Abstract
Cardiac injuries, like heart attacks, drive the secondary pathology with advanced heart failure. In this process, non-resolving inflammation is a prime component of accelerated cardiovascular disease and subsequent fatal events associated with imbalanced diet, physical inactivity, disrupted circadian rhythms, neuro-hormonal stress, and poly- or co-medication. Laboratory rodents have established that splenic leukocyte-directed resolution mechanisms are essential for cardiac repair after injury. Here, we discuss the impact of three life-style related factors that are prime causes of derailed cardiac healing, putative non-resolving inflammation-resolution mechanisms in cardiovascular diseases and progressive heart failure after cardiac injury. The presented review resurfaces the lifestyle-related risks and future research directions required to understand the molecular and cellular mechanisms between the causes of cardiovascular disease and their related consequences of non-resolving inflammation.
Keywords: cardiovascular disease, chronic inflammation, cardiac repair, heart failure, leukocytes
1. One Dimensional View of Cardiovascular Disease and Heart Failure
Cardiometabolic risk-enriched lifestyles including an imbalance of nutrition, physical inactivity or overactivity, disturbed sleep/wake cycles, high stress environments or jobs, and increased use of medications are major contributors to different cardiovascular diseases, including heart failure (HF). HF is one of the major causes of cardiovascular morbidity and mortality in western countries and worldwide [1]. Approximately 1–3% of the population is diagnosed with a heart disease, and one in five people will develop HF during their lifetime, increasing in risk with age [2, 3]. The incidence of patients suffering from HF with preserved ejection fraction (HFpEF) relative to HF with reduced ejection fraction (HFrEF) is increasing at a rate of 1% per year, leading to increased hospitalization [4]. A one-dimensional view of HF is classified into three types: 1) Atrophic HF, 2) Ischemic HF, and 3) Non-ischemic HF [5]. Atrophic HF is common in prolonged bedrest patients and astronauts due to the muscular atrophy caused by the absence of movement or a gravitational pull on bones and muscles during time spent in space; which leads to biochemical and structural changes of muscle fibers, decreasing muscle size and increasing protein degradation, driving a decrease in heart function [6, 7]. Ischemic HF, commonly associated with coronary artery disease, is caused by a build-up of plaque that restricts oxygen and blood flow, inducing a heart attack and leading to HF [8]. Non-ischemic HF is commonly caused by an underlying medical condition, such as obesity or hypertension, and often is associated with diseases affecting other organs [9–11]. The multi-dimensional, multi-factorial, and multi-organ MOGE(S) cardiomyopathies classification is introduced for precise classification and treatment of HF patients [12, 13]. HF is the end stage of heart disease pathology in humans, beginning with endothelial dysfunction, then progressing to plaque formation, hypertrophy and/or hypertension, atherosclerosis, coronary artery disease, myocardial infarction, stroke, arrhythmias, atrial fibrillation, sudden cardiac death, and cardiac arrest [14, 15] (Figure 1).
Figure 1. Change of historical and short-term inflammation perspectives to the current view of lifestyle related non-resolving and chronic inflammation in cardiovascular diseases and heart failure.
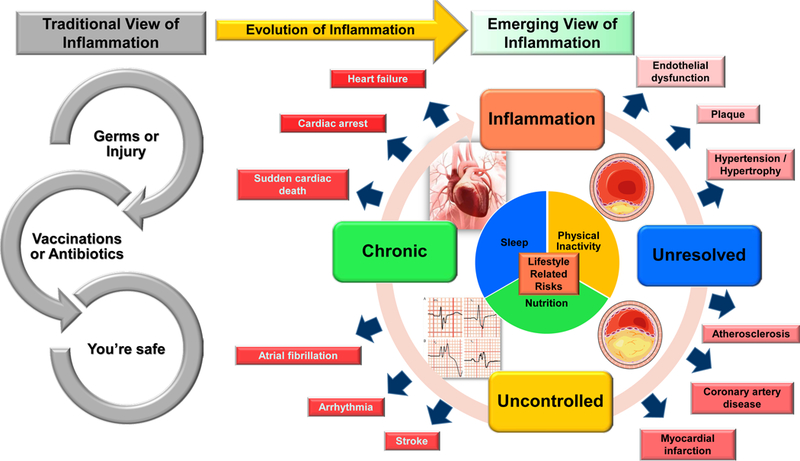
Historically, inflammation was thought to be caused by germs or work related injuries and a vaccination or a simple round of antibiotics was all that necessary for resolution or cure. Now, we know that inflammation can be caused by a variety of lifestyle choices, including unbalanced nutrition, lack of physical activity, and disturbed sleep. If life style-related inflammation is left unresolved, it can become uncontrolled and morph into chronic inflammation. This process of amplified inflammation is relatable to coronary artery disease by the development of dysfunctions and overlapping symptoms that can build a pro-inflammatory environment and lead to heart failure syndrome.
2. Cardiac Inflammation-Resolution Mechanisms after Myocardium Injury
Traditionally, inflammatory responses were believed to only be associated with injuries and germs (infection); now, in the 21st century, we know there are various sources of inflammation that change depending on the individual and their lifestyle (Figure 1). With revised sanitization practices and the development of antibiotics, germs are not considered a major contributor to cardiovascular disease, unless the immune system is compromised or an infection is acquired due to hospitalization or aging. Inflammation in HF may be triggered by wall stress, signals released by infarct-induced or hypotrophy-related stressed malfunctioning, or dead muscle cells secondary to HF [16, 17]. In response to an injury during inflammation, cytokines, chemokines, and eicosanoids, such as tumor necrosis factor (TNF-α), interleukin (IL-1β, IL-6), CCL2, and leukotrienes, are elevated to initiate the infiltration of polymorphonuclear neutrophils (PMNs) to the site of injury [18, 19]. Along with initiation of acute inflammation, the resolution of inflammation is also triggered to monitor and regulate the time-dependent inflammation process, which is critical for maintaining tissue homeostasis [20, 21]. Apoptotic PMNs attract macrophages to the site of injury to limit neutrophil activity. If PMN infiltration remains unchecked or if there is a prolonged persistence of inflammatory triggers at the site of injury, it can create a negative feedback loop, inducing non-resolving and chronic inflammation [22]. This kind of non-resolving and chronic inflammation is common when obesity is superimposed on aging and can lead to multi-organ failure [23]. It has been discovered that in healthy individuals, leukocytes continuously monitor for threats by “crawling” on the luminal side of the endothelium [24, 25]. Once a threat is detected, these patrolling monocytes release intracellular components, such as DAMPs, or damage associated molecular patterns, to alert neutrophils and initiate an immune response [24, 26]. Once the threat has been eliminated, the body initiates an active process to limit neutrophil infiltration, including the production of lipoxins, for the resolution of inflammation, ensuring minimal tissue damage [24]. One of these lipoxins, LXA4, increases the migration of monocytes to the site of inflammation and inhibits neutrophil activity to aid in their removal [24, 27].
Aging is one of the primary risk factors for HF and humans are five times more likely to develop HF as they age into their 70s and 80s [28]. The likelihood of HF rises from 1.9% of men and 1.4% of women at 40 to 59 years, to 14.7% of men and 12.8% of women at 80+ years [28]. This could be attributed to the decline of tissues, organs, and bodily functions that are associated with age [29]. Cardiac myocytes increase in size and become hypertrophic as an individual ages [29]. During chronic, inflammatory conditions, like in aging patients, the infiltration of neutrophils is weakened, there is a delayed in the initiation of chemokines and cytokines, and there is an upregulation of pro-inflammatory leukocytes (CD11b+/F4/80+/Ly6Chigh) that leads to defective cardiac healing and, therefore, non-resolving inflammation [29, 30]. After MI (myocardial infarction; heart attack) in an elderly patient, aged fibroblasts are less effective in permeating the infarcted area, resulting in larger infarcts, HF, and increased mortality [28].
In a clinical setting, cardiovascular disease is studied as the pathophysiological changes of the coronary artery and left ventricle (LV) [31]. This myocardium centric approach focuses on the patient initially developing signs of endothelial dysfunction and, with sustained, unhealthy lifestyle choices, differential stages of atherosclerosis with hypertension (Figure 1). In most patients, the MI event most likely occurs because of an inter-organ, systemic, pro-inflammatory environment, unstable plaque, and lifestyle-related other universal risk factors, like aging (Figure 2) [10]. In contrast to atherosclerotic or HF patients, the majority of laboratory experiments are performed in essentially healthy, adult rodents (mice/rats) or large animals, like rabbits and pigs, which are continuously monitored and regulated for optimal health (Figure 2). The result of this continued monitoring is no mortality after reperfusion in rodents (except peri-operative mortality); however, the mortality rate of humans is more than 40–50% within one year, with or without reperfusion [1]. Post-MI, 50–60% mice survive end-stage HF without physiological discomfort despite advanced, irreversible fibrotic remodeling, and extensive wall-thinning [32]. This could be attributed to laboratory rodents ability to streamline splenic leukocytes and produce specialized pro-resolving mediators (SPMs), such as resolvin (Rv) D1, RvD3, RvD4, RvD5, RvD6, AT-RvD1, PD1, maresin (Ma)R1, 7S,14S-diHDHA and 4S,14S-diHDHA, at the site of injury effectively post-MI [33–36]. Certain strains of monogenic and polygenic adult mice are able to biosynthesize differential bioactive resolving mediators (cypoxins), thereby improved cardiac healing, limited heart dysfunction, and increased survival [37–41].
Figure 2. Differential perspectives of cardiac healing in mice and humans after myocardium infarction (MI) leading to the progressive development of heart failure syndrome.
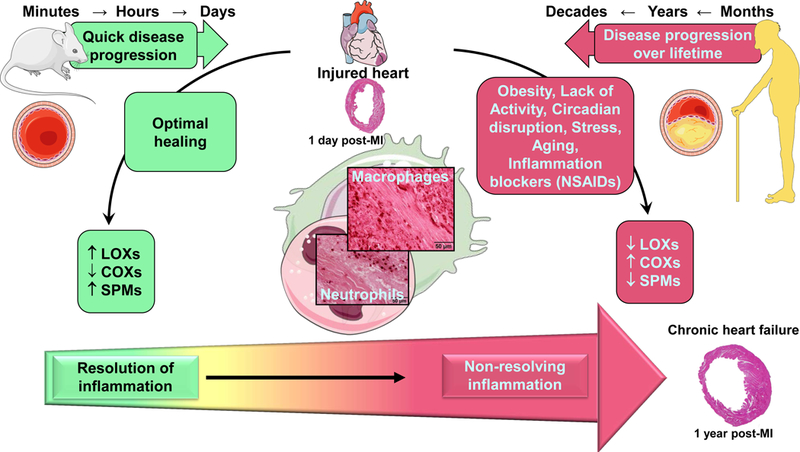
In humans, chronic diseases are developed over months, years, and decades of lifestyle related choices, neuro-hormonal stress, and consequences. Laboratory rodents, such as mice that are subjected to myocardial infarction surgery in order to replicate human heart failure over the course of minutes to days. Cardiac healing is optimal in mice due to absence of risk factor for heart disease. Optimal cardiac healing is facilitated by lipoxygenase (LOX) enzymes with biosynthesis of specialized pro-resolving mediators (SPMs) at the site of infarction. If mice are introduced to setting of diet-induced obesity, aging, co-medications like NSAIDs (nonsteroidal anti-inflammatory drugs), or combinations of these risk factors, cardiac healing is impaired leading to non-resolving inflammation with dysregulation of LOXs. Likewise, in age-related obese individuals and/or co-medications perturb healing marked with non-resolving inflammation. This inability to heal after cardiac injury is associated with amplified non-resolving inflammation and chronic heart failure.
Unresolved inflammation morphing into chronic inflammation can lead to HF and might present as ischemic, viral heart disease, hypertensive heart disease, or secondary to obesity and diabetes [42, 43]. Prolonged inflammation to the site of injury in acute sustained coronary occlusion in ischemic HF causes irreparable damage to the cardiomyocytes and disruption in heart remodeling [32, 44]. Several studies showed that chronic over-activation of inflammatory networks could result in hypertrophy and eventually, with a relatively higher degree of inflammation, HF [43]. Chronic inflammation along with comorbidities like diabetes, obesity, and hypertension-mediated endothelial dysfunction overlap with atherosclerosis and can lead to progressive HF [42, 45].
3. Obesity-mediated Immune Dysfunction and Non-resolving Inflammation
Obesity, recognized by an increase of adipose tissue and fat mass, is described as chronic, low-grade, mild inflammation. Fat is essential for maintaining the metabolism and various bodily functions, such as the integumentary and reproductive systems, but, at increased levels, can become a primary cause of obesity and non-resolving inflammation [46–51]. The World Health Organization (WHO) determined people can be divided into four categories, based on body mass index (BMI): underweight (15–19.9), normal (20–24.9), overweight (25–29.9), and obese (30–35+). The International Obesity Task Force divided obesity further to include pre-obesity, or overweight, (25–29.9), class I obesity (30–34.9), class II obesity (35–39.9), and class III obesity (40+) [52]. The International Diabetes Federation and WHO have said that of the approximately 600 million individuals suffering from obesity, 370 million people are also diagnosed with Type 2 Diabetes [53–56].
Since obesity is often paired with Type 2 Diabetes, insulin resistance can play a role in the accumulation of inflammation. Fat, particularly saturated fatty acids, commonly found in butter, increases the risk of insulin resistance and inflammation via diacylglycerol-mediated activation of protein kinase C and activation of toll-like receptors in humans [57]. It has been suggested that various adipose tissue macrophages (ATMs) existing in the same area within the body cause the inflammation associated with obesity in humans [58]. Adipose tissue is partially composed of activated immune cells, aiding in both inflammation activation and inhibition [59]. Increased adipose tissue, which is present during obesity, promotes inflammation, leading to adverse adipose function and remodeling [10, 59].
The overconsumption of a additive/preservative-enriched, packaged, processed, frozen, premade canned, and fried foods combined with a sedentary lifestyle is believed to be, at least in part, an underlying reason for the widespread obesity epidemic and HF in young individuals [9, 54, 60]. The belief that overconsumption of food leads to obesity was recently broken down to discover if protein, carbohydrates, or fat would lead to an increase in body weight in laboratory rodents. Hu et al. found that a diet high in fat content, not carbohydrates or sugar, caused an increase in the body weight and obesity of laboratory mice [54]. A study using mice with MI found that when obesity is superimposed on aging, there are dysregulated levels of COX-2, 5-LOX, and HO-1 enzymes within the injured heart, causing increased inflammation and reduced resolution resulting in adverse remodeling and chronic inflammation [23]. Humans with a BMI within the range of obesity (30+), have a higher risk of developing hypertension, coronary artery disease and stroke [61]. Hypertension has been associated with obesity because of the need for increased blood volumes and cardiac outputs to compensate for the increased size of affected individuals [61]. Individuals suffering from obesity have also been shown to have an increase in the secretion of angiotensinogen from fat cells, causing increased blood pressure resulting in hypertension [61]. Systemic insulin resistance can be caused by a reduction of insulin activities in visceral white adipose tissue (WAT) after prolonged and continuous activation of both the stress and inflammation pathways [62]. This alteration has been associated with changes in both innate and adaptive immune cells within the WAT [62]. Innate immune cells as neutrophils, reparative M2 macrophages with impairment in activation, such as a myeloid-specific deletion of peroxisome proliferator activated receptor-γ (PPAR-γ), PPAR-δ, or Kruppel Like Factor 4 (Klf4), and inflammatory macrophages (Ccr2+Ly6Chi) migrate into WAT and promote adipose tissue inflammation and insulin resistance [62]. Neutrophils via secretion of elastase augment adipose tissue inflammation and insulin resistance [62]. In the conditions within obese individuals, adaptive cells, such as Treg’s (T-cells), are relatively higher in the WAT compared to spleen and lymph nodes [62]. These adaptive cells express the adipogenic transcription factor PPARγ and can lead to adipose tissue inflammation and insulin resistance when inhibited [62]. Obesity superimposed on aging can have a negative impact on the resolution of inflammation from both internal and external sources, caused by the combination of excess abdominal adipose tissue and these altered cellular functions [61, 62]. This adverse effect can aid in the promotion and continuation of heart damage and disease.
4. Role of Action and Inaction in Cardiovascular Disease
Exercise is commonly considered a beneficial activity for both cardiovascular and overall health. In humans, light physical activity has been shown to positively affect cholesterol levels, improve cardiac rhythms, and lower blood pressure [63, 64]. There is, however, a ‘sweet spot’ that outlines the amount of exercise needed to obtain optimal health benefits. Based on the current literature, this ‘sweet spot’ for the average person has been found to be 75 minutes of strenuous exercise or 150 minutes of moderate exercise per week [65]. The effects of going over, or under, this range of time has been shown to increase the risk of cardiovascular disease, coronary heart disease, type 2 diabetes, insulin resistance, arrhythmias, and even death [65–69].
Individuals who do not exercise are at a higher risk for mortality and weight gain compared to their active counterparts [61, 70]. Insufficient exercise is associated with adverse LV remodeling and diastolic dysfunction [71, 72]. Physical inactivity can change the secretion of myokines from skeletal muscle, causing the body to become immune to their effects [66]. Lifestyle or work-related inactivity can cause issues in the balance of myokines normally secreted during exercise, resulting in inflammation [66]. Extensive sedentary activities, such as sitting, have been shown to disrupt the normal production of lipoprotein lipase and leads to an increase in the levels of sugars and fatty acids within the bloodstream [73]. This elevation can also create an increase in the inflammatory profile of C-reactive protein (CRP) and leukocytes mediators, which can cause endothelial dysfunction [73].
On the other end of the spectrum, too much physical activity can result in burst of dormant coronary artery plaque and sudden cardiac death in athletes over the age of 35, predominately males [74]. These athletes are more likely to participate in frequent, rigorous activities which cause their increased risk [74]. The risk of developing an illness increases with frequent, rigorous physical activity without adequate rest periods, also known as “overtraining” [75]. During physical activity, there is an increase in the number of neutrophils and monocytes present within the body; during the rest, or recovery, period, the number of circulating leukocytes decreases [75]. High-intensity, interval exercise has been shown to negatively affect the leukocyte, lymphocyte, and neutrophil numbers during the physical activity and immediately after [76]. This change could be the cause of increased stress, both hormonal and metabolic, after high-intensity exercise [76]. It is important to find a healthy balance between exercise and recovery to ensure optimal health of the heart, mind, and body.
5. Influence of Sleep and Wake Cycle on Cardiac Health
The sleep and wake cycle, or the circadian rhythm, is vital for maintaining overall health. The circadian cycle ensures the body’s organs are functioning properly by regulating energy storage, cardiac output, heart rate, and blood pressure around a specific ‘time-of-day’ pattern [63, 77, 78]. Frequent disruptions of the circadian cycle could result in stress to the body, which can trigger a neuroendocrine and metabolic response [79]. This response could cause a spike in the levels of hormones normally secreted from the adrenal gland, activating the sympathetic nervous system [79]. Continuous activation of the sympathetic nervous system can create suppression of thyroid-steroidal hormonal axis, leading to metabolic disturbances, obesity, and hypertension, which can increase the risk of heart disease [79]. Numerous genes focus on the circadian cycle and regulating bodily functions based on the time-of-day, including Brain and Muscle ARNT-Like 1 (BMAL1) and Circadian Locomotor Output Cycles Kaput (CLOCK), two circadian cycle transcription factors. BMAL1 plays a role in metabolism, inflammation-resolution, and signaling of the heart and CLOCK is involved in contractile function and ischemia/reperfusion tolerance [77, 80, 81]. Genetic deletion of BMAL1 or CLOCK in mouse cardiomyocytes or other tissues increases the risk of cardiovascular disease [77, 80].
Working the ‘night shift,’ which disrupts the typical sleep-wake pattern, can result in alterations in the cardiometabolism and arterial pressure [82]. Long-term “shift workers” have an increased risk of hypertension and myocardial infarction which can lead to cardiovascular disease [82, 83]. Prolonged disruption of the circadian cycle can cause an increase in the number of pro-inflammatory macrophages, driving inflammation and, as a result, cardiovascular disease [84]. In typical situations, immune cells, such as hematopoietic stem and progenitor cells (HSPCs), and a majority of mature leukocytes, are most active during the night, or the ‘rest period’ and least active during the day, or the ‘active period’ [85]. In certain situations, exposure to a dietary or pharmaceutical allergen at the beginning of the ‘active period’ can result in the immune system over-activating, increasing the likelihood of a negative outcome, such as death [85]. In laboratory rodents, alterations of sleeping patterns have shown to affect the levels of consumption and activity, which disrupts the metabolism of laboratory rodents, leading to obesity [86]. Individuals working the “night shift,” on average, are considered at an increased risk for overeating, obesity, type 2 diabetes, high blood pressure and glucose, and psychosocial stress, increasing the likelihood of developing heart disease [87, 88].
6. Stress and Heart Health
Recently, psychological stress has been more closely examined for potentially negative implications on human health. In today’s society, stress is widespread and comes in many forms including, but not limited to: financial strains, marriage or divorce, workplace deadlines and hours, depression, anxiety, social pressures, personality traits, and grief [89]. Stress can be acute, episodic, or chronic. According to the Centre for Studies on Human Stress (CSHS), acute stress is a release of hormones due to new or important events and situations and chronic stress occurs when these hormones are continually triggered to be released [90]. Stress causes the activation of hormones, such as corticosterone, epinephrine, and norepinephrine, that control the migration of lymphocytes, neutrophils, and monocytes [91]. Different body mineral levels affected by continual activation of stress hormones, such as salt, water, and cholesterol, can also increase the risk of a cardiac event [90]. These hormones can also cause an increase in blood pressure and heart rate, which, when activated chronically, could also be a cause for the escalated risk of a cardiac event [92]. Psychological stress has been linked to myocardial infarction (MI), hypertension, stroke, and atherosclerotic disease, which increase the risk of CVD [93]. A study involving 600,000+ individuals from the United States, Europe, and Japan found evidence to suggest a high-stress work environment, i.e. job insecurity, increased pressure, and long hours, can escalate the risk of employees to develop coronary heart disease and stroke by 10–40% [94]. About 20% of patients suffering from coronary artery disease or cardiovascular disease are depressed, which is nearly three times higher than the general population [95]. Prolonged stress can cause stress-induced heart disease clinically known as Takotsubo cardiomyopathy and has a mortality rate comparable to acute coronary syndrome [96, 97].
7. Proresolution Pathways, Nutritional, and Dietary Supplements
To treat non-resolving inflammation and improve cardiac health, emerging proresolution pathways for cardiovascular diseases under intense development and is novel frontier in resolution physiology [98–103]. With an increased focus on personal health, nutritional and dietary supplements have become more prevalent, with 6% rise per year through 2018 [104]. These supplements most often include multivitamins, Vitamin D, fish oil, probiotics, and coenzyme Q10 (CoQ10) [104]. Although there is no definitive evidence that multivitamins aid in the prevention of cardiovascular disease, there is an increase in the death rate of non-vitamin users compared to individuals who consume multivitamins regularly [104]. Vitamin D is most closely associated with thrombosis, but it has been discovered that insufficient levels of Vitamin D can increase the likelihood of cardiovascular disease, diabetes, multiple sclerosis, and cancer [104]. Fish oil and other ω−3 fatty acids have shown to improve cardiac health, endothelial function, exercise tolerance, and cognitive function and have anti-arrhythmic, anti-thrombotic and anti-inflammatory effects [105, 106]. A study involving 11,324 individuals who experienced an MI within three months recorded a 41% decline in mortality and a 53% decline in sudden cardiac death (SCD) with the supplementation of fish oil into their daily routines [105]. Probiotics became popular because of their range of proposed contributions to health, including improved blood pressure, digestion, lipid metabolism, and immune system function and decreased cancer risk [104]. Despite these potential benefits, most of the benefits focusing on cardiovascular disease have been discovered using in vitro studies, so the exact contributions to blood pressure reduction and lipid metabolism are still unknown [104, 107]. A deficiency of CoQ10 has been associated with an increase in cardiac events and death, and a decrease in heart function [104, 108]. More research is needed to adequately determine the benefits of dietary supplements and their effect on cardiovascular disease before they can be definitively used as a means of risk reduction.
8. Non-Resolving Therapeutics
Use of more than one pharmaceutical agent is obvious in non-resolving inflammation and multi-organ chronic health conditions particularly in the older population [103, 109–111]. Pain limiting agents, such as nonsteroidal anti-inflammatory drugs (NSAIDs), are mainly COX inhibitors that have been used for decades as an analgesic and anti-inflammatory agents. However, because of the adverse effects caused by NSAIDs, they should be cautiously administered to patients diagnosed with HF [112, 113]. NSAIDs exacerbate the risk of hospitalization approximately four times in chronic HF patients [114]. Recently, it was discovered that the subacute treatment of carprofen before MI in mice caused pre-activation of neutrophils and increased expression of CD47 (the ‘don’t eat me’ signal) on neutrophils, leading to a neutrophil swarming in the spleen and heart, driving non-resolving inflammation [115]. High doses of NSAIDs (ibuprofen (>1200 mg/day), naproxen (>750 mg/day), and rofecoxib (>25 mg/day) increase the likelihood of myocardial infarction after subacute exposure (1 week) and are exceptionally harmful after prolonged exposure (1 month) [112]. Canakinumab, recently marketed to limit single cytokine IL-1β (out of total 33 cytokines), increases the cases of fetal infection after prolonged treatment, suggestive of immune suppression rather than anti-inflammatory benefits [116]. Even aspirin, the most commonly recommended over-the-counter drug for preventing cardiovascular disease, increases the risk of major hemorrhaging and fails to lower the risk of cardiovascular disease, particularly in elderly patients [117]. Other drugs that are used in disease treatments can also induce HF. Cancer therapies can cause a variety of cardiac issues, including ischemia, cardiomyopathy, HF, myocarditis, arrhythmias, vascular disease, hypertension, and hyperlipidemia [118]. An anti-cancer drug, doxorubicin, causes dysregulation of the lipoxygenase (LOX) and cyclooxygenase (COX) pathways leading to splenic contraction and cardiac cachexia in mice [119]. Anti-diabetic drugs like sulphonylureas, glitazones, and some DPP4 inhibitors cause an increased risk of HF in patients with type 2 diabetes [120]. Amphetamines induce critical coronary artery stenosis, ischemia, hypoxia and myocardial necrosis [121]. Long term exposure of amphetamines results in arterial blood pressure elevation, resulting in damage to the blood vessel endothelium and myocardial infarction at the anterolateral wall of the heart [122]. Therapeutics having unclear immunometabolic profiles that directly or indirectly interfere with LOX and COX pathways alter the inflammation-resolution axis, contributing to defective remodeling of the heart [115]. More than century old safe medicine, aspirin failed to lower risk of cardiovascular disease mortality in aging adults and in fact significantly elevated the risk of major hemorrhage compared to placebo controls [117, 123, 124].
9. Future Perspectives
Based on diversified and idiopathic sources of inflammation, such as an imbalance of nutrient intake and energy expenditure and disruptions to the sleep/wake cycle, possible causes of non-resolving inflammation in HF can be attributed to human metabolic evolution or the over-use of labor-saving technology. This expansion of technology and consequential luxury has divided the future perspective of HF syndrome and inflammation management into four different categories: prevention, prognosis, precision, and personalization.
Identify wearable or continuous monitoring Prevention strategies that will help to balance the diet, energy expenditure, and circadian rhythm while maintaining the quality of life to help limit lifestyle-related low-grade, chronic inflammation, and subsequent cardio-metabolic diseases.
Discover the Prognostic or diagnostic molecular or cellular markers that will help to define both non-resolving and resolving inflammation and the classification of cardio-metabolic diseases.
Precise management of any dysregulated factor (diet, physical over-activity or under-activity, and/or circadian rhythm) would limit the possible causes of cardio-metabolic pathologies.
Identify the Personalized requirements of various individuals, depending on sex, ethnicity, and environment to accurately define cardio-metabolic risk factors that will aid within the prevention strategy.
10. Conclusion
Non-resolving and chronic inflammation is an enormous cause of cardio-metabolic health issues impacting patients across a wide spectrum of ages, from womb to tomb, due to the burden of cardiovascular diseases. Balancing the everyday lifestyle-related primary risks by precise alignments of diet, daily action-based energy expenditure, and circadian routines would help to prevent the adverse consequences of non-resolving inflammation and cardiovascular diseases. With advancements in antibiotics and sanitization techniques, low-grade, chronic inflammation sources have varied. We propose the primary causes of low-grade chronic inflammation and related consequences leading to non-resolving inflammation-related cardiovascular diseases and end-stage HF. Future research that focuses on the alignment of these causes of non-resolving inflammation for the prevention of early cardiovascular diseases therefore is necessary to reduce the number of deaths associated with HF.
Supplementary Material
Acknowledgement:
This work was supported by National Institutes of Health (AT006704 and HL132989) and The University of Alabama at Birmingham (UAB) Pittman scholar award to G.V.H.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest- none.
References
- 1.Ponikowski P, et al. , Heart failure: preventing disease and death worldwide. ESC Heart Fail, 2014. 1(1): p. 4–25. [DOI] [PubMed] [Google Scholar]
- 2.Nabeebaccus A, Zheng S, and Shah AM, Heart failure—potential new targets for therapy. British Medical Bulletin, 2016. 119(1): p. 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Driscoll A, et al. , What is the impact of systems of care for heart failure on patients diagnosed with heart failure: a systematic review. BMC Cardiovascular Disorders, 2016. 16: p. 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.A, A.O. and Shah SJ, Diagnosis and Management of Heart Failure with Preserved Ejection Frac-tion: 10 Key Lessons. Current Cardiology Reviews, 2015. 11(1): p. 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inamdar AA and Inamdar AC, Heart Failure: Diagnosis, Management and Utilization. Journal of clinical medicine, 2016. 5(7): p. 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delp MD, et al. , Apollo Lunar Astronauts Show Higher Cardiovascular Disease Mortality: Possible Deep Space Radiation Effects on the Vascular Endothelium. Scientific Reports, 2016. 6: p. 29901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ade CJ, et al. , Incidence Rate of Cardiovascular Disease End Points in the National Aeronautics and Space Administration Astronaut Corps. Journal of the American Heart Association, 2017. 6(8): p. e005564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross R, The pathogenesis of atherosclerosis--an update. N Engl J Med, 1986. 314(8): p. 488–500. [DOI] [PubMed] [Google Scholar]
- 9.Tromp J, et al. , Heart Failure With Preserved Ejection Fraction in the Young. Circulation, 2018. 138(24): p. 2763–2773. [DOI] [PubMed] [Google Scholar]
- 10.Halade GV and Kain V, Obesity and Cardiometabolic Defects in Heart Failure Pathology. Compr Physiol, 2017. 7(4): p. 1463–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tourki B and Halade GV, The failing of the obesity paradox in the failing heart. Am J Physiol Heart Circ Physiol, 2018. [DOI] [PMC free article] [PubMed]
- 12.Westphal JG, et al. , The MOGE(S) classification for cardiomyopathies: current status and future outlook. Heart Fail Rev, 2017. 22(6): p. 743–752. [DOI] [PubMed] [Google Scholar]
- 13.Arbustini E, et al. , The MOGE(S) classification for a phenotype-genotype nomenclature of cardiomyopathy: endorsed by the World Heart Federation. J Am Coll Cardiol, 2013. 62(22): p. 2046–72. [DOI] [PubMed] [Google Scholar]
- 14.Segovia Cubero J, et al. , Heart Failure: Etiology and Approach to Diagnosis. Revista Española de Cardiología (English Edition), 2004. 57(03): p. 250–259. [PubMed] [Google Scholar]
- 15.Follath F, Ischemic versus non-ischemic heart failure: should the etiology be determined? Heart Fail Monit, 2001. 1(4): p. 122–5. [PubMed] [Google Scholar]
- 16.Van Linthout S and Tschöpe C, Inflammation – Cause or Consequence of Heart Failure or Both? Current Heart Failure Reports, 2017. 14(4): p. 251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westman PC, et al. , Inflammation as a Driver of Adverse Left Ventricular Remodeling After Acute Myocardial Infarction. J Am Coll Cardiol, 2016. 67(17): p. 2050–60. [DOI] [PubMed] [Google Scholar]
- 18.Dick SA and Epelman S, Chronic Heart Failure and Inflammation: What Do We Really Know? Circ Res, 2016. 119(1): p. 159–76. [DOI] [PubMed] [Google Scholar]
- 19.Yndestad A, et al. , Systemic inflammation in heart failure--the whys and wherefores. Heart Fail Rev, 2006. 11(1): p. 83–92. [DOI] [PubMed] [Google Scholar]
- 20.Sansbury BE and Spite M, Resolution of Acute Inflammation and the Role of Resolvins in Immunity, Thrombosis and Vascular Biology. Circulation research, 2016. 119(1): p. 113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kain V, Prabhu SD, and Halade GV, Inflammation revisited: inflammation versus resolution of inflammation following myocardial infarction. Basic Res Cardiol, 2014. 109(6): p. 444. [DOI] [PubMed] [Google Scholar]
- 22.Hotamisligil GS, Inflammation, metaflammation and immunometabolic disorders. Nature, 2017. 542: p. 177. [DOI] [PubMed] [Google Scholar]
- 23.Lopez EF, et al. , Obesity superimposed on aging magnifies inflammation and delays the resolving response after myocardial infarction. American journal of physiology. Heart and circulatory physiology, 2015. 308(4): p. H269–H280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soehnlein O and Lindbom L, Phagocyte partnership during the onset and resolution of inflammation. Nature Reviews Immunology, 2010. 10: p. 427. [DOI] [PubMed] [Google Scholar]
- 25.Auffray C, et al. , Monitoring of Blood Vessels and Tissues by a Population of Monocytes with Patrolling Behavior. Science, 2007. 317(5838): p. 666. [DOI] [PubMed] [Google Scholar]
- 26.Kono H and Rock KL, How dying cells alert the immune system to danger. Nature reviews. Immunology, 2008. 8(4): p. 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maddox JF, et al. , Lipoxin A4 Stable Analogs Are Potent Mimetics That Stimulate Human Monocytes and THP-1 Cells via a G-protein-linked Lipoxin A4 Receptor. Journal of Biological Chemistry, 1997. 272(11): p. 6972–6978. [DOI] [PubMed] [Google Scholar]
- 28.Shih H, et al. , The aging heart and post-infarction left ventricular remodeling. Journal of the American College of Cardiology, 2010. 57(1): p. 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woulfe KC and Bruns DR, From pediatrics to geriatrics: Mechanisms of heart failure across the life-course. J Mol Cell Cardiol, 2018. 126: p. 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halade GV, et al. , Aging dysregulates D- and E-series resolvins to modulate cardiosplenic and cardiorenal network following myocardial infarction. Aging, 2016. 8(11): p. 2611–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dzau V and Braunwald E, Resolved and unresolved issues in the prevention and treatment of coronary artery disease: a workshop consensus statement. Am Heart J, 1991. 121(4 Pt 1): p. 1244–63. [DOI] [PubMed] [Google Scholar]
- 32.Halade GV, Kain V, and Ingle KA, Heart functional and structural compendium of cardiosplenic and cardiorenal networks in acute and chronic heart failure pathology. Am J Physiol Heart Circ Physiol, 2018. 314(2): p. H255–h267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halade GV, et al. , Splenic leukocytes define the resolution of inflammation in heart failure. Science signaling, 2018. 11(520): p. eaao1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kain V, et al. , Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J Mol Cell Cardiol, 2015. 84: p. 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halade GV, Kain V, and Serhan CN, Immune responsive resolvin D1 programs myocardial infarction-induced cardiorenal syndrome in heart failure. Faseb j, 2018. 32(7): p. 3717–3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jadapalli JK and Halade GV, Unified nexus of macrophages and maresins in cardiac reparative mechanisms. Faseb j, 2018. 32(10): p. 5227–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halade GV, et al. , Lipoxygenase drives Lipidomic and metabolic reprogramming in ischemic heart failure. Metabolism, 2019. [DOI] [PubMed]
- 38.Kain V, et al. , Genetic deletion of 12/15 lipoxygenase promotes effective resolution of inflammation following myocardial infarction. J Mol Cell Cardiol, 2018. 118: p. 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halade GV, et al. , Interaction of 12/15-lipoxygenase with fatty acids alters the leukocyte kinetics leading to improved postmyocardial infarction healing. Am J Physiol Heart Circ Physiol, 2017. 313(1): p. H89–h102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neckar J, et al. , Epoxyeicosatrienoic acid analog EET-B attenuates post-myocardial infarction remodeling in spontaneously hypertensive rats. Clin Sci (Lond), 2019. 133(8): p. 939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neckar J, et al. , Infarct size-limiting effect of epoxyeicosatrienoic acid analog EET-B is mediated by hypoxia-inducible factor-1alpha via downregulation of prolyl hydroxylase 3. Am J Physiol Heart Circ Physiol, 2018. 315(5): p. H1148–h1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brake R and Jones ID, Chronic heart failure part 1: pathophysiology, signs and symptoms. Nurs Stand, 2017. 31(19): p. 54–63. [DOI] [PubMed] [Google Scholar]
- 43.Heymans S, et al. , Inflammation as a therapeutic target in heart failure? A scientific statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. European Journal of Heart Failure, 2009. 11(2): p. 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frantz S, Bauersachs J, and Ertl G, Post-infarct remodelling: contribution of wound healing and inflammation. Cardiovasc Res, 2009. 81(3): p. 474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altabas V, Diabetes, Endothelial Dysfunction, and Vascular Repair: What Should a Diabetologist Keep His Eye on? International Journal of Endocrinology, 2015. 2015: p. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burr GO and Burr MM, Nutrition classics from The Journal of Biological Chemistry 82:345–67, 1929. A new deficiency disease produced by the rigid exclusion of fat from the diet. Nutr Rev, 1973. 31(8): p. 248–9. [DOI] [PubMed] [Google Scholar]
- 47.Hu S, et al. , Dietary Fat, but Not Protein or Carbohydrate, Regulates Energy Intake and Causes Adiposity in Mice. Cell Metab, 2018. 28(3): p. 415–431.e4. [DOI] [PubMed] [Google Scholar]
- 48.Kain V, et al. , Obesogenic diet in aging mice disrupts gut microbe composition and alters neutrophil:lymphocyte ratio, leading to inflamed milieu in acute heart failure. Faseb j, 2019: p. fj201802477R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kain V, et al. , Excess omega-6 fatty acids influx in aging drives metabolic dysregulation, electrocardiographic alterations, and low-grade chronic inflammation. Am J Physiol Heart Circ Physiol, 2018. 314(2): p. H160–h169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Halade GV, et al. , Obesity-mediated inflammatory microenvironment stimulates osteoclastogenesis and bone loss in mice. Exp Gerontol, 2011. 46(1): p. 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kain V and Halade GV, Metabolic and Biochemical Stressors in Diabetic Cardiomyopathy. Front Cardiovasc Med, 2017. 4: p. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nuttall FQ, Body Mass Index: Obesity, BMI, and Health A Critical Review. Nutrition Today, 2015. 50(3): p. 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lai M, Chandrasekera PC, and Barnard ND, You are what you eat, or are you? The challenges of translating high-fat-fed rodents to human obesity and diabetes. Nutrition & Diabetes, 2014. 4(9): p. e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu S, et al. , Dietary Fat, but Not Protein or Carbohydrate, Regulates Energy Intake and Causes Adiposity in Mice. Cell Metabolism, 2018. 28(3): p. 415–431.e4. [DOI] [PubMed] [Google Scholar]
- 55.Obesity and overweight 2016. [cited 2018; World Health Organization (WHO)]. Available from: (http://www.who.int/mediacentre/factsheets/fs311/en/.
- 56.International Diabetes Federation 2012. [cited 2018; 5th Edition:[Available from: http://www.idf.org/diabetesatlas. [Google Scholar]
- 57.DiNicolantonio JJ and O’Keefe JH, Good Fats versus Bad Fats: A Comparison of Fatty Acids in the Promotion of Insulin Resistance, Inflammation, and Obesity. Missouri Medicine, 2017. 114(4): p. 303–307. [PMC free article] [PubMed] [Google Scholar]
- 58.Russo L and Lumeng CN, Properties and functions of adipose tissue macrophages in obesity. Immunology 0(ja). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dadvar S, et al. , The weight of nutrients: kynurenine metabolites in obesity and exercise. Journal of Internal Medicine, 2018. 284(5): p. 519–533. [DOI] [PubMed] [Google Scholar]
- 60.Westerterp KR and Speakman JR, Physical activity energy expenditure has not declined since the 1980s and matches energy expenditures of wild mammals. International Journal Of Obesity, 2008. 32: p. 1256. [DOI] [PubMed] [Google Scholar]
- 61.Haslam DW and James WPT, Obesity. The Lancet, 2005. 366(9492): p. 1197–1209. [DOI] [PubMed] [Google Scholar]
- 62.Carvalheira JBC, Qiu Y, and Chawla A, Blood spotlight on leukocytes and obesity. Blood, 2013. 122(19): p. 3263–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hower IM, Harper SA, and Buford TW, Circadian Rhythms, Exercise, and Cardiovascular Health. Journal of Circadian Rhythms, 2018. 16: p. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lavie CJ, et al. , Exercise and the Cardiovascular System: Clinical Science and Cardiovascular Outcomes. Circulation research, 2015. 117(2): p. 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Keefe JH, O’Keefe EL, and Lavie CJ, The Goldilocks Zone for Exercise: Not Too Little, Not Too Much. Missouri Medicine, 2018. 115(2): p. 98–105. [PMC free article] [PubMed] [Google Scholar]
- 66.Díaz BB, et al. , Myokines, physical activity, insulin resistance and autoimmune diseases. Immunology Letters, 2018. 203: p. 1–5. [DOI] [PubMed] [Google Scholar]
- 67.Eijsvogels TMH, Thompson PD, and Franklin BA, The “Extreme Exercise Hypothesis”: Recent Findings and Cardiovascular Health Implications. Current Treatment Options in Cardiovascular Medicine, 2018. 20(10): p. 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pedersen BK and Febbraio MA, Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nature Reviews Endocrinology, 2012. 8: p. 457. [DOI] [PubMed] [Google Scholar]
- 69.Lavie CJ, et al. , Exercise is Medicine – The Importance of Physical Activity, Exercise Training, Cardiorespiratory Fitness and Obesity in the Prevention and Treatment of Type 2 Diabetes. European Endocrinology, 2014. 10(1): p. 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fletcher GF, et al. , Promoting Physical Activity and Exercise: JACC Health Promotion Series. Journal of the American College of Cardiology, 2018. 72(14): p. 1622–1639. [DOI] [PubMed] [Google Scholar]
- 71.Cattadori G, et al. , Exercise and heart failure: an update. ESC heart failure, 2017. 5(2): p. 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brinker SK, et al. , Association of cardiorespiratory fitness with left ventricular remodeling and diastolic function: the Cooper Center Longitudinal Study. JACC. Heart failure, 2014. 2(3): p. 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.León-Latre M, et al. , Sedentary Lifestyle and Its Relation to Cardiovascular Risk Factors, Insulin Resistance and Inflammatory Profile. Revista Española de Cardiología (English Edition), 2014. 67(06): p. 449–455. [DOI] [PubMed] [Google Scholar]
- 74.Morrison BN, et al. , Assessment of cardiovascular risk and preparticipation screening protocols in masters athletes: the Masters Athlete Screening Study (MASS): a cross-sectional study. BMJ Open Sport — Exercise Medicine, 2018. 4(1): p. e000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peake JM, et al. , Recovery of the immune system after exercise. Journal of Applied Physiology, 2017. 122(5): p. 1077–1087. [DOI] [PubMed] [Google Scholar]
- 76.Jamurtas AZ, et al. , The Effects of Acute Low-Volume HIIT and Aerobic Exercise on Leukocyte Count and Redox Status. Journal of Sports Science & Medicine, 2018. 17(3): p. 501–508. [PMC free article] [PubMed] [Google Scholar]
- 77.Young ME, et al. , Cardiomyocyte-specific BMAL1 Plays Critical Roles in Metabolism, Signaling, and Maintenance of Contractile Function of the Heart. Journal of biological rhythms, 2014. 29(4): p. 257–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marcheva B, et al. , Disruption of the Clock Components CLOCK and BMAL1 Leads to Hypoinsulinemia and Diabetes. Nature, 2010. 466(7306): p. 627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang XS, et al. , Shift work and chronic disease: the epidemiological evidence. Occupational Medicine (Oxford, England), 2011. 61(2): p. 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ingle KA, et al. , Cardiomyocyte-specific Bmal1 deletion in mice triggers diastolic dysfunction, extracellular matrix response, and impaired resolution of inflammation. Am J Physiol Heart Circ Physiol, 2015. 309(11): p. H1827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Colas RA, et al. , Impaired Production and Diurnal Regulation of Vascular RvDn-3 DPA Increase Systemic Inflammation and Cardiovascular Disease. Circ Res, 2018. 122(6): p. 855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scheer FAJL, et al. , Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences of the United States of America, 2009. 106(11): p. 4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hermansson J, et al. , Shift work, parental cardiovascular disease and myocardial infarction in males. Occupational Medicine, 2018. 68(2): p. 120–125. [DOI] [PubMed] [Google Scholar]
- 84.Sato S, et al. , A Circadian Clock Gene, Rev-erbα, Modulates the Inflammatory Function of Macrophages through the Negative Regulation of Ccl2 Expression. The Journal of Immunology, 2014. 192(1): p. 407–417. [DOI] [PubMed] [Google Scholar]
- 85.Scheiermann C, Kunisaki Y, and Frenette PS, Circadian control of the immune system. Nature Reviews Immunology, 2013. 13: p. 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Turek FW, et al. , Obesity and Metabolic Syndrome in Circadian Clock Mutant Mice. Science (New York, N.Y.), 2005. 308(5724): p. 1043–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abu Farha R and Alefishat E, Shift Work and the Risk of Cardiovascular Diseases and Metabolic Syndrome Among Jordanian Employees. Oman Medical Journal, 2018. 33(3): p. 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Poggiogalle E, Jamshed H, and Peterson CM, Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism, 2018. 84: p. 11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wirtz PH and von Känel R, Psychological Stress, Inflammation, and Coronary Heart Disease. Current Cardiology Reports, 2017. 19(11): p. 111. [DOI] [PubMed] [Google Scholar]
- 90.(CSHS), C.f.S.o.H.S. Acute vs Chronic Stress 2017. 05/17/2019]; Available from: https://humanstress.ca/stress/understand-your-stress/acute-vs-chronic-stress/.
- 91.Lagraauw HM, Kuiper J, and Bot I, Acute and chronic psychological stress as risk factors for cardiovascular disease: Insights gained from epidemiological, clinical and experimental studies. Brain, Behavior, and Immunity, 2015. 50: p. 18–30. [DOI] [PubMed] [Google Scholar]
- 92.Dimsdale JE, Psychological stress and cardiovascular disease. Journal of the American College of Cardiology, 2008. 51(13): p. 1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Slopen N, et al. , Job strain, job insecurity, and incident cardiovascular disease in the Women’s Health Study: results from a 10-year prospective study. PloS one, 2012. 7(7): p. e40512–e40512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kivimäki M and Kawachi I, Work Stress as a Risk Factor for Cardiovascular Disease. Current cardiology reports, 2015. 17(9): p. 630-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cohen BE, Edmondson D, and Kronish IM, State of the Art Review: Depression, Stress, Anxiety, and Cardiovascular Disease. American journal of hypertension, 2015. 28(11): p. 1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang L and Piña IL, Stress-Induced Cardiomyopathy. Heart Failure Clinics, 2019. 15(1): p. 41–53. [DOI] [PubMed] [Google Scholar]
- 97.Templin C, et al. , Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med, 2015. 373(10): p. 929–38. [DOI] [PubMed] [Google Scholar]
- 98.Fullerton JN and Gilroy DW, Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov, 2016. 15(8): p. 551–67. [DOI] [PubMed] [Google Scholar]
- 99.Heinz J, Marinello M, and Fredman G, Pro-resolution therapeutics for cardiovascular diseases. Prostaglandins Other Lipid Mediat, 2017. 132: p. 12–16. [DOI] [PubMed] [Google Scholar]
- 100.Serhan CN, Pro-resolving lipid mediators are leads for resolution physiology. Nature, 2014. 510(7503): p. 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Serhan CN, et al. , Resolution of inflammation: state of the art, definitions and terms. Faseb j, 2007. 21(2): p. 325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Serhan CN, Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. Faseb j, 2017. 31(4): p. 1273–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Serhan CN, Chiang N, and Van Dyke TE, Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol, 2008. 8(5): p. 349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bronzato S and Durante A, Dietary Supplements and Cardiovascular Diseases. International journal of preventive medicine, 2018. 9: p. 80–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goel A, et al. , Fish, Fish Oils and Cardioprotection: Promise or Fish Tale? International journal of molecular sciences, 2018. 19(12): p. 3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Halade GV, et al. , Fish oil decreases inflammation and reduces cardiac remodeling in rosiglitazone treated aging mice. Pharmacol Res, 2011. 63(4): p. 300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kain V and Halade GV, Immune responsive resolvin D1 programs peritoneal macrophages and cardiac fibroblast phenotypes in diversified metabolic microenvironment. J Cell Physiol, 2019. 234(4): p. 3910–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aggarwal M, et al. , Lifestyle Modifications for Preventing and Treating Heart Failure. Journal of the American College of Cardiology, 2018. 72(19): p. 2391–2405. [DOI] [PubMed] [Google Scholar]
- 109.Salive ME, Multimorbidity in older adults. Epidemiol Rev, 2013. 35: p. 75–83. [DOI] [PubMed] [Google Scholar]
- 110.Forman DE, et al. , Multimorbidity in Older Adults With Cardiovascular Disease. J Am Coll Cardiol, 2018. 71(19): p. 2149–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Leelakanok N, et al. , Association between polypharmacy and death: A systematic review and meta-analysis. J Am Pharm Assoc (2003), 2017. 57(6): p. 729–738.e10. [DOI] [PubMed] [Google Scholar]
- 112.Arfè A, et al. , Non-steroidal anti-inflammatory drugs and risk of heart failure in four European countries: nested case-control study. BMJ, 2016. 354. [DOI] [PubMed] [Google Scholar]
- 113.Huang SP, et al. , Nonsteroidal Anti-Inflammatory Drugs and Risk of First Hospitalization for Heart Failure in Patients with No History of Heart Failure: A Population-Based Case-Crossover Study. Drug Saf, 2018. [DOI] [PubMed] [Google Scholar]
- 114.Rotunno R, et al. , NSAIDs and heart failure: A dangerous relationship. Monaldi Arch Chest Dis, 2018. 88(2): p. 950. [DOI] [PubMed] [Google Scholar]
- 115.Halade GV, et al. , Subacute treatment of carprofen facilitate splenocardiac resolution deficit in cardiac injury. J Leukoc Biol, 2018. [DOI] [PMC free article] [PubMed]
- 116.Ridker PM, et al. , Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. New England Journal of Medicine, 2017. 377(12): p. 1119–1131. [DOI] [PubMed] [Google Scholar]
- 117.McNeil JJ, et al. , Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly. N Engl J Med, 2018. 379(16): p. 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dong J and Chen H, Cardiotoxicity of Anticancer Therapeutics. Frontiers in Cardiovascular Medicine, 2018. 5: p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jadapalli JK, et al. , Doxorubicin triggers splenic contraction and irreversible dysregulation of COX and LOX that alters inflammation-resolution program in the myocardium. American Journal of Physiology-Heart and Circulatory Physiology, 2018. [DOI] [PMC free article] [PubMed]
- 120.Linhart A and Belohlavek J, [Type 2 diabetes mellitus and heart failure]. Vnitr Lek 62(7–8): p. 592–7. [PubMed] [Google Scholar]
- 121.Waksman J, et al. , Acute Myocardial Infarction Associated With Amphetamine Use. Mayo Clinic Proceedings, 2001. 76(3): p. 323–326. [DOI] [PubMed] [Google Scholar]
- 122.Smedra A, Szustowski S, and Berent J, Amphetamine-related myocardial infarction in a 42-year old man. Arch Med Sadowej Kryminol, 2015. 65(3): p. 173–81. [DOI] [PubMed] [Google Scholar]
- 123.Valgimigli M, The remarkable story of a wonder drug, which now comes to an end in the primary prevention setting: say bye-bye to aspirin! Eur Heart J, 2018. [DOI] [PubMed] [Google Scholar]
- 124.McNeil JJ, et al. , Effect of Aspirin on All-Cause Mortality in the Healthy Elderly. N Engl J Med, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


