Abstract
OBJECTIVE
Seizure outcome after mesial temporal lobe epilepsy (mTLE) surgery is complex and diverse, even across patients with homogeneous presurgical clinical profiles. The authors hypothesized that this is due in part to variations in network connectivity across the brain before and after surgery. Although presurgical network connectivity has been previously characterized in these patients, the objective of this study was to characterize presurgical to postsurgical functional network connectivity changes across the brain after mTLE surgery.
METHODS
Twenty patients with drug-refractory unilateral mTLE (5 left side, 10 female, age 39.3 ± 13.5 years) who underwent either selective amygdalohippocampectomy (n = 13) or temporal lobectomy (n = 7) were included in the study. Presurgical and postsurgical (36.6 ± 14.3 months after surgery) functional connectivity (FC) was measured with 3-T MRI and compared with findings in age-matched healthy controls (n = 44, 21 female, age 39.3 ± 14.3 years). Postsurgical connectivity changes were then related to seizure outcome, type of surgery, and presurgical disease parameters.
RESULTS
The results demonstrated significant decreases of FC from control group values across the brain after surgery that were not present before surgery, including many contralateral hippocampal connections distal to the surgical site. Postsurgical impairment of contralateral precuneus to ipsilateral occipital connectivity was associated with seizure recurrence. Presurgical impairment of the contralateral precuneus to contralateral temporal lobe connectivity was associated with those who underwent selective amygdalohippocampectomy compared to those who had temporal lobectomy. Finally, changes in thalamic connectivity after surgery were linearly related to duration of epilepsy and frequency of consciousness-impairing seizures prior to surgery.
CONCLUSIONS
The widespread contralateral hippocampal FC changes after surgery may be a reflection of an ongoing epileptogenic progression that has been altered by the surgery, rather than a direct result of the surgery itself. This network evolution may contribute to long-term seizure outcome. Therefore, the combination of presurgical network mapping with the understanding of the dynamic effects of surgery on the networks may ultimately be used to create predictors of the likelihood of long-term seizure recurrence in individual patients after mTLE surgery.
Keywords: temporal lobe epilepsy, functional neuroimaging, epilepsy surgery, MRI, connectivity, functional neurosurgery
TEMPORAL lobe epilepsy is one of the most common forms of epilepsy.8 Antiepileptic medication is successful in treating approximately 60%–70% of these patients.9,22 In mesial temporal lobe epilepsy (mTLE) the gold standard clinical assessments identify the hippocampus as the seizure focus. Resection of this region is a potential treatment for individuals in whom the disease is drug refractory. However, even in these seemingly homogeneous patients with a well-defined seizure focus, seizure freedom rates after surgery range from approximately 58%32 to 80%10 of patients. Thus, predicting seizure outcome after mTLE surgery remains a significant clinical challenge.
Electrophysiological and imaging evidence suggests that widespread brain networks are impaired in mTLE,1,11,12,18 and that network characterization may improve seizure outcome prediction in these patients. Moreover, investigations of presurgical structural and functional network properties quantified by MRI have identified relationships between network properties and postsurgical seizure freedom in mTLE.3,15,19 Clinically, however, seizure outcome is complex, with the possibility of recurrence days to years after surgery at varying levels of severity.5,17 Although certain recurrences may reflect an inaccurate localization or incomplete resection of the seizure focus, it is believed that others may be due in part to the development of epileptogenicity in other regions of the network.21,28 From this perspective, we have developed the following set of working hypotheses: that 1) there is at least one presurgical brain network topology (“model”) that is associated with significant seizure improvement after mTLE surgery, and early recurrence of severe, disabling seizures indicates a network inconsistent with the model; 2) variations within the presurgical network model contribute to mild seizure recurrence after surgery; and 3) these presurgical networks and their evolution are altered by surgery, which then influences the ultimate seizure freedom or recurrence.
We have recently shown evidence of a presurgical functional and structural network model required for seizure freedom or improvement.25 In that work, we identified a model mTLE connectivity network that was present in those patients who were seizure free or showed worthwhile clinical improvement at 1 year after surgery. Alternatively, patients with networks that were not similar to the model experienced multiple disabling seizures in the first year after surgery. More recently, we identified variations in the presurgical network model related to mild recurrence.26 Specifically, greater contralateral hippocampal connectivity impairment in the model network prior to surgery was associated with mild seizure recurrence in the first 3 years after surgery. Therefore, the objectives of the present work were designed to examine our third hypothesis. First, we characterized and quantified postsurgical functional connectivity (FC) in mTLE. Second, we compared postsurgical FC network changes to seizure outcome to identify possible regions responsible for postsurgical seizure recurrence. Third, we compared postsurgical network changes to the type of surgery performed. Last, we determined the relationship between postsurgical FC change and presurgical disease parameters to identify possible regions where postsurgical changes may reflect continuation of presurgical disease processes.
Methods
Study Subjects
This study includes 20 patients diagnosed with unilateral mTLE after a standard presurgical evaluation, with lateralizing long-term video EEG, seizure semiology consistent with mTLE, mesial temporal hypometabolism on interictal PET scans, and possible hippocampal sclerosis on structural MRI. Exclusion criteria included structural abnormalities on MRI outside mesial temporal lobe structures and presurgical intracranial monitoring. Patients underwent either selective amygdalohippocampectomy (n = 13) or temporal lobectomy (n = 7) at Vanderbilt University Medical Center between 2012 and 2017. Each individual had at least 1 year of postoperative follow-up at our institution, with seizure outcome assigned by the treating epileptologist using the Engel outcome score.7 Patient characteristics are given in Table 1. In addition, 44 healthy controls were also enrolled (21 females, mean age ± SD: 39.3 ± 14.3 years) to allow normalization of connectivity to age-matched controls. The Vanderbilt University Institutional Review Board has approved the use of human subjects for this study.
TABLE 1.
Characteristics of 20 patients with mTLE
| Variable | All (n = 20) | Seizure Free (n = 9) | Mild Recurrence (n = 7) | Failure (n = 4) | Statistic | p Value |
|---|---|---|---|---|---|---|
| Age (yrs)* | 39.3 ± 13.5 | 38.3 ± 15.6 | 42.8 ± 13.5 | 35.5 ± 9.5 | F = 0.39 | 0.68 |
| Sex (% female) | 50 | 77.8 | 42.8 | 0 | E = 6.53 | 0.03 |
| Duration of epilepsy (yrs)* | 19.3 ± 16.2 | 22.6 ± 12.9 | 17.4 ± 19.5 | 15.5 ± 20.1 | F = 0.33 | 0.71 |
| Side of op (% It) | 25 | 22.2 | 28.5 | 25 | E = 0.40 | 1.00 |
| Hippocampal sclerosis on clinical MRI (%) | 75 | 77.8 | 71.4 | 75 | E = 0.40 | 1.00 |
| Ictal EEG localization in ipsilat mTL (%) | 95 | 100 | 100 | 75 | E = 3.10 | 0.20 |
| PET localization in ipsilat mTL (%) | 80 | 89 | 57 | 100 | E = 2.92 | 0.21 |
| Frequency of preop consciousness-impairing seizures (no./mo) | 7.4 ± 8.3 | 3.6 ± 3.2 | 10.8 ± 9.4 | 9.9 ± 12.3 | F = 1.85 | 0.18 |
| Op type | E = 1.18 | 1.00 | ||||
| Lt Sel AH (%) | 25 | 22.2 | 28.6 | 25 | ||
| Rt Sel AH (%) | 40 | 44.5 | 42.8 | 25 | ||
| Rt temporal lobectomy (%) | 35 | 33.3 | 28.6 | 50 | ||
| Time of postop scan (mos since op) | 36.6 ± 14.3 | 34.5 ± 15.2 | 40.0 ± 16.4 | 35.2 ± 9.6 | F = 0.28 | 0.75 |
| Postop medication | E = 1.82 | 1.00 | ||||
| Off all AEDs (%) | 5 | 44.5 | 0 | 0 | ||
| Decrease in AEDs (%) | 45 | 44.5 | 43 | 50 | ||
| No change or increase in AEDs (%) | 50 | 11 | 57 | 50 |
AEDs = antiepileptic drugs; E = Fisher’s exact test value; Sel AH = selective amygdalohippocampectomy.
Values are expressed as the mean ± SD unless otherwise specified. Outcome group definitions are described in the text. Statistics and p values compare the variable across the 3 outcome groups.
At time of presurgical MRI session.
Imaging Protocol
All patients underwent a presurgical and a postsurgical MRI session. The postsurgical session was at least 1 year after surgery (mean ± SD: 36.6 ± 14.3 months). All healthy control subjects had one imaging session. All imaging sessions were performed using a 3-T MRI scanner (Philips Healthcare) with a 32-channel head coil. Each session included the following scans: 1) T1-weighted, 3D scan for intersubject normalization and regional and tissue segmentation (1 mm3); 2) T1-weighted scan for spatial normalization acquired in the same slice orientation as the functional images (1 mm × 1 mm × 3.5 mm with 0.5-mm gap); and 3) T2*-weighted functional MRI (fMRI) blood oxygen level–dependent scan at rest with eyes closed for FC (34 axial slices, TE 35 msec, TR 2 seconds, 3 mm × 3 mm × 3.5 mm with a 0.5-mm gap, 10 minutes). Simultaneous physiological monitoring of cardiac and respiratory fluctuations was performed using the MRI scanner integrated pulse oximeter and the respiratory belt at a sampling frequency of 500 Hz.
Functional Connectivity
The preprocessing of the fMR images was done using SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) and MATLAB (The MathWorks, Inc.). The protocol included slice timing correction, motion correction, physiological noise correction using the retrospective correction of physiological motion effects in functional MRI (RETROICOR) protocol16 using the pulse oximeter and respiratory belt time series, spatial normalization to the Montreal Neurological Institute template via the T1-weighted datasets, and spatial smoothing using a 6 mm × 6 mm × 6 mm full width, half-maximum gaussian kernel. The fMRI time series were then temporally band-pass filtered at 0.0067 Hz to 0.1 Hz.4
The 1-mm3 isotropic T1-weighted images were regionally segmented using the Multi-Atlas20 algorithm. The networks of interest for this investigation were defined using a 12 × 87 set of connections. The 12 regions (6 in each hemisphere, called “seeds”) were chosen as those most contributing to mTLE seizure propagation:25 hippocampus, thalamus, anterior insula, posterior insula, precuneus, and mid-cingulate gyrus. The 87 regions included the 12 seed regions described above, as well as 75 regions covering the rest of the supratentorial brain (called “targets”) (Table 2). The average fMRI time series was computed across each region. Then the FC between pairs of regions was computed as the partial correlation of the average time series of the two regions adjusted for mean white matter time series and motion.31 These values were then transformed using the Fisher z-transformation.14 This was computed for each of the 12 seeds to each of the total 87 regions, yielding a 12 × 87 set of FC values.
TABLE 2.
Regions of interest in mTLE
| Region of Interest | Lobe/Grouping |
|---|---|
| Seeds | |
| Hippocampus | |
| Thalamus | |
| Precuneus | |
| Anterior insula | |
| Posterior insula | |
| Mid-cingulate gyrus | |
| Targets | |
| Anterior cingulate gyrus | Prefrontal |
| Central operculum | Prefrontal |
| Frontal operculum | Prefrontal |
| Frontal pole | Prefrontal |
| Middle frontal gyrus | Prefrontal |
| Superior frontal gyrus, medial segment | Prefrontal |
| Inferior frontal gyrus (pars opercularis) | Prefrontal |
| Superior frontal gyrus | Prefrontal |
| Inferior frontal gyrus (pars triangularis) | Prefrontal |
| Angular gyrus | Parietal |
| Posterior cingulate gyrus | Parietal |
| Parietal operculum | Parietal |
| Supramarginal gyrus | Parietal |
| Superior parietal lobule | Parietal |
| Calcarine cortex | Occipital |
| Cuneus | Occipital |
| Inferior occipital gyrus | Occipital |
| Lingual gyrus | Occipital |
| occipital gyrus | Occipital |
| Occipital fusiform gyrus | Occipital |
| Superior occipital gyrus | Occipital |
| Fusiform gyrus | Temporal |
| Parahippocampal gyrus | Temporal |
| Planum polare | Temporal |
| Superior temporal gyrus | Temporal |
| Temporal pole | Temporal |
| Transverse temporal gyrus | Temporal |
| Amygdala* | Temporal |
| Precentral gyrus, medial segment | Motor |
| Precentral gyrus | Motor |
| Supplementary motor cortex | Motor |
| Postcentral gyrus, medial segment | Somatosensory |
| Postcentral gyrus | Somatosensory |
| Putamen | Subcortical |
| Basal forebrain | Subcortical |
| Caudate | Subcortical |
| Ventral diencephalon | Subcortical |
| Brainstem† | Subcortical |
The amygdala was originally part of the subcortical grouping in the template; however, it was moved to the temporal lobe grouping so that the ipsilateral amygdala could be excluded from postsurgical analyses with the rest of the ipsilateral temporal lobe.
All regions were identified separately in left and right hemispheres, except for the brainstem, which was one bilateral region.
Using only the healthy controls, the FC across each connection was fit to age. The linear fit and the standard deviation across the healthy controls was then used to normalize the FC of each patient across each pair of regions to units of standard deviations from an age-matched healthy control, with zero being equal to age-matched control. After correction, left and right regions were then transformed to ipsilateral and contralateral in relation to the seizure focus in all patients. For an additional larger spatial scale summary, the FC values for each region pair were averaged across groups of regions (which are subsequently referred to as “lobes”) that contained the target region. The “lobes” used were prefrontal, parietal, occipital, temporal, motor, somatosensory, and subcortical (Table 2). Results will be presented for both regional and lobe level analyses.
Statistical Analyses
The FC values acquired across the network prior to and after surgery were characterized and compared to outcome and presurgical disease parameters. It is clear that the surgery will impact regions and white matter tracts directly involved in the resection, presumably the seizure focus. However, quantifying these direct functional network changes noninvasively is inhibited by the inaccurate segmentation of remaining tissue, in which functional integrity may be poorly characterized and highly dependent on the individual surgery. Consequently, the ipsilateral hippocampus seed region and the target regions involving the ipsilateral temporal lobe were excluded from postsurgical analyses.
To characterize the FC across the group of all mTLE patients before surgery relative to age-matched control, we used a 1-sample t-test to compare the FC across each connection across all patients versus zero. Note that an FC of zero is equal to the age-matched control. The p value and the mean of the 95% confidence interval of the t-test were computed for each connection. The t-tests were repeated using the postsurgical FC values.
In order to identify individual regional connections that have a significant change from presurgery to postsurgery, we used a linear model with change in FC (presurgical FC - postsurgical FC) as the response and the time of postsurgical scan in months after surgery and a constant as the predictors. The statistical significance of the constant in the model represents the significance of the difference between presurgical and postsurgical FC, adjusting for the number of months after surgery at which the postsurgical scan was obtained. The procedure was repeated using the lobe-level connections.
To investigate the change in FC related to seizure outcome we identified 3 outcome groups based on the Engel outcome classification.7 Group 1 included those patients who were seizure free (Engel Ia–b) at 1 year postsurgery, and who remained seizure free at the time of the postsurgical scan (seizure free, n = 9). Group 2 included those we categorized as having some improvement. These patients had Engel I–II outcome at the first year after surgery, but were Engel Ic–III at the time of the postsurgical scan (mild recur, n = 7). Group 3 included those patients who were Engel III–IV with no improvement at 1 year post-surgery (failure, n = 4). All patients in groups 2 and 3 had experienced some type of seizure recurrence prior to the postsurgical scan. To identify those connections in which FC change was related to seizure outcome (adjusting for time of postsurgical scan), the time of postsurgical scan in months after surgery was linearly regressed from the change in FC across all patients. Then the residuals of this regression were analyzed using an ANOVA. The procedure was performed using the regional and lobe-level connections. Similarly, to investigate the relationship between postsurgical FC (rather than change in FC) and the seizure outcome, an ANOVA was performed on the postsurgical FC measures by using the same 3 outcome groups.
The patients were then divided into 3 groups based on type of surgery: left selective amygdalohippocampectomy (n = 5), right selective amygdalohippocampectomy (n = 8), or right temporal lobectomy (n = 7). As detailed above, the time of postsurgical scan was linearly regressed from the FC change, and then an ANOVA was performed on the residuals. This identified connections where FC change was related to type of surgery, adjusting for time of postsurgical scan. The analysis was repeated using only the postsurgical FC measures.
The linear relationship between change in FC and presurgical disease parameters was computed using the Pearson correlation after linear regression of the number of months after surgery at which the postsurgical scan was acquired and the change in FC. Two presurgical disease parameters were investigated: 1) the frequency of presurgical seizures defined as the total number of consciousness-impairing focal seizures and consciousness-impairing focal to bilateral tonicclonic seizures per month; and 2) the duration of epilepsy prior to the presurgical acquisition, measured in years.
Statistical significance for all analyses was determined as p < 0.05 or p < 0.01 with Bonferroni correction for multiple comparisons based on each seed region. In the regional analysis there were a total of 171 region pairs involving each set of bilateral seed regions (87 for ipsilateral seed + 87 for contralateral seed − 2 seeds to themselves − 1 ipsilateral to contralateral seed, which is the same as contralateral to ipsilateral seed). In the lobe-level analysis there were 26 unique region-to-lobe pairs for each seed region (14 lobes for ipsilateral seed + 14 lobes for contralateral seed − 2 for each seed to ipsilateral temporal lobe).
Results
Table 1 provides the patient characteristics in total and separated by seizure outcome group. None of the patient variables were statistically associated with seizure outcome except sex (Fisher’s exact test = 6.53, p = 0.03, uncorrected). In our cohort, females tended to have a better outcome than males.
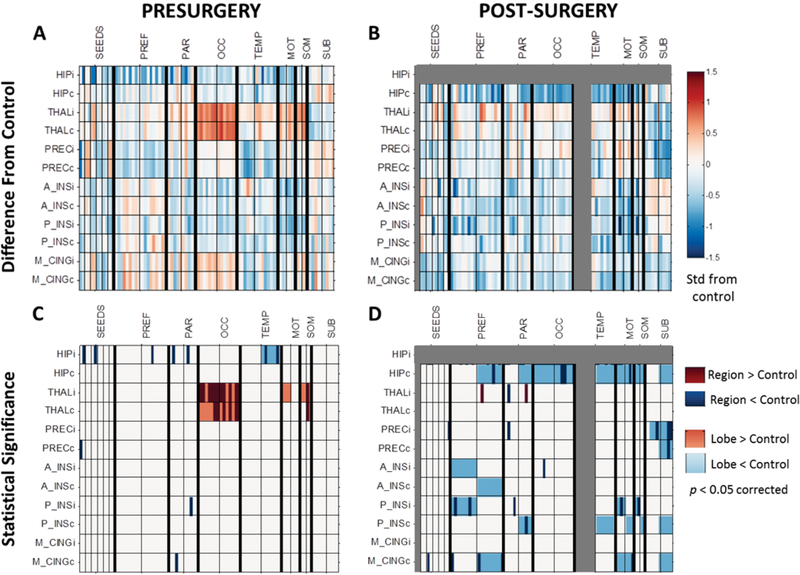
Prior to surgery the patients with mTLE had lower FC relative to the age-matched healthy controls from the ipsilateral hippocampus to many regions and from the ipsilateral anterior and posterior insula to parietal lobe regions (Fig. 1A, C). Presurgical FC was higher relative to controls from the bilateral thalamus to the bilateral occipital regions and contralateral somatosensory regions, and from the ipsilateral thalamus to ipsilateral motor and somatosensory regions (Fig. 1A, C). After surgery (excluding ipsilateral temporal lobe regions), patients with mTLE had lower FC relative to controls throughout the network, including from the contralateral hippocampus to contralateral prefrontal, parietal, occipital, temporal, and subcortical regions (Fig. 1B, D). Lower FC was also seen across several other connections, including the bilateral precuneus to contralateral subcortical regions; from the ipsilateral anterior and posterior insula to the ipsilateral prefrontal lobe; and from the mid-cingulate gyrus to contralateral prefrontal, motor, and subcortical regions (Fig. 1B, D).
FIG. 1.
FC in patients with mTLE before and after surgery related to age-matched healthy controls. A: FC in mTLE before surgery compared to age-matched healthy controls (represented by zero). B: FC in mTLE after surgery compared to age-matched healthy controls. Values in panels A and B reflect the mean of the 95% confidence interval of the t-test between FC and zero. Units are standard deviations from age-matched healthy controls. C: Presurgical regions (dark colors) and lobes (light colors) were significantly different from age-matched controls (p < 0.05, Bonferroni correction). D: Postsurgical regions (dark colors) and lobes (light colors) were significantly different from age-matched controls (p < 0.05, Bonferroni correction). Seed regions are represented by each row and the first 12 columns. The remaining columns are arranged according to lobes of the brain, separated by thick black lines. Within lobes, all regions ipsilateral and contralateral to seizure focus are on the left and right side of the thin black lines, respectively. Connections involving the ipsilateral hippocampus and temporal lobe are gray to indicate that these were excluded from postsurgical analyses. i (following abbreviation) = ipsilateral to seizure focus; c (following abbreviation) = contralateral to seizure focus; HIP = hippocampus; THAL = thalamus; PREC = precuneus; A_INS = anterior insula; P_INS = posterior insula; M_CING = mid-cingulate; PREF = prefrontal; PAR = parietal; OCC = occipital; TEMP = temporal; MOT = motor; SOM = somatosensory; SUB = subcortical.
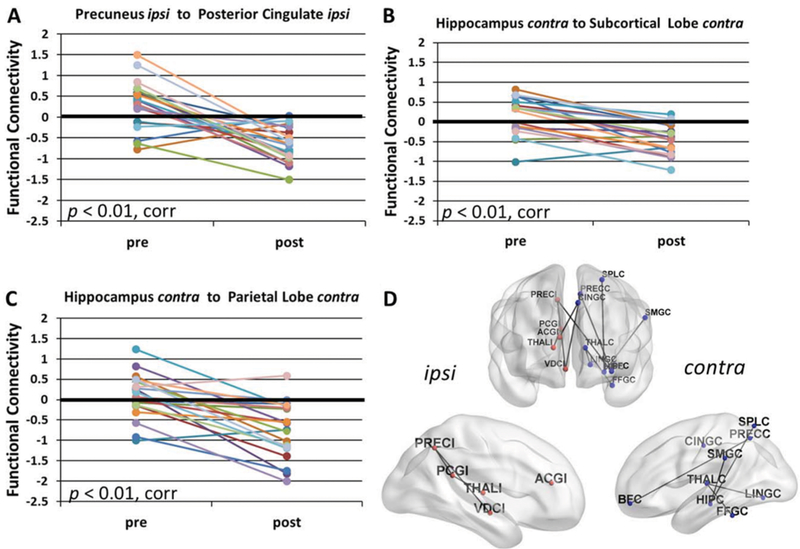
Next, direct comparisons were made between presurgical and postsurgical FC. No significant increases in postsurgical FC were detected, whereas postsurgical FC decreases were identified across many connections. Postsurgical FC from the ipsilateral precuneus to the ipsilateral posterior cingulate was decreased after surgery (p < 0.01, corrected) (Fig. 2A). Before surgery this connection was not different from control (Fig. 1C), but after surgery it was less than control (Fig. 1D). At the lobe level, postsurgical FC decreases were detected from the contralateral hippocampus to the parietal lobe (p < 0.01, corrected) (Fig. 2B) and to the subcortical lobe (p < 0.01, corrected) (Fig. 2C). These connections were not different from control before surgery (Fig. 1C), but were less than control after surgery (Fig. 1D). A decrease was also detected from the contralateral thalamus to the contralateral occipital lobe after surgery (p < 0.01, corrected) (not shown). This connection was greater than control before surgery (Fig. 1C), but not different after surgery (Fig. 1D). All significant regional decreases in FC from pre- to postsurgery (p < 0.05, corrected) are illustrated in Fig. 2D.
FIG. 2.
FC decreases after surgery in mTLE. Postsurgical decreases in FC compared to presurgical FC are found (A) from the ipsilateral precuneus to the ipsilateral posterior cingulate (p < 0.01, Bonferroni correction); (B) from the contralateral hippocampus to the contralateral subcortical lobe (p < 0.01, Bonferroni correction); and (C) from the contralateral hippocampus to the contralateral parietal lobe (p < 0.01, Bonferroni correction). D: All regional connections with a postsurgical decrease in FC compared to presurgical FC (p < 0.05, Bonferroni correction) are depicted on the brain to indicate the spatial distribution of the changes, excluding those involving the ipsilateral temporal lobe. All statistics are adjusted for months after surgery at which postsurgical scan was obtained. Value of FC = 0 represents age-matched healthy control. ipsi, I (following abbreviation) = ipsilateral to seizure focus; contra, C (following abbreviation) = contralateral to seizure focus; corr = Bonferroni correction; ACG = anterior cingulate gyrus; BF = basal forebrain; CING = mid-cingulate gyrus; FFG = fusiform gyrus; HIP = hippocampus; LING = lingual gyrus; PCG = posterior cingulate gyrus; PREC = precuneus; SMG = supramarginal gyrus; SPL = superior parietal lobule; THAL = thalamus; VDC = ventral diencephalon.
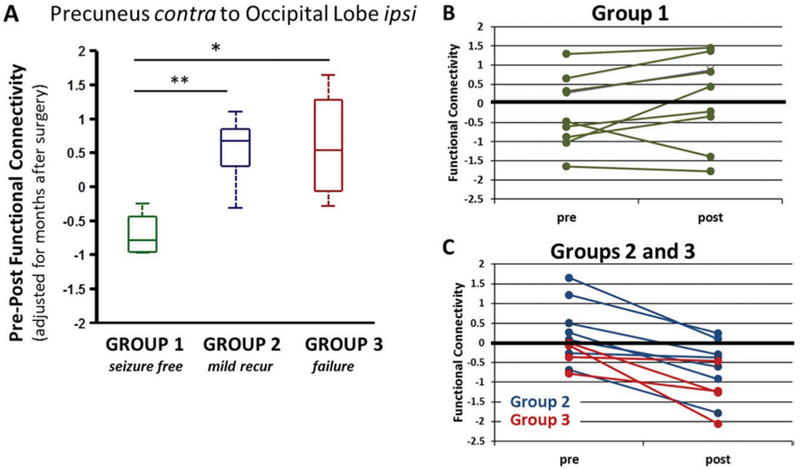
Presurgical to postsurgical change in FC was then compared to seizure outcome. When considering FC at the regional level, there were no individual regional connections with FC change after surgery that differed between groups (ANOVA, p > 0.05, corrected). However, in the lobe-level analysis, the postsurgical change in FC from contralateral precuneus to ipsilateral occipital lobe was lower in the seizure-free group compared to the groups with mild recurrence or failure (ANOVA, p < 0.05, corrected) (Fig. 3A). In the seizure-free group (group 1) both the presurgical and postsurgical FC was not different from control (1-sample t-test, p > 0.05) (Fig. 3B). Whereas the two groups of patients who continue to have seizures after surgery (group 2, mild recur; group 3, failure) have no detectable difference from control before surgery (1-sample t-test, p > 0.05), after surgery they have less FC than control (1-sample t-test, p < 0.01, uncorrected) (Fig. 3C). Furthermore, none of the regional and lobe-level connections that were significantly reduced from pre- to postsurgery were differentially associated with outcome. When comparing postsurgical FC (rather than pre- to postsurgical change in FC), there were no regional or lobe-level statistically significant associations with seizure outcome group.
FIG. 3.
Postsurgical change in FC between the contralateral precuneus and the ipsilateral occipital lobe is related to seizure outcome. A: Presurgical - postsurgical FC is lower in those patients who are seizure free (group 1) compared to those with recurring seizures (groups 2 and 3) at postsurgical scan (ANOVA, p < 0.05, Bonferroni correction). Change in FC is adjusted for months after surgery at which postsurgical scan was obtained. B: Group 1 patients (n = 9) show no difference from control before or after surgery (1-sample t-test, p > 0.05). C: Groups 2 and 3 patients (n = 11) show no difference from control prior to surgery (1-sample t-test, p > 0.05), but a decrease from control after surgery (1-sample t-test, p < 0.01, uncorrected). Value of FC = 0 represents age-matched healthy control. contra = contralateral to seizure focus.
* 2-sample t-test (p < 0.05, uncorrected); ** 2-sample t-test (p < 0.001, uncorrected).
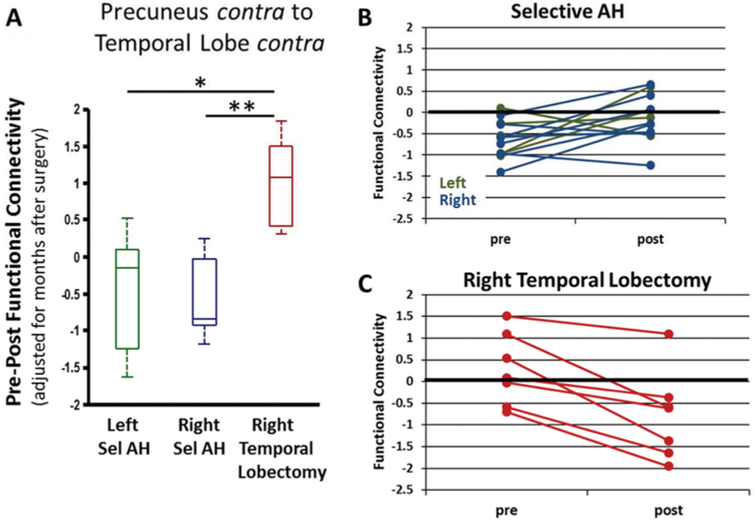
Presurgical to postsurgical change in FC was also compared to type of surgery. Due to the low numbers of patients with left selective amygdalohippocampectomy, we focused on results in which the patients with right temporal lobectomy had a significantly different change in FC from those with the left and right selective amygdalohippocampectomy. There were no significant findings for the region-to-region analyses, but the FC change from the contralateral precuneus to the contralateral temporal lobe was significantly greater in the patients with right temporal lobectomy than in both groups with selective amygdalohippocampectomy (ANOVA, p < 0.05, corrected) (Fig. 4A). In the patients with selective amygdalohippocampectomy, the FC prior to surgery was less than that in controls (1-sample t-test, p < 0.01, uncorrected) (Fig. 4B), whereas postsurgery the FC was not different from control (1-sample t-test, p > 0.05). In those with temporal lobectomy, there was no difference in FC from control prior to or after surgery (1-sample t-test, p > 0.05) (Fig. 4C). When considering the postsurgical FC (not the change in FC), FC from the ipsilateral anterior insula to the cuneus, lingual gyrus, and superior occipital gyrus had increased postsurgically in patients with right temporal lobectomy compared to the other patients (ANOVA, p < 0.05, corrected) (not shown). In the lobe-level analyses, the postsurgical FC in the patients with right temporal lobectomy was greater than in the other patients from the ipsilateral anterior insula to the contralateral and ipsilateral occipital lobe, and from the ipsilateral posterior insula to the ipsilateral occipital lobe (ANOVA, p < 0.05, corrected) (not shown).
FIG. 4.
Postsurgical change in FC between the contralateral precuneus and the contralateral temporal lobe is related to type of surgery. A: Presurgical - postsurgical FC is higher in those patients who underwent right temporal lobectomy compared to those who underwent selective amygdalohippocampectomy (Sel AH) (ANOVA, p < 0.05, Bonferroni correction). Change in FC is adjusted for months after surgery at which postsurgical scan was obtained. B: Patients with selective AH (n = 13) show a decrease from control before (1-sample t-test, p < 0.01, uncorrected) but not after surgery. C: Patients with temporal lobectomy (n = 13) show no difference from control prior to or after surgery (1-sample t-test, p > 0.05). Value of FC = 0 represents age-matched healthy control.
* 2-sample t-test (p < 0.01, uncorrected); ** 2-sample t-test (p < 0.001, uncorrected).
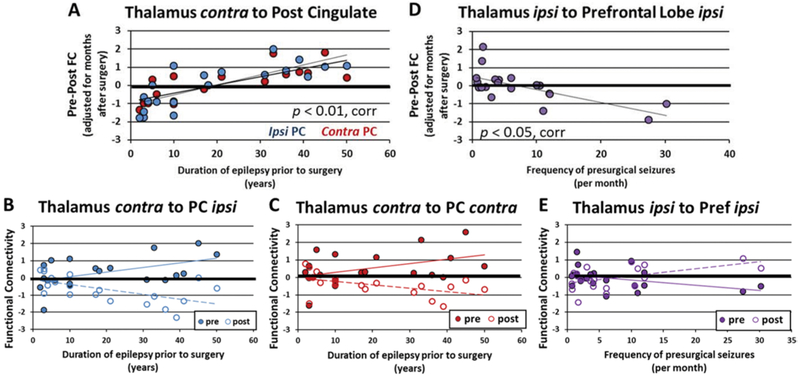
Last, FC change after surgery was compared to presurgical duration of disease and seizure frequency. All significant relationships detected involved the thalamus. First, there was a positive correlation between duration of disease prior to surgery and FC change from contralateral thalamus to ipsilateral and contralateral posterior cingulate (Pearson correlation, p < 0.01, corrected) (Fig. 5A–C).
FIG. 5.
Thalamic postsurgical changes in FC related to presurgical disease parameters. A: Positive correlation between duration of disease prior to surgery and FC change from contralateral thalamus to ipsilateral and contralateral posterior cingulate (Pearson correlation, p < 0.01, corrected). B: Presurgical and postsurgical FC between contralateral thalamus and ipsilateral posterior cingulate related to duration of disease prior to surgery. C: Presurgical and postsurgical FC between contralateral thalamus and contralateral posterior cingulate related to duration of disease prior to surgery. D: Negative correlation between frequency of consciousness-impairing seizures per month prior to surgery and FC change from the ipsilateral thalamus to the ipsilateral prefrontal lobe (Pearson correlation, p < 0.05, corrected). E: Presurgical and postsurgical FC between ipsilateral thalamus and ipsilateral prefrontal lobe related to frequency of presurgical seizures. Value of FC = 0 represents age-matched healthy control. Linear trend lines are shown. corr = Bonferroni correction; PC = posterior cingulate; Pref = prefrontal lobe.
Second, at the lobe level, the FC change from the ipsilateral thalamus to the contralateral parietal lobe increased as duration of disease prior to surgery increased (Pearson correlation, p < 0.05, corrected) (not shown). Third, there was a negative correlation between frequency of consciousness-impairing seizures per month and FC change from the ipsilateral thalamus to the ipsilateral prefrontal lobe (Pearson correlation, p < 0.05, corrected) (Fig. 5D, E).
Discussion
There are important clinical differences between early surgical failure leading to frequent disabling seizures, and late recurrence of less frequent mild seizures. In previous work, we demonstrated divergent presurgical network properties that were uniquely associated with these disparate outcomes.26 We hypothesized that mTLE surgery alters these presurgical networks to influence the ultimate seizure freedom or recurrence. In order to investigate these postsurgical networks, FC from 12 seed regions in a predetermined mTLE network to regions across the brain was quantified before and after surgery in 20 patients with mTLE. The patients were well characterized by standard presurgical clinical assessments with clear diagnosis of unilateral mTLE (Table 1). Surgical outcome varied across the cohort, with 45% being seizure free at the time of postsurgical MRI. No clinical variable except sex was associated with outcome group.
Many studies have investigated presurgical FC in mTLE from the hippocampus and across other networks, with mixed results of both increases and decreases compared to controls when using group comparisons.2,18,29 Our results show that prior to surgery (Fig. 1A, C) the patients with mTLE had lower FC, mostly from the ipsilateral hippocampus, compared to age-matched controls. Other reductions were found involving the ipsilateral posterior insula and the contralateral mid-cingulate. Only from thalamus seed regions did patients with mTLE have higher FC than controls before surgery. In healthy controls there is a known negative correlation between thalamus and occipital regions in the eyes-closed condition (as used here).33 This may be a reflection of the differential relationship between the fMRI signal in these regions and the posterior alpha EEG power during wakefulness.23 In mTLE prior to surgery we found a loss of this negative correlation detected as an increase in (or less negative) FC.
After surgery we saw an overall shift to widespread decreases compared to age-matched controls (Fig. 1B, D), including most connections to the contralateral hippocampus, when excluding the ipsilateral hippocampus and temporal lobe. When pre- and postsurgical FC were directly compared, no statistically significant increases were detected. However, significant decreases in regional (Fig. 2A, D) and lobe-level (Fig. 2B, C) FC were detected in ipsilateral parietal regions and across widespread contralateral regions, mostly involving the contralateral hippocampus. Many of these same connections were found to be significantly lower than in controls after surgery. This suggests postsurgical changes in regions across the brain that are not anatomically directly involved in the surgery. The mechanism for these changes is unknown, but the implications for distal effects may be significant. These results also complement our recent findings that presurgical FC from the contralateral hippocampus to precuneus was related to mild seizure recurrence after surgery.26
The investigation of FC changes after surgery that are associated with postsurgical outcome provides some evidence that contralateral postsurgical FC impairment or decrease from control may be related to seizure recurrence. The FC from the contralateral precuneus to the ipsilateral temporal lobe (Fig. 3) shows no difference from controls prior to or after surgery in those who become seizure free (group 1). But in those who have seizure recurrence (groups 2 and 3), the FC is not different from controls before surgery, but is less than in controls after surgery.
Although the investigation of FC changes related to type of surgery detected a statistically significant change in the contralateral hemisphere (from precuneus to temporal lobe) between those with temporal lobectomy versus those with selective amygdalohippocampectomy, this appeared to be due more to differences prior to surgery rather than after (Fig. 4). This suggests a potential selection bias in those patients referred to the different types of surgeries. However, in this sample the ANOVA and Fisher’s exact tests did not reveal statistically significant relationships between presurgical parameters shown in Table 1 and the type of surgery performed (data not shown).
Next, the relationship between postsurgical FC change and presurgical disease parameters was investigated to identify possible regions where postsurgical changes may reflect continuation of presurgical disease processes. Interestingly, in our cohort the connections identified with postsurgical impairment across the brain were not those related to presurgical duration of disease or seizure frequency. Alternatively, postsurgical change in thalamic FC was found to be positively associated with duration of disease, and negatively associated with seizure frequency (Fig. 5). Generally, the larger FC changes were found in those patients with more severe disease, which may suggest that the thalamus is an important node in the propagation of seizures before and after surgery.
Overall, these results showed that at least 1 year after mTLE surgery there are extensive FC differences from controls that are not present before surgery. The wide-spread nature of the changes and their distance from the surgical site suggest that these changes may not be anatomically directly related to the surgical procedure itself. The concentration of many of the postsurgical changes in the contralateral hippocampus implies these may be due in part to postsurgical evolution of the epileptogenic process, because the ipsilateral to contralateral hippocampal FC is known to be significantly impaired presurgically in these patients.27,30 This is also consistent with findings of progressive postsurgical contralateral hippocampal atrophy in mTLE.6,13 The fact that these connections were not related to presurgical parameters of duration of disease and seizure frequency (only thalamic connections were detected) suggests that this postsurgical evolution in FC may be altered or separate from the presurgical disease progression. However, these results cannot confirm if FC changes might represent potential facilitators of seizure recurrence, or may be possible compensatory consequences that aid in seizure inhibition after successful surgeries. Longitudinal functional and structural connectivity studies with longer-term outcomes are needed to examine this in more depth.
The overall results presented in this work are not obviously consistent with a recent publication by Maccotta et al.,24 who concluded that postoperative seizure freedom was not associated with postsurgical FC changes compared to preoperative FC in 17 patients with mTLE. Our study involves patients with different seizure outcomes. If only the seizure-free patients (group 1, n = 9) are considered here, significant postsurgical FC decreases were detected from the contralateral hippocampus to the ipsilateral medial superior frontal gyrus (p < 0.05, Bonferroni corrected). However, Maccotta et al. excluded both the ipsilateral and contralateral temporal lobes from their analyses, and so would not have investigated the contralateral hippocampus.
Although this study is unique in the ways described, one important limitation is in its small sample size. In addition, although it was possible to generalize the patients with regard to medication changes after surgery (Table 1), individual medication details were not considered. However, no FC changes after surgery were identified that related to using the general medication categories (data not shown). The variability of the timing of the postsurgical scan after surgery is relatively large, and these data do not allow the determination of the specific timing of these postsurgical changes. By using age correction and adjusting for time after surgery, some of these effects may be reduced. However, adjustments for time after surgery at which the postsurgical scan was obtained assumed a linear relationship, which is not proven and may vary across different connections. Similarly, network changes in healthy controls over time are not considered in this work, but the effect of change in age from presurgical to postsurgical scan is included in the age normalization. Future studies involving a larger patient cohort with longitudinal postsurgical network mapping in addition to longitudinal investigations of healthy controls will address many of these limitations.
Conclusions
This work presents a systematic quantification of presurgical to postsurgical FC changes in patients with mTLE. Our results demonstrated significant FC decreases from control across the brain after surgery that were not present before surgery, including many contralateral hippocampal connections distal to the surgical site. This suggests that these changes may be a reflection of an ongoing epileptogenic progression that has been altered by the surgery, rather than a direct result of the surgery itself. Further-more, this interaction between surgical treatment and network evolution may play a significant role in the long-term divergent outcomes after surgery in patients with mTLE. Although in previous work we identified presurgical network properties, including contralateral hippocampus connections, associated with these disparate outcomes,25,26 we did not consider the potential for variability in postsurgical network evolution, which may also affect long-term seizure outcome. We now propose that the combination of presurgical network mapping with the understanding of the dynamic effects of surgery on the networks may be used to create predictors of the likelihood of long-term seizure recurrence in individual patients after mTLE surgery. To do this, however, requires the quantification of individual longitudinal, postsurgical network evolution and its relation to presurgical networks and parameters. Finally, the detection and characterization of the regions and networks involved in seizure recurrence may identify alternative or additional targets for treatment of drug-refractory mTLE.
Acknowledgments
This work was supported by NIH R01 NS075270 (V.L.M.), R01 NS110130 (V.L.M.), and R00 NS097618 (D.J.E.).
ABBREVIATIONS:
- FC
functional connectivity
- fMRI
functional MRI
- mTLE
mesial temporal lobe epilepsy
Footnotes
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Besson P, Dinkelacker V, Valabregue R, Thivard L, Leclerc X, Baulac M, et al. : Structural connectivity differences in left and right temporal lobe epilepsy. Neuroimage 100:135–144, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Bettus G, Bartolomei F, Confort-Gouny S, Guedj E, Chauvel P, Cozzone PJ, et al. : Role of resting state functional connectivity MRI in presurgical investigation of mesial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 81:1147–1154, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Bonilha L, Jensen JH, Baker N, Breedlove J, Nesland T, Lin JJ, et al. : The brain connectome as a personalized biomarker of seizure outcomes after temporal lobectomy. Neurology 84:1846–1853, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, et al. : Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol 22:1326–1333, 2001 [PMC free article] [PubMed] [Google Scholar]
- 5.Deleo F, Garbelli R, Milesi G, Gozzo F, Bramerio M, Villani F, et al. : Short- and long-term surgical outcomes of temporal lobe epilepsy associated with hippocampal sclerosis: relationships with neuropathology. Epilepsia 57:306–315, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Elliott CA, Gross DW, Wheatley BM, Beaulieu C, Sankar T: Progressive contralateral hippocampal atrophy following surgery for medically refractory temporal lobe epilepsy. Epilepsy Res 125:62–71, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Engel J, Van Ness PC, Rasmussen TB, Ojemann LM: Outcome with respect to epileptic seizures, in Engel J (ed): Surgical Treatment of the Epilepsies, ed 2. New York: Raven Press, 1993, pp 609–621 [Google Scholar]
- 8.Engel J Jr: Mesial temporal lobe epilepsy: what have we learned? Neuroscientist 7:340–352, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Engel J Jr: What can we do for people with drug-resistant epilepsy? The 2016 Wartenberg Lecture. Neurology 87:2483–2489, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Englot DJ, Chang EF: Rates and predictors of seizure freedom in resective epilepsy surgery: an update. Neurosurg Rev 37:389–405, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Englot DJ, Konrad PE, Morgan VL: Regional and global connectivity disturbances in focal epilepsy, related neurocognitive sequelae, and potential mechanistic underpinnings. Epilepsia 57:1546–1557, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Englot DJ, Mishra AM, Mansuripur PK, Herman P, Hyder F, Blumenfeld H: Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci 28:9066–9081, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandes DA, Yasuda CL, Lopes TM, Enrico G, Alessio A, Tedeschi H, et al. : Long-term postoperative atrophy of contralateral hippocampus and cognitive function in unilateral refractory MTLE with unilateral hippocampal sclerosis. Epilepsy Behav 36:108–114, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Fisher RA: Frequency distribution of the values of the correlation coefficient in samples from an indefinitely large population. Biometrika 10:507–521, 1915 [Google Scholar]
- 15.Gleichgerrcht E, Munsell B, Bhatia S, Vandergrift WA III, Rorden C, McDonald C, et al. : Deep learning applied to whole-brain connectome to determine seizure control after epilepsy surgery. Epilepsia 59:1643–1654, 2018 [DOI] [PubMed] [Google Scholar]
- 16.Glover GH, Li TQ, Ress D: Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 44:162–167, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Goellner E, Bianchin MM, Burneo JG, Parrent AG, Steven DA: Timing of early and late seizure recurrence after temporal lobe epilepsy surgery. Epilepsia 54:1933–1941, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Haneef Z, Lenartowicz A, Yeh HJ, Levin HS, Engel J Jr, Stern JM: Functional connectivity of hippocampal networks in temporal lobe epilepsy. Epilepsia 55:137–145, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He X, Doucet GE, Pustina D, Sperling MR, Sharan AD, Tracy JI: Presurgical thalamic “hubness” predicts surgical outcome in temporal lobe epilepsy. Neurology 88:2285–2293, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huo Y, Plassard AJ, Carass A, Resnick SM, Pham DL, Prince JL, et al. : Consistent cortical reconstruction and multi-atlas brain segmentation. Neuroimage 138:197–210, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jehi LE, Silveira DC, Bingaman W, Najm I: Temporal lobe epilepsy surgery failures: predictors of seizure recurrence, yield of reevaluation, and outcome following reoperation. J Neurosurg 113:1186–1194, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Laxer KD, Trinka E, Hirsch LJ, Cendes F, Langfitt J, Delanty N, et al. : The consequences of refractory epilepsy and its treatment. Epilepsy Behav 37:59–70, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, de Zwart JA, Yao B, van Gelderen P, Kuo LW, Duyn JH: Finding thalamic BOLD correlates to posterior alpha EEG. Neuroimage 63:1060–1069, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maccotta L, Lopez MA, Adeyemo B, Ances BM, Day BK, Eisenman LN, et al. : Postoperative seizure freedom does not normalize altered connectivity in temporal lobe epilepsy. Epilepsia 58:1842–1851, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan VL, Englot DJ, Rogers BP, Landman BA, Cakir A, Abou-Khalil BW, et al. : Magnetic resonance imaging connectivity for the prediction of seizure outcome in temporal lobe epilepsy. Epilepsia 58:1251–1260, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan VL, Rogers BP, Anderson AW, Landman BA, Englot DJ: Divergent network properties that predict early surgical failure versus late recurrence in temporal lobe epilepsy. J Neurosurg [epub ahead of print April 5, 2019. DOI: 10.317½019.1.JNS182875] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan VL, Rogers BP, Sonmezturk HH, Gore JC, Abou-Khalil B: Cross hippocampal influence in mesial temporal lobe epilepsy measured with high temporal resolution functional magnetic resonance imaging. Epilepsia 52:1741–1749, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Najm I, Jehi L, Palmini A, Gonzalez-Martinez J, Paglioli E, Bingaman W: Temporal patterns and mechanisms of epilepsy surgery failure. Epilepsia 54:772–782, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Pereira FRS, Alessio A, Sercheli MS, Pedro T, Bilevicius E, Rondina JM, et al. : Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: evidence from resting state fMRI. BMC Neurosci 11:66, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pittau F, Grova C, Moeller F, Dubeau F, Gotman J: Patterns of altered functional connectivity in mesial temporal lobe epilepsy. Epilepsia 53:1013–1023, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers BP, Morgan VL, Newton AT, Gore JC: Assessing functional connectivity in the human brain by fMRI. Magn Reson Imaging 25:1347–1357, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiebe S, Blume WT, Girvin JP, Eliasziw M: A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 345:311–318, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Zou Q, Long X, Zuo X, Yan C, Zhu C, Yang Y, et al. : Functional connectivity between the thalamus and visual cortex under eyes closed and eyes open conditions: a resting-state fMRI study. Hum Brain Mapp 30:3066–3078, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]