Significance
We present a major advancement in our ability to bring the physiological laboratory to the open ocean through the noninvasive use of a suction cup-attached tag equipped with surface electrodes. Our study provides heart rate data of a large, free-diving whale (blue whale) without prior capture or restraint. We recorded a wide range of heart rates from the tag, reaching only several beats per minute during deep foraging dives (bradycardia) and nearly 40 beats per minute at the sea surface (tachycardia) as the whale recovered from its oxygen debt. The latter likely represents maximal heart rate given the measured duration of the heart beat itself, thereby demonstrating the greatest dynamic range in cardiac activity at this scale.
Keywords: scaling, heart rate, cardiac function, blue whale, diving
Abstract
The biology of the blue whale has long fascinated physiologists because of the animal’s extreme size. Despite high energetic demands from a large body, low mass-specific metabolic rates are likely powered by low heart rates. Diving bradycardia should slow blood oxygen depletion and enhance dive time available for foraging at depth. However, blue whales exhibit a high-cost feeding mechanism, lunge feeding, whereby large volumes of prey-laden water are intermittently engulfed and filtered during dives. This paradox of such a large, slowly beating heart and the high cost of lunge feeding represents a unique test of our understanding of cardiac function, hemodynamics, and physiological limits to body size. Here, we used an electrocardiogram (ECG)-depth recorder tag to measure blue whale heart rates during foraging dives as deep as 184 m and as long as 16.5 min. Heart rates during dives were typically 4 to 8 beats min−1 (bpm) and as low as 2 bpm, while after-dive surface heart rates were 25 to 37 bpm, near the estimated maximum heart rate possible. Despite extreme bradycardia, we recorded a 2.5-fold increase above diving heart rate minima during the powered ascent phase of feeding lunges followed by a gradual decrease of heart rate during the prolonged glide as engulfed water is filtered. These heart rate dynamics explain the unique hemodynamic design in rorqual whales consisting of a large-diameter, highly compliant, elastic aortic arch that allows the aorta to accommodate blood ejected by the heart and maintain blood flow during the long and variable pauses between heartbeats.
The widely recognized importance of scale in determining function in mammals has led researchers to investigate physiological processes at the extremes of body mass. From the smallest shrews to the largest whales, physiological performance at the extremes may shed light on constraints to body size. In particular, understanding cardiac function at these extremes remains a central challenge in physiology, especially as it relates to energetic demand in the natural environment.
At the largest scale, marine mammals exhibit a suite of anatomical and physiological adaptations to enhance diving ability and improve foraging capacity during a breath hold. The decrease in heart rate associated with the cardiovascular dive response in marine mammals results in heart rates during dives that are lower than resting values, whereas at the sea surface, the heart rates are higher (1, 2). A slower heart rate during dives decreases oxygen delivery to tissues and slows the overall depletion of blood oxygen stores while also decreasing the oxygen consumed by the heart itself (2, 3). The hypothesis (4, 5) that exercise modulates the dive response and increases heart rate during a dive is particularly relevant to the extreme nature of blue whales, because feeding lunges have an estimated metabolic rate 50 times the basal value (6). The lunges are considered to accelerate the depletion of body oxygen stores and account for shorter than expected dive durations (7). Elevated heart rates and increased blood-to-muscle oxygen transfer during a lunge may be critical because of the metabolic cost of a lunge and the low myoglobin (Mb) concentration (0.8 g Mb 100 g−1 muscle) of blue whales (Balaenoptera musculus) relative to Mb concentrations 5 to 10 times higher in most marine mammals (1, 8–10). Exercise, depth, and volitional/anticipatory control have been postulated to modify heart rate during the dive response via the autonomic nervous system (4, 5, 11). Increased heart rate with exercise may occur via withdrawal of parasympathetic tone or increased sympathetic tone. Lung compression during descent and expansion during ascent may also contribute to a decrease or increase, respectively, in heart rate via pulmonary stretch receptor reflexes (12).
Any, even minimal, supplementation of aerobic muscle metabolism due to an increased heart rate and blood oxygen delivery to muscle would result in faster depletion of the blood oxygen stores. As a result, the potential duration of aerobic metabolism would be decreased in essential organs, such as the brain and heart, thereby limiting routine dive durations. Such a crucial role for blood oxygen depletion in baleen whales is supported by 5- to 15-fold elevations (relative to a terrestrial control, the cow [Bos Taurus]) in minke whale (Balaenoptera acutorostrata) gene expression of neuroglobin, the brain oxygen-binding protein that is associated with increased hypoxemic tolerance (13).
Heart rates during surface and dive periods are also relevant to models of arterial hemodynamics. The biomechanical properties and dimensions of the aortic wall in fin whales (Balaenoptera physalus) have led to the hypothesis that, at heart rates ≤10 beats min−1 (bpm) during dives, the highly compliant aortic arch acts as a windkessel that maintains blood flow during the long diastolic periods between heart beats and reduces pulsatility of blood inflow into the rigid distal aorta (14, 15). During surface intervals, it has been postulated that the mechanical properties and dimensions of the aorta in combination with the wavelengths of pressure waves generated with heart rates above 20 bpm allow for destructive interference of reflected and outgoing pressure waves (14). This process decreases the impedance against which the heart must work to eject blood during the cardiac cycle.
Results
To date, no heart rate profile data have been collected for any large whale at sea. Therefore, to address these questions of routine heart rate, the dive response, oxygen store management, and hemodynamics in the blue whale, we applied a suction cup-attached electrocardiogram (ECG)-depth recorder to a single free-diving whale in Monterey Bay, California. Photo identification and a prior biopsy revealed that this whale was a male, at least 15 y old, first sighted in 2003, and observed previously in Monterey Bay, off Southern California, and in the Gulf of California (Cascadia Research Collective Photo ID Database). We obtained an 8.5-h ECG-depth record from which we constructed the heart rate profile (Fig. 1). The shape of the ECG signal was similar to those recorded in smaller whales in captivity with the same recorder (16). Dive behavior was characteristic of this species (7, 17), with foraging dives as long as 16.5 min and as deep as 184 m. Surface intervals typically ranged from 1 to 4 min.
Fig. 1.
(A) An 8.5-h record of heart rate and depth profiles of a blue whale. (B) A 1-h view of heart rate and depth profiles during deep foraging dives revealing lunge feeding events at depth. (C) A 1-h view of heart rate and depth profiles during shallow nighttime nonforaging dives. (D) Details of one deep foraging dive (green, surface; dark blue, descent; light orange, lunge prior to engulfment; light blue, gliding filter phase; dark orange, ascent) consistent with a modulated bradycardia during lunges. (E) Summary of heart rate by activity state and phase of dive for deep foraging dives (color as above). Insets show ECG signals of a blue whale at depth and at the surface with characteristic QRS complexes (ventricular excitation; time from onset of Q wave to end of S wave) and T waves (ventricular relaxation). QRS intervals (361 ± 51.2 ms) and QT intervals (1,021 ± 103.0 ms) were measured for 20 heartbeats at 32 ± 2.0 bpm. The PR interval (atrial excitation and a–v node conduction; time from onset of P wave to onset of R wave) was not calculable, because P waves were not discernible. Based on the measured QT interval (time of ventricular excitation and relaxation; time from onset of Q wave to end of T wave) and an allometrically predicted (25) PR interval of 771 ms in a 70,000-kg animal, the duration of a heart beat at the surface would be about 1.8 s, resulting in an approximate upper limit of 33 bpm for heart rate. This analysis suggests that surface heart rates after deep dives of the blue whale were near maximal. Illustrations courtesy of Alex Boersma (artist). (F) In 60 dives with movement artifact gaps in the ECG record of 9.6 ± 12.0% of total dive duration, dive heart rate and minimum instantaneous heart rate declined with dive duration (black circles, r = 0.31 and blue squares, r = 0.66, respectively), while maximum instantaneous heart rate at the surface increased (green triangles, r = 0.45).
The heart rate profile, consistent with the cardiovascular dive response, revealed that beat-to-beat heart rates of 4 to 8 bpm dominated the bottom phase of foraging dives, regardless of dive duration or maximum depth. Dive heart rate (calculated over the entire dive duration) and minimum instantaneous heart rate during a dive decreased with dive duration, while maximum after-dive surface heart rate increased with dive duration (Fig. 1). Based on mammalian allometric equations (18–20), a 70,000-kg whale has a 319-kg heart, a stroke volume (volume of blood ejected per beat) of about 80 L, and a resting heart rate of 15 (bpm). Dive heart rates were below the allometrically predicted resting heart rate of 15 bpm assuming an adult blue whale of average body length (23 m) and an estimated body mass of ∼70,000 kg (21).
During the bottom phases of dives, instantaneous heart rates were about 1/3 to 1/2 the predicted resting heart rate. Indeed, most of these bottom-phase heart rates were less than the predicted resting heart rate (11 bpm) for even the largest reported blue whale (200,000 kg) (21). Similar to other divers (1), an increase in heart rate occurred during the ascent of the dive. During surface intervals, heart rates were about twice the predicted resting heart rate and predominantly ranged from 30 to 37 bpm after deep dives (>125-m depth) and from 20 to 30 bpm after shallower dives. Based on the calculated duration of a heartbeat (Fig. 1), blue whales seem to be near maximal heart rate in order to accomplish the necessary respiratory gas exchange and reperfusion of tissues between deep dives. Shallow, short-duration dives at night have been associated with rest (17) and are, therefore, more representative of a less active state. Typical heart rates observed for a 5-min dive (8 bpm) and an accompanying 2-min surface interval (25 bpm) at night would result in a heart rate for both the dive and surface intervals of about 13 bpm, remarkably close to the allometrically predicted 15-bpm rate (19).
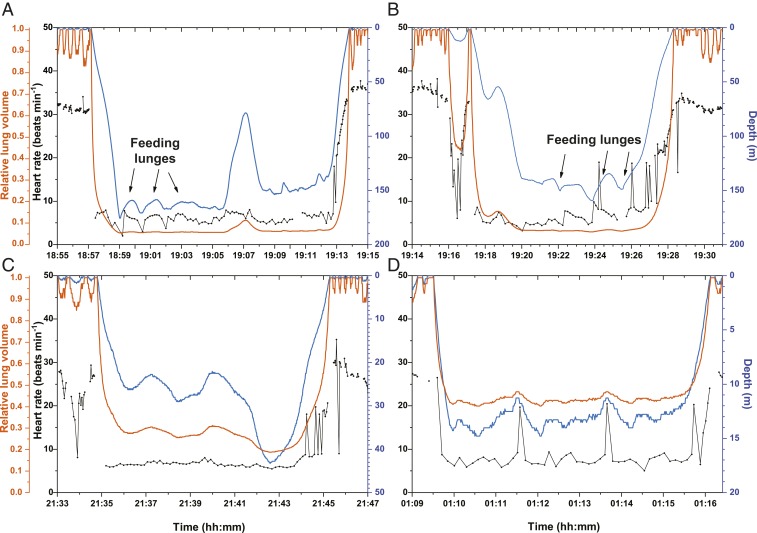
Heart rate, depth, and relative lung volume profiles of 4 individual dives (Fig. 2) were constructed to examine potential effects of exercise and depth on heart rate regulation. During feeding lunges of deep dives, active stroking of the fluke occurs during the ascent phase of the lunge (feeding occurs at the apex of this ascent), while the descent phase is associated with gliding as the engulfed water mass is purged out of the oropharyngeal cavity (17). The 2.5-fold increase in heart rate during the ascent phase of the lunge feeding and the gradual decrease during the descent phase of the lunge (i.e., filtration phase) parallel these substantial differences in locomotory activity that are characteristic and stereotypical of these foraging dives (17). Such heart rate patterns may reflect modulation of heart rate by exercise (Fig. 2). It is unlikely that pulmonary stretch receptor reflexes contributed to these changes in heart rate, as there were no large changes in relative lung volumes at these depths. However, in the bottom phases of shallow dives, transient increases in heart rate (Fig. 2) were associated with changes in relative lung volume and may have been associated with pulmonary stretch receptor activation as well as changes in locomotory activity, the latter of which was not determined in this study.
Fig. 2.
Two deep dives (A: a 16.5-min, 176-m dive; B: a 10.5-min, 143-m dive) and 2 shallow dives (C: a 11.0-min, 43-m dive; D, a 7.6-min, 14-m dive) illustrate potential contributions of exercise and pulmonary stretch receptor reflexes to changes in the cardiovascular dive response and heart rate (5, 12, 26). Relative lung volume was calculated as 1/(1 + [depth/10]), with depth in meters. In all cases, the heart rate profiles generally paralleled changes in depth and relative lung volume during descent and ascent. Increases in heart rate during the active ascent phases of feeding lunges of deep dives were not associated with changes in relative lung volume but were more likely related to exercise and the locomotory cost of the lunge. In artifact-free heart rate profiles of 13 lunges during deep dives, heart rate increased 2.5 ± 1.00 times above the before-ascent minima. Peak heart rates during the 13 lunges averaged 8.5 ± 3.53 bpm. During shallow dives, changes in activity as well as in relative lung volume potentially contributed to the increases in heart rate observed during transient ascents during the bottom phases of the dives. The common, often single-beat oscillations in heart rate observed during ascents in all of the dives are typical in other marine mammals and penguins (1).
Discussion
Provided that stroke volume was constant, the small increases in heart rate during the feeding lunges of deep dives represented, on average, a transient 2.5-fold maximum increase in cardiac output and peripheral oxygen delivery from the prelunge value. However, average peak heart rates during the lunges were still only about half the predicted value at rest. These data were consistent with the hypothesis that the compliant aortic arches of large balaenopterid whales act as windkessels during the slow heart rates of the dive. Similarly, the range of higher heart rates in the after-dive period supported the hypothesis that aortic impedance and cardiac workload decrease during the surface interval through destructive interference of outgoing and reflected pressure waves in the aorta (14).
The extreme bradycardia observed in this study may be unexpected given the high cost of lunge feeding. However, the metabolic cost of a lunge may not be matched to heart rate or convective oxygen transport due in part to the short duration of feeding events and possible recruitment of glycolytic, fast-twitch muscle fibers (22). During a lunge, blue whales accelerate to high speed and engulf a volume of prey-laden water that is larger than their own body (7, 23). The high drag and energy required for lunge feeding have been hypothesized to rapidly deplete total body oxygen stores and limit the dive time available for foraging (7). The mechanical power required to engulf large volumes of water likely far exceeds the aerobic metabolic power available at this body size (6). Although we observed modulation of heart rate during lunges at depth, the distribution of regional blood flow has not been determined during these very transient increases in heart rate. Consequently, the magnitudes of any increases in muscle blood flow, blood oxygen delivery to muscle, and the depletion rate of blood oxygen remain undocumented. Therefore, it is unknown if blood oxygen decreases more rapidly during these transient elevations in heart rate. Indeed, if blood oxygen delivery to muscle remains low or is nil, the metabolic cost of the lunge may be covered primarily by Mb-bound oxygen depletion, phosphocreatine depletion, and glycolysis. In this scenario, muscle oxygen store depletion and lactate accumulation, but not blood oxygen depletion, may contribute most to the relatively short-duration foraging dives of blue whales and other large rorqual whales, despite their large body sizes (7). Much longer foraging dives are regularly observed in other large whale species, such as sperm whales (Physeter macrocephalus) and bowhead whales (Balaena mysticetus), which exhibit comparatively less costly feeding strategies (24) and also, have higher Mb concentrations (1). Documentation of heart rates during dives now provides a step toward evaluation of oxygen store management in all of these large cetacean species.
Regardless of the pattern of blood and muscle oxygen store depletion in blue whales during dives, it seems that near-maximal heart rates are required for gas exchange and reperfusion during their short surface intervals. Because rorqual engulfment capacity exhibits positive allometry (7), whereby larger whales must do relatively more work in less time to feed during a dive, blue whales likely face several physiological constraints during both the dive interval and the surface interval that may have limited the evolution of maximum body size.
Methods and Materials
The ECG of the whale was recorded with use of a custom-built ECG recorder/housing (UFI/Meer Instruments) incorporated into a custom suction cup-attached tag (CATS Diary WiFi; Customized Animal Tracking Solutions). Surface ECG electrodes (E-244 electrode; In Vivo Metrics) were embedded in the bases of 2 suction cups (16). The tag was attached with use of a 6-m carbon fiber pole and a 6.3-m inflatable boat in Monterey Bay, California following the tagging procedures described previously (17). Analysis of ECG data followed established protocols described previously (16), with data expressed as mean ± SD.
Acknowledgments
This research was funded by Office of Naval Research Grant N000141912455 and a Terman Fellowship from Stanford University; and permitted by National Marine Fisheries Service (NMFS) 16111, National Oceanic and Atmospheric Administration Office of National Marine Sanctuaries Multiple Sanctuary Permit (MULTI)-2017-007-009, and Administrative Panel on Laboratory Animal Care (APLAC) 30123. The custom ECG recorder tag was funded by the John B. McKee Fund (2018) at Scripps Institution of Oceanography. We thank the veterinarians, trainers, and animal care staff of SeaWorld San Diego for their support and cooperation in development of the ECG heart rate recorder.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The data for this paper are available at Stanford University’s Data Repository (sdr.stanford.edu): purl.stanford.edu/zp260dk8787.
References
- 1.Ponganis P. J., Diving Physiology of Marine Mammals and Seabirds (Cambridge University Press, 2015). [Google Scholar]
- 2.Scholander P. F., Experimental investigations on the respiratory function in diving mammals and birds. Hvalradets Skrifter 22, 1–131 (1940). [Google Scholar]
- 3.Irving L., Scholander P., Grinnell S., Significance of the heart rate to the diving ability of seals. J. Cell. Comp. Physiol. 18, 283–297 (1941). [Google Scholar]
- 4.Williams T. M., et al. , Exercise at depth alters bradycardia and incidence of cardiac anomalies in deep-diving marine mammals. Nat. Commun. 6, 6055 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Davis R. W., Williams T. M., The marine mammal dive response is exercise modulated to maximize aerobic dive duration. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 198, 583–591 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Potvin J., Goldbogen J. A., Shadwick R. E., Metabolic expenditures of lunge feeding rorquals across scale: Implications for the evolution of filter feeding and the limits to maximum body size. PLoS One 7, e44854 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldbogen J. A., et al. , Scaling of lunge feeding performance in rorqual whales: Mass-specific energy expenditure increases with body size and progressively limits diving capacity. Funct. Ecol. 26, 216–226 (2012). [Google Scholar]
- 8.Lawrie R. A., The activity of the cytochrome system in muscle and its relation to myoglobin. Biochem. J. 55, 298–305 (1953). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kooyman G. L., Ponganis P. J., The physiological basis of diving to depth: Birds and mammals. Annu. Rev. Physiol. 60, 19–32 (1998). [DOI] [PubMed] [Google Scholar]
- 10.Noren S. R., Williams T. M., Body size and skeletal muscle myoglobin of cetaceans: Adaptations for maximizing dive duration. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 126, 181–191 (2000). [DOI] [PubMed] [Google Scholar]
- 11.Ponganis P. J., McDonald B. I., Tift M. S., Williams C. L., Heart rate regulation in diving sea lions: The vagus nerve rules. J. Exp. Biol. 220, 1372–1381 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Angell-James J. E., Elsner R., De Burgh Daly M., Lung inflation: Effects on heart rate, respiration, and vagal afferent activity in seals. Am. J. Physiol. 240, H190–H198 (1981). [DOI] [PubMed] [Google Scholar]
- 13.Schneuer M., et al. , Neuroglobin of seals and whales: Evidence for a divergent role in the diving brain. Neuroscience 223, 35–44 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Shadwick R. E., Gosline J. M., Arterial mechanics in the fin whale suggest a unique hemodynamic design. Am. J. Physiol. 267, R805–R818 (1994). [DOI] [PubMed] [Google Scholar]
- 15.Lillie M. A., Piscitelli M. A., Vogl A. W., Gosline J. M., Shadwick R. E., Cardiovascular design in fin whales: High-stiffness arteries protect against adverse pressure gradients at depth. J. Exp. Biol. 216, 2548–2563 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Bickett N. J., Tift M. S., Leger J. St., Ponganis P. J., Heart rates, heart rate profiles, and electrocardiograms in three killer whales, a beluga, and a pilot whale: An exploratory investigation. Mar. Mamm. Sci. 35, 1112–1132 (2019). [Google Scholar]
- 17.Goldbogen J. A., et al. , Mechanics, hydrodynamics and energetics of blue whale lunge feeding: Efficiency dependence on krill density. J. Exp. Biol. 214, 131–146 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Innes S., Lavigne D., Earle W., Kovacs K., Estimating feeding rates of marine mammals from heart mass to body mass ratios. Mar. Mamm. Sci. 2, 227–229 (1986). [Google Scholar]
- 19.Stahl W. R., Scaling of respiratory variables in mammals. J. Appl. Physiol. 22, 453–460 (1967). [DOI] [PubMed] [Google Scholar]
- 20.Holt J. P., Rhode E. A., Kines H., Ventricular volumes and body weight in mammals. Am. J. Physiol. 215, 704–715 (1968). [DOI] [PubMed] [Google Scholar]
- 21.Lockyer C., Body weights of some species of large whales. ICES J. Mar. Sci. 36, 259–273 (1976). [Google Scholar]
- 22.Rivero J.-L. L., Locomotor muscle fibre heterogeneity and metabolism in the fastest large-bodied rorqual: The fin whale (Balaenoptera physalus). J. Exp. Biol. 221, jeb177758 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Goldbogen J. A., et al. , How baleen whales feed: The biomechanics of engulfment and filtration. Annu. Rev. Mar. Sci. 9, 367–386 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Goldbogen J. A., Madsen P. T., The evolution of foraging capacity and gigantism in cetaceans. J. Exp. Biol. 221, jeb166033 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Noujaim S. F., et al. , From mouse to whale: A universal scaling relation for the PR Interval of the electrocardiogram of mammals. Circulation 110, 2802–2808 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Davis R. W., et al. , The diving paradox: New insights into the role of the dive response in air-breathing vertebrates. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 138, 263–268 (2004). [DOI] [PubMed] [Google Scholar]