Abstract
Aims
Growing data suggest that antibiotic exposure is associated with a long-lasting alteration in gut microbiota, and may be related to subsequent cardiovascular disease (CVD). We investigated associations of life-stage and duration of antibiotic exposure during adulthood with subsequent CVD events.
Methods and results
This study included 36 429 women initially free of CVD and cancer from the Nurses’ Health Study. We estimated hazard ratios (HRs) for CVD (a composite endpoint of coronary heart disease or stroke) according to duration of antibiotic use in young (age 20–39), middle (age 40–59), and late (age 60 and older) adulthood. During an average of 7.6 years of follow-up, 1056 participants developed CVD. Women with long-term use of antibiotics (for ≥2 months) in late adulthood had a significantly increased risk of CVD (HR 1.32, 95% confidence interval 1.03–1.70) after adjustment for covariates (such as demographic factors, diet and lifestyle, reasons for antibiotic use, overweight or obesity, disease status, and other medication use), as compared to women who did not use antibiotics in this life-stage. Longer duration of antibiotic use in middle adulthood was also related to higher risk of CVD (P trend = 0.003) after controlling for these covariates. There was no significant relationship between the use in young adulthood and the risk of CVD.
Conclusion
In this study which examined the antibiotic use in different life-stages, longer duration of exposure to antibiotics in the middle and older adulthood was related to an increased risk of future CVD events among elderly women at usual risk.
Keywords: Risk factors, Cardiovascular disease, Antibiotics
Introduction
A recent study suggests that a substantial proportion of antibiotics are not prescribed appropriately in an outpatient clinical setting.1 Specific antibiotic classes have been linked to increased risk of prolongation of the QT interval and the potentially deadly rhythm, Torsades de Pointes,2 and some antibiotics also stimulate proliferation and activity of macrophages3,4 which may induce atherosclerosis.5 Also, antibiotic exposure has been found to affect balance and composition of gut microbiota,6–8 e.g. increasing gut pathogens9–11 and decreasing the abundance of probiotic bacteria,12,13 which are related to cardiometabolic abnormalities.14
Some, though not all, previous studies have reported positive associations between macrolide antibiotics and cardiovascular and sudden cardiac death15–26 particularly among patients with a pre-existing disease such as coronary heart disease (CHD), peripheral artery disease, infections, or pneumonia. Studies have examined associations for antibiotics of this class with cardiovascular disease (CVD) events.15,17,21,26–32 A meta-analysis has shown that macrolide antibiotic use (for 3 days to 1 year) is associated with sudden cardiac death, ventricular tachyarrhythmias, and cardiovascular death.15 A study reported that clarithromycin use was associated with myocardial infarction (MI) incidence during 2 weeks after the first antibiotic prescription, as compared to amoxicillin; however, there was no significantly increased risk of MI for 2 weeks to 3 years after the start of antibiotic treatment.26 Another recent meta-analysis also showed that use of macrolide antibiotics was related to increased risks of cardiovascular outcomes for a limited follow-up period (<30 days); however, there was no significant relationship between macrolide antibiotics and cardiovascular outcomes among studies with a longer follow-up time (>30 days to >3 years).17 These observations suggest that the adverse cardiovascular effect of antibiotics may be weakening with time; however, associations of antibiotic use and long-term cardiovascular events has yet to be established. In addition, an evaluation of duration of antibiotic exposure for subsequent risk of CVD would be important. According to a study of patients with respiratory tract infections, a 7-day-or-more treatment of clarithromycin was associated with more cardiovascular events than a shorter-term (<3-day) treatment.21 To the best of our knowledge, no longitudinal study has investigated associations of duration of antibiotic use in different phases of adulthood (young, middle, and late adulthood) with the CVD incidence in a population at usual risk.
Therefore, in the Nurses’ Health Study (NHS) which has collected detailed information on cumulative antibiotic use during adulthood, we investigated associations of duration and life-stage of antibiotic use with CVD risk over 8 years.
Methods
Study participants
The NHS is an ongoing cohort study established in 1976 of 121 701 female registered nurses in the USA who were 30–55 years of age at enrolment. Information on demographics, lifestyle factors, medical history, and disease status was collected through a self-administered questionnaire in 1976 and has been updated every 2 years through follow-up questionnaires.
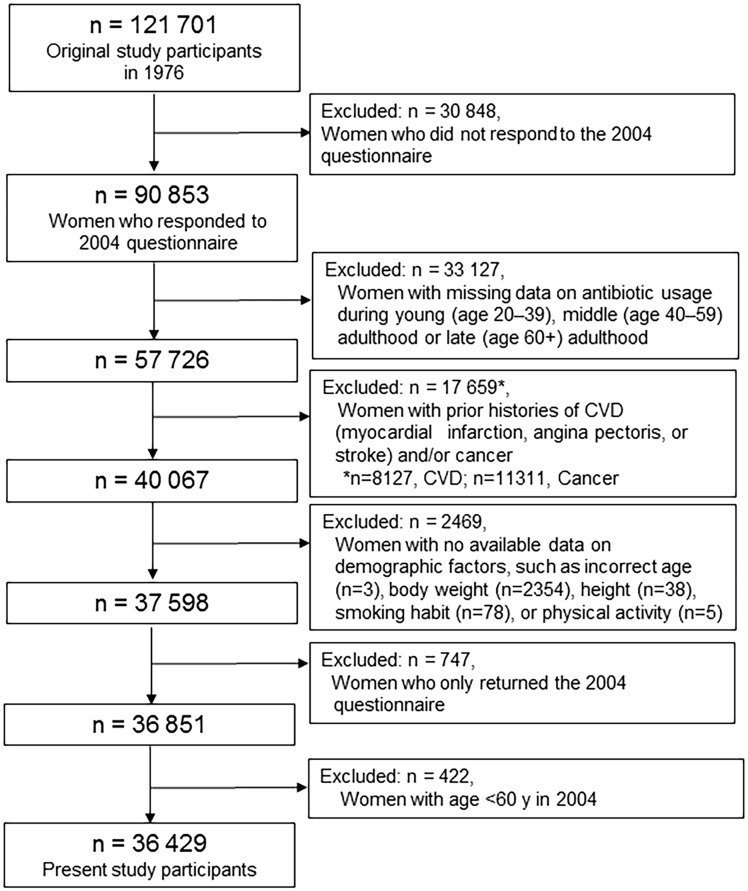
The baseline year for the present analysis was set as 2004 when information on antibiotic use was assessed; a total of 90 853 women returned the 2004 questionnaire33 (Figure 1). Of the 90 853 women, 57 726 reported data on antibiotic usage during young (age 20–39), middle (age 40–59), and late (age 60+) adulthood in life. We excluded women with prior histories of MI, angina pectoris, or stroke (n = 8127) or cancer (n = 11 311) in 2004, and those with no available data on demographic factors, such as body weight, height, smoking habit, or physical activity (n = 2469). Women who only returned the 2004 questionnaire were also excluded, leaving 36 851 women for our analyses. Among the 36 851 participants, 422 were aged <60 y in 2004, and they did not have the ability to provide information on antibiotics during late adulthood. After we excluded these 422 women to reduce the potential for detection bias, a total of 36 429 women aged ≥60 years were included in this study. Additional information on the study design and participants are described in Supplementary material online, Methods. The study was approved by the institutional review board at the Brigham and Women’s Hospital.
Figure 1.
Selection of study participants.
Assessment of duration and life-stage of antibiotic use
In the 2004 questionnaire, women were asked to indicate the total time using antibiotics with eight categories ranging from none to 5+ years (excluding skin creams, mouthwash, or isoniazid) for time periods between age 20–39, 40–59, and age 60+. We combined participants with 2 months or more use, and categorized participants into four groups (none, <15 days, 15 days to <2 months, 2 months or more) to have a reasonable sample size for each category. Antibiotic use for long-term was defined as ≥2 months based on the previous analysis in the NHS.33 The most common reason for the antibiotic use (respiratory infection, urinary tract infection, acne/rosacea, chronic bronchitis, dental, or other) was also assessed. Information on specific type of antibiotics or daily dosage was not available.
Ascertainment of cardiovascular disease
Incident CVD was defined as a composite endpoint of CHD (non-fatal MI or fatal CHD) and total stroke (non-fatal or fatal).34,35 Details about outcome ascertainment are fully described in Supplementary material online, Methods. Deaths were reported by the next of kin or the postal system or identified by searching the National Death Index. Causes of death were primarily confirmed by review of autopsy reports, medical records, and death certificates.36
Statistical analysis
We calculated person-years of follow-up from the return date of the 2004 questionnaire until date of CVD diagnosis, date of death, or end of follow-up (30 June 2012), whichever occurred first. Cox proportional hazard regression, including age as the time scale and stratified by calendar time in 2-year intervals, was performed to calculate hazard ratios (HRs) for CVD according to categories of duration of antibiotic use (none, <15 days, 15 days to <2 months, and ≥2 months) in each young, middle, or late adulthood. Tests of linear trend (Ptrend) across categories of duration of antibiotic use were conducted with the use of the Wald test of a continuous variable on the basis of midpoint day for each category; the highest category (≥2 months) conservatively set to 60 days. We tested the proportional hazard assumption using interaction terms between antibiotic use and follow-up time, and the assumption was unlikely violated (P > 0.05). Covariates of multivariate-adjusted Model 1 included reasons for antibiotic use and traditional risk factors for CVD [demographic, diet, lifestyle factors, and body mass index (BMI)] based on previous publications of the NHS and guidelines on CVD prevention.37,38 Multivariate-adjusted Model 2 included additional covariates to examine whether other metabolic risk factors (hypercholesterolaemia, hypertension, and diabetes) and medication use [aspirin, non-aspirin non-steroidal anti-inflammatory drugs (NSAIDs) or COX-2 inhibitors, calcium channel blockers, statin, H2 blocker, proton pump inhibitors, and steroid]39,40 influenced the associations.
The antibiotic use in each adulthood was treated separately; sensitivity analyses were performed considering usage in prior life-stages. Other sensitivity analyses were also conducted to examine whether competing risks41 of non-cardiovascular events (i.e. all cancers) were present. We also compared HRs for CVD during the first 4 years vs. the last 4 years of the follow-up period to investigate whether associations were different over 8 years of total follow-up. We performed stratified analyses to assess potential effect modification according to disease status and indications for antibiotic use. The P-values for interactions between antibiotic use and these factors were calculated by the log likelihood ratio test which compared models with and without cross-product interaction terms. Further, being obesity is one of important modifiable risk factors for CVD,37,38 and obese individuals may have altered gut microbiome compared with lean individuals.42 To examine whether the presence of obesity may augment the effect of antibiotic exposure for CVD, we calculated HRs for CVD according to a joint classification of antibiotics and BMI. Statistical analyses were performed with SAS version 9.4; P-value <0.05 was considered statistically significant.
Results
Women with longer duration of antibiotic use were more likely to have unfavourable cardiovascular risk profiles including having a family history of MI, higher BMI, metabolic abnormalities (such as hypertension, hypercholesterolaemia, and diabetes); also they were more likely to use other medications (Supplementary material online, Table S1). The most common indication for using antibiotics was for a respiratory infection, with urinary tract infections, and dental indications also being common indications. Study participant characteristics were similar according to categories of use during middle- (40–59 years) and young adulthood (20–39 years) (Supplementary material online, Table S2).
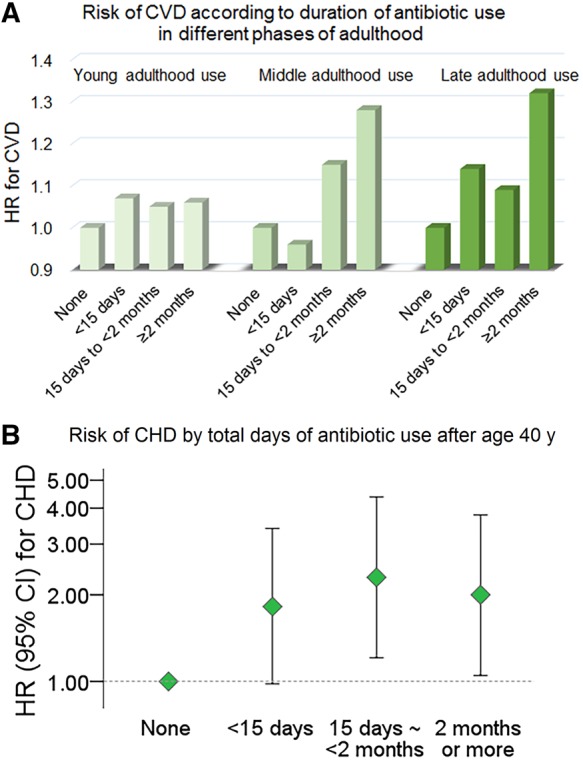
Over 276 409 person-years of follow-up [average 7.6 (SD 1.0) years], 1056 participants developed CVD. A longer duration of exposure to antibiotics in late- (Ptrend = 0.03) and middle adulthood (Ptrend = 0.001) was significantly associated with higher risk of CVD in age-adjusted model (Table 1). The association remained significant after adjustment for covariates in the multivariate-adjusted Model 1 (Ptrend = 0.03 for the use in late adulthood; Ptrend < 0.001 for the use in middle adulthood). In the Model 1, as compared to women did not use antibiotics, those with long-term use (for ≥2 months) in late- or middle adulthood had an adjusted HR of 1.44 [95% confidence interval (CI) 1.13–1.85] or HR 1.39 (95% CI 1.04–1.85) for CVD, respectively. After additional adjustment for covariates in Model 2, the long-term use in late adulthood was significantly associated with an increased risk of CVD (HR 1.32, 95% CI 1.03–1.70). Women with long-term use in middle adulthood showed a multivariate-adjusted HR of 1.28 (95% CI 0.95–1.70) for CVD in the Model 2 (Ptrend = 0.003). Antibiotic use in young adulthood was not significantly associated with the CVD incidence. Results were similar when we performed sensitivity analysis excluding women with a prior history of major diseases before the baseline (2004) (Supplementary material online, Table S3). There was no significant effect modification by follow-up time on associations of antibiotics use with the CVD incidence (Supplementary material online, Table S4). Also, results of the competing risk analyses considering risks of non-CVD events (Supplementary material online, Table S5) were identical to results presented in Table 1.
Table 1.
Hazard ratios for CVD according to duration of antibiotic use in late-, middle-, and young adulthood
| Total time using antibiotics |
Ptrend | ||||
|---|---|---|---|---|---|
| None | <15 days | 15 days to <2 months | 2 months or more | ||
| Late-adulthood (60 years and older) use | |||||
| Incident cases/person-years | 141/52 312 | 515/134 255 | 264/65 720 | 136/24 123 | |
| Age-adjusted HR | 1.00 | 1.14 (0.94–1.37) | 1.11 (0.90–1.37) | 1.42 (1.12–1.81) | 0.03 |
| Multivariate-adjusted HR, Model 1 | 1.00 | 1.15 (0.95–1.40) | 1.14 (0.92–1.41) | 1.44 (1.13–1.85) | 0.03 |
| Multivariate-adjusted HR, Model 2 | 1.00 | 1.14 (0.93–1.38) | 1.09 (0.88–1.36) | 1.32 (1.03–1.70) | 0.17 |
| Middle-adulthood (during age 40–59) use | |||||
| Incident cases/person-years | 104/23 523 | 565/155 872 | 284/72 309 | 103/24 705 | |
| Age-adjusted HR | 1.00 | 0.95 (0.77–1.17) | 1.13 (0.90–1.42) | 1.28 (0.97–1.69) | 0.001 |
| Multivariate-adjusted HR, Model 1 | 1.00 | 0.99 (0.80–1.24) | 1.20 (0.95–1.52) | 1.39 (1.04–1.85) | <0.001 |
| Multivariate-adjusted HR, Model 2 | 1.00 | 0.96 (0.77–1.20) | 1.15 (0.90–1.46) | 1.28 (0.95–1.70) | 0.003 |
| Young-adulthood (during age 20–39) use | |||||
| Incident cases/person-years | 203/43 502 | 601/157 044 | 187/55 733 | 65/20 131 | |
| Age-adjusted HR | 1.00 | 1.07 (0.90–1.26) | 1.03 (0.84–1.27) | 1.07 (0.80–1.42) | 0.91 |
| Multivariate-adjusted HR, Model 1 | 1.00 | 1.10 (0.93–1.29) | 1.09 (0.88–1.34) | 1.13 (0.84–1.51) | 0.60 |
| Multivariate-adjusted HR, Model 2 | 1.00 | 1.07 (0.91–1.27) | 1.05 (0.85–1.30) | 1.06 (0.79–1.42) | 0.94 |
Model 1: age, menopausal status and postmenopausal hormone use, race, family history of myocardial infarction, reasons for using antibiotics, smoking (never, former, or current), physical activity (quintiles), alcohol consumption (none, 0–4.9, 5–14.9, or ≥15.0 g/day), Alternate Healthy Eating Index without alcohol (quintiles), and BMI (<25, 25–<30, or 30 kg/m2). Model 2: Model 1 + hypertension, hypercholesterolaemia, diabetes, aspirin, NSAIDs or COX-2 inhibitors, calcium channel blockers, statin, H2 blocker, proton pump inhibitors, and steroid.
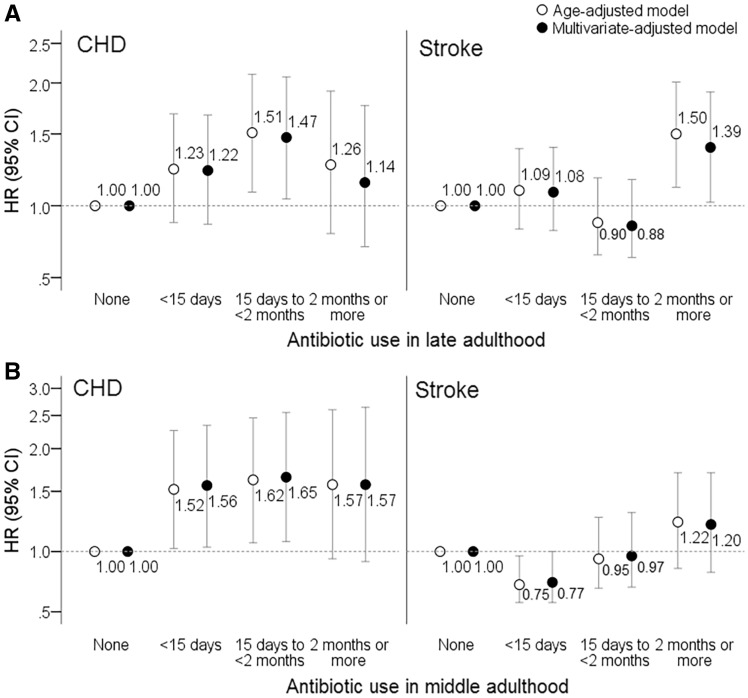
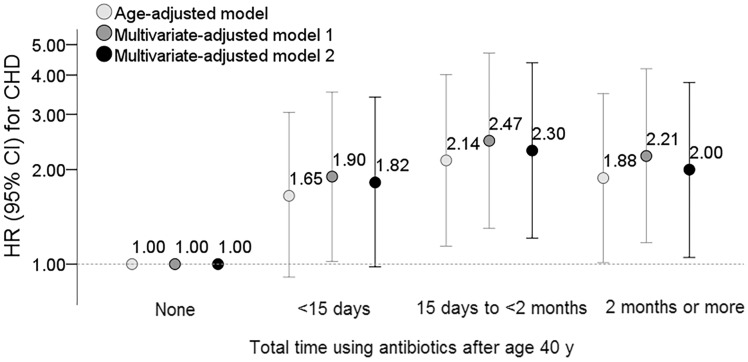
Figure 2 shows results when outcomes of stroke and CHD were examined separately. Women who used antibiotics for <15 days (HR 1.56, 95% CI 1.03–2.34) or 15 days to <2 months (HR 1.65, 95% CI 1.07–2.55) during middle adulthood (B) showed an increased risk of CHD as compared to those who did not use in this period. Furthermore, we assessed risks of total exposure to antibiotics for stroke and CHD based on the sum of average days using antibiotics after age 40. Since the use of antibiotics during young adulthood was not significantly associated with the outcomes, we assessed the total exposure of antibiotics especially after age 40. Compared with non-users, women who used antibiotics for average 15 days to <2 months, and average ≥2 months had an adjusted HR of 2.30 (95% CI 1.21–4.38) and HR 2.00 (95% CI 1.05–3.79) for CHD (Figure 3). The total exposure of antibiotics after age 40 did not show a significant association with stroke (data not shown).
Figure 2.
Hazard ratios for coronary heart disease or stroke according to antibiotic use in late- (A) or middle adulthood (B). Multivariate-adjusted model included covariates of Model 2 in Table 1.
Figure 3.
Hazard ratios for coronary heart disease according to total time using antibiotics after age 40 years. Multivariate-adjusted models included the same covariates in Table 1.
We did not observe significant interactions of the antibiotic use with overweight or obesity, dyslipidaemia, emphysema or chronic bronchitis, postmenopausal hormone use, or reasons of antibiotic use for respiratory infections on the CVD risk (Pinteraction > 0.05) (Supplementary material online, Tables S6 and S7). Associations of antibiotic use in late adulthood with CVD appeared to be stronger in women without diabetes (Pinteraction = 0.08); those who reported the common indication of using antibiotics for urinary tract infections (Pinteraction = 0.02); not for dental indications (Pinteraction = 0.048) (Supplementary material online, Table S6). The positive association of antibiotic use in middle adulthood with CVD appeared to be stronger in non-hypertensive than in hypertensive participants (Pinteraction = 0.06) (Supplementary material online, Table S7). If we calculated HRs for CVD considering the use in prior life-stages, the positive relationship between antibiotic use in middle- or late adulthood and CVD risk was observed for women who also used antibiotics in prior life-stages (Supplementary material online, Figures S1 and S2).
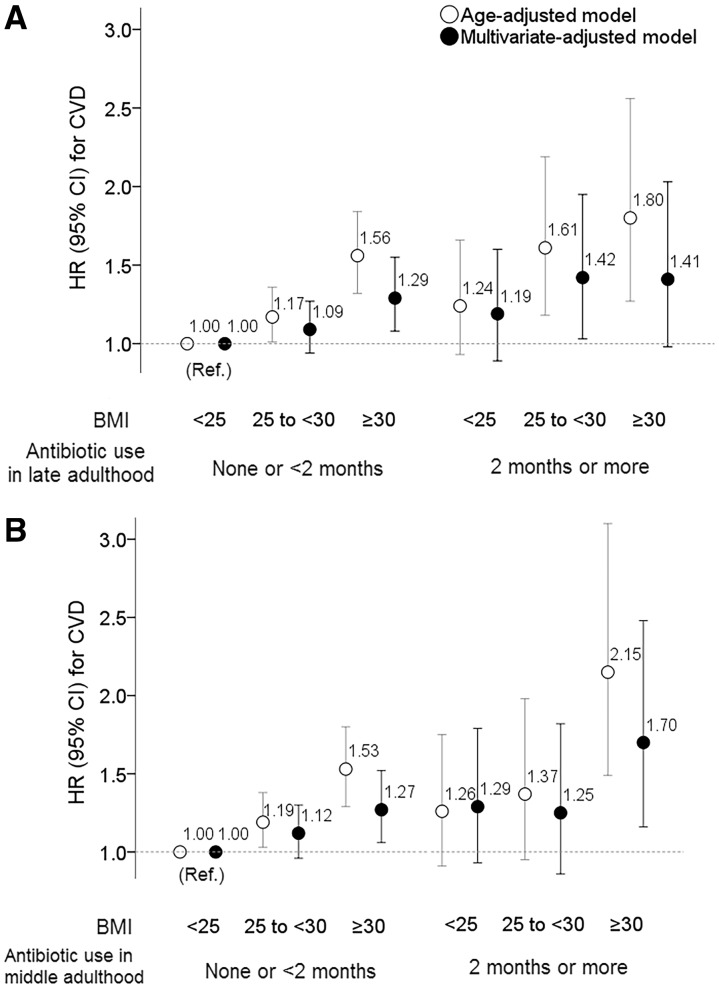
Figure 4 shows results assessing joint associations of antibiotic use in late or middle adulthood and BMI with CVD. When compared with a reference group of women with ‘BMI <25.0 kg/m2 and antibiotic use <2 months’, long-term antibiotic use in late adulthood (≥2 months) was not associated with an elevated risk for CVD among women with BMI <25 kg/m2 (A); the risk was lower than that of obese (BMI ≥30 kg/m2) women without long-term antibiotic use. We observed a similar joint association of BMI categories with antibiotic use during middle adulthood for CVD events (B).
Figure 4.
Joint associations of body mass index and antibiotic use in late- or middle adulthood with the risk of cardiovascular disease. Participants were categorized into six groups in each panel. The category with ‘body mass index <25.0 kg/m2’ and ‘none or <2 months antibiotic use’ in late- (A) or middle adulthood (B) was used as a reference. Multivariate-adjusted model included covariates of Model 2 (except for body mass index) in Table 1.
Take home figure.

Associations of longer duration of exposure to antibiotics in middle and older adulthood with increased risks of cardiovascular events.
Discussion
We found that longer duration of antibiotic use in middle adulthood was significantly associated with subsequent risk of CVD, independent of traditional risk factors for CVD. Also, women with long-term (≥2 months) use of antibiotics in late adulthood had a significantly increased risk of CVD. Our findings were not influenced by the presence of major diseases, or other medication use.
Several previous studies found that antibiotic use was related to increased risks of cardiovascular deaths and MI,15 especially during a limited period (<30 days) after the use.17 Nonetheless, inconsistent results were also reported, probably due to heterogeneous study designs and differences in study populations, duration of antibiotic exposure, antibiotic classes examined, outcome definitions, and follow-up time. A retrospective study of older patients hospitalized with pneumonia showed that azithromycin use was significantly associated with 90-day MI event risk.31 In a study among antibiotic users, clarithromycin use was associated with an increased risk of MI compared with amoxicillin, although no long-term effect of clarithromycin for MI was observed.26 According to follow-up analyses of a randomized clinical trial of patients with stable CHD, a 2-week treatment of clarithromycin was related to a risk of cerebrovascular disease over 10 years after the treatment.32 On the other hand, meta-analyses showed no increased risk of stroke.15,17 We introduced a different approach and investigated the duration of antibiotic use in various stages of adulthood with the incidence of CHD and stroke over 8 years. Our results are in line with a study of patients with respiratory tract infections which reported that longer clarithromycin use (for ≥7 days) might be related to more cardiovascular events during the next year than shorter-term use (<3-day).21 Our results, together with findings from other studies, suggest that use of antibiotics especially long-term use during more recent adulthood may adversely affect the CVD incidence in later life.
There are several potential explanations for the observed associations. Antibiotic treatment may induce prolongation of the QT interval and the Torsades de Pointes, and sudden cardiac death.2 Antibiotics can stimulate proliferation and activity of macrophages3,4 which may induce accumulation of lipids and atherosclerosis in long-term.5 A recent animal study suggests an unexpected effect of antibiotics for promoting inflammation.43 Also, antibiotic exposure might affect cardiovascular risk by influencing the abundance and composition of gut microbiota, which has been associated with atherosclerotic CVD in humans.14 Evidence has shown that effects of a single course of antibiotics on the specific microbial populations can persist for years.44–46 The gut microbe-related metabolites may also have roles in increasing platelet hyperreactivity and propensity to thrombosis that are risk factors for CVD.47 Microbiota disruption caused by antibiotics may also lead to weight gain and greater adiposity by increasing energy harvest or altering metabolic signals and inflammation.48,49 The presence of obesity augmented the association of long-term antibiotic exposure with CVD risk in this study.
Our study has several important strengths. We used a well-established cohort with high follow-up rates and well-validated assessment of CVD events, which minimized selection and ascertainment biases. A large sample size and a long-term follow-up provided the statistical power to detect relevant associations. Comprehensive information on demographics, disease status, lifestyle and diet minimized the potential for residual confounding. Our study has also several potential limitations. First, information on antibiotic use was self-reported, leading to potential misclassification particularly for exposure to antibiotics during early life periods. Nonetheless, all participants were health professionals who were able to provide more accurate information on medication use than general populations. Second, we did not have information on specific types of antibiotics, precluding assessment of whether our findings were specific to certain types of antibiotics. On the other hand, the most common type of prescription largely depends on the cause, and we considered this information in our analysis. Indeed, if the CVD risks were attributable to only specific antibiotics, our analysis of antibiotic use might have underestimated the magnitude of the association. Furthermore, men were not included in this study, and our study population consisted of midlife and elderly women. Also, the present analysis only included 30% of the original study participants. Whether our results would apply to other populations needs to be further investigated. Lastly, our observational study cannot determine a causal effect for our observations. Women who reported antibiotic use might be sicker in other unmeasured ways, and there might be residual or unmeasured confounding factors in the associations, although we carefully considered traditional risk factors, lifestyles, other medication use, indications for antibiotic use, and disease status.
Conclusion
In this study which examined antibiotic use in different life-stages, duration of antibiotic use in the middle- and older adulthood, but not in young adulthood, showed significant associations with the development of CVD in later life. Cumulative antibiotic use during different stages of adulthood may be linked to CVD incidence among elderly women.
Supplementary Material
Acknowledgements
We appreciate all the participants of the NHS and the HPFS for their continued cooperation. We thank Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School for their assistance.
Funding
The study was supported by NIH grants from the National Cancer Institute (UM1 CA186107), the National Heart, Lung, and Blood Institute (R01 HL034594, R01 HL088521, HL071981, HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718, DK100383, DK078616), the Boston Obesity Nutrition Research Center (DK46200), and United States–Israel Binational Science Foundation (2011036). Y.H. was a recipient of a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science.
Conflict of interest: none declared.
See page 3846 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz383)
References
- 1. Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM Jr, Finkelstein JA, Gerber JS, Hyun DY, Linder JA, Lynfield R, Margolis DJ, May LS, Merenstein D, Metlay JP, Newland JG, Piccirillo JF, Roberts RM, Sanchez GV, Suda KJ, Thomas A, Woo TM, Zetts RM, Hicks LA.. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA 2016;315:1864–1873. [DOI] [PubMed] [Google Scholar]
- 2. Nachimuthu S, Assar MD, Schussler JM.. Drug-induced QT interval prolongation: mechanisms and clinical management. Ther Adv Drug Saf 2012;3:241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kita E, Sawaki M, Mikasa K, Oku D, Hamada K, Maeda K, Narita N, Kashiba S.. Proliferation of erythromycin-stimulated mouse peritoneal macrophages in the absence of exogenous growth factors. Nat Immun 1993;12:326–338. [PubMed] [Google Scholar]
- 4. Xu G, Fujita J, Negayama K, Yuube K, Hojo S, Yamaji Y, Kawanishi K, Takahara J.. Effect of macrolide antibiotics on macrophage functions. Microbiol Immunol 1996;40:473–479. [DOI] [PubMed] [Google Scholar]
- 5. Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK.. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med 2013;19:1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ianiro G, Tilg H, Gasbarrini A.. Antibiotics as deep modulators of gut microbiota: between good and evil. Gut 2016;65:1906–1915. [DOI] [PubMed] [Google Scholar]
- 7. Modi SR, Collins JJ, Relman DA.. Antibiotics and the gut microbiota. J Clin Invest 2014;124:4212–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reijnders D, Goossens GH, Hermes GD, Neis EP, van der Beek CM, Most J, Holst JJ, Lenaerts K, Kootte RS, Nieuwdorp M, Groen AK, Olde Damink SW, Boekschoten MV, Smidt H, Zoetendal EG, Dejong CH, Blaak EE.. Effects of gut microbiota manipulation by antibiotics on host metabolism in obese humans: a randomized double-blind placebo-controlled trial. Cell Metab 2016;24:63–74. [DOI] [PubMed] [Google Scholar]
- 9. Kelly CP, Pothoulakis C, LaMont JT.. Clostridium difficile colitis. N Engl J Med 1994;330:257–262. [DOI] [PubMed] [Google Scholar]
- 10. Faber F, Tran L, Byndloss MX, Lopez CA, Velazquez EM, Kerrinnes T, Nuccio SP, Wangdi T, Fiehn O, Tsolis RM, Baumler AJ.. Host-mediated sugar oxidation promotes post-antibiotic pathogen expansion. Nature 2016;534:697–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baumler AJ, Sperandio V.. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016;535:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Korpela K, Salonen A, Virta LJ, Kekkonen RA, Forslund K, Bork P, de Vos WM.. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat Commun 2016;7:10410.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tanaka S, Kobayashi T, Songjinda P, Tateyama A, Tsubouchi M, Kiyohara C, Shirakawa T, Sonomoto K, Nakayama J.. Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol Med Microbiol 2009;56:80–87. [DOI] [PubMed] [Google Scholar]
- 14. Jie Z, Xia H, Zhong SL, Feng Q, Li S, Liang S, Zhong H, Liu Z, Gao Y, Zhao H, Zhang D, Su Z, Fang Z, Lan Z, Li J, Xiao L, Li J, Li R, Li X, Li F, Ren H, Huang Y, Peng Y, Li G, Wen B, Dong B, Chen JY, Geng QS, Zhang ZW, Yang H, Wang J, Wang J, Zhang X, Madsen L, Brix S, Ning G, Xu X, Liu X, Hou Y, Jia H, He K, Kristiansen K.. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun 2017;8:845.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng YJ, Nie XY, Chen XM, Lin XX, Tang K, Zeng WT, Mei WY, Liu LJ, Long M, Yao FJ, Liu J, Liao XX, Du ZM, Dong YG, Ma H, Xiao HP, Wu SH.. The role of macrolide antibiotics in increasing cardiovascular risk. J Am Coll Cardiol 2015;66:2173–2184. [DOI] [PubMed] [Google Scholar]
- 16. Li X, Wang M, Liu G, Ma J, Li C.. Association of macrolides with overall mortality and cardiac death among patients with various infections: a meta-analysis. Eur J Intern Med 2016;28:32–37. [DOI] [PubMed] [Google Scholar]
- 17. Wong AYS, Chan EW, Anand S, Worsley AJ, Wong I.. Managing cardiovascular risk of macrolides: systematic review and meta-analysis. Drug Saf 2017;40:663–677. [DOI] [PubMed] [Google Scholar]
- 18. Bin Abdulhak AA, Khan AR, Garbati MA, Qazi AH, Erwin P, Kisra S, Aly A, Farid T, El-Chami M, Wimmer AP.. Azithromycin and risk of cardiovascular death: a meta-analytic review of observational studies. Am J Ther 2015;22:e122–e129. [DOI] [PubMed] [Google Scholar]
- 19. Ray WA, Murray KT, Meredith S, Narasimhulu SS, Hall K, Stein CM.. Oral erythromycin and the risk of sudden death from cardiac causes. N Engl J Med 2004;351:1089–1096. [DOI] [PubMed] [Google Scholar]
- 20. Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM.. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012;366:1881–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schembri S, Williamson PA, Short PM, Singanayagam A, Akram A, Taylor J, Singanayagam A, Hill AT, Chalmers JD.. Cardiovascular events after clarithromycin use in lower respiratory tract infections: analysis of two prospective cohort studies. BMJ 2013;346:f1235.. [DOI] [PubMed] [Google Scholar]
- 22. Svanstrom H, Pasternak B, Hviid A.. Use of azithromycin and death from cardiovascular causes. N Engl J Med 2013;368:1704–1712. [DOI] [PubMed] [Google Scholar]
- 23. Svanstrom H, Pasternak B, Hviid A.. Use of clarithromycin and roxithromycin and risk of cardiac death: cohort study. BMJ 2014;349:g4930.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khosropour CM, Capizzi JD, Schafer SD, Kent JB, Dombrowski JC, Golden MR.. Lack of association between azithromycin and death from cardiovascular causes. N Engl J Med 2014;370:1961–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chou HW, Wang JL, Chang CH, Lai CL, Lai MS, Chan KA.. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and beta-lactam/beta-lactamase inhibitors: a Taiwanese nationwide study. Clin Infect Dis 2015;60:566–577. [DOI] [PubMed] [Google Scholar]
- 26. Wong AY, Root A, Douglas IJ, Chui CS, Chan EW, Ghebremichael-Weldeselassie Y, Siu CW, Smeeth L, Wong IC.. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ 2016;352:h6926. [DOI] [PubMed] [Google Scholar]
- 27. Karter AJ, Thom DH, Liu J, Moffet HH, Ferrara A, Selby JV.. Use of antibiotics is not associated with decreased risk of myocardial infarction among patients with diabetes. Diabetes Care 2003;26:2100–2106. [DOI] [PubMed] [Google Scholar]
- 28. Grayston JT, Kronmal RA, Jackson LA, Parisi AF, Muhlestein JB, Cohen JD, Rogers WJ, Crouse JR, Borrowdale SL, Schron E, Knirsch C.. Azithromycin for the secondary prevention of coronary events. N Engl J Med 2005;352:1637–1645. [DOI] [PubMed] [Google Scholar]
- 29. Cannon CP, Braunwald E, McCabe CH, Grayston JT, Muhlestein B, Giugliano RP, Cairns R, Skene AM.. Antibiotic treatment of Chlamydia pneumoniae after acute coronary syndrome. N Engl J Med 2005;352:1646–1654. [DOI] [PubMed] [Google Scholar]
- 30. Hutson JR, Fischer HD, Wang X, Gruneir A, Daneman N, Gill SS, Rochon PA, Anderson GM.. Use of clarithromycin and adverse cardiovascular events among older patients receiving donepezil: a population-based, nested case-control study. Drugs Aging 2012;29:205–211. [DOI] [PubMed] [Google Scholar]
- 31. Mortensen EM, Halm EA, Pugh MJ, Copeland LA, Metersky M, Fine MJ, Johnson CS, Alvarez CA, Frei CR, Good C, Restrepo MI, Downs JR, Anzueto A.. Association of azithromycin with mortality and cardiovascular events among older patients hospitalized with pneumonia. JAMA 2014;311:2199–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Winkel P, Hilden J, Hansen JF, Kastrup J, Kolmos HJ, Kjoller E, Jensen GB, Skoog M, Lindschou J, Gluud C;CLARICOR trial group. Clarithromycin for stable coronary heart disease increases all-cause and cardiovascular mortality and cerebrovascular morbidity over 10years in the CLARICOR randomised, blinded clinical trial. Int J Cardiol 2015;182:459–465. [DOI] [PubMed] [Google Scholar]
- 33. Cao Y, Wu K, Mehta R, Drew DA, Song M, Lochhead P, Nguyen LH, Izard J, Fuchs CS, Garrett WS, Huttenhower C, Ogino S, Giovannucci EL, Chan AT.. Long-term use of antibiotics and risk of colorectal adenoma. Gut 2018;67:672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mendis S, Thygesen K, Kuulasmaa K, Giampaoli S, Mahonen M, Ngu Blackett K, Lisheng L; Writing group on behalf of the participating experts of the WHO consultation for revision of WHO definition of myocardial infarction . World Health Organization definition of myocardial infarction: 2008-09 revision. Int J Epidemiol 2011;40:139–146. [DOI] [PubMed] [Google Scholar]
- 35. Walker AE, Robins M, Weinfeld FD.. The National Survey of Stroke. Clinical findings. Stroke 1981;12:I13–I44. [PubMed] [Google Scholar]
- 36. Stampfer MJ, Willett WC, Speizer FE, Dysert DC, Lipnick R, Rosner B, Hennekens CH.. Test of the National Death Index. Am J Epidemiol 1984;119:837–839. [DOI] [PubMed] [Google Scholar]
- 37. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S; ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu E, Rimm E, Qi L, Rexrode K, Albert CM, Sun Q, Willett WC, Hu FB, Manson JE.. Diet, lifestyle, biomarkers, genetic factors, and risk of cardiovascular disease in the nurses' health studies. Am J Public Health 2016;106:1616–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Charlot M, Grove EL, Hansen PR, Olesen JB, Ahlehoff O, Selmer C, Lindhardsen J, Madsen JK, Kober L, Torp-Pedersen C, Gislason GH.. Proton pump inhibitor use and risk of adverse cardiovascular events in aspirin treated patients with first time myocardial infarction: nationwide propensity score matched study. BMJ 2011;342:d2690.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leary PJ, Tedford RJ, Bluemke DA, Bristow MR, Heckbert SR, Kawut SM, Krieger EV, Lima JA, Masri CS, Ralph DD, Shea S, Weiss NS, Kronmal RA.. Histamine H2 receptor antagonists, left ventricular morphology, and heart failure risk: the MESA Study. J Am Coll Cardiol 2016;67:1544–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang M, Spiegelman D, Kuchiba A, Lochhead P, Kim S, Chan AT, Poole EM, Tamimi R, Tworoger SS, Giovannucci E, Rosner B, Ogino S.. Statistical methods for studying disease subtype heterogeneity. Stat Med 2016;35:782–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI.. A core gut microbiome in obese and lean twins. Nature 2009;457:480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Knoop KA, McDonald KG, Kulkarni DH, Newberry RD.. Antibiotics promote inflammation through the translocation of native commensal colonic bacteria. Gut 2016;65:1100–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sjolund M, Wreiber K, Andersson DI, Blaser MJ, Engstrand L.. Long-term persistence of resistant Enterococcus species after antibiotics to eradicate Helicobacter pylori. Ann Intern Med 2003;139:483–487. [DOI] [PubMed] [Google Scholar]
- 45. Jernberg C, Lofmark S, Edlund C, Jansson JK.. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J 2007;1:56–66. [DOI] [PubMed] [Google Scholar]
- 46. Jernberg C, Lofmark S, Edlund C, Jansson JK.. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 2010;156:3216–3223. [DOI] [PubMed] [Google Scholar]
- 47. Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WHW, DiDonato JA, Brown JM, Lusis AJ, Hazen SL.. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 2016;165:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, Li H, Alekseyenko AV, Blaser MJ.. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012;488:621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cox LM, Blaser MJ.. Pathways in microbe-induced obesity. Cell Metab 2013;17:883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.