Abstract
Gut-brain axis signaling is critical for maintaining health and homeostasis. Stressful life events can impact gut-brain signaling, leading to altered mood, cognition and intestinal dysfunction. Here we identify nucleotide binding oligomerization domain (Nod)-like receptors (NLR), Nod1 and Nod2, as novel regulators for gut-brain signaling. NLR are innate immune pattern recognition receptors expressed in the gut and brain, important in the regulation of gastrointestinal (GI) physiology. We found that mice deficient in both Nod1 and Nod2 (NodDKO) demonstrate signs of stress-induced anxiety, cognitive impairment and depression in the context of a hyperactive hypothalamic-pituitary-adrenal axis. These deficits were coupled with impairments in the serotonergic pathway in the brain, decreased hippocampal cell proliferation and immature neurons, as well as reduced neural activation. In addition, NodDKO mice had increased GI permeability and altered serotonin signaling in the gut following exposure to acute stress. Administration of the selective serotonin reuptake inhibitor, fluoxetine, abrogated behavioral impairments and restored serotonin signaling. We also identified that intestinal epithelial cell-specific deletion of Nod1 (VilCre+Nod1f/f), but not Nod2, increased susceptibility to stress-induced anxiety-like behavior and cognitive impairment following exposure to stress. Together these data suggest that intestinal epithelial NLR are novel modulators of gut-brain communication and may serve as potential novel therapeutic targets for the treatment of gut-brain disorders.
Keywords: Microbiota-gut-brain axis, NLR, stress, anxiety, depression, cognition, neurogenesis, intestinal physiology, 5-HT system, HPA-axis
INTRODUCTION
The hypothalamic-pituitary-adrenal (HPA) axis is a critical regulator of the stress response. It is one of the main pathways through which the gut-brain axis signals, with stress negatively impacting intestinal function, including permeability, motility (Huerta-Franco et al., 2012) and visceral sensitivity (Theodorou, 2013). Stress can also induce disease relapse in patients with inflammatory bowel disease (IBD) (Mawdsley & Rampton, 2005; Martin et al., 2015) and cause symptom flare-ups in patients with irritable bowel syndrome (IBS) (Qin et al., 2014). Stressful life events also represent risks factors for the development of psychiatric disorders, including anxiety and major depressive disorder (McEwen, 2000). Additionally, there is significant genetic pleiotropy for psychiatric and immune system disorders, suggesting an underlying common pathway of regulation (Wang et al., 2015). While the precise mechanisms for this susceptibility to stress remain to be fully understood, it is thought to involve a complex interplay between environmental, biological, and genetic risk factors.
The immune system plays a pivotal role in brain function and stress responses (Pruett, 2003; Pariante, 2016). Mice deficient in T- and B-cells display anxiety-like behavior and cognitive deficits compared to wild type (WT) controls (Smith et al., 2014). More recently, mice deficient for the innate immune pattern recognition receptor (PRR), peptidoglycan (PGN) recognition protein (PGLYRP) 2 gene, showed alterations in social behavior in a sex dependent manner (Arentsen et al., 2017). Together, these findings suggest a role for adaptive and innate immune pathways in regulating gut-brain communication. Nucleotide binding oligomerization domain (Nod)-like receptors (NLR), Nod1 and Nod2, are intracellular PRRs that recognize specific moieties of PGN and are important in maintaining gut homeostasis by eliciting protective immune responses to bacteria (Clarke et al., 2010; Claes et al., 2015). In addition to their well-established expression in the periphery, including in intestinal epithelial cells (IEC) and mucosal immune cells (Ogura et al., 2003; Franchi et al., 2009), Nod1 and Nod2 are expressed in several brain areas, including the hippocampus and diverse cell types, such as pyramidal neurons, astrocytes, and microglia (Arentsen et al., 2017). Despite this evidence of central nervous system (CNS) expression, the precise role of Nod1 and Nod2 in the brain remains to be fully elucidated.
Given their physiological distribution, we hypothesized that NLR may represent a novel potential therapeutic target for the treatment of stress-related disorders and a novel modulator of gut-brain communication. Using mice deficient in both Nod1 and Nod2 (NodDKO), we assessed behavior and brain function as well as intestinal physiology in the context of acute psychological stress.
METHODS
Mice.
Adult (6–8 week) male and female mice (Jackson Labs; bred in-house) were used in the study. Nod1/Nod2 double knockout (NodDKO; constitutive knockout; C57BL/6 background) mice and WT (C57BL/6) controls were maintained at UC Davis and in-bred for at least 11 generations (Keestra-Gounder et al., 2016). Nod1 floxed mice were created using a targeting construct for Nod1 generated using C57BL/6-derived bacterial artificial chromosome clones. The construct was designed with loxP sites flanking exons 2 and 3 of Nod1 and an EGFP kanamycin/neomycin cassette was introduced for selection in Escherichia coli and embryonic stem (ES) cells, respectively. The targeting construct was verified by sequencing and electroporated into C57BL/6 ES cells. G418-resistant ES cell clones were picked and expanded. DNA was extracted and screened for targeting by PCR. ES cell clones that amplified a product of the expected size were further characterized by Southern blot analysis to confirm targeting at both the 5’ and 3’ ends of the targeting construct. Correctly targeted ES cell clones were karyotyped and two independently targeted clones with a normal chromosome count were prepared for microinjection into blastocysts. Chimeric mice were prepared by microinjecting gene targeted ES cells into recipient blastocysts (Balb/c) and transferred to pseudo-pregnant recipients. The targeted ES cells were derived from C57BL/6 mice. Male chimeric mice were mated with WT C57BL/6 females to obtain germline transmission of the targeted allele. Animals that were heterozygous for the targeted allele (as determined by coat color) were bred to generate Nod1f/f animals which were bred and maintained under specific-pathogen-free (SPF) conditions in the Monash Medical Centre Animal Facilities. Generation of floxed mice was performed according to the guidelines of Monash University’s Institutional Biosafety and Animal Ethics Committees. Nod2f/f mice were created as described elsewhere (Kim et al., 2016). Once generated, both Nod1f/f and Nod2f/f mice were bred at UC Davis where all experiments were performed.
Nod1f/f and Nod2f/f mice were crossed with IEC-specific Cre-expressing (VilCre) mice (Jackson Labs; bred in-house). Cages consisted of either VilCre−Nod1f/f and VilCre+Nod1f/f or VilCre−Nod2f/f and VilCre+Nod2f/f genotypes.
Mice were housed in cages lined with chip bedding and had free access to food and water throughout the study. The vivarium lighting schedule allowed 12 hours of light and 12 hours of darkness each day with temperature maintained at 21 ± 1 °C. All behavioral tests were performed between 9am and 6pm and all procedures and protocols were reviewed and approved by the Institutional Animal Care and Use Committee at the University of California, Davis (IACUC protocol #20072). Mice were euthanized by hypoxia via CO2 followed by cervical dislocation following AAALAC guidelines.
Fluoxetine treatment.
Fluoxetine hydrochloride (18 mg/Kg per day; Sigma-Aldrich) was administered ad libitum in the drinking water for 4 weeks in opaque bottles to protect it from light. Selective serotonin reuptake inhibitors typically require several weeks to alleviate depressive symptoms due to unknown mechanisms (Perez-Caballero et al., 2014). The drinking water containing fluoxetine was changed every 3 days to prevent any possible degradation. Control animals received plain drinking water as vehicle. Animals were treated immediately starting after weaning for a period of 28 days. On day 28, animals underwent the one-day behavioral testing protocol (as described below), plus the forced swim test (FST) 2h later to assess depressive-like behaviors.
WAS.
Mice were placed on small platforms (inverted 50ml beakers) in a clean, standard housing cage filled with approximately 2 cm of room temperature water for 1 hour. Water avoidance stress (WAS) is a well-established model for inducing stress in rodents (Gareau et al., 2011), which can impact intestinal mucosal barrier function for up to 4h (Gareau et al., 2008). After exposure to stress, mice immediately underwent (1) behavioral testing or were euthanized for (2) perfusion (immunofluorescence) or (3) gut physiology (Ussing chambers) (Fig 1A).
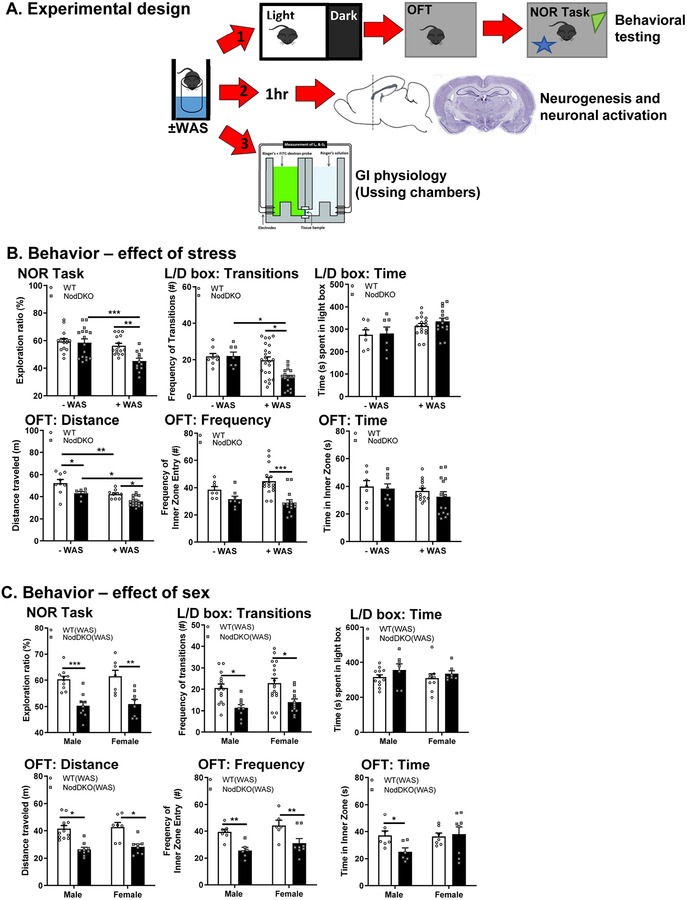
Figure 1. NodDKO(+WAS) mice display behavioral deficits.
(A) Experimental timeline: adult C57BL/6 male and female WT and NodDKO mice ± water avoidance stress (WAS) underwent 1 of 3 study paradigms: 1) ± WAS +behavioral testing; or 2) ± WAS + 1 hr wait followed by perfusion for hippocampal neurogenesis and neural activation analysis; or 3) ± WAS + gut physiology assessed by Ussing chambers. Behavior: (B) stress effect on: novel object recognition (NOR) task, light/dark (L/D) box and open field test (OFT) (N = 10–16); (C) Sex effect on NOR task, L/D box, and OFT (N = 7–18). Data are presented as mean ± SEM. (*P < 0.05; **P < 0.01; ***P < 0.001, 2-way ANOVA).
Behavioral testing.
Light/dark (L/D) box, open field task (OFT) and novel object recognition (NOR) task were performed as described (Gareau et al., 2011; Smith et al., 2014; Emge et al., 2016) ± WAS (Fig 1A). For mice exposed to WAS, behavior was commenced immediately following exposure. All behavior testing was completed within a 2 h block of time (10 min L/D + 10 min OFT + 55 min NOR [5 min training + 45 min recovery + 5 min testing]). Preliminary studies (performed by MS and MGG), coupled with published protocols (Crawley, 2008), confirmed that performing these tests alone or together in the same mouse does not affect outcome (data not shown).
L/D Box Test:
To measure anxiety-like behavior, the L/D box test was used as described previously (Gareau et al., 2011). Briefly, mice were placed in a box with a light (2/3) and a dark (1/3) compartment for 10 minutes, during which behavior of the mouse was video-recorded. These videos were analyzed by a digital tracking system (Ethovision; XT 8.5, Noldus, Leesburg, VA) for the total time spent by the mouse in the lit portion of the box, with lower values ascribed to anxiety-like behavior (Bourin & Hascoet, 2003). In addition, the number of times the mouse transitioned from the dark compartment to the light compartment of the box were quantified to indicate the mouse’s activity and exploratory level.
OFT:
Animal behavior for the OFT was recorded for 10 minutes in the novel arena during acclimation for the NOR task. Both locomotor activity and anxiety-like behavior were determined as quantification of total distance moved, time spent in the inner zone, and frequency of inner zone entries (Ethovision).
NOR Task:
The NOR task was performed as previously described (Gareau et al., 2011; Smith et al., 2014). Briefly, mice were subjected to 10 min of acclimation to the novel arena (30 × 30 cm white plexiglass box) followed by 5 min of habituation to two identical objects (training phase). After a rest and recovery period (45 minutes) one of the two original objects was replaced with a novel object and interactions were monitored for an additional 5 min (testing phase). Direct contacts with the objects, including any contact with nose or paw, or an approach within 2 cm, were recorded and scored automatically (Ethovision) and verified manually. Any close contact in which the animal was not directly interacting with the object but rather exploring the chamber were not interpreted as direct contact with the object and omitted. Results are expressed as a ratio quantifying preference for exploration of a novel object rather than a familiar object during the testing phase. An exploration ratio of greater than 50% indicates that the mouse investigated the novel object more than the familiar object, indicating memory recall for the latter (Gareau et al., 2011). Mice that did not pass the training phase (less than 40% or greater than 60% exploration ratio [suggesting bias towards one object]) were omitted.
FST.
The forced swim test (FST) was used to assess depressive-like behavior in mice following fluoxetine or vehicle treatment. Briefly, mice were placed individually in Pyrex cylinders (40 cm tall × 20 cm in diameter) filled with water (24.5 °C) to a 30 cm depth for 6 minutes. The last 4 minutes of the test were analyzed using a tracking system (Ethovision). During the behavioral analysis, mobility time, intended as any movements other than those necessary to balance the body and keep the head above the water, was measured. FST was performed after a 2h rest in their home cage following completion of the NOR task.
Immunofluorescence.
Brains (WT[+WAS] and NodDKO[+WAS]) were collected following anesthesia (5% isoflurane) and transcardial perfusion with 4% PFA. Brains were post-fixed overnight at 4°C and placed in 30% (w/v) sucrose for 4 days before embedding in optimal cutting temperature (OCT) medium in ice-cold isopentane followed by storage at −80°C. Samples were cut using a cryostat (Leica Microsystem, Germany) into 20 μm-thick coronal sections. Sections were serially collected and stored at −20°C until use. Immunofluorescence was performed as previously described (Murray et al., 2017). Briefly, sections underwent an antigen retrieval step using citrate buffer (10 mM, pH 6.0, 1h, 95°C). After blocking in 5% BSA normal goat serum (1h, room temperature), samples were incubated with primary antibody overnight (16h, 4°C). The primary antibodies used were: rabbit Anti-c-Fos (2250S, Cell Signaling, Danvers, MA), guinea pig anti-DCX [(AB2253, Millipore, Burlington, MA)], and rabbit anti-Ki67 (LS-C141898, Lifespan Biosciences, Seattle, WA). Slides were washed (3 × 5mins) and incubated with appropriately labeled secondary antibodies (1 h at room temperature), then washed and mounted in Prolong diamond (Invitrogen, Carlsbad, CA). The secondary antibodies used were Alexa 647 goat anti-rabbit (ab150155, Abcam, Cambridge, UK) for both c-Fos and Ki67, and Alexa 555 goat anti-guinea pig for DCX (A21435, Invitrogen, Carlsbad, CA). DAPI was used as a nuclear stain.
Image analysis and cell quantification.
Confocal imaging was performed on a Leica SP8 STED 3X microscope. Immunofluorescent Z-stack images with a 1.04 μm step size were collected using a 20x objective. Systematic random sampling was used for the dorsal hippocampus by counting the cells in both hemispheres of each section in 1:6 series (120 μm apart). Every second section, for a total of three sections, was used either for doublecortin (DCX)/Ki67 co-staining [dentate gyrus (DG) area] or for c-Fos staining [cornus ammonium (CA)3 and DG areas]. Cell quantification and calculation of the volume of the DG and CA3 were performed using the image processing software package Imaris x64_8.2.1. The ratio between the cell numbers and either the DG or CA3 volumes are expressed as an average of 3 sections per animal and presented as cells/μM3 × 10−6. The dorsal hippocampus was defined as AP −0.94 to −2.30 according to the Paxinos and Franklin atlas of the mouse brain (Franklin & Paxinos, 1996).
Serum corticosterone.
Blood samples were collected via cardiac puncture following CO2 exposure. Blood was centrifuged and serum aspirated and stored at −80°C. Corticosterone levels were assayed using a commercially-available enzyme immune assay (EIA) kit (Corticosterone EIA Kit, ADI-900–097, Enzo Life Sciences) according to the manufacturer’s instructions. Absorbance was read with a multi-mode plate reader (Synergy H1, BioTek Instruments, Inc.) at 405 nm.
Quantitative PCR.
Tissues [hippocampus, prefrontal cortex (PFC), colon and ileum] were collected and frozen at −80°C before homogenization in Trizol (Invitrogen). RNA was isolated according to the manufacturer’s protocol (Invitrogen), treated with DNase 1 (Invitrogen) and transcribed into cDNA (BioRad, iSCRIPT cDNA Synthesis kit; Proflex PCR System, Applied Biosystem). qPCR was performed using SYBR green and β-actin used as a housekeeping gene on a QuantStudio 6 Flex Real time PCR machine (Applied Biosystem). Data were presented as ΔΔCT. All primer sequences used in this study are shown in Table 1.
Table 1.
Primer sequences
| Gene | Primer sequences (5’->3’) | |
|---|---|---|
| GR | Forward | TCCGATGAAGCTTCGGGATG |
| Reverse | AATTGTGCTGTCCTTCCACTG | |
| MR | Forward | TGGCCAAGGCAGCTATGGA |
| Reverse | CATTGGTCCTCTCTGCAGGT | |
| Arc | Forward | GGAGGGAGGTCTTCTACCGT |
| Reverse | CTACAGAGACAGTGTGGCGG | |
| Tph2 | Forward | TCGAAATCTTCGTGGACTGC |
| Reverse | CGGATTCAGGGTCACAATG | |
| Tph1 | Forward | AACAAAGACCATTCCTCCGA |
| Reverse | TGTAACAGGCTCACATGATT | |
| 5HTr1a | Forward | CTAATGGGGCGGTGAGACAG |
| Reverse | GGAGGTAGCTCCTGATTCGC | |
| 5HTr2c | Forward | GCATAGCCGGTTCAATTCGC |
| Reverse | TTGCTTTCGTCCCTCAGTCC | |
| SERT | Forward | GGCGCGAGGGTCGAG |
| Reverse | ATCTGCCAAGGACCCTGACT | |
| Bdnf | Forward | TGCAGGGGCATAGACAAAAGG |
| Reverse | CTTATGAATCGCCAGCCAATTCTC | |
| GABAA1A | Forward | TCCCAAGTCTCCTTCTGGCT |
| Reverse | TTCGGGAGGGAATTTCTGGC | |
| GABAA2A | Forward | GCCACTGGAGGAAAACATCTACT |
| Reverse | GATGTTAGCCAGCACCAACCT | |
| GABAB1A | Forward | CACTGCCAGGTGAATCGAAC |
| Reverse | TTAACGTCCTCCAGCGCCAT | |
| GABAB1B | Forward | GGCCTCTCACTCCCCTCATCT |
| Reverse | TCATGGGAAACAGCGCCCC | |
| GABAB2B | Forward | CTTCTTCGGAGTCACGGGTC |
| Reverse | CGACCTTCACCTCTCTGCTG | |
Brain serotonin (5-HT) and tryptophan (Trp) quantification.
The hippocampus and brain stem were freshly dissected and stored at −80°C until analysis. Tissues were homogenized in 20 mM HEPES buffer (pH 7.5) containing 0.25M sucrose and a cocktail of protease inhibitors (Calbiochem, Burlington, MA) followed by ultracentrifugation at 100,000g for 45 min. Membrane-free tissue supernatants (100 μl) were transferred to 96-well plates for analysis. Liquid chromatography-mass spectrometry (LC/MS) analysis was performed on an Agilent Infinity 1290 ultra-high-performance LC coupled to a triple quadropole MS. Chromatographic separation was carried out on an Agilent Pursuit 3 Pentafluorophenyl (PFP) stationary phase (2 × 150 mm, 3 mm) column. The mobile phases were 100% methanol (B) or water with 0.25% formic acid (A). The analytical gradient was as follows: 2%−60% B in 2 min, 100% B from 2–4 min, 2% B for 4 min. Flow rate was 300 μl/min. Samples were held at 4°C in the autosampler, and the column was operated at 40°C. The MS was operated in positive and selected reaction monitoring (SRM) mode. A 5-HT standard was used for optimization and to generate the calibration curve for quantification. Data acquisition and processing was done using Mass Hunter Qualitative and Quantitative Analysis (Version B.06.00) and Quantitative Analysis (Version B.08.00).
Serum Trp quantification.
Serum proteins were removed by ultrafiltration using Amicon Ultra Centrifugal Filters (molecular weight cutoff 3KDa). Briefly, the upper chambers of the centrifugal devices were rinsed with water (400 μL) twice and centrifuged at 10,000 g for 5 min before individual mouse serum samples (50 μl) were loaded. Water (150 μl) was added to the samples and they were centrifuged at 10,000 g for 5 min. Protein-free serum flow-throughs were collected in 1.5 mL tubes and aliquots (50 μl) were transferred into vials for MS-analysis as described above.
Ussing chambers.
Segments of GI tract (distal ileum and proximal colon) were excised and cut along the mesenteric border and mounted in Ussing chambers (Physiologic Instruments, San Diego, CA), exposing 0.1 cm2 of tissue area to 4 ml of circulating oxygenated Ringer’s buffer maintained at 37°C. The buffer consisted of (in mM) of: 115 NaCl, 1.25 CaCl2, 1.2 MgCl2, 2.0 KH2PO4 and 25 NaHCO3 at pH 7.35±0.02. Additionally, glucose (10 mM) was added to the serosal buffer as a source of energy, which was balanced osmotically by mannitol (10 mM) in the mucosal buffer. Agar–salt bridges were used to monitor potential differences across the tissue and to inject the required short‐circuit current (Isc) to maintain the potential difference at zero as registered by an automated voltage clamp. A computer connected to the voltage clamp system recorded Isc and voltage continuously and analyzed using acquisition software (Acquire and Analyze; Physiologic Instruments). Baseline Isc values were obtained at equilibrium, approximately 15 min after the tissues were mounted. Isc, an indicator of active ion transport, was expressed in μA/cm2. Conductance (G) was used to assess tight junction permeability and mucosal to serosal flux of 4KDa FITC-labeled dextran (Sigma) over time (sampled every 30 minutes for 2 hours) was used to assess macromolecular permeability. After completion of the FITC flux measurements, tissues were treated with forskolin (FSK, 20 μM) to assess viability.
Study Design:
Three sets of animals were used for three parallel experiments.
1). Behavioral testing.
Mice (±WAS) were exposed to the L/D box, OFT, and NOR task (Fig 1A). Animals were habituated to the testing room by allowing them to acclimate in their home-cages in the testing room for at least 30 min prior to testing. Once the behavioral tests were completed, mice were euthanized by CO2 inhalation followed by cervical dislocation. PFC, hippocampus, colon and ileum were collected for qPCR analysis. A subset of mice was used for the collection of serum, hippocampus, and brain stem for 5-HT, and Trp analysis.
2). Brain imaging.
Mice (+WAS) were returned to their home-cages for 1 hour followed by perfusion (4% PFA) and measurement of c-Fos, Ki67 and DCX expression levels in the hippocampus (Reichmann et al., 2013). The 1h period is necessary to allow for c-Fos expression to occur without impacting cell proliferation or immature neuron expression, which takes multiple weeks, therefore allowing us to use the same animals for both imaging studies (Piatti et al., 2011).
3). Ussing chambers.
Mice (±WAS) were euthanized by CO2 inhalation followed by cervical dislocation and both ileum and colon were collected to measure ion transport and permeability in Ussing chambers. Serum was also collected for corticosterone analysis.
Statistical analysis.
Results are expressed as means ± standard error (SEM). Unpaired Student’s t-test or two-way ANOVA followed by Tukey’s post hoc test were performed as appropriate using Prism 8 GraphPad (San Diego, CA). A P-value of less than 0.05 was selected as the threshold of statistical significance. N represents a single mouse.
RESULTS
NodDKO mice display stress-induced behavioral deficits.
To study the role of NLR in regulating stress responses, behavior was assessed in NodDKO mice ±WAS. NOR task was used to assess recognition memory. All the mice used for the NOR task successfully performed the training phase with no differences seen in success rate based on genotype, treatment, or sex (data not shown). While baseline cognitive functions in NodDKO mice were intact, as determined by the ratio of interactions between a known and a novel object, exposure to a single session of acute WAS for 1h led to a cognitive deficit compared to WT(+WAS) mice and NodDKO(-WAS) control mice (Fig 1B). In the L/D box test, used to assess anxiety-like behavior, NodDKO(-WAS) mice did not demonstrate evidence of baseline anxiety-like behavior, as indicated by total number of transitions between the L/D boxes and time spent in the light box compared to WT(-WAS) controls (Fig 1B). In contrast, NodDKO(+WAS) mice displayed a significant decrease in the number of transitions between the L/D compartments compared to WT(+WAS) mice without an impact on time spent in the light box, indicative of anxiety-like behavior. The OFT was used to measure exploratory behavior and general well-being along with serving as a secondary assessment of anxiety-like behavior. NodDKO(+WAS) mice displayed significantly reduced frequency of entries into the inner zone in the OFT compared to WT mice, without a difference in the total time spent in the inner zone (Fig 1B). Exploratory behavior, as determined by total distance travelled in the OFT, was significantly reduced in NodDKO(-WAS) mice compared to WT, which was further decreased in NodDKO(+WAS). Stress also caused decreased exploratory behavior in WT(+WAS) mice. These findings suggest that deficiency in NLR increases susceptibility to acute stress, profoundly impacting behavior.
Cognitive function and anxiety-like behaviors were similar in male and female mice for both WT(±WAS) and NodDKO(±WAS) groups, therefore, all subsequent data are derived from combined sexes, which were equally distributed (Fig 2C).
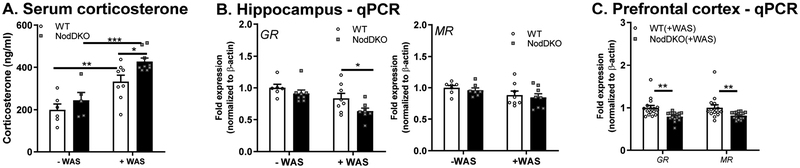
Figure 2. NodDKO(+WAS) mice display HPA-axis hyperactivation.
HPA-axis: (A) serum corticosterone levels (N = 6–9), (B) hippocampal and (C) prefrontal cortex (PFC) glucocorticoid (GR) and mineralcorticoid (MR) receptor mRNA expression levels (N = 6–16) ± WAS. Data are presented as mean ± SEM. (*P < 0.05; **P < 0.01; ***P < 0.001, 2-way ANOVA).
NodDKO mice display hyperactivation of the HPA-axis.
Dysregulation of the HPA-axis is associated with the development of anxiety-like behavior (Kolber et al., 2008) and cognitive impairments (Avital et al., 2006). Given that exposure to WAS impaired behavior in NodDKO mice, we measured serum corticosterone levels and expression of glucocorticoid receptors (GR) and mineralocorticoid receptors (MR) in the hippocampus, which serves as the primary site for feedback inhibition of HPA-axis signaling. As expected, WAS increased the concentration of serum corticosterone in WT mice, with this stress response being further increased in NodDKO(+WAS) compared to WT(+WAS) mice (Fig 2A). As basal corticosterone serum concentrations were similar between WT(-WAS) and NodDKO(-WAS) mice, these data suggest NodDKO mice are susceptible to stress-induced hyperactivation of the HPA axis (Fig 2A).
Corticosterone induces physiological and behavioral effects by binding to GR and MR, which are expressed in the hippocampus and serve to limit HPA-axis activation. Glucocorticoids are proposed to cause stress-induced depression, with decreased GR function and expression implicated in the stress response and have been associated with depression (Zhu et al., 2014). Hippocampal mRNA expression of GR was significantly reduced in NodDKO(+WAS) mice compared to WT(+WAS) mice, whereas no differences in MR expression were observed (Fig 2B). Decreases in GR and MR in NodDKO(+WAS) mice were also seen in the PFC region (Fig 2C).
NodDKO mice have impaired stress-induced neural activation.
Neural activation is critical for memory formation with acute stress-induced increases in glucocorticoids necessary for hippocampal neuronal survival, memory acquisition, and consolidation (Uchoa et al., 2014). To identify the neural circuitry underlying the differential stress susceptibility between NodDKO(+WAS) and WT(+WAS) mice, we measured expression of the immediate early genes (IEGs) Arc and c-Fos, indicative of neural activation (Tzingounis & Nicoll, 2006; Santos et al., 2018). IEG expression, associated with transcription factors, proteases, enzymes, etc., in neurons is extremely low at resting, generally below the limit of detection, but can be rapidly induced following exposure to a stimulus, such as stress (Guzowski et al., 2005). Arc mRNA expression was significantly increased in the hippocampus of WT(+WAS) but not NodDKO(+WAS) mice compared with WT(-WAS) or NodDKO(-WAS) mice respectively (Fig 3A). This was specific for the hippocampus, with no changes in Arc seen in the PFC (data not shown). These findings suggest that Nod1/2 are required for stress-induced activation of hippocampal neurons and subsequent memory consolidation. In contrast, brain-derived neurotrophic factor (BDNF), which is important for maintaining neuronal survival, was not impacted in either the hippocampus or PFC in NodDKO(+WAS) mice (data not shown).
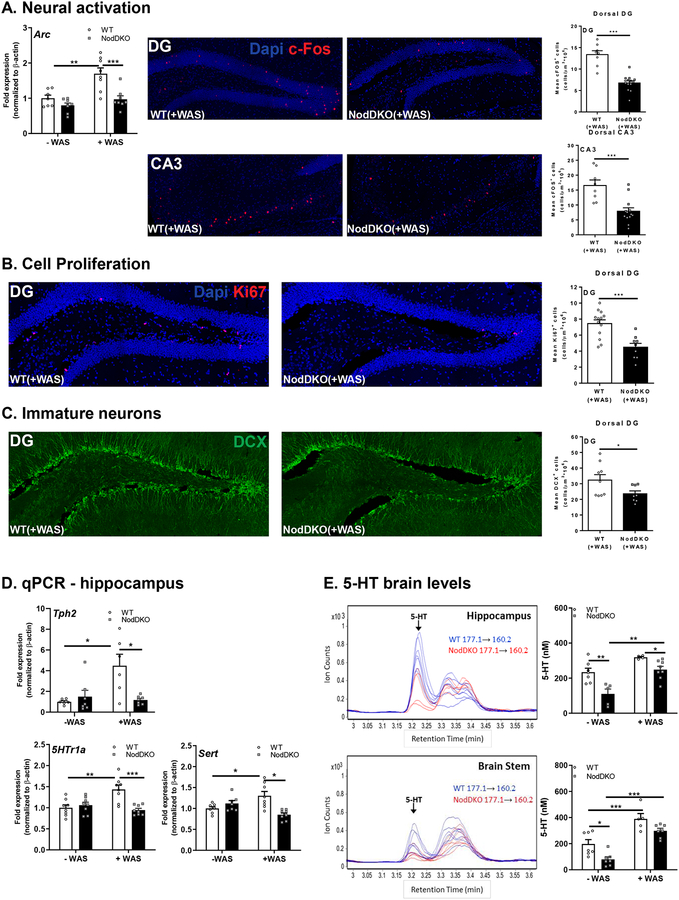
Figure 3. NodDKO(+WAS) mice display decreased neural activation, cell proliferation, and immature neurons, with 5-HT signaling impairments.
(A) Hippocampal Arc mRNA expression levels (N = 7–8), and c-Fos+ cells in the dentate gyrus (DG) (N = 8–13) and the cornus ammonis (CA) 3 regions (N = 8–13) and representative images of c-Fos immunofluorescence; (B) Ki67+ cells (N = 8–13) and representative images of Ki67 immunofluorescence in the DG. (C) DCX+ cells (N = 6–7) and representative images of DCX immunofluorescence in the DG; (D) Tph2, 5HTr1a and Sert hippocampal mRNA expression levels (N = 6–16); (E) serotonin (5-HT) protein levels in the hippocampus (N = 5–8) and brain stem (N = 5–7) detected by LC/MS and multiple reaction monitoring chromatogram of 5-HT in cytoplasmic extracts of both the hippocampus and brain stem: m/z 177.1 correspond to 5-HT precursor ion mass and m/z 160.2 correspond to fragment ion mass. Data are presented as mean ± SEM. (*P < 0.05; **P < 0.01; ***P < 0.001, 2-way ANOVA and unpaired Student’s t-test).
Neuronal activation was further assessed by quantification of c-Fos-positive cells in the DG and CA3 regions of the dorsal hippocampus by confocal microscopy in mice exposed to WAS. Given that the peak of c-Fos expression occurs 1–3 h following exposure to an acute stimulus (Reichmann et al., 2013), c-Fos positive cells were enumerated in hippocampal sections collected at 1 h post-WAS. The total number of c-Fos-positive cells, as quantified using Imaris, was significantly lower in NodDKO(+WAS) mice compared to WT(+WAS) in both the DG and CA3 region of the dorsal hippocampus (Fig 3A). Taken together, these results suggest that neuronal activation following WAS is impaired in NodDKO(+WAS) mice, leading to lack of consolidation of hippocampal-dependent memory, and subsequently leading to cognitive deficits compared to WT(+WAS) mice.
NodDKO(+WAS) mice exhibit reduced cell proliferation and decreased immature neurons in the dorsal hippocampus.
Cell proliferation as well as the abundance of immature neurons in the adult hippocampus are involved in the process of neurogenesis, which is thought to play an important role in the stress response (Snyder et al., 2011), consolidation of memory (Jessberger et al., 2009), and regulation of mood (Hill et al., 2015). Given the impaired recognition memory and increased anxiety-like behavior observed in NodDKO(+WAS) mice, we assessed if hippocampal cell proliferation and the abundance of immature neurons were impacted by Nod1/2 deficiency. Cell proliferation was measured by staining for the proliferation marker Ki67 and the abundance of immature neurons was assessed by quantifying DCX+ cells in the dorsal hippocampus. Confocal image analysis revealed significantly fewer Ki67-positive cells (Fig 3B) and lower numbers of DCX-positive cells in the DG of NodDKO(+WAS) mice compared to WT(+WAS) controls (Fig 3C). These findings suggest that altered hippocampal neurogenesis may be involved in the cognitive and emotional impairments found in NodDKO(+WAS) mice.
NodDKO mice exhibit down-regulation of the serotonergic system in the brain.
Changes in the central 5-HT system can regulate adult hippocampal neurogenesis in rodents (Malberg et al., 2000; Song et al., 2017). To investigate the potential molecular mechanisms underlying the behavioral deficits observed in NodDKO(+WAS) mice, we first assessed components of the 5-HT signaling pathway by qPCR in the hippocampus and PFC. Expression of tryptophan hydroxylase (Tph)2, the rate limiting enzyme for 5-HT synthesis, the 5-HT receptor (5HTr)1a, which regulates 5-HT release in different brain areas including the hippocampus (Bravo et al., 2014), and the 5-HT transporter (Sert) was quantified. In the hippocampus, but not the PFC (data not shown), increased expression of Tph2, 5HTr1a and Sert was demonstrated in WT mice following exposure to WAS, which was impaired in NodDKO(+WAS) mice (Fig 3D). In contrast, expression of 5HTr2c was not impacted in either hippocampus or PFC (data not shown).
To determine the impact of reduced Tph2 expression on 5-HT concentrations in the hippocampus and brain stem, LC/MS was used. The brain stem was assessed as it represents the primary site of 5-HT synthesis in the brain (Charnay & Leger, 2010). Quantification of 5-HT revealed significantly reduced baseline concentrations in the hippocampus and brain stem of NodDKO(-WAS) mice compared to WT(-WAS) controls (Fig 3E). 5-HT was increased by WAS in WT (brain stem) and NodDKO (hippocampus and brain stem) mice, with WT(+WAS) having higher hippocampal 5-HT levels compared to NodDKO(+WAS). These data demonstrate that Nod1/2 participate in an important control mechanism that regulates 5-HT concentration in the brain and impairs stress-induced 5-HT receptor expression.
Other neurotransmitters such as gamma-aminobutyric acid (GABA), are involved in the regulation of stress, anxiety, and cognition (O’Leary et al., 2014). Overall, no overt alterations were observed in GABA signaling either in the hippocampus or PFC with only Gabab1b found to be increased in the PFC of NodDKO(+WAS) mice compared to WT(+WAS) (data not shown). These findings suggest a specific interaction between NLR and serotonergic signaling.
NodDKO mice exhibit stress-induced impairments in intestinal physiology.
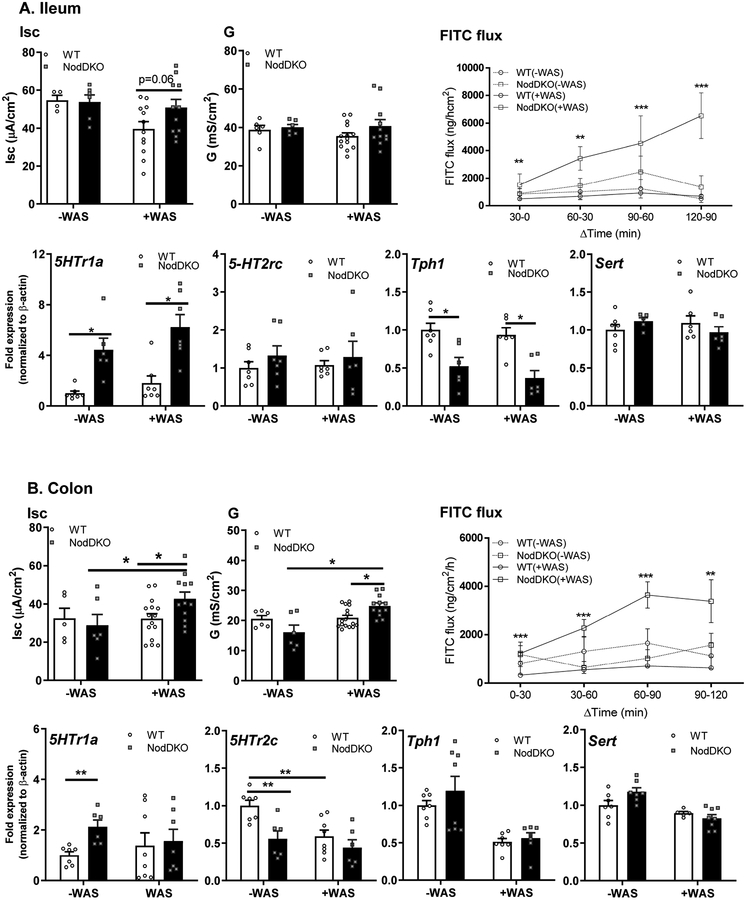
Psychological stress and the resulting HPA-axis activation can detrimentally impact intestinal physiology, in part by causing elevated intestinal ion transport and increased intestinal permeability (Gareau et al., 2008). Given previous findings identifying a role for Nod1 and Nod2 in regulating intestinal mucosal barrier function (Natividad et al., 2012), we assessed intestinal physiology in ileal and colonic tissues from WT and NodDKO mice using Ussing chambers. Active ion transport was assessed by measuring Isc in ileal and colonic segments, with NodDKO(-WAS) mice displaying normal baseline values compared to WT(-WAS) mice (Fig 4A–B). Intestinal permeability was assessed by measuring G for tight junction permeability and flux of FITC-labeled dextran (4KDa) for macromolecular permeability. Similarly to ion transport, G and FITC flux were both normal in NodDKO(-WAS) mice compared to WT(-WAS) mice. In contrast, both ion transport (Isc [colon]) and permeability (G [colon] and FITC-dextran flux [colon/ileum]) were increased in in NodDKO(+WAS) mice compared to WT(+WAS) controls (Fig 4A–B).
Figure 4. NodDKO(+WAS) mice exhibit altered GI physiology and impaired 5-HT signaling.
(A) Ileum and (B) colon basal short circuit current [Isc], basal conductance [G] and FITC dextran flux assessment (N = 11–16) as well as 5HTr1a, 5HTr2c, Tph1 and Sert mRNA expression levels (N = 6–8). Data are presented as mean ± SEM. (*P < 0.05; **P < 0.01; ***P < 0.001, unpaired Student’s t-test and 2-way ANOVA).
Given the important role of 5-HT signaling in regulating gut physiology (Mawe & Hoffman, 2013), and our evidence of altered 5-HT signaling in the hippocampus in NodDKO(+WAS) mice, qPCR analysis for 5-HT signaling in ileum and colon were performed. Increased 5HTr1a and reduced Tph1 expression were observed in the ileum of both NodDKO(±WAS) mice when compared to WT(±WAS) controls (Fig 4A). In the colon, baseline expression of 5HTr1a and 5HTr2c was increased and decreased, respectively, whereas Tph1 was unaffected (Fig 4B). Expression of the serotonin reuptake transporter (Sert), which terminates 5-HT activity, was unaffected in both ileum and colon (Fig 4A–B). Taken together, these findings identify that Nod1 and Nod2 deficiency leads to dysregulated peripheral 5-HT signaling, similar to findings in the hippocampus.
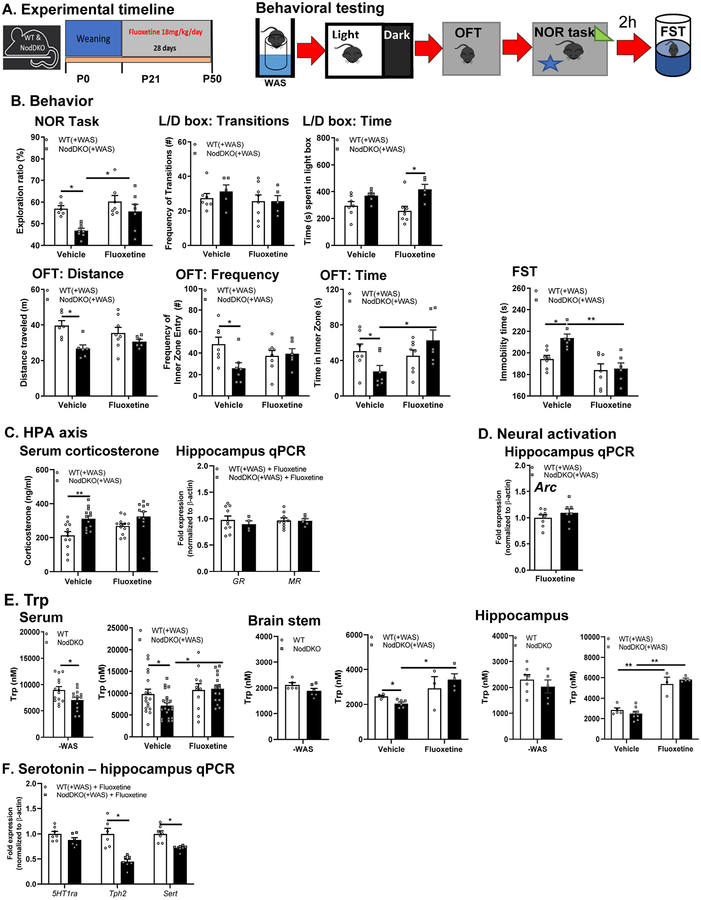
Stress-induced behavioral impairments in NodDKO mice are restored by chronic fluoxetine administration.
The selective serotonin reuptake inhibitor (SSRI), fluoxetine, is one of the most commonly prescribed anti-depressant drugs that ameliorates cognitive impairments and depression both in rodents (David et al., 2009) and humans (Jaykaran et al., 2009), mainly by acting on the 5-HT system (Malagie et al., 2002; Taylor et al., 2005). Chronic fluoxetine administration is also known to attenuate acute stress reactivity in mice (McVey Neufeld et al., 2018). In accordance with our observation of reduced 5-HT in the brain and down-regulation of the 5-HT signaling in NodDKO(+WAS) mice, mice were treated chronically with fluoxetine for 28 days starting at weaning to restore hippocampal 5-HT levels. Behavior (L/D box, OFT, and NOR) was performed as described previously (Fig 1A) followed by a 2h rest and finally the FST to assess depressive-like behavior (Fig 5A). Cognitive impairments seen in NodDKO(+WAS) mice in the NOR task relative to WT(+WAS) were reversed by fluoxetine administration when compared to vehicle (water)-treated control NodDKO(+WAS) mice (Fig 5B). Fluoxetine administration also improved anxiety-like behavior by increasing the time spent in the L/D box, but not affecting the number of transitions, in NodDKO(+WAS) mice compared to WT(+WAS) fluoxetine-treated mice (Fig 5B). Similarly, deficits in total distance traveled, time spent, and frequency of entries into the inner zone of the OFT in NodDKO(+WAS) mice were reversed by fluoxetine treatment compared to vehicle controls (Fig 5B). Given fluoxetine’s anti-depressant properties, depressive-like behavior was assessed using the FST. The total time spent immobile was increased in NodDKO(+WAS) mice compared to WT(+WAS) mice administered vehicle, suggesting the presence of depressive-like behavior. This was reversed by chronic fluoxetine administration to NodDKO(+WAS) mice (Fig 5B). Chronic fluoxetine administration also normalized the decreased GR expression (Fig 5C) but did not reduce the elevation of serum corticosterone levels that had previously been seen in NodDKO(+WAS) mice compared to WT(+WAS) mice (Fig 5C) suggesting the ability of fluoxetine to partially modulate HPA-axis activation. In addition, fluoxetine inhibited the decreased expression of hippocampal Arc seen in NodDKO(+WAS), suggesting restoration of neuronal activation (Fig 5D).
Figure 5. Chronic fluoxetine administration restored behavioral impairments as well as HPA-axis activity, Trp and 5HT signaling in NodDKO(+WAS) mice.
(A) Experimental timeline showing fluoxetine treatment and behavioral testing. Fluoxetine effect on (B) NOR task, L/D box, OFT and the forced swim test (FST) (N = 6–8), (C) corticosterone levels in serum (N = 11–13), and hippocampal GR, MR and (D) Arc mRNA expression levels (N = 4–10); (E) tryptophan levels in serum (N = 11–20), brain stem (N = 3–6) and hippocampus (N = 4–5) detected by LC/MS; (F) hippocampal 5HT1ra, Tph2 and Sert mRNA expression levels (N = 6–7). Data are presented as mean ± SEM. (*P < 0.05; **P < 0.01, unpaired Student’s t-test and 2-way ANOVA).
Nod1/2 deficiency reduces serum and tissue tryptophan levels, which can be restored by chronic fluoxetine administration.
Trp is the sole precursor of peripherally and centrally produced 5-HT (Richard et al., 2009). Trp exists in the circulation as both free or bound to albumin, with only free Trp able to cross the blood brain barrier. However, given the high affinity for Trp to the transporter, versus to albumin, approximately 75% of bound Trp is thought to be able to cross the blood brain barrier, making almost all of it accessible for 5HT synthesis within the CNS (Richard et al., 2009). Furthermore, given that reduced serum Trp levels correlate with alterations in mood and cognition (Young & Leyton, 2002; Toker et al., 2010), serum and hippocampal Trp concentrations were assessed by LC/MS. Lower free Trp levels were found in serum of both NodDKO(-WAS) (Fig 5E) and NodDKO(+WAS) compared to their control WT(±WAS) counterparts (Fig 5E), suggesting a role for Nod1/2 in regulating Trp transport. Interestingly, chronic fluoxetine treatment restored serum Trp concentration to WT levels in NodDKO(+WAS) mice (Fig 5E). In the brain stem, Trp levels were decreased in NodDKO(+WAS) compared to WT(+WAS) which was restored by administration of fluoxetine (Fig 5E). While hippocampal Trp was not impaired in NodDKO(±WAS) (Fig 5E), fluoxetine administration significantly increased hippocampal levels in both WT(+WAS) and NodDKO(+WAS) mice (Fig 5E). Despite the ability to beneficially impact Trp levels, fluoxetine was not able to restore the decreased hippocampal Tph2 or Sert expression levels in NodDKO(+WAS) mice, but it did normalize 5HTr1a levels (Fig 5F). Given that the brain stem is the major area of 5-HT synthesis in the brain, which can then be used by other regions, signaling in the brain stem may indirectly impact hippocampus function and behavior.
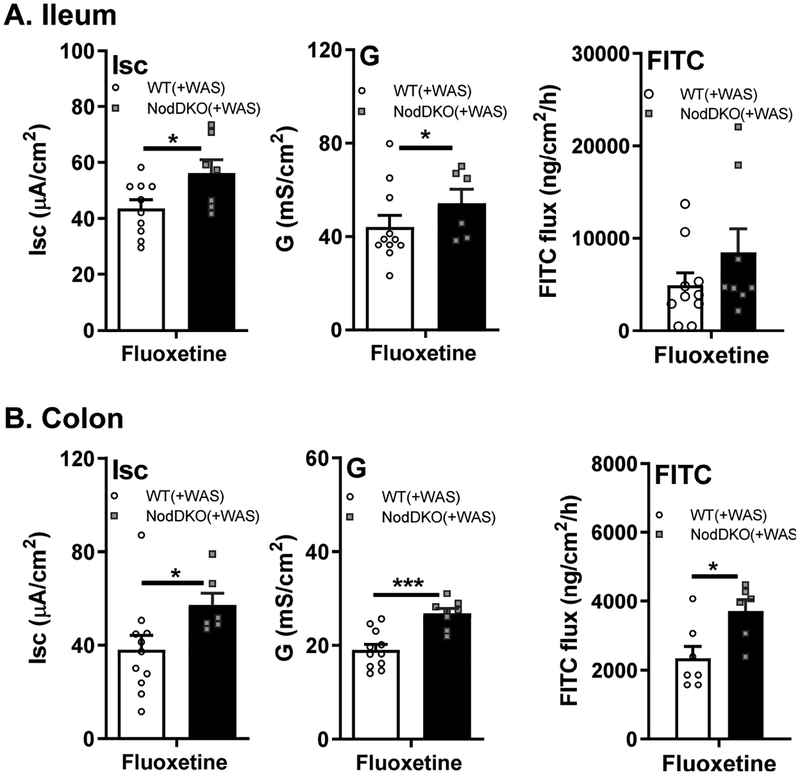
Chronic fluoxetine administration did not restore intestinal physiology in NodDKO(+WAS).
Given the impairments in 5-HT signaling found in the ileum and colon of NodDKO(+WAS) mice, we assessed if chronic fluoxetine administration could restore the altered intestinal physiology we observed in these animals (Fig 6). In contrast to amelioration of behavioral deficits, the elevations in secretory state (Isc) and gut permeability (G and FITC flux) seen in NodDKO(+WAS) in colon were not reversed by chronic fluoxetine administration (Fig 6B) and may even be slightly potentiated in the ileum (Fig 6A). These findings suggest that while intestinal alterations of selected genes of the 5-HT system were found, the mechanism by which fluoxetine normalizes behavior is likely independent of changes in intestinal physiology.
Figure 6. Chronic fluoxetine administration did not restore intestinal physiology in NodDKO(+WAS) mice.
(A) Ileum and (B) colon basal short circuit current [Isc], basal conductance [G] and FITC dextran flux assessment (N = 6–8). Data are presented as mean ± SEM. (*P < 0.05; **P < 0.01; ***P < 0.001, unpaired Student’s t-test).
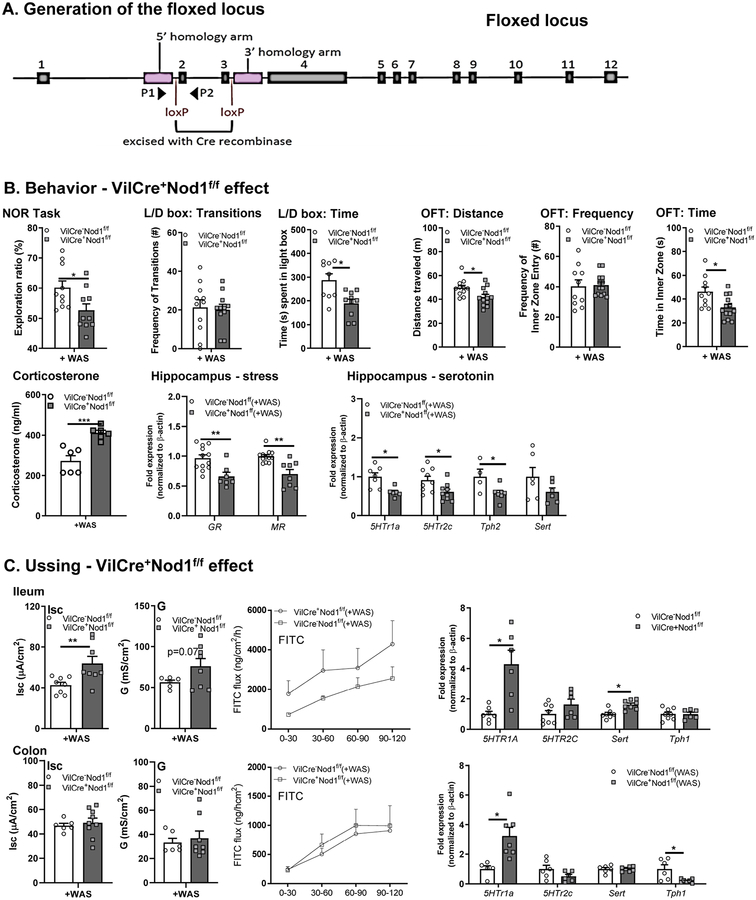
Stress-induced behavioral deficits are dependent on intestinal epithelial Nod1 receptors.
To determine whether intestinal NLR account for the behavioral and biochemical effects observed in NodDKO mice, we utilized a conditional knockout (cKO) strategy (Fig 7A). Behavior and intestinal physiology were assessed in IEC cKO mice (VilCre+Nod1f/f and VilCre+Nod2f/f). VilCre+Nod1f/f(+WAS) mice demonstrated significantly reduced recognition of the novel object in the NOR task compared to control mice (VilCre−Nod1f/f[+WAS]; Fig 7B). Furthermore, VilCre+Nod1f/f(+WAS) mice displayed anxiety-like behavior as determined by the L/D box (reduced time spent in the light compartment) and OFT (reduced total distance traveled and time spent into the inner zone) (Fig 7B). Similar to findings in NodDKO mice, VilCre+Nod1f/f mice also had increased serum corticosterone levels and decreased GR and MR expression in the hippocampus (Fig 7B). These changes in HPA axis signaling were accompanied by decrease 5-HT signaling, with decreased expression of 5HTr1a, 5HTr2c, Tph2 in the hippocampus (Fig 7B). Taken together, these findings suggest that IEC Nod1 expression can regulate CNS mechanisms, including the HPA axis and 5HT signaling, and impact behavior.
Figure 7. Behavioral deficits in NodDKO(+WAS) mice are dependent on intestinal epithelial Nod1 receptor expression.
(A) Schematic representing the floxed locus for the generation of Nod1f/f mice. (B) Effect of intestinal epithelial Nod1 deletion on behavior: NOR task, L/D box and OFT (N = 7–12), serum corticosterone and hippocampal mRNA expression levels of stress genes (GR, MR) and serotonin signaling (5HTr1a, 5HTr2c, Tph1 and Sert); (C) Ileum and colon basal short circuit current [Isc], basal conductance [G] and FITC dextran flux assessment (N = 6–9) as well as 5HTr1a, 5HTr2c, SERT and Tph1 mRNA expression levels (N = 6–8). Data are presented as mean ± SEM. (*P < 0.05; **P < 0.01, ***P<0.001 unpaired Student’s t-test).
In the GI tract, significantly increased ion transport (Isc) was found in the ileum of VilCre+Nod1f/f(+WAS) mice compared to their WT control littermates (Fig 7C). In contrast, no changes in physiology were observed in the colon, with similar ion transport and permeability seen in both WT and VilCre+Nod1f/f(+WAS) mice, suggesting a differential role for Nod1 in the ileum versus the colon in response to stress. qPCR analysis for 5-HT signaling in ileum identified increased 5HTr1a and Sert mRNA expression levels in VilCre+Nod1f/f(+WAS) mice when compared to VilCre−Nod1f/f(+WAS) controls (Fig 7C). In the colon, 5HTr1a was increased whereas Tph1 mRNA levels were decreased (Fig 7C).
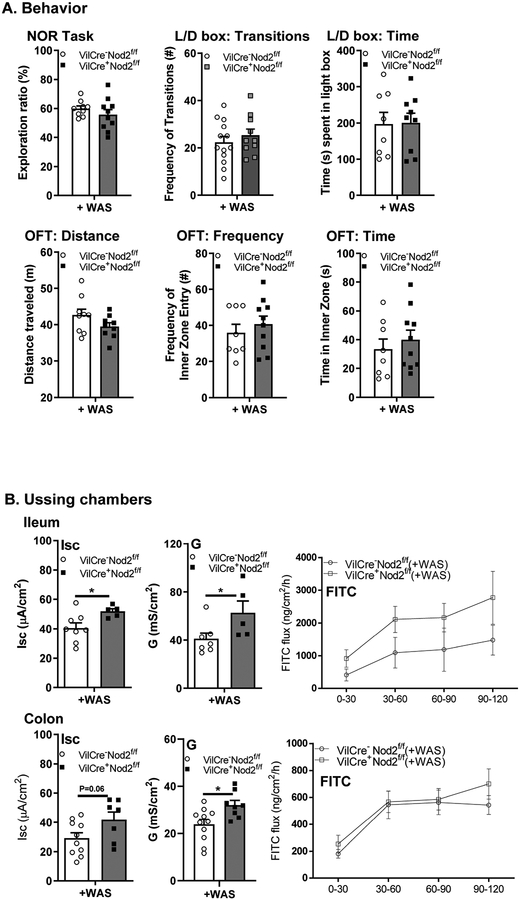
In contrast to VilCre+Nod1f/f(+WAS) mice, VilCre+Nod2f/f(+WAS) mice had normal cognitive function and anxiety-like behavior when compared to littermate controls (Fig 8A). In the GI tract, VilCre+Nod2f/f(+WAS) mice displayed significantly increased Isc in the ileum, but not the colon, as well as increased G in both ileum and colon compared to their WT control littermates (Fig 8B). These findings suggest a unique role for IEC Nod1 receptors in the regulation of behavior through the GI tract with a possible involvement of the peripheral 5-HT system.
Figure 8. Behavioral, but not GI, deficits are independent of intestinal epithelial Nod2 receptor expression.
(A) Effect of intestinal Nod2 deletion on NOR task, L/D box, and OFT (N = 9–12); (B) Ileum and colon basal short circuit current [Isc], basal conductance [G] and FITC dextran flux assessment (N = 5–11). Data are presented as mean ± SEM (unpaired Student’s t-test).
DISCUSSION
Understanding the molecular mechanisms underlying stress susceptibility is key for the identification of novel pharmacological treatments for stress-related gut-brain disorders. Here we demonstrated that mice deficient in Nod1 and Nod2 have altered serotonergic signaling in the gut and brain, which makes them susceptible to hyperactivation of the HPA-axis following exposure to acute psychological stress (Figure 9). This HPA-axis activation leads to GI pathophysiology, cognitive deficits, anxiety-like behavior and depressive-like behavior. Highlighting a previously unappreciated role of Nod1/2 in regulating HPA-axis activation and serotonergic signaling, behavioral deficits (but not abnormalities in GI physiology) in NodDKO(+WAS) mice were normalized by chronic fluoxetine administration. Using a cKO approach, we further identified that mice lacking Nod1 in IEC and exposed to WAS recapitulated the changes in behavior and intestinal physiology seen in NodDKO(+WAS) mice. Deficiency of Nod1 in IEC in these mice resulted in stress-induced impairments in cognitive function and anxiety-like behavior. These effects appear to reflect a specific function of Nod1 in IEC, as there was no impact of loss of IEC Nod2 expression on behavior or GI physiology. Together, these results identify Nod1 as a novel factor that regulates the stress response, 5-HT biosynthesis, and signaling. These results further indicate that Nod1 may contribute to a previously unrecognized signaling pathway in the gut-brain axis.
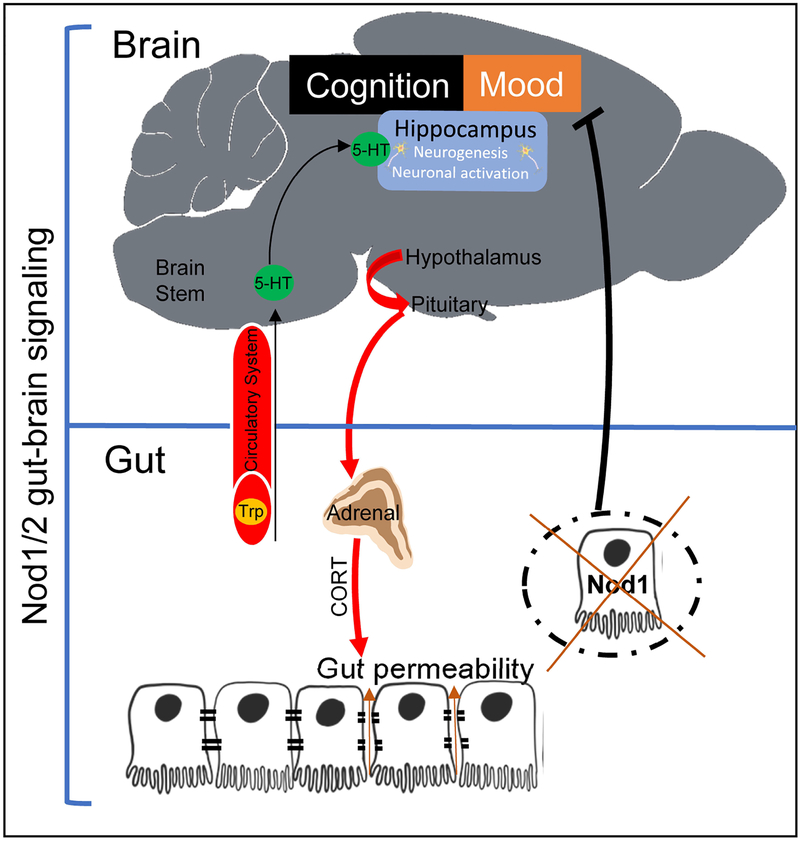
Figure 9. Graphical summary.
Constitutive knockout of Nod1 and Nod2 induces gut dysbiosis and altered behavior through the 5-HT system. Intestinal epithelial Nod1 receptor regulates susceptibility to cognitive impairment, anxiety-like and depressive-like behaviors.
NLR are expressed in both the brain and periphery, and function as receptors that detect bacterial PGN, therefore we hypothesized that NLR could modulate gut-brain signaling. Highlighting this novel role, mice deficient in Nod1/2 subjected to WAS had pronounced cognitive dysfunction, as well as anxiety-like and depressive-like behaviors, compared to WT(+WAS) controls. These findings complement previous studies by Farzi et al., who showed a synergistic effect between agonists for Nod1/2 and the toll-like receptor (TLR) 4 agonist lipopolysaccharide (LPS) in causing impairments in brain activity and sickness behavior (Farzi et al., 2015), demonstrating a role for NLR in regulating behavior. In accordance, our study identified a role for Nod1/2 in regulating mood and cognitive behavior which was uncovered after exposure to an acute stressor. This was associated with alterations in HPA-axis signaling, due to increased serum corticosterone levels and decreased hippocampal GR expression in NodDKO(+WAS) mice. This finding may suggest a novel NLR-stress dependent mechanism of action for the regulation of brain and behavior.
Given the association between stress, hippocampal neuronal activation, and behavior (Finsterwald & Alberini, 2014; O’Leary et al., 2014; Kim et al., 2015), hippocampal neuronal activation was assessed by Arc expression and c-Fos activation. The increase in Arc expression that would otherwise be induced by stress was inhibited, and the numbers of c-Fos positive neurons were reduced in NodDKO(+WAS) mice. This indicates a lack of neuronal activation following exposure to WAS. Stress is also known to be detrimental for adult hippocampal neurogenesis (Baptista & Andrade, 2018), which has been shown to reduce memory recognition in rodents, as assessed by NOR tasks (Jessberger et al., 2009; Denny et al., 2012). Similarly, both the number of immature neurons and hippocampal cell proliferation were impaired in NodDKO(+WAS) mice. Taken together, these findings demonstrate that behavioral impairments driven by NLR appear to involve alterations in hippocampal neurons, including reduced neuronal activation and decreased numbers of immature neurons, which may lead to impaired consolidation of hippocampal-dependent memories and cognitive deficits. However, additional studies focused on the role of NLRs in regulating behavior through neurogenesis-dependent and independent mechanisms are warranted.
Given the interaction between stress and hippocampal serotonergic signaling (Mahar et al., 2014) we wanted to assess 5-HT biology in NodDKO mice. In the hippocampus, a lack of Nod1/Nod2 receptors resulted in significantly decreased 5-HT at both baseline and following exposure to acute stress compared to WT controls. This finding was associated with significantly reduced expression of both Tph2 (the rate-limiting enzyme for neuronal 5-HT synthesis) and the heteroreceptor 5HTr1a in NodDKO(+WAS) mice. While Tph2 expression was not reduced in NodDKO(-WAS) vs. WT(-WAS), significantly decreased hippocampal 5-HT concentrations in NodDKO(-WAS) mice compared to WT(-WAS) controls were detected. These data suggest that reduced Tph2 expression may be sufficient to cause profound deficits in neurotransmitter production in the brain. Interestingly, both stress and elevated corticosterone levels have been shown to downregulate the expression and the functionality of 5HTr1a through the alteration of GR expression in the hippocampus of both rats and subjects with a history of depression (Meijer & de Kloet, 1994; Lopez et al., 1998). Highlighting a role for 5-HT in mediating cognitive function, administration of the selective post-synaptic 5HTr1a agonist F15599 can ameliorate cognitive performance during the NOR task in a rat model of cognitive impairment (Horiguchi & Meltzer, 2012). Moreover, pharmacological blockade of 5HTr1a can reduce hippocampal neurogenesis in adult rats (Radley & Jacobs, 2002), and regulate memory (Saxe et al., 2006) as well as emotional behavior (Snyder et al., 2011). In addition to hippocampal changes, significantly lower 5-HT concentrations were observed in the brain stem of NodDKO(±WAS) mice, suggesting lower release of 5-HT to the hippocampus (Charnay & Leger, 2010). Although reduced production of 5-HT was observed in both NodDKO(-WAS) and NodDKO(+WAS) mice, exposure to acute stress was necessary to uncover the behavioral deficits found in NodDKO(+WAS) mice. These data suggest a critical interaction between stress, NLR, and the 5-HT system in the regulation of memory and emotional behavior. Future studies characterizing the long-lasting effects of Nod1/Nod2 receptors on serotonergic signaling in response to chronic stress could support the concept that NLR play a crucial role in the pathophysiology of stress-related disorders, with implications for treatment.
To determine if the behavioral deficits observed in NodDKO(+WAS) mice were dependent on the impaired serotonergic system seen in the hippocampus, behavior and intestinal physiology were assessed in mice treated chronically with fluoxetine to increase 5-HT availability in the CNS. Restoration of cognitive impairment and reduced anxiety- and depressive-like behaviors were observed after chronic fluoxetine treatment, further supporting a role for 5-HT in mediating the behavioral deficits seen in NodDKO(+WAS) mice. In addition to increasing 5-HT concentration in the synaptic cleft by inhibiting SERT, the antidepressant effects of fluoxetine have been demonstrated to involve other mechanisms such as neurogenesis (Malberg et al., 2000), increased BDNF levels (Nibuya et al., 1996), regulation of HPA-axis activity (Pariante, 2004) and modulation of long-term potentiation (Rubio et al., 2013). Therefore, while NLR appear to regulate serotonergic signaling in the brain, it remains to be determined at which point in the signaling pathway they are critical for maintaining 5-HT levels in the brain and periphery.
Since NLR are important in maintaining intestinal physiology (Natividad et al., 2012) that can be detrimentally impacted by exposure to stress (Demaude et al., 2009; Hattay et al., 2017), we assessed ileal and colonic mucosal barrier function in WT(±WAS) and NodDKO(±WAS) mice. While baseline ion transport (Isc) and permeability (G, FITC flux) were intact in NodDKO(-WAS), stress significantly increased Isc, G, and FITC flux in the ileum and/or the colon of NodDKO(+WAS) mice vs WT(+WAS) controls. These changes in GI physiology were associated with altered 5-HT signaling, with increases in 5HTr1a and decreased Tph1 expression seen in the ileum. Mucosal barrier deficits in NodDKO(+WAS) mice were not restored by fluoxetine administration, even potentiating effects in the ileum, suggesting that fluoxetine ameliorates behavioral deficits via a mechanism independent of GI physiology. This may be due, in part, to the dual function of 5-HT in the GI tract, depending on whether it is released from enteric nerves or enteroendocrine cells (EC) in the mucosa (Mawe & Hoffman, 2013), although future studies would be necessary to discern the specific contribution of each source in maintaining GI physiology when NLR are absent. Interestingly, activation of Nod1 has been shown to downregulate the activity and expression of SERT in epithelial Caco-2/T7 cells (Layunta et al., 2018). Although these findings need to be confirmed in vivo, this suggests a potential interaction between NLR and the serotonergic system in the GI tract.
To assess if Nod1/2 deficient mice have reduced peripheral availability of Trp, the biochemical precursor for 5-HT, the concentration of this amino acid was quantified in the serum. The concentration of serum Trp was significantly reduced in NodDKO(+WAS) mice but could be restored by chronic fluoxetine treatment. Since CNS concentrations of Trp are predominantly determined by availability in the periphery (Ruddick et al., 2006), our data suggests a reduced supply of this amino acid precursor of 5-HT to the CNS as a potential mechanism by which peripheral Nod1/Nod2 receptors can influence the CNS. Indeed, measurement of Trp in hippocampus and brain stem, with elevations in both regions following fluoxetine administration, confirm its ability to increase available Trp in the CNS for 5-HT synthesis.
Given our findings of altered physiology and 5-HT signaling in the GI tract in NodDKO mice, the role of IEC NLR expression in regulating gut-brain communication was assessed using cKO mice. Cognitive deficits and anxiety-like behavior were observed in VilCre+Nod1f/f(+WAS), but not VilCre+Nod2f/f(+WAS) mice, suggesting that Nod1 expression in IEC can regulate cognition and mood in response to stress. While the anxiety-like behavior parameters in our cKO mice did not identically phenocopy the changes seen in NodDKO(+WAS) mice, likely in part due to slight changes in vivarium conditions over the timing of the multiple experiments, the presence of anxiety-like behavior is consistent and supportive of a common behavioral feature between the two strains. While our findings highlight a crucial role for IEC Nod1 receptors in maintaining behavior, it also suggests a potential additional role of brain Nod1/2 receptors in the regulation of memory and emotional mood. Thus, the use of selective antagonists as well as cKO mice targeting Nod1/2 receptors in the brain would be valuable to further elucidate the role of NLR in regulating mood and cognition.
In conclusion, Nod1/Nod2 receptors represent novel mediators of gut-brain axis signaling. Deficiencies in NLR signaling in mice causes increased susceptibility to stress-induced behavioral deficits, including cognitive function, anxiety-like behavior, and depressive-like behavior, as well as intestinal mucosal barrier defects. These effects were coupled with alterations in the serotonergic system that could be restored by chronic fluoxetine administration, suggesting a link between 5-HT signaling and NLR. Finally, these findings also indicate that Nod1/Nod2 receptors may be novel targets for the treatment of stress-related gut-brain disorders.
Key points:
Nod-like receptors regulate cognition, anxiety, and HPA-axis activation
Nod-like receptors regulate central and peripheral serotonergic biology
Nod-like receptors are important for maintenance of GI physiology
Intestinal epithelial cell expression of Nod1 receptors regulate behavior
Acknowledgments
The authors would like to thank Mr Dirk Truman (Monash Gene Targeting Facility, Monash University) for designing the Nod1 targeting strategy and for generating the heterozygote animals. This research was supported by the NIH (1R01AT009365-01 and 5R21MH108154-01 to MGG) and by the NHMRC (Senior Research Fellowships APP1079904, 606476 and Project grants APP1011303, 1079930 to RF). Research at the Hudson Institute of Medical Research is supported by the Victorian Government’s Operational Infrastructure Support Program.
Footnotes
Competing interests
Authors declare no conflict of interest.
References
- Arentsen T, Qian Y, Gkotzis S, Femenia T, Wang T, Udekwu K, Forssberg H & Diaz Heijtz R. (2017). The bacterial peptidoglycan-sensing molecule Pglyrp2 modulates brain development and behavior. Mol Psychiatry 22, 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avital A, Segal M & Richter-Levin G. (2006). Contrasting roles of corticosteroid receptors in hippocampal plasticity. J Neurosci 26, 9130–9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista P & Andrade JP. (2018). Adult Hippocampal Neurogenesis: Regulation and Possible Functional and Clinical Correlates. Front Neuroanat 12, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin M & Hascoet M. (2003). The mouse light/dark box test. Eur J Pharmacol 463, 55–65. [DOI] [PubMed] [Google Scholar]
- Bravo JA, Dinan TG & Cryan JF. (2014). Early-life stress induces persistent alterations in 5-HT1A receptor and serotonin transporter mRNA expression in the adult rat brain. Front Mol Neurosci 7, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnay Y & Leger L. (2010). Brain serotonergic circuitries. Dialogues Clin Neurosci 12, 471–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes AK, Zhou JY & Philpott DJ. (2015). NOD-Like Receptors: Guardians of Intestinal Mucosal Barriers. Physiology (Bethesda) 30, 241–250. [DOI] [PubMed] [Google Scholar]
- Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y & Weiser JN. (2010). Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med 16, 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. (2008). Behavioral phenotyping strategies for mutant mice. Neuron 57, 809–818. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED & Hen R. (2009). Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62, 479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaude J, Leveque M, Chaumaz G, Eutamene H, Fioramonti J, Bueno L & Ferrier L. (2009). Acute stress increases colonic paracellular permeability in mice through a mast cell-independent mechanism: involvement of pancreatic trypsin. Life Sci 84, 847–852. [DOI] [PubMed] [Google Scholar]
- Denny CA, Burghardt NS, Schachter DM, Hen R & Drew MR. (2012). 4- to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning. Hippocampus 22, 1188–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emge JR, Huynh K, Miller EN, Kaur M, Reardon C, Barrett KE & Gareau MG. (2016). Modulation of the microbiota-gut-brain axis by probiotics in a murine model of inflammatory bowel disease. American journal of physiology Gastrointestinal and liver physiology 310, G989–998. [DOI] [PubMed] [Google Scholar]
- Farzi A, Reichmann F, Meinitzer A, Mayerhofer R, Jain P, Hassan AM, Frohlich EE, Wagner K, Painsipp E, Rinner B & Holzer P. (2015). Synergistic effects of NOD1 or NOD2 and TLR4 activation on mouse sickness behavior in relation to immune and brain activity markers. Brain, behavior, and immunity 44, 106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterwald C & Alberini CM. (2014). Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: from adaptive responses to psychopathologies. Neurobiol Learn Mem 112, 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Warner N, Viani K & Nunez G. (2009). Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev 227, 106–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin K & Paxinos G. (1996). The Mouse Brain in Stereotaxic Coordinates, Compact 3rd Edition. [Google Scholar]
- Gareau MG, Silva MA & Perdue MH. (2008). Pathophysiological mechanisms of stress-induced intestinal damage. Curr Mol Med 8, 274–281. [DOI] [PubMed] [Google Scholar]
- Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, Macqueen G & Sherman PM. (2011). Bacterial infection causes stress-induced memory dysfunction in mice. Gut 60, 307–317. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Timlin JA, Roysam B, McNaughton BL, Worley PF & Barnes CA. (2005). Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr Opin Neurobiol 15, 599–606. [DOI] [PubMed] [Google Scholar]
- Hattay P, Prusator DK, Tran L & Greenwood-Van Meerveld B. (2017). Psychological stress-induced colonic barrier dysfunction: Role of immune-mediated mechanisms. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society 29. [DOI] [PubMed] [Google Scholar]
- Hill AS, Sahay A & Hen R. (2015). Increasing Adult Hippocampal Neurogenesis is Sufficient to Reduce Anxiety and Depression-Like Behaviors. Neuropsychopharmacology 40, 2368–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi M & Meltzer HY. (2012). The role of 5-HT1A receptors in phencyclidine (PCP)-induced novel object recognition (NOR) deficit in rats. Psychopharmacology (Berl) 221, 205–215. [DOI] [PubMed] [Google Scholar]
- Huerta-Franco MR, Vargas-Luna M, Montes-Frausto JB, Morales-Mata I & Ramirez-Padilla L. (2012). Effect of psychological stress on gastric motility assessed by electrical bio-impedance. World J Gastroenterol 18, 5027–5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaykaran, Bhardwaj P, Kantharia ND, Yadav P & Panwar A. (2009). Effect of fluoxetine on some cognitive functions of patients of depression. Indian J Psychol Med 31, 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD Jr., Consiglio A, Lie DC, Squire LR & Gage FH. (2009). Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem 16, 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keestra-Gounder AM, Byndloss MX, Seyffert N, Young BM, Chavez-Arroyo A, Tsai AY, Cevallos SA, Winter MG, Pham OH, Tiffany CR, de Jong MF, Kerrinnes T, Ravindran R, Luciw PA, McSorley SJ, Baumler AJ & Tsolis RM. (2016). NOD1 and NOD2 signalling links ER stress with inflammation. Nature 532, 394–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kim YG, Seo SU, Kim DJ, Kamada N, Prescott D, Chamaillard M, Philpott DJ, Rosenstiel P, Inohara N & Nunez G. (2016). Nod2-mediated recognition of the microbiota is critical for mucosal adjuvant activity of cholera toxin. Nat Med 22, 524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Pellman B & Kim JJ. (2015). Stress effects on the hippocampus: a critical review. Learn Mem 22, 411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolber BJ, Wieczorek L & Muglia LJ. (2008). Hypothalamic-pituitary-adrenal axis dysregulation and behavioral analysis of mouse mutants with altered glucocorticoid or mineralocorticoid receptor function. Stress 11, 321–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layunta E, Latorre E, Forcen R, Grasa L, Plaza MA, Arias M, Alcalde AI & Mesonero JE. (2018). NOD1 downregulates intestinal serotonin transporter and interacts with other pattern recognition receptors. J Cell Physiol 233, 4183–4193. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Chalmers DT, Little KY & Watson SJ. (1998). A.E. Bennett Research Award. Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychiatry 43, 547–573. [DOI] [PubMed] [Google Scholar]
- Mahar I, Bambico FR, Mechawar N & Nobrega JN. (2014). Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neuroscience and biobehavioral reviews 38, 173–192. [DOI] [PubMed] [Google Scholar]
- Malagie I, David DJ, Jolliet P, Hen R, Bourin M & Gardier AM. (2002). Improved efficacy of fluoxetine in increasing hippocampal 5-hydroxytryptamine outflow in 5-HT(1B) receptor knock-out mice. Eur J Pharmacol 443, 99–104. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ & Duman RS. (2000). Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20, 9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TD, Chan SS & Hart AR. (2015). Environmental factors in the relapse and recurrence of inflammatory bowel disease: a review of the literature. Dig Dis Sci 60, 1396–1405. [DOI] [PubMed] [Google Scholar]
- Mawdsley JE & Rampton DS. (2005). Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut 54, 1481–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawe GM & Hoffman JM. (2013). Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nature reviews Gastroenterology & hepatology 10, 473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. (2000). The neurobiology of stress: from serendipity to clinical relevance. Brain Res 886, 172–189. [DOI] [PubMed] [Google Scholar]
- McVey Neufeld KA, Kay S & Bienenstock J. (2018). Mouse Strain Affects Behavioral and Neuroendocrine Stress Responses Following Administration of Probiotic Lactobacillus rhamnosus JB-1 or Traditional Antidepressant Fluoxetine. Front Neurosci 12, 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer OC & de Kloet ER. (1994). Corticosterone suppresses the expression of 5-HT1A receptor mRNA in rat dentate gyrus. Eur J Pharmacol 266, 255–261. [DOI] [PubMed] [Google Scholar]
- Murray K, Godinez DR, Brust-Mascher I, Miller EN, Gareau MG & Reardon C. (2017). Neuroanatomy of the spleen: Mapping the relationship between sympathetic neurons and lymphocytes. PLoS One 12, e0182416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad JM, Petit V, Huang X, de Palma G, Jury J, Sanz Y, Philpott D, Garcia Rodenas CL, McCoy KD & Verdu EF. (2012). Commensal and probiotic bacteria influence intestinal barrier function and susceptibility to colitis in Nod1−/−; Nod2−/− mice. Inflamm Bowel Dis 18, 1434–1446. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ & Duman RS. (1996). Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci 16, 2365–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary OF, Felice D, Galimberti S, Savignac HM, Bravo JA, Crowley T, El Yacoubi M, Vaugeois JM, Gassmann M, Bettler B, Dinan TG & Cryan JF. (2014). GABAB(1) receptor subunit isoforms differentially regulate stress resilience. Proc Natl Acad Sci U S A 111, 15232–15237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y, Lala S, Xin W, Smith E, Dowds TA, Chen FF, Zimmermann E, Tretiakova M, Cho JH, Hart J, Greenson JK, Keshav S & Nunez G. (2003). Expression of NOD2 in Paneth cells: a possible link to Crohn’s ileitis. Gut 52, 1591–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM. (2004). Glucocorticoid receptor function in vitro in patients with major depression. Stress 7, 209–219. [DOI] [PubMed] [Google Scholar]
- Pariante CM. (2016). Neuroscience, mental health and the immune system: overcoming the brain-mind-body trichotomy. Epidemiol Psychiatr Sci 25, 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Caballero L, Torres-Sanchez S, Bravo L, Mico JA & Berrocoso E. (2014). Fluoxetine: a case history of its discovery and preclinical development. Expert Opin Drug Discov 9, 567–578. [DOI] [PubMed] [Google Scholar]
- Piatti VC, Davies-Sala MG, Esposito MS, Mongiat LA, Trinchero MF & Schinder AF. (2011). The timing for neuronal maturation in the adult hippocampus is modulated by local network activity. The Journal of neuroscience: the official journal of the Society for Neuroscience 31, 7715–7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett SB. (2003). Stress and the immune system. Pathophysiology 9, 133–153. [DOI] [PubMed] [Google Scholar]
- Qin HY, Cheng CW, Tang XD & Bian ZX. (2014). Impact of psychological stress on irritable bowel syndrome. World J Gastroenterol 20, 14126–14131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ & Jacobs BL. (2002). 5-HT1A receptor antagonist administration decreases cell proliferation in the dentate gyrus. Brain Res 955, 264–267. [DOI] [PubMed] [Google Scholar]
- Reichmann F, Painsipp E & Holzer P. (2013). Environmental enrichment and gut inflammation modify stress-induced c-Fos expression in the mouse corticolimbic system. PLoS One 8, e54811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard DM, Dawes MA, Mathias CW, Acheson A, Hill-Kapturczak N & Dougherty DM. (2009). L-Tryptophan: Basic Metabolic Functions, Behavioral Research and Therapeutic Indications. Int J Tryptophan Res 2, 45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio FJ, Ampuero E, Sandoval R, Toledo J, Pancetti F & Wyneken U. (2013). Long-term fluoxetine treatment induces input-specific LTP and LTD impairment and structural plasticity in the CA1 hippocampal subfield. Front Cell Neurosci 7, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddick JP, Evans AK, Nutt DJ, Lightman SL, Rook GA & Lowry CA. (2006). Tryptophan metabolism in the central nervous system: medical implications. Expert Rev Mol Med 8, 1–27. [DOI] [PubMed] [Google Scholar]
- Santos PL, Brito RG, Matos J, Quintans JSS & Quintans-Junior LJ. (2018). Fos Protein as a Marker of Neuronal Activity: a Useful Tool in the Study of the Mechanism of Action of Natural Products with Analgesic Activity. Mol Neurobiol 55, 4560–4579. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R & Drew MR. (2006). Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A 103, 17501–17506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Emge JR, Berzins K, Lung L, Khamishon R, Shah P, Rodrigues DM, Sousa AJ, Reardon C, Sherman PM, Barrett KE & Gareau MG. (2014). Probiotics normalize the gut-brain-microbiota axis in immunodeficient mice. American journal of physiology Gastrointestinal and liver physiology 307, G793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J & Cameron HA. (2011). Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476, 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song NN, Huang Y, Yu X, Lang B, Ding YQ & Zhang L. (2017). Divergent Roles of Central Serotonin in Adult Hippocampal Neurogenesis. Front Cell Neurosci 11, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C, Fricker AD, Devi LA & Gomes I. (2005). Mechanisms of action of antidepressants: from neurotransmitter systems to signaling pathways. Cell Signal 17, 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodorou V. (2013). Susceptibility to stress-induced visceral sensitivity: a bad legacy for next generations. Neurogastroenterol Motil 25, 927–930. [DOI] [PubMed] [Google Scholar]
- Toker L, Amar S, Bersudsky Y, Benjamin J & Klein E. (2010). The biology of tryptophan depletion and mood disorders. Isr J Psychiatry Relat Sci 47, 46–55. [PubMed] [Google Scholar]
- Tzingounis AV & Nicoll RA. (2006). Arc/Arg3.1: linking gene expression to synaptic plasticity and memory. Neuron 52, 403–407. [DOI] [PubMed] [Google Scholar]
- Uchoa ET, Aguilera G, Herman JP, Fiedler JL, Deak T & de Sousa MB. (2014). Novel aspects of glucocorticoid actions. Journal of neuroendocrinology 26, 557–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Yang C, Gelernter J & Zhao H. (2015). Pervasive pleiotropy between psychiatric disorders and immune disorders revealed by integrative analysis of multiple GWAS. Hum Genet 134, 1195–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SN & Leyton M. (2002). The role of serotonin in human mood and social interaction. Insight from altered tryptophan levels. Pharmacol Biochem Behav 71, 857–865. [DOI] [PubMed] [Google Scholar]
- Zhu LJ, Liu MY, Li H, Liu X, Chen C, Han Z, Wu HY, Jing X, Zhou HH, Suh H, Zhu DY & Zhou QG. (2014). The different roles of glucocorticoids in the hippocampus and hypothalamus in chronic stress-induced HPA axis hyperactivity. PloS one 9, e97689. [DOI] [PMC free article] [PubMed] [Google Scholar]