Abstract
Purpose
To assess the effect of assisted hatching (AH) on live birth rate (LBR) in first cycle, fresh in vitro fertilization (IVF) in good and poor prognosis patients.
Methods
Retrospective cohort using cycles reported to the Society for Assisted Reproductive Technology Clinic Outcomes Reporting System. Live birth rate was compared in women who underwent first cycle, autologous, fresh IVF cycles with (n = 48,858) and without (n = 103,413) AH from 2007 to 2015.
Results
The propensity-weighted LBR was 39.2% with AH versus 43.9% without AH in all patients. The rate difference (RD) with AH was − 4.7% ([CI − 0.053, − 0.040], P < 0.001) with the calculated number needed to harm being 22. AH affected live birth in both good prognosis and poor prognosis patients. The propensity-weighted monozygotic twinning (MZT) rate was 2.3% in patients treated with AH as compared to 1.2% patients that did not receive AH. The RD with AH on MZT in fresh, first IVF cycles was 1.1% ([0.008, 0.014], P < 0.001).
Conclusion
AH may affect LBR across all patients and in poor prognosis patients in fresh IVF cycles. Caution should be exercised when applying this technology. More prospective research is needed.
Electronic supplementary material
The online version of this article (10.1007/s10815-019-01619-2) contains supplementary material, which is available to authorized users.
Keywords: Assisted hatching, Live birth rate, In vitro fertilization, Poor prognosis
Introduction
Success rates with in vitro fertilization (IVF) continue to improve, yet some embryos still fail to implant. Human embryos naturally “hatch” during the physiologic process of development and implantation. Assisted hatching (AH), or artificially thinning/drilling/breaching the zona pellucida, has been proposed as a technique in assisted reproduction to improve the capacity for the embryo to implant. A variety of AH techniques have been employed in the past including mechanical, chemical, and laser-assisted hatching [1].
Data regarding AH and its effect on live birth rate (LBR) is limited. There have only been a few studies looking at LBR and AH with just 255 live births reported from these small trials [2]. In a Cochrane Review from 2012, even though the clinical pregnancy rate was improved with AH, the LBR was not different [3]. ASRM concluded in 2014 that due to a limited number of studies, there is insufficient evidence at this time to conclude that AH improves LBR [2]. A recent review highlighted that many adjuncts used in the IVF laboratory, including AH, are utilized in the absence of evidence based medicine and often at an additional fee [4].
Some studies have shown an improvement in clinical pregnancy rate with AH specifically in poor prognosis patients [3]. These poor prognosis patients have been defined as those that have 2 or more failed IVF cycles, poor embryo quality, 38 years of age or older, elevated follicle-stimulating hormone (FSH) value, and/or have a diagnosis of diminished ovarian reserve (DOR) [3, 5, 6]. The Cochrane review identified 4 randomized control trials with 567 women with poor prognosis patients and found that there was no significant difference in LBR in those that underwent AH and those that did not (OR 1.46, 95% CI 0.99–2.15, P = 0.6) [3]. More recent data has shown that AH in first cycle autologous frozen cycles is not beneficial and may actually decrease LBR, especially in patients 38 and older [7]. The ASRM and SART practice committee opinion states that “until data about LBR are available and in the context of increased risk of multiple pregnancy, it is premature to recommend AH in all patients with poor prognosis.” [2].
Here, we describe, in a large retrospective series, the effect of AH on LBR in first cycle, fresh IVF in all patient populations and specifically in poor prognosis patients. We aim to add to the literature in AH to help physicians deliver evidence-based care.
Methods
Ethical approval
The study was reviewed by the Institutional Review Board at the University of Texas Health Science Center and was determined to be exempt.
Data source and outcome measures
Data used in this study was obtained from the Society for Assisted Reproductive Technology Clinic Outcomes Reporting System (SART CORS) between 2007 and 2015 and was approved by the SART research committee. The SART CORS contains comprehensive data from more than 90% of clinics performing ART in the US. The data is reported to the Centers for Disease Control and Prevention, in compliance with the Fertility Clinic Success Rate and Certification Act of 1992 (Public Law 102- 493) and is validated annually [8]. All data was de-identified.
The primary outcome measure was LBR. Secondary outcomes were pregnancy rate (PR), spontaneous abortion rate, and the rate of monozygotic twinning (MZT) [9]. Data analysis included all fresh, autologous first IVF cycles where transfer occurred between 2007 and 2015. We choose to use first cycles to yield the purest evaluation of the effect of AH on pregnancy outcomes. Outcomes of ART are multifactorial with multiple cycles and embryo transfers adding confounding variables. Primary and secondary outcomes were compared in the embryo groups that all received AH versus those groups without AH (no-AH). IVF cycles where AH data was not entered or AH on only some embryos were excluded. Cycles with an incomplete data set, defined as missing a covariate described below, or those that included preimplantation genetic testing (PGT) were also excluded from the analysis. A total of 48,694 cycles were excluded with AH, and 98,689 cycles that received AH. The covariates with the most missing data included body mass index (BMI), parity, and maximum FSH.
Primary and secondary outcomes were calculated separately in poor prognosis and good prognosis patients. Poor prognosis patients included patients with one of the following poor prognosis criteria defined by: age 38 years or older, no history of a live birth, or poor-quality embryos. Good prognosis patients included patients with all of the following: age 37 years and younger, history of a live birth, and no poor-quality embryos. Embryo quality was based on morphologic features recorded in the SART database. To evaluate the most recent year-specific effects of AH, primary outcomes were also compared per year in the last 5 years in the data set (2011–2015).
Statistical methods
Factors associated with receiving AH, such as age and etiology of infertility, were associated with cycle outcomes. To account for these confounding factors, we conducted inverse probability of treatment weighting using the covariate balancing propensity score methodology, which balances the means of covariates in the propensity-weighted data [10]. The effect of receiving AH on each cycle outcome was measured by the average treatment effect (ATE). For this study, the ATE compares the probabilities of two counterfactual cycle outcomes of the recipients that would have been observed if all the recipients had been treated with and without AH. The propensity score was defined as the probability of receiving AH given the following covariates: reporting year, prior birth history, etiology of infertility, age at retrieval, day 3 or day 5 transfer, maximum FSH level, BMI, total embryos transferred, and quality of embryos. Therefore, the ATEs estimated with propensity score weights represent the causal effects of AH on cycle outcomes with the above covariates being controlled. In addition, using all patients, the ATEs were estimated per year from 2011 to 2015. Logistic regression models were used to estimate the propensity score. The data set used for estimating the propensity was summarized in terms of demographics and confounding factors as described before. AH and no-AH groups were compared with chi-squared tests for categorical variables, t tests for continuous variables, and standardized mean differences. Standardized mean differences (SMD) are an indication of how closely potential confounding variables are balanced between intervention and comparison groups. To assess effect modification by prognosis status, separate propensity score analyses were conducted for good and poor prognosis groups as described above. Theses stratifications were motivated from the study of Kissin [5]. Regarding LBR, analysis of diagnosis of DOR and day of transfer were performed separately. R software (R Foundation for Statistical Computing, Vienna, Austria) was used for all analyses and the threshold for significance was a two-sided P value of 0.05.
Results
Demographics
The study population from 2007 to 2015 included 152,271 fresh, autologous, first IVF cycles. Overall, AH occurred in 48,858 cycles and no-AH occurred in 103,413 cycles. The AH versus no-AH study groups were statistically different in terms of age at retrieval, race, gravidity, parity, prior birth history, infertility diagnosis, and markers of ovarian reserve (FSH). The AH group was older at time of retrieval, less parous, with a higher FSH compared with the no-AH group. The AH group also had a higher number of embryos transferred and more day 3 transfers as compared with the no-AH group (Table 1). The embryo quality and prognosis were also different between the study groups with the AH group having a higher percentage of poor prognosis patients compared to the no-AH group (Table 2). Due to differences in demographics and prognostic indicators, propensity score analysis was performed, and standard mean differences were evaluated.
Table 1.
Description of study groups from complete cases, before and after propensity score weighting
| Study groups before propensity score weighting | Study groups after propensity score weighting | |||||||
|---|---|---|---|---|---|---|---|---|
| Factor | Fresh transfer with AH (48,858 cycles) | Fresh transfer without AH (103,413 cycles) | P value | SMD | Fresh transfer with AH (148,723 cycles) | Fresh transfer without AH (153,114 cycles) | P value | SMD |
| Age at retrieval | < 0.001 | 0.796 | 0.201 | 0.015 | ||||
| < 35 | 15,978 (32.7) | 64,654 (62.5) | 78,955 (53.1) | 81,861 (53.5) | ||||
| 35–37 | 9445 (19.3) | 23,136 (22.4) | 31,847 (21.4) | 32,197 (21.0) | ||||
| 38–40 | 13,664 (28.0) | 11,195 (10.8) | 24,054 (16.2) | 24,361 (15.9) | ||||
| 41–42 | 6448 (13.2) | 3169 (3.1) | 9310 (6.3) | 9805 (6.4) | ||||
| ≥ 43 | 3323 (6.8) | 1259 (1.2) | 4557 (3.1) | 4890 (3.2) | ||||
| Race | < 0.001 | 0.151 | < 0.001 | 0.188 | ||||
| White | 24,679 (50.5) | 49,815 (48.2) | 78,135 (52.5) | 71,798 (46.9) | ||||
| Asian | 4942 (10.1) | 8847 (8.6) | 14,239 (9.6) | 12,660 (8.3) | ||||
| Hispanic Latino | 3257 (6.7) | 5672 (5.5) | 9671 (6.5) | 8502 (5.6) | ||||
| African American | 2993 (6.1) | 5040 (4.9) | 8346 (5.6) | 7665 (5.0) | ||||
| American Indian | 114 (0.2) | 206 (0.2) | 350 (0.2) | 307 (0.2) | ||||
| Unknown | 12,873 (26.3) | 33,833 (32.7) | 37,983 (25.5) | 52,182 (34.1) | ||||
| BMI (mean (SD)) | 26.08 (29.32) | 25.80 (29.99) | 0.082 | 0.01 | 25.94 (26.65) | 25.94 (32.98) | 0.982 | < 0.001 |
| Gravidity | < 0.001 | 0.154 | 0.36 | 0.016 | ||||
| 0 | 25,464 (52.1) | 61,032 (59.0) | 84,431 (56.8) | 86,871 (56.7) | ||||
| 1 | 11,543 (23.6) | 22,734 (22.0) | 33,819 (22.7) | 34,362 (22.4) | ||||
| 2 | 5920 (12.1) | 10,457 (10.1) | 15,847 (10.7) | 16,618 (10.9) | ||||
| 3 | 3197 (6.5) | 5103 (4.9) | 8212 (5.5) | 8296 (5.4) | ||||
| 4 | 1502 (3.1) | 2312 (2.2) | 3497 (2.4) | 3911 (2.6) | ||||
| 5 | 704 (1.4) | 1038 (1.0) | 1739 (1.2) | 1820 (1.2) | ||||
| > 5 | 528 (1.1) | 737 (0.7) | 1178 (0.8) | 1237 (0.8) | ||||
| Parity | < 0.001 | 0.085 | < 0.001 | 0.04 | ||||
| 0 | 12,266 (38.4) | 25,801 (42.5) | 35,423 (39.5) | 38,748 (41.4) | ||||
| ≥ 1 | 19,706 (61.6) | 34,881 (57.5) | 54,345 (60.5) | 54,818 (58.6) | ||||
| Infertility diagnosis | < 0.001 | 0.354 | 0.004 | 0.031 | ||||
| Diminished ovarian reserve | 9663 (19.8) | 8754 (8.5) | 18,554 (12.5) | 19,268 (12.6) | ||||
| Endometriosis | 3832 (7.8) | 7941 (7.7) | 11,853 (8.0) | 11,991 (7.8) | ||||
| Male factor | 17,642 (36.1) | 41,654 (40.3) | 57,439 (38.6) | 59,331 (38.7) | ||||
| PCOS | 3508 (7.2) | 11,688 (11.3) | 15,059 (10.1) | 15,309 (10.0) | ||||
| Tubal | 1142 (2.3) | 2677 (2.6) | 3483 (2.4) | 4051 (2.8) | ||||
| Unexplained | 6730 (13.8) | 17,622 (17.0) | 23,173 (15.6) | 23,940 (15.6) | ||||
| Uterine | 784 (1.6) | 1405 (1.4) | 2374 (1.6) | 2130 (1.4) | ||||
| Other | 5557 (11.4) | 11,672 (11.3) | 16,788 (11.3) | 17,094 (11.2) | ||||
| Maximum FSH (mean mIU/mL (SD)) | 8.45 (26.19) | 7.32 (29.99) | < 0.001 | 0.04 | 7.58 (17.07) | 7.64 (53.49) | 0.27 | 0.009 |
| Number of embryos transferred (mean (SD)) | 2.35 (0.98) | 1.90 (0.65) | < 0.001 | 0.55 | 2.05 (0.82) | 2.06 (0.80) | 0.319 | 0.007 |
| Day of transfer | < 0.001 | 0.785 | 0.027 | 0.015 | ||||
| 3 | 35,995 (73.7) | 38,630 (37.4) | 72,970 (49.1) | 74,407 (48.3) | ||||
| 5 | 12,863 (26.3) | 64,783 (62.6) | 75,753 (50.9) | 79,107 (51.7) | ||||
P value < 0.05 denotes a difference in groups; SMD, standard mean difference; SMD < 0.1 implies balance; AH, assisted hatching; BMI, body mass index; FSH, follicle-stimulating hormone; SD, standard deviation
Table 2.
Description of prognostic factors used to define good and poor prognosis groups in complete cases, before and after propensity score weighting
| Study groups before propensity score weighting | Study groups after propensity score weighting | |||||||
|---|---|---|---|---|---|---|---|---|
| Factor | Fresh transfer with AH (48,858 cycles) | Fresh transfer without AH (103,413 cycles) | P value | SMD | Fresh transfer with AH (148,723 cycles) | Fresh transfer without AH (153,114 cycles) | P value | SMD |
| Age | < 0.001 | 0.756 | 0.969 | < 0.001 | ||||
| < 38 | 25,423 (52.0) | 87,790 (84.9) | 110,802 (74.5) | 114,058 (74.5) | ||||
| ≥ 38 | 23,435 (48.0) | 15,623 (15.1) | 37,921 (25.5) | 39,056 (25.5) | ||||
| Live birth history | < 0.001 | 0.139 | 0.916 | 0.001 | ||||
| No | 25,464 (52.1) | 61,032 (59.0) | 84,431 (56.8) | 86,871 (56.7) | ||||
| Yes | 23,394 (47.9) | 42,381 (41.0) | 64,293 (43.2) | 66,244 (43.3) | ||||
| Embryo quality | < 0.001 | 0.187 | 0.018 | 0.018 | ||||
| Poor | 2803 (5.7) | 3755 (3.6) | 6528 (4.4) | 6440 (4.2) | ||||
| Fair | 12,788 (26.2) | 20,778 (20.1) | 34,259 (23.0) | 34,370 (22.4) | ||||
| Good | 33,267 (68.1) | 78,880 (76.3) | 107,936 (72.6) | 112,305 (73.3) | ||||
| Good or poor prognosis* | < 0.001 | 0.272 | 0.778 | 0.002 | ||||
| Good | 9591 (19.6) | 32,429 (31.4) | 41,192 (27.7) | 42,277 (27.6) | ||||
| Poor | 39,267 (80.4) | 70,984 (68.6) | 107,531 (72.3) | 110,837 (72.4) | ||||
P value < 0.05 denotes a difference in groups; SMD, standard mean difference; SMD < 0.1 implies balance; AH, assisted hatching
*Poor prognosis: one of the following poor prognosis criteria defined by: age 38 years or older, no history of a live birth, or poor-quality embryos. *Good prognosis: all of the following: age 37 years and younger, history of a live birth, and no poor-quality embryos
Pregnancy outcomes in all patient populations
The propensity-weighted LBR was 39.2% with AH versus 43.9% without AH in all patients. The rate difference (RD) with AH was − 4.7% ([CI − 0.053, − 0.040], P < 0.001) with the calculated number needed to harm being 22 (Table 3). The RD with AH on pregnancy rate was − 5.0% ([CI − 0.057, − 0.044], P < 0.001). AH did not change spontaneous abortion rate (7.7% AH compared to 7.7% no-AH, RD − 0.001 [CI − 0.004, 0.003], P = 0.772). Pregnancy outcomes with AH compared to no-AH without adjusting for confounders have a larger rate difference (Supplemental Table 1).
Table 3.
Pregnancy outcomes and assisted hatching
| Group (sample size) | AH | No-AH | Rate difference [CI] | P value |
|---|---|---|---|---|
| A. Pregnancy rate and AH | ||||
| All patients (152,271) | 56.51% | 61.55% | − 0.050 [− 0.057, − 0.044] | < 0.001 |
| Poor prognosis (110,251) | 54.37% | 59.65% | − 0.053 [− 0.06, − 0.045] | < 0.001 |
| Good prognosis (42,020) | 62.17% | 66.88% | − 0.047 [− 0.059, − 0.035] | < 0.001 |
| B. Spontaneous abortion rate and AH | ||||
| All patients (152,271) | 7.66% | 7.72% | − 0.001 [− 0.004, 0.003] | 0.772 |
| Poor prognosis (110,251) | 7.83% | 7.76% | 0.001 [− 0.003, 0.005] | 0.723 |
| Good prognosis (42,020) | 7.18% | 7.48% | − 0.003 [− 0.001, 0.004] | 0.380 |
| C. Live birth rate and AH | ||||
| All patients (152,271) | 39.16% | 43.85% | − 0.047 [− 0.053, − 0.040] | < 0.001 |
| Poor prognosis (110,251) | 36.97% | 41.90% | − 0.049 [− 0.057, − 0.042] | < 0.001 |
| Good prognosis (42,020) | 45.16% | 49.49% | − 0.043 [− 0.056, − 0.030] | < 0.001 |
| D. Monozygotic twinning and AH | ||||
| All patients (76,759) | 2.29% | 1.22% | 0.011 [0.008, 0.014] | < 0.001 |
| Poor prognosis (53,194) | 2.26% | 1.14% | 0.011 [0.007, 0.015] | < 0.001 |
| Good prognosis (23,565) | 2.41% | 1.43% | 0.01 [0.004, 0.015] | < 0.001 |
P value < 0.05 denotes a difference in groups; AH, assisted hatching; CI, confidence interval
Pregnancy outcomes in subgroups by patient prognosis
From 2007 to 2015, there were 42,020 fresh, autologous, first IVF cycles with a good prognosis (AH in 9591 and no-AH in 32,429) and 110,251 fresh, autologous, first IVF cycles with a poor prognosis (AH in 39,267 and no-AH in 70,984). AH was performed in 35.6% of the poor prognosis group and 22.8% of the good prognosis. After adjusting for confounders, LBR was 47.35% in the good prognosis group and 39.47% in the poor prognosis group. In good prognosis cycles, the RD with AH on LBR was − 4.3 ([CI − 0.056, − 0.030], P < 0.001). In poor prognosis cycles, the RD with AH on LBR was − 4.9% ([− 0.057, − 0.042], P < 0.001) (Table 3).
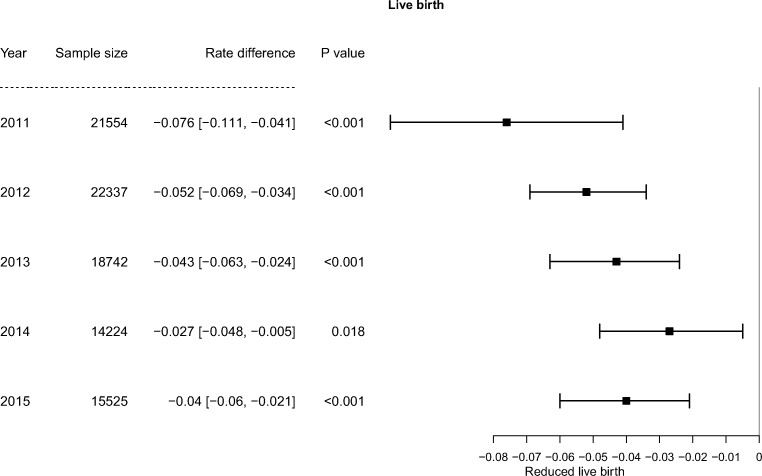
AH analysis per year
From the most recent years 2011 to 2015, AH consistently had a negative effect on LBR when other factors were controlled (Fig. 1). In the most recent analysis year (2015) including 15,525 cycles, the rate difference with AH was − 4.0% ([CI − 0.060, − 0.021], P < 0.001). On average, in the past recent 5 years, the rate difference with AH was − 6.0% ([CI − 0.089, − 0.032], P < 0.001), which did not differ significantly from the − 4.5% rate difference ([CI − 0.055, − 0.034], P < 0.001), in the previous years (2007–2010) (P = 0.32). AH also significantly decreased PR across these years and did not affect spontaneous abortion rate (data not shown).
Fig. 1.
Effect of AH on LBR per year. AH consistently decreased LBR from 2011 to 2015
Monozygotic twinning
The propensity-weighted MZT rate was 2.3% in patients treated with AH as compared with 1.2% patients that did not receive AH. The RD with AH on MZT in fresh, first IVF cycles was 1.1% ([0.008, 0.014], P < 0.001). AH increased the MZT rate in both good and poor prognosis patients (Table 3).
Day of transfer
Even though day of transfer was a variable controlled by propensity scores, a further analysis of AH and LBR was completed according to day of embryo transfer. AH consistently affected live birth outcomes with both day 3 and day 5 embryo transfers when other factors were controlled across all patients, both good and poor prognoses. Across all patients undergoing day 3 transfers, AH reduced LBR by 3.8% (rate difference (RD) − 0.038 [CI − 0.045, − 0.03], P < 0.001). Across all patients undergoing day 5 transfers, AH reduced LBR by 5.3% (rate difference (RD) − 0.053[CI − 0.063, − 0.043], P < 0.001) (Table 4).
Table 4.
Live birth rate and assisted hatching in propensity-weighted populations with day 3 and day 5 embryo transfer
| Group (sample size) | AH | AH | Rate difference [CI] | P value |
|---|---|---|---|---|
| Day 3 transfer | ||||
| All patients (74,625) | 31.54% | 35.33% | − 0.038 [− 0.045, − 0.03] | < 0.001 |
| Poor prognosis (56,754) | 29.32% | 33.3% | − 0.04 [− 0.048, − 0.031] | < 0.001 |
| Good prognosis (17,871) | 38.2% | 43.57 | − 0.054 [− 0.073, − 0.035] | < 0.001 |
| Day 5 transfer | ||||
| All patients (77,646) | 46.46% | 51.74% | − 0.053 [− 0.063, − 0.043] | < 0.001 |
| Poor prognosis (53,497) | 44.58% | 50.77% | − 0.062 [− 0.074, − 0.05] | < 0.001 |
| Good prognosis (24,149) | 50.8% | 53.92% | − 0.031 [− 0.05, − 0.012] | < 0.001 |
P value < 0.05 denotes a difference in groups; AH, assisted hatching; CI, confidence interval
Diagnosis of diminished ovarian reserve
Another analysis was performed based on the diagnosis of DOR as reported to SART. AH consistently affected LBR in both patients with and without a diagnosis of DOR. Across patients with a diagnosis of DOR, AH reduced LBR by 5.2% (rate difference (RD) − 0.052 [CI − 0.067, − 0.037], P < 0.001). In patients without a diagnosis of DOR, AH reduced LBR by 5.1% (rate difference (RD) − 0.051 [CI − 0.058, − 0.044], P < 0.001).
Discussion
We found that AH is still being used and more women in the poor prognosis category had AH than those without AH. The use of AH in first, autologous fresh IVF cycles may affect live birth outcomes in both good and poor prognosis patients. Usage of AH continues despite the lack of definitive data supporting and directing its use [7]. This analysis highlights the need for more prospective data on the use AH as an adjunct in IVF.
This study offers a more recent analysis of AH and pregnancy outcomes. In previous studies, Kissin et al. analyzed AH in a large retrospective analysis of the National Assisted Reproductive Technology Surveillance System from 2000 to 2010 and showed that AH was not associated with improved outcomes [5]. We did a sub-analysis of the most recent 5 years available in SART (2011–2015) and found that AH was consistently not beneficial. This 5-year period was chosen to evaluate the time period since the analysis by Kissin et al. [5].
AH usage has increased and there has also been a change in the type of hatching method over time [5, 11]. Laser-mediated technology has been more widespread in the last 10 years as compared to mechanical or chemical procedures [11]. Although SART does not differentiate the type of AH procedure, our recent analysis is more reflective of current techniques. Our sub-analysis of the most recent 5 years available in SART (2011–2015) did not show any difference in rate of harm as compared to previous years (2007–2010) suggesting that although AH techniques have changed, the outcome is not improving LBR as intended. In addition to a more recent analysis, this analysis includes embryo morphology in poor prognosis patients. SART morphologic grading system reports overall grade of the embryo at time of transfer. Grading is a subjective assessment of the overall quality of the embryo as good, fair, or poor, and is based on the assessment of the embryo, such as fragmentation, symmetry, inner cell mass quality, or trophectoderm quality. SART CORS morphological measures of embryos have been previously shown to be predictive of live birth after IVF [12]. Previous analyses have used a lack of embryos left for cryopreservation as a surrogate marker for embryo quality in poor prognosis patients [5]. However, it has since been shown that not having cryopreservation did not reliably indicate poor quality [13]. We choose to use SART morphologic embryo grading as a more accurate marker for poor-quality embryos as compared to just utilizing lack of embryos for cryopreservation. Therefore, our subgroup analysis of poor prognosis patients included either increased age, patients without a history of live birth, or poor embryo quality as depicted in SART. Although this definition of poor prognosis does not include patients with failed prior IVF cycles, it does allow one to consider the population that presents for their first IVF cycle with these patient characteristics.
Although definitions for poor prognosis have varied, it has been proposed that AH is most beneficial in this patient subgroup. However, our analysis shows that poor prognosis patients reap no benefit from AH and in fact, AH does not improve LBR in this patient population. The risk difference with AH is less in poor prognosis patients compared to good prognosis patients, yet it still affects both groups. Based on this data, we do not recommend patients with a poor prognosis be targeted as a group that would benefit from AH. These conclusions do not extend to frozen/thawed embryos; however, this was recently published in separate analysis which did not find a benefit to AH in frozen embryo cycles [7].
Increased manipulation of the embryo, such as with AH, may be harmful to the embryo. This may offer an explanation to the reduction in live birth. Specifically, the created hole, by AH, in the zona pellucida may decrease protection of the embryo from toxins. It has also been speculated that interrupting the natural hatching process of the blastomeres may impair implantation [14].
Whether AH is associated with an increased risk for MZT continues to be controversial and may vary based on the type of embryo transfer. Previous data supports that AH increases the risk for MZT, especially in fresh transfers and day 2–3 transfers [15, 16]. Conversely, analysis of frozen embryo transfers (FET) and AH from the SART database did not reveal an increased risk [7]. Whether there is only an increased risk of MZT with AH in fresh cycles as compared to FET is still not clear due to limited studies. Because of the possible associated risk, use of AH for all patients undergoing IVF, including those with poor prognosis, is not recommended by ASRM [2]. Based on our analysis, there may be an increased risk, although the risk is still small, for MZT in fresh, first cycle IVF cycles with AH. Larger prospective, multicenter studies are needed in the future to confirm this finding.
Using retrospective data is a limitation but allows us to look at large groups of certain patient subsets that we could not otherwise do prospectively. The SART database is designed to enter patients prospectively during their cycle to limit any misclassification of exposure or recall bias; however, there is still a potential source of bias. SART tracks clinics instead of specific patients, so it is possible that a patient was not truly going through their first cycle, but had switched from a different clinic after a failed cycle. Although there are limitations to the database, SART collects information from > 95% of IVF cycles in the US and has been instrumental in supplying the data and feedback leading to well documented improvements in quality and care [17]. The lack of information on method of AH in SART limits our ability to be more specific in analysis and subsequent recommendations as we are forced to consider AH as a homogenous technique. Moreover, clinical outcomes could be affected by culture condition or differences in biopsy technique utilized in each lab. Selection bias could have impacted our results because patients with a poor prognosis were more likely to have AH. Even with propensity score weighting, residual confounds could have affected the results. Evidence of MZT was assumed as number of fetal heart beats greater than the number of embryos transferred as described in previous papers [18]. This methodology does have some limitations as recently described; although the large majority of twins after single embryo transfer are monozygotic, not all are [19]. The SART database is a reporting system for IVF cycles in the United States (US), and therefore results may not be generalizable to IVF patients outside of the US.
Other limitations include those intrinsic to using the SART database as discussed previously, including missing data [9]. The number of cycles excluded due to missing data was large, but necessary to best assess the effect of AH by the use of propensity weighting using the covariates. Propensity weighting was used to account for more poor prognosis patients receiving AH and the multiple covariates that influence prognosis. Using this statistical method allowed for a more balanced comparison of outcomes from an intervention.
Conclusion
AH may affect live birth outcomes in all patient populations in fresh IVF cycles. Lower live birth rates are seen in good and poor prognosis patients that received AH. These findings are consistent with previously published studies; however, AH is still being performed. Caution should be exercised when liberally applying this technology as a major tool to improve clinical outcomes.
Electronic supplementary material
(DOCX 12 kb)
Acknowledgments
We wish to thank all SART members for providing clinical information to the SART CORS database for use by patients and researchers. Without the efforts of SART members, this research would not have been possible.
Author’s roles
Study design: JM, JK, RR
Statistical analysis: BC, QL, JG
Data analysis: JM, JK, BC, JG
Manuscript: JM, JK, RR, BC, TC
Funding information
The project described was financially supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2 TR001118 (JFK) and by the Eunice Kennedy Shriver National Institute for Children Health and Development, National Institutes of Health, through Grant K23 HD097307 (JFK).
Compliance with ethical standards
The study was reviewed by the Institutional Review Board at the University of Texas Health Science Center and was determined to be exempt. This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
The material contained in the manuscript has not been published, has not been submitted, or is not being submitted elsewhere for publication.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hammadeh ME, Fischer-Hammadeh C, Ali KR. Assisted hatching in assisted reproduction: a state of the art. J Assist Reprod Genet. 2011;28(2):119–128. doi: 10.1007/s10815-010-9495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Practice Committee of the American Society for Reproductive, M. and T. Practice Committee of the Society for Assisted Reproductive Role of assisted hatching in in vitro fertilization: a guideline. Fertil Steril. 2014;102(2):348–351. doi: 10.1016/j.fertnstert.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 3.Carney SK, Das S, Blake D, Farquhar C, Seif MM, Nelson L. Assisted hatching on assisted conception (in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI). Cochrane Database Syst Rev 2012;12:CD001894 [DOI] [PMC free article] [PubMed]
- 4.Harper J, et al. Adjuncts in the IVF laboratory: where is the evidence for ‘add-on’ interventions? Hum Reprod. 2017;32(3):485–491. doi: 10.1093/humrep/dex004. [DOI] [PubMed] [Google Scholar]
- 5.Kissin DM, et al. Assisted hatching: trends and pregnancy outcomes, United States, 2000-2010. Fertil Steril. 2014;102(3):795–801. doi: 10.1016/j.fertnstert.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansour RT, et al. Transfer of zona-free embryos improves outcome in poor prognosis patients: a prospective randomized controlled study. Hum Reprod. 2000;15(5):1061–1064. doi: 10.1093/humrep/15.5.1061. [DOI] [PubMed] [Google Scholar]
- 7.Knudtson JF, et al. Assisted hatching and live births in first-cycle frozen embryo transfers. Fertil Steril. 2017;108(4):628–634. doi: 10.1016/j.fertnstert.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention, A.S.f.R.M., Society for Assisted Reproductive Technology . 2012 assisted reproductive technology success rates: national summary and fertility clinic reports. Washington, DC: US Department of Health and Human Services; 2014. [Google Scholar]
- 9.Stern JE, et al. Validation of birth outcomes from the Society for Assisted Reproductive Technology Clinic Outcome Reporting System (SART CORS): population-based analysis from the Massachusetts Outcome Study of Assisted Reproductive Technology (MOSART) Fertil Steril. 2016;106(3):717–722. doi: 10.1016/j.fertnstert.2016.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imai Kosuke, Ratkovic Marc. Covariate balancing propensity score. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2013;76(1):243–263. doi: 10.1111/rssb.12027. [DOI] [Google Scholar]
- 11.Alteri A, et al. Revisiting embryo assisted hatching approaches: a systematic review of the current protocols. J Assist Reprod Genet. 2018;35(3):367–391. doi: 10.1007/s10815-018-1118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luke B, et al. Using the Society for Assisted Reproductive Technology Clinic Outcome System morphological measures to predict live birth after assisted reproductive technology. Fertil Steril. 2014;102(5):1338–1344. doi: 10.1016/j.fertnstert.2014.07.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stern JE, et al. Is cryopreservation of embryos a legitimate surrogate marker of embryo quality in studies of assisted reproductive technology conducted using national databases? Fertil Steril. 2012;97(4):890–893. doi: 10.1016/j.fertnstert.2011.12.050. [DOI] [PubMed] [Google Scholar]
- 14.al-Nuaim LA, Jenkins JM. Assisted hatching in assisted reproduction. BJOG. 2002;109(8):856–862. doi: 10.1111/j.1471-0528.2002.t01-1-01005.x. [DOI] [PubMed] [Google Scholar]
- 15.Schieve LA, et al. Does assisted hatching pose a risk for monozygotic twinning in pregnancies conceived through in vitro fertilization? Fertil Steril. 2000;74(2):288–294. doi: 10.1016/S0015-0282(00)00602-6. [DOI] [PubMed] [Google Scholar]
- 16.Kanter JR, et al. Trends and correlates of monozygotic twinning after single embryo transfer. Obstet Gynecol. 2015;125(1):111–117. doi: 10.1097/AOG.0000000000000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain T, et al. 30 years of data: impact of the United States in vitro fertilization data registry on advancing fertility care. Fertil Steril. 2019;111(3):477–488. doi: 10.1016/j.fertnstert.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Luke B, et al. Factors associated with monozygosity in assisted reproductive technology pregnancies and the risk of recurrence using linked cycles. Fertil Steril. 2014;101(3):683–689. doi: 10.1016/j.fertnstert.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vega M, et al. Not all twins are monozygotic after elective single embryo transfer: analysis of 32,600 elective single embryo transfer cycles as reported to the Society for Assisted Reproductive Technology. Fertil Steril. 2018;109(1):118–122. doi: 10.1016/j.fertnstert.2017.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 12 kb)