiMartino DL, Prasad MR. Direct effects of reactive oxygen species on cochlear outer hair cell shape in vitro. Hearing research 1995; 84:30–40. [DOI] [PubMed] [Google Scholar]
- 6.Rybak LP, Whitworth CA. Ototoxicity: therapeutic opportunities. Drug discovery today 2005; 10:1313–1321. [DOI] [PubMed] [Google Scholar]
- 7.Sachs RE, Ginsburg PB, Goldman DP. Encouraging New Uses for Old Drugs. JAMA : the journal of the American Medical Association 2017; 318:2421–2422. [DOI] [PubMed] [Google Scholar]
- 8.Naples JG. Calcium-channel blockers as therapeutic agents for acquired sensorineural hearing loss. Med Hypotheses 2017; 104:121–125. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda K, Sunose H, Takasaka T. Effects of free radicals on the intracellular calcium concentration in the isolated outer hair cell of the guinea pig cochlea. Acta oto-laryngologica 1993; 113:137–141. [DOI] [PubMed] [Google Scholar]
- 10.So HS, Park C, Kim HJ et al. Protective effect of T-type calcium channel blocker flunarizine on cisplatin-induced death of auditory cells. Hearing research 2005; 204:127–139. [DOI] [PubMed] [Google Scholar]
- 11.Naples JG, Parham K. Cisplatin-Induced Ototoxicity and the Effects of Intratympanic Diltiazem in a Mouse Model. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 2016; 154:144–149. [DOI] [PubMed] [Google Scholar]
- 12.Naples J, Cox R, Bonaiuto G, Parham K. Prestin as an Otologic Biomarker of Cisplatin Ototoxicity in a Guinea Pig Model. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 2017:194599817742093. [DOI] [PubMed] [Google Scholar]
- 13.Salt AN, Plontke SK. Pharmacokinetic principles in the inner ear: Influence of drug properties on intratympanic applications. Hearing research 2018; 368:28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salt AN, Plontke SK. Principles of local drug delivery to the inner ear. Audiology & neuro-otology 2009; 14:350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lajud SA, Nagda DA, Qiao P et al. A novel chitosan-hydrogel-based nanoparticle delivery system for local inner ear application. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 2015; 36:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paulson DP, Abuzeid W, Jiang H, Oe T, O’Malley BW, Li D. A novel controlled local drug delivery system for inner ear disease. The Laryngoscope 2008; 118:706–711. [DOI] [PubMed] [Google Scholar]
- 17.Lajud SA, Han Z, Chi FL et al. A regulated delivery system for inner ear drug application. J Control Release 2013; 166:268–276. [DOI] [PubMed] [Google Scholar]
- 18.Roehm P, Hoffer M, Balaban CD. Gentamicin uptake in the chinchilla inner ear. Hearing research 2007; 230:43–52. [DOI] [PubMed] [Google Scholar]
- 19.Montgomery SC, Cox BC. Whole Mount Dissection and Immunofluorescence of the Adult Mouse Cochlea. J Vis Exp 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berndtson WE. A simple, rapid and reliable method for selecting or assessing the number of replicates for animal experiments. Journal of animal science 1991; 69:67–76. [DOI] [PubMed] [Google Scholar]
- 21.Hill GW, Morest DK, Parham K. Cisplatin-induced ototoxicity: effect of intratympanic dexamethasone injections. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 2008; 29:1005–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kujawa SG, Liberman MC. Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hearing research 2015; 330:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. The Journal of neuroscience : the official journal of the Society for Neuroscience 2013; 33:13686–13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waissbluth S, Daniel SJ. Cisplatin-induced ototoxicity: transporters playing a role in cisplatin toxicity. Hearing research 2013; 299:37–45. [DOI] [PubMed] [Google Scholar]
- 25.Sheth S, Mukherjea D, Rybak LP, Ramkumar V. Mechanisms of Cisplatin-Induced Ototoxicity and Otoprotection. Front Cell Neurosci 2017; 11:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borse V, Al Aameri RFH, Sheehan K et al. Epigallocatechin-3-gallate, a prototypic chemopreventative agent for protection against cisplatin-based ototoxicity. Cell Death Dis 2017; 8:e2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh S, Sheth S, Sheehan K et al. The Endocannabinoid/Cannabinoid Receptor 2 System Protects Against Cisplatin-Induced Hearing Loss. Front Cell Neurosci 2018; 12:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikulec AA, Plontke SK, Hartsock JJ, Salt AN. Entry of substances into perilymph through the bone of the otic capsule after intratympanic applications in guinea pigs: implications for local drug delivery in humans. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 2009; 30:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proceedings of the National Academy of Sciences of the United States of America 2002; 99:14298–14302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breglio AM, Rusheen AE, Shide ED et al. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat Commun 2017; 8:1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez K, Wafa T, Fitzgerald TS, Cunningham LL. An optimized, clinically relevant mouse model of cisplatin-induced ototoxicity. Hearing research 2019; 375:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sockalingam R, Freeman S, Cherny TL, Sohmer H. Effect of high-dose cisplatin on auditory brainstem responses and otoacoustic emissions in laboratory animals. The American journal of otology 2000; 21:521–527. [PubMed] [Google Scholar]
- 33.Harrison RT, Seiler BM, Bielefeld EC. Ototoxicity of 12 mg/kg cisplatin in the Fischer 344/NHsd rat using multiple dosing strategies. Anticancer Drugs 2016; 27:780–786. [DOI] [PubMed] [Google Scholar]
- 34.Sluyter S, Klis SF, de Groot JC, Smoorenburg GF. Alterations in the stria vascularis in relation to cisplatin ototoxicity and recovery. Hearing research 2003; 185:49–56. [DOI] [PubMed] [Google Scholar]
- 35.Hamers FP, Wijbenga J, Wolters FL, Klis SF, Sluyter S, Smoorenburg GF. Cisplatin ototoxicity involves organ of Corti, stria vascularis and spiral ganglion: modulation by alphaMSH and ORG 2766. Audiology & neuro-otology 2003; 8:305–315. [DOI] [PubMed] [Google Scholar]
- 36.Sekiya T, Yagihashi A, Asano K, Suzuki S. Nimodipine ameliorates trauma-induced cochlear neuronal death. Neurol Res 2002; 24:775–780. [DOI] [PubMed] [Google Scholar]
- 37.Sheehy JL. Vasodilator therapy in sensory-neural hearing loss. The Laryngoscope 1960; 70:885–914. [DOI] [PubMed] [Google Scholar]
- 38.Uemaetomari I, Tabuchi K, Nakamagoe M, Tanaka S, Murashita H, Hara A. L-type voltage-gated calcium channel is involved in the pathogenesis of acoustic injury in the cochlea. The Tohoku journal of experimental medicine 2009; 218:41–47. [DOI] [PubMed] [Google Scholar]
- 39.Rizzuto R, Pinton P, Ferrari D et al. Calcium and apoptosis: facts and hypotheses. Oncogene 2003; 22:8619–8627. [DOI] [PubMed] [Google Scholar]
- 40.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nature reviews Molecular cell biology 2003; 4:552–565. [DOI] [PubMed] [Google Scholar]
- 41.Kondratskyi A, Kondratska K, Skryma R, Prevarskaya N. Ion channels in the regulation of apoptosis. Biochimica et biophysica acta 2014. [DOI] [PubMed] [Google Scholar]
- 42.Mak IT, Zhang J, Weglicki WB. Protective effects of dihydropyridine Ca-blockers against endothelial cell oxidative injury due to combined nitric oxide and superoxide. Pharmacol Res 2002; 45:27–33. [DOI] [PubMed] [Google Scholar]
- 43.Mak IT, Boehme P, Weglicki WB. Antioxidant effects of calcium channel blockers against free radical injury in endothelial cells. Correlation of protection with preservation of glutathione levels. Circ Res 1992; 70:1099–1103. [DOI] [PubMed] [Google Scholar]
- 44.Mak IT, Weglicki WB. Comparative antioxidant activities of propranolol, nifedipine, verapamil, and diltiazem against sarcolemmal membrane lipid peroxidation. Circ Res 1990; 66:1449–1452. [DOI] [PubMed] [Google Scholar]
- 45.Murillo-Cuesta S, Vallecillo N, Cediel R et al. A Comparative Study of Drug Delivery Methods Targeted to the Mouse Inner Ear: Bullostomy Versus Transtympanic Injection. J Vis Exp 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katzung BG, Masters SB, Trevor AJ. Basic & clinical pharmacology McGraw-Hill's AccessMedicine. New York, N.Y.: McGraw-Hill Education LLC,, 2012:xiii, 1,229 p. [Google Scholar]
- 47.Murphy D, Daniel SJ. Intratympanic dexamethasone to prevent cisplatin ototoxicity: a guinea pig model. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 2011; 145:452–457. [DOI] [PubMed] [Google Scholar]
- 48.Hellberg V, Wallin I, Ehrsson H, Laurell G. Cochlear pharmacokinetics of cisplatin: an in vivo study in the guinea pig. The Laryngoscope 2013; 123:3172–3177. [DOI] [PubMed] [Google Scholar]
- 49.Guan X, Gan RZ. Effect of middle ear fluid on sound transmission and auditory brainstem response in guinea pigs. Hearing research 2011; 277:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu BZ, Saleh J, Isgrig KT, Cunningham LL, Chien WW. Hearing Loss after Round Window Surgery in Mice Is due to Middle Ear Effusion. Audiology & neuro-otology 2016; 21:356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hughes AL, Hussain N, Pafford R, Parham K. Dexamethasone otoprotection in a multidose cisplatin ototoxicity mouse model. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 2014; 150:115–120. [DOI] [PubMed] [Google Scholar]
- 52.Stengs CH, Klis SF, Huizing EH, Smoorenburg GF. Cisplatin-induced ototoxicity; electrophysiological evidence of spontaneous recovery in the albino guinea pig. Hearing research 1997; 111:103–113. [DOI] [PubMed] [Google Scholar]
- 53.Cardinaal RM, de Groot JC, Huizing EH, Veldman JE, Smoorenburg GF. Cisplatin-induced ototoxicity: morphological evidence of spontaneous outer hair cell recovery in albino guinea pigs? Hearing research 2000; 144:147–156. [DOI] [PubMed] [Google Scholar]


