Abstract
Though sleep disturbance constitutes the risk factor for Alzheimer’s disease (AD), the underlying mechanism is still unclear. This study aims to explore the interaction between sleep disturbances and AD on brain function. We included 192 normal controls, 111 mild cognitive impairment (MCI), and 30 AD patients, with either poor or normal sleep (PS, NS, respectively). To explore the strength and stability of brain activity, we used static amplitude of low-frequency fluctuation (sALFF) and dynamic ALFF (dALFF) variance. Further, we examined white matter hyperintensities (WMH) and amyloid PET deposition, representing the vascular risk factor and AD-related hallmark, respectively. We observed that sleep disturbance significantly interacted with disease severity, exposing distinct effects on sALFF and dALFF variance. Interestingly, PS groups showed the dALFF variance trajectory of initially increased, then decreased and finally increased along the AD spectrum, while showing the opposite trajectory of sALFF. Further correlation analysis showed that the WMH burden correlates with dALFF variance in PS groups. Conclusively, our study suggested that sleep disturbance interacts with AD severity, expressing as effects of compensatory in MCI and de-compensatory in AD, respectively. Further, vascular impairment might act as important pathogenesis underlying the interaction effect between sleep and AD.
Subject terms: Sleep, Alzheimer's disease
Introduction
Alzheimer’s disease (AD) is the most common form of dementia, clinically characterized by progressive memory and other cognitive ability deficits. According to the 2018 World Alzheimer Report, there have been about 50 million AD patients worldwide, and this number will more than triple to 152 million by 20501. Given that cure lifestyle factors might reduce or increase the risk of developing dementia, clinicians are paying more attention to modifying the reversible high-risk factors to delay the onset and progression of AD2. Recently, sleep disturbance was implicated as a risk factor for AD and has received much attention. About 25–40% of patients with AD or mild cognitive impairment (MCI) had abnormal sleep architecture3–7. Moreover, emerging evidence suggests that sleep disturbance may precede dementia onset and exacerbate cognitive symptoms8,9. Understanding the underlying neural mechanism of the sleep disturbance interacting with AD may shed light on future clinical interventions.
Fundamental research proposed that the pathological mechanism of interaction between sleep disturbance and AD may be the increased AD pathology deposit-amyloid beta (Aβ)10,11 or vascular impairments like the blood-brain barrier (BBB) dysfunction12–14. Such vascular risk factors may always appear as white matter hyperintensities (WMH) in neuroimage15,16. One previous study found that self-reported sleep-disordered breathing is associated with larger WMH volumes, suggesting that vascular impairments may mediate the sleep problem-related cognition decline17. Additionally, previous neuroimaging studies hinted that sleep disturbance might lead to the impairments of intrinsic brain activity, involving the hippocampal-neocortical, thalamo-cortical circuits, and further lead to cognitive dysfunction in normal subjects18–24. However, there is still a lack of in vivo study that aims to tackle the question of how sleep disturbances influence brain function in AD subjects.
Amplitude of low-frequency fluctuation (ALFF), as a high-sensitive brain measure reflecting the intrinsic brain activity, can be used to explore possible mechanisms. Specifically, the dynamic ALFF (dALFF) could reflect the temporal stability of the intrinsic brain activity25 while the static ALFF (sALFF) could reflect the strength of intrinsic brain activity. The increased temporal variance (decreased stability) or decreased temporal variance (excessive stability) may occur at the different pathological states and changed cognitive functions26,27. Previous studies have used the sALFF and dALFF variance in neuropsychiatric disorders, like AD and major depression disorder, and proved their effectiveness in reflecting the brain function and further clinical symptoms28–30. Thus, combining dynamic and static intrinsic brain activity information could help understand the neural mechanism underlying the interaction between sleep disturbances and dementia in different aspects31,32.
Taken together, the goal of our study aims to explore the interactions between sleep disturbances and AD severity on brain function in vivo. We used sALFF and dALFF to reflect the strength and stability of intrinsic brain activity, respectively. Moreover, we evaluated the WMH and amyloid PET deposit data to explore the possible mechanism.
Methods and Materials
Alzheimer’s disease neuroimaging and initiative
Data used in this study were obtained from the Alzheimer’s disease Neuroimaging Initiative (ADNI) database (http://adni.loni.usc.edu). The ADNI was initially launched in 2003 (ADNI-1) by the National Institute on Aging (NIA), the Food and Drug Administration (FDA), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), and additional recruitment was made through ADNI-GO in 2009, ADNI-2 in 2010 and ADNI-3 in 2016. The primary goal of ADNI has been to identify serial magnetic resonance imaging (MRI), positron emission tomography (PET), biomarkers, and genetic characteristics that would support the early detection and tracking of AD, and improved clinical trial design. For up-to-date information, see http://www.adni-info.org. Similar information was described in a previous study33.
Study participants
The ADNI project was approved by the Institutional Review Boards of all participating institutions, and all participants signed the informed written consent (http://adni.loni.usc.edu/wp-content/themes/freshnews-dev-v2/documents/policy/ADNI_Acknowledgement_List%205-29-18.pdf). We identified 192 normal controls (NC), 111 MCI subjects and 30 AD patients from the ADNI database (Supplementary Material 1, Flowchart). Each subject underwent 3D T1 weighted structural scan, resting-state functional MRI (rsfMRI), and comprehensive neuropsychological assessments.
We defined the NC as subjects had a Clinical Dementia Rating scale (CDR) score of 0, an Mini-Mental State Examination (MMSE) between 24 and 30 (inclusive), Wechsler Memory Scale Logical Memory, WMS-LM, delay recall performance ≥9 for subjects with 16 or more years of education; ≥5 for subjects with 8–15 years of education; and ≥3 for 0–7 years of education; non clinical depression (Geriatric Depression Scale-15, GDS-15 score < 6) and absence of dementia34. Regarding MCI inclusion criteria, subjects had preserved activities of daily living, non-dementia, and objective cognitive impairment as shown on the delayed recall test of the WMS-LM as well as a CDR score of 0.535. As for AD, patients have MMSE of ≤26, CDR of ≥0.5, as well as met the NINCDS/ADRDA criteria for probable AD36. Exclusion criteria are listed below: (a) significant medical, neurological, and psychiatric illness; (b) obvious head trauma history; (c) use of non-AD-related medication known to influence cerebral function; (d) clinical depression; (e) alcohol or drug abuse. Similar criteria were described in previous studies33,37.
As an explorative investigation, we defined sleep state using medical history and night-time behavior scale in Neuropsychiatric Inventory (NPI) or a brief questionnaire form of NPI (NPI-Q), as previously described (Supplementary Material 2)4,38–40. Specifically, subjects with sleep disturbance (including sleep problems recorded in the medical history or abnormal NPI/NPI-Q (the scale score of the 9th item: night-time behavioral disturbances ≥1)) were defined as the poor sleeper (PS); by contrast, subjects with both normal medical history and NPI/NPI-Q were defined as the normal sleeper (NS).
Neuropsychological assessment
Each subjects finished comprehensive neuropsychological tests, including assessment of general mental status (MMSE) and other cognitive domains, involving episodic memory (Auditory Verbal Learning Test, AVLT; Wechsler Memory Scale Logical Memory, WMS-LM, immediate and delayed memory), attention (Trail-Making Test part A, TMT-A), visuospatial function (Clock-Drawing Test, CDT), decision-making function (Trail-Making Test part B, TMT-B), and language ability (Category verbal fluency).
MRI acquisition and pre-processing
The structural images were obtained based on 3D Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) T1W sequence, with following parameters: voxel size = 1.1 × 1.1 × 1.2 mm3; echo time (TE) = 2.98 ms; inversion time (TI) = 900 ms; repetition time (TR) = 2300 ms; 170 sagittal slices; within plane FOV = 256 × 240 mm2. The FLAIR scans were obtained using an echo-planar imaging sequence: TE = 90 ms, TR = 9000 ms. Meanwhile, the rsfMRI images were obtained using an echo-planar imaging sequence: TE = 30 ms; TR = 3000 ms; the number of slices = 48; slice thickness = 3.3 mm; spatial resolution = 3.31 × 3.31 × 3.31 mm3. According to the ADNI scanning protocol, all subjects were instructed to open their eyes, focusing on a cross, and keep at rest calmly during the scan.
Neuroimaging data preprocessing was performed using the Data Processing Assistant and Resting-State fMRI (DPARSF; Yan and Zang; www.rfmri.org/DPASFA)41 based on the platform of Statistical Parametric Mapping 12 (SPM12; www.fil.ion.ucl.ac.uk/spm). The First 5 image volumes of rsfMRI scans were discarded for the signal equilibrium and subject’s adaptation to the scanning noise41. The remaining 135 images were corrected for timing differences and head motion (Friston 24 parameters)42. The image data with more than 3 mm maximum displacement in any of the x, y, or z directions or 3° of any angular motion were discarded. Then, T1-weighted images were co-registered to the mean rsfMRI image and spatially normalized to the Montreal Neurological Institute (MNI) standard space based on rigid-body transformation, then re-sampled into 3 mm × 3 mm × 3 mm cubic voxel. Finally, scrubbing was performed to reduce motion-related artifacts by using a framewise displacement (FD) threshold of 0.5, interpolating bad time points with cubic spline43,44. To control the residual effects of motion and other non-neuronal factors, we concluded covariates, including 24 head motion parameters and signals of white matter and cerebrospinal fluid (CSF)41,42.
sALFF and dALFF variance calculation
The sALFF calculated the averaged ALFF across the scanning session and can be used to explore the strength of intrinsic brain activity. We calculated sALFF using the DPARSF software. Firstly, the time series were converted into the frequency domain using a fast Fourier transform at each voxel. Then, across 0.01–0.1 Hz, we computed and averaged the square root of the power spectrum. This averaged square root was taken as the sALFF at the given voxel45.
The dALFF was raised based on the theory that resting-state brain is a highly dynamic system on a variety of time scales31,32,46–49. Specifically, dALFF could distinguish the time course into several scales and calculate the variance value, to reflect the temporal stability of the intrinsic brain activity25. We calculated dALFF using the DynamicBC software (www.restfmri.net/forum/DynamicBC)49. The window size was set at 14 TR (42 s), and window step at 1TR based on previous studies, which suggested the window size in the range of 40 s to 100 s capturing brain dynamics reasonably50,51. The sliding-window was used for time-variant connectivity analysis. For each sliding window, the ALFF map was obtained. To study the temporal stability of intrinsic brain activity, we computed the dALFF variance. To test the reliability of the dALFF results, we repeatedly analyzed the dALFF with other window sizes (20, 26, 33 TR) (Supplementary Material 7).
Assessment of WMH and amyloid deposit
To assess the cerebral vascular risk factor, we assessed the WMH burden. For each subject, the WMH lesion map was automatically created based on the T2 FLAIR image using Lesion Segmentation Toolbox in SPM12 (www.applied-statistics.de/lst)52. The masks were then manually corrected by two experienced neuroradiologists (MMZ, HPY). The WMH volume was subsequently calculated by multiplying voxel numbers by voxel size.
Regarding the amyloid PET, we acquired the composite Aβ deposition value (i.e., SUVR, standard uptake value ratio) from the ADNI database (UCBERKELEYAV45). To reflect the local amyloid deposition, we extracted the SUVRs based on the region of interest (ROIs). Notably, not all subjects had an amyloid PET scan. Thus 122 out of 176 NS and 145 out of 157 PS with amyloid PET data were included in the subsequent neuropathological correlation analysis.
Statistical analysis
We used a Chi-squared test and analysis of variance (ANOVA) for categorical (gender) and continuous data (age, education), respectively (SPSS, version 19.0). Then, post-hoc analysis using a two-sample t-test was performed to reveal the source of ANOVA difference (significant at p < 0.05).
The interactions between sleep disturbance and disease severity on brain function (both sALFF and dALFF in a whole-brain voxel-wise way) were assessed with a 3 × 2 full factorial design, with groups (NC, MCI, and AD) and sleep state (NS and PS) as between-participant factors. Age, gender, education, and FD were employed as covariates (Supplementary Material 3, design matrix of the full factorial model). Firstly, F test was performed by setting the threshold at P < 0.001 at a voxel level, with P < 0.05 at the cluster level, corrected for multiple comparisons using the Gaussian random field (GRF) method53–55. Then, post-hoc analysis using a two-sample t-test was performed to explore the difference between groups by setting the threshold at P < 0.05. Moreover, the main effects of sleep quality, disease severity were showed in Supplementary Material 5. To explore the possible effect of GDS, we repeated our full factorial analysis by adding GDS as covariate (Supplementary Material 8).
Moreover, we investigated the associations between neuroimaging metrics (sALFF and dALFF variance in ROIs) and neuropathological as well as neuropsychological results using Pearson correlation analysis.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance by the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all participants and authorized representatives, and the study partners before any protocol-specific procedures were carried out in the ADNI study. More details in http://www.adni-info.org.
Results
Demographic and neuropsychological data
Finally, we defined six groups combining the disease severity (NC, MCI, AD) and sleep state (normal sleeper-NS, poor sleeper-PS): 123 NC with normal sleep (NC-NS); 69 NC with poor sleep (NC-PS); 39 MCI with normal sleep (MCI-NS); 72 MCI with poor sleep (MCI-PS); 14 AD with normal sleep (AD-NS); 16 AD with poor sleep (AD-PS).
We presented descriptive data as the mean ± standard deviation and percentage for continuous and dichotomous variables, respectively. All counterpart groups matched well for age and education and cognitive profile (Table 1 and Supplementary Material 4).
Table 1.
Demographic and neuropsychological data.
| Demographic characteristics | NC | MCI | AD | P-value | F-value | |||
|---|---|---|---|---|---|---|---|---|
| NS (N = 123) | PS (N = 69) | NS (N = 39) | PS (N = 72) | NS (N = 14) | PS (N = 16) | |||
| Age,y, mean (SD) | 74.02 ± 7.13 | 74.77 ± 6.57 | 73.41 ± 8.07 | 73.78 ± 6.72 | 70.76 ± 8.24 | 74.66 ± 8.50 | 0.54 | 0.82 |
| Female, n/N | 70/123 | 46/69 | 19/39 | 30/72 | 5/14 | 9/16 | 0.04g | 11.62 |
| Education, y, mean (SD) | 16.52 ± 2.16 | 16.39 ± 2.43 | 16.23 ± 3.17 | 16.10 ± 2.67 | 15.57 ± 2.77 | 16.38 ± 2.58 | 0.74 | 0.54 |
| Neuropsychiatric Scores | ||||||||
| GDS | 0.65 ± 0.98 | 1.20 ± 1.15 | 1.10 ± 1.27 | 1.94 ± 1.76 | 1.86 ± 0.95 | 1.75 ± 1.57 | <0.001abdfg | 10.79 |
| General mental status | ||||||||
| MMSE | 29.20 ± 1.06 | 29.12 ± 1.04 | 27.77 ± 2.64 | 27.03 ± 3.89 | 22.93 ± 2.67 | 20.81 ± 4.42 | <0.001defghi | 50.94 |
| CDR global | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.42 ± 0.24 | 0.49 ± 0.32 | 0.75 ± 0.26 | 1.00 ± 0.55 | <0.001defghi | 127.96 |
| CDR sum | 0.01 ± 0.00 | 0.00 ± 0.00 | 1.21 ± 1.45 | 1.53 ± 2.07 | 3.79 ± 1.12 | 5.50 ± 3.46 | <0.001defghi | 72.06 |
| Memory function | ||||||||
| WMS-LM immediate | 14.39 ± 3.54 | 15.13 ± 3.17 | 9.74 ± 4.60 | 10.43 ± 4.39 | 4.25 ± 2.99 | 4.27 ± 2.99 | <0.001defghi | 46.90 |
| WMS-LM delay | 13.38 ± 3.73 | 14.03 ± 3.46 | 7.71 ± 5.59 | 8.10 ± 4.98 | 1.75 ± 2.22 | 1.07 ± 1.83 | <0.001defghi | 56.37 |
| AVLT sum of trials 1–5 | 13.06 ± 2.11 | 13.36 ± 2.29 | 11.11 ± 3.63 | 10.42 ± 3.79 | 5.13 ± 3.36 | 4.13 ± 3.67 | <0.001defghi | 43.05 |
| AVLT 30 min | 7.77 ± 4.23 | 8.65 ± 3.54 | 4.82 ± 3.97 | 4.76 ± 5.57 | 0.50 ± 0.93 | 0.31 ± 1.25 | <0.001defghi | 19.08 |
| Attention | ||||||||
| Log-transformed TMT-A | 1.49 ± 0.13 | 1.46 ± 0.11 | 1.54 ± 0.15 | 1.56 ± 0.18 | 1.76 ± 0.24 | 1.86 ± 0.20 | <0.001efghi | 24.45 |
| Decision-making function | ||||||||
| Log-transformed TMT-B | 1.86 ± 0.20 | 1.83 ± 0.14 | 1.97 ± 0.22 | 1.96 ± 0.24 | 2.10 ± 0.18 | 2.35 ± 0.17 | <0.001cdfghi | 18.40 |
| Language | ||||||||
| Category verbal fluency | 21.57 ± 5.30 | 21.97 ± 4.70 | 18.44 ± 5.50 | 17.69 ± 6.14 | 10.50 ± 4.87 | 12.44 ± 5.81 | <0.001defghi | 18.06 |
| Visuospatial processing | ||||||||
| CDT | 4.75 ± 0.45 | 4.74 ± 0.53 | 4.32 ± 1.02 | 4.39 ± 1.00 | 3.14 ± 2.12 | 3.25 ± 1.57 | <0.001dghi | 15.19 |
Data are presented as means ± standard deviations.
Abbreviation: NC: normal control; MCI: mild cognitive impairment; AD: Alzheimer’s disease; NS: normal sleeper; PS: poor sleeper; GDS: Geriatric Depression Scale; MMSE, Mini-Mental State Examination; CDR: Clinical Dementia Rating; WMS-LM, Wechsler Memory Scale Logical Memory; AVLT, Auditory Verbal Learning Test; TMT, Trail-Making Test; CDT, Clock Drawing Test.
a–iPost-hoc analysis further revealed the source of ANOVA difference (aNC-NS vs NC-PS; bMCI-NS vs MCI-PS; cAD-NS vs AD-PS; dNC-NS vs MCI-NS; eMCI-NS vs AD-NS; fNC-NS vs AD-NS; gNC-PS vs MCI-PS; hMCI-PS vs AD-PS; iNC-PS vs AD-PS) (p < 0.05, significant difference between groups).
sALFF results
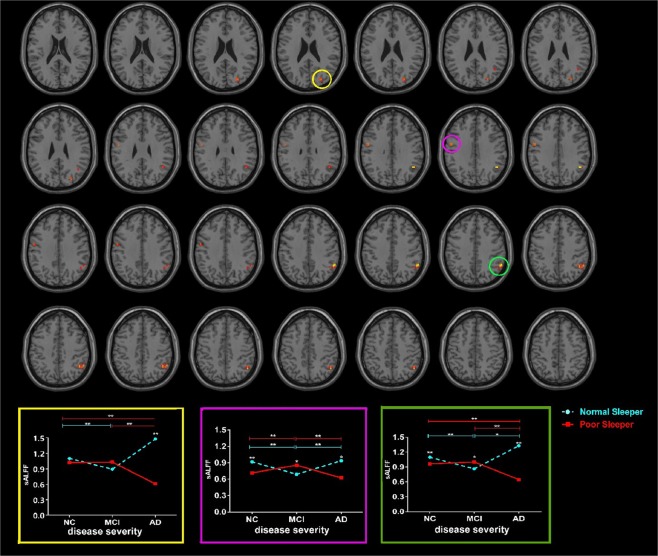
The interaction effects between sleep quality and disease severity were primarily located in the left precentral gyrus, right cuneus and right IPG (Fig. 1; Table 2: F test, threshold at voxel P < 0.001 and cluster P < 0.05, corrected by GRF; Tables 3 and 4: two-sample t-test, threshold at P < 0.05). To be specific, NC-PS had decreased sALFF in the precentral gyrus and IPG; MCI-PS had increased sALFF in the precentral gyrus and IPG; and AD-PS had decreased sALFF in the precentral gyrus, IPG, and cuneus when compared to their NS counterpart groups.
Figure 1.
The sALFF differences between groups using F test by setting the threshold at voxel P < 0.001 and cluster P < 0.05, corrected by GRF. The interaction effects between sleep quality and disease severity were primarily located in the left precentral gyrus, right cuneus and right IPG (Full factorial model, voxel P < 0.001 and cluster P < 0.05, corrected by GRF, minimum cluster size is 8 voxels.). To be specific, NC-PS showed decreased sALFF in precentral gyrus and IPG when compared to NC-NS; MCI-PS showed increased sALFF in precentral gyrus and IPG when compared to MCI-NS; AD-PS showed decreased sALFF in precentral gyrus, IPG and cuneus when compared to AD-NS. Abbreviation: sALFF, static amplitude of low-frequency fluctuation; NC, normal control; MCI: mild cognitive impairment; AD: Alzheimer’s disease; NS: normal sleeper; PS: poor sleeper; IPG, inferior parietal region; GRF, Gaussian random field.
Table 2.
The difference of sALFF and dALFF variance between groups using F test by setting the threshold at voxel P < 0.001 and cluster P < 0.05, corrected by GRF.
| Neuroimaging Metrics | Cluster/peak regions | MNI coordinates | Extent | Max T | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| dALFF variance | Cerebelum_Crus1_R | 45 | −57 | −30 | 10 | 8.53 |
| Cerebelum_8_R | 24 | −45 | −45 | 10 | 9.45 | |
| Insula_R | 39 | −3 | 6 | 10 | 10.68 | |
| Insula_L | −39 | −3 | 3 | 10 | 10.62 | |
| Thalamus_L | −6 | −6 | 0 | 9 | 9.38 | |
| Temporal_Pole_Sup_L | −36 | 15 | −30 | 14 | 14.27 | |
| Hippocampal_R | 21 | −9 | −21 | 8 | 10.74 | |
| Rectus_R | 6 | 27 | −18 | 15 | 10.42 | |
| SupraMarginal_R | 57 | −42 | 27 | 9 | 10.17 | |
| sALFF | Cuneus_R | 27 | −81 | 24 | 8 | 9.80 |
| Precentral_L | −51 | −6 | 33 | 10 | 9.58 | |
| Parietal_Inf_R | 48 | −54 | 33 | 35 | 12.49 | |
Abbreviation: sALFF, static amplitude of low-frequency fluctuation; dALFF, dynamic amplitude of low-frequency fluctuation; GRF: Gaussian random field correction.
Table 3.
Post-hoc analysis results within NC/MCI/AD subjects using two-sample t-test by setting the threshold at p < 0.05.
| Post-hoc analysis | dALFF variance | sALFF |
|---|---|---|
| NC-PS > NC-NS |
Cerebelum_8_R; Insula_R/L |
No |
| NC-PS < NC-NS | NO |
Parietal_Inf_R; Precentral_L |
| MCI-PS > MCI-NS | SupraMarginal_R |
Parietal_Inf_R; Precentral_L |
| MCI-PS < MCI-NS |
Insula_R/L; Temporal_Pole_Sup_L; Hippocampal_R |
No |
| AD-PS > AD-NS |
Cerebelum_8_R; Temporal_Pole_Sup_L; Rectus_R; Hippocampal_R |
No |
| AD-PS < AD-NS | SupraMarginal_R |
Precentral_L; Parietal_Inf_R; Cuneus |
Abbreviation: NC: normal control; MCI: mild cognitive impairment; AD: Alzheimer’s disease; NS: normal sleeper; PS: poor sleeper; sALFF, static amplitude of low-frequency fluctuation; dALFF, dynamic amplitude of low-frequency fluctuation.
Table 4.
Post-hoc analysis results between NC/MCI/AD subjects using two-sample t-test by setting the threshold at p < 0.05.
| Post-hoc analysis | dALFF variance | sALFF |
|---|---|---|
| NC-PS < MCI-PS |
Cerebelum_Crus1_R; Rectus_R; Temporal_Pole_Sup_L |
Precentral_L |
| NC-PS > MCI-PS | No | No |
| MCI-PS < AD-PS |
Cerebelum_8_R; Insula_R/L; Rectus_R; Temporal_Pole_Sup_L; Hippocampal_R; Thalamus_L |
No |
| MCI-PS > AD-PS | No |
Precentral_L; Parietal_Inf_R; Cuneus |
| NC-PS < AD-PS |
Cerebelum_8_R; Insula_L; Rectus_R; Temporal_Pole_Sup_L; Hippocampal_R; Thalamus_L |
No |
| NC-PS < AD-PS |
Cerebelum_8_R; Insula_L; Rectus_R; Temporal_Pole_Sup_L; Hippocampal_R; Thalamus_L |
No |
| NC-PS > AD-PS | No |
Parietal_Inf_R; Cuneus |
Abbreviation: NC: normal control; MCI: mild cognitive impairment; AD: Alzheimer’s disease; NS: normal sleeper; PS: poor sleeper; sALFF, static amplitude of low-frequency fluctuation; dALFF, dynamic amplitude of low-frequency fluctuation.
The main effect of sleep primarily located in the IPG, cerebellum, and motor-related region (Supplementary Material 5).
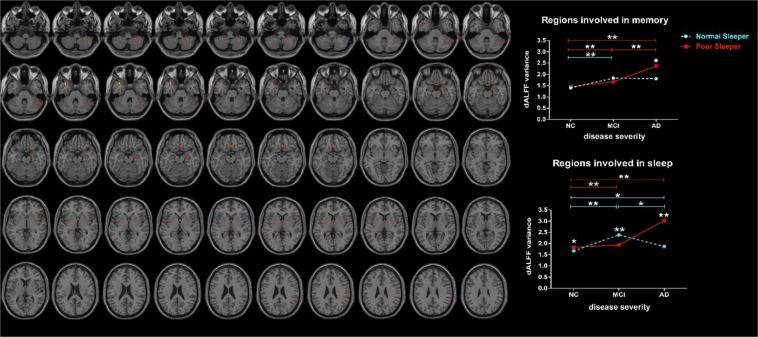
dALFF variance results
The interaction effects between sleep quality and disease severity located in the insula, thalamus, cerebellum, superior temporal gyrus (STG), HP, gyrus rectus and supramarginal gyrus (Fig. 2, Table 2: F test, threshold at voxel P < 0.001 and cluster P < 0.05, corrected by GRF; Tables 3 and 4: two-sample t-test, threshold at P < 0.05). According to the previous studies about the hippocampus-dependent memories56 combined with the function of the brain region, we hypothetically classify these results into regions involved in sleep (insula, thalamus, cerebellum)57–60 and regions involved in memory (STG, HP, gyrus rectus and supramarginal gyrus)61–64. We noted that distributions of dALFF variance formed a biphasic trajectory along the AD spectrum. To be specific, relative to counterpart NS groups, NC-PS showed an increased dALFF variance in the regions involved in sleep; MCI-PS showed a decreased dALFF variance in the regions involved in sleep; while AD-PS showed an increased dALFF variance in both regions involved in sleep and memory. Moreover, we showed the functional change in each ROI in Supplementary Material 6.
Figure 2.
The dALFF variance differences between groups using F test by setting the threshold at voxel P < 0.001 and cluster P < 0.05, corrected by GRF. The interaction effects between sleep quality and disease severity were primarily located in regions involved in sleep and memory (Full factorial model, voxel P < 0.001 and cluster P < 0.05, corrected by GRF). To be specific, NC-PS showed increased dALFF variance in regions involved in sleep when compared to NC-NS. MCI-PS showed decreased dALFF variance in regions involved in sleep when compared to MCI-NS. AD-PS showed increased dALFF variance in both regions involved in sleep and memory when compared to AD-NS. Abbreviation: dALFF, dynamic amplitude of low-frequency fluctuation; NC, normal control; MCI: mild cognitive impairment; AD: Alzheimer’s disease; NS: normal sleeper; PS: poor sleeper; GRF, Gaussian random field.
The main effect of sleep primarily located in the cerebellum, gyrus rectus, and superior frontal gyrus (Supplementary Supplementary Material 5).
Correlation analysis
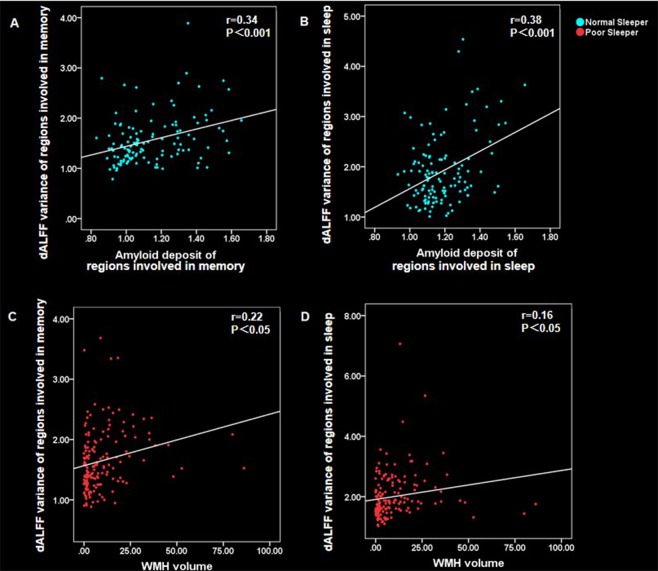
To explore the possible mechanism underlying intrinsic brain activity alterations, we related ROI-derived ALFF values to the WMH and SUVR of amyloid PET in NS and PS separately. According to the Bonferroni correction criterion, the threshold was set at 0.0017 (p < 0.05/30). Based on this criterion, subjects with NS showed a significant association between dALFF variance and amyloid deposit. As for the subjects with PS, the dALFF variance showed a trend of positive association with WMH. To be specific, in subjects with PS, dALFF variance correlated with WMH in regions involved in memory (r = 0.22, P = 0.005) and sleep (r = 0.16, P = 0.041). In subjects with NS, dALFF variance correlated with amyloid deposit in regions involved in memory (r = 0.34, P = 0.00014) and sleep (r = 0.38, P = 0.000018) (Fig. 3).
Figure 3.
Scatter plot diagram of the correlation between ALFF values and WMH/amyloid. In subjects with normal sleep, the dALFF variance positively associated with amyloid deposit: (A) the dALFF variance of regions involved in memory was positively correlated with amyloid deposit (r = 0.34, p < 0.001); (B) the dALFF variance of regions involved in sleep was positively correlated with amyloid deposit (r = 0.38, p < 0.001). In subjects with poor sleep, the dALFF variance positively associated with WMH burden: (C) the dALFF variance of regions involved in memory was positively correlated with WMH burden (r = 0.22, P < 0.05); (D) the dALFF variance of regions involved in sleep was positively correlated with WMH burden (r = 0.16, P < 0.05). Abbreviation: dALFF, dynamic amplitude of low-frequency fluctuation; WMH, white matter hyperintensities.
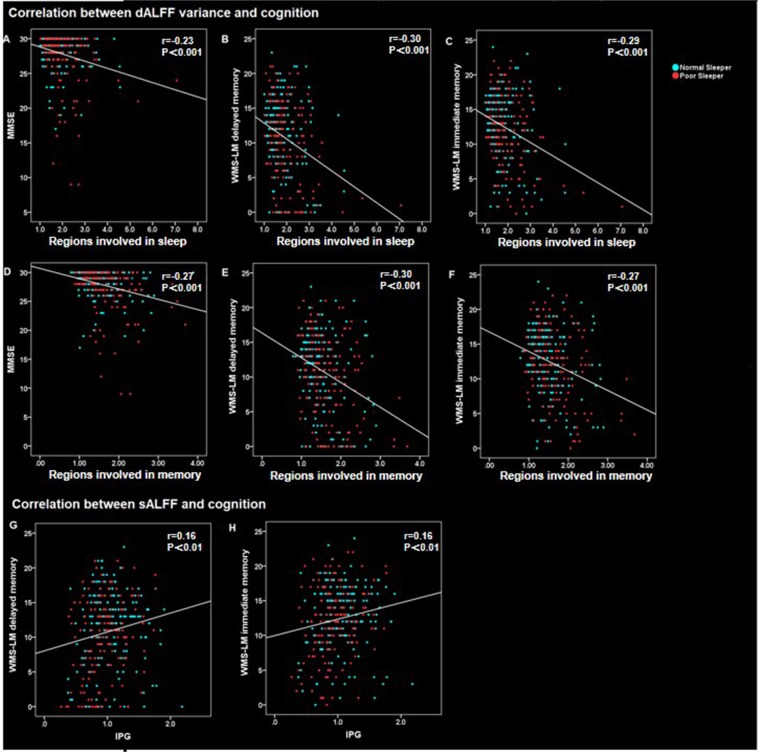
Additionally, to prove the physiological signification of the ALFF, we correlated the functional changes to cognition. According to the Bonferroni correction criterion, the threshold should be set at 0.003 (p < 0.05/15). Based on this criterion, our results showed significant association between dALFF and cognition as well as a trend of a significant association between sALFF and cognition. To be specific, as for dALFF, we found a negative correlation between dALFF variance and cognition, in both regions involved in memory and sleep across groups. Detailed results were: dALFF variance of regions involved in memory and cognition (MMSE: r = −0.27, P = 4.72E-7; WMS-LM immediate memory: r = −0.27, P = 7.10E-7; MS-LM delayed memory: r = −0.30, P = 3.08E-8); dALFF variance of regions involved in sleep and cognition (MMSE: r = −0.23, P = 2.28E-5; WMS-LM immediate memory: r = −0.29, P = 2.14E-7; WMS-LM delayed memory: r = −0.30, P = 6.77E-8). As for sALFF analysis, we found a positive correlation between sALFF and cognition in IPG across groups (WMS-LM immediate memory: r = 0.16, P = 0.005; MS-LM delayed memory: r = 0.16, P = 0.005) (Fig. 4).
Figure 4.
Scatter plot diagram of the correlation between ALFF values and cognition. Across groups, the dALFF variance negatively associated with cognition. (A) The dALFF variance of regions involved in sleep was negatively correlated with MMSE(r = −0.23, P < 0.001); (B) the dALFF variance of regions involved in sleep was negatively correlated with WMS-LM delayed memory (r = −0.30, P < 0.001); (C) the dALFF variance of regions involved in sleep was negatively correlated with WMS-LM immediate memory (r = −0.29, P < 0.001); (D) the dALFF variance of regions involved in memory was negatively correlated with MMSE (r = −0.27, P < 0.001); (E) the dALFF variance of regions involved in memory was negatively correlated with WMS-LM delayed memory (r = −0.30, P < 0.001); (F) the dALFF variance of regions involved in memory was negatively correlated with WMS-LM immediate memory (r = −0.27, P < 0.001). Across groups, we found a positive correlation between sALFF and cognition in IPG. (G) the sALFF of IPG was positively correlated with MS-LM delayed memory (r = 0.16, P < 0.05); (H) the sALFF of IPG was positively correlated with WMS-LM immediate memory (r = 0.16, P < 0.05). Abbreviation: dALFF, dynamic amplitude of low-frequency fluctuation; sALFF, static amplitude of low-frequency fluctuation; MMSE, Mini-Mental State Examination; WMS-LM, Wechsler memory scale-logical memory; IPG, inferior parietal gyrus.
Discussion
Our study explored the interactions between sleep disturbance and AD on intrinsic brain activity in vivo. We found that distributions of sALFF and dALFF variance formed a biphasic trajectory along the AD spectrum, mainly locating in the regions involved in memory and sleep. Further, our study suggested that vascular impairment might act as important pathogenesis underlying the interaction effect between sleep and AD.
Stable intrinsic brain activity enables advanced cognitive function
The current study found that the dALFF variance negatively correlated with cognition, while sALFF positively correlated with cognition, including general cognition and memory. These results suggested that stable brain activity sustains the successful cognitive process. In this study, the interaction effect on the stability of intrinsic brain activity located in regions involved in memory (STG, HP, gyrus rectus, supramarginal gyrus)61–64 and sleep (insula, thalamus, cerebellum)57–60. These regions are involved in hippocampal-neocortical, thalamo-cortical, and cortico-thalamic circuits, which play an essential role in memory-consolidation during sleep65–69. Thereinto, the cerebellum participates in the sleep-wake cycle, and its malfunctions can lead to sleep disorders70. The thalamus selected information to be projected to the cortex while the cortex is asleep to protect sleep from external perturbations71, while the insula works to initiate and maintain sleep state72. On the other hand, HP plays a vital role in memory encoding and memory consolidation; STG is a substrate for auditory short-term memory73; gyrus rectus and supramarginal gyrus are also reported an association with memory loss in AD patients61–64. These regions interact with the cerebral cortex during sleep and contribute to the consolidation of memories70,74–77.
Distributions of sALFF and dALFF variance formed a biphasic trajectory in subjects with poor sleep along the AD spectrum
At the stage of NC, NC-PS showed decreased stability of intrinsic brain activity in regions involved in sleep (especially the cerebellum and insula) compared to the NC-NS. This is in line with previous studies that reported that insula, thalamus, and cerebellum significantly contribute to sleep generation and stability57–60. Moreover, abnormal brain activity in thalamus and insula have also been reported in subjects with sleep problem78,79. As for the brain activity strength, NC-PS showed decreased functional strength in the precentral gyrus and IPG when compared to the NC-NS. Similarly, another study found sleep-related disrupted functional connectivity in IPG in older adults80. Moreover, evidence also comes from structural analysis, which found the association between poor sleep quality and reduced GM volume in the parietal regions and postcentral gyrus72,81,82. Conclusively, NC-PS showed both decreased stability and strength of intrinsic brain activity.
As the disease severity progresses, MCI-PS showed increased stability of intrinsic brain activity in regions involved in sleep when compared to the MCI-NS. We hypothesized that the increased functional stability is a compensatory enhancement, temporarily maintaining the brain function. The possible underlying mechanism may be the relatively severer pathological changes caused by sleep disturbance10,11. Such a compensatory hypothesis can partially be supported by the increased strength of intrinsic brain activity in the precentral gyrus and IPG in MCI-PS. Additionally, the well-matched cognitive profile between MCI-PS and MCI-NS also suggested the cognition maintain processes at the MCI stage.
Interestingly, MCI-PS also showed a trend of increased brain activity stability in regions involved in memory (especially the HP). Notably, HP is regarded as the critical structure in memory consolidation during sleep56 which involves information reactivation and hippocampo-neocortical transfer, based on the wide connection with other networks, including the hippocampo-striato-thalamo-cortical networks83, the hippocampus–amygdala system84, and hippocampal-cortical communication85. Accordingly, we speculated that the effect of sleep disturbance on brain activity in AD subjects might start from regions involved in sleep, then to the regions involved in memory, which may start from HP. Such impaired function will keep spreading as disease progression and ultimately lead to the irreversible cognition decline.
In the final disease stage, AD-PS showed decreased stability of intrinsic brain activity in both regions involved in sleep and memory when compared to AD-NS. The widespread functional alternations might reflect the de-compensation processes in the “biphasic model” in the AD spectrum. The decreased functional stability may suggest the overactivated functional oscillation and decreased synchronization, which leads to ineffective brain activity and finally causes cognition decline86,87. This decompensation hypothesis can also be proved by the decreased intrinsic brain activity strength in the precentral gyrus, IPG, and cuneus in AD-PS. Moreover, our analysis also found the relatively lower cognition in AD-PS, suggesting the severer cognition impairment.
Taken together, PS groups showed the dALFF variance trajectory of initially increased, then decreased and finally increased along the AD spectrum, while showing the opposite trajectory of sALFF. These changes may suggest the dynamic trajectory of an interaction effect between sleep disturbance and AD, expression as effects of compensatory in MCI and de-compensatory in AD, respectively.
Vascular impairment might be an important pathogenesis underlying sleep-related impairments in AD
Different neural mechanisms may contribute to the opposite ALFF changes pattern in the NS and PS groups. In NS, we found the greater dALFF variance correlated with more amyloid deposition, rather than WMH burden, suggesting amyloid dominates the intrinsic brain activity. This is consistent with the amyloid hypothesis in previous AD studies. On the other hand, in PS, we found the greater dALFF variance correlated between severer WMH burden, rather than amyloid, indicating that vascular impairment works more prominently. This is partially in line with previous studies which reported that sleep might impair the function of BBB (including tight junction proteins loss and endothelial loss), which is a key pathway mediating the relationship between sleep disturbances and AD pathologies aggravation12–14. Similarly, previous studies also reported the link between sleep disturbance and vascular risk factors like subcortical infarcts and WMH, which may interfere with the sleep-wake cycle88,89.
Collectively, our results suggested the interaction effect between sleep disturbance and disease severity on brain function. These findings suggested that improving sleep quality4,90 or prevent cerebral vascular risk91 may achieve potentially better clinical prognosis for patients along the AD continuum.
Limitation
There exist several limitations to our study. First, as an explorative investigation, our definition of sleep problem included insomnia, sleep apnea, and other self-reported information, which is somewhat heterogeneous. Further studies with sleep monitor equipment and standard quantitative sleep grades are urgent to verify our results. Moreover, our finding on NC is similar to previous studies, which suggested the feasibility of our result to some extent. Second, some amyloid PET data are missing (54 out of 176 NS and 2 out of 157 PS with amyloid PET data missing), which may reduce statistical power. Further studies with larger PET sample sizes are urgent. Thirdly, the sample size of the AD group is relatively small. Further studies with more AD subjects should be performed. Moreover, the observed effect might be affected by different sleep profiles during the scan since NS and PS may have a different risk to fall asleep in the scanner92. Further study should use the accurate eye-tracker system to reduce the possible effect. Finally, some medicine like acetylcholinesterase inhibitors may affect the sleep state. Further study should consider detailed medicine information to decrease the possible effect.
Conclusion
Our study demonstrated that sleep disturbances interact with AD severity on static and dynamic intrinsic activity alteration, mainly locating in the regions involved in the memory and sleep. Moreover, the distributions of functional change are more likely to be a biphasic trajectory along the AD spectrum, with a compensatory enhancement in the MCI stage but decompensation in the AD stage. The mechanism underlying the interaction effects between sleep disturbances and AD may attribute to cerebral vascular-related risk factors.
Supplementary information
Acknowledgements
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found http://adni.loni.usc.edu/wp-content/themes/freshnews-dev-v2/documents/policy/ADNI_Acknowledgement_List%205-29-18.pdf. Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www. fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. Samples from the National Cell Repository for AD (NCRAD), which receives government support under a cooperative agreement grant (U24 AG21886) awarded by the National Institute on Aging (AIG), were used in this study. Additional support for data analysis was provided by NLM R01 LM012535, NIA R03 AG054936, and the Pennsylvania Department of Health (#SAP 4100070267). The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. This research was also supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education [NRF- 2016R1D1A1B03933875]. This study was funded by the 13th Five-year Plan for National Key Research and Development Program of China (Grant No. 2016YFC1306600), Zhejiang Provincial Natural Science Foundation of China (Grant No LSZ19H180001), Natural Science Foundation of China (grant number: 81271530 & 81771820), Young Research Talents Fund, Chinese Medicine Science, and Technology Project of Zhejiang Province (Grant No. 2018ZQ035), the Fundamental Research Funds for the Central Universities (No.2017XZZX001-01), Zhejiang Medicine and Health Science and Technology Program (2018KY418 and 2016KYA099).
Author contributions
K.L. and X.L. contributed equally to this work. K.L. designed the study and wrote the first draft of the manuscript. X.L. analysed the MRI data and wrote the protocol. Q.Z. and J.X. collected the clinical and MRI data. J.Z., C.W., X.X., P.H., S.W. and M.Z. assisted with study design and interpretation of findings. All authors have contributed to and approved the final manuscript. All authors read and approved the final manuscript.
Data availability
The datasets generated and/or analysed during the current study are available in the ADNI study. More details in www.adni-info.org.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Kaicheng Li and Xiao Luo.
Contributor Information
Peiyu Huang, Email: hpyzju@foxmail.com.
Minming Zhang, Email: zhangminming@zju.edu.cn.
Supplementary information
is available for this paper at 10.1038/s41598-019-55452-9.
References
- 1.Prince, M. W. A., Guerchet M., Ali, G., Wu, Y & Prina M. An analysis of prevalence, incidence, cost & trends. Alzheimer’s Disease International; London: 2015. World Alzheimer Report 2015., Available at, https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf and https://www.alz.co.uk/research/world-report-2018.
- 2.Livingston G, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 3.McCurry SM, et al. Characteristics of sleep disturbance in community-dwelling Alzheimer’s disease patients. J Geriatr Psychiatry Neurol. 1999;12:53–59. doi: 10.1177/089198879901200203. [DOI] [PubMed] [Google Scholar]
- 4.Moran M, et al. Sleep disturbance in mild to moderate Alzheimer’s disease. Sleep Med. 2005;6:347–352. doi: 10.1016/j.sleep.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Westerberg CE, et al. Concurrent impairments in sleep and memory in amnestic mild cognitive impairment. J Int Neuropsychol Soc. 2012;18:490–500. doi: 10.1017/S135561771200001X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonanni E, et al. Daytime sleepiness in mild and moderate Alzheimer’s disease and its relationship with cognitive impairment. J Sleep Res. 2005;14:311–317. doi: 10.1111/j.1365-2869.2005.00462.x. [DOI] [PubMed] [Google Scholar]
- 7.Rauchs G, et al. Retrieval of Recent Autobiographical Memories is Associated with Slow-Wave Sleep in Early AD. Front Behav Neurosci. 2013;7:114. doi: 10.3389/fnbeh.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology–a bidirectional relationship. Nat Rev Neurol. 2014;10:115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bubu, O. M et al. Sleep, Cognitive impairment, and Alzheimer’s disease: A Systematic Review and Meta-Analysis. Sleep40, 10.1093/sleep/zsw1032 (2017). [DOI] [PubMed]
- 10.Roh JH, et al. Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med. 2012;4:150ra122. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y, et al. Effects of age and amyloid deposition on Abeta dynamics in the human central nervous system. Arch Neurol. 2012;69:51–58. doi: 10.1001/archneurol.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez-Gonzalez B, et al. REM sleep loss and recovery regulates blood-brain barrier function. Curr Neurovasc Res. 2013;10:197–207. doi: 10.2174/15672026113109990002. [DOI] [PubMed] [Google Scholar]
- 13.Bien-Ly N, et al. Lack of Widespread BBB Disruption in Alzheimer’s Disease Models: Focus on Therapeutic Antibodies. Neuron. 2015;88:289–297. doi: 10.1016/j.neuron.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 14.Winkler EA, Sagare AP, Zlokovic BV. The pericyte: a forgotten cell type with important implications for Alzheimer’s disease? Brain Pathol. 2014;24:371–386. doi: 10.1111/bpa.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wardlaw JM, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurology. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiaerken Yeerfan, Luo Xiao, Yu Xinfeng, Huang Peiyu, Xu Xiaojun, Zhang Minming. Microstructural and metabolic changes in the longitudinal progression of white matter hyperintensities. Journal of Cerebral Blood Flow & Metabolism. 2018;39(8):1613–1622. doi: 10.1177/0271678X18761438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rostanski SK, et al. Sleep Disordered Breathing and White Matter Hyperintensities in Community-Dwelling Elders. Sleep. 2016;39:785–791. doi: 10.5665/sleep.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackwell T, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–410. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 19.Xu L, et al. Short or long sleep duration is associated with memory impairment in older Chinese: the Guangzhou Biobank Cohort Study. Sleep. 2011;34:575–580. doi: 10.1093/sleep/34.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yaffe K, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: A Novel Mechanistic Pathway, Biomarker, and Treatment Target in the Pathology of Alzheimer’s Disease? Trends Neurosci. 2016;39:552–566. doi: 10.1016/j.tins.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landry, G. J. & Liu-Ambrose, T. Buying time: a rationale for examining the use of circadian rhythm and sleep interventions to delay progression of mild cognitive impairment to Alzheimer’s disease. Front Aging Neurosci6, 10.3389/fnagi.2014.00325. eCollection 02014 (2014). [DOI] [PMC free article] [PubMed]
- 23.Canessa N, et al. Sleep apnea: Altered brain connectivity underlying a working-memory challenge. Neuroimage Clin. 2018;19:56–65. doi: 10.1016/j.nicl.2018.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z, et al. Disrupted brain network topology in chronic insomnia disorder: A resting-state fMRI study. Neuroimage Clin. 2018;18:178–185. doi: 10.1016/j.nicl.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bassett DS, Sporns O. Network neuroscience. Nature Neuroscience. 2017;20:353–364. doi: 10.1038/nn.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christoff K, et al. Mind-wandering as spontaneous thought: a dynamic framework. Nature Reviews Neurosciences. 2016;17:718–731. doi: 10.1038/nrn.2016.113. [DOI] [PubMed] [Google Scholar]
- 27.Preti MG, Bolton TA, Van DVD. The dynamic functional connectome: state-of-the-art and perspectives. Neuroimage. 2017;160:41–54. doi: 10.1016/j.neuroimage.2016.12.061. [DOI] [PubMed] [Google Scholar]
- 28.Cha J, et al. Assessment of Functional Characteristics of Amnestic Mild Cognitive Impairment and Alzheimer’s Disease Using Various Methods of Resting-State FMRI Analysis. Biomed Res Int. 2015;2015:907464. doi: 10.1155/2015/907464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, et al. Abnormal amplitude of low-frequency fluctuations of intrinsic brain activity in Alzheimer’s disease. J Alzheimers Dis. 2014;40:387–397. doi: 10.3233/JAD-131322. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Duan X, Cui Q, Chen H, Liao W. More than just statics: temporal dynamics of intrinsic brain activity predicts the suicidal ideation in depressed patients. Psychol Med. 2019;49:852–860. doi: 10.1017/S0033291718001502. [DOI] [PubMed] [Google Scholar]
- 31.Allen EA, et al. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex. 2014;24:663–676. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutchison RM, et al. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li K, et al. Gray matter structural covariance networks changes along the Alzheimer’s disease continuum. Neuroimage Clin. 2019;23:101828. doi: 10.1016/j.nicl.2019.101828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheikh, J. I. & Yesavage, J. A. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. In: Brink, T. L, ed. Clinical Gerontology: A Guide to Assessment and Intervention. New York, NY: The Haworth Press;, 165–173 (1986).
- 35.Bondi MW, et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis. 2014;42:275–289. doi: 10.3233/JAD-140276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKhann G, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- 37.Li K, et al. Aberrant functional connectivity network in subjective memory complaint individuals relates to pathological biomarkers. Transl Neurodegener. 2018;7:27. doi: 10.1186/s40035-018-0130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mecca AP, et al. Sleep Disturbance and the Risk of Cognitive Decline or Clinical Conversion in the ADNI Cohort. Dement Geriatr Cogn Disord. 2018;45:232–242. doi: 10.1159/000488671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benedict C, et al. Self-reported sleep disturbance is associated with Alzheimer’s disease risk in men. Alzheimers Dement. 2015;11:1090–1097. doi: 10.1016/j.jalz.2014.08.104. [DOI] [PubMed] [Google Scholar]
- 40.Kaufer DI, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 41.Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Front Syst Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- 43.Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics. 2016;14:339–351. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- 44.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zang YF, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Marusak HA, et al. Dynamic functional connectivity of neurocognitive networks in children. Hum Brain Mapp. 2017;38:97–108. doi: 10.1002/hbm.23346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allen EA, et al. A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci. 2011;5:2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hutchison RM, Morton JB. Tracking the Brain’s Functional Coupling Dynamics over Development. J Neurosci. 2015;35:6849–6859. doi: 10.1523/JNEUROSCI.4638-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liao W, et al. DynamicBC: a MATLAB toolbox for dynamic brain connectome analysis. Brain Connect. 2014;4:780–790. doi: 10.1089/brain.2014.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zalesky A, Breakspear M. Towards a statistical test for functional connectivity dynamics. Neuroimage. 2015;114:466–470. doi: 10.1016/j.neuroimage.2015.03.047. [DOI] [PubMed] [Google Scholar]
- 51.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt P, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage. 2012;59:3774–3783. doi: 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 53.Poline JB, Worsley KJ, Evans AC, Friston KJ. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage. 1997;5:83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- 54.Zhang H, Nichols TE, Johnson TD. Cluster mass inference via random field theory. Neuroimage. 2009;44:51–61. doi: 10.1016/j.neuroimage.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li M, et al. Altered gray matter volume in primary insomnia patients: a DARTEL-VBM study. Brain Imaging Behav. 2018;12:1759–1767. doi: 10.1007/s11682-018-9844-x. [DOI] [PubMed] [Google Scholar]
- 56.Sara SJ. Sleep to remember. J Neurosci. 2017;37:457–463. doi: 10.1523/JNEUROSCI.0297-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schabus M, et al. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci USA. 2007;104:13164–13169. doi: 10.1073/pnas.0703084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dang-Vu TT, McKinney SM, Buxton OM, Solet JM, Ellenbogen JM. Spontaneous brain rhythms predict sleep stability in the face of noise. Curr Biol. 2010;20:R626–627. doi: 10.1016/j.cub.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 59.Koenigs M, Holliday J, Solomon J, Grafman J. Left dorsomedial frontal brain damage is associated with insomnia. J Neurosci. 2010;30:16041–16043. doi: 10.1523/JNEUROSCI.3745-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Andres I, Garzon M, Reinoso-Suarez F. Functional Anatomy of Non-REM Sleep. Front Neurol. 2011;2:70. doi: 10.3389/fneur.2011.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Supekar K, Menon V, Rubin D, Musen M, Greicius MD. Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLoS Comput Biol. 2008;4:e1000100. doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer’s disease: an fMRI study. Human Brain Mapping. 2005;26:231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zald DH, Andreotti C. Neuropsychological assessment of the orbital and ventromedial prefrontal cortex. Neuropsychologia. 2010;48:3377–3391. doi: 10.1016/j.neuropsychologia.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 65.Latchoumane CV, Ngo HV, Born J, Shin HS. Thalamic Spindles Promote Memory Formation during Sleep through Triple Phase-Locking of Cortical, Thalamic, and Hippocampal Rhythms. Neuron. 2017;95:424–435 e426. doi: 10.1016/j.neuron.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 66.Wang DV, et al. Mesopontine median raphe regulates hippocampal ripple oscillation and memory consolidation. Nat Neurosci. 2015;18:728–735. doi: 10.1038/nn.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011;35:1154–1165. doi: 10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 68.Logothetis NK, et al. Hippocampal-cortical interaction during periods of subcortical silence. Nature. 2012;491:547–553. doi: 10.1038/nature11618. [DOI] [PubMed] [Google Scholar]
- 69.Xu W, Sudhof TC. A neural circuit for memory specificity and generalization. Science. 2013;339:1290–1295. doi: 10.1126/science.1229534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Canto CB, Onuki Y, Bruinsma B, van der Werf YD, De Zeeuw CI. The Sleeping Cerebellum. Trends Neurosci. 2017;40:309–323. doi: 10.1016/j.tins.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 71.Del Felice A, Formaggio E, Storti SF, Fiaschi A, Manganotti P. The gating role of the thalamus to protect sleep: an f-MRI report. Sleep Med. 2012;13:447–449. doi: 10.1016/j.sleep.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 72.Branger P, et al. Relationships between sleep quality and brain volume, metabolism, and amyloid deposition in late adulthood. Neurobiol Aging. 2016;41:107–114. doi: 10.1016/j.neurobiolaging.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 73.Leff AP, et al. The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: evidence from 210 patients with stroke. Brain. 2009;132:3401–3410. doi: 10.1093/brain/awp273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tagliazucchi E, et al. Large-scale brain functional modularity is reflected in slow electroencephalographic rhythms across the human non-rapid eye movement sleep cycle. Neuroimage. 2013;70:327–339. doi: 10.1016/j.neuroimage.2012.12.073. [DOI] [PubMed] [Google Scholar]
- 75.Chow HM, et al. Rhythmic alternating patterns of brain activity distinguish rapid eye movement sleep from other states of consciousness. Proc Natl Acad Sci USA. 2013;110:10300–10305. doi: 10.1073/pnas.1217691110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rauchs G, et al. Is there a link between sleep changes and memory in Alzheimer’s disease? Neuroreport. 2008;19:1159–1162. doi: 10.1097/WNR.0b013e32830867c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rauchs G, Harand C, Bertran F, Desgranges B, Eustache F. Sleep and episodic memory: a review of the literature in young healthy subjects and potential links between sleep changes and memory impairment observed during aging and Alzheimer’s disease. Rev Neurol (Paris) 2010;166:873–881. doi: 10.1016/j.neurol.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 78.Telzer EH, Fuligni AJ, Lieberman MD, Galvan A. The effects of poor quality sleep on brain function and risk taking in adolescence. Neuroimage. 2013;71:275–283. doi: 10.1016/j.neuroimage.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu CH, et al. Reduced spontaneous neuronal activity in the insular cortex and thalamus in healthy adults with insomnia symptoms. Brain Res. 2016;1648(Pt A):317–324. doi: 10.1016/j.brainres.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 80.Liu YR, et al. Sleep-related brain atrophy and disrupted functional connectivity in older adults. Behav Brain Res. 2018;347:92–299. doi: 10.1016/j.bbr.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 81.Sexton CE, Storsve AB, Walhovd KB, Johansen-Berg H, Fjell AM. Poor sleep quality is associated with increased cortical atrophy in community-dwelling adults. Neurology. 2014;83:967–973. doi: 10.1212/WNL.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sanchez-Espinosa MP, Atienza M, Cantero JL. Sleep deficits in mild cognitive impairment are related to increased levels of plasma amyloid-β and cortical thinning. Neuroimage. 2014;98:395–404. doi: 10.1016/j.neuroimage.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 83.Boutin A, et al. Transient synchronization of hippocampo-striato-thalamo-cortical networks during sleep spindle oscillations induces motor memory consolidation. Neuroimage. 2018;169:419–430. doi: 10.1016/j.neuroimage.2017.12.066. [DOI] [PubMed] [Google Scholar]
- 84.Girardeau G, Inema I, Buzsaki G. Reactivations of emotional memory in the hippocampus-amygdala system during sleep. Nat Neurosci. 2017;20:1634–1642. doi: 10.1038/nn.4637. [DOI] [PubMed] [Google Scholar]
- 85.Alger SE, Chambers AM, Cunningham T, Payne JD. The role of sleep in human declarative memory consolidation. Curr Top Behav Neurosci. 2015;25:269–306. doi: 10.1007/7854_2014_341. [DOI] [PubMed] [Google Scholar]
- 86.Stam CJ, et al. Generalized synchronization of MEG recordings in Alzheimer’s Disease: evidence for involvement of the gamma band. J Clin Neurophysiol. 2002;19:562–574. doi: 10.1097/00004691-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 87.Montez T, et al. Altered temporal correlations in parietal alpha and prefrontal theta oscillations in early-stage Alzheimer disease. Proc Natl Acad Sci USA. 2009;106:1614–1619. doi: 10.1073/pnas.0811699106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zuurbier LA, et al. Cerebral small vessel disease is related to disturbed 24-h activity rhythms: a population-based study. Eur J Neurol. 2015;22:1482–1487. doi: 10.1111/ene.12775. [DOI] [PubMed] [Google Scholar]
- 89.Lim A, et al. Sleep fragmentation, cerebral arteriolosclerosis, and brain infarct pathology in community-dwelling older people. Stroke. 2016;47:516–518. doi: 10.1161/STROKEAHA.115.011608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peter-Derex L, Yammine P, Bastuji H, Croisile B. Sleep and Alzheimer’s disease. Sleep Med Rev. 2015;19:29–38. doi: 10.1016/j.smrv.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 91.Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. 2015;11:157–165. doi: 10.1038/nrneurol.2015.10. [DOI] [PubMed] [Google Scholar]
- 92.Tagliazucchi E, Helmut L. Decoding wakefulness levels from typical fMRI resting-state data reveals reliable drifts between wakefulness and sleep. Neuron. 2014;82:695–708. doi: 10.1016/j.neuron.2014.03.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the ADNI study. More details in www.adni-info.org.