Abstract
Background & Aims:
Gastric cancer is the leading cause of infection-related cancer death and the third-leading cause of cancer death worldwide. The effect of immigration on gastric cancer risk is not well-defined but might be helpful for screening or surveillance endeavors. We performed a systematic review and meta-analysis to define the risk of gastric cancer in immigrants from high-incidence regions to low-incidence regions (Western Europe, Australia, Brazil, Canada, Israel, and the United States).
Methods:
We searched MEDLINE and EMBASE databases, from January 1980 to January 2019, for studies that identified immigrants from high-incidence regions of gastric cancer, provided clear definitions of immigrant and reference populations, and provided sufficient data to calculate gastric cancer incidence and gastric cancer-related mortality. We performed meta-analyses of standardized incidence ratios (SIR) for first-generation immigrants from high- to low-incidence regions, stratified by immigrant generation, sex, and anatomic and histologic subtype, when data were available.
Results:
We identified 38 cohort studies that met our inclusion criteria. Thirteen studies of 21 distinct populations reported significantly increased SIRs for gastric cancer in first-generation foreign-born immigrants (men SIR range, 1.24–4.50 and women SIR range, 1.27–5.05). The pooled SIR for immigrants with all types of gastric cancer was 1.66 (95% CI, 1.52–1.80) for men and 1.83 (95% CI, 1.69–1.98) for women. Nine studies from 2 high-incidence populations (the former Soviet Union and Japan) reported an increased gastric cancer standardized mortality ratio in first-generation immigrants who migrated to regions of low incidence (former Soviet Union immigrants, 1.44–1.91 for men and 1.40–2.56 for women).
Conclusions:
Immigrants from regions with a high incidence of gastric cancer in regions of low incidence maintain a higher risk of gastric cancer and related mortality, based on a comprehensive systematic review and meta-analysis. Assessment of immigrant generation along with other risk factors might help identify high-risk populations for prevention and therapeutic interventions.
Keywords: H. pylori, stomach, tumor, environment
INTRODUCTION
Globally, gastric cancer is the leading cause of infection-related cancer mortality and the third leading cause of cancer-related mortality overall.1–4 Approximately one million incident cases are projected annually, with the majority of these cases occurring in East Asia, Latin America, and Eastern Europe. The United States (U.S.) is considered a low incidence nation cancer overall, with an estimated 28,000 new cases occurring in 2017; however, there are clear racial and ethnic differences in disease burden, with incidence rates nearly double in Hispanics and non-Hispanic blacks, and potentially much higher in immigrants from countries where gastric cancer is endemic.5 This is particularly relevant since the U.S. is home to over 43 million foreign-born individuals with diverse ethnic backgrounds, an estimated 29% of whom are from countries of high gastric cancer incidence. 6
H. pylori is the most common chronic bacterial infection in the world, with approximately half of the world’s population being infected (~4.4 billion)7, and is the strongest known risk factor for gastric cancer. Infection prevalence ranges from 20–35% in most high-income countries to 60–90% in low resource settings.8,9 Given the marked geographic variability of gastric cancer, as manifest by the African, Asian, and Latin American enigmas -- it is plausible that region of origin is an important determinant of gastric cancer risk for foreign-born immigrants who migrate to low-incidence regions. 10–12 Individuals may “import” their synergistic host (e. g., genetic),13,14 and H. pylori strain-associated (e.g., cagA, vacAs1m1) gastric cancer risks,15 the latter of which are established most often in early childhood years in endemic regions and reflect the timing of H. pylori acquisition. This risk is further modulated by diet, the microbiome, behavioral, and environmental factors, and may be influenced by the age at immigration and by the extent of individual acculturation.16,17
The risk of gastric cancer among individuals who immigrate from high- to low-gastric cancer incidence regions is not known, but might have important implications for informing screening and surveillance practices in otherwise low-gastric cancer incidence areas, such as the U.S. Indeed, it was recently shown that gastric cancer screening and surveillance if gastric preneoplasia is detected is cost-effective for racial/ethnic minorities residing in the U.S.18 We conducted a systematic review and meta-analysis analyzing gastric cancer risk in immigrants from regions of high- to low-gastric cancer incidence, stratified by immigrant generation, gender, and anatomic/histologic subtype where possible.
METHODS
Data Sources and Searches
We conducted a systematic review of the published literature from January 1980 to January 2019 using MEDLINE and EMBASE (initial search November 6th, 2017, repeat search January 12th, 2019) to identify studies reporting the risk of gastric cancer in immigrants from areas of high to areas of low gastric cancer incidence, as defined below. The following search string was used in EMBASE: “stomach cancer.mp. or exp stomach cancer” AND “ exp migrant/ or migrant.mp.” with a filter of “limit to (human)”. The following search string was used in MEDLINE: “stomach cancer.mp. or exp Stomach Neoplasms” AND “immigrant.mp. or exp “Emigrants and Immigrants”“ with a filter of “limit 3 to (humans)”. References from studies meeting inclusion criteria and relevant reviews which were identified via the search string were also reviewed for eligibility.
Inclusion and exclusion criteria
Predefined inclusion criteria were: 1) clear documentation of the risk of gastric cancer in immigrants from a high incidence country relative to native born inhabitants of a low incidence country; and 2) clear definition of immigrant and reference populations. High incidence countries were defined as countries with an age standardized rate (ASR) in males and females greater than 10 per 100,000 of the world standard population, as defined by the International Agency for Research on Cancer (IARC) 2012 data, while low incidence countries were defined as an ASR less than, or equal to 10.19 The Preferred Items for Systematic Reviews and Meta-Analysis (PRISMA) methodology was followed for this study. 20
Data extraction
Two authors (BP and SCS) reviewed all abstracts for eligibility according to the inclusion criteria defined above, with discrepancies resolved by DRM. Studies were assessed for quality using the Newcastle-Ottawa Scale (NOS) Quality Assessment Form for cohort studies. The following study characteristics were abstracted for each study meeting inclusion criteria using a standardized data collection form: study design, publication year, basic population demographics (age, sex), duration of follow-up and/or time interval of the study, immigrant country of origin, immigrant generation, and the measure(s) of association (e.g. relative risk, odds ratio). Data were stratified by sex, immigrant country of origin, and immigrant generation (where available). Anatomic location (cardia vs. non-cardia) and histologic classification of gastric cancer (intestinal-type vs. diffuse-type) were documented when provided. Age of immigration was abstracted when provided. Multi-country immigrant groups were classified as being high incidence if at least one country in the group was a high-incidence country as previously defined.
Qualitative Synthesis and Quantitative Statistical Analysis
Details of each study were qualitatively summarized. Meta-analysis of standardized incidence ratios (SIR) stratified by immigrant generation, gender, and anatomic/histologic subtype were planned a priori whenever possible. For the other measures of association of incidence and mortality, results were summarized in tables. Heterogeneity was estimated with chi-squared and I2 test statistics. The chi-squared test suggests heterogeneity between studies when the P-value is less than 0.15. We further used I2 cut-offs of <30%, 30–59%, 60–75%, and >75% to for low, moderate, substantial, and considerable heterogeneity, respectively. 21 Random effects models were used to calculate aggregated estimates. Publication bias was investigated by visual inspection of funnel plots. All statistical analyses were performed with Stata version SE 13 (Stata-Corp, College Station, TX , USA).
RESULTS
The initial search yielded 188 studies, with 159 unique studies after removal of duplicates. Eighty-five studies were excluded based on title and abstract screening. Of the 74 full texts reviewed, 36 were excluded for the reasons detailed in Figure 1. No additional studies were identified based on review of the cited references. The remaining 38 full texts met all predefined inclusion criteria. (PRISMA Flow Diagram, Figure 1)
Figure 1.
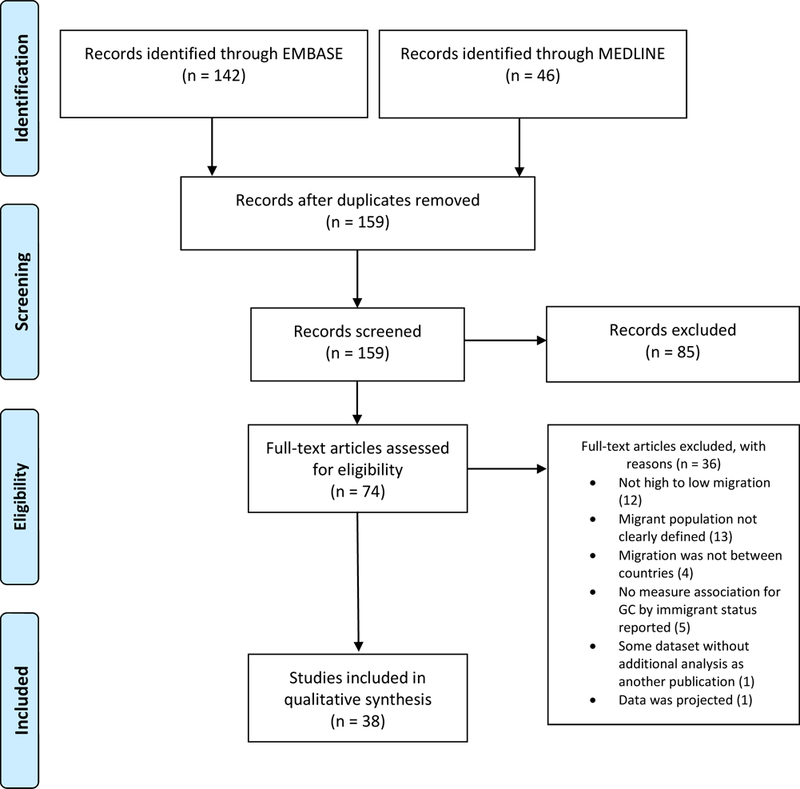
PRISMA Flow Diagram
All 38 studies were cohort studies published between 1984–2019 and included data collected from 1958 to 2014. The details of each of the included studies categorized by destination country are provided in Tables 1 and 2. Twenty (52.6%) studies reported some form of gastric cancer incidence rates, twenty (52.6%) studies reported a measure or estimate of mortality rates, and three (7.9%) studies reported both incidence and mortality rates. The data sources for these studies included national registries (n=17, 44.7%), state registries (n=14, 36.8%), city registries (n=4, 10.5%) or a combination of state and national registries (n=3, 7.9%).
Table 1:
Immigrant Gastric Cancer Risk Study Characteristics
| Country | Authors and Year | Group | Type of Ratio | Increase | Magnitude | ||
|---|---|---|---|---|---|---|---|
| Country/Region | Sex (M,F) | Generation (1st, 2nd) | |||||
| Canada | Sutradhar et al., 2018 72 | All immigrants, East Asia and Pacific, Europe and Central Asia, China, Korea | Both | 1st | Hazard Ratio | Yes | 1.10–4.44 |
| Denmark | Ducarroz et al., 2015 24 | All immigrants, Former Yugoslavia, Europe | Men | 1st | Standardized Incidence Ratio | Yes | 1.28–2.23 |
| Germany | Kaucher, Kajüter, Becher, & Winkler, 2018 27 | Former Soviet Union | Men | 1st | Standardized Incidence Ratio | Yes | 1.62 |
| Cho, Jaehn, Holleczek, Becher, & Winkler, 2018 28 | Former Soviet Union | Both | 1st | Standardized Incidence Ratio | Yes | 2.22–2.32 | |
| Jaehn et al., 2016 43 | Former Soviet Union | Both | 1st | Standardized Incidence Ratio | Yes | 1.98–3.04 | |
| Winkler, Holleczek, Stegmaier, & Becher, 2014 73 | Former Soviet Union | Both | 1st | Standardized Incidence Ratio | Yes | 2.68–3.04 | |
| Winkler, Ott, Holleczek, Stegmaier, & Becher, 2009 29 | Former Soviet Union | Both | 1st | Standardized Incidence Ratio | Yes | 1.44–2.81 | |
| Netherlands | Arnold et al., 2013 44 | Turkey | Both | 1st | Standardized Incidence Ratio | Yes | 1.7–2.2 |
| Arnold et al., 2011 45 | Turkey | Both | 1st | Standardized Incidence Ratio | Yes | 1.4–1.9 | |
| Norway | Hjerkind et al., 2017 74 | Eastern Europe | Both | 1st | Age Standardized Rate | Yes | 7.5–13.4 |
| Sweden | Mousavi & Hemminki, 2015 40 | All immigrants, High Risk Countries, South America, Other East Europe, Asian Arab, Southeast Asia, East Asia, Former Yugoslavia, Iran, Russia, Turkey | Both | Both | Standardized Incidence Ratio | Yes | 1.20–4.18 |
| Mousavi, Sundquist, & Hemminki, 2013 75 | Chile | Men | 1st | Age Standardized Incidence Rate | Yes | 17.6 | |
| Mousavi et al., 2012 23 | High Risk Countries, Other Countries, All Immigrants | Both | 1st | Standardized Incidence Ratio | Yes | 1.77–2.33 | |
| Hemminki & Li, 2002 41 | Eastern Europe, Asia, All descendants | Both | 2nd | Standardized Incidence Ratio | No | .78–4.37 | |
| Hemminki, Li, & Czene, 2002 76 | All immigrants, Eastern Europe, Estonia, Yugoslavia, Asia, Turkey, Romania | Both | 1st | Standardized Incidence Ratio | Yes | 1.24 – 5.05 | |
| Nilsson et al., 1993 77 | Estonia | Both | 1st | Standardized Incidence Ratio | Yes | 1.56–2.04 | |
| United States | Ziadeh et al., 2017 38 | Middle East | Both | Both | Proportional Incidence Ratio | Yes | 1.46–3.13 |
| Kamineni et al., 1999 39 | Japan, China | Both | Both | Rate Ratio | Yes | 2.2–5.8 | |
| Cho et al., 199628 | Korea | Both | 1st | 3 year Age Adjusted Cumulative Incidence Rate | Yes | 172 per 100000 | |
| Gregorio et al.., 1992 22 | Portugal | Both | 1st | Standardized Incidence Ratio | Yes | 4.27 | |
| Maskarinec & Noh, 2004 18 | Japan | Both | 1st | Migration Effect* | Yes | 2.06–2.77 |
• Not an incidence rate.
Table 2.
Immigrant gastric cancer mortality data
| Country | Authors and Year | Group | Type of Ratio | Increase | Magnitude | ||
|---|---|---|---|---|---|---|---|
| Country/ Region | Sex (M,F) | Generation (1st, 2nd) | |||||
| Australia | McCredie, Williams, & Coates, 1999 78 | Eastern Europe | Both | 1st | Relative Risk of Death from Cancer | Yes | 2.0–3.1 |
| Belgium | Hemelrijck, Valk, & Vandenheede, 2017 79 | Turkey | Female | 1st | Indirectly Standardized Mortality Rate | Yes | 27.6 (18.6–39.4)a |
| Brazil | Iwasaki et al., 200832 | Japan | Both | 1st | Standardized Mortality Ratio | Yes | 49–88b |
| Iwasaki et al., 200433 | Japan | Both | 1st, 2nd for male only | StandardizedMortality Ratio and Standardized Proportional Mortality Ratio | Yes | 91–118b | |
| Canada | Newman & Spengler, 1984 26 | Soviet Union | Both | 1st | Standardized Mortality Ratio | Yes | 161–256c |
| Germany | Kaucher, Kajüter, Becher, & Winkler, 2018 27 | Former Soviet Union | Both | 1st | Standardized Mortality Ratio | Yes | 1.52–1.62 |
| Cho, Jaehn, Holleczek, Becher, & Winkler, 2018 28 | Former Soviet Union | Both | 1st | Standardized Mortality Ratio | Yes | 1.91–2.15 | |
| Winkler, Ott, Holleczek, Stegmaier, & Becher, 2009 29 | Former Soviet Union | Both | 1st | Standardized Mortality Ratio | Yes | 1.44–2.81 | |
| Kyobutungi, Ronellenfitsch, Razum, & Becher, 2006 25 | Former Soviet Union | Both | 1st | Standardized Mortality Ratio | No | 1.44–1.45 | |
| Israel | Jördis Jennifer Ott, Paltiel, & Becher, 2009 80 | Former Soviet Union | Both | 1st | Standardized Mortality Ratio | Yes | 1.85–1.86 |
| Netherlands | Siemerink, van der Aa, Siesling, Hospers, & Mulder, 2011 46 | Non-western | Both | 1st | Relative Excess Risk of Dying | No | 0.55 (0.43–0.70)d |
| Stirbu et al., 200642 | All migrants | Both | 1st | Relative Risk of Cancer Mortality | No | 1.05–1.35 | |
| Sweden | Nilsson et al., 199748 | Estonia | Both | 1st | Excess Mortality Rate | No | 12.3–42.2%e |
| United States | Singh & Miller, 2004 81 | All immigrant s, China, Japan | Both | 1st | Average Annual Age Adjusted Death Rates | Yes | 1.61–2.19 |
| Fang, Madhavan, & Alderman, 1996 34 | China | Both | 1st | Relative Risk of Age Adjusted Annual Death Rate | Yes | 1.4–1.5 | |
| Stellman & Wang, 1994 35 | China | Both | 1st | Standardized Proportional Cancer Mortality Ratio | Yes | .44-.60f | |
| Multiple: Denmark, England & Wales, France, Netherlands, Scotland, Spain | Ikram et al., 2016 36 | Eastern Europe, Turkey | Both | 1st | Mortality Rate Ratio | Yes | 1.88–1.97 |
| Multiple: Belgium, Denmark, France, Netherlands | Spallek et al., 201237 | Turkey | Both | 1st | Mortality Rate Ratio | Yes | 1.35–1.69 |
| Multiple: Israel and Germany | Ronellenfitsch et al., 2009 30 | Former Soviet Union | Both | 1st | Standardized Mortality Ratio | Yes | 1.44–1.83 |
| Multiple: Israel and Germany | Jördis J Ott, Paltiel, Winkler, & Becher, 2008 31 | Former Soviet Union | Both | 1st | Standardized Mortality Ratio | Yes | 1.42–1.83 |
Notes:
• Belgium female ISMR was 8.9 with 95% CI of 8.5–9.4
• Japanese mortality rate in Japan used as baseline with reference 100
• Canadian mortality ratio of 100 is reference
• 1 was reference for both western immigrants and native patients
• Reference values were given for dichotomous age groups and two categories of follow up (1, 5 years). The authors noted that these were not statistically significant but did not provide confidence intervals for this estimate
• Chinese mortality rate in China used as a baseline with a reference of 1.0
• Statistics listed if they reached statistical and clinical significance as specified by the authors. All statistically significance ratios were greater than the reference low incidence country when considering confidence intervals of at least 95%. Region counted as high risk if at least one of the countries in the region included a high risk country.
The majority (n=33/38, 86.8%) of studies found statistically significant (p < 0.05) increases in either the incidence or mortality of gastric cancer in immigrants from certain high incidence countries or regions immigrating to low incidence countries. The low incidence destination regions included those in Western Europe, Australia, Brazil, Canada, Israel, and the U.S. High incidence countries of emigration included those in Eastern Asia, the former Soviet Union, Eastern Europe, and South America. Studies which were scored as high-quality and demonstrated increased standardized incidence and mortality ratios among immigrants from high- to low-gastric cancer incidence countries are detailed in Figure 2.
Figure 2. Increased gastric cancer incidence and mortality observed in first generation immigrants.
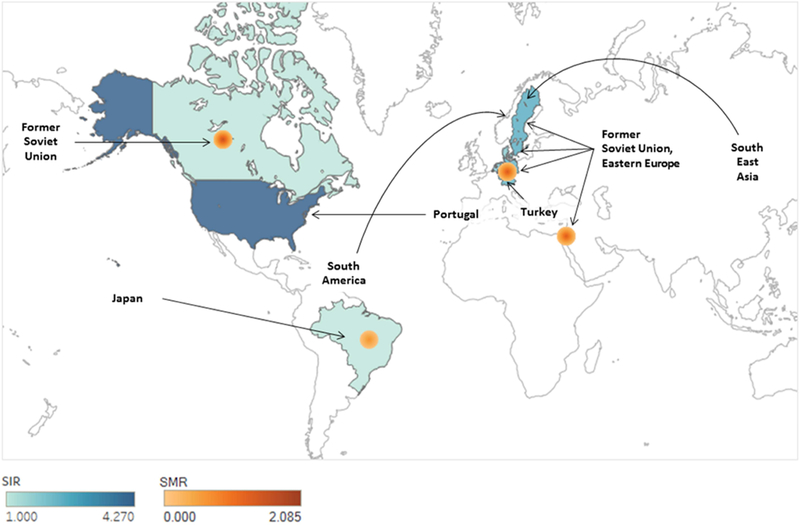
Graphic summary of 26 studies considered to be higher quality reporting standardized incidence ratios (SIR) and standardized mortality ratios (SMR). Details and references available on Table 1 and 2. With the exception of Sweden, all data is specific for first generation immigrants. Migration from Latin America to the United States is not diagramed due to absence of SIR or SMR estimates.
Incidence of gastric cancer among immigrants from high-to low-incidence regions
All thirteen studies that reported SIRs in first generation foreign-born immigrants from high incidence countries demonstrated significantly increased gastric cancer incidence rates compared to the native population. These studies represented 21 distinct populations in 8 nations (Chile, Estonia, Iran, Portugal, Romania, the Former Soviet Union (FSU) /Russia, Turkey, the former Yugoslavia) and 8 broader regions (Asia, Asian Arab countries, East Asia, Eastern Europe, Europe, Other East Europe - Romania, Slovakia, Czechoslovakia, Czech Republic, Hungry, Bulgaria; Other Europe – a broad group including Portugal, Albania, former Macedonia, Moldova, Slovenia; South America, Southeast Asia). These studies found statistically significant increases for both males (SIR range: 1.24–4.50) and females (SIR range: 1.27–5.05) (Table 1). Combined ratios for males and females were reported in two papers (SIR range: 1.77–4.27 22,23), and one study only included males in the analysis (SIR range: 1.28–2.23 24).
The pooled SIR when gastric cancer subtype (anatomic, histologic) was not specified was 1.66 (95% CI 1.52–1.80) for men and 1.83 (95% CI 1.69–1.98), with high risk gastric cancer, defined as noncardia gastric adenocarcinoma (NCGA), intestinal type gastric cancer, adenocarcinoma, or unspecified gastric cancer, calculated as 1.66 (95% CI 1.52–1.81) and 1.83 (95% CI 1.68–1.97) for males and females respectively (Table 3).
Table 3:
Pooled estimates of standardized incidence ratios (SIR) using a random effects meta-analysis model for first generation immigrants from all high risk countries or areas by anatomic and histologic subtypes where data is available
| Pooled SIR | 95% CI | I2 | |
|---|---|---|---|
| All Gastric Cancer | |||
| Female (n=10) 27, 28, 29, 40, 43, 44, 45, 73, 76, 77 | 1.83 | (1.69–1.98) | 29.5 |
| Male (n=11) 24, 27, 28, 29, 40, 43, 44, 45, 73, 76, 77 | 1.66 | (1.52–1.80) | 70.1 |
| High risk Gastric Cancer* | |||
| Female (n=9) 27, 28, 29, 40, 43, 44, 45, 73, 76 | 1.83 | (1.68–1.97) | 29.7 |
| Male (n=10) 24, 27, 28, 29, 40, 43, 44, 45, 73, 76 | 1.66 | (1.52–1.81) | 70.7 |
| Anatomic Subtypes | |||
| Noncardia Gastric Cancer or Unspecified | |||
| Female (n=9) 27, 28, 29, 40, 44, 45, 73, 76, 77 | 1.80 | (1.65–1.95) | 33.9 |
| Male (n=10) 24, 27, 28, 29, 40, 44, 45, 73, 76, 77 | 1.62 | (1.47–1.76) | 70.2 |
| Noncardia Gastric Cancer | |||
| Female (n=3) 40,44,45 | 1.80 | (1.56–2.05) | 10.4 |
| Male (n=3) 40,44,45 | 1.56 | (1.21–1.91) | 80.8 |
| Cardia Gastric Cancer | |||
| Female (n=3) 40,44,45 | 1.69 | (1.13–2.25) | 68.5 |
| Male (n=3) 40,44,45 | 1.67 | (1.17–2.18) | 66.3 |
• Abbreviations: Standardized Incidence Ratio (SIR)
• Non-cardia, unspecified, intestinal type, or adenocarcinoma
Gastric cancer-related mortality among immigrants from high-to low-incidence regions
Twenty studies reported gastric cancer-related mortality, which was reported as standardized mortality ratio (SMR) in half of the studies (n=10/20). Nine of these ten studies reporting SMR reported an increase in gastric cancer-related mortality, and 7 included populations from the FSU. One of these seven studies did not report a statistically significant increased SMR in the FSU immigrant population to Germany25, but the other six did report statistically significant increases when migrating to Germany, Canada, and Israel; sex-specific rates varied from 1.44–1.91 among males, 1.40–2.56 among females, and 1.40–2.56 for combined sexes (Table 2). 26–31 Two studies reported increased SMR for first generation Japanese immigrants to Brazil. One study reported ranges of 61–88 and 49–72 for men and women from 1980 – 2000 respectively (Japanese mortality in Japan used as a reference, with the rates listed found to be statistically significant when compared to the Brazilian born population). 32 The second study reported elevated rates of 97 (95% CI 82–113) and 91 (95% CI 70–117) for males and females respectively (Japanese mortality in Japan used as a reference) compared to Brazilian born rates of 58 (95% CI 56–60) and 54 (95% CI 51–58) for men and women respectively from the period of time analyzed. 33 Additional populations where mortality in first generation immigrants was found to be increased in included Eastern Europe, China, Turkey, and an “all immigrants” category in one study. 34–37 The considerable differences in the standards and methods to determine mortality estimates precluded meta-analysis.
Subsequent immigrant generations
Six (15.8%) of the included studies provided data on multiple generations of immigrants from areas of high to areas of low gastric cancer incidence or mortality. 33,38–42 Four of the six studies reported a persistently elevated incidence or mortality rate in second-generation immigrants compared to the reference population; however, these rates were lower than in first-generation immigrants in all studies. 33,38–40 Of two studies reporting SIR in second generation immigrants, one demonstrated a retained significant SIR above the reference population in the “all immigrant” broad subgroup (SIR 1.20, 95% CI 1.02–1.41). 40 One study of 4,824 (2182 males, 2642 females) second-generation Middle Eastern immigrants (total population 900,854) to the United States reported a proportion incidence ratio of 1.18 (95% CI 0.86 – 1.59) and 1.46 (95% CI 1.01 – 2.05) among second-generation males and females respectively. 38 One study of 281 cases of gastric cancer in second-generation Japanese immigrants to U.S. reports increased rates of gastric cancer with rate ratios 2.80 ( 95% CI 2.20 – 3.60) and 2.90 (95% CI 2.03 – 3.70) for males and females respectively. 39 One study of Japanese descendants in Brazil reported a retained elevated mortality rate for second-generation male immigrants but not females based on standardized proportional mortality ratios. (Table 2). 33
Age at the time of Immigration
Only one study provided sufficiently detailed information regarding age of immigration for immigrant populations. This study reported an increased risk of gastric cancer (SIR 2.79; 95% CI 1.23–6.31) for all immigrants age 0–14 years at the time of immigration to the Netherlands; however, there was no increased risk of gastric cancer (SIR 1.22 95% CI 0.86–1.73 and 1.07 95% 0.54–1.35) among immigrants who immigrated at age 15–29 and >=30 years old. 42
Histologic and anatomic subtypes
Only one study distinguished histologic subtypes of gastric cancer based on the Lauren classification of intestinal- versus diffuse-type gastric cancer. 43 Intestinal-type gastric cancer was increased in immigrants from the FSU for males (SIR 3.04; 95% CI 2.05–4.50) and females (SIR 2.78; 95% CI 1.61–4.79). Whether diffuse-type gastric cancer was increased could not be determined based on the data provided.
One study distinguished histologic subtypes of gastric cancer based on adenocarcinoma versus signet cell-type gastric cancer. 40 Estimated SIRs for adenocarcinoma varied, but ranged from 1.4–3.3 fold higher (p<0.05) among the following high risk groups: total combined immigrants, Former Yugoslavia, Other East Europe, South America, East Asia, Russia, Asian Arab countries, Southeast Asia, South America, and East Asia. Other individual high-risk immigrant groups (Other Europe, Turkey, Iran) did not have a statistically significantly elevated risk of gastric cancer compared to the destination country population (Sweden).
Anatomic classification of gastric cancer was available in five of the included studies (13.2%) 23,39,40,44,45 with the distinction made based on ICD codes in all of these studies (Table 4). The majority (n=4/5) of these studies reported an increase in NCGA among high-risk male and female immigrants. One study showed a suggestive trend towards increased risk of NCGA among high-risk immigrants, though the results of which anatomic subtype conferred the highest risk were mixed among several immigrant groups. 40 The pooled SIR among first generation immigrants with gastric cancer types of NCGA or unspecified resulted in a pooled SIR of 1.62 (95% CI 1.47–1.76) and 1.80 (95% CI 1.65–1.95) for males and females respectively (Table 3). A restricted analysis to only NCGA demonstrated pooled SIRs of 1.56 (95% CI 1.21–1.91) and 1.80 (95% CI 1.56–2.05), while an analysis of only CGA demonstrated pooled SIRs of 1.67 (95% CI 1.17–2.18) and 1.69 (95% CI 1.13–2.25) for males and females respectively (Table 3).
Table 4:
Distribution of Risk by Anatomic Location
| Study | Ratio | Group | CGC | NCGA | ||
|---|---|---|---|---|---|---|
| Female | Male | Female | Male | |||
| Arnold et al., 201344 | SIR | Turkey | 0.8 (.4–1.6) | 0.7 (0.5–1.0) | 2.1 (1.7–2.6) | 2.2 (1.9–2.6) |
| Mousavi & Hemminki, 2015 40 | SIR | All Immigrants | 1.39 (1.28–1.50) | 1.46 (1.36–1.57) | 1.40 (1.30–1.52) | 1.31 (1.23–1.40) |
| High Risk | 1.26 (0.80–1.89) | 2.33 (1.90–2.83) | ||||
| South America | 3.32 (1.97–5.25) | 3.95 (2.60–5.75) | 1.57 (.83–2.68) | 1.62 (1.05–2.38) | ||
| Other East Europe | 2.00 (1.38–2.81) | 2.51 (1.92–3.22) | 2.32 (1.74–3.02) | 2.18 (1.80–2.62) | ||
| Asian Arab | 4.08 (2.33–6.63) | 1.96 (1.04–3.35) | .98 (.40–2.03) | 1.01 (0.61–1.57) | ||
| Southeast Asia | 3.83 (1.75–7.27) | n/a | 2.46 (1.18–4.52) | 1.11 (0.36–2.58) | ||
| East Asia | 4.18 (2.09–7.48) | 1.48 (0.54–3.23) | 2.53 (1.22–4.66) | 2.00 (1.12–3.30) | ||
| Mousavi et al. 2012 23 | SIR | High Risk Countries | 1.26 (0.8–1.89) | 2.23 (1.90–2.83) | ||
| Other Countries | 0.89 (0.18–2.61) | 2.15 (1.25–3.44) | ||||
| All immigrants | 1.18 (0.77–1.72) | 2.25 (1.86–2.70) | ||||
| Arnold et al., 2011 45 | SIR | Turkey | 0.6 (0.2–1.5) | 0.6 (0.4–0.9) | 1.7 (1.3–2.3) | 1.9 (1.6–2.3) |
| Kamineni et al.,1999 39 | Rate Ratio | Japan | 1.7 (0.77–3.9) | 5.6–6.5* | ||
| China | 0.37 (0.16–0.86) | 1.8–7.9* | ||||
Notes:
• Abbreviations: CGC (cardia gastric cancer), NCGA (non-cardia gastric adenocarcinoma), SIR (standardized incidence ratio)
• Pylorus, body, and unknown are distinguished in the manuscript.
• Male and female gender were grouped into one cell if they were analyzed together.
Heterogeneity and risk of bias assessment
There was significant heterogeneity among studies included for the meta-analysis (I2 range 29.7 to 80.8, Table 3). This is more likely secondary to true differences in immigration populations (biology and environmental factors), background cancer incidence, and study design, as opposed to random sampling variation.21 Inconsistency was higher for estimates in males compared to females (66–80% vs. 10–68% variation across studies, respectively), which might reflect the wide confidence intervals for the sex-specific SIRs reported from one Swedish study, which was heavily weighted in meta-analysis, and less likely true differences in biologic variability to this high degree.40 Based on the NOS for cohort studies, 34 (89.5%) were scored as good quality (Supplemental Table S1 NOS). These studies controlled for major confounders such as age and sex in reporting rates of gastric cancer and contained data from well-maintained registries. Four studies were score as poor quality and were limited by their duration of follow-up and small numbers of patients from high-risk countries. 26,33,46,47
Funnel plot analysis limited to the twelve studies that reported SIR (see Supplement Figure 1) demonstrated moderate risk of publication bias, with absence of large studies with SIR > 3 (high effect) and paucity of small sample studies in general.
Negative Studies
In the five studies showing no significant increase in gastric cancer risk in immigrants from high to low incidence regions), four assessed disease specific mortality by various methodologies, and one study was restricted to second-generation immigrants.25,41,42,46,48
DISCUSSION
In this comprehensive systematic review of the literature and meta-analysis we identified a consistently higher gastric cancer risk among foreign-born immigrants who emigrate from countries of high gastric cancer incidence to countries of low incidence. Thirteen studies reported SIR and represented 21 distinct populations in the 8 nations and 8 broader regions, with all reporting significantly higher gastric cancer risk in the immigrant populations ranging from 1.08 to 5.05-fold higher compared to the reference low-incidence destination country populations. Pooled SIRs stratified by sex and anatomic subtypes revealed a 1.56 to 1.83-fold higher risk for gastric cancer among immigrants, with NCGA risk calculated to be 1.56 (95% CI 1.21–1.91) and 1.80 (95% CI 1.56–2.05) for males and females respectively. Gastric cancer-related mortality was also overall increased among immigrants irrespective of sex. Importantly, elevated risk was retained in second-generation immigrants, albeit slightly lower compared to the first-generation counterparts. Collectively, these data suggest that foreign-born immigrants from regions where gastric cancer is endemic remain a high-risk population within otherwise low incidence countries. This may have important implications for focused gastric cancer screening and the surveillance among high-risk immigrant groups, as well as other targeted preventative or therapeutic interventions.
The phenomenon of importing risk with infection-associated malignancies has been observed with other cancers, including hepatocellular carcinoma in Asian immigrants (hepatitis B), and cervical cancer in Latino immigrants (human papilloma virus). 49–52 Gastric cancer pathogenesis is multifactorial and is the result of microbial, environmental, behavioral and host (genetic) determinants. For NCGA, which accounts for vast majority of gastric cancer cases globally, H. pylori infection plays a leading role in pathogenesis, with an attributable risk of 80–90%. 53,54 While an estimated 4.4 billion people worldwide are infected with H. pylori, there are marked global and regional variations in prevalence. In high prevalence areas, colonization generally occurs in early in childhood7 , which is important since it is the cumulative time of exposure to H. pylori infection is a strong determinant of cancer risk. 55
While traditional risk factors such as male sex, age, family history, and tobacco use contribute to the risk of gastric cancer, the level of acculturation following emigration also influences gastric cancer risk. Using the California Cancer Registry data from 1988–2004, Chang, et.al found that NCGA was increased in Hispanics with the following characteristics: foreign-born, lower socioeconomic status, and residence in higher enclave neighborhoods, the latter of which is a surrogate marker of less acculturation. 56 Dietary habits also parallel acculturation and may be one important factor for the observed decrease in gastric cancer rates with subsequent generations living in low incidence regions. 17,57,58 Level of acculturation might explain why some groups have a more rapid decrease in gastric cancer rates upon emigration (e.g. Japanese Americans) compared to other groups (e.g. Korean Americans). 59 Recognizing the differential cancer risk profile in immigrant groups is essential for optimizing preventative care.
Lee et.al. noted that the incidence of gastric cancer in Korean-Americans is similar to the incidence of colorectal cancer in the U.S. population, a cancer routinely screened for in the U.S., and is estimated to be over five times higher than the incidence of NCGA among U.S.-born whites. 60 For this reason, some consensus recommendations advise a test and treat strategy for H. pylori among immigrants from endemic countries. 61,62 Furthermore, the American Society of Gastrointestinal Endoscopy (ASGE) Standards of Practice Committee suggest considering screening for gastric cancer for first generation U.S. immigrants over 40 years old who are from certain high-risk areas, including Japan, China, Russia, and South America. 63 Our study emphasizes the importance of further investigations to determine if an evidence-based recommendation generalizing this statement to immigrants from any high-incidence area can be made.
What are the implications for the U.S. immigrant populations? There are 43 million foreign-born individuals who reside in the U.S. using data from IARC GLOBOCAN, the Migration Policy Institute, and the U.S. Census Bureau. 64,65 We estimate that 29.4% or 12.73 million of these individuals have emigrated from high incidence areas (Supplemental Table S-2). It is estimated that 7.53 million (59.3%) of foreign-born immigrants are over age 40. 66 The largest U.S. immigrant populations from high incidence regions include China, El Salvador, Vietnam, Korea, and Guatemala. Other populations from particularly high incidence regions, yet with modest populations in the U.S. include: Colombia, Ecuador, Iran, Russia, Ukraine, Japan, Portugal, Turkey, Chile, Costa Rica, Bulgaria, Belarus, Uruguay, Lithuania, and Latvia. The foreign-born immigrant populations have settled in diverse locations, lending a “geographic variability” of high risk populations in the U.S., with the state of California and New York City hosting large at-risk populations (Supplement Table S3). While Mexico is considered a low incidence nation overall according to IARC data, there are regions of Mexico, primarily those states which are contiguous with Central America (e.g., Chiapas), that are considered high incidence and mortality areas based on the highest quality data. 67–69 This is particularly relevant when we acknowledge that Mexican immigrants represent the most populous U.S. immigrant group (approximately 11.6 million). We calculate that the seven high-risk states in Mexico account for 25.2% of the Mexican foreign-born immigrants for the years 2004–2015, the most recent data (Supplemental Table S4). 70
While our comprehensive systematic review of the published literature identified supportive evidence that immigration status and country of origin are important metrics for gastric cancer risk stratification, our study is not without limitations. The principal limitations were the heterogeneous nature of existing studies and in some cases insufficient details for formal meta-analysis (e.g. age of immigration). The magnitude of the effect size of the SIR was possibly diluted by the inclusion of broad groups which included immigrants from countries that were not high risk, such as groups like “Middle East”. Because we focused our present analysis on immigrant status we are unable to comment on specific racial and ethnic variations in gastric cancer risk within countries since studies were only included if place of birth was provided. We also acknowledge that reliance on ICD coding can sometimes be problematic in discriminating histologic and anatomic subtypes of gastric cancer. The fact that all data included in this systematic review came from well-maintained registries and that a consistent increase in risk was observed across studies supports the validity of our findings. The majority of studies had large sample size and adequate patient-years follow-up. Cancer incidence, more so than mortality and survival, is most closely linked to etiology and risk factors, to identify at-risk populations, which helps explain the lack of positive findings in the studies that looked at cancer specific mortality. 71 Publication bias is important to consider as cohort studies that did not find an association between stomach cancer and immigrant populations might be underreported. This concern is somewhat mitigated by the fact that several of the studies were large population studies that reported results for multiple malignancies.
Conclusion
Foreign-born immigrants from high gastric cancer incidence regions, who immigrate to low incidence regions, such as the U.S., remain at increased risk for gastric cancer and potentially gastric cancer-related mortality. Further research regarding the efficacy and effectiveness of focused screening for early gastric cancer and/or surveillance of premalignant lesions is warranted.
Supplementary Material
What You Need to Know.
Background:
The effect of immigration on gastric cancer risk is not well-defined but might be helpful for screening or surveillance endeavors. We performed a systematic review and meta-analysis to define the risk of gastric cancer in immigrants from high-incidence regions to low-incidence regions (Western Europe, Australia, Brazil, Canada, Israel, and the United States).
Findings:
Immigrants from regions with a high incidence of gastric cancer in regions of low incidence maintain a higher risk of gastric cancer and related mortality.
Implications for Patient Care:
Assessment of immigrant generation along with other risk factors might help identify high-risk populations for prevention and therapeutic interventions.
Acknowledgments
Grant Support
The following funding provided support in part: US National Institutes of Health grants P30 CA068485 (Vanderbilt Ingram Cancer Center), P01CA028842, R01CA190612, PAR-15–155, and P30 DK058404 (Vanderbilt Digestive Disease Research Center).
Abbreviations:
- (U.S.)
United States
- SIR
(standardized incidence ratio)
- SMR
(standardized mortality ratio)
- (ASR)
Age Standardized Rate
- (IARC)
International Agency for Research on Cancer
- (NCGA)
Non-cardia gastric adenocarcinoma
- (CGC)
Cardia gastric cancer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
Writing Assistance: None
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 3.Plummer M, de Martel C, Vignat J, et al. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Heal 2016;4:e609–e616. [DOI] [PubMed] [Google Scholar]
- 4.Bray F, Jemal A, Grey N, et al. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol 2012;13:790–801. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 6.United States Census Bureau / American FactFinder. US Census Bureau, 2016 American Community Survey 1-Year Estimates. Dig Educ Stat 2012.
- 7.Hooi JKY, Lai WY, Ng WK, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017;153:420–429. [DOI] [PubMed] [Google Scholar]
- 8.Feldman RA, Eccersley AJP, Hardie JM. Epidemiology of Helicobacter pylori: Acquisition, transmission, population prevalence and disease-to-infection ratio. Br Med Bull 1998;54:39–53. [DOI] [PubMed] [Google Scholar]
- 9.Mégraud F, Brassens-Rabbé MP, Denis F, et al. Seroepidemiology of Campylobacter pylori infection in various populations. J Clin Microbiol 1989;27:1870–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holcombe C. Helicobacter pylori: the African enigma. Gut 1992;33:429–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fock KM, Ang TL. Epidemiology of Helicobacter pylori infection and gastric cancer in Asia. J Gastroenterol Hepatol 2010;25:479–86. [DOI] [PubMed] [Google Scholar]
- 12.Torres J, Correa P, Ferreccio C, et al. Gastric cancer incidence and mortality is associated with altitude in the mountainous regions of Pacific Latin America. Cancer Causes Control 2013;24:249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kupfer SS. Gaining Ground in the Genetics of Gastric Cancer. Gastroenterology 2017;152:926–928. [DOI] [PubMed] [Google Scholar]
- 14.Persson C, Canedo P, MacHado JC, et al. Polymorphisms in inflammatory response genes and their association with gastric cancer: A HuGE systematic review and meta-analyses. Am J Epidemiol 2011;173:259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nomura A, Stemmermann GN, Chyou P-H, et al. Helicobacter pylori Infection and Gastric Carcinoma among Japanese Americans in Hawaii. N Engl J Med 1991;325:1132–1136. [DOI] [PubMed] [Google Scholar]
- 16.Wu GD, Chen J, Hoffmann C, et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science (80- ) 2011;334:105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maskarinec G, Noh JJ. The effect of migration on cancer incidence among Japanese in Hawaii. Ethn Dis 2004;14:431–439. [PubMed] [Google Scholar]
- 18.Saumoy M, Schneider Y, Shen N, et al. Cost Effectiveness of Gastric Cancer Screening According to Race and Ethnicity. Gastroenterology 2018. [DOI] [PubMed]
- 19.Ang TL, Fock KM. Clinical epidemiology of gastric cancer. Singapore Med J 2014;55:621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregorio D, Flannery J, Hansen H. Stomach cancer patterns in European immigrants to Conneticut, United States. Cancer Causes Control 199AD;3:215–221. [DOI] [PubMed] [Google Scholar]
- 23.Mousavi SM, Sundquist K, Hemminki K. Does the risk of stomach cancer remain among second-generation immigrants in Sweden? Gastric Cancer 2012;15:213–215. [DOI] [PubMed] [Google Scholar]
- 24.Ducarroz S, Leon ME, Schott A-M, et al. Are male immigrants in Denmark at lower or higher risk of tobacco-related cancers? A Danish nationwide cohort study. Acta Oncol (Madr) 2015;54:1128–1135. [DOI] [PubMed] [Google Scholar]
- 25.Kyobutungi C, Ronellenfitsch U, Razum O, et al. Mortality from cancer among ethnic German immigrants from the Former Soviet Union, in Germany. Eur J Cancer 2006;42:2577–2584. [DOI] [PubMed] [Google Scholar]
- 26.Newman AM, Spengler RF. Cancer mortality among immigrant populations in Ontario, 1969 through 1973. Can Med Assoc J 1984;130:399–405. [PMC free article] [PubMed] [Google Scholar]
- 27.Kaucher S, Kajüter H, Becher H, et al. Cancer Incidence and Mortality Among Ethnic German Migrants From the Former Soviet Union. Front Oncol 2018;8:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho A Bin, Jaehn P, Holleczek B, et al. Stage of cancer diagnoses among migrants from the former Soviet Union in comparison to the German population – are diagnoses among migrants delayed? BMC Public Health 2018;18:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winkler V, Ott JJ, Holleczek B, et al. Cancer profile of migrants from the Former Soviet Union in Germany: Incidence and mortality. Cancer Causes Control 2009;20:1873–1879. [DOI] [PubMed] [Google Scholar]
- 30.Ronellenfitsch U, Kyobutungi C, Ott JJ, et al. Stomach cancer mortality in two large cohorts of migrants from the Former Soviet Union to Israel and Germany: are there implications for prevention? Eur J Gastroenterol Hepatol 2009;21:319–326. [DOI] [PubMed] [Google Scholar]
- 31.Ott JJ, Paltiel AM, Winkler V, et al. Chronic disease mortality associated with infectious agents: A comparative cohort study of migrants from the Former Soviet Union in Israel and Germany. BMC Public Health 2008;8:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwasaki M, Mameri CP, Hamada GS, et al. Secular trends in cancer mortality among Japanese immigrants in the state of Sao Paulo, Brazil, 1979–2001. Eur J Cancer Prev 2008;17:1–8. [DOI] [PubMed] [Google Scholar]
- 33.Iwasaki M, Mameri CP, Hamada GS, et al. Cancer mortality among Japanese immigrants and their descendants in the State of São Paulo, Brazil , 1999–2001. Jpn J Clin Oncol 2004;34:673–680. [DOI] [PubMed] [Google Scholar]
- 34.Fang J, Madhavan S, Alderman MH. Cancer mortality of Chinese in New York City 1988–1992. Int J Epidemiol 1996;25:907–912. [DOI] [PubMed] [Google Scholar]
- 35.Stellman SD, Wang Q‐S. Cancer mortality in chinese immigrants to New York city. Comparison with chinese in tianjin and with United States‐born whites. Cancer 1994;73:1270–1275. [DOI] [PubMed] [Google Scholar]
- 36.Ikram UZ, Mackenbach JP, Harding S, et al. All-cause and cause-specific mortality of different migrant populations in Europe. Eur J Epidemiol 2016;31:655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spallek J, Arnold M, Razum O, et al. Cancer mortality patterns among Turkish immigrants in four European countries and in Turkey. Eur J Epidemiol 2012;27:915–921. [DOI] [PubMed] [Google Scholar]
- 38.Ziadeh C, Ziogas A, Anton-Culver H. Cancer risk in different generations of Middle Eastern immigrants to California, 1988–2013. Int J Cancer 2017;141:2260–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamineni A, Williams MA, Schwartz SM, et al. The incidence of gastric carcinoma in Asian migrants to the United States and their descendants. Cancer Causes Control 1999;10:77–83. [DOI] [PubMed] [Google Scholar]
- 40.Mousavi SM, Hemminki K. Cancer incidence, trends, and survival among immigrants to Sweden: a population-based study 2015. [DOI] [PubMed]
- 41.Hemminki K, Li X. Cancer risks in second-generation immigrants to Sweden. Int J Cancer 2002;99:229–237. [DOI] [PubMed] [Google Scholar]
- 42.Stirbu I, Kunst AE, Vlems FA, et al. Cancer mortality rates among first and second generation migrants in the Netherlands: Convergence toward the rates of the native Dutch population. Int J Cancer 2006;119:2665–2672. [DOI] [PubMed] [Google Scholar]
- 43.Jaehn P, Holleczek B, Becher H, et al. Histologic types of gastric cancer among migrants from the former Soviet Union and the general population in Germany. Eur J Gastroenterol Hepatol 2016;28:863–870. [DOI] [PubMed] [Google Scholar]
- 44.Arnold M, Aarts MJ, Siesling S, et al. Diverging breast and stomach cancer incidence and survival in migrants in The Netherlands, 1996–2009. Acta Oncol 2013;52:1195–1201. [DOI] [PubMed] [Google Scholar]
- 45.Arnold M, Aarts MJ, Siesling S, et al. Breast and stomach cancer incidence and survival in migrants in the Netherlands, 1996–2006. Eur J Cancer Prev 2011;20:150–156. [DOI] [PubMed] [Google Scholar]
- 46.Siemerink EJM, van der Aa MA, Siesling S, et al. Survival of non-Western first generations immigrants with stomach cancer in North East Netherlands. Br J Cancer 2011;104:1193–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho NH, Moy CS, Davis F, et al. Ethnic variation in the incidence of stomach cancer in Illinois, 1986–1988. Am J Epidemiol 1996;144:661–664. [DOI] [PubMed] [Google Scholar]
- 48.Nilsson B, Gustavson-Kadaka E, Hakulinen T, et al. Cancer Survival in Estonian Migrants to Sweden. J Epidemiol Community Heal 1997;51:418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh GK, Hiatt RA. Trends and disparities in socioeconomic and behavioural characteristics, life expectancy, and cause-specific mortality of native-born and foreign-born populations in the United States, 1979–2003. Int J Epidemiol 2006;35:903–919. [DOI] [PubMed] [Google Scholar]
- 50.Kem R, Chu KC. Cambodian cancer incidence rates in California and Washington, 1998–2002. Cancer 2007;110:1370–1375. [DOI] [PubMed] [Google Scholar]
- 51.Chen Y, Yi Q, Mao Y. Cluster of liver cancer and immigration: A geographic analysis of incidence data for Ontario 1998–2002. Int J Health Geogr 2008;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siegel RL, Fedewa SA, Miller KD, et al. Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J Clin 2015;65:457–480. [DOI] [PubMed] [Google Scholar]
- 53.Plummer M, Franceschi S, Vignat J, et al. Global burden of gastric cancer attributable to pylori. Int J Cancer 2015;136:487–490. [DOI] [PubMed] [Google Scholar]
- 54.Ford AC, Forman D, Hunt R, et al. Helicobacter pylori eradication for the prevention of gastric neoplasia. Moayyedi P, ed. Cochrane database Syst Rev 2015:CD005583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mera RM, Bravo LE, Camargo MC, et al. Dynamics of Helicobacter pylori infection as a determinant of progression of gastric precancerous lesions: 16-year follow-up of an eradication trial. Gut 2018;67:1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang ET, Gomez SL, Fish K, et al. Gastric cancer incidence among hispanics in California: Patterns by time, nativity, and neighborhood characteristics. Cancer Epidemiol Biomarkers Prev 2012;21:709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lunet N, Valbuena C, Vieira AL, et al. Fruit and vegetable consumption and gastric cancer by location and histological type: case-control and meta-analysis. Eur J Cancer Prev 2007;16:312–327. [DOI] [PubMed] [Google Scholar]
- 58.Zhou Y, Zhuang W, Hu W, et al. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology 2011;141:80–89. [DOI] [PubMed] [Google Scholar]
- 59.Kim GH, Liang PS, Bang SJ, et al. Screening and surveillance for gastric cancer in the United States: Is it needed? Gastrointest Endosc 2016;84:18–28. [DOI] [PubMed] [Google Scholar]
- 60.Lee E, Liu L, Zhang J, et al. Stomach Cancer Disparity among Korean Americans by Tumor Characteristics: Comparison with Non-Hispanic Whites, Japanese Americans, South Koreans, and Japanese. Cancer Epidemiol Biomarkers Prev 2017;26:587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siao D, Somsouk M. Helicobacter pylori: Evidence-based review with a focus on immigrant populations. J Gen Intern Med 2014;29:520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Talley NJ, Ming Fock K, Moayyedi P. Gastric Cancer Consensus Conference Recommends Helicobacter pylori Screening and Treatment in Asymptomatic Persons From High-Risk Populations to Prevent Gastric Cancer. Am J Gastroenterol J Gastroenterol 2008;103:510–514. [DOI] [PubMed] [Google Scholar]
- 63.Wang A, Shaukat A, Acosta RD, et al. Race and ethnicity considerations in GI endoscopy 2015. [DOI] [PubMed]
- 64.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v.1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No.11 2013.
- 65.United States Census Bureau. State Demographics Data 2016.
- 66.Ruggles S, Alexander JT, Genadek K, et al. Age-Sex Pyramids of U.S. Immigrant and Native-Born Populations, 1970-Present | migrationpolicy.org 2017.
- 67.Villalobos J, Ávila M, Domínguez M, et al. Perfil epidemiológico de los tumores malignos en México México, Distrito Federa; 2011. [Google Scholar]
- 68.Gómez-Dantés H, Lamadrid-Figueroa H, Cahuana-Hurtado L, et al. The burden of cancer in Mexico, 1990–2013. Salud Publica Mex 2016;58:118–31. [DOI] [PubMed] [Google Scholar]
- 69.Sánchez-Barriga JJ. Mortality trends and years of potential life lost from gastric cancer in Mexico, 2000–2012. Rev Gastroenterol Mex 2016;81:65–73. [DOI] [PubMed] [Google Scholar]
- 70.Ministry of Foreign Affairs. Origins of Mexican Migrants to the United States by Mexican State of Residence, Number, and Share, 2004–2015 | migrationpolicy.org Surv Migr North Bord Mex
- 71.Ellis L, Woods LM, Estève J, et al. Cancer incidence, survival and mortality: Explaining the concepts. Int J Cancer 2014;135:1774–1782. [DOI] [PubMed] [Google Scholar]
- 72.Sutradhar R, Asidianya N, Lee F, et al. Higher risk of gastric cancer among immigrants to Ontario: a population-based matched cohort study with over 2 million individuals. Gastric Cancer 2018;21:588–597. [DOI] [PubMed] [Google Scholar]
- 73.Winkler V, Holleczek B, Stegmaier C, et al. Cancer incidence in ethnic German migrants from the Former Soviet Union in comparison to the host population. Cancer Epidemiol 2014;38:22–27. [DOI] [PubMed] [Google Scholar]
- 74.Hjerkind KV, Qureshi SA, Møller B, et al. Eth nic differences in the incidence of cancer in Norway. Int J Cancer 2017;140:1770–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mousavi SM, Sundquist J, Hemminki K. Cancer incidence among Turkish, Chilean, and North African first-generation immigrants in Sweden compared with residents in the countries of origin and native Swedes. Eur J Cancer Prev 2013;22:1–7. [DOI] [PubMed] [Google Scholar]
- 76.Hemminki K, Li X, Czene K. Cancer risks in first-generation immigrants to Sweden. Int J Cancer 2002;99:218–228. [DOI] [PubMed] [Google Scholar]
- 77.Nilsson B, Gustavson‐Kadaka E, Rotstein S, et al. Cancer incidence in estonian migrants to Sweden. Int J Cancer 1993;55:190–195. [DOI] [PubMed] [Google Scholar]
- 78.McCredie M, Williams S, Coates M. Cancer mortality in migrants from the British Isles and continental Europe to New South Wales, Australia, 1975–1995. Int J Cancer 1999;83:179. [DOI] [PubMed] [Google Scholar]
- 79.Van Hemelrijck WMJ, Valk HAG d., Vandenheede H. Cancer mortality by migrant background in Belgium during the 2000s: Patterns and social determinants. Cancer Treat Res Commun 2017;12:19–24. [Google Scholar]
- 80.Ott JJ, Paltiel AM, Becher H. Noncommunicable disease mortality and life expectancy in immigrants to Israel from the former Soviet Union: Country of origin compared with host country. Bull World Health Organ 2009;87:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh GK, Miller BA. Health, life expectancy, and mortality patterns among immigrant populations in the United States. Can J Public Heal 2004;95:I14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


