Abstract
Oxalobacter sp. promotion of enteric oxalate excretion, correlating with reductions in urinary oxalate excretion, was previously reported in rats and mice but the mechanistic basis for this affect has not been described. The main objective of the present study was to determine whether the apical oxalate transport proteins, PAT1 (slc26a6) and DRA (slc26a3) are involved in mediating the Oxalobacter-induced net secretory flux across colonized mouse cecum and distal colon. We measured unidirectional and net fluxes of oxalate across tissues removed from colonized PAT1 and DRA knockout (KO) mice and also across two double knockout (dKO) mouse models with Primary Hyperoxaluria, type 1 (i.e. deficient in alanine-glyoxylate aminotransferase; AGT KO), including PAT1/AGT dKO and DRA/AGT dKO mice compared to non-colonized mice. In addition, urinary oxalate excretion was measured before and after the colonization procedure. The results demonstrate that Oxalobacter can induce enteric oxalate excretion in the absence of either apical oxalate transporter and urinary oxalate excretion was reduced in all colonized genotypes fed a 1.5% oxalate-supplemented diet. We conclude that there are other, as yet unidentified, oxalate transporters involved in mediating the directional changes in oxalate transport across the Oxalobacter-colonized mouse large intestine.
Keywords: PAT-1, DRA, AGT, cecum, distal colon, urinary oxalate
INTRODUCTION
It has been generally acknowledged in recent years that the absence of intestinal commensal oxalatedegrading bacteria can pose as a risk factor for the development of hyperoxaluria leading to calcium oxalate kidney stone disease [23, 28, 30, 31, 35, 37, 38]. We have consistently reported the beneficial effect of reducing urinary oxalate excretion by colonizing the rat and mouse intestinal tract with Oxalobacter sp. [10-13] despite inconsistent results from similar studies conducted in humans [4, 16-19, 24-26, 29, 36, 37, 39]. While we observed that the “generalist oxalotrophs”, including Bifidobacterium animalis, Lactobacillus acidophilus and Lactobacillus gasseri degraded luminal oxalate sources thereby reducing the amount of oxalate absorbed from intestinal contents, these bacterial strains were not as effective as the “specialist oxalotroph” Oxalobacter formigenes in lowering urinary oxalate excretion [10, 12, 21, 39]. The superior efficacy of Oxalobacter sp. in participating in oxalate homeostasis can be attributed to the dual action of this bacteria in not only degrading luminal oxalate and reducing its absorption but, additionally, by promoting enteric oxalate excretion into the gut lumen [10-13, 39]. While we were the first investigators [11] to suggest that Oxalobacter maybe elaborating a secretagogue that possibly initiates directional changes in intestinal oxalate transport resulting in enteric oxalate secretion and excretion, the mechanistic basis for this affect has yet to be described. Whether the purported secretagogue directly or indirectly targets any of the known epithelial transport proteins that contribute to the movements of oxalate across native tissue is presently unknown.
Apically expressed PAT1 (Slc26a6) and DRA (Slc26a3) have been shown to contribute to apical oxalate efflux and uptake, respectively, across mouse intestinal epithelia [6, 7, 20]. In this study, we sought to determine whether PAT1 or DRA play a role in mediating Oxalobacter-induced enteric oxalate excretion by employing PAT1 and DRA knockout (KO) mice and also by examining a double KO (dKO) mouse model of Primary Hyperoxaluria, type 1 (having a deficiency of alanine glyoxylate aminotransferase, AGT) cross-bred with either PAT1 or DRA KO mice. We conclude from this study that Oxalobacter-induced enteric oxalate excretion does not require the presence of either apical oxalate transporter.
MATERIALS AND METHODS
Animals:
The animal studies were conducted in accordance with the University of Florida and the NIH Guide for the Care and Use of Laboratory Animals using protocols approved by the University of Florida Institutional Animal Care and Use Committee. Male and female mice of the different genotypes included in this study were all bred in the University of Florida Animal Care Facility. Heterozygous breeding pairs of PAT1 (Slc26a6 +/−) and DRA (Slc26a3 +/−) were initially, generously provided by Dr. Soleimani (The University of Cincinnati, OH, USA) approximately 10 years ago and used to generate homozygous knockout PAT −/− and DRA −/− animals on a C57BL/6 background over many generations in the interim. Wild type C57BL/6 mice (WT) were originally purchased from Charles River Laboratories (Raleigh, NC, USA) and then bred in-house as “controls” for the AGT knockout mice which are bred on the same background strain. The original breeding pairs of homozygous AGT knockout mice (AGT −/−) were also generously provided by Dr. Salido (University La Laguna, Tenerife, Spain) over 10 years ago and these were cross-bred in-house with the PAT1 and DRA knockouts (KO) to produce two double knockout (dKO) mouse models for the studies presented here. It is notable here that in order to generate n=5 DRA KO mice, we have to generate n=100 pups by mating heterozygous DRA males and females. This clearly required an intensive breeding protocol. Then, female (males are infertile) DRA KO were crossed with male AGT KO to produce that dKO genotype which took significant time to generate sufficient adult mice to be placed in the study groups. Contemporary controls were included over this lengthy time period (data provided in three Supplemental Tables). All mice were given free access to water and fed a standard chow TD.7912 (Harlan Teklad, Indianapolis, IN) until recruitment into the study as adult animals aged between 4 and 8 months in order to examine the effects of Oxalobacter colonization on intestinal oxalate transport.
Colonization studies:
Prior to study, all of the mice were tested for the fecal presence of Oxalobacter as previously described [13] and all were confirmed Oxalobacter-negative. A wild rat strain of Oxalobacter formigenes (OXWR) was used in these colonization studies which was delivered by esophageal gavage with a 0.5 ml inoculum containing approximately 50 mg wet weight of bacteria from a 24-hour culture. Prior to the gavage, the mice were provided with a 1.5% oxalate-supplemented diet (0.5% calcium / 0.4% phosphate; diet TD.89222.PWD (Harlan Teklad) for 3-5 days and a 24-h urine collection was obtained prior to the gavage procedure. Following esophageal gavage with viable bacteria, there is a “wash-out” period of bacteria moving out of the intestine for a period of time. So typically, fecal samples are collected from gavaged mice 5 days after gavage in order to avoid a false-positive result for colonization status which could simply be due to Oxalobacter shedding following the bolus delivery of viable bacteria. The freshly collected fecal samples are inoculated into anaerobic media vials containing 20 mM oxalate and after 7 days incubation at 37°C, the oxalate concentration of the media is determined. Oxalobacter is a slow-grower but if the mice have been successfully colonized the concentration of oxalate in the inoculated media vials will be zero mM after 7 days. However, this entire confirmation process takes at least 12 days. Since the flux experiments are labor-intensive, it is useful to know in advance, rather than after the fact, that gavaged mice are confirmed colonized. In addition, at the time of the flux studies, luminal contents from the two intestinal segments (cecum and distal colon) are also inoculated into anaerobic media vials and these are also incubated for 7 days before we have absolute confirmation that the tissue segments were colonized at the time of the flux studies which adds another 7+ days to the experimental design. Animals can lose colonization between the two testing time points as we have seen. Thus we necessarily have to wait for the colonization results at the time of flux studies in order to analyze data collected from confirmed colonized animals of different genotypes. A second 24-h urine collection was obtained 12-15 days post gavage immediately prior to euthanizing the mice for the flux studies in Ussing chambers [13]. This dietary regimen has been shown to have no influence on the magnitude of oxalate fluxes across intestine [11].
Urine collection and analytical methods:
Urine was collected from mice housed individually in metabolic cages for a 24-h period into receptacles containing 10 μl of 2% sodium azide, as a preservative, and under 75μl hydrated mineral oil to prevent evaporative volume loss. Urinary oxalate was determined as previously described using an oxalate kit assay (#591, Trinity Biotech, St. Louis, MO) and creatinine was measured using a modification of the Jaffe reaction [8].
Intestinal oxalate flux studies:
Intestinal tissues were removed from mice euthanized by inhalation of 100% CO2 followed by exsanguination via cardiac puncture. Transmural fluxes of oxalate across isolated, short-circuited segments of the mouse cecum and distal colon were determined using 14C-oxalate (Amersham, Piscataway, NJ, USA) as previously described [6]. The magnitude and direction of the net flux was determined by calculating the difference between two measured unidirectional fluxes (mucosal to serosal, and serosal to mucosal, ) determined for a period of 45 min at 15 min intervals, under short-circuit conditions. A positive is indicative of a net absorptive flux whereas a negative indicates transmural net oxalate secretion. The specific activity of 14C-oxalate, which was constant throughout the flux period, was determined both at the beginning and end of the experiment by sampling (50 μl) from the labeled (“hot”) compartment. At each 15 min interval, a 1 ml sample was removed from the opposing (“cold”) compartment with replacement of 1 ml of unlabeled buffer and a correction factor was applied for this dilution effect. The electrical parameters of the tissue were also recorded at 15 min intervals throughout the entire experiment. Tissue conductance (GT, mS·cm−2) was calculated as the ratio of the open-circuit potential (mV) to the short-circuit current (lsc, μA·cm−2), according to Ohm’s Law and net fluxes were determined on conductance matched (GT ≤ 20%) tissues.
Statistical analyses:
Results are presented as the mean ± one standard error (SE) for “n” the number of matched tissue pairs as well as for the urinary oxalate analyses for the different groups of animals. A statistical comparison of more than two means was performed by a one-way analysis of variance (ANOVA) followed by Bonferroni’s t-test for multiple comparisons with the control group. An un-paired t-test was used for the comparison of two means. In both cases, differences were considered significant if p ≤ 0.05 and notated with an asterisk in the Figures. The graphics were drawn using SigmaPlot 10 (Systat Software, San Jose, CA).
RESULTS
Mouse urinary phenotypes:
A hyperoxaluric phenotype was previously reported for the PAT1 KO (of 5.1 ± 0.6 μmol/24 h compared with an excretion of 1.3 ± 0.2 μmol/24 h in their contemporary WT littermates [6]. In contrast, urinary oxalate excretion in DRA KO mice was reported at 0.5 ± 0.1 μmol/24 h compared with an excretion of 1.6 ± 0.2 μmol/24 h in their contemporary WT littermates [7]. In the AGT KO mouse on a C57BL/6 background, oxalate excretion was reported at a significantly higher level of 1.3 ± 0.1 μmol/24 h compared with 0.5 ± 0.1 μmol/24 h in C57BL/6 WT controls [13] purchased from Charles River Laboratories. These reference values were obtained from all of the above animals fed standard mouse chow containing 1% calcium (TD.7912; Harlan Teklad). The urinary oxalate excretion values reported in this study (see Supplemental Table 1) on the n=84 mice involved were obtained from all genotypes fed a 0.5% calcium diet (TD.89222.PWD) supplemented with 1.5% oxalate which is used routinely to prime the mice for gavage with Oxalobacter [10]. Mean urinary creatinine (3.5 ± 0.1 μmol/24 h, n=84, respectively) was normal [6, 12] ranging across all genotypes included in this study and kidney stone formation was not observed in any mouse.
Oxalobacter colonization of PAT1 KO and PAT1/AGT dKO mice:
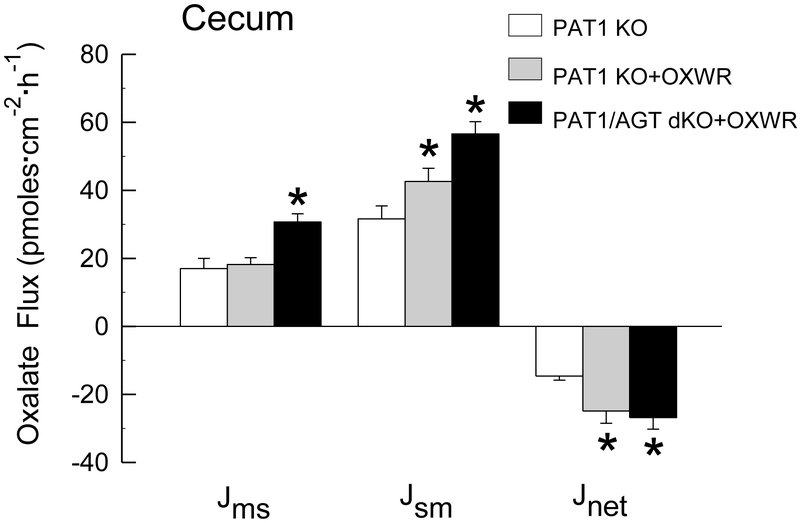
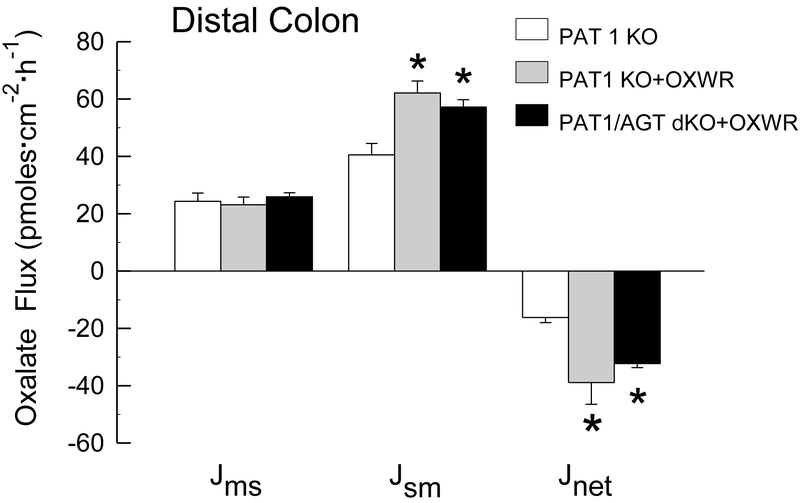
It is notable, at the outset, that studies examining the effects of colonizing AGT KO [13] and WT [12, 13] mice with Oxalobacter have already been conducted and published so these questions and experiments are not duplicated in the present study. In summary, colonization of AGT KO and WT mice resulted in significant increases in net oxalate secretion across the cecum and distal colon into the intestinal lumen when compared to oxalate transport across non-colonized mice [13]. In addition, both the hyperoxaluria and hyperoxalemia characteristic of the AGT KO were normalized following Oxalobacter colonization [13]. The results of oxalate transport studies across the cecum and distal colon of colonized PAT1 KO and PAT1/AGT dKO, compared to PAT1 KO mice that are not colonized (shown in Figure 1 and Figure 2) illustrate a similar pattern. It is clear from the results of the flux studies that even in the absence of the apical transport protein PAT 1, Oxalobacter colonization significantly promotes a net secretory flux of oxalate into the intestinal lumen primarily via an increase in . Net oxalate secretion across the cecum was enhanced 1.7 - fold and 1.8 - fold in PAT1 KO +OXWR and in PAT1/AGT dKO + OXWR, respectively, compared to non-colonized PAT1 KO. Similarly, in the distal colon, net oxalate secretion across the cecum was enhanced 2.4 - fold and 2.0 - fold in PAT1 KO +OXWR and in PAT1/AGT dKO + OXWR, respectively, compared to non-colonized PAT1 KO. These changes in large intestinal oxalate movements in the two PAT1 KO groups were accompanied by significant reductions in urinary oxalate excretion in both groups of colonized mice. Urinary oxalate excretion in PAT1 KO was reduced from 3.6 ± 0.6 μmol/24 h, (n=9) to 0.5 ± 0.1 μmol/24 h, (n=9) and in PAT/AGT dKO from 4.8 ± 0.2 μmol/24 h, (n=9) to 0.4 ± 0.1 μmol/24 h, (n=12) 12-15 days following esophageal gavage with Oxalobacter and during which time the mice were continuously fed the oxalate-supplemented diet.
Figure 1.
Unidirectional fluxes of oxalate (mucosal to serosal, and serosal to mucosal, and net flux, in pmoles·cm−2·hr−1) measured across the isolated, short-circuited cecum removed from non-colonized PAT1 KO and in colonized PAT1 KO and PAT1/AGT dKO mice (n=8, n=10, and n=6 in each group, respectively) fed a 1.5% oxalate-supplemented diet for 12-15 days. An asterisk indicates a significant difference between colonized and non-colonized groups. Transepithelial conductance GT was comparable in all groups (GT = 15.7 ± 1.2 mS·cm−2 and GT = 18.5 ± 0.9 mS·cm−2 and GT = 19.5 ± 1.1 mS·cm−2 in non-colonized and colonized groups, respectively). Short-circuit current (Isc) was significantly higher in the colonized PAT1/AGT dKO group (Isc = 3.3 ± 0.3 μEq·cm−2·h−1) compared to either the non-colonized group (Isc = 1.1 ± 0.2 μEq·cm−2·h−1) or colonized PAT1 KO (Isc = 1.0 ± 0.2 μEq·cm−2·h−1).
Figure 2.
Unidirectional fluxes of oxalate (mucosal to serosal, and serosal to mucosal, and net flux, in pmoles·cm−2·hr−1) measured across the isolated, short-circuited distal colon removed from non-colonized PAT1 KO and in colonized PAT1 KO and PAT1/AGT dKO mice (n=7, n=7, and n=10 in each group, respectively) fed a 1.5% oxalate-supplemented diet for 12-15 days. An asterisk indicates a significant difference between groups. Transepithelial conductance GT was comparable in all groups (GT = 14.5 ± 1.5 mS·cm−2 and GT = 13.1 ± 1.2 mS·cm−2 and GT = 11.6 ± 0.5 mS·cm−2 in non-colonized and colonized groups, respectively). Short-circuit current (Isc) was also comparable in all groups (Isc = 1.9 ± 0.3 μEq·cm−2·h−1 and Isc = 1.3 ± 0.2 μEq·cm−2·h−1 and Isc = 1.7 ± 0.2 μEq·cm−2·h−1).
Oxalobacter colonization of DRA KO and DRA/AGT dKO mice:
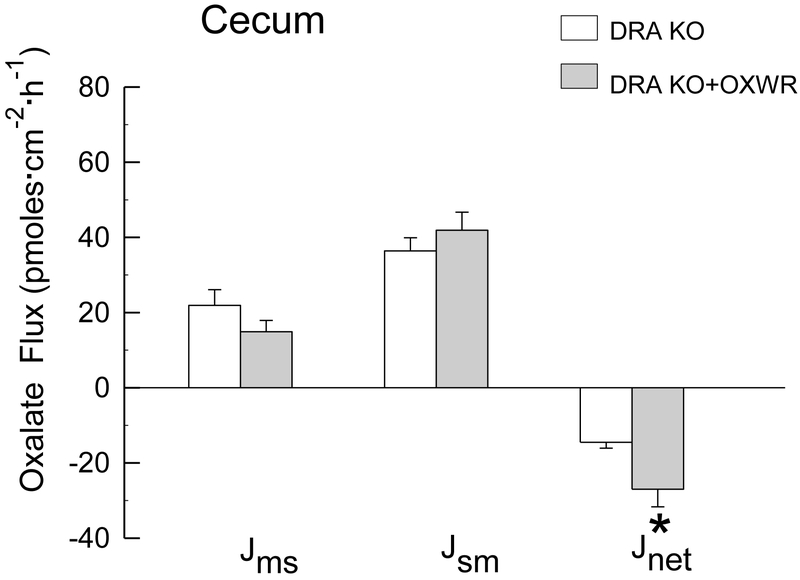
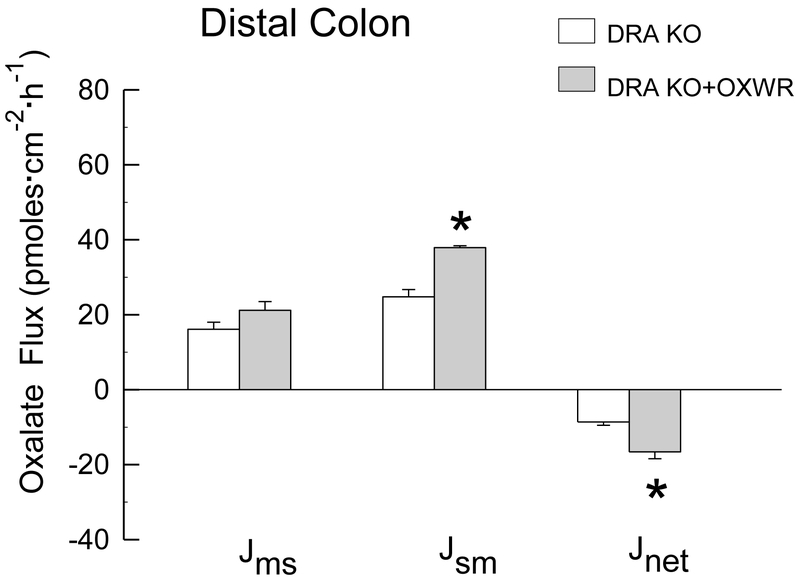
In contrast to PAT1 KO and PAT1/AGT dKO mice, which were 100% colonized at the time of the flux studies, only 45% of DRA KO mice sustained colonization at the time the flux studies were conducted (12 - 15 days following gavage). In the DRA/AGT dKO group, Oxalobacter was detected in only 2 out of 11 dKO distal colon segments, and not at all in the cecum, at the time the intestinal segments were prepared for the flux studies. Thus, only the results of oxalate transport studies across the cecum and distal colon of colonized DRA KO compared to DRA KO mice that are not colonized are shown in Figure 3 and Figure 4. The results presented are from confirmed colonized tissues and show that Oxalobacter colonization significantly promotes a net secretory flux of oxalate into the large intestinal lumen in the absence of the apical transport protein DRA. Net oxalate secretion across both the cecum and distal colon was enhanced 1.9- fold in DRA KO +OXWR compared to non-colonized DRA KO. An average of the distal colon fluxes in the n=2 colonized DRA/AGT dKO (not graphically presented in Figure 4) revealed a secretory net flux of −20. 6 ± 5.7 pmoles·cm−2·hr−1 which is comparable to the magnitude of the net flux across the colonized DRA KO group of −16.6 ± 1.8 pmoles·cm−2·hr−1, shown in Figure 4. In addition, the unidirectional fluxes are also very comparable between colonized DRA KO and the n=2 colonized DRA/AGT dKO (not shown). The changes in oxalate movements across the cecum and distal colon in the colonized DRA KO mice were accompanied by significant reductions in urinary oxalate excretion from 0.8 ± 0.2 μmol/24 h, (n=7) to 0.5 ± 0.03 μmol/24 h, (n=16) 12-15 days following esophageal gavage with Oxalobacter. In the DRA/AGT dKO group, mean urinary oxalate excretion of 1.44 ± 0.2 μmol/24 h, (n=11) was not different from the gavaged dKO group (1.64 ± 0.2 μmol/24 h, (n=11) because the majority (~ 82%) of this dKO group had lost colonization by the time the pre-flux urinary collections were conducted.
Figure 3.
Unidirectional fluxes of oxalate (mucosal to serosal, and serosal to mucosal, and net flux, in pmoles·cm−2·hr−1) measured across the isolated, short-circuited cecum removed from non-colonized and colonized DRA KO mice (n=9 in each group) fed a 1.5% oxalate-supplemented diet for 12-15 days. An asterisk indicates a significant difference between groups. Transepithelial conductance GT was comparable in both groups (GT = 18.9 ± 1.7 mS·cm−2 and GT = 20.8 ± 1.8 mS·cm−2 in non-colonized and colonized groups, respectively). Short-circuit current (Isc) was significantly higher in the colonized group (Isc = 4.4 ± 0.3 μEq·cm−2·h−1) compared to the non-colonized group (Isc = 2.2 ± 0.3 μEq·cm−2·h−1).
Figure 4.
Unidirectional fluxes of oxalate (mucosal to serosal, and serosal to mucosal, and net flux, in pmoes·cm−2·hr−1) measured across the isolated, short-circuited distal colon removed from non-colonized and colonized DRA KO mice (n=12 and n=9 in each group, respectively) fed a 1.5% oxalate-supplemented diet for 12-15 days. An asterisk indicates a significant difference between groups. Transepithelial conductance GT was comparable in both groups (GT = 11.5 ± 1.5 mS·cm−2 and GT = 13.0 ± 0.8 mS·cm−2 in non-colonized and colonized groups, respectively). Short-circuit current (Isc) was also comparable in both groups (Isc = 1.7 ± 0.2 μEq-·cm−2·h−1 and Isc = 1.8 ± 0.3 μEq·cm−2·h−1).
DISCUSSION
The focus of the present study was to determine whether the apical oxalate transport proteins PAT1 or DRA participate in mediating Oxalobacter-induced enteric secretion of oxalate. Since PAT1 has been shown to mediate a significant component of apical oxalate efflux across the distal ileum [6] and duodenum [20] in mice, it could reasonably be expected that Oxalobacter-induced net oxalate secretion might be reduced or abolished in the colonized PAT1 KO intestine, if PAT1 was integral in this response to Oxalobacter colonization. Similarly, since DRA has been reported to mediate a predominance of apical uptake of oxalate across the mouse small and large intestine [7], the question of whether Oxalobacter down-regulates the functional activity of DRA in reducing oxalate absorption, possibly unmasking a net secretory flux, also needs to be examined. In order to address these simple questions we measured bidirectional movements of oxalate across the cecum and distal colon of Oxalobacter-colonized PAT1 and DRA KO mice and also in two dKO mouse models of Primary Hyperoxaluria, type 1 (PAT1/AGT and DRA/AGT) compared to their non-colonized counterparts. We find, unexpectedly, that the induction of enteric net oxalate secretion by Oxalobacter formigenes in mouse large intestine does not require the presence of either apical oxalate transport proteins PAT1 or DRA.
Oxalobacter interaction with PAT1 and DRA apical oxalate transporters:
The conclusion from the present study that the PAT1 anion exchanger does not participate in Oxalobacter-induced oxalate secretion in mouse cecum and distal colon was surprising and in sharp contrast with earlier observations of Arvans et al [2] who used an siRNA approach to knockdown PAT1 in cultured Caco2-BBE cells (C2). This group reported that the PAT1 knockdown significantly decreased the stimulation of C2 oxalate uptake induced by Oxalobacter conditioned media (CM) by 49% with a concomitant reduction in PAT1 mRNA expression of 61%. It is notable, however that CM did not affect PAT1 protein expression in the C2 cells despite the functionally significant reduction in C2 oxalate uptake. Although the studies conducted by these investigators did not employ a PAT1 KO mouse model, they did show that CM, rectally administered to AGT KO mice twice daily for 21 days, resulted in ~ 4-fold increase in net oxalate secretion across the distal colon suggesting that Oxalobacter CM contained the purported secretagogue(s). Pertinent to the discussion of CM secretagogue activity, however, is the discrepancy between several reports from the Hassan et al group [2, 3, 9] and with a similar study conducted in our laboratory using Oxalobacter CM also applied to C2 monolayers [39]. The Hassan group initially reported that C2 cells exposed to Oxalobacter CM (1:50 dilution for 6-24 hours) had a 4-fold increase in DRA expression but no changes in PAT1 expression despite a stimulation of oxalate uptake [3, 9]. This would appear to suggest that DRA, rather than PAT1, was involved in this response especially since DRA has been shown [7] to mediate intestinal oxalate uptake and these investigators came to the same conclusion in a second report [3]. Nonetheless, these authors ultimately concluded in their most recent third report that PAT1 mediates Oxalobacter CM effects [2]. A further discrepancy is the fact that we repeated this same experiment using 1:50 and 1:25 dilution of Oxalobacter CM applied to C2 monolayers for 18-24 h followed by the measurement of bidirectional fluxes of oxalate across the monolayers in Ussing chambers and we found no effects of Oxalobacter CM on oxalate transport [39]. Consequently, it is difficult to reconcile these disparate results from the two laboratories except to note that the Hassan group employed a sheep rumen strain of Oxalobacter (OxB, ATTC #35274) and we employed CM from a human strain of Oxalobacter (HC-1) that has been shown to promote net oxalate secretion across Oxalobacter-colonized mouse intestinal tissues [12]. In addition, our approach was different in that we simultaneously measured two unidirectional fluxes across C2 monolayers bathed by symmetrical physiological saline in Ussing chambers under short-circuit conditions whereas Hassan et al measured uptake of oxalate (one unidirectional flux) with an outward Cl− gradient imposed.
With respect to the involvement of DRA in mediating Oxalobacter induction of enteric oxalate excretion, the results here clearly show a significant ~ 2-fold increase in net oxalate secretion across both the colonized cecum and distal colon in the absence of DRA. In the cecum, the increase in occurred via coordinated changes in the unidirectional fluxes with a small reduction in and a small increase in that was not statistically significant. In the distal colon, net oxalate secretion was increased solely via a significant increase in in colonized DRA KO compared to non-colonized KO (Figs 3 and 4). It was clear that the mechanism of Oxalobacter-mediated effects on was not due to any significant reduction in oxalate absorption leading to an unmasking of a net secretory flux. Again, however, these results from the native mouse tissues showing Oxalobacter induces net oxalate secretion in the large intestine in the absence of DRA sharply contrasts with the initial interpretation by the Hassan group of their studies using C2 cells [3, 9] implicating a significant role for DRA in mediating changes in oxalate transport attributable to Oxalobacter.
While the conclusions of the present study seem straight-forward and clear, there are limitations in interpreting these results appropriately. Given both PAT1 and DRA contribute significantly to transmural oxalate movements across the intestine under basal conditions in the WT mouse [6, 7, 20], it is possible that in the absence of either transport protein, a compensatory adaptation (either up- or down-regulation in terms of function) of another, as yet unidentified oxalate transporter(s), could occur. Another candidate, slc26a2 (DTDST: Diastrophic Dysplasia Sulfate Transporter), reported to be located in the apical membrane and expressed abundantly along the mouse intestine, has also been shown to transport oxalate [14, 32, 34]. However, the lethality of DTDST KO prevents conducting the colonization and flux experiments described herein to determine if DTDST contributes to basal oxalate movements or the enteric oxalate secretion promoted by Oxalobacter. Moreover, attempts to acquire information regarding the expression of the slc26a transport proteins has been fraught with difficulties in our hands and, after years of effort, we have reached an impasse. Simply stated, no specific commercial antibodies exist for any of the slc26a proteins of interest and even our efforts in custom synthesis have yielded no satisfactory working antibodies to address either upregulation or downregulation of these proteins precluding an effort to correlate this aspect with the functional studies.
Thus, the results strongly indicate that there are other apical oxalate transporters not yet identified that may be direct/indirect targets of the Oxalobacter purported secretagogue and basolateral oxalate transporters need to be examined in this regard as well. It is noteworthy here that a recent study from our lab [40] examining the contribution of basolateral SAT1 (slc26a1) to transmural fluxes of oxalate in mouse intestine unexpectedly showed that the absence of this transport protein (i.e. SAT1 KO) had no effect on unidirectional oxalate fluxes across the distal ileum, cecum, or distal colon compared to WT. Indeed, an earlier report using the same SAT1 KO mouse model also concluded that “SAT1 is dispensable for active oxalate secretion in mouse duodenum” [22]. This was a surprising finding given SAT1 which is expressed in intestine, liver, and kidney has generally been considered, to date, to be an important oxalate transporter [1, 5, 15, 22, 27, 33]. Thus, consistent with the present results, other unidentified oxalate transport proteins clearly contribute to oxalate movements across both the apical and basolateral membranes of the mouse intestinal tract.
Oxalobacter colonization and urinary oxalate excretion:
The results of the present study confirm our previous findings that Oxalobacter colonized mice excrete lower amounts of urinary oxalate compared to non-colonized mice of the same genotype [10, 12, 13]. With the exception of DRA/AGT dKO group, all colonized knockout mice had significant reductions in urinary oxalate while they were being fed the oxalate-supplemented diet. In the PAT1 KO and PAT1/AGT dKO, 24-h urinary oxalate was reduced 85% and 91%, respectively. In the DRA KO mouse, which excretes much lower basal amounts of oxalate [7], 24-h urinary excretion was lowered by 37% in the colonized DRA KO group on the oxalate-supplemented diet which is comparable to a WT mouse urinary excretion on regular mouse chow. The reason there was no reduction in mean urinary oxalate excretion in the DRA/AGT dKO group was that these mice had lost colonization by the time the collections were obtained and at the time the flux experiments were conducted (which is the reason the flux data were not included in the analyses or graphically presented in Figs 3 and 4). Previously, we reported that AGT KO mice, which are easily colonized, begin to lose colonization about 12 days after oral gavage (G+12) with the bacteria and by ~ 20-35 days 100% of all AGT KO lost colonization [13]. In the process of conducting the present study we discovered that the colonized DRA KO mouse also lost colonization in a similar pattern. Between G+12 and G+15 days after oral gavage, ~55% of DRA KO lost colonization necessitating the addition of more gavaged DRA KO mice to the study. Thus, crossing AGT KO with DRA KO produces a dKO that cannot sustain Oxalobacter colonization despite the fact that in this study these two groups were maintained on an oxalate-supplemented diet. In contrast the PAT1 KO and the PAT1/AGT dKO mice sustained 100% colonization over the same time course of the study. As we stated before in the previous report with the AGT mouse model, and now with a similar issue with the DRA KO model, little or nothing is currently known about the nature of Oxalobacter colonization of the intestinal tract or about the intraluminal factors impacting the colonization process and/or duration of colonization. While we have no good explanation, DRA KO mice exhibit persistent chloride diarrhea which we speculate could influence Oxalobacter retention of colonization.
In summary, this study is the first to show that Oxalobacter-induced enteric oxalate excretion does not require the presence of the known apical oxalate exchangers, namely PAT1 or DRA. The findings strongly implicate the contribution, or the potential recruitment, of other as yet unidentified oxalate transporters in mediating the interaction between Oxalobacter and the host enterocyte and in altering the transmural movements of its substrate. This study also highlights major gaps in our knowledge regarding the suite of transport proteins that potentially move oxalate across the intestine and also regarding intraluminal factors influencing intestinal colonization with the commensal probiotic Oxalobacter formigenes.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Shreya Mishra, Heran Getachew, and Tisha Van Pelt for excellent technical assistance and animal husbandry.
GRANTS
This work was supported by NIH grants DK088892 and DK081624 in addition to a grant from the Oxalosis and Hyperoxaluria Foundation.
Footnotes
Ethical Approval: All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted. This article does not contain any studies with human participants performed by the author.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Alper SL, Sharma AK (2013) The SLC26 gene family of anion transporters and channels. Mol Aspects Med 34: 494–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arvans D, Jung YC, Antonopoulos D, Koval J, Granja I, Bashir M, Karrar E, Roy-Chowdhury J, Musch M, Asplin J, Chang E, Hassan H (2017) Oxalobacter formigenes-Derived Bioactive Factors Stimulate Oxalate Transport by Intestinal Epithelial Cells. J Am Soc Nephrol 28: 876–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvans D, Musch M, Chang E, Hassan H, Cheng M (2012) Oxalobacter formigenes conditioned medum stimulates oxalate transport by human intestinal cells. J Investigative Medicine 60: 738 [Google Scholar]
- 4.Campieri C, Campieri M, Bertuzzi V, Swennen E, Matteuzzi D, Stefoni S, Pirovano F, Centi C, Ulisse S, Famularo G, De Simone C (2001) Reduction of oxaluria after an oral course of lactic acid bacteria at high concentration. Kidney Int 60: 1097–1105 [DOI] [PubMed] [Google Scholar]
- 5.Dawson PA, Russell CS, Lee S, McLeay SC, van Dongen JM, Cowley DM, Clarke LA, Markovich D (2010) Urolithiasis and hepatotoxicity are linked to the anion transporter Sat1 in mice. J Clin Invest 120: 706–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freel RW, Hatch M, Green M, Soleimani M (2006) Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am J Physiol Gastrointest Liver Physiol 290: G719–728 [DOI] [PubMed] [Google Scholar]
- 7.Freel RW, Whittamore JM, Hatch M (2013) Transcellular oxalate and Cl- absorption in mouse intestine is mediated by the DRA anion exchanger Slc26a3, and DRA deletion decreases urinary oxalate. Am J Physiol Gastrointest Liver Physiol 305: G520–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green ML, Hatch M, Freel RW (2005) Ethylene glycol induces hyperoxaluria without metabolic acidosis in rats. Am J Physiol Renal Physiol 289: F536–543 [DOI] [PubMed] [Google Scholar]
- 9.Hassan H, Arvans D, Cheng M, Musch M, Chang E (2011) Oxalobacter formigenes conditioned medium stimulates oxalate transport by human intestinal cells. J Am Soc Nephrol 22: 383A [Google Scholar]
- 10.Hatch M (2017) Gut microbiota and oxalate homeostasis. Ann Transl Med 5: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatch M, Cornelius J, Allison M, Sidhu H, Peck A, Freel RW (2006) Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int 69: 691–698 [DOI] [PubMed] [Google Scholar]
- 12.Hatch M, Freel RW (2013) A human strain of Oxalobacter (HC-1) promotes enteric oxalate secretion in the small intestine of mice and reduces urinary oxalate excretion. Urolithiasis 41: 379–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatch M, Gjymishka A, Salido EC, Allison MJ, Freel RW (2011) Enteric oxalate elimination is induced and oxalate is normalized in a mouse model of primary hyperoxaluria following intestinal colonization with Oxalobacter. Am J Physiol Gastrointest Liver Physiol 300: G461–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heneghan JF, Akhavein A, Salas MJ, Shmukler BE, Karniski LP, Vandorpe DH, Alper SL (2010) Regulated transport of sulfate and oxalate by SLC26A2/DTDST. Am J Physiol Cell Physiol 298: C1363–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heneghan JF, Alper SL (2011) This, too, shall pass--like a kidney stone: a possible path to prophylaxis of nephrolithiasis? Focus on “Cholinergic signaling inhibits oxalate transport by human intestinal T84 cells”. Am J Physiol Cell Physiol 302: C18–20 [DOI] [PubMed] [Google Scholar]
- 16.Hoppe B, Beck B, Gatter N, von Unruh G, Tischer A, Hesse A, Laube N, Kaul P, Sidhu H (2006) Oxalobacter formigenes: a potential tool for the treatment of primary hyperoxaluria type 1. Kidney Int 70: 1305–1311 [DOI] [PubMed] [Google Scholar]
- 17.Hoppe B, Groothoff JW, Hulton SA, Cochat P, Niaudet P, Kemper MJ, Deschenes G, Unwin R, Milliner D (2011) Efficacy and safety of Oxalobacter formigenes to reduce urinary oxalate in primary hyperoxaluria. Nephrol Dial Transplant 26: 3609–3615 [DOI] [PubMed] [Google Scholar]
- 18.Hoppe B, von Unruh G, Laube N, Hesse A, Sidhu H (2005) Oxalate degrading bacteria: new treatment option for patients with primary and secondary hyperoxaluria? Urol Res 33: 372–375 [DOI] [PubMed] [Google Scholar]
- 19.Jiang J, Knight J, Easter LH, Neiberg R, Holmes RP, Assimos DG (2011) Impact of dietary calcium and oxalate, and Oxalobacter formigenes colonization on urinary oxalate excretion. J Urol 186: 135–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS (2006) Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 38: 474–478 [DOI] [PubMed] [Google Scholar]
- 21.Klimesova K, Whittamore JM, Hatch M (2015) Bifidobacterium animalis subsp. lactis decreases urinary oxalate excretion in a mouse model of primary hyperoxaluria. Urolithiasis 43: 107–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko N, Knauf F, Jiang Z, Markovich D, Aronson PS (2012) Sat1 is dispensable for active oxalate secretion in mouse duodenum. Am J Physiol Cell Physiol 303: C52–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwak C, Kim HK, Kim EC, Choi MS, Kim HH (2003) Urinary oxalate levels and the enteric bacterium Oxalobacter formigenes in patients with calcium oxalate urolithiasis Eur Urol 44: 475–481 [DOI] [PubMed] [Google Scholar]
- 24.Liebman M, Al-Wahsh IA (2011) Probiotics and other key determinants of dietary oxalate absorption. Adv Nutr 2: 254–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieske JC, Goldfarb DS, De Simone C, Regnier C (2005) Use of a probiotic to decrease enteric hyperoxaluria. Kidney Int 68: 1244–1249 [DOI] [PubMed] [Google Scholar]
- 26.Lieske JC, Tremaine WJ, De Simone C, O'Connor HM, Li X, Bergstralh EJ, Goldfarb DS (2010) Diet, but not oral probiotics, effectively reduces urinary oxalate excretion and calcium oxalate supersaturation. Kidney Int 78: 1178–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markovich D (2012) Slc13a1 and Slc26a1 KO models reveal physiological roles of anion transporters. Physiology (Bethesda) 27: 7–14 [DOI] [PubMed] [Google Scholar]
- 28.Mikami K, Akakura K, Takei K, Ueda T, Mizoguchi K, Noda M, Miyake M, Ito H (2003) Association of absence of intestinal oxalate degrading bacteria with urinary calcium oxalate stone formation. Int J Urol 10: 293–296 [DOI] [PubMed] [Google Scholar]
- 29.Milliner D, Hoppe B, Groothoff J (2018) A randomised Phase II/III study to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Urolithiasis 46: 313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mittal RD, Kumar R, Mittal B, Prasad R, Bhandari M (2003) Stone composition, metabolic profile and the presence of the gut-inhabiting bacterium Oxalobacter formigenes as risk factors for renal stone formation. Med Princ Pract 12: 208–213 [DOI] [PubMed] [Google Scholar]
- 31.Neuhaus TJ, Belzer T, Blau N, Hoppe B, Sidhu H, Leumann E (2000) Urinary oxalate excretion in urolithiasis and nephrocalcinosis. Arch Dis Child 82: 322–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohana E, Shcheynikov N, Park M, Muallem S (2012) Solute carrier family 26 member a2 (Slc26a2) protein functions as an electroneutral SOFormula/OH-/Cl- exchanger regulated by extracellular Cl J Biol Chem 287: 5122–5132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robijn S, Hoppe B, Vervaet BA, D'Haese PC, Verhulst A (2011) Hyperoxaluria: a gut-kidney axis? Kidney Int 80: 1146–1158 [DOI] [PubMed] [Google Scholar]
- 34.Satoh H, Susaki M, Shukunami C, lyama K, Negoro T, Hiraki Y (1998) Functional analysis of diastrophic dysplasia sulfate transporter. Its involvement in growth regulation of chondrocytes mediated by sulfated proteoglycans. J Biol Chem 273: 12307–12315 [DOI] [PubMed] [Google Scholar]
- 35.Sidhu H, Schmidt ME, Cornelius JG, Thamilselvan S, Khan SR, Hesse A, Peck AB (1999) Direct correlation between hyperoxaluria/oxalate stone disease and the absence of the gastrointestinal tract-dwelling bacterium Oxalobacter formigenes: possible prevention by gut recolonization or enzyme replacement therapy. J Am Soc Nephrol 10 Suppl 14: S334–340 [PubMed] [Google Scholar]
- 36.Siener R, Bade DJ, Hesse A, Hoppe B (2013) Dietary hyperoxaluria is not reduced by treatment with lactic acid bacteria. J Transl Med 11: 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siener R, Bangen U, Sidhu H, Honow R, von Unruh G, Hesse A (2013) The role of Oxalobacter formigenes colonization in calcium oxalate stone disease. Kidney Int 83: 1144–1149 [DOI] [PubMed] [Google Scholar]
- 38.Troxel SA, Sidhu H, Kaul P, Low RK (2003) Intestinal Oxalobacter formigenes colonization in calcium oxalate stone formers and its relation to urinary oxalate. J Endourol 17: 173–176 [DOI] [PubMed] [Google Scholar]
- 39.Whittamore JM, Hatch M (2017) The role of intestinal oxalate transport in hyperoxaluria and the formation of kidney stones in animals and man. Urolithiasis 45: 89–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whittamore JM, Stephens CE, Hatch M (2018) Absence of the sulfate transporter SAT-1 (Slc26a1) has no impact on oxalate handling by mouse intestine and does not cause hyperoxaluria or hyperoxalemia. Am J Physiol Gastrointest Liver Physiol [E-Pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.