Abstract
Epidemiological studies report associations between air pollution (AP) exposures and several neurodevelopmental disorders including autism, attention deficit disorder and cognitive delays. Our studies in mice of postnatal (human 3rd trimester brain equivalent) exposures to concentrated ambient ultrafine particles (CAPs) provide biological plausibility for these associations, producing numerous neuropathological and behavioral features of these disorders, including male-biased vulnerability. These findings raise questions about the specific components of AP that underlie its neurotoxicity which our studies suggest could involve trace elements as candidate neurotoxicants. X-ray fluorescence analyses of CAPs chamber filters confirms contamination of AP exposures by multiple elements, including iron (Fe) and sulfur (S). Correspondingly, laser ablation inductively coupled plasma-mass spectrometry (ICP-MS) of brains of male mice indicate marked post-exposure elevations of Fe and S and other elements. Elevations of brain Fe and S in particular are consistent with potential ferroptotic, oxidative-stress and altered antioxidant capacity based mechanisms of CAPs-induced neurotoxicity, supported by observations of increased serum oxidized glutathione and increased neuronal cell death in nucleus accumbens with no corresponding significant increase in caspase-3, in male brains following postnatal CAPs exposures. Understanding the role of trace element contaminants of particulate matter AP as a source of neurotoxicity is critical for public health protection.
Keywords: Ultrafine particles, Iron, Sulfur, Glutathione, Ventriculomegaly, Corpus callosum, Nucleus accumbens
Introduction
Numerous epidemiological studies report associations of various metrics of air pollution (AP) exposures with characteristics of neurodevelopmental disorders and neurodegenerative diseases. These have included reports of increased risk for autism spectrum disorder (ASD), attention deficit hyperactivity disorder (ADHD), slower cognitive development in children, schizophrenia and a more rapid cognitive decline with age in adults, as summarized in a series of recent reviews.1-10 Several studies have also reported neuropathological changes in response to air pollution exposures, which to date, include reductions in brain white matter (myelin) and corresponding brain disconnectivity, reductions in gray matter volume, and altered basal ganglia structure.11-16 AP as a risk factor for neurodevelopmental disorders and neurodegenerative diseases would carry heavy social and economic burdens. Annual U.S. costs just for ASD are $11–60 billion according to the Centers for Disease Control, while estimated costs for Alzheimer’s are $290 billion (Alzheimer’s Association).
Biological plausibility for the neurotoxic effects of AP has also begun to emerge from studies using animal models6,17-19. Based on the fact that for different particle sizes of the same chemisty, ultrafine particulate (UFP) matter is considered the most reactive component of AP per unit mass because of its greater surface area/mass ratio available for adsorption of organic and inorganic contaminants,20 we have examined the impact of developmental exposures to Concentrated Ambient UFPs (CAPs) on brain and behavior in mice since 2013 as reviewed here. Exposures were carried out from postnatal days (PND) 4–7 and 10–13 for 4 hr/day. This period is considered analogous to human third trimester brain development21,22 and is characterized by significant neuro- and gliogenesis.23 Mass CAPs concentrations across these studies to date have ranged from 22–121 ug/m3; levels at the lower-mid part of this range are similar to PM2.5 levels in global cities. 24-26
These postnatal CAPs exposures in mice resulted in marked neuropathological effects, as well as behavioral dysfunctions, with these effects occurring predominantly in males, as previously reported27-33. One dramatic neuropathological effect was a male-specific persistent ventriculomegaly (i.e., enlarged lateral ventricles) occurring at 96 ug/m3 and observed even at postnatal day 270, i.e., the last time point examined. Subsequent exposure cohorts revealed that postnatal CAPs produced male-specific ventriculomegaly even at 44 μg/m3, the lowest levels yet examined with sufficient sample sizes to date. In addition, marked reductions in size and myelination of the corpus callosum (CC), the largest white matter tract of the brain and a structure critical to interhemispheric connectivity, were found. Male biased microglial activation (inflammation) was found in corpus callosum (340% of control) as late as postnatal day 270, the last time point examined to date. A profile of male-specific elevated brain glutamate, with consequent excitatory-inhibitory imbalance was also observed. Behavioral impairments, as assessed to date, reveal increased impulsivity/cognitive inflexibility and altered social interactions, including reduced social preference for a novel mouse vs. a cage-mate28,31,34,35.
Collectively, these findings following postnatal CAPs exposures suggest that AP can reproduce numerous characteristics of a number of neurodevelopmental disorders, including the enhanced vulnerability of males to such conditions. While ASD has unique features, it also shares features with several other neurodevelopmental disorders 36-41. ASD and early childhood onset schizophrenia 42-45 share abnormalities of glutamatergic systems 46-48, inflammatory mechanisms 49 and neuropathological features, including ventriculomegaly and disconnectivity 50-52. Attention deficit hyperactivity disorder (ADHD) occurs at higher rates in children with ASD 53. Both involve increases in impulsive behavior and are considered disorders of hemispheric disconnectivity 37. Like ASD, ADHD is male-biased and its rates have increased significantly from 7.8% in 2003 to 9.5% in 2007 and 11.0% in 2011–2012 according to CDC. ADHD can also include ventriculomegaly 54, and alterations in glutamatergic function 55, brain white matter 56, brain connectivity 57 and inflammation 58. Thus, the neurotoxic consequences of AP seen in these experimental models may contribute risk not only for ASD, but for other neurodevelopmental disorders as well. Differences in timing of AP exposure during pregnancy could contribute to the heterogeneity and sub-groups of ASD 59 or other neurodevelopmental disorders.
A collaborative study carried out at NYU (Tuxedo, NY)60-62 obtained brains of mice following gestational (GE, days 0.5–16.5) fine/UFP CAPs exposures, i.e., a 1st+2nd trimester human brain equivalent time point. GE exposures produced ventriculomegaly in both sexes (see 34,60,63) at PND 11–15. In contrast to postnatal CAPs, GE exposures increased, rather than decreased CC size and myelination, effects that were still present at PND 57–61, the last time point available for examination. Increased CC size and myelination are actually seen early in the ASD brain64, followed only later by reductions in size and myelination, as seen with our PN CAPs exposures65,66. The findings produced by gestational CAPs exposures to date confirm that AP-induced neurotoxicity is not unique to exposures carried out in Rochester, and despite the heterogeneity of AP compositions, similarities exist in their potential to disrupt white matter development in the CNS. In addition, they reconfirm sex differences in outcome, and also underscore the importance of timing of AP exposures during brain development.
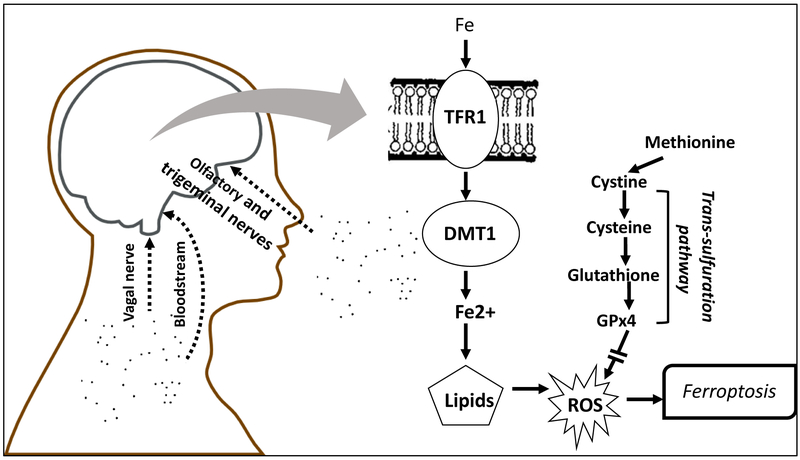
The observations of neuropathological effects from particulate AP exposures at two different sites also suggested that similar AP components could be involved in this neurotoxicity. Consequently, we carried out studies to determine whether the carbon-based nanoparticle itself, i.e., the base of UFPs, or perhaps a known inflammatory component of AP, e.g., endotoxin 67 could produce such effects, by generating atmospheres of each and exposing mice to them alone or in combination. However, neither exposure alone, nor the two combined were able to reproduce the developmental neurotoxicity of postnatal exposure to ambient CAPs.68 Trace element contaminants were subsequently considered another potential source of CAPs neurotoxicity. Subsequent analyses, elaborated here, reveal that trace element contaminants of AP may be a likely source of its neurotoxic consequences, based on the presence of known elemental neurotoxicants in the exposures and in excess in brain following such exposures. The increases in these elements, which can be taken up into brain from blood stream but also via the olfactory and trigeminal nerves 69-71 moreover, suggest specific mechanisms of CAPs-induced neurotoxicity via ferroptosis, as schematized in Figure 1, and their actions in brain would be likely to influence pathways consistent with reported biomarkers of neurodevelopmental disorders.72-78 In addition, data across different exposures were utilized to determine preliminary concentration-effect functions for various outcomes where available.
Figure 1.
Schematic diagram showing entry routes of ultrafine particles to brain via olfactory and trigeminal nerves, vagal nerve and blood stream. The consequent elevation of brain metals as contaminants of ultrafine particles, e.g., Fe, raise the potential for ferroptotic mechanisms of brain neuropathology associated with air pollution. Both transferrin bound and non-transferrin (labile) Fe can be released into cells with Fe3+ (ferric iron) reduced to Fe2+ (ferrous iron) followed by movement into cytosol via the divalent metal transporter-1. Potential increases in the redox active Fe2+ can lead to reactive oxygen species via the Fenton reaction and lipid peroxidation. Ferroptosis is negatively regulated by glutathione and glutathione peroxidase 4, derived from the trans-sulfuration pathway, which if reduced can facilitate ferroptosis, an iron- and lipid peroxidation-dependent form of cell death. TRF1 = transferrin; DMT1 = divalent metal transporter 1; ROS = reactive oxygen species; GPx4 = glutathione peroxidase 4.
Methods and Materials
Animals and Postnatal CAPs Exposures
Male and female C57BL6/J mice 8 weeks of age from Jackson Laboratories (Bar Harbor, ME) were acclimated to a housing room for 1 week and then bred monogamously for 3 days. Pregnant dams were singly housed with litters until weaning on PND21. To preclude litter-specific effects, only single pups/sex/litter were used in all of these studies. All mice used in this study were treated humanely and with regard for alleviation of suffering and approved by the University of Rochester Institutional Animal Care and Use Committee.
Pups were placed singly in compartmentalized whole-body exposure chambers. They were exposed to filtered air (Air) or Concentrated Ambient ultrafine Particles (CAPs) using the Harvard University Concentrated Ambient Particle System (HUCAPS) fitted with a size-selective inlet and a high-volume ultrafine particle (UFPs, ≤100nm) concentrator (10–20x) that takes in outdoor air at 5000 liters per minute and concentrates ambient UFP, as previously described.27-30 Exposures lasted for 4 hours per day from 0700–1100 for 4 days per week from PND 4–7 and PND10–13, with exposure timing corresponding to peak vehicular traffic outside the intake valve of the HUCAPS instrumentation. A condensation particle counter (TSI 3022A) provided particle counts. Mass concentration was calculated using idealized particle density (1.5g/cm3). A Scanning Mobility Particle Sizer (SMPS) was used to determine particle size distribution and median particle diameter + geometric standard deviation. Flow of CAPs-enriched and filtered air was maintained at 35–40% relative humidity and 77–79° F. Average exposure mass concentrations from these exposures were 22, 44, 53, 96 and 121 μg/m3. Teflon filter samples from the breathing zone inside the exposure chamber were taken for chemical analysis of the constituents. Teflon filter samples were analyzed by the Desert Research Institute (DRI, Reno, NV, USA) in a temperature-controlled package at below 4˚ for elemental analyses by an energy dispersive x-ray fluorescence analyzer (Epsilon 5, PANalytical Company, the Netherlands) as previously described. 79
Brain Pathology
To preclude any effect of anesthetics on measured endpoints, brains were extracted following decapitation without sedation and placed into 4% paraformaldehyde for 24 hours and then cryoprotected in 30% sucrose until they sank. Brains were stored at 4°C until they were sectioned, and then stored in sucrose- and ethylene glycol containing cryoprotectant and kept at −20°C.
Corpus Callosum (CC) Area Determination:
The size of the CC (approximate adult-equivalent Bregma range 0.74mm-0.2mm) was determined by tracing the area of interest in at least 3 adjacent coronal sections of slide-mounted brain using Neurolucida (MBF, Villiston, VT). Software enumerated the area of interest in μm2.
Myelin Basic Protein Immunostaining & Image analysis:
To determine the extent of myelination, every 4th section (PND14) or every 6th section (PND270) was stained for myelin basic protein (MBP). Briefly, brain sections were washed of cryoprotectant and placed into primary antibody for MBP (EMD Millipore; MAB386). Tissue was then placed into biotinylated secondary antibody (Vector labs; BA-9401; 1:200 dilution) for 1 hour and the stain was visualized using 3–3’-diaminobenzidine (DAB). Expression of MBP was analyzed using Image Pro Plus 7.0 (Mediacybernetics) using segmentation and thresholding to estimate a percent area of the region of interest that was positive for MBP expression.
Laser Ablation Inductively Coupled Plasma Mass Spectrometry
Brain thin sections of 40 μm were prepared by cryosectioning and mounted onto a microscope slide for elemental imaging by LA-ICP-MS. Slides were not subbed (coated with chromium potassium sulfate to prevent metal contamination. The LA-ICP-MS system used was an ESI Lasers NWR 213 laser ablation unit coupled to an Agilent 8900 ICP-MS operated in O2 reaction mode to enhance detection of S by mass shifting to SO at m/z 48. The laser ‘spot’ size was 50 μm square and the laser scan speed was 250 μm/sec and the laser shot frequency was 20 Hz. The sample was analyzed in a series of line scans with a spacing of 50 μm. The ICP-MS collected data for Al, S, Ca, Cr, Mn, Fe, Ni, Cu, Zn, Se, Cd, Pb with a total acquisition time of 0.2 sec. The raw ICP-MS data was concatenated into an XL sheet, and relative X and Y co-ordinates for each data point were added. The resulting file was then imported into SMAK elemental imaging software to generate 2D elemental images of each section 80. The images show the relative concentration differences in each element across the thin section as no quantitative calibration was performed during this analysis.
Atomic Absorption Spectrophotometry
Atomic absorption spectrophotometry was used to measure levels of metals in olfactory bulb. After wet weights were recorded, tissue were digested with a 1:1 mixture of nitric and perchloric acids, samples brought to a constant volume, and determination of metal levels performed by graphite AAS (Hitachi 170–70). For each analysis, controls (blanks) and standards were carried in 0.7% nitric acid and calibration curves determined at the beginning and end of a sample run.
Serum Oxidized Glutathione
Blood was collected during sacrifice into pre-chilled tubes and spun at 1500 g for 20 mins and serum removed. Serum oxidized glutathione was measured using a commercially available colorimetric assay kit (Arbor Assays, Ann Arbor MI) according to the manufacturer’s instructions. Quality controls fell within appropriate ranges with intraassay sample variability below 15% variance.
Immunohistochemistry
Caspase-3:
Briefly, brain sections incubated in primary antibody solution (1:2,000; 9662S, Cell Signaling Technology, Danvers, MA 01923) at 4°C for 24 hours. Sections were then washed and incubated with biotinylated secondary antibody (1:200, anti-rabbit; BA1000, Vector, Burlingame, CA 94010) for 1 hour; the stain was visualized using DAB (3–3’ diaminobenzidine) and quantified using ImageJ with established thresholding methods81.
Silver Staining:
was carried out on brain sections using the FD NeuroSilver Kit II (FD NeuroTechnologies, Inc., Columbia, MD) according to manufacturer’s instructions.
Statistical Analyses
Statistical analysis was carried out using JMP11 (Cary, NC) with initial between groups ANOVAs that included all experimental factors (sex and treatment) in full factorial designs. Main effects and interactions were then pursued in post-hoc t- testing as appropriate. P values ≤0.05 were considered statistically significant.
Results
Concentration Effect Functions for Postnatal UFPs
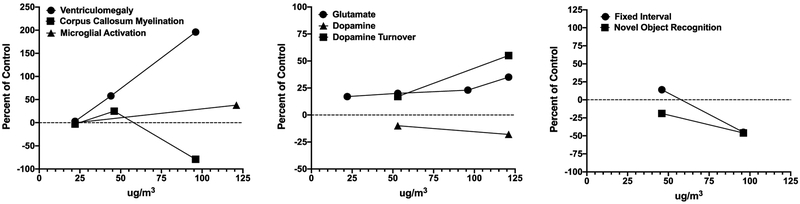
Postnatal CAPs exposure concentrations across our studies to date have ranged from mass concentrations of 22–121 μg/m3. In order to ascertain the impact of concentration, we plotted magnitude of effect against exposure concentrations for outcomes in males that were measured at two or more of the exposure concentrations. The results are depicted in Figure 2. As shown in the left panel, ventriculomegaly was not observed in preliminary assessments following a 22 μg/m3 exposure, but trending increases in size of the lateral ventricle were observed at 44 μg/m3 (F(1,17)= 3.98. p=0.062) in males, and significantly further increased at 96 μg/m3 as previously reported and shown in Figure 3. 28,31 No changes in myelination were evident at 22 or 44 μg/m3, while, as previously reported, marked reductions occurred as concentrations increased to 96 μg/m3. 28,31
Figure 2.
Concentration-effect functions for neuropathological (left), neurochemical (middle) and behavioral (right) consequences for CAPs exposure. Data are from males only and presented as percent of the corresponding filtered air control data and the CAPs concentration (x axis). Data from 27-32,82
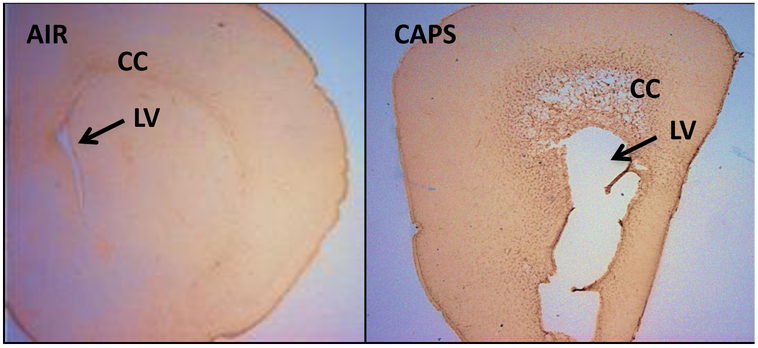
Figure 3.
Coronal unstained brain sections from a male brain exposed to filtered air (left) or to 96 ug/m3 CAPs (right) during the postnatal period representing the extreme of ventriculomegaly observed. The enlarged lateral ventricle (LV) of the CAPs-exposed brain, below the corpus callosum (CC) is shown by arrows. 28
The middle panel depicts alterations in dopamine and glutamate levels and function. Even at the lowest concentrations generated in our studies to date (22 μg/m3), increases in levels of glutamate have been observed, with slight increases in the magnitude of elevation at the highest concentration generated to date (121 μg/m3). 28-30,82 Findings also suggest increases in levels of dopamine turnover in brain, again increasing with increasing CAPS exposure concentration 29,30. With increases in dopamine turnover, levels of dopamine appear to decline with increasing CAPS exposure concentration.29,30
The right panel depicts two behavioral baselines that have been measured in two different exposure cohorts, i.e., behavior on a fixed interval (FI) schedule of food reward, as a measure of temporal learning, and novel object recognition, an index of short-term memory, as previously described. 30,83 Slight increases in rates of response on the fixed interval schedule (delayed learning) at 44 μg/m3 were observed, while at 96 μg/m3, marked reductions in response rates (delayed learning and delayed reward) were found that persisted across sessions. Novel object recognition was already significantly impaired at 44 μg/m3, with even further impairments in performance observed at 96 μg/m3.
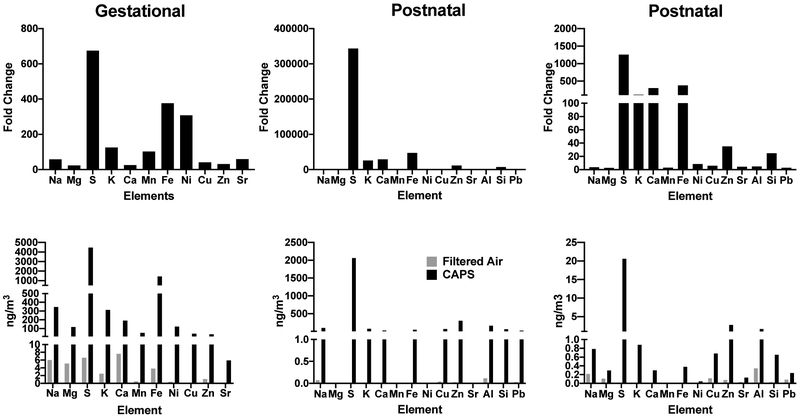
Assessment of Trace Elements in CAPs Exposures
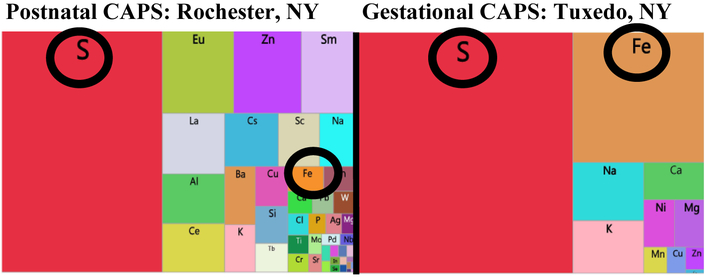
XRF analyses were utilized to examine the elemental exposures associated with various CAPs exposure studies. As shown in the treemaps for one such exposure from Rochester, NY and another from Tuxedo, NY in Figure 4, all such analyses revealed multiple trace elements were contaminants of CAPs, regardless of the site of the exposure. In addition, as expected, the profile of elevation of trace elements in CAPs differed somewhat by exposure site, with S being the highest in Tuxedo with a relatively high Fe levels as well; S was also highest in Rochester, but Fe was not as high as in Tuxedo.
Figure 4.
Tree-maps of elements from ambient UFP exposures via XRF analysis of filters during postnatal (left; Rochester, NY) 28-30 and gestational exposures (right; Tuxedo NY). 60,62,63 Both S and Fe are circled.
Elemental contaminants of the AP exposures are further characterized in Figure 5 where the top row depicts fold changes in various elements relative to the filtered air chamber exposure levels for three different exposures. As it shows, levels of sulfur (S) showed dramatic increases in virtually all these exposures. In addition, increases in iron (Fe) levels were also consistently evident. Potassium levels likewise showed increases. Increases in numerous other elements were also observed, but fold-changes were less systematic across such exposures.
Figure 5.
Top row shows fold changes in various elements relative to levels in filtered air control chambers for gestational (left column) and for two postnatal exposures (middle and right columns) following XRF analyses. Corresponding data in ng/m3 are depicted along the bottom row.
Total concentrations in ng/m3 are depicted in the bottom row of Figure 5 relative to filtered air controls for these same exposure cohorts. As these plots show, S levels ranged from 2000 to 4000 ng/m3, and Fe levels from approximately 500–1000 ng/m3. Other metals of note in these exposures included zinc (Zn: range from 2.7 to 297 ng/m3), copper (Cu: range from 0.68 to 69 ng/m3), aluminum (Al: range from 1.65 to 164 ng/m3), potassium (K: range from 0.88 to 313 ng/m3) and silica (Si: range from 0.65 to 64 ng/m3).
Assessment of Trace Elements in Brain
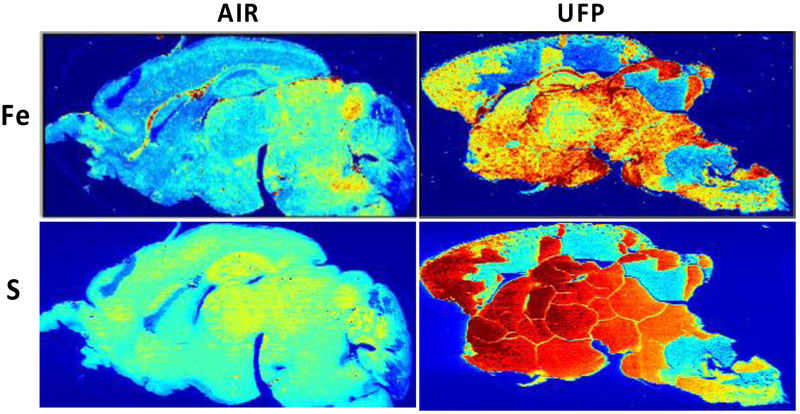
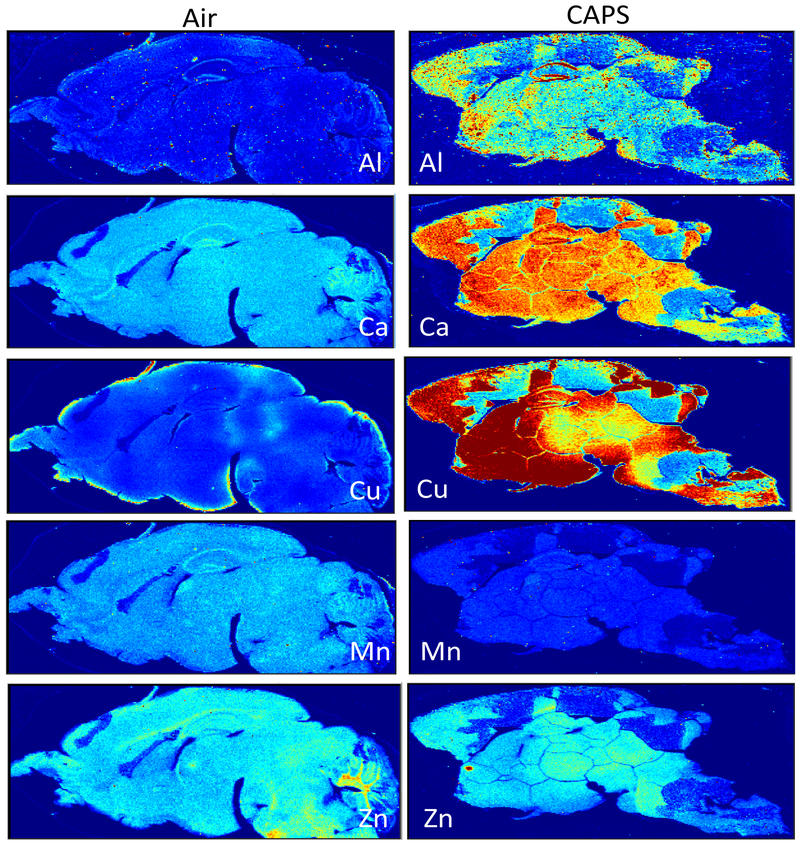
Subsequently, laser ablation ICP-MS was carried out on 40 μm sagittal sections after postnatal CAPs exposure from a randomly chosen filtered air control male and a CAPs-exposed male brain. Figure 6 contrasts the levels of Fe and S in these brains at postnatal day 14, i.e., 24 hr after completion of exposures. As it shows, postnatal CAPs markedly elevated the levels of both elements in brain relative to levels in filtered air control brain. These elevations, moreover, were seen diffusely across the brain rather than regionally, as would be consistent with the broad functions of Fe and S in brain. As would be suggested by the analyses of filters from exposure chambers, other metals were likewise elevated in CAPs brain post exposure (Figure 7), in particular levels of Cu and to some degree calcium (Ca). Notably, increases in Al were also seen that were somewhat diffuse across regions, but showed a selectively high accumulation in hippocampus. In contrast, levels of two essential metals in brain appeared to be of a lower concentration in brain, specifically manganese (Mn) and Zn. Collectively, these findings suggest brain metal dyshomeostasis.
Figure 6.
Brain Fe and S Levels are increased in postnatal CAPs-exposed males after analysis by ICPMS in one randomly chosen filtered air control male and one male exposed to concentrated ambient ultrafine particles. These images show a relatively diffuse pattern of increases across brain regions. Brains from females not yet examined. Exposure concentrations averaged 45 ug/m3.
Figure 7.
Brain levels of aluminum (Al), calcium (Ca), copper (Cu), manganese (Mn) and zinc (Zn) from a randomly chosen male (Air) and CAPs-exposed (CAPS) male following postnatal exposures. Exposure concentrations averaged 45 ug/m3.
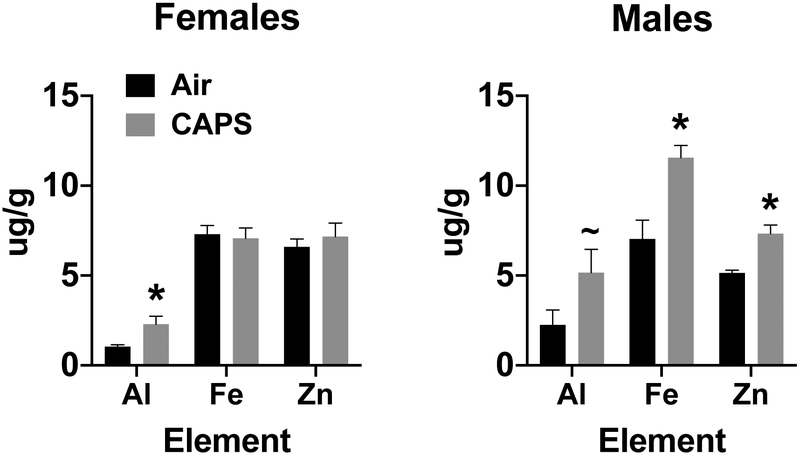
Given the demonstrated olfactory and trigeminal nerve uptake of elemental contaminants of UFPs, and since the laser ablation ICP-MS analysis is not quantitative, olfactory bulb tissue from postnatal day 14 CAPs exposed and filtered air exposed brains of males and females were analyzed by atomic absorption spectrophotometry for levels of Al, Fe and Zn. As can be seen from Figure 8. In support of the ICP-MS images, significantly increased levels of both Fe (65%, F(1,11)=47.58, p<0.0001) and Zn (43%, F(1,11)=20.09, p=0.0008) were found in males, with a similar trend for Al increases (F(1,11)=3.495, p=0.086) relative to filtered air controls after exposures to 44 ug/m3. Females showed a significant increase only in levels of Al by 120% (F(1,11)=6.56, p=0.0265), but not in Fe or Zn.
Figure 8.
Mean ± standard error levels (ug/g) of aluminum (Al), iron (Fe) and zinc (Zn) in olfactory bulb following postnatal CAPs exposure concentrations of 45 ug/m3 in females (left) and males (right).
Potential Ferroptotic Mechanisms
Increases in Fe and S in brain suggested the potential for ferroptotic mechanisms could underlie the neuropathological changes observed. 84-87 Ferroptosis is a non-apoptotic fatal combination of Fe toxicity, antioxidant depletion due to disruption of glutathione peroxidase-4 (GPx4), i.e., trans-sulfuration, and lipid peroxidation-induced membrane damage88. Ferroptosis is negatively regulated by glutathione (GSH), a product of the trans-sulfuration pathway, making S levels critical. An inverse relationship between Fe overload and GSH, hydrogen peroxide and reactive oxygen species (ROS) are well documented 89-91. Thus, high brain Fe, and low GSH or GPx4 would facilitate ferroptosis.
Oxidized Glutathione
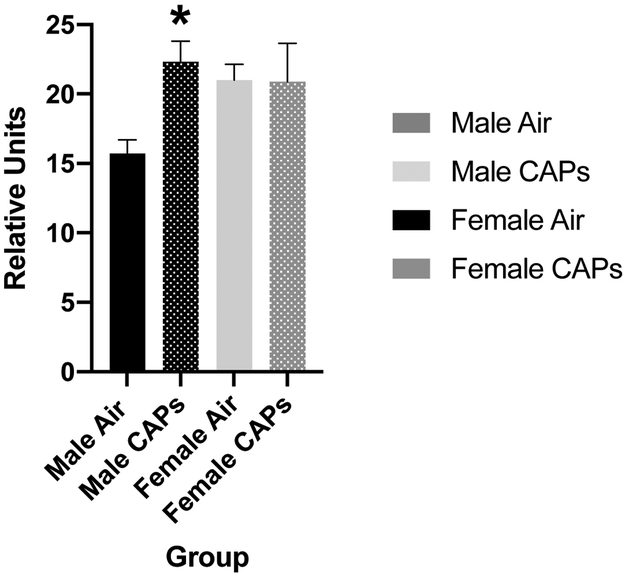
To determine the potential involvement of ferroptotic mechanisms in these effects, levels of serum oxidized glutathione were measured and revealed male-specific increases as shown in Figure 9 (F(1,8)=10.9, p=0.0108). These effects were seen following postnatal CAPS exposures where the exposure concentrations averaged only 22 ug/m3.
Figure 9.
Mean ± standard error levels of oxidized glutathione in serum from males (black) and females (gray) exposed postnatally to filtered air or to 45 ug/m3 CAPs. Statistically significant increases in serum oxidized glutathione were seen in males after CAPs, but not in females.
Nucleus Accumbens Neuronal Cell Death
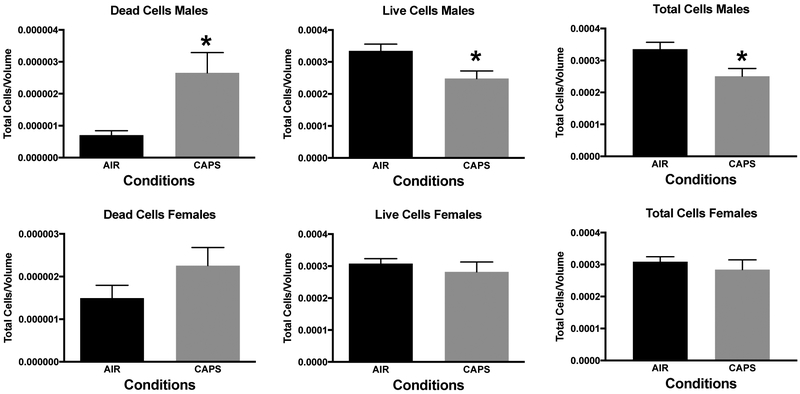
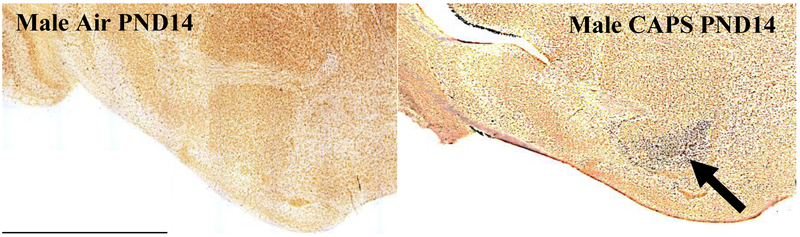
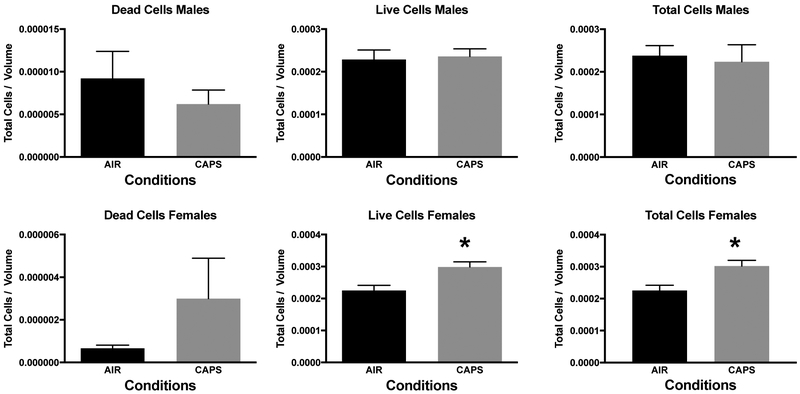
In addition, measures of neuronal death were examined at postnatal day 14 in nucleus accumbens (NAc), a key region of the mesocorticolimbic circuit of brain critical to executive functions, after exposure concentrations averaging 44 ug/m3. For this purpose, tissue was silver stained as well as examined by caspase immunohistochemistry. Males exhibited significant increases in numbers of dead cells, and reductions in numbers of live cells and total numbers of cells (Figure 10; males: dead cells, F(1,8)=8.92, p=0.0174; live cells, F(1,8)=7.46, p=0.0258; total cells, F(1,8)=6.97, p=0.0297) as measured via silver staining. While a similar trend towards an increase in dead cells was seen in females at postnatal day 14, effects were not statistically significant. Figure 11 shows a photomicrograph depicting the increased silver staining in CAPS-exposed male nucleus accumbens; importantly these increases were not seen with caspase staining. When measured at postnatal day 25 (Figure 12), effects were no longer evident in males, whereas significant increases in live cells (F(1,6)=8.99, p=0.024) and total cells (F(1,6)=8.95, p=0.043) were seen in females at this time point.
Figure 10.
Mean ± standard error levels of dead cells (left column), live cells (middle column) and total cells (right column) in nucleus accumbens at postnatal day 14 as evaluated by silver staining of males (top row) and females (bottom row) exposed postnatally to filtered air (Air) or to 45 ug/m3 CAPs. Males, but not females exhibited increases in dead cells, and reductions in both live cells and total cells.
Figure 11.
Male-specific degeneration of neurons in nucleus accumbens (arrow) as measured by silver staining as described in the methods at PND14 following postnatal exposure to 45 ug/m3 CAPs. No comparable effects were seen in males exposed to filtered air, nor did caspase staining show increases.
Figure 12.
Mean ± standard error levels of dead cells (left column), live cells (middle column) and total cells (right column) in nucleus accumbens at postnatal day 25 as evaluated by silver staining of males (top row) and females (bottom row) exposed postnatally to filtered air (Air) or to 45 ug/m3 CAPs. At this time point, changes were no longer evident in males, whereas females showed increases in both live cells and total cells.
Discussion
Collectively, our studies of postnatal and gestational CAPs exposures provide biological plausibility supporting an impact of AP on brain development and behavior.17,27-31,60,62,63 Importantly, these studies demonstrate that the impacts of ultrafine particulate matter exposures are sex-dependent and differ by the specific developmental window of time in which it occurs. Further, they suggest that the adverse impacts are not geographically unique to Rochester, and that similar components of air pollution could be responsible for the observed neuropathological consequences.
Similarities of Effects of Postnatal CAPs Exposures in Mice to Features and Hypothesized Mechanisms of Autism Spectrum Disorder and other Neurodevelopmental Disorders in Humans
The similarity of the effects of CAPs exposures to characteristics and hypothesized mechanisms of ASD is striking as depicted for postnatal CAPs exposures in Table 1. ASD is a male-biased disorder diagnosed on the basis of impaired social behaviors and restricted/repetitive behaviors 49,92,93. Neuropathology of ASD in humans is dynamic across time, and can include persistent ventriculomegaly,66,94-96 initial increases followed by sustained loss of white matter 65,66 and gray matter, 97-101 loss of myelin connectivity across the hemispheres 102-104 particularly in CC, 64 persistent brain inflammation 105,106 and excitatory (glutamate)-inhibitory (GABA) imbalance 107 that correlates with IQ reductions and social and cognitive deficits. Notably, in mice, increases in white matter were seen following gestational CAPs exposures, whereas postnatal exposures markedly reduced white matter. 65,66 Nucleus accumbens dysfunction in ASD has been related to social and repetitive behavior deficits and response inhibition 100,101,108-110 and to altered self-control in ADHD. 111,112 While ASD etiology remains unknown, inflammation is considered key, and biomarkers include altered trans-sulfuration pathways and increased oxidative stress. 72,113-118
Table 1.
| ASD | PN CAPs | |
|---|---|---|
| Male-biased | ✓ | ✓ |
| Ventriculomegaly | ✓ | ✓ |
| Reductions in white matter | ✓ | ✓ |
| Reduction in Corpus Callosum | ✓ | ✓ |
| Interhemispheric disconnectivity | ✓ | ✓ |
| Persistent inflammation | ✓ | ✓ |
| Excitatory/inhibitory imbalance | ✓ | ✓ |
| Oxidative stress | ✓ | ✓ |
| Neuronal cell death | ✓ | ✓ |
It is important to recognize, however, that many of the phenotypic features and hypothesized mechanisms of ASD are also seen in other neurodevelopmental disorders 36-41. ASD and early childhood onset schizophrenia 42-45 share abnormalities of glutamatergic systems, 46-48 inflammatory mechanisms 49 and neuropathological features, including ventriculomegaly and disconnectivity. 50-52 Attention deficit hyperactivity disorder (ADHD) occurs at higher rates in children with ASD. 53 Both involve increases in impulsive behavior and are considered disorders of hemispheric disconnectivity. 37 Like ASD, ADHD is male-biased and its rates have increased significantly from 7.8% in 2003 to 9.5% in 2007 and 11.0% in 2011–2012 according to CDC. ADHD can also include ventriculomegaly, 54 and alterations in glutamatergic function, 55 brain white matter, 56 brain connectivity 57 and inflammation. 58 Thus, the neurotoxic consequences of AP seen in our experimental models may contribute risk not only for ASD, but for other neurodevelopmental disorders as well.
Olfactory Uptake of Elemental Contaminants of AP
ICP-MS analyses of brains from mice after CAPs exposures suggest that AP exposures could produce brain metal dyshomeostasis, with increases in several brain metals, e.g., Fe and S, and suggestive depletion of others. The observed increases in olfactory bulb Fe levels in males is consistent with the assertion that some component of this uptake occurred via nasal olfactory uptake after postnatal CAPs exposures. 119,120 Similarly, increases in brain Fe were seen in cerebellum of both sexes following gestational CAPs exposures.121 Nasal olfactory uptake of elemental AP particles has been demonstrated in both rats and concluded from studies in humans, 122 including Fe, but also Mn, Cd, Ni, Hg, Al, Co, Zn, Cu, 119,122-128 across olfactory epithelial barriers in the nasal passages followed by transport to olfactory bulb, and movement to other brain regions. 129 In one study, intranasal instillation of either ferric or ferrous iron subsequently increased blood Fe levels in rats, with uptake relying on divalent metal transporter-1.119 Accumulation of ferric iron in olfactory bulb was found following inhalation exposures to iron soot (40 ug/m3 Fe oxide nanoparticles) in adult female mice. 130
Inhaled exposures to SO2 show that virtually all of it is removed from air by the nose. 131 Early studies indicate that subsequently, its derivatives enter the blood and dissociates to form the derivatives bisulfite and sulfite, 132 both of which have been found in hippocampal slices from rat brain. 133
With olfactory nerve uptake, elements bypass the blood brain barrier such that increased levels of trace elements in brain may not be reflected in peripheral markers. In addition, sensory nerves in the upper and lower respiratory tract can translocate particles 134 that reach e.g., the trigeminal ganglion or the vagal nerve. 69,71,135,136 As Fe regulatory proteins 1 and 2, and Fe transporters such as the transferrin receptor and divalent metal transporter-1, critical to tight brain regulation of Fe levels, are not fully expressed until postnatal days 15–20 in rodents, 137,138 an extended window is open during which excess Fe or S can reach and directly influence brain development. Similarly, homeostatic regulation of Fe absorption appears in human infants at about 6–9 mos of age. 139
Plausibility of Elemental Contaminants of AP for the Postnatal CAPs-Induced Phenotype
Notably, reported consequences of excess brain Fe or SO2 are consistent with ASD and other neurodevelopmental disorders. Normally, Fe2+ oxidation to Fe3+ releases a free radical quenching electron, with nanoparticulate Fe3+ existing in a biomineralized solid or particle. However, excess Fe2+, i.e., the labile pool, (like other ionic species, e.g., Ce3+), has greater bioactivity than Fe3+ and produces oxidative stress and inflammation, reductions in antioxidant capacity, increased cell death markers and altered prefrontal cortex and hippocampal glutamate function. 140 Fe(III)Cl injections into rodent brain produce ventriculomegaly. 141,142 Developing brain is particularly susceptible to excess ionic Fe2+ given its immature antioxidant defense systems, low total Fe binding capacity, and low concentrations of Fe2+ binding proteins. 143 Indeed, increased non-protein bound Fe in umbilical cord blood is an early predictive marker of neonatal brain damage. 144 The uptake, transport and sequestration of extrinsic FexOy nanoparticles may be accompanied by breakdown and in vivo processing, making it ultimately critical to distinguish extrinsic FexOy from intrinsic ferritin nanoparticle formation.
In what might seem contradictory to our findings, some studies have reported reduced, rather than increased, serum Fe levels in ASD, 145-147 although this was not confirmed by a recent meta-analysis. 148 Even if such a finding was confirmed, it does not preclude a concurrent elevation of brain Fe. For example, neuroferritinopathy resulting from a mutation in the ferritin light chain gene results in elevated brain Fe and ferritin levels, but reduced serum ferritin. 149 Additionally, intranasal instillation of Fe2O3 nanoparticles to rats over 7 days increased Fe brain levels, while significantly reducing serum Fe levels. 150 Such discrepancies are also seen in neurodegenerative diseases that include elevated brain Fe, e.g., Parkinson’s disease, 151-153 suggesting a broader metal dyshomeostasis under such conditions.
Further exacerbating the potential toxicity of elevated brain Fe, studies have demonstrated its extremely slow turnover in rodent brain (half-life of ca. 9 mos), with estimates on the order of decades when extrapolated to humans; 154-156 such slow turnover may relate to the potential role of Fe in multiple neurodegenerative diseases, including Alzheimer’s and Parkinson’s disease, 88,157 given that such exposures are cumulative across the life-span.
Sulfur is critical for Fe-S cluster proteins, amino acid metabolism and brain trans-sulfuration pathways critical to glutathione (GSH) production necessary to maintain cellular redox states and scavenge free radicals. GSH activity is very low in fetal brain, reaching adult levels only at PND 14 in rat, indicating incomplete regulation of this pathway during brain development. 143,158 Alterations in the trans-sulfuration pathway adversely influence brain and behavior. 159,160 Sulfite, taken up during SO2 inhalation, 161 can auto-oxidize, as mediated by O2 or Fe3+ (the most stable such complex) to produce sulfite radicals and reactive oxygen species (ROS). 162-164 Inhaled SO2 increases levels of brain sulfite 165, hippocampal neuronal death, protein oxidation, lipid peroxidation 166, brain inflammation and oxidative damage in brain; it also alters glutamate function, and reduces GSH and glutathione peroxidase-4 (GPx4) levels. 167 These features correspond to the key role that chronic neuroinflammation is considered to play in ASD. 168 Of direct relevance to our findings, recent studies report that 1 ppb increases in SO2 in AP increase the odds ratios for ASD by 17%. 169
Elevated Brain Fe and S Suggest Potential Ferroptotic and Oxidative Stress Based Mechanisms for Postnatal CAPs Effects
Elevated brain Fe and S also suggest ferroptosis and oxidative stress mechanisms of postnatal CAPs effects. Ferroptosis is a non-apoptotic fatal combination of Fe toxicity, antioxidant depletion due to disruption of glutathione peroxidase-4, i.e., trans-sulfuration, and lipid peroxidation-induced membrane damage. 88 It is negatively regulated by glutathione, a product of the trans-sulfuration pathway, making S levels critical. An inverse relationship between Fe overload and glutathione, hydrogen peroxide and reactive oxygen species are well documented. 89-91 Thus, high brain Fe, and low GSH or GPx4 would facilitate ferroptosis, as proposed in Figure 1. These mechanisms would be consistent with our observations of postnatal CAPs-induced increases in NAc neuronal cell death that were not revealed by caspase staining (Fig. 11), as well as the increases in levels of serum oxidized GSH (Fig. 9). While ferroptosis is seen in multiple neurodegenerative diseases, 86 its role in neurodevelopmental disorders has not been investigated, although it is seen in periventricular leukomalacia of prematurity, i.e., brain white matter injury arising from free radical damage to oligodendrocytes. 87,170
Male Vulnerability to Postnatal CAPs Exposures and Heterogeneity of Neurodevelopmental Disorders
Although numerous different and perhaps even multiple mechanisms could account for the enhanced vulnerability of the male brain to postnatal CAPs exposures, one attractive hypothesis relates to differences in the trajectory of colonizing activated brain microglia. Specifically, males have both a greater number of, as well as more activated (amoeboid) microglia than females in early postnatal development, 171 which could impact early neurogenesis and oligodendrogenesis and late cell toxicity. Additionally, it is interesting to consider that differences not only in the timing but also in the components of AP exposure during development could contribute to the heterogeneity and sub-groups of ASD 59 or other neurodevelopmental disorders.
But Our Air is Getting Cleaner?
One seeming inconsistency relates to the fact that U.S. air quality has improved, whereas rates of ASD have continued to increase. AP health effects are related to particulate matter (PM) size, designated as PM10 (coarse, <10μm), PM2.5 (fine, <2.5μm), or PM0.1 (ultrafine:UFPs; <100nm). UFPs, being the subject of our studies, and the same size as engineered nanoparticles, are considered the most reactive component of AP, 20 with greater surface area/mass for adsorption. Levels of PM10 and PM2.5, but not UFPs, are regulated by EPA. While it might be anticipated that UFP levels would decline with PM2.5 regulation, this has not necessarily been the case, with several studies reporting no correlations between levels of PM2.5 and UFPs. 172-176 UFPs are part of PM2.5 and PM10, due to the fact that UFPs agglomerate rapidly into the PM2.5 mode 177 and PM10 mode: as larger agglomerated particles they have a high deposition efficiency on nasal mucosa, where de-agglomeration occurs on epithelial lining fluid so that neuronal uptake and CNS translocation takes place (olfactory and trigeminal pathways). Further understanding of how ultrafine particulate matter, and its most toxic components, alter sex-differentiated brain development in children, and alters risk for neurobehavioral disorders, is critical for public health protection.
Limitations of Animal Models for Human Risk and Research Needs
Of course rodent models have limitations in regard to characterizing human risk. Among these is the fact that rodents are altricial and thus the 3rd trimester human equivalent of brain development occurs postnatally. Therefore, rodents are nose-breathing rather than being exposed in utero during this period. There are also differences in the anatomy of the nasal cavity between rodents and humans that can influence extent of deposition and thus transfer of particulates to brain via olfactory routes. 178-180
To date, our studies have only begun to examine the neurotoxicity of particulate AP on brain development, but they have raised innumerable research questions that will be critical to ultimately understanding the impacts and consequences of AP. Our studies have focused primarily on white matter to date, leaving many open questions about developmental AP exposures and changes in gray matter as well as susceptibility of various brain regions. In addition, more complete characterization of effects during different windows of development will be required and would be assisted by a more complete assessment of the trajectory of effects across time; such information will also be critical to the ultimate understanding of the mechanisms by which neuropathological consequences occur. For example, with gestational CAPs, we see a correlation between increased brain Fe and hypermyelination in females and a premature maturationals shift in oligodendrocytes, the myelinating cell of the central nervous system and suggest an impact on oligodendrocyte precursor cells. 61
Certainly interventions would be desirable. In that regard, the elevation of brain metals always raises the suggestion by some to employ chelating agents that would bind metals and enhance their excretion. As noted by others, 181 such approaches are problematic for multiple reasons, including the complexity of brain metal homeostaisis and its differences within different cellular components, and the lack of specificity of most chelating agents. Other approaches may be devised following a more complete understanding of the dynamics of particle chemistry and tissue interactions in vivo, i.e., their in vivo bioprocessing. Aside from primary prevention, an understanding of the specific components of AP that contribute to risk could be used to alter regulatory policies to minimize exposures. For example, while levels of Pb in air are currently regulated by EPA, should this be extended to other metals?
Acknowledgements and Resource Sharing
Supported in part by NIH Grants P30 ES001247 and R01 ES025541. Data will be made available to any qualified individuals within the scientific community to the extent that resources are possible to accommodate the nature of the request.
Footnotes
Conflicts of Interest
The authors author(s) declare that they have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- 1.Sunyer J, Dadvand P. Pre-natal brain development as a target for urban air pollution. Basic & Clinical Pharmacology & Toxicology. 2019;0(0). [DOI] [PubMed] [Google Scholar]
- 2.de Prado Bert P, Mercader EMH, Pujol J, Sunyer J, Mortamais M. The Effects of Air Pollution on the Brain: a Review of Studies Interfacing Environmental Epidemiology and Neuroimaging. Current environmental health reports. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sram RJ, Veleminsky M Jr., Veleminsky M Sr., Stejskalova J The impact of air pollution to central nervous system in children and adults. Neuro Endocrinol Lett. 2017;38(6):389–396. [PubMed] [Google Scholar]
- 4.Palacios N Air pollution and Parkinson’s disease - evidence and future directions. Rev Environ Health. 2017. [DOI] [PubMed] [Google Scholar]
- 5.Jayaraj RL, Rodriguez EA, Wang Y, Block ML. Outdoor Ambient Air Pollution and Neurodegenerative Diseases: the Neuroinflammation Hypothesis. Current environmental health reports. 2017;4(2):166–179. [DOI] [PubMed] [Google Scholar]
- 6.Costa LG, Chang YC, Cole TB. Developmental Neurotoxicity of Traffic-Related Air Pollution: Focus on Autism. Current environmental health reports. 2017;4(2):156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu X, Ha SU, Basnet R. A Review of Epidemiological Research on Adverse Neurological Effects of Exposure to Ambient Air Pollution. Front Public Health. 2016;4:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Power MC, Adar SD, Yanosky JD, Weuve J. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: A systematic review of epidemiologic research. Neurotoxicology. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clifford A, Lang L, Chen R, Anstey KJ, Seaton A. Exposure to air pollution and cognitive functioning across the life course--A systematic literature review. Environ Res. 2016;147:383–398. [DOI] [PubMed] [Google Scholar]
- 10.Suades-Gonzalez E, Gascon M, Guxens M, Sunyer J. Air Pollution and Neuropsychological Development: A Review of the Latest Evidence. Endocrinology. 2015:en20151403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilker EH, Preis SR, Beiser AS, et al. Long-term exposure to fine particulate matter, residential proximity to major roads and measures of brain structure. Stroke. 2015;46(5):1161–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pujol J, Martinez-Vilavella G, Macia D, et al. Traffic pollution exposure is associated with altered brain connectivity in school children. NeuroImage. 2016;129:175–184. [DOI] [PubMed] [Google Scholar]
- 13.Pujol J, Fenoll R, Macia D, et al. Airborne copper exposure in school environments associated with poorer motor performance and altered basal ganglia. Brain and behavior. 2016;6(6):e00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Power MC, Lamichhane AP, Liao D, et al. The Association of Long-Term Exposure to Particulate Matter Air Pollution with Brain MRI Findings: The ARIC Study. Environ Health Perspect. 2018;126(2):027009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen JC, Wang X, Wellenius GA, et al. Ambient air pollution and neurotoxicity on brain structure: Evidence from women’s health initiative memory study. Annals of neurology. 2015;78(3):466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alemany S, Vilor-Tejedor N, Garcia-Esteban R, et al. Traffic-Related Air Pollution, APOEepsilon4 Status, and Neurodevelopmental Outcomes among School Children Enrolled in the BREATHE Project (Catalonia, Spain). Environ Health Perspect. 2018;126(8):087001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cory-Slechta DA, Allen JL, Conrad K, Marvin E, Sobolewski M. Developmental exposure to low level ambient ultrafine particle air pollution and cognitive dysfunction. Neurotoxicology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque P. Neurotoxicants are in the air: convergence of human, animal, and in vitro studies on the effects of air pollution on the brain. Biomed Res Int. 2014;2014:736385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heusinkveld HJ, Wahle T, Campbell A, et al. Neurodegenerative and neurological disorders by small inhaled particles. Neurotoxicology. 2016;56:94–106. [DOI] [PubMed] [Google Scholar]
- 20.Lin CC, Chen SJ, Huang KL, Hwang WI, Chang-Chien GP, Lin WY. Characteristics of metals in nano/ultrafine/fine/coarse particles collected beside a heavily trafficked road. Environ Sci Technol. 2005;39(21):8113–8122. [DOI] [PubMed] [Google Scholar]
- 21.Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28(5):931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007;5(1):79–94. [DOI] [PubMed] [Google Scholar]
- 23.Bandeira F, Lent R, Herculano-Houzel S. Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(33):14108–14113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur S, Nieuwenhuijsen M, Colvile R. Personal exposure of street canyon intersection users to PM2.5, ultrafine particle counts and carbon monoxide in Central London, UK. Atmospheric Environment. 2005;39(20):3629–3641. [Google Scholar]
- 25.Cattaneo A, Garramone G, Taronna M, Peruzzo C, Cavallo DM. Personal exposure to airborne ultrafine particles in the urban area of Milan. Journal of Physics: Conference Series. 2009;151:012039. [Google Scholar]
- 26.Zhang J Appendix F. Intensive Characterization of Air Pollution in Oxford Street and Hyde Park. Boston, MA: Health Effects Institute; 2009. [Google Scholar]
- 27.Allen JL, Conrad K, Oberdorster G, Johnston CJ, Sleezer B, Cory-Slechta DA. Developmental exposure to concentrated ambient particles and preference for immediate reward in mice. Environmental Health Perspectives. 2013;121(1):32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen JL, Liu X, Pelkowski S, et al. Early postnatal exposure to ultrafine particulate matter air pollution: persistent ventriculomegaly, neurochemical disruption, and glial activation preferentially in male mice. Environmental Health Perspectives. 2014;122(9):939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen JL, Liu X, Weston D, Conrad K, Oberdorster G, Cory-Slechta DA. Consequences of developmental exposure to concentrated ambient ultrafine particle air pollution combined with the adult paraquat and maneb model of the Parkinson’s disease phenotype in male mice. Neurotoxicology. 2014;41:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen JL, Liu X, Weston D, et al. Developmental exposure to concentrated ambient ultrafine particulate matter air pollution in mice results in persistent and sex-dependent behavioral neurotoxicity and glial activation. Toxicological Sciences. 2014;140(1):160–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen JL, Oberdorster G, Morris-Schaffer K, et al. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: Parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris-Schaffer K, Sobolewski M, Allen JL, et al. Effect of neonatal hyperoxia followed by concentrated ambient ultrafine particle exposure on cumulative learning in C57Bl/6J mice. Neurotoxicology. 2018;67:234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris-Schaffer K, Sobolewski M, Welle K, et al. Cognitive flexibility deficits in male mice exposed to neonatal hyperoxia followed by concentrated ambient ultrafine particles. Neurotoxicol Teratol. 2018;70:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen JL, Oberdorster G, Morris-Schaffer K, et al. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: Parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology. 2017;59:140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sobolewski M, Anderson T, Conrad K, et al. Developmental exposures to ultrafine particle air pollution reduces early testosterone levels and adult male social novelty preference: Risk for children’s sex-biased neurobehavioral disorders. Neurotoxicology. 2018;68:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamshere ML, Stergiakouli E, Langley K, et al. Shared polygenic contribution between childhood attention-deficit hyperactivity disorder and adult schizophrenia. Br J Psychiatry. 2013;203(2):107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kern JK, Geier DA, King PG, Sykes LK, Mehta JA, Geier MR. Shared Brain Connectivity Issues, Symptoms, and Comorbidities in Autism Spectrum Disorder, Attention Deficit/Hyperactivity Disorder, and Tourette Syndrome. Brain Connect. 2015;5(6):321–335. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell RH, Goldstein BI. Inflammation in children and adolescents with neuropsychiatric disorders: a systematic review. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(3):274–296. [DOI] [PubMed] [Google Scholar]
- 39.Paul LK. Developmental malformation of the corpus callosum: a review of typical callosal development and examples of developmental disorders with callosal involvement. Journal of neurodevelopmental disorders. 2011;3(1):3–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rommelse NN, Geurts HM, Franke B, Buitelaar JK, Hartman CA. A review on cognitive and brain endophenotypes that may be common in autism spectrum disorder and attention-deficit/hyperactivity disorder and facilitate the search for pleiotropic genes. Neurosci Biobehav Rev. 2011;35(6):1363–1396. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi Y, Yu Z, Sakai M, Tomita H. Linking Activation of Microglia and Peripheral Monocytic Cells to the Pathophysiology of Psychiatric Disorders. Front Cell Neurosci. 2016;10:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheung C, Yu K, Fung G, et al. Autistic disorders and schizophrenia: related or remote? An anatomical likelihood estimation. PloS one. 2010;5(8):e12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.King BH, Lord C. Is schizophrenia on the autism spectrum? Brain research. 2011;1380:34–41. [DOI] [PubMed] [Google Scholar]
- 44.Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatric research. 2011;69(5 Pt 2):26R–33R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rapoport J, Chavez A, Greenstein D, Addington A, Gogtay N. Autism spectrum disorders and childhood-onset schizophrenia: clinical and biological contributions to a relation revisited. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(1):10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown MS, Singel D, Hepburn S, Rojas DC. Increased glutamate concentration in the auditory cortex of persons with autism and first-degree relatives: a (1)H-MRS study. Autism Res. 2013;6(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimmura C, Suzuki K, Iwata Y, et al. Enzymes in the glutamate-glutamine cycle in the anterior cingulate cortex in postmortem brain of subjects with autism. Molecular autism. 2013;4(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sodhi MS, Sanders-Bush E. Serotonin and brain development. International review of neurobiology. 2004;59:111–174. [DOI] [PubMed] [Google Scholar]
- 49.van Rees GF, Lago SG, Cox DA, et al. Evidence of microglial activation following exposure to serum from first-onset drug-naive schizophrenia patients. Brain Behav Immun. 2018;67:364–373. [DOI] [PubMed] [Google Scholar]
- 50.Palmen SJ, Hulshoff Pol HE, Kemner C, et al. Increased gray-matter volume in medication-naive high-functioning children with autism spectrum disorder. Psychological medicine. 2005;35(4):561–570. [DOI] [PubMed] [Google Scholar]
- 51.Sanfilipo M, Lafargue T, Arena L, et al. Fine volumetric analysis of the cerebral ventricular system in schizophrenia: further evidence for multifocal mild to moderate enlargement. Schizophrenia bulletin. 2000;26(1):201–216. [DOI] [PubMed] [Google Scholar]
- 52.Shen MD, Nordahl CW, Young GS, et al. Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain : a journal of neurology. 2013;136(Pt 9):2825–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taurines R, Schwenck C, Westerwald E, Sachse M, Siniatchkin M, Freitag C. ADHD and autism: differential diagnosis or overlapping traits? A selective review. Atten Defic Hyperact Disord. 2012;4(3):115–139. [DOI] [PubMed] [Google Scholar]
- 54.Ball JD, Abuhamad AZ, Mason JL, Burket J, Katz E, Deutsch SI. Clinical outcomes of mild isolated cerebral ventriculomegaly in the presence of other neurodevelopmental risk factors. J Ultrasound Med. 2013;32(11):1933–1938. [DOI] [PubMed] [Google Scholar]
- 55.Bauer J, Werner A, Kohl W, et al. Hyperactivity and impulsivity in adult attention-deficit/hyperactivity disorder is related to glutamatergic dysfunction in the anterior cingulate cortex. World J Biol Psychiatry. 2016:1–9. [DOI] [PubMed] [Google Scholar]
- 56.Aoki Y, Yoncheva YN, Chen B, et al. Association of White Matter Structure With Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder. JAMA Psychiatry. 2017;74(11):1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bos DJ, Oranje B, Achterberg M, et al. Structural and functional connectivity in children and adolescents with and without attention deficit/hyperactivity disorder. J Child Psychol Psychiatry. 2017;58(7):810–818. [DOI] [PubMed] [Google Scholar]
- 58.Donfrancesco R, Nativio P, Borrelli E, et al. Serum cytokines in paediatric neuropsychiatric syndromes: focus on Attention Deficit Hyperactivity Disorder. Minerva Pediatr. 2016. [DOI] [PubMed] [Google Scholar]
- 59.Cholemkery H, Medda J, Lempp T, Freitag CM. Classifying Autism Spectrum Disorders by ADI-R: Subtypes or Severity Gradient? J Autism Dev Disord. 2016;46(7):2327–2339. [DOI] [PubMed] [Google Scholar]
- 60.Klocke C, Allen JL, Sobolewski M, et al. Neuropathological Consequences of Gestational Exposure to Concentrated Ambient Fine and Ultrafine Particles in the Mouse. Toxicological sciences : an official journal of the Society of Toxicology. 2017;156(2):492–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klocke C, Allen JL, Sobolewski M, Blum JL, Zelikoff JT, Cory-Slechta DA. Exposure to fine and ultrafine particulate matter during gestation alters postnatal oligodendrocyte maturation, proliferation capacity, and myelination. Neurotoxicology. 2018;65:196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klocke C, Sherina V, Graham UM, et al. Enhanced cerebellar myelination with concomitant iron elevation and ultrastructural irregularities following prenatal exposure to ambient particulate matter in the mouse. Inhal Toxicol. 2018:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klocke C, Allen JL, Sobolewski M, Blum JL, Zelikoff JT, Cory-Slechta DA. Exposure to fine and ultrafine particulate matter during gestation alters postnatal oligodendrocyte maturation, proliferation capacity, and myelination. Neurotoxicology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levman J, Vasung L, MacDonald P, et al. Regional volumetric abnormalities in pediatric autism revealed by structural magnetic resonance imaging. International Journal of Developmental Neuroscience. 2018;71:34–45. [DOI] [PubMed] [Google Scholar]
- 65.Ouyang M, Cheng H, Mishra V, et al. Atypical age-dependent effects of autism on white matter microstructure in children of 2–7 years. Hum Brain Mapp. 2016;37(2):819–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lange N, Travers BG, Bigler ED, et al. Longitudinal volumetric brain changes in autism spectrum disorder ages 6–35 years. Autism Res. 2015;8(1):82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morris-Schaffer K, Merrill A, Jew K, et al. Effects of neonatal inhalation exposure to ultrafine carbon particles on pathology and behavioral outcomes in C57BL/6J mice. Particle and fibre toxicology. 2019;16(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morris-Schaffer K, Merrill A, Jew K, et al. Effects of neonatal inhalation exposure to ultrafine carbon particles on pathology and behavioral outcomes in C57BL/6J mice. Particle and fibre toxicology. 2019;16(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Y, Wang C, Zong S, et al. The Trigeminal Pathway Dominates the Nose-to-Brain Transportation of Intact Polymeric Nanoparticles: Evidence from Aggregation-Caused Quenching Probes. J Biomed Nanotechnol. 2019;15(4):686–702. [DOI] [PubMed] [Google Scholar]
- 70.Lochhead JJ, Wolak DJ, Pizzo ME, Thorne RG. Rapid transport within cerebral perivascular spaces underlies widespread tracer distribution in the brain after intranasal administration. J Cereb Blood Flow Metab. 2015;35(3):371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kozlovskaya L, Abou-Kaoud M, Stepensky D. Quantitative analysis of drug delivery to the brain via nasal route. J Control Release. 2014;189:133–140. [DOI] [PubMed] [Google Scholar]
- 72.Bjorklund G, Meguid NA, El-Ansary A, et al. Diagnostic and Severity-Tracking Biomarkers for Autism Spectrum Disorder. J Mol Neurosci. 2018;66(4):492–511. [DOI] [PubMed] [Google Scholar]
- 73.Leffa DT, Torres ILS, Rohde LA. A Review on the Role of Inflammation in Attention-Deficit/Hyperactivity Disorder. Neuroimmunomodulation. 2018;25(5–6):328–333. [DOI] [PubMed] [Google Scholar]
- 74.Fuentes-Albero M, Cauli O. Homocysteine Levels in Autism Spectrum Disorder: A Clinical Update. Endocr Metab Immune Disord Drug Targets. 2018;18(4):289–296. [DOI] [PubMed] [Google Scholar]
- 75.Prata J, Santos SG, Almeida MI, Coelho R, Barbosa MA. Bridging Autism Spectrum Disorders and Schizophrenia through inflammation and biomarkers - pre-clinical and clinical investigations. J Neuroinflammation. 2017;14(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Al-Yafee YA, Al-Ayadhi LY, Haq SH, El-Ansary AK. Novel metabolic biomarkers related to sulfur-dependent detoxification pathways in autistic patients of Saudi Arabia. BMC Neurol. 2011;11:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.El-Ansary A, Al-Ayadhi L. Neuroinflammation in autism spectrum disorders. J Neuroinflammation. 2012;9:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.El-Ansary A, Al-Ayadhi L. GABAergic/glutamatergic imbalance relative to excessive neuroinflammation in autism spectrum disorders. J Neuroinflammation. 2014;11:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen X-C, Chow JC, Ward TJ, et al. Estimation of personal exposure to fine particles (PM2.5) of ambient origin for healthy adults in Hong Kong. Science of The Total Environment. 2019;654:514–524. [DOI] [PubMed] [Google Scholar]
- 80.Webb SM. The MicroAnalysis Toolkit: X-ray Fluorescence Image Processing Software. AIP Conference Proceedings. 2011;1365:196–199. [Google Scholar]
- 81.Montgomery SL, Narrow WC, Mastrangelo MA, Olschowka JA, O’Banion MK, Bowers WJ. Chronic Neuron- and Age-Selective Down-Regulation of TNF Receptor Expression in Triple-Transgenic Alzheimer Disease Mice Leads to Significant Modulation of Amyloid- and Tau-Related Pathologies. The American Journal of Pathology. 2013;182(6):2285–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morris-Schaffer K, Sobolewski M, Welle K, et al. Cognitive flexibility deficits in male mice exposed to neonatal hyperoxia followed by concentrated ambient ultrafine particles. Neurotoxicology and Teratology. 2018;70:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cory-Slechta DA, Allen JL, Conrad K, Marvin E, Sobolewski M. Developmental exposure to low level ambient ultrafine particle air pollution and cognitive dysfunction. Neurotoxicology. 2018;69:217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bertrand RL. Iron accumulation, glutathione depletion, and lipid peroxidation must occur simultaneously during ferroptosis and are mutually amplifying events. Med Hypotheses. 2017;101:69–74. [DOI] [PubMed] [Google Scholar]
- 85.Latunde-Dada GO. Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta. 2017;1861(8):1893–1900. [DOI] [PubMed] [Google Scholar]
- 86.Masaldan S, Bush AI, Devos D, Rolland AS, Moreau C. Striking while the iron is hot: Iron metabolism and ferroptosis in neurodegeneration. Free radical biology & medicine. 2019;133:221–233. [DOI] [PubMed] [Google Scholar]
- 87.Weiland A, Wang Y, Wu W, et al. Ferroptosis and Its Role in Diverse Brain Diseases. Mol Neurobiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morris G, Berk M, Carvalho AF, Maes M, Walker AJ, Puri BK. Why should neuroscientists worry about iron? The emerging role of ferroptosis in the pathophysiology of neuroprogressive diseases. Behav Brain Res. 2018;341:154–175. [DOI] [PubMed] [Google Scholar]
- 89.Radu M, Munteanu MC, Petrache S, et al. Depletion of intracellular glutathione and increased lipid peroxidation mediate cytotoxicity of hematite nanoparticles in MRC-5 cells. Acta Biochim Pol. 2010;57(3):355–360. [PubMed] [Google Scholar]
- 90.Hohnholt MC, Dringen R. Iron-dependent formation of reactive oxygen species and glutathione depletion after accumulation of magnetic iron oxide nanoparticles by oligodendroglial cells. Journal of Nanoparticle Research. 2011;13(12):6761–6774. [Google Scholar]
- 91.Viktorinova A, Ursinyova M, Trebaticka J, Uhnakova I, Durackova Z, Masanova V. Changed Plasma Levels of Zinc and Copper to Zinc Ratio and Their Possible Associations with Parent- and Teacher-Rated Symptoms in Children with Attention-Deficit Hyperactivity Disorder. Biol Trace Elem Res. 2016;169(1):1–7. [DOI] [PubMed] [Google Scholar]
- 92.Mintz M Evolution in the Understanding of Autism Spectrum Disorder: Historical Perspective. Indian J Pediatr. 2017;84(1):44–52. [DOI] [PubMed] [Google Scholar]
- 93.Goldson E Advances in Autism-2016. Adv Pediatr. 2016;63(1):333–355. [DOI] [PubMed] [Google Scholar]
- 94.Turner AH, Greenspan KS, van Erp TGM. Pallidum and lateral ventricle volume enlargement in autism spectrum disorder. Psychiatry Res. 2016;252:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Damasio H, Maurer RG, Damasio AR, Chui HC. Computerized tomographic scan findings in patients with autistic behavior. Archives of Neurology. 1980;37(8):504–510. [DOI] [PubMed] [Google Scholar]
- 96.Lyall AE, Woolson S, Wolfe HM, et al. Prenatal isolated mild ventriculomegaly is associated with persistent ventricle enlargement at ages 1 and 2. Early Hum Dev. 2012;88(8):691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Riva D, Bulgheroni S, Aquino D, Di Salle F, Savoiardo M, Erbetta A. Basal Forebrain Involvement in Low-Functioning Autistic Children: A Voxel-Based Morphometry Study. American Journal of Neuroradiology. 2011;32(8):1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wegiel J, Flory M, Kuchna I, et al. Brain-region–specific alterations of the trajectories of neuronal volume growth throughout the lifespan in autism. Acta Neuropathologica Communications. 2014;2(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wegiel J, Flory M, Kuchna I, et al. Stereological study of the neuronal number and volume of 38 brain subdivisions of subjects diagnosed with autism reveals significant alterations restricted to the striatum, amygdala and cerebellum. Acta Neuropathologica Communications. 2014;2(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.D’Cruz AM, Mosconi MW, Ragozzino ME, Cook EH, Sweeney JA. Alterations in the functional neural circuitry supporting flexible choice behavior in autism spectrum disorders. Translational Psychiatry. 2016;6:e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shafritz KM, Bregman JD, Ikuta T, Szeszko PR. Neural systems mediating decision-making and response inhibition for social and nonsocial stimuli in autism. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2015;60:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Current Opinion in Neurobiology. 2007;17(1):103–111. [DOI] [PubMed] [Google Scholar]
- 103.Frith C Is autism a disconnection disorder? Lancet Neurol. 2004;3(10):577. [DOI] [PubMed] [Google Scholar]
- 104.Blackmon K, Ben-Avi E, Wang X, et al. Periventricular white matter abnormalities and restricted repetitive behavior in autism spectrum disorder. NeuroImage: Clinical. 2016;10:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pardo CA, Vargas DL, Zimmerman AW. Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry. 2005;17(6):485–495. [DOI] [PubMed] [Google Scholar]
- 106.Zimmerman AW, Jyonouchi H, Comi AM, et al. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatric Neurology. 2005;33(3):195–201. [DOI] [PubMed] [Google Scholar]
- 107.Rojas DC. The role of glutamate and its receptors in autism and the use of glutamate receptor antagonists in treatment. Journal of Neural Transmission. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Akkermans SEA, Rheinheimer N, Bruchhage MMK, et al. Frontostriatal functional connectivity correlates with repetitive behaviour across autism spectrum disorder and obsessive-compulsive disorder. Psychol Med. 2018:1–9. [DOI] [PubMed] [Google Scholar]
- 109.Supekar K, Kochalka J, Schaer M, et al. Deficits in mesolimbic reward pathway underlie social interaction impairments in children with autism. Brain. 2018;141(9):2795–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shafritz KM, Bregman JD, Ikuta T, Szeszko PR. Neural systems mediating decision-making and response inhibition for social and nonsocial stimuli in autism. Prog Neuropsychopharmacol Biol Psychiatry. 2015;60:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.von Rhein D, Cools R, Zwiers MP, et al. Increased neural responses to reward in adolescents and young adults with attention-deficit/hyperactivity disorder and their unaffected siblings. J Am Acad Child Adolesc Psychiatry. 2015;54(5):394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Costa Dias TG, Wilson VB, Bathula DR, et al. Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol. 2013;23(1):33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Frye RE, Melnyk S, Fuchs G, et al. Effectiveness of methylcobalamin and folinic Acid treatment on adaptive behavior in children with autistic disorder is related to glutathione redox status. Autism Res Treat. 2013;2013:609705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chauhan A, Audhya T, Chauhan V. Brain region-specific glutathione redox imbalance in autism. Neurochem Res. 2012;37(8):1681–1689. [DOI] [PubMed] [Google Scholar]
- 115.Frye RE, James SJ. Metabolic pathology of autism in relation to redox metabolism. Biomark Med. 2014;8(3):321–330. [DOI] [PubMed] [Google Scholar]
- 116.Puig-Alcaraz C, Fuentes-Albero M, Calderon J, Garrote D, Cauli O. Increased homocysteine levels correlate with the communication deficit in children with autism spectrum disorder. Psychiatry Res. 2015;229(3):1031–1037. [DOI] [PubMed] [Google Scholar]
- 117.Nagiah S, Phulukdaree A, Naidoo D, et al. Oxidative stress and air pollution exposure during pregnancy: A molecular assessment. Hum Exp Toxicol. 2015;34(8):838–847. [DOI] [PubMed] [Google Scholar]
- 118.Li Y, Nie J, Beyea J, et al. Exposure to traffic emissions: associations with biomarkers of antioxidant status and oxidative damage. Environ Res. 2013;121:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ruvin Kumara VM, Wessling-Resnick M. Olfactory ferric and ferrous iron absorption in iron-deficient rats. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2012;302(12):L1280–L1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang B, Feng WY, Wang M, et al. Transport of intranasally instilled fine Fe2O3 particles into the brain: micro-distribution, chemical states, and histopathological observation. Biol Trace Elem Res. 2007;118(3):233–243. [DOI] [PubMed] [Google Scholar]
- 121.Klocke C, Sherina V, Graham UM, et al. Enhanced cerebellar myelination with concomitant iron elevation and ultrastructural irregularities following prenatal exposure to ambient particulate matter in the mouse. Inhalation Toxicology. 2018;30(9–10):381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tjalve H, Henriksson J. Uptake of metals in the brain via olfactory pathways. Neurotoxicology. 1999;20(2–3):181–195. [PubMed] [Google Scholar]
- 123.Maher BA, Ahmed IA, Karloukovski V, et al. Magnetite pollution nanoparticles in the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(39):10797–10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gonzalez-Maciel A, Reynoso-Robles R, Torres-Jardon R, Mukherjee PS, Calderon-Garciduenas L. Combustion-Derived Nanoparticles in Key Brain Target Cells and Organelles in Young Urbanites: Culprit Hidden in Plain Sight in Alzheimer’s Disease Development. J Alzheimers Dis. 2017;59(1):189–208. [DOI] [PubMed] [Google Scholar]
- 125.Calderón-Garcidueñas L, Gónzalez-Maciel A, Reynoso-Robles R, et al. Hallmarks of Alzheimer disease are evolving relentlessly in Metropolitan Mexico City infants, children and young adults. APOE4 carriers have higher suicide risk and higher odds of reaching NFT stage V at ≤ 40 years of age. Environmental Research. 2018;164:475–487. [DOI] [PubMed] [Google Scholar]
- 126.Persson E, Henriksson J, Tjalve H. Uptake of cobalt from the nasal mucosa into the brain via olfactory pathways in rats. Toxicol Lett. 2003;145(1):19–27. [DOI] [PubMed] [Google Scholar]
- 127.Ibanez C, Suhard D, Tessier C, et al. Intranasal exposure to uranium results in direct transfer to the brain along olfactory nerve bundles. Neuropathology and Applied Neurobiology. 2014;40(4):477–488. [DOI] [PubMed] [Google Scholar]
- 128.Sunderman FW Jr. Nasal toxicity, carcinogenicity, and olfactory uptake of metals. Ann Clin Lab Sci. 2001;31(1):3–24. [PubMed] [Google Scholar]
- 129.Oberdörster G, Sharp Z, Atudorei V, et al. Translocation of inhaled ultrafine particles to the brain. Inhalation toxicology. 2004;16(6–7):437–445. [DOI] [PubMed] [Google Scholar]
- 130.Hopkins LE, Laing EA, Peake JL, et al. Repeated Iron-Soot Exposure and Nose-to-brain Transport of Inhaled Ultrafine Particles. Toxicol Pathol. 2018;46(1):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Speizer FE, Frank NR. The uptake and release of SO2 by the human nose. Arch Environ Health. 1966;12(6):725–728. [DOI] [PubMed] [Google Scholar]
- 132.Du Z, Meng Z. Modulation of sodium currents in rat dorsal root ganglion neurons by sulfur dioxide derivatives. Brain Res. 2004;1010(1–2):127–133. [DOI] [PubMed] [Google Scholar]
- 133.Li G, Chen Y, Wang J, et al. Direct imaging of biological sulfur dioxide derivatives in vivo using a two-photon phosphorescent probe. Biomaterials. 2015;63:128–136. [DOI] [PubMed] [Google Scholar]
- 134.Hunter DD, Dey RD. Identification and neuropeptide content of trigeminal neurons innervating the rat nasal epithelium. Neuroscience. 1998;83(2):591–599. [DOI] [PubMed] [Google Scholar]
- 135.Oberdorster G, Sharp Z, Atudorei V, et al. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16(6–7):437–445. [DOI] [PubMed] [Google Scholar]
- 136.Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64(7):614–628. [DOI] [PubMed] [Google Scholar]
- 137.Moos T, Morgan EH. A morphological study of the developmentally regulated transport of iron into the brain. Dev Neurosci. 2002;24(2–3):99–105. [DOI] [PubMed] [Google Scholar]
- 138.Siddappa AJ, Rao RB, Wobken JD, Leibold EA, Connor JR, Georgieff MK. Developmental changes in the expression of iron regulatory proteins and iron transport proteins in the perinatal rat brain. J Neurosci Res. 2002;68(6):761–775. [DOI] [PubMed] [Google Scholar]
- 139.Lonnerdal B, Georgieff MK, Hernell O. Developmental Physiology of Iron Absorption, Homeostasis, and Metabolism in the Healthy Term Infant. J Pediatr. 2015;167(4 Suppl):S8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Han M, Kim J. Effect of dietary iron loading on recognition memory in growing rats. PLoS One. 2015;10(3):e0120609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Strahle JM, Garton T, Bazzi AA, et al. Role of hemoglobin and iron in hydrocephalus after neonatal intraventricular hemorrhage. Neurosurgery. 2014;75(6):696–705; discussion 706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gao C, Du H, Hua Y, Keep RF, Strahle J, Xi G. Role of red blood cell lysis and iron in hydrocephalus after intraventricular hemorrhage. J Cereb Blood Flow Metab. 2014;34(6):1070–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cheng C, Juul S. Iron balance in the neonate. NeoReviews. 2011;12:148–157. [Google Scholar]
- 144.Buonocore G, Perrone S, Longini M, et al. Non protein bound iron as early predictive marker of neonatal brain damage. Brain. 2003;126(Pt 5):1224–1230. [DOI] [PubMed] [Google Scholar]
- 145.Schmidt RJ, Tancredi DJ, Krakowiak P, Hansen RL, Ozonoff S. Maternal intake of supplemental iron and risk of autism spectrum disorder. American journal of epidemiology. 2014;180(9):890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sidrak S, Yoong T, Woolfenden S. Iron deficiency in children with global developmental delay and autism spectrum disorder. J Paediatr Child Health. 2014;50(5):356–361. [DOI] [PubMed] [Google Scholar]
- 147.Chen M-H, Su T-P, Chen Y-S, et al. Association between psychiatric disorders and iron deficiency anemia among children and adolescents: a nationwide population-based study. BMC Psychiatry. 2013;13(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tseng P-T, Cheng Y-S, Chen Y-W, et al. Peripheral iron levels in children with autism spectrum disorders vs controls: a systematic review and meta-analysis. Nutrition Research. 2018;50:44–52. [DOI] [PubMed] [Google Scholar]
- 149.Ke Y, Ming Qian Z. Iron misregulation in the brain: a primary cause of neurodegenerative disorders. Lancet Neurol. 2003;2(4):246–253. [DOI] [PubMed] [Google Scholar]
- 150.Askri D, Ouni S, Galai S, et al. Intranasal instillation of iron oxide nanoparticles induces inflammation and perturbation of trace elements and neurotransmitters, but not behavioral impairment in rats. Environ Sci Pollut Res Int. 2018. [DOI] [PubMed] [Google Scholar]
- 151.Costa-Mallen P, Gatenby C, Friend S, et al. Brain iron concentrations in regions of interest and relation with serum iron levels in Parkinson disease. Journal of the Neurological Sciences. 2017;378:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jin L, Wang J, Zhao L, et al. Decreased serum ceruloplasmin levels characteristically aggravate nigral iron deposition in Parkinson’s disease. Brain. 2011;134(1):50–58. [DOI] [PubMed] [Google Scholar]
- 153.Logroscino G, Marder K, Graziano J, et al. Altered systemic iron metabolism in Parkinson’s disease. Neurology. 1997;49(3):714–717. [DOI] [PubMed] [Google Scholar]
- 154.Chen JH, Singh N, Tay H, Walczyk T. Imbalance of iron influx and efflux causes brain iron accumulation over time in the healthy adult rat. Metallomics. 2014;6(8):1417–1426. [DOI] [PubMed] [Google Scholar]
- 155.Banks WA, Kastin AJ, Fasold MB, Barrera CM, Augereau G. Studies of the slow bidirectional transport of iron and transferrin across the blood-brain barrier. Brain Res Bull. 1988;21(6):881–885. [DOI] [PubMed] [Google Scholar]
- 156.Dallman PR, Spirito RA. Brain iron in the rat: extremely slow turnover in normal rats may explain long-lasting effects of early iron deficiency. J Nutr. 1977;107(6):1075–1081. [DOI] [PubMed] [Google Scholar]
- 157.Apostolakis S, Kypraiou AM. Iron in neurodegenerative disorders: being in the wrong place at the wrong time? Rev Neurosci. 2017;28(8):893–911. [DOI] [PubMed] [Google Scholar]
- 158.Volpe JJ, Laster L. Transsulfuration in fetal and postnatal mammalian liver and brain. Cystathionine synthase, its relation to hormonal influences, and cystathionine. Biol Neonate. 1972;20(5):385–403. [DOI] [PubMed] [Google Scholar]
- 159.Marseglia LM, Nicotera A, Salpietro V, et al. Hyperhomocysteinemia and MTHFR polymorphisms as antenatal risk factors of white matter abnormalities in two cohorts of late preterm and full term newborns. Oxidative medicine and cellular longevity. 2015;2015:543134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Makhro AV, Mashkina AP, Solenaya OA, et al. Prenatal hyperhomocysteinemia as a model of oxidative stress of the brain. Bulletin of experimental biology and medicine. 2008;146(1):33–35. [DOI] [PubMed] [Google Scholar]
- 161.Gunnison AF. Sulphite toxicity: a critical review of in vitro and in vivo data. Food Cosmet Toxicol. 1981;19(5):667–682. [DOI] [PubMed] [Google Scholar]
- 162.Hayon E, Treinin A, Wilf J. Electronic spectra, photochemistry, and autoxidation mechanism of the sulfite-bisulfite-pyrosulfite systems. SO2-, SO3-, SO4-, and SO5- radicals. Journal of the American Chemical Society. 1972;94(1):47–57. [Google Scholar]
- 163.Eatough DJ, Major T, Ryder J, et al. THE FORMATION AND STABILITY OF SULFITE SPECIES IN AEROSOLS In: Husar RB, Lodge JP, Moore DJ, eds. Sulfur in the Atmosphere. Pergamon; 1978:263–271. [Google Scholar]
- 164.Gunnison AF, Sellakumar A, Currie D, Snyder EA. Distribution, metabolism and toxicity of inhaled sulfur dioxide and endogenously generated sulfite in the respiratory tract of normal and sulfite oxidase-deficient rats. J Toxicol Environ Health. 1987;21(1–2):141–162. [DOI] [PubMed] [Google Scholar]
- 165.Meng Z, Li R, Zhang X. Levels of sulfite in three organs from mice exposed to sulfur [corrected] dioxide. Inhal Toxicol. 2005;17(6):309–313. [DOI] [PubMed] [Google Scholar]
- 166.Sang N, Hou L, Yun Y, Li G. SO(2) inhalation induces protein oxidation, DNA-protein crosslinks and apoptosis in rat hippocampus. Ecotoxicol Environ Saf. 2009;72(3):879–884. [DOI] [PubMed] [Google Scholar]
- 167.Meng Z Oxidative damage of sulfur dioxide on various organs of mice: sulfur dioxide is a systemic oxidative damage agent. Inhal Toxicol. 2003;15(2):181–195. [DOI] [PubMed] [Google Scholar]
- 168.Petrelli F, Pucci L, Bezzi P. Astrocytes and Microglia and Their Potential Link with Autism Spectrum Disorders. Frontiers in cellular neuroscience. 2016;10:21–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jung CR, Lin YT, Hwang BF. Air pollution and newly diagnostic autism spectrum disorders: a population-based cohort study in Taiwan. PloS one. 2013;8(9):e75510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Inder T, Mocatta T, Darlow B, Spencer C, Volpe JJ, Winterbourn C. Elevated Free Radical Products in the Cerebrospinal Fluid of VLBW Infants with Cerebral White Matter Injury. Pediatric Research. 2002;52:213. [DOI] [PubMed] [Google Scholar]
- 171.Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. Journal of Neurochemistry. 2012;120(6):948–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ruuskanen J, Tuch T, Brink T, et al. Concentrations of ultrafine, fine and PM2.5 particles in three European cities. Atmospheric Environment. 2001;35:3729–3728. [Google Scholar]
- 173.Tuch T, Brand P, Wichmann HE, Heyder J. Variation of particle number and mass concentration in various size ranges of ambient aerosols in Eastern Germany. Atmospheric Environment. 1997;31(24):4193–4197. [Google Scholar]
- 174.Knibbs LD, de Dear RJ. Exposure to ultrafine particles and PM2.5 in four Sydney transport modes. Atmospheric Environment. 2010;44(26):3224–3227. [Google Scholar]
- 175.Ebelt S, Brauer M, Cyrys J, et al. Air quality in postunification Erfurt, East Germany: associating changes in pollutant concentrations with changes in emissions. Environ Health Perspect. 2001;109(4):325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pitz M, Kreyling WG, Hölscher B, Cyrys J, Wichmann HE, Heinrich J. Change of the ambient particle size distribution in East Germany between 1993 and 1999. Atmospheric Environment. 2001;35(25):4357–4366. [Google Scholar]
- 177.K.T. W, Charlson RJ Aerosols: Characteristics, Behavior and Measurement In: Airborne Particles. University Park Press; 1979:1–19. [Google Scholar]
- 178.Chamanza R, Wright JA. A Review of the Comparative Anatomy, Histology, Physiology and Pathology of the Nasal Cavity of Rats, Mice, Dogs and Non-human Primates. Relevance to Inhalation Toxicology and Human Health Risk Assessment. J Comp Pathol. 2015;153(4):287–314. [DOI] [PubMed] [Google Scholar]
- 179.Dong J, Shang Y, Inthavong K, et al. From the Cover: Comparative Numerical Modeling of Inhaled Nanoparticle Deposition in Human and Rat Nasal Cavities. Toxicological sciences : an official journal of the Society of Toxicology. 2016;152(2):284–296. [DOI] [PubMed] [Google Scholar]
- 180.Tian L, Shang Y, Chen R, et al. Correlation of regional deposition dosage for inhaled nanoparticles in human and rat olfactory. Particle and fibre toxicology. 2019;16(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Drew SC. The Case for Abandoning Therapeutic Chelation of Copper Ions in Alzheimer’s Disease. Frontiers in Neuroscience. 2017;11:317. [DOI] [PMC free article] [PubMed] [Google Scholar]