Abstract
Purpose
To report the effects of blastocyst stage aneuploidy testing on clinical, gestational, and neonatal outcomes for patients of advanced maternal age undergoing IVF.
Methods
This is a single-center observational-cohort study with 2 years follow-up. The study includes a total of 2538 couples undergoing 2905 egg collections (control group), 308 (PGT-A), and 106 (drop-out group, consenting for PGT-A but withdrawing due to poor embryological outcome)
Results
Compared with control group, PGT-A showed improved clinical outcomes (live-birth rate per transferred embryo, LBR 40.3% vs 11.0%) and reduced multiple pregnancy rate (MPR, 0% vs 11.1%) and pregnancy loss (PL, 3.6% vs 22.6%). Drop-out group showed the worst clinical outcomes suggesting that abandoning PGT-A due to poor response to ovarian stimulation is not a favorable option. Cytogenetic analysis of product of conceptions and CVS/amniocentesis showed higher aneuploid pregnancy rates for control group regardless of embryo transfer strategy (0%, 17.9%, and 19.9%, for PGT-A, control day 5 and day 3, respectively). Multivariate analysis showed no negative impact of PGT-A-related interventions on cumulative delivery rate (26.3%, 95% CI 21.5–31.6 vs 24.0%, 95% CI 22.5–25.6 for PGT-A and control, respectively) and on neonatal outcomes.
Conclusion
PGT-A improves clinical outcomes, particularly by reducing pregnancy loss and chromosomally abnormal pregnancy for patients of advanced maternal age, with no major impact on cumulative live-birth rate (CLBR) per egg retrieval.
Electronic supplementary material
The online version of this article (10.1007/s10815-019-01609-4) contains supplementary material, which is available to authorized users.
Keywords: Preimplantation genetic testing, Advanced maternal age, Clinical outcome, Miscarriage, Aneuploidy
Introduction
The use of preimplantation genetic testing (PGT-A) for aneuploidies has been under debate for the last two decades [1–3]. Despite its wide application, it has been suggested that not only embryo biopsy may be detrimental to embryo development, but also that knowledge of embryo’s chromosomal composition does not provide crucial information able to positively affect transfer outcomes in IVF cycles [4]. Similarly, the introduction of extended embryo culture has encountered resistance from part of the scientific community. It is argued that the long exposure of embryos to artificial conditions may impair their natural epigenetic patterns, potentially affecting both fetal and post-natal development [5, 6]. Although this hypothesis has theoretical fundamentals and warrants concern from several standpoints, it still requires confirmation through properly designed clinical studies and long-term follow-ups. Meanwhile, the vast majority of IVF laboratories employing blastocyst culture report increased pregnancy rates and similar obstetrical/neonatal outcomes compared with cleavage rate embryo transfer (ET) procedures [7, 8]. Additionally, blastocyst culture has facilitated the implementation of elective single embryo transfer (eSET) practice by ensuring high implantation rates even when a single embryo was transferred [9, 10]. Consequently, this approach has drastically reduced multiple pregnancy rates and risks associated to its complications. Due to the high benefits produced by extended embryo culture in the short-term, clinical follow-up and immediate outcomes may be used to define the extent of its impact on IVF treatments. Moreover, blastocyst stage biopsy for PGT-A purposes was demonstrated to be the most clinically efficient strategy from both technical and medical standpoints [11–16]. Indeed, elective transfer of euploid blastocysts was shown to be an effective strategy to further facilitate SET policies, especially in advanced maternal age (AMA) women and to minimize miscarriages and aneuploid pregnancies [12, 17]. In fact, while multiple pregnancies are obvious iatrogenic limitations of IVF itself that can be mitigated by broaden SET policies, miscarriages and aneuploid gestations are intrinsically related to woman’s age (e.g., aneuploid conception), where the only effective prevention during an IVF treatment is PGT-A [18, 19]. The ongoing debate on overall effectiveness of PGT-A has so far been limited by the lack of extensive follow-up data from IVF-derived pregnancies and by potential commercial interests of IVF clinics in offering PGT-A. Furthermore, cumulative data with prolonged follow-up are still required to evaluate the impact of blastocyst culture, embryo biopsy and aneuploidy testing on cumulative live-birth rate (LBR), particularly in AMA women.
In this paper, we report clinical performance of a single, large IVF center where both extended embryo culture and PGT-A were offered to AMA patients attending for infertility and infertility-related conditions. This substantial and comprehensive dataset allowed for meaningful clinical outcomes analysis thanks to prolonged follow-up of patients (2-year period from oocyte retrieval) and a low treatment drop-out rate (full public treatment subsidy). Statistical comparison of clinical outcomes across treatment groups was performed to identify any differences derived from the interventions employed (e.g., blastocyst culture and PGT-A), taking into consideration pre- and post- embryo transfer outcomes, pregnancy follow-up (including product of conception (POC) and prenatal diagnosis (PND) analyses), as well as perinatal results. The presence of the obstetrics division within the same hospital premises as the IVF unit granted a detailed gestational and neonatal follow-up in this study.
Material and methods
Study design and patients’ population
This prospective observational-cohort study included all couples with female patients between 38 and 44 years of age attending Humanitas Fertility Center in Rozzano, Italy between January 2015 and May 2017. Only patients with FSH levels < 12 mIU/mL and/or AMH levels > 0.5 ng/mL were considered in an attempt to homogenize the population and exclude the outliers. Due to their advanced maternal age, all patients were counseled regarding the possibility to submit their embryos to PGT-A in order to de-select chromosomally abnormal embryos from transfer.
Two study groups were formed based on patients’ will to undergo PGT-A analysis. A total of 370 and 2168 couples were enrolled in the PGT-A and control group, respectively (Fig. 1). The PGT-A group was further subdivided into the PGT-A group, which consisted of patients completing the blastocyst culture and PGT-A, and drop-out group with patients producing poor embryological outcome and, due to the low number of fertilized oocytes to start with, did not receive trophectoderm biopsy/genetic testing. In particular, on the day of fertilization check, patients with less than 5 normally fertilized zygotes were counseled about the low chances of obtaining euploid blastocysts and let decide whether to continue or withdraw PGT-A in favor of standard embryo transfer on day 3. Although this general guideline for treatment decision, some patients decided to pursue PGT-A despite low number of fertilized oocytes (74/308, 24.0%) and, on the other hand, a few patients dropped out from PGT-A analysis regardless having more than 4 normally fertilized zygotes (11/106, 10.4%).
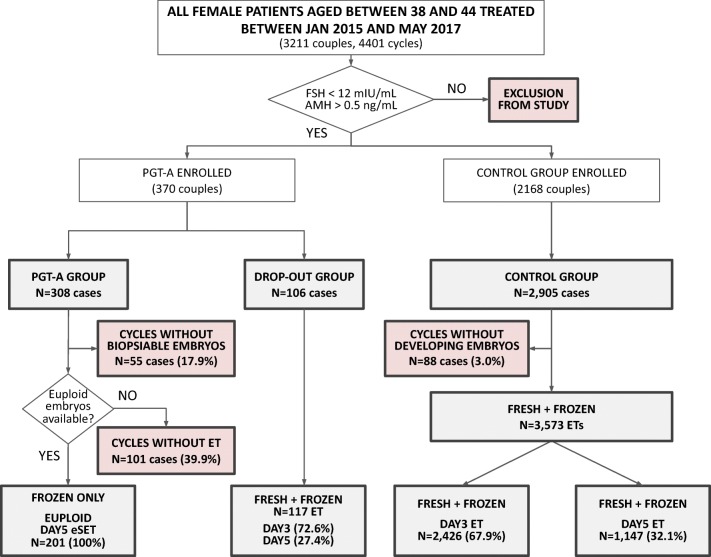
Fig. 1.
Flow chart of the study. This chart shows general enrolment (white boxes) and points of exclusion (red boxes) of patients into the study
Eventually, our study population included 2905 egg collections in the control group, 308 in the PGT-A group and 106 in the drop-out group, leading to 3575, 201, and 117 embryo transfers, respectively. Additionally, one patient was removed from the PGT-A group as her pregnancy was generated from spontaneous conception instead of as a result of the IVF treatment undertaken as ascertained by DNA fingerprinting [20].
Approval for this study was obtained from an independent Ethical Committee from IRCCS Istituto Clinico Humanitas.
Ovarian stimulation, egg collection, and embryo transfer
The controlled ovarian stimulation (COS) protocols employed in this study involved the use of recombinant FSH (rFSH), human menopausal gonadotropin (hMG) or rFSH + recombinant LH (rFSH + rLH). The gonadotropin starting dose was determined according to ovarian reserve parameters (e.g., AMH, AFC, and BMI).
COS was performed using four different protocols: GnRH agonist long protocol; GnRH agonist short protocol; GnRH antagonist protocol; and Flare-up GnRH agonist protocol. Most of antagonist COSs involved pretreatment with combined oral contraceptives [21].
The COS protocol and the dose of gonadotropins administered were tailored on an individual basis according to patient’s age, serum hormonal levels, and AFC. Transvaginal ultrasonography, estradiol and progesterone determinations were performed during COS. When at least three follicles with a mean diameter > 18 mm were observed, 250 mcg of recombinant hCG (Ovitrelle, Merck Serono) was administered subcutaneously. Oocyte retrieval was performed transvaginally 36 h after hCG injection. Embryo transfer was performed on day 3 or day 5 after oocyte collection. Luteal phase was supported in all patients with vaginal progesterone (Crinone 8%, Merck Serono or Prometrium, Rottapharm). Serum hCG was assessed 2 weeks after embryo transfer and then every 48 h until a value over 1000 mIU was detected and a vaginal ultrasound was scheduled 4 weeks after the embryo transfer to confirm pregnancy. Endometrial preparation and transfer procedures were performed as previously described elsewhere [22].
Laboratory procedures
Oocyte collection and denudation were performed as previously described [23]. Metaphase 2 (MII) oocytes were subjected to intracytoplasmic sperm injection (ICSI) between 36 and 38 hours after human chorionic gonadotropin administration. All injected oocytes were cultured in single wells in a time-lapse incubator (Embryoscope, Vitrolife) under humidified atmosphere containing 5% O2 and 5% CO2 up to the blastocyst stage (days 5–7). A two-step culture was performed: Quinn’s AdvantageTM Cleavage Medium (Sage, Origio) until day 3 and Quinn’s AdvantageTM Blastocyst Medium (Sage, Origio) until day 7. Presence of pronuclei was assessed between 16 and 18 hours after ICSI. In the PGT-A groups, expanded blastocysts underwent trophectoderm biopsy and were subsequently vitrified using the Kitazato protocol [24]. Quantitative PCR (qPCR) was employed for comprehensive chromosome testing at Igenomix Italy laboratory. Only uniform aneuploidies were considered for genetic data analysis and issued in the diagnostic report [25]. Euploid blastocysts were selected for elective single embryo transfer (eSET) and were warmed and cultured at 37 °C (6% CO2 and 5% O2) 2 h before replacement.
Outcome measures
Primary outcome measure of this study was cumulative live-birth rate per oocyte retrieval (CLBR), defined as the number of deliveries per oocyte collection cycle. Secondary outcomes included biochemical pregnancy loss (BPL) defined as serum βHCG levels ≥ 50 IU/L in at least two pregnancy tests (2–4 days elapsed between consecutive examinations), but not associated with any ultrasonographical evidence of intrauterine or extrauterine pregnancy 20–25 days after ET. Clinical pregnancy was defined as the presence of a gestational sac and fetal heartbeat at week 8 after embryo transfer. Ongoing implantation rate (OIR) was defined as the proportion of sacs with fetal heartbeat over transferred embryos. Miscarriage was defined as the absence of fetal heartbeat after initial confirmation of pregnancy. Miscarriage was further divided in first trimester miscarriage (< 12 weeks) that is usually associated with higher aneuploidies occurrence and late miscarriage (> 12 weeks). Clinically recognizable chromosomally abnormal pregnancy rates were identified by abnormal standard cytogenetic analysis following CVS/amniocentesis (prenatal diagnosis specimens, PND) and, when available, product of conception (POC) cytogenetic analysis, or at birth. Neonatal outcomes analyzed included birth weight, height and cranial circumference, as well as associated rates. Preterm birth (PTB) and low birth weight (LBR) rate were defined as delivery before 37 weeks of gestation and as birth weight below 2500 g. Time-to-pregnancy was defined as the number of embryo transfer procedures required achieving a delivery for all cycles where at least one transferrable embryo (i.e., euploid blastocysts for PGT-A group) was available.
Statistical analysis
Categorical variables are shown as percentages with 95% confidence interval (CI) and continuous variables as mean ± standard deviation (SD). Statistical analysis was conducted through a two-tailed Chi-square test for categorical variables and ANOVA with Bonferroni’s correction for continuous variables. Logistic regression analysis was used to correct the main analysis for the basal and cycle parameters related to the likelihood of achieving a live birth per egg retrieval and to control for confounding factors in the main comparisons. The post-hoc power analysis for the primary outcome measure was performed on the website http://www.powerandsamplesize.com using actual data from this study and considering a beta and alfa error of 80% and 5%, respectively. Kaplan-Meier statistical analysis was performed to calculate the effect on time-to-pregnancy between PGT-A and control group using as primary outcome the cumulative live-birth rate in function of number of transfers required. P value < 0.05 was considered statistically significant.
Results and discussion
PGT-A vs drop-out and control groups
A total of 3211 couples with female patients between 38 and 44 years old were initially enrolled in this study (Fig. 1). Basal characteristics, indications for IVF treatment, indications for PGT analysis (PGT-A and drop-out only), stimulation protocol, semen parameters and stimulation outcomes are reported on Table 1. Of note, female age was significantly higher in the drop-out group, while the control group showed a significantly lower average female age compared with both the other two groups. Similarly, FSH and AMH level parameters were significantly worse in the drop-out group compared with the other two. On the contrary, patients enrolled in the PGT-A and control groups had similar hormonal values. Other differences were present across groups in regards of medical indications for IVF treatment (Table 1). All these variables were controlled in multivariate models when performing the main statistical comparisons (Supplementary data).
Table 1.
Basal characteristics and stimulation outcomes of the studied populations
| Demographic data of study population | ||||||
|---|---|---|---|---|---|---|
| PGT-A | Drop-out | Control | P value | |||
| PGT-A vs drop-out | PGT-A vs control | Drop-out vs control | ||||
| No. of patients | 269 | 101 | 2168 | |||
| No. of cycles | 308 | 106 | 2905 | |||
| Mean female age (SD) | 40.4 (± 3.3) | 41.1 (± 3.0) | 39.7 (± 2.4) | P = 0.047 | P < 0.001 | P = 0.001 |
| BMI female | 21.9 (± 3.0) | 21.8 (± 3.0) | 22.4 (± 3.3) | NS | NS | NS |
| Duration of infertility, months (SD) | 57 (± 32.7) | 53 (± 36.4) | 51 (± 31.8) | NS | P = 0.004 | NS |
| FSH (mIU/mL) | 7.3 (± 2.3) | 8 (± 3.0) | 7.4 (± 2.5) | P = 0.017 | NS | P = 0.020 |
| AMH (ng/mL) | 2.7 (± 2.1) | 1.8 (± 1.7) | 2.4 (± 2.9) | P < 0.001 | NS | P = 0.039 |
| Indication to PGT-A per cycle | ||||||
| AMA (%) | 124/308 (40.3%) | 48/106 (45.3%) | NS | |||
| AMA + RIF (%) | 128/308 (41.6%) | 28/106 (26.4%) | P = 0.006 | |||
| AMA + RPL (%) | 49/308 (15.9%) | 28/106 (26.4%) | P = 0.016 | |||
| AMA + RIF+ RPL (%) | 6/308 (1.9%) | 1/106 (0.9%) | NS | |||
| AMA + other (%) | 1/308 (0.3%) | 1/106 (0.9%) | NS | |||
| Indication for infertility treatment per cycle | ||||||
| Male Factor | 71/308 (23.05%) | 15/106 (14.1%) | 823/2905 (28.3%) | NS | P = 0.049 | P = 0.001 |
| Idiopathic | 73/308 (23.7%) | 18/106 (17.0%) | 376/2905 (12.9%) | NS | P < 0.001 | NS |
| Mixed male and female factors | 53/308 (17.21%) | 26/106 (24.5%) | 713/2905 (24.5%) | NS | P = 0.004 | NS |
| Poor ovarian reserve | 41/308 (13.32%) | 26/106 (24.5%) | 466/2905 (16.0%) | P = 0.007 | NS | P = 0.020 |
| Recurrent miscarriage | 32/308 (10.39%) | 6/106 (5.7%) | 17/2905 (0.6%) | NS | P < 0.001 | P < 0.001 |
| Multiple female factors | 15/308 (4.87%) | 6/106 (5.7%) | 171/2905 (5.9%) | NS | NS | NS |
| Tubal factor | 14/308 (4.54%) | 4/106 (3.8%) | 230/2905 (7.9%) | NS | P = 0.034 | NS |
| Endometriosis | 6/308 (1.95%) | 3/106 (2.8%) | 70/2905 (2.4%) | NS | NS | NS |
| Abnormal ovulation | 3/308 (0.97%) | 2/106 (1.9%) | 39/2905 (1.3%) | NS | NS | NS |
| Protocol per cycle | ||||||
| Antagonist | 255/308 (82.8%) | 81/106 (76.5%) | 2235/2905 (76.9%) | NS | NS | NS |
| Flare-up | 9/308 (2.9%) | 12/106 (11.3%) | 290/2905 (10%) | P = 0.001 | P < 0.001 | NS |
| Agonist | 44/308 (14.3%) | 13/106 (12.3%) | 380/2905 (13.1%) | NS | NS | NS |
| Semen | ||||||
| Ejaculated (%) | 284/308 (92.2%) | 97/106 (91.5%) | 2609/2905 (89.8%) | NS | NS | NS |
| Surgical (%) | 24/308 (7.8%) | 9/106 (8.5%) | 296/2905 (10.2%) | NS | NS | NS |
| Stimulation outcome | ||||||
| Mean aspirated follicles (SD) | 13.4 (± 6.2) | 7.9 (± 4.4) | 12.8 (± 5.3) | P = 0.001 | NS | P = 0.001 |
| Mean retrieved oocyte (SD) | 10.9 (± 5.8) | 6.6 (± 4.1) | 10.2 (± 5.0) | P = 0.001 | P = 0.021 | P = 0.001 |
| Mean mature oocytes (SD) | 8.3 (± 4.1) | 4.6 (± 3.0) | 7.9 (± 3.7) | P = 0.001 | NS | P = 0.001 |
Of the 308 cases started in the PGT-A group, 55 did not generate blastocysts suitable for biopsy (17.9%, 95% CI 13.7–22.6; Fig. 1) and did not receive an embryo transfer. In 101 cases of the remaining 253 (40%, 95% CI 33.8–46.2), no euploid embryos were identified following PGT-A analysis (Table 2), therefore no embryo transfer was performed. Overall euploidy rate calculated on the total number of embryos biopsied and analyzed in this group was 40.8% (95% CI 37.3–44.3). Similarly, in the control group 88 oocyte collections of the initial 2905 did not lead to developing embryos by the time of transfer (3.0%, 95% CI 2.4–3.7). These patients also did not receive an embryo transfer. In total, 201, 117 and 3573 embryo transfers (ET) were performed in each group (PGT-A, drop-out and control, respectively). All ETs performed in the PGT-A group were eSET, while both in the drop-out and control groups a two-embryo strategy was commonly adopted (Table 2).
Table 2.
Treatment outcomes of main study groups
| Cumulative clinical outcomes of complete study group | ||||||
|---|---|---|---|---|---|---|
| PGT-A | Drop-out | Control | P value | |||
| PGT-A vs drop-out | PGT-A vs control | Drop-out vs control | ||||
| Treatment cycles started | 308 | 106 | 2905 | |||
| Embryo transfers | 201 | 117 | 3573 | |||
| Cases with embryos suitable for ET* or FZ* or biopsy** (%) | 253/308 (82.1%) | 106/106 (100%) | 2817/2905 (97.0%) | P < 0.001 | P < 0.001 | NS |
| Biopsied embryo (mean; + SD) | 770 (2.5 ± 2.0) | |||||
| Euploid embryos (%) | 314/770 (40.8%) | |||||
| Ongoing cases with one or more euploid blastocysts (%) | 152/253 (60%) | |||||
| Transferred embryos (mean; ± SD) | 201 (1 ± 0) | 195 (1.67 ± 0.68) | 6373 (1.78 ± 0.68) | P < 0.001 | P < 0.001 | P = 0.026 |
| Positive βhCG per transfer (%) | 93/201 (46.3%) | 22/117 (18.8%) | 1112/3573 (31.1%) | P < 0.001 | P = 0.004 | P < 0.001 |
| Biochemical pregnancy per βhCG +ve (%) | 5/93 (5.4%) | 2/22 (9.1%) | 84/1112 (7.5%) | NS | NS | NS |
| Ongoing implantation (%)§ | 84/201 (41.8%) | 19/195 (9.7%) | 1006/6373 (15.8%) | P < 0.001 | P < 0.001 | P = 0.021 |
| Ongoing clinical pregnancy per transfer (%) | 84/201 (41.8%) | 18/117 (15.4%) | 902/3573 (25.2%) | P < 0.001 | P < 0.001 | P = 0.016 |
| Ongoing clinical pregnancy per cycle started (%) | 84/308 (27.3%) | 18/106 (17.0%) | 902/2905 (31.0%) | P = 0.034 | NS | P = 0.002 |
| Multiple pregnancy rate (%) | 0/84 (0.0%) | 1/18 (5.6%) | 100/902 (11.1%) | NS | P < 0.001 | NS |
| Overall clinical pregnancy loss (%) | 3/84 (3.6%) | 8/18 (44.4%) | 204/902 (22.6%) | P < 0.001 | P < 0.001 | P = 0.044 |
| Miscarriages < 12 week (%) | 2/84 (2.4%) | 8/18 (44.4%) | 152/902 (16.8%) | P < 0.001 | P < 0.001 | P = 0.006 |
| Miscarriages > 12 week (%) | 0/84 (0.0%) | 0/18 (0.0%) | 35/902 (3.9%) | NS | NS | NS |
| Therapeutic abortion (%) | 0/84 (0.0%) | 0/18 (0.0%) | 13/902 (1.4%) | NS | NS | NS |
| Ectopic pregnancies (%) | 1/84 (1.2%) | 0/18 (0.0%) | 4/902 (0.4%) | NS | NS | NS |
| Aneuploid pregnancy per prenatal screening test (%)♯ | 0/24 (0.0%)° | 0/9 (0.0%) | 65/339 (19.2%) | NS | P = 0.012 | NS |
| Delivery per ongoing clinical pregnancy (%) | 81/84 (96.4%) | 9/18 (50.0%) | 698/902 (77.4%) | P < 0.001 | P < 0.001 | P = 0.011 |
| Singleton delivery (%) | 81/84 (100%) | 8/9 (88.9%) | 623/698 (89.3%) | NS | P = 0.034 | NS |
| Twin delivery (%) | 0/84 (0.0%) | 1/9 (11.1%) | 73/698 (10.5%) | NS | P < 0.001 | NS |
| Triplet delivery (%) | 0/84 (0.0%) | 0/9 (0.0%) | 2/698 (0.3%) | NS | NS | NS |
| Total multiple delivery (%) | 0/84 (0.0%) | 1/9 (11.1%) | 75/698 (10.8%) | NS | P < 0.001 | NS |
| Modeled drop-out rate (%)∞ | 5/233 (2.1%) | 5/96 (5.2%) | 264/2207 (11.9%) | NS | P < 0.0001 | 0.0495 |
| Outcome summary, delivery | ||||||
| Per cycle started (%) | 81/308 (26.3%) | 9/106 (8.5%) | 698/2905 (24.0%) | P < 0.001 | NS | P < 0.001 |
| Per embryo transfer (%) | 81/201 (40.3%) | 9/117 (7.7%) | 698/3573 (19.5%) | P < 0.001 | P < 0.001 | P < 0.001 |
| Per βhCG (%) | 81/93 (87.1%) | 9/22 (40.1%) | 698/1112 (62.8%) | P < 0.001 | P < 0.001 | P = 0.045 |
| Per ongoing clinical pregnancy (%) | 81/84 (96.4%) | 9/18 (50.0%) | 698/902 (77.4%) | P < 0.001 | P < 0.001 | P = 0.010 |
| Per cycle with transferrable embryos (%) | 81/152 (53.3%) | 9/106 (8.5%) | 698/2817 (24.8%) | P < 0.001 | P < 0.001 | P < 0.001 |
*Control and drop-out
**PGT-A
§Sacs with FHB/transferred embryos
♯Calculated on prenatal and POC analyses available
°One aneuploid pregnancy identified in the group removed due to spontaneous conception (Bettio et al., 2016)
∞Number of patients without live births with frozen embryos left/number of patients without live births
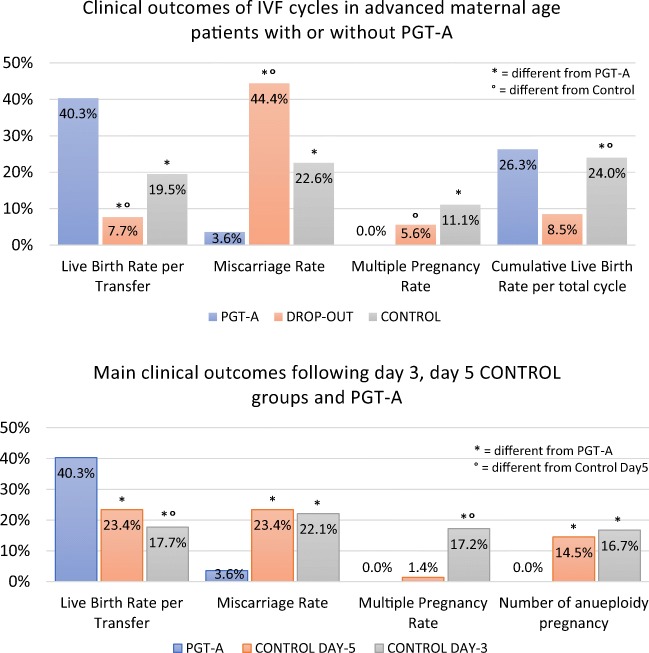
In this dataset, the PGT-A group achieved significantly higher positive βhCG (β + ve) and ongoing implantation (OIR) rates (46.3% and 41.8%, respectively) compared with both drop-out (18.8% and 9.7%, respectively) and control (31.1% and 15.8%, respectively) groups (Table 2). Additionally, despite the low overall multiple pregnancy rate, in the PGT-A group a significantly reduced multiple pregnancy rate was observed compared with the control group (0% and 11.1%, respectively; P < 0.001).
Remarkably, the most important direct effect of PGT-A was observed in the significant reduction of pregnancy loss. Both drop-out and control groups showed an increased rate of total pregnancy loss (44.4%, 95% CI 21.5–69.2 and 22.6%, 95% CI 19.9–25.5, respectively) compared with the PGT-A group (3.6%; 95% CI 0.7–10.1; Fig. 2 and Table 2). These results can be further identified as an effect of genetic testing when first trimester miscarriage rates (< 12 weeks) were assessed (2.4%, 95% CI 0.3–8.3; 44.4%, 95% CI 21.5–69.2, and 16.8%, 95% CI 14.5–19.5, in the PGT-A, drop-out, and control groups, respectively). Indeed, several chromosomal abnormalities allow blastocyst development and embryo implantation, however, rarely allow the fetus to grow in utero past the 12/16 weeks [19]. Logistic regression analysis adjusted for potential confounding factors confirmed this effect and showed around 80% relative reduction in the miscarriage risk following the transfer of euploid embryos (OR = 0.19, 95% CI 0.09–0.41; Supplementary table 1). It is crucial to note that in the drop-out group, abandonment of PGT-A analysis had drastic effects on miscarriage rate. These patients were initially enrolled to undergo chromosomal testing, however, they abandoned due to low number of embryos available. Due to their advanced maternal age, this decision critically exposed them to a high risk of transferring aneuploid embryos, thus increasing the chance of miscarriage or abnormal pregnancy. These data suggest that, in order to minimize the risk of miscarriage, AMA patients should not withdraw from PGT-A assessment even if few normally fertilized zygotes are produced. Similar negative effects of withdrawal from PGT-A due to poor embryological outcomes were also previously reported [26, 27]. Overall, this dataset shows that PGT-A improves clinical outcomes in terms of baby delivery per embryo transfer and that embryo chromosomal testing has a positive effect on the ability to successfully maintain the pregnancy to term. Compared with both drop-out and control groups, the PGT-A group also showed higher delivery rates per transfer cycle where transferrable embryos were available (i.e., euploid). Indeed, time-to-pregnancy was shorter in the PGT-A group (Fig. 3), with fewer embryos transferred and less ET procedures performed overall (Table 2).
Fig. 2.
Comparison of clinical outcomes across study groups. a Results of the main study groups (PGT-A, drop-out, and control group). b Results of control subgroups (day 5 and day 3)
Fig. 3.
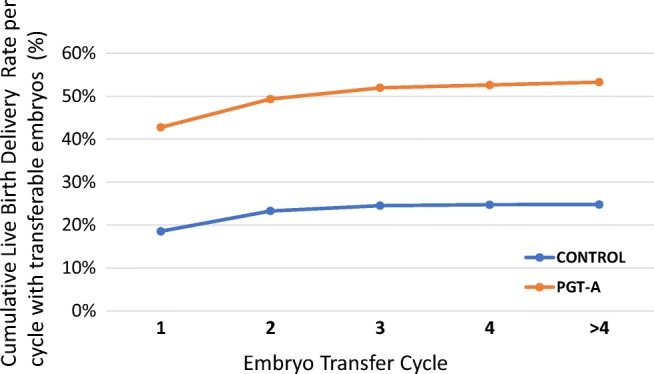
Progression of cumulative birth rates across embryo transfer cycles for patients with transferrable embryos (euploid embryos for PGT-A group)
Cumulative live-birth rate in PGT-A and control group is similar
Regarding the primary outcome measure of this study, the differences in delivery rate per egg retrieval between PGT-A and control groups were not significant (Table 2). The logistic regression model adjusted for the main potential confounding factors, confirmed the lack of a detrimental effect of PGT-A on the CLBR (Supplementary table 2). These data are extremely reassuring, considering that chromosomal testing does not offer the possibility to repair a genetically defective embryo, therefore any intervention performed on the embryo can have detrimental effects on its developmental ability. Regarding the possibility that biopsy procedures could both reduce embryo viability, and that normal embryos could be discarded as a result of false-positive diagnoses, these data provide evidence that PGT-A-related interventions have no long-lasting effect on embryo’s reproductive potential and no negative impact on the overall outcome of a treatment. As expected, female age, FSH, AMH and number of MII retrieved were variables associated with the likelihood of delivery per cycle. Furthermore, post-hoc analysis showed that this study is sufficiently powered to rule out a true reduction of 4% in the CLBR per started cycle in the PGT-A group. This data is extremely important to highlight that cumulative delivery rate per cycle with 2 years follow-up is not compromised by embryo biopsy intervention and the potential for false-positive aneuploidy calls when PGT-A is performed in optimal conditions by highly experienced IVF and preimplantation genetics laboratories. The value of this observation is particularly relevant in this study, which encompasses a two-year follow-up period comprising the transfer of almost all embryos obtained in each cycle and in the context of a reimbursed IVF treatment scheme allowing for minimal patients’ drop-out rate (Table 2).
PGT-A vs day 3 and day 5 control groups
In order to identify potential variations within the control group, we subsequently subdivided the control group according to embryo transfer strategy (e.g., day 3 and day 5) and compared the outcomes from these subsets with those produced by the PGT-A group. Similarly to the previous dataset, PGT-A group performed significantly better than the other two groups in terms of positive βhCG and ongoing implantation rates (Fig. 2b and Table 3). Notably, the control day 5 group received an average number of embryos per transfer (1.04 ± 0.19) comparable with the PGT-A group (1 ± 0), while the control day 3 group received a significantly higher number of embryos per transfer (2.14 ± 0.53). This difference had an impact on the multiple pregnancy rate observed for the three groups, with the latter performing significantly worse than the first two (0%; 1.4%, 17.2%, for PGT-A, control day 5, and control day 3, respectively; Table 3). Indeed, the employment of a strict SET policy on day 5 transfers also allowed the control group to significantly minimize the occurrence of multiple gestations. However, despite comparable multiple pregnancy rate achieved by the blastocyst ET groups (e.g., PGT-A and control day 5), the lack of aneuploidy testing permitted the transfer of abnormal embryos to the control groups. This reflected on significantly increased pregnancy loss rates in both day 5 and day 3 control groups (23.4%, 95% CI 19.1–28.2 and 22.1%, 95% CI 18.7–25.8, respectively) compared with the PGT-A group (3.6%, 95% CI 0.7–10.1; Table 3). Prenatal genetic testing results were also collected and analyzed when available. These included results from amniocentesis, chorionic villus sampling (CVS) and POCs. Results from this dataset showed that chromosomal abnormalities were equally detected in both day 3 and day 5 control groups (Table 3), while no recognizable aneuploid pregnancy event has been recorded in the PGT-A group. Among the several chromosomal abnormalities identified, the most common ones were those involving chromosome 21 and chromosome 16. A detailed listing of the aneuploidies identified through PND and POC analysis is available in Supplementary table 3.
Table 3.
Treatment outcomes of study subgroups
| Cumulative clinical outcomes of PGT-A group and day 3/day 5 control group | ||||||
|---|---|---|---|---|---|---|
| PGT-A | Control day 5 | Control day 3 | P value | |||
| PGT-A vs control (day 5 only) | PGT-A vs control (day 3 only) | Day 5 only vs day 3 only | ||||
| Embryo transfers | 201 | 1147 | 2426 | |||
| Transferred embryos (mean; ± SD) | 201 (1 ± 0) | 1188 (1.04 ± 0.19) | 5185 (2.14 ± 0.53) | P = 0.013 | P < 0.001 | P < 0.001 |
| Positive βhCG per transfer (%) | 93/201 (46.3%) | 433/1147 (37.7%) | 679/2426 (28.0%) | P = 0.023 | P < 0.001 | P < 0.001 |
| Biochemical pregnancy per βhCG + ve (%) | 5/93 (5.4%) | 31/433 (7.2%) | 53/679 (7.8%) | NS | NS | NS |
| Ongoing implantation (%)§ | 84/201 (41.8%) | 355/1188 (29.9%) | 651/5185 (12.55%) | P = 0.001 | P < 0.001 | P < 0.001 |
| Ongoing clinical pregnancy per transfer (%) | 84/201 (41.8%) | 350/1147 (30.5%) | 552/2426 (22.8%) | P = 0.002 | P < 0.001 | P < 0.001 |
| Multiple pregnancy rate (%) | 0/84 (0.0%) | 5/350 (1.4%) | 95/552 (17.2%) | NS | P < 0.001 | P < 0.001 |
| Overall clinical pregnancy loss (%) | 3/84 (3.6%) | 82/350 (23.4%) | 122/552 (22.1%) | P < 0.001 | P < 0.001 | NS |
| Miscarriages < 12 week (%) | 2/84 (2.4%) | 63/350 (18.0%) | 89/552 (16.1%) | P < 0.001 | P < 0.001 | NS |
| Miscarriages > 12 week (%) | 0/84 (0.0%) | 16/350 (4.6%) | 19/552 (3.4%) | P = 0.05 | NS | NS |
| Therapeutic abortion (%) | 0/84 (0.0%) | 2/350 (0.6%) | 11/552 (2.0%) | NS | NS | NS |
| Ectopic pregnancies (%) | 1/84 (1.2%) | 1/350 (0.3%) | 3/552 (0.5%) | NS | NS | NS |
| Aneuploid pregnancy per POC or prenatal screening test (%)♯ | 0/24 (0.0%)° | 17/117 (14.5%) | 34/203(16.7%) | P = 0.046 | P = 0.030 | NS |
| Delivery per ongoing clinical pregnancy (%) | 81/84 (96.4%) | 268/350 (76.6%) | 430/552 (77.9%) | P < 0.001 | P < 0.001 | NS |
| Singleton delivery (%) | 81/81 (100%) | 264/268 (98.5%) | 359/430 (83.5%) | NS | P < 0.001 | P < 0.001 |
| Twin delivery (%) | 0/81 (0.0%) | 4/268 (1.5%) | 69/430 (16.0%) | NS | P < 0.001 | P < 0.001 |
| Triplet delivery (%) | 0/81 (0.0%) | 0/268 (0.0%) | 2/430 (0.5%) | NS | NS | NS |
| Total multiple delivery (%) | 0/81 (0.0%) | 4/268 (1.5%) | 71/430 (16.5%) | NS | P < 0.001 | P < 0.001 |
| Outcome summary, delivery | ||||||
| Per embryo transfer (%) | 81/201 (40.3%) | 268/1147 (23.4%) | 430/2426 (17.7%) | P < 0.001 | P < 0.001 | P < 0.001 |
| Per βhCG (%) | 81/93 (87.1%) | 268/433 (61.9%) | 430/679 (63.3%) | P < 0.001 | P < 0.001 | NS |
| Per ongoing clinical pregnancy (%) | 81/84 (96.4%) | 268/350 (76.6%) | 430/552 (77.9%) | P < 0.001 | P < 0.001 | NS |
§Sacs with FHB/transferred embryos
♯Calculated on prenatal and POC analyses available
°One aneuploid pregnancy identified in the group removed due to spontaneous conception (Bettio et al., 2016)
In summary, data analysis across control groups suggest that, although single blastocyst transfer leads to higher implantation rates and lower multiple pregnancy rates compared with double cleavage stage embryo transfer, the risk of pregnancy loss and aneuploid gestation is comparable between the two strategies. On the other hand, PGT-A leads to significantly better outcomes in terms of implantation, multiple pregnancy risk minimization and sustained pregnancy compared with all other embryo transfer strategies.
Neonatal outcomes in PGT-A, day 3, and day 5 control groups
Further, we followed up the pregnancies until birth to investigate whether there were differences in standard obstetrical/neonatal parameters across the groups. Although no significant variations were recorded across PGT-A, drop-out, and total control groups (Table 4(a)), day 3 control group showed a significantly higher preterm rate and a higher proportion of babies born at low birth weight (Table 4(b)). As shown in the logistic regression models, these differences were primarily the consequence of the use of multiple embryo transfer strategy, which resulted in multiple pregnancies (Supplementary table 4). These results suggest that neither blastocyst culture nor PGT-A assessment (including trophectoderm biopsy) lead to suboptimal obstetrical and neonatal outcomes. As previously demonstrated by other studies [28, 29], multiple gestations are responsible for poor neonatal outcomes at different levels. In this context, PGT-A demonstrated to be a powerful technology to facilitate the implementation of SET policies, even when treating poor prognosis patients [12, 30].
Table 4.
Obstetrical outcomes and neonatal parameters of study groups. For neonatal parameters, not all characteristics were accessible for each baby and analyses were based on available data (reported as N)
| Cumulative neonatal outcomes | ||||||
|---|---|---|---|---|---|---|
| (a) | PGT-A | Drop-out | Control | P value | ||
| PGT-A vs drop-out | PGT-A vs control | Drop-out vs control | ||||
| Babies delivered | 81 | 10 | 775 | |||
| Still-birth (%) | 0/81 (0.0%) | 0/10 (0.0%) | 9/775 (1.2%) | NS | NS | NS |
| Chromosomal abnormality (%) | 0/81 (0.0%) | 0/10 (0.0%) | 7/775 (0.8%) | NS | NS | NS |
| Low birthweight (%) | 5/81 (6.2%) | 2/10 (20.0%) | 106/775 (13.7%) | NS | NS | NS |
| Preterm birth (%) | 6/81 (7.4%) | 1/10 (10.0%) | 109/775 (14.1%) | NS | NS | NS |
| Average length at birth (SD) (N) | 50 (± 2.5) (70) | 49.6 (± 1.8) (8) | 49.4 (± 3.2) (651) | NS | NS | NS |
| Average head circumference at birth (SD) (N) | 33.8 (± 1.5) (19) | 34 (± 1.4) (2) | 34.1 (± 2.5) (87) | NS | NS | NS |
| Average weight at birth (SD) (N) | 3203 (± 604) (55) | 3071 (± 489) (11) | 3087 (± 654) (528) | NS | NS | NS |
| (b) | PGT-A | Control (day 5 only) | Control (day 3 only) | P value | ||
| PGT-A vs control (day 5 only) | PGT-A vs control (day 3 only) | Day 5 vs day 3 | ||||
| Babies delivered | 81 | 272 | 503 | |||
| Still-birth (%) | 0/81 (0.0%) | 2/272 (0.7%) | 7/503 (1.4%) | NS | NS | NS |
| Chromosomal abnormality (%) | 0/81 (0.0%) | 1/272 (0.4%) | 6/503 (1.2%) | NS | NS | NS |
| Low Birthweight (%) | 5/82 (6.2%) | 9/272 (3.3%) | 97/503 (19.3%) | NS | NS | P < 0.001 |
| Preterm birth (%) | 6/82 (7.4%) | 11/272 (4.0%) | 98/503 (19.5%) | NS | P = 0.005 | P < 0.001 |
| Average length at birth (SD) (N) | 50 (± 2.5) (70) | 50.1 (± 2.5) (238) | 48.5 (± 3.9) (413) | NS | NS | 0.003 |
| Average head circumference at birth (SD) (N) | 33.8 (± 1.5) (19) | 34.4 (± 1.9) (36) | 33.7 (± 2.8) (51) | NS | NS | NS |
| Average weight at birth (SD) (N) | 3203 (± 604) (55) | 3305 (± 500) (185) | 2964 (± 693) (343) | NS | P = 0.016 | P < 0.001 |
Study limitations
Being an observational-cohort study, patients could not be randomly allocated to receive the specific intervention before monitoring the outcome. Hence, it is possible that some differences in the study populations might contribute to the observed outcomes. However, logistic regression model adjusted for all possible confounding factors recorded was used to minimize the impact of sampling bias and group heterogeneity for the main comparisons. Nonetheless, our results and conclusions are based on an exceptionally large IVF clinical dataset, completed with extensive prenatal and neonatal follow-up, thus providing meaningful comparisons.
Conclusions
Our study shows that PGT-A analysis offers significant improvement in transfer outcomes for patients of advanced maternal age undergoing IVF treatment without compromising cumulative live-birth rate. In particular, post-hoc analysis showed that this study is sufficiently powered to rule out a true reduction of 4% in the CLBR per started cycle in the PGT-A group for this patient population. Although further evidence is required from RCTs, this study with 2-years observation from egg retrieval suggests that no major impact on CLBR is expected when PGT-A is performed by standardized genetic technologies and experienced IVF laboratories. Enhancement of transfer outcomes was evident in all clinical parameters, including implantation and ongoing clinical pregnancy rates. Crucially, pregnancy loss was significantly reduced in the PGT-A group, compared with all other comparative groups. This finding suggests that patients qualifying for PGT-A assessment should undergo chromosomal testing procedures despite low number of embryos available as this strategy may minimize their risk of miscarriage. These data were further supported by the evidence that no aneuploid pregnancy was detected in PGT-A pregnancies compared with all other embryo transfer policies that did not employ aneuploidy testing at blastocyst stage. Additionally, our results show that neonatal parameters deriving from PGT-A cycles are comparable with singletons deliveries from the control group.
These results instill further confidence in the application of PGT-A strategies, demonstrating its safe and beneficial applicability, especially for those patients at increased risk of generating aneuploid embryos, as women of advanced reproductive age.
Electronic supplementary material
(DOCX 51 kb)
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mastenbroek S., Repping S. Preimplantation genetic screening: back to the future. Human Reproduction. 2014;29(9):1846–1850. doi: 10.1093/humrep/deu163. [DOI] [PubMed] [Google Scholar]
- 2.Dahdouh EM, Balayla J, Garcia-Velasco JA. Comprehensive chromosome screening improves embryo selection: a meta-analysis. Fertil Steril. 2015;104:1503–1512. doi: 10.1016/j.fertnstert.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 3.Geraedts J, Sermon K. Preimplantation genetic screening 2.0: the theory. Mol Hum Reprod [Internet]. Oxford University Press; 2016;22:839–44. Available from: https://academic.oup.com/molehr/article-lookup/doi/10.1093/molehr/gaw033 [DOI] [PMC free article] [PubMed]
- 4.Gleicher N, Orvieto R. Is the hypothesis of preimplantation genetic screening (PGS) still supportable? A review. J Ovarian Res. 2017:1–7. [DOI] [PMC free article] [PubMed]
- 5.Cox GF, Bürger J, Lip V, Mau UA, Sperling K, Wu B-L, Horsthemke B. Intracytoplasmic sperm injection may increase the risk of imprinting defects. Am J Hum Genet. 2002;71:162–164. doi: 10.1086/341096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeBaun MR, Niemitz EL, Feinberg AP. Association of in vitro fertilization with beckwith-wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet. 2003;72:156–160. doi: 10.1086/346031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos F, Hyslop L, Stojkovic P, Leary C, Murdoch A, Reik W, Stojkovic M, Herbert M, Dean W. Evaluation of epigenetic marks in human embryos derived from IVF and ICSI. Hum Reprod. 2010;25:2387–2395. doi: 10.1093/humrep/deq151. [DOI] [PubMed] [Google Scholar]
- 8.Martins W. P., Nastri C. O., Rienzi L., van der Poel S. Z., Gracia C., Racowsky C. Blastocyst vs cleavage-stage embryo transfer: systematic review and meta-analysis of reproductive outcomes. Ultrasound in Obstetrics & Gynecology. 2017;49(5):583–591. doi: 10.1002/uog.17327. [DOI] [PubMed] [Google Scholar]
- 9.Tannus S, Cohen Y, Son WY, Shavit T, Dahan MH. Cumulative live-birth rate following elective single blastocyst transfer compared with double blastocyst transfer in women aged 40 years and over. Reprod BioMed Online. 2017;35:733–738. doi: 10.1016/j.rbmo.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Styer AK, Wright DL, Wolkovich AM, Veiga C, Toth TL. Single-blastocyst transfer decreases twin gestation without affecting pregnancy outcome. Fertil Steril. 2008;89:1702–1708. doi: 10.1016/j.fertnstert.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 11.Capalbo A., Bono S., Spizzichino L., Biricik A., Baldi M., Colamaria S., Ubaldi F. M., Rienzi L., Fiorentino F. Sequential comprehensive chromosome analysis on polar bodies, blastomeres and trophoblast: insights into female meiotic errors and chromosomal segregation in the preimplantation window of embryo development. Human Reproduction. 2012;28(2):509–518. doi: 10.1093/humrep/des394. [DOI] [PubMed] [Google Scholar]
- 12.Rubio C, Bellver J, Rodrigo L, Castillón G, Guillén A, Vidal C, et al. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertil Steril. 2017;107:1122–1129. doi: 10.1016/j.fertnstert.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Forman Eric J., Hong Kathleen H., Ferry Kathleen M., Tao Xin, Taylor Deanne, Levy Brynn, Treff Nathan R., Scott Richard T. In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertility and Sterility. 2013;100(1):100-107.e1. doi: 10.1016/j.fertnstert.2013.02.056. [DOI] [PubMed] [Google Scholar]
- 14.Somigliana E, Busnelli A, Paffoni A, Vigano P, Riccaboni A, Rubio C, et al. Cost-effectiveness of preimplantation genetic testing for aneuploidies. Fertil Steril. 2019;111:1169–1176. doi: 10.1016/j.fertnstert.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 15.Scott RT, Jr, Ferry K, Su J, Tao X, Scott K, Treff NR. Comprehensive chromosome screening is highly predictive of the reproductive potential of human embryos: a prospective, blinded, nonselection study. Fertil Steril. 2012;97:870–875. doi: 10.1016/j.fertnstert.2012.01.104. [DOI] [PubMed] [Google Scholar]
- 16.Scott RT, Jr, Treff NR, Stevens J, Forman EJ, Hong KH, Katz-Jaffe MG, et al. Delivery of a chromosomally normal child from an oocyte with reciprocal aneuploid polar bodies. J Assist Reprod Genet. 2012;29:533–537. doi: 10.1007/s10815-012-9746-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ubaldi Filippo Maria, Cimadomo Danilo, Capalbo Antonio, Vaiarelli Alberto, Buffo Laura, Trabucco Elisabetta, Ferrero Susanna, Albani Elena, Rienzi Laura, Levi Setti Paolo E. Preimplantation genetic diagnosis for aneuploidy testing in women older than 44 years: a multicenter experience. Fertility and Sterility. 2017;107(5):1173–1180. doi: 10.1016/j.fertnstert.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Capalbo Antonio, Hoffmann Eva R, Cimadomo Danilo, Maria Ubaldi Filippo, Rienzi Laura. Human female meiosis revised: new insights into the mechanisms of chromosome segregation and aneuploidies from advanced genomics and time-lapse imaging. Human Reproduction Update. 2017;23(6):706–722. doi: 10.1093/humupd/dmx026. [DOI] [PubMed] [Google Scholar]
- 19.Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- 20.Bettio D, Capalbo A, Albani E, Rienzi L, Achille V, Venci A, et al. 45,X product of conception after preimplantation genetic diagnosis and euploid embryo transfer: evidence of a spontaneous conception confirmed by DNA fingerprinting. Reprod Biol Endocrinol. 2016;14. [DOI] [PMC free article] [PubMed]
- 21.Levi-Setti PE, Zerbetto I, Baggiani A, Zannoni E, Sacchi L, Smeraldi A, et al. An observational retrospective cohort trial on 4,828 IVF cycles evaluating different low prognosis patients following the Poseidon criteria. Front Endocrinol (Lausanne). 2019;10. [DOI] [PMC free article] [PubMed]
- 22.Levi-Setti Paolo Emanuele, Menduni Francesca, Smeraldi Antonella, Patrizio Pasquale, Morenghi Emanuela, Albani Elena. Artificial shrinkage of blastocysts prior to vitrification improves pregnancy outcome: analysis of 1028 consecutive warming cycles. Journal of Assisted Reproduction and Genetics. 2016;33(4):461–466. doi: 10.1007/s10815-016-0655-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tedeschi G, Albani E, Borroni EM, Parini V, Brucculeri AM, Maffioli E, Negri A, Nonnis S, Maccarrone M, Levi-Setti PE. Proteomic profile of maternal-aged blastocoel fluid suggests a novel role for ubiquitin system in blastocyst quality. J Assist Reprod Genet. 2017;34:225–238. doi: 10.1007/s10815-016-0842-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cobo Ana, de los Santos María José, Castellò Damià, Gámiz Pilar, Campos Pilar, Remohí José. Outcomes of vitrified early cleavage-stage and blastocyst-stage embryos in a cryopreservation program: evaluation of 3,150 warming cycles. Fertility and Sterility. 2012;98(5):1138-1146.e1. doi: 10.1016/j.fertnstert.2012.07.1107. [DOI] [PubMed] [Google Scholar]
- 25.Capalbo Antonio, Treff Nathan R, Cimadomo Danilo, Tao Xin, Upham Kathleen, Ubaldi Filippo Maria, Rienzi Laura, Scott Richard T. Comparison of array comparative genomic hybridization and quantitative real-time PCR-based aneuploidy screening of blastocyst biopsies. European Journal of Human Genetics. 2014;23(7):901–906. doi: 10.1038/ejhg.2014.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murugappan G, Shahine LK, Perfetto CO, Hickok LR, Lathi RB. Intent to treat analysis of in vitro fertilization and preimplantation genetic screening versus expectant management in patients with recurrent pregnancy loss. Hum Reprod. 2016. [DOI] [PubMed]
- 27.Rienzi Laura, Capalbo Antonio, Vajta Gabor, Ubaldi Filippo Maria. PGS for recurrent pregnancy loss: still an open question. Human Reproduction. 2016;32(2):476–477. doi: 10.1093/humrep/dew311. [DOI] [PubMed] [Google Scholar]
- 28.Levi Setti PE, Moioli M, Smeraldi A, Cesaratto E, Menduni F, Livio S, Morenghi E, Patrizio P. Obstetric outcome and incidence of congenital anomalies in 2351 IVF/ICSI babies. J Assist Reprod Genet. 2016;33:711–717. doi: 10.1007/s10815-016-0714-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castillo CM, Horne G, Fitzgerald CT, Johnstone ED, Brison DR, Roberts SA. The impact of IVF on birthweight from 1991 to 2015: a cross-sectional study. Hum Reprod. 2019;34:920–931. doi: 10.1093/humrep/dez025. [DOI] [PubMed] [Google Scholar]
- 30.Ubaldi Filippo Maria, Capalbo Antonio, Colamaria Silvia, Ferrero Susanna, Maggiulli Roberta, Vajta Gábor, Sapienza Fabio, Cimadomo Danilo, Giuliani Maddalena, Gravotta Enrica, Vaiarelli Alberto, Rienzi Laura. Reduction of multiple pregnancies in the advanced maternal age population after implementation of an elective single embryo transfer policy coupled with enhanced embryo selection: pre- and post-intervention study. Human Reproduction. 2015;30(9):2097–2106. doi: 10.1093/humrep/dev159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 51 kb)