Significance
Phagocytic receptors collaboratively regulate innate immune responses against infections. It has been proposed that receptor synergy depends on their spatial distribution on phagosomes. However, studies to reveal the relationship between phagocytic receptor organization and their collaborative signaling have been impeded by the lack of methods to access receptors on the intracellular membranes of organelles. Here, we report a technique to directly manipulate the spatial organization of receptors and signaling proteins on phagosomes inside living cells. We provide direct evidence demonstrating that Dectin-1 and TLR2 must be within nanoscale proximity on phagosomes to elicit synergistic antifungal responses in macrophages. This approach delineates receptor interactions and signaling through physical manipulation and is applicable to other phagocytic receptors of innate immune cells.
Keywords: membrane organization, phagosome membrane, nanocluster proximity, receptor synergy, phagocytosis
Abstract
Receptors of innate immune cells function synergistically to detect pathogens and elicit appropriate immune responses. Many receptor pairs also appear “colocalized” on the membranes of phagosomes, the intracellular compartments for pathogen ingestion. However, the nature of the seemingly receptor colocalization and the role it plays in immune regulation are unclear, due to the inaccessibility of intracellular phagocytic receptors. Here, we report a geometric manipulation technique to directly probe the role of phagocytic receptor “colocalization” in innate immune regulation. Using particles with spatially patterned ligands as phagocytic targets, we can decouple the receptor pair, Dectin-1 and Toll-like receptor (TLR)2, to opposite sides on a single phagosome or bring them into nanoscale proximity without changing the overall membrane composition. We show that Dectin-1 enhances immune responses triggered predominantly by TLR2 when their centroid-to-centroid proximity is <500 nm, but this signaling synergy diminishes upon receptor segregation beyond this threshold distance. Our results demonstrate that nanoscale proximity, not necessarily colocalization, between Dectin-1 and TLR2 is required for their synergistic regulation of macrophage immune responses. This study elucidates the relationship between the spatial organization of phagocytic receptors and innate immune responses. It showcases a technique that allows spatial manipulation of receptors and their signal cross-talk on phagosomes inside living cells.
The stimulation of immune responses relies on more than just individual receptor–ligand recognition. It also depends on the larger-scale spatial organization of membrane receptors and signaling molecules. An extensively studied example is the formation of an immune synapse in T-cell activation, where receptors are segregated into concentric ring patterns (1–4). By contrast, the spatial organization of receptors on intracellular membranes involved in immune functions has scarcely been explored due to the lack of a technical means of doing so. Phagosomes, the intracellular membrane compartments where pathogens are ingested, are known to contain a variety of receptors for regulating innate immune responses to infections. Many of these receptors function in synergistic pairs (5–13). Some of them are known to exhibit organized spatial arrangements, such as colocalization on membranes (14). While the spatial organization of these receptors seems likely to be important, there is, as of yet, no direct evidence of a causal relationship between this phenomenon and the receptor synergy in immune regulation.
Many phagocytic receptor pairs are known to collaborate in signaling. One example is the pair consisting of Dectin-1 and Toll-like receptor (TLR)2. Both of these receptors play important roles in the innate immune defense against fungal infections, which can cause life-threatening inflammatory reactions in children and immunocompromised individuals (15–19). Dectin-1 is a pattern-recognition receptor that recognizes the β-1,3- and β-1,6-glucans on the cell wall of fungi and bacteria (20, 21). TLR2 is activated by lipoproteins found on fungi, bacteria, and viruses (22–27). Genetic knockout of either receptor was shown to cause a defective immune response, with abnormalities for both the ingestion of microbes by immune cells and the secretion of proinflammatory cytokines (6, 15, 22, 28–30). This observation provides important evidence that Dectin-1 and TLR2 work together to coordinate the antifungal immune responses. Further, immunofluorescence studies have suggested that both receptors colocalize on phagosome membranes, although the image resolutions were insufficient to resolve if the receptors truly colocalize on the nanoscale (31–33). Importantly, whether or not the seemingly colocalization of the receptors plays any role in their signaling synergy is still a matter of debate. Coimmunoprecipitation studies have suggested physical interactions between Dectin-1 and TLR2 (31, 34). However, some other studies have shown that the synergistic function of the 2 receptors is due to shared signaling pathways rather than to direct interaction between the 2 receptors (6).
The controversy revolving around the biophysical mechanism of Dectin-1 and TLR2 synergy highlights the more general technical challenges involved in probing receptors on the intracellular membranes of organelles inside living cells. Phagosomes, once formed, are inaccessible from the cell’s surface. Existing methods of manipulating the spatial organization of receptors, such as the patterning of signaling ligands on flat surfaces external to the cell, are only useful for studying receptors on the cell surface (3, 35–38). The current study introduces a method to directly manipulate the spatial organization of receptors on phagosome membranes inside the cell. With this method, it is possible to precisely determine the functional significance of the spatial organization of phagocytic receptors in immune signaling.
Our method is a geometric manipulation strategy using anisotropic particles as phagocytic targets. We designed particles with varied spatial presentations of ligands. One type displayed 2 different ligands spatially segregated on its 2 opposite hemispheres. The other type displayed homogeneously mixed ligands. Once these particles are encapsulated inside phagosomes, the spatial presentation of the 2 ligands on their surfaces forces the receptors on the phagosome membranes to reorganize with no changes to the overall constitution of the membrane. Corresponding changes in immune responses caused by this geometric manipulation indicate the function of this spatial organization of receptors.
To demonstrate the geometric manipulation approach, we combine it with superresolution fluorescence microscopy to directly assess the role of the proximity of Dectin-1 and TLR2 during phagocytosis in macrophages. We confirmed that the distribution of both receptors on phagosome membranes reorganizes following the spatial presentation of their ligands on the surfaces of the encapsulated engineered particles. We showed that Dectin-1 enhances the immune responses triggered predominantly by TLR2 activation, including the secretion of cytokines, the translocation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and the production of reactive oxygen species (ROS). However, this signaling synergy requires the nanoscale proximity between the receptor pairs. Immune responses reached their maximum when the centroid-to-centroid distances between Dectin-1 and TLR2 pairs were <500 nm on phagosomes. When the receptor pairs were segregated beyond this threshold distance on the same phagosomes, the immune responses decreased to the same levels as if only TLR2 receptors were stimulated. These results provide direct evidence demonstrating that nanoscale proximity between Dectin-1 and TLR2 is required for their synergistic immune functions. More generally, this study showcases the potential of a methodology that allows direct spatial manipulation of receptors and their signal cross-talk inside phagosome membranes in living cells.
Results
Fabrication of Particles with Spatially Mixed or Segregated Ligands.
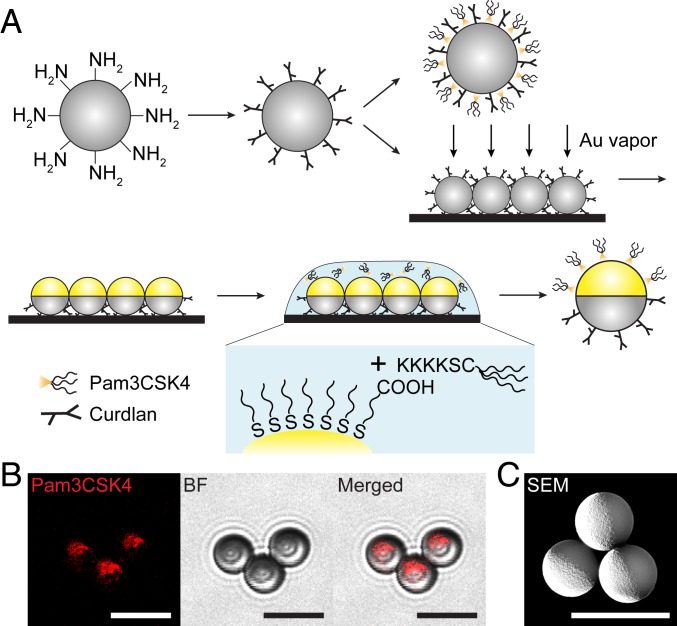
Our first step was to create particles that presented the ligands for Dectin-1 and TLR2 in 2 different sorts of spatial arrangements. The Dectin-1 specific ligand used was curdlan, a linear β-1,3-glucan polymer derived from the bacterium Alcaligenes faecalis (21, 39), and the TLR2 specific ligand was Pam3CSK4, a synthetic triacylated lipopeptide that mimics the acylated amino terminus of bacterial lipopeptides (40–42). The ligands were presented on particles in 2 different ways intended to cause the mixing or the decoupling of Dectin-1 and TLR2 receptors. For the type of particle designed to decouple the receptors, curdlan and Pam3CSK4 were spatially segregated onto the 2 opposite hemispheres of individual particles. We refer to these particles as Janus curdlan-Pam3CSK4 (jCPam) particles, a terminology used in the colloidal community to highlight a surface morphology reminiscent of the 2-faced Roman god (43). These Janus particles were made through a partial-masking technique, in which a thin coating of gold was used to mask one hemisphere of curdlan-coated particles and simultaneously to provide a hemispheric surface for attaching Pam3CSK4 (Fig. 1A). The resulting particles were conjugated with Pam3CSK4 on their gold-coated hemisphere and curdlan on their noncoated side. We confirmed that there was negligible cross-contamination of Pam3CSK4 on the curdlan side of particles (Fig. 1B). Given that the hemispheric gold coating was done after curdlan conjugation (Fig. 1C), no curdlan is expected on the Pam3CSK4 side. For the type of particle designed to induce mixing of Dectin-1 and TLR2 on phagosomes, particles were functionalized with a homogenous mixture of curdlan and Pam3CSK4 (Fig. 1A). These particles were referred to as uniform curdlan-Pam3 particles (uCPam). Abbreviations for different particle types are listed in Table 1.
Fig. 1.
Fabrication and characterization of particles. (A) Schematic illustration of fabrication procedures for particles displaying either uniformly mixed or spatially segregated curdlan and Pam3CSK4. The first step is the conjugation of the ligand curdlan onto aliphatic amine latex particles (amine groups indicated), resulting in the production of uniform curdlan particles. To prepare bifunctional uniform curdlan-Pam3CSK4 particles (Upper, second step), Pam3CSK4 is physically adsorbed onto uniform curdlan particles. To prepare bifunctional Janus curdlan-Pam3CSK4 particles (jCPam) (Lower, second step), uniform curdlan particles were coated on one hemisphere with vaporized gold (Au). Pam3CSK4 was then conjugated onto the gold-coated hemisphere. The result is a jCPam particle coated with the ligand curdlan on one hemisphere and Pam3CSK4 on the other. (B) Fluorescence, BF, and merged images showing the spatial distribution of rhodamine-labeled Pam3CSK4 on the gold-coated hemisphere of Janus particles. (C) Scanning electron microscopy (SEM) image illustrating the gold coating on one hemisphere of Janus particles. (Scale bars in all images: 5 μm.)
Table 1.
List of engineered particles and the abbreviations
| Abbreviation | Description of surface functionalization |
| bareP | Particles without ligands |
| jP | Janus particles without ligands |
| uC | Particles with uniformly coated curdlan |
| jC | Janus particles with curdlan on one side and gold coating on the other |
| uPam | Particles with uniformly coated Pam3CSK4 |
| jPam | Janus particles with Pam3CSK4 on the gold-coated side |
| uCPam | Particles with uniformly coated curdlan and Pam3CSK4 |
| jCPam | Janus particles with curdlan on one side and Pam3CSK4 on the gold-coated side |
| uCIgG | Particles with uniformly coated curdlan and IgG |
| jCIgG | Janus particles with curdlan on one side and IgG on the gold-coated side |
Dectin-1, TLR2, and Downstream Signaling Proteins Are Reorganized on Phagosome Membranes by the Spatial Organization of Ligands on Particles.
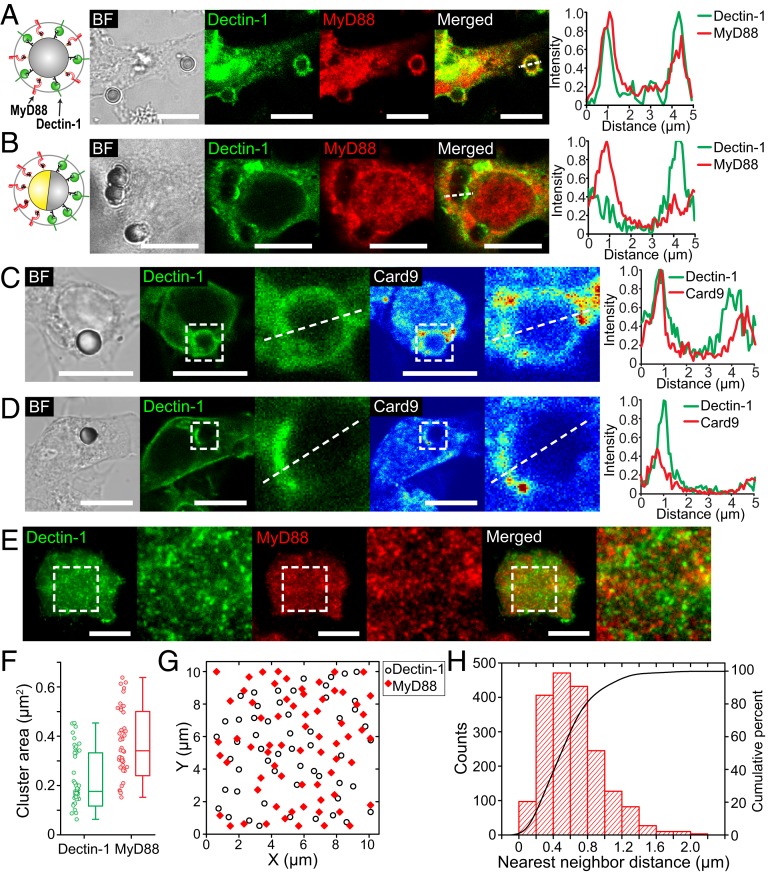
The working principle of our geometric manipulation strategy is that the arrangement of ligands on particles dictates the spatial distribution of their corresponding receptors on phagosome membranes. To demonstrate this, we stimulated RAW264.7 macrophages expressing GFP-Dectin-1 with the 2 different types of particles, uCPam and jCPam, and examined the resulting spatial distribution of Dectin-1 and TLR2 on phagosomes encapsulating each type of particles. Because it has been suggested previously that TLR2 receptors are present on phagosome membranes even without stimulatory ligands (44), we sought to visualize activated TLR2 receptors by immunostaining the adaptor protein called myeloid differentiation primary response 88 (MyD88). MyD88 is recruited to the cytoplasmic domains of activated TLR1 and TLR2 receptors (45), so its distribution on phagosome membranes serves as a marker to indicate the distribution of activated TLR2 receptors. On phagosomes that encapsulate uCPam particles, both Dectin-1 and MyD88 were enriched on the entire phagosome membrane, as shown in the fluorescence confocal images in Fig. 2A. In contrast, phagosomes encapsulating jCPam particles exhibited clear segregation of Dectin-1 and MyD88 (Fig. 2B). Dectin-1 accumulated on the side of the phagosome near the curdlan-coated hemisphere of the particle, which appeared bright in the bright-field (BF) image due to the absence of gold coating. Meanwhile, MyD88 was concentrated on the other side of the phagosome membrane near the Pam3CSK4-coated hemisphere. The spatial segregation of Dectin-1 and MyD88 was further shown in intensity line-scan plots.
Fig. 2.
Spatial reorganization of receptors and signaling molecules on phagosome membranes. (A and B) Representative confocal images and line-scan plots showing the recruitment and distribution of Dectin-1 (green) and MyD88 (red) on phagosomes encapsulating uCPam particles (A) and jCPam particles (B) in RAW264.7 macrophages. Dectin-1 was labeled with GFP, and MyD88 was immunostained. (C and D) Representative confocal images and line-scan plots showing the recruitment and distribution of Dectin-1 and Card9 (shown in intensity-based jet color) on phagosomes of a uCPam particle (C) and jCPam particle (D). (E) Representative TIRF images showing Dectin-1 and MyD88 nanoclusters in the cell membrane at the junction between RAW264.7 cells and a glass substrate uniformly coated with curdlan and Pam3CSK4. (F) Statistic analysis result showing the average area of single nanoclusters shown in the zoomed-in TIRF images in E. In the scattered plot, each data point represents the average value obtained from a single cell. Results from a total of 92 cells are shown. Each box plot indicates the maximum to minimum, the median (horizontal line), and the SD of the corresponding dataset. (G) Positions of Dectin-1 and MyD88 nanoclusters were identified using a single-particle tracking analysis. Each point in the graph represents the centroid position of a single receptor cluster. (H) Statistic analysis result showing the nearest-neighbor distance between Dectin-1 and MyD88 cluster pairs. (Scale bars in all images: 10 μm.)
The enrichment of Dectin-1 in phagosome membranes is a strong but indirect indicator of its activation (20, 34). To confirm that Dectin-1 was activated by the particle-bound curdlan, we examined the activation of phosphorylated spleen tyrosine kinase (pSyk) downstream of Dectin-1 activation. In macrophages, Syk is recruited to the phosphorylated cytoplasmic tail of Dectin-1 after activation and subsequently becomes phosphorylated to facilitate downstream signaling (46). Syk phosphorylation plays an important role in synergistic cytokine induction by Dectin-1 and TLR2 (11, 47). After stimulating macrophage cells with particles either uniformly or half-coated with curdlan (uC and jC particles, respectively), we immunostained cells and imaged Dectin-1 and pSyk using structured illumination microscopy (SIM). Dectin-1 and pSyk were distributed over entire phagosomes containing uC particles but were found only on the curdlan-coated side of phagosomes containing jC particles (SI Appendix, Fig. S1 A and C). This shows that the distribution of pSyk follows the spatial arrangement of curdlan on particles. In both types of samples, Dectin-1 and pSyk appeared as small clusters colocalized on phagosome membranes (SI Appendix, Fig. S1 B and D), which indicates successful activation of Dectin-1 by curdlan on the particles.
In addition to pSyk, we also examined the spatial reorganization of caspase recruitment domain-containing protein 9 (Card9). Card9 is an adaptor protein in Dectin-1 signal transduction that plays a critical role in linking tyrosine kinase to NF-κB activation and mitogen-activated protein kinases (MAPKs) (48–51). It has also been proposed that Card9 is where Dectin-1 and TLR2 signaling pathways converge (49, 52, 53). We found, in immunofluorescence images, that Card9 was recruited to the entire phagosome membrane containing uCPam particles but only on the side of the phagosome near the curdlan-coated hemisphere of jCPam particles (Fig. 2 C and D). When only one type of ligand was present on particles, Card9 was recruited only to activated Dectin-1 receptors, not to TLR2 receptors (SI Appendix, Fig. S2). Like pSyk, the spatial distribution of Card9 on phagosomes is controlled by the presentation of curdlan on particles.
In fluorescence confocal images, as shown in Fig. 2 A and B, we noticed that both Dectin-1 and MyD88 appeared in puncta with high intensity on phagosome membranes. We confirmed with SIM imaging that those puncta are in fact receptor nanoclusters (SI Appendix, Fig. S3A). To quantify the possible colocalization between the clusters, we incubated macrophage cells on glass substrates that were uniformly coated with curdlan and Pam3CSK4, and imaged Dectin-1 and MyD88 at the cell–substrate interface by using total internal reflection fluorescence (TIRF) microscopy (Fig. 2E). We chose TIRF over SIM in this experiment because TIRF allows us to image MyD88 in the membrane with reduced interference from the cytoplasmic population. Culturing phagocytic cells on opsonized flat substrates is a common approach to study early phagocytic events, such as ligand–receptor binding, that are difficult to measure on the 3-dimensional phagosome membranes (54). Because the bifunctional glass substrates used here were functionalized the same way as for the uCPam particles, the formation of receptor clusters at the cell–substrate interface is expected to represent that on the phagosomes. Image analysis revealed that the area of individual receptor clusters is within the range of 0.1 to 0.4 µm2 for Dectin-1 and 0.2 to 0.6 µm2 for MyD88 (Fig. 2F). That translates to cluster radius of 200 to 350 nm for Dectin-1 and 250 to 450 nm for TLR2, assuming the clusters have circular shapes. It is important to note that the cluster size may be overestimated due to the optical diffraction limit. We confirmed that the observed receptor clusters were formed after binding to ligands, as cells incubated on bare glass substrates exhibited few clusters of high intensity (SI Appendix, Fig. S3B). To quantify the possible colocalization between Dectin-1 and TLR2, we identified the centroid position of each cluster (Fig. 2G) and calculated the nearest-neighbor distance of Dectin-1 clusters to MyD88 clusters (Fig. 2H). We found that the median centroid-to-centroid proximity from Dectin-1 nanoclusters to their nearest neighboring MyD88 clusters is ∼590 nm, and >80% of the cluster pairs are within distances below 800 nm. The inaccuracy of this distance measurement was estimated to be ∼21 nm (SI Appendix, Fig. S4). This result shows that Dectin-1 and TLR2 do not colocalize during their synergistic stimulation by the uniformly mixed curdlan and Pam3SCK4 ligands. Instead, they form distinct clusters with nanoscale proximity. Considering that the average radius of the Dectin-1 and MyD88 clusters was estimated ∼260 and 340 nm, respectively, the centroid-to-centroid proximity of ∼590 nm implies that some Dectin-1 and MyD88 nanoclusters may overlap at the interfaces. Moreover, it is unlikely that the observed cluster segregation was caused by the way ligands are mixed on the glass substrates, as the ligand spacing is expected to be only a few nanometers at the ligand density used in experiments.
The results together demonstrate the validity of the working principle of our geometric manipulation strategy. Dectin-1 and TLR2 receptors together with their downstream signaling proteins on single phagosomes are brought into nanoscale proximity when their ligands are homogeneously mixed on uCPam particles but are forced to segregate to opposite sides of jCPam particles. The next question is: how does the spatial reorganization of the Dectin-1/Syk and TLR2/MyD88 signaling units influence the immune responses of the macrophage?
Synergistic Induction of Tumor Necrosis Factor α Secretion Requires Proximity of Dectin-1 and TLR2 on Phagosomes.
Tumor necrosis factor α (TNFα) is a key cytokine secreted by activated macrophages that is known to play an important role in systemic inflammation (55). Previous studies have shown that the production of TNFα and other cytokines by innate immune cells requires a synergy between Dectin-1 and TLRs via unknown mechanisms (6, 19, 29, 47, 56, 57). Here, we applied the geometric manipulation strategy to investigate whether the secretion of TNFα requires the proximity of Dectin-1 and TLR2.
To enable direct comparison of TNFα measurements from different samples, we controlled the density of both ligands on particles and the total number of particles added to cells and kept those experimental parameters consistent for all experiments unless specified otherwise. The surface density of curdlan on particles was 2.1 × 10−8 μg/µm2, measured using a phenol-sulfuric acid assay (58), and that of Pam3CSK4 was 5.0 × 10−9 μg/µm2, measured using a Bradford protein assay (59). Given the molecular mass of 180 kDa for curdlan and 1.5 kDa for Pam3CSK4, the number density of curdlan and Pam3CSK4 on the particle surface is 7.2 × 104/µm2 and 20 × 105/µm2, respectively. Surface density of ligands was the same for both uniformly coated particles and Janus particles. The total number of particles added in each sample was kept at an average of 5 particles per cell. At this particle-to-cell ratio, about 2 particles were internalized per cell after a 24-h incubation, which was the same condition used for TNFα measurements (SI Appendix, Fig. S5). We found no significant difference in particle internalization for different types of particles at this incubation time.
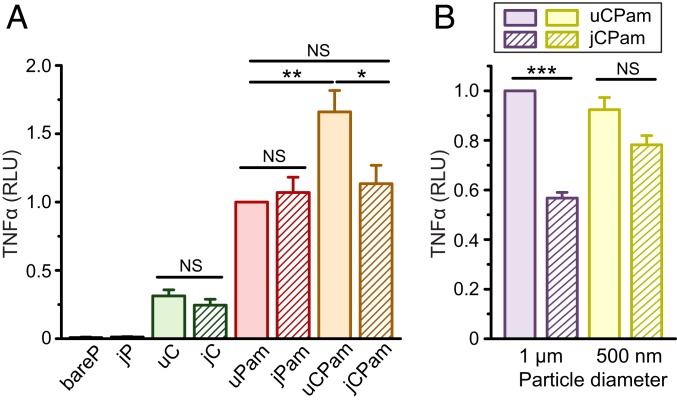
To first investigate if Dectin-1 or TLR2 alone can elicit TNFα secretion in macrophage cells, we stimulated the cells with 3-µm particles uniformly coated with either curdlan (uC) or Pam3SCK4 (uPam). We found that both uPam and uC particles can stimulate TNFα secretion in cells, compared to the negligible TNFα level in cells incubated with bare particles without ligands (bareP). However, TNFα secretion induced by uPam particles was at a significantly higher level than that by uC particles (Fig. 3A). This result implies that TLR2 activation is predominately responsible for the production of TNFα in macrophages, with Dectin-1 playing a minor role. Of course, the difference in TNFα secretion could also be influenced by differential receptor expression levels on RAW264.7 macrophages. Moreover, we observed that TNFα secretion was similar in cells stimulated by Janus particles and their uniform particle counterparts, as shown by the comparison between jC and uC or jPam and uPam (Fig. 3A). Because Janus particles display the same density of ligands as uniform ones but with only half the total ligand amount, this implies that the absolute amount of ligands presented on particles has a negligible effect on TNFα secretion. In addition, the gold coating on one hemisphere of jCPam particles has no effect on the TNFα response, as shown by the similarly low level of TNFα induced by either bare Janus particles (jP) or bare particles (bareP) (Fig. 3A). It is important to note that all TNFα results presented were normalized to the measured values from uPam particle samples. This was because we noticed that TNFα measurements were consistent across all duplicate samples prepared on the same day but could vary slightly on different days, possibly due to variation in cell-seeding density and other factors. The normalization was done to enable direct comparison of TNFα results from all experiments.
Fig. 3.
Measurements of TNFα secretion in RAW264.7 macrophages upon stimulation by particles of different types (A) and diameters (B); 3-µm particles were used in A; 1-µm and 500-nm particles were used in B. The average particle-to-cell ratio was 5:1 in all measurements. In all plots, each data bar represents the mean and SE obtained from 3 independent experiments, each performed in triplicate. Statistical significance is highlighted by P values (Student’s t test) as follows: ***P < 0.001; **P < 0.01; *P < 0.05; NS, not significant.
We next studied how Dectin-1 and TLR2 proximity affects TNFα secretion by stimulating macrophage cells with jCPam and uCPam particles. We found that uCPam particles induced the highest level of TNFα secretion, and jCPam and uPam particles induced similar levels of TNFα (Fig. 3A). This result indicates a few important points. First, the higher TNFα induction by uCPam particles than that by uPam particles shows that activation of Dectin-1 enhances the function of TLR2 in triggering cytokine secretion, in agreement with what has been reported previously (6, 19, 29, 60). Second, and more importantly, uCPam particles induced more TNFα secretion than jCPam particles. Both types of particles display the same ligands at the same surface density; they differ in the spatial presentation of ligands. Their differential effects in inducing TNFα secretion demonstrate that the synergistic signaling between Dectin-1 and TLR2 requires the nanoscale proximity of both receptors on phagosome membranes. In fact, the similar level of TNFα induced by jCPam and uPam particles indicates that this receptor synergy is diminished by the physical separation of the 2 kinds of receptors onto the opposite sides of phagosomes. However, is there a threshold distance required for the Dectin-1 and TLR2 synergy?
We have shown that the centroid-to-centroid distance between Dectin-1 and MyD88 nanoclusters is on average ∼590 nm upon synergistic stimulation. Based on this result, we hypothesized that this receptor proximity determines the distance threshold required for the receptor synergy. To test this hypothesis, we fabricated uCPam and jCPam particles of 1 µm and 500 nm in diameter and measured the TNFα secretion upon particle stimulation. We found that 1-µm jCPam particles stimulated significantly less TNFα than uCPam particles, an observation similar to that of 3-µm particles (Fig. 3B). However, when the particle size was decreased to 500 nm, jCPam and uCPam particles were equally effective in TNFα induction; both resulted in secretion levels similar to that by 1-µm uCPam particles. This result indicates that the signaling synergy between Dectin-1 and TLR2 requires receptor proximity below 500 nm. The spatial segregation of Dectin-1 and TLR2 receptors at distances below this threshold has negligible effects on their collaborative signaling.
Because Dectin-1 enhances the TLR2-induced TNFα response, we further investigated how this enhancement effect depends on the density of its ligand curdlan. As the surface density of curdlan on particles was reduced from 2.1 × 10−8 μg/µm2 to 7.3 × 10−9 μg/µm2, equivalent to a number surface density of 2.5 × 104/µm2, TNFα signaling appears to be mostly induced through TLR2 activation (SI Appendix, Fig. S6A). The results show that Dectin-1 signaling and its collaborative effect on the synergistic induction of TNFα is dependent on the density of the ligand on particles. We also varied the particle-to-cell ratio from 5:1 to 10:1. At the 10:1 particle-cell ratio, TNFα secretion appeared to have reached saturation as no difference in its level was found upon simulation by all 4 types of particles containing Pam3CSK4 (SI Appendix, Fig. S6B). This agrees with previous reports that high concentrations of Pam3CSK4 can lead to saturated secretion of cytokines in macrophages and monocytes (56, 61–63).
To further confirm that the Dectin-1 and TLR2 synergy is a result of their proximity, we performed a negative-control experiment in which TNFα secretion was measured upon activation of Dectin-1 and Fcγ receptors (FcγRs) in macrophages. Like Dectin-1, FcγR signals via tyrosine-based activation motifs and activation of Src and Syk family kinases (64). Due to their similar signaling mechanisms, synergy between Dectin-1 and FcγR is unlikely. In the control experiments, we fabricated uniform and Janus particles coated with curdlan and IgG (ligand for FcγR). The 2 types of particles were referred to as uCIgG and jCIgG, respectively. Ligand density was the same for both types of particles. We found that both uCIgG and jCIgG stimulated low levels of TNFα secretion as uC and jC particles (SI Appendix, Fig. S6C). This result confirms that Dectin-1 and FcγR have no synergistic signaling and that our geometric manipulation strategy is applicable for studying different phagocytic receptors.
The results from all of the TNFα measurements taken together demonstrate that TLR2 activation plays a major role in inducing TNFα secretion in macrophages, in agreement with previous findings (6, 19). Dectin-1 activation enhances the function of TLR2 when their nanoclusters are <500 nm apart. This receptor synergy in TNFα induction also seems to require an optimal range of ligand density and amount. Too low a concentration of curdlan fails to provide a synergy effect on TNFα secretion. However, too high a concentration of Pam3CSK4 saturates the TNFα response so that the collaborative effect from Dectin-1 becomes insignificant.
Synergistic Regulation of NF-κB Activation Is Time-Dependent and Requires Proximity of Dectin-1 and TLR2 on Phagosomes.
We next investigated the role of Dectin-1 and TLR2 proximity in regulating the activation of the NF-κB, a key step required for the expression and signal transduction of several downstream cytokines, including TNFα (65). The NF-κB activation is commonly indicated by its translocation from the cell cytoplasm to the nucleus.
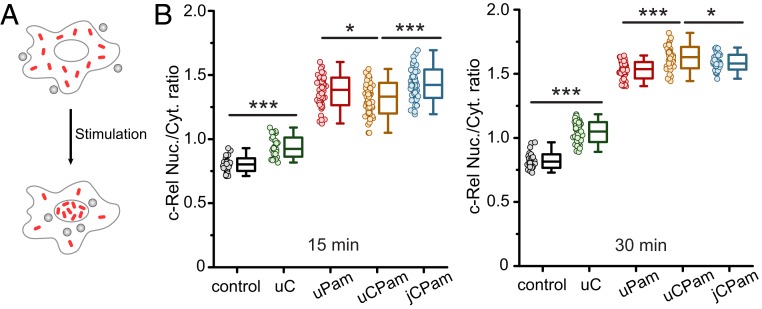
To quantify NF-κB translocation, we stimulated macrophage cells with different types of particles at a 5:1 particle-to-cell ratio and immunostained the cells for c-Rel, which is one of NF-κB family proteins (66). The intensity of c-Rel in cell nuclei increased upon cell stimulation (SI Appendix, Fig. S7 A and B). The c-Rel translocation was quantified as the fluorescence intensity ratio of c-Rel in the nuclei to that in the cytoplasm (c-Rel nuc./cyto. ratio) (SI Appendix, Fig. S7C), following a previously reported assay (67). We observed that the uPam particles caused more c-Rel translocation than uC particles, indicating that TLR2 activation plays a more dominant role in regulating the NF-κB pathway than Dectin-1 (Fig. 4B). When both Dectin-1 and TLR2 were activated, c-Rel translocation exhibited an interesting time dependence. uCPam particles induced less c-Rel translocation than either uPam or jCPam particles 15 min after stimulation but significantly more at 30 min after stimulation. This implies a negative synergy between Dectin-1 and TLR2 at an early stage of stimulation and a positive synergy at a later stage. Our observation is in agreement with a previous study in which Dectin-1 activation was found to trigger 2 independent signaling pathways of opposite modulatory effects on TLR signaling (68). An important observation, however, is that regardless whether their synergy is negative or positive, the spatial decoupling of Dectin-1 and TLR2 resulted in similar c-Rel translocation ratios as with uPam stimulation in both cases. This demonstrates that the proximity of Dectin-1 and TLR2 is required for their synergy in regulating NF-κB activation.
Fig. 4.
Measurement of c-Rel translocation in RAW264.7 macrophages after stimulation by different types of particles. (A) Schematic of nuclear translocation of c-Rel after cell activation. (B) Scatter plots showing nuclear-to-cytoplasmic ratio (nuc./cyt.) of c-Rel in cells after stimulation for 15 min (Left) and 30 min (Right). Each data point (dot) represents the result from one image containing ∼50 cells. For each particle type, a total of ∼3,750 cells were analyzed from 3 independent experiments, each performed in triplicate. Each box plot indicates the maximum to minimum, the median (horizontal line), and the SD of the corresponding dataset. Statistical significance is highlighted by P values (Student’s t test) as follows: ***P < 0.001; *P < 0.05.
Synergistic Production of ROS Depends on Dectin-1 and TLR2 Proximity on Phagosomes.
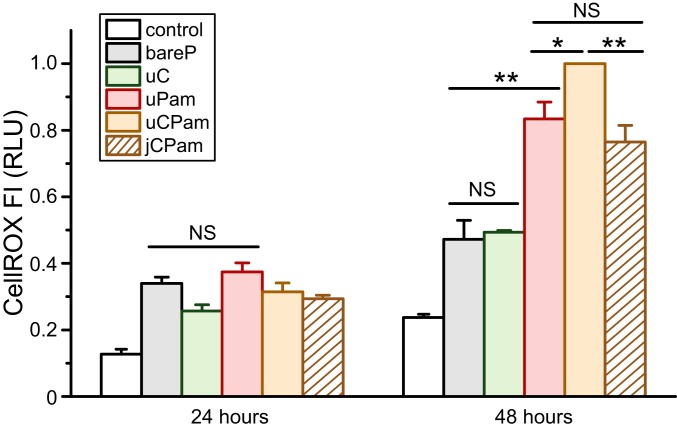
We then studied the role of Dectin-1 and TLR2 proximity in the production of ROS, which play a critical regulatory role in many cellular signaling pathways (69). The intracellular level of ROS, after stimulation by various types of particles, was measured by using a fluorescent indicator CellROX Red. To optimize the experimental condition, we stimulated cells with soluble ligands and found that the CellROX signal became most pronounced 48 h after cell stimulation (SI Appendix, Fig. S8A). Both soluble Pam3CSK4 (1.84 μg/mL) and soluble curdlan (19.05 μg/mL) led to significant CellROX signal, but Pam3CSK4 resulted in higher signals. This result agrees with previous reports that either TLR2 or Dectin-1 stimulation alone can trigger ROS production in macrophages (6, 70). We found that the mixture of soluble Pam3CSK4 and curdlan led to an increased level of CellROX signal (SI Appendix, Fig. S8A). This is somewhat surprising, as it suggests that even the soluble form of both ligands can stimulate the synergistic function of TLR2 and Dectin-1 in ROS. In cells stimulated with various types of particles, there was a considerable level of cell cytotoxicity, as indicated by the increased CellROX signal in bare particle samples compared to the control sample without any particles. Nevertheless, our results clearly show that ROS production reached highest upon uCPam stimulation, and, importantly, it decreased to similar levels for jCPam and uPam stimulation (Fig. 5). Therefore, it is evident that the proximity of Dectin-1 and TLR2 is required for optimal ROS production in macrophages. To further confirm this phenomenon, we also used the Griess assay to measure the production of nitric oxide (NO). We found that uCPam induced more NO than jCPam particles (SI Appendix, Fig. S8B).
Fig. 5.
Measurement of ROS production in RAW264.7 macrophages upon stimulation with different particles and stained after 24 and 48 h. Each bar represents the mean and SE obtained from 3 independent experiments, each performed in triplicate. Statistical significance is highlighted by P values (Student’s t test) as follows: **P < 0.01; *P < 0.05; NS, not significant.
Previous reports on whether Dectin-1 and TLR2 act individually in ROS production in macrophages have been contradictory. Some have reported that Dectin-1 signaling alone is sufficient to drive ROS production and that TLR2 activation is not required but can prime ROS production triggered by Dectin-1 (6, 34, 47, 71, 72). However, others maintained that TLR2 plays an indispensable role in ROS production (70, 73–75). Our results support the latter conclusion. More importantly, our results show that Dectin-1 must be in proximity to TLR2 to enhance the synergistic production of ROS in macrophages.
Discussion
In this study, we developed a geometric manipulation strategy to directly dissect the role of phagocytic receptor proximity in innate immune responses. In this approach, we engineered surface-patterned particles as phagocytic targets on which 2 types of ligands were either homogeneously mixed or spatially separated onto opposite hemispheres. As a result of the spatial arrangement of ligands, we directly manipulated the spatial segregation and mixing of the receptor pair and their downstream signaling proteins on single phagosomes and simultaneously measured the consequent changes in the innate immune responses of macrophages. In essence, this method works by the same principles as the ligand-patterning method used to study the spatial organization of cell-surface receptors. However, our study here demonstrates an example of spatially reorganizing receptors and signaling proteins on intracellular membrane organelles.
Using the geometric manipulation strategy, we tackled an important and unresolved question: what is the role of Dectin-1 and TLR2 spatial organization on phagosomes? The collaborative signaling between this receptor pair in antifungal innate immunity is well established (6, 29, 76). Both receptors had been shown in previous studies to colocalize on phagosomes encapsulating fungi or fungal cell-wall extracts (31, 34). However, whether or not these 2 phenomena are connected has been controversial (6, 31). This matter has remained unresolved due to a lack of methods to access phagosome membranes inside the cell without biochemical interference. In contrast, our geometric manipulation strategy has allowed us to obtain direct evidence demonstrating the significance of Dectin-1 and TLR2 proximity in regulating immune responses in macrophage phagocytosis. We have made 2 key findings in this study. First, Dectin-1 and TLR2 during collaborative signaling do not colocalize but instead form distinct clusters with a median centroid-to-centroid distance of ∼590 nm. This contrasts with previous reports that Dectin-1 and TLR2 colocalize on phagosomes encapsulating fungi or fungal cell wall extracts (31, 34). While the spatial distribution of receptors may depend on the properties of phagocytic targets, the discrepancy between previous studies and ours is likely caused by the different spatial resolutions of imaging techniques used. As shown in Fig. 2A, the receptors appear “colocalized” in images obtained from fluorescence confocal microscopy, a technique used in previous studies mentioned above. However, superresolution imaging and single-particle localization analysis in this study revealed that Dectin-1 and TLR2 are in fact concentrated in separate clusters with nanoscale proximity. Second, and more importantly, we demonstrated that this nanoscale proximity between Dectin-1 and TLR2 clusters is required for their synergistic signaling. TLR2, compared to Dectin-1, plays a more significant role in inducing the activation of the transcription factor NF-κB, the secretion of cytokine TNFα, and the production of ROS. We showed that these TLR2-dependent responses are enhanced by the activation of Dectin-1, as long as both receptors are located within distances below 500 nm. When the receptor pair on the same phagosomes is spatially segregated beyond this distance threshold, the immune responses decrease to a level similar to the case when only TLR2 receptor is activated. These results provide direct evidence demonstrating that the nanoscale proximity, not necessarily colocalization, of Dectin-1 and TLR2 is required for their synergistic regulation of immune responses in macrophages.
An important question that warrants further investigation is why the nanoscale proximity between Dectin-1 and TLR2 clusters is required for their synergistic signaling. The general hypothesis regarding the role of signaling clusters in plasma membrane is that the clustering of macromolecules compartmentalizes signaling pathways and effectively reduces the “reaction space” to enhance the probability of protein interactions (77, 78). In our observation, Dectin-1 and TLR2 clusters do not colocalize but are within nanoscale proximity and may overlap at the interfaces. The “island” structures of receptor clusters may provide large interfacial surfaces for protein interactions and signal amplifications, a mechanism that was proposed to explain the observation of distinct clusters of T-cell receptors (TCRs) and the coreceptor cluster of differentiation 4 (79). Meanwhile, the receptor proximity on phagosomes may be necessary for sharing signaling molecules to converge their signals. There is currently no known molecule that interacts with both receptors. Some studies suggested that Dectin-1 and TLR2 signals converge on adaptor protein Card9 (48, 49, 52, 53). However, our results showed that Card9 associates with Dectin-1 but not with TLR2 during receptor decoupling (Fig. 2C). Other possible candidates for future explorations include extracellular signal regulated kinase (ERK)/MAPK and protein kinase C (PKC) (48, 80).
Finally, findings from this study have general implications regarding the structure–function relationship in the pathogen–host interactions. Because Dectin-1 and TLR2 recognize different main components of the fungal cell wall (6), our results here imply that the structure and spatial presentation of cell-wall components on microbes might have a direct influence on the innate immune responses. More generally, emerging evidence indicates that receptor cross-talk on phagosomes is a key signaling mechanism shared by many different receptors (7, 11, 81, 82) and various types of immune cells, including myeloid cells (83), natural killer cells (84, 85), dendritic cells (86), and mast cells (87). The geometric manipulation strategy established in this study can be applied more generally to investigate the spatial organization of a wide range of phagocytic receptors and thereby elucidate the general physical mechanisms by which the immune system recognizes and distinguishes invaders.
Materials and Methods
Details of reagents, cells, and experimental methods are provided in SI Appendix.
Particle Fabrication and Characterization.
Bifunctional Janus curdlan-Pam3CSK4 particles (jCPam) were fabricated using a previously reported procedure with modifications (88). Curdlan and Pam3CSK4 were selectively and covalently conjugated to the aminated and gold-coated hemispheres, respectively. The surface density of curdlan and Pam3CSK4 on particles was estimated using the phenol-sulfuric acid method (58) and a Bradford protein assay, respectively.
Fluorescence Imaging.
Fluorescence confocal images were acquired with a Leica TCS SP8 confocal microscope. TIRF images were acquired using a Nikon Eclipse-Ti inverted microscope. Superresolution SIM images were acquired using a DeltaVision OMX SR imaging system. TIRF images were realigned to correct shift between 2 imaging channels before analysis. The size of protein clusters in TIRF images were analyzed using the Analyze Particles plugin in ImageJ. The x,y coordinates of individual cluster centroids were identified using a single-particle tracking algorithm for the calculation of nearest neighbor distances.
Measurement of Immune Responses.
TNFα secretion was measured using enzyme-linked immunosorbent assay after cells were stimulated for 24 h. For NF-κB measurements, cells were immunostained for c-Rel and the nuclei. The nuclear-to-cytoplasmic c-Rel fluorescence intensity ratio was calculated using ImageJ following a previously reported method (67). ROS production was measured using CellROX Deep Red. NO production was measured using the Griess assay. Phagocytosis probability was measured using a BD LSR II flow cytometer. The average number of internalized particles per cell was estimated from differential interference contrast images.
Data Availability.
The raw data that were used to generate Figs. 1 B and C, 2 A–E, 3 A and B, 4B, and 5 have been deposited on Figshare (89). These data include: Fig. 1B, raw fluorescence and BF images; Fig. 1C, raw SEM image; Fig. 2 A–E, raw BF and fluorescence images; Fig. 3, raw histogram workbook; Fig. 4B, raw data used for the plot; and Fig. 5, raw histogram workbook.
Supplementary Material
Acknowledgments
We thank Dr. Jim Power at the Light Microscopy Imaging Center (LMIC) of Indiana University, Dr. Yi Yi at the Nanoscale Characterization Center, Dr. Christiane Hassel at the Flow Cytometry Core Facility, and Dr. Giovanni Gonzalez-Gutierrez at the Physical Biochemistry Instrumentation Facility for assistance with instrument use. The 3-dimensional structured illumination microscope at LMIC was provided by NIH Award 1S10OD024988-01. This work was supported by NIH Awards R03AI130751 and R35GM124918 (to Y.Y.); and R01CA213990 and P01CA163223 (to J.Y.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The raw data that were used to generate Figs. 1–5 have been deposited on Figshare (https://figshare.com/s/53dcafcdecac2c604094).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1909870116/-/DCSupplemental.
References
- 1.Grakoui A., et al. , The immunological synapse: A molecular machine controlling T cell activation. Science 285, 221–227 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Huppa J. B., Davis M. M., T-cell-antigen recognition and the immunological synapse. Nat. Rev. Immunol. 3, 973–983 (2003). [DOI] [PubMed] [Google Scholar]
- 3.DeMond A. L., Mossman K. D., Starr T., Dustin M. L., Groves J. T., T cell receptor microcluster transport through molecular mazes reveals mechanism of translocation. Biophys. J. 94, 3286–3292 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaizuka Y., Douglass A. D., Varma R., Dustin M. L., Vale R. D., Mechanisms for segregating T cell receptor and adhesion molecules during immunological synapse formation in Jurkat T cells. Proc. Natl. Acad. Sci. U.S.A. 104, 20296–20301 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blander J. M., Medzhitov R., Regulation of phagosome maturation by signals from toll-like receptors. Science 304, 1014–1018 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Gantner B. N., Simmons R. M., Canavera S. J., Akira S., Underhill D. M., Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197, 1107–1117 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Egmond M., Vidarsson G., Bakema J. E., Cross-talk between pathogen recognizing Toll-like receptors and immunoglobulin Fc receptors in immunity. Immunol. Rev. 268, 311–327 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Kawai T., Akira S., The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat. Immunol. 11, 373–384 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Kawai T., Akira S., Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–650 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Trinchieri G., Sher A., Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 7, 179–190 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Dennehy K. M., et al. , Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur. J. Immunol. 38, 500–506 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodridge H. S., Underhill D. M., Touret N., Mechanisms of Fc receptor and dectin-1 activation for phagocytosis. Traffic 13, 1062–1071 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Thaiss C. A., Levy M., Itav S., Elinav E., Integration of innate immune signaling. Trends Immunol. 37, 84–101 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Goodridge H. S., et al. , Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature 472, 471–475 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gersuk G. M., Underhill D. M., Zhu L., Marr K. A., Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J. Immunol. 176, 3717–3724 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Viriyakosol S., Fierer J., Brown G. D., Kirkland T. N., Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on Toll-like receptor 2 and Dectin-1. Infect. Immun. 73, 1553–1560 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Netea M. G., et al. , Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J. Immunol. 172, 3712–3718 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Dennehy K. M., Brown G. D., The role of the β-glucan receptor Dectin-1 in control of fungal infection. J. Leukoc. Biol. 82, 253–258 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Yadav M., Schorey J. S., The β-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood 108, 3168–3175 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown G. D., Gordon S., Immune recognition. A new receptor for β-glucans. Nature 413, 36–37 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Goodridge H. S., Wolf A. J., Underhill D. M., β-glucan recognition by the innate immune system. Immunol. Rev. 230, 38–50 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Underhill D. M., et al. , The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401, 811–815 (1999). [DOI] [PubMed] [Google Scholar]
- 23.Underhill D. M., Ozinsky A., Smith K. D., Aderem A., Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl. Acad. Sci. U.S.A. 96, 14459–14463 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brightbill H. D., et al. , Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285, 732–736 (1999). [DOI] [PubMed] [Google Scholar]
- 25.Aliprantis A. O., et al. , Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285, 736–739 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi O., et al. , Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11, 443–451 (1999). [DOI] [PubMed] [Google Scholar]
- 27.Cuevas C. D., Ross S. R., Toll-like receptor 2-mediated innate immune responses against Junín virus in mice lead to antiviral adaptive immune responses during systemic infection and do not affect viral replication in the brain. J. Virol. 88, 7703–7714 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dillon S., et al. , Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J. Clin. Invest. 116, 916–928 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferwerda G., Meyer-Wentrup F., Kullberg B. J., Netea M. G., Adema G. J., Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 10, 2058–2066 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Eberle M. E., Dalpke A. H., Dectin-1 stimulation induces suppressor of cytokine signaling 1, thereby modulating TLR signaling and T cell responses. J. Immunol. 188, 5644–5654 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Shin D. M., et al. , Mycobacterium abscessus activates the macrophage innate immune response via a physical and functional interaction between TLR2 and dectin-1. Cell Microbiol. 10, 1608–1621 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Inoue M., Shinohara M. L., Clustering of pattern recognition receptors for fungal detection. PLoS Pathog. 10, e1003873 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoue M., et al. , Cutting edge: Critical role of intracellular osteopontin in antifungal innate immune responses. J. Immunol. 186, 19–23 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown G. D., et al. , Dectin-1 mediates the biological effects of β-glucans. J. Exp. Med. 197, 1119–1124 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torres A. J., Wu M., Holowka D., Baird B., Nanobiotechnology and cell biology: Micro- and nanofabricated surfaces to investigate receptor-mediated signaling. Annu. Rev. Biophys. 37, 265–288 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Doh J., Irvine D. J., Immunological synapse arrays: Patterned protein surfaces that modulate immunological synapse structure formation in T cells. Proc. Natl. Acad. Sci. U.S.A. 103, 5700–5705 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deeg J., et al. , T cell activation is determined by the number of presented antigens. Nano Lett. 13, 5619–5626 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su X., et al. , Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 352, 595–599 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McIntosh M., Stone B. A., Stanisich V. A., Curdlan and other bacterial (1–>3)-β-D-glucans. Appl. Microbiol. Biotechnol. 68, 163–173 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Jin M. S., et al. , Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130, 1071–1082 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Hamley I. W., Lipopeptides: From self-assembly to bioactivity. Chem. Commun. (Camb.) 51, 8574–8583 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Schromm A. B., et al. , Physicochemical and biological analysis of synthetic bacterial lipopeptides: Validity of the concept of endotoxic conformation. J. Biol. Chem. 282, 11030–11037 (2007). [DOI] [PubMed] [Google Scholar]
- 43.de Gennes P. G., Soft matter. Science 256, 495–497 (1992). [DOI] [PubMed] [Google Scholar]
- 44.Ozinsky A., et al. , The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. U.S.A. 97, 13766–13771 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akira S., Takeda K., Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 (2004). [DOI] [PubMed] [Google Scholar]
- 46.Plato A., Willment J. A., Brown G. D., C-type lectin-like receptors of the dectin-1 cluster: Ligands and signaling pathways. Int. Rev. Immunol. 32, 134–156 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rogers N. C., et al. , Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22, 507–517 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Colonna M., All roads lead to CARD9. Nat. Immunol. 8, 554–555 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Hara H., et al. , The adaptor protein CARD9 is essential for the activation of myeloid cells through ITAM-associated and Toll-like receptors. Nat. Immunol. 8, 619–629 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Bertin J., et al. , CARD9 is a novel caspase recruitment domain-containing protein that interacts with BCL10/CLAP and activates NF-κ B. J. Biol. Chem. 275, 41082–41086 (2000). [DOI] [PubMed] [Google Scholar]
- 51.Zhong X., Chen B., Yang L., Yang Z., Molecular and physiological roles of the adaptor protein CARD9 in immunity. Cell Death Dis. 9, 52 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goodridge H. S., et al. , Differential use of CARD9 by dectin-1 in macrophages and dendritic cells. J. Immunol. 182, 1146–1154 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gross O., et al. , Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 442, 651–656 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Mularski A., Marie-Anaïs F., Mazzolini J., Niedergang F., “Observing Frustrated phagocytosis and phagosome formation and closure using total internal reflection fluorescence microscopy (TIRFM)” in Macrophages, Rousselet G., Ed. (Humana Press, 2018), pp. 165–176. [DOI] [PubMed] [Google Scholar]
- 55.Parameswaran N., Patial S., Tumor necrosis factor-α signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 20, 87–103 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dennehy K. M., Willment J. A., Williams D. L., Brown G. D., Reciprocal regulation of IL-23 and IL-12 following co-activation of Dectin-1 and TLR signaling pathways. Eur. J. Immunol. 39, 1379–1386 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gerosa F., et al. , Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J. Exp. Med. 205, 1447–1461 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DuBois M., Gilles K. A., Hamilton J. K., Rebers P. A., Smith F., Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956). [Google Scholar]
- 59.Zor T., Selinger Z., Linearization of the Bradford protein assay increases its sensitivity: Theoretical and experimental studies. Anal. Biochem. 236, 302–308 (1996). [DOI] [PubMed] [Google Scholar]
- 60.Huang H., et al. , Distinct patterns of dendritic cell cytokine release stimulated by fungal beta-glucans and toll-like receptor agonists. Infect. Immun. 77, 1774–1781 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noll F., et al. , Self-extracellular RNA acts in synergy with exogenous danger signals to promote inflammation. PLoS One 12, e0190002 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu B., et al. , RP105 involved in activation of mouse macrophages via TLR2 and TLR4 signaling. Mol. Cell. Biochem. 378, 183–193 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Yanai S., et al. , Diabetic pregnancy activates the innate immune response through TLR5 or TLR1/2 on neonatal monocyte. J. Reprod. Immunol. 117, 17–23 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Nimmerjahn F., Ravetch J. V., Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 8, 34–47 (2008). [DOI] [PubMed] [Google Scholar]
- 65.Dev A., Iyer S., Razani B., Cheng G., “NF-κB and innate immunity” in NF-κB in Health and Disease, Karin M., Ed. (Springer, 2010), pp. 115–143. [Google Scholar]
- 66.Oeckinghaus A., Ghosh S., The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 1, a000034 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noursadeghi M., et al. , Quantitative imaging assay for NF-kappaB nuclear translocation in primary human macrophages. J. Immunol. Methods 329, 194–200 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gringhuis S. I., et al. , Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat. Immunol. 10, 203–213 (2009). [DOI] [PubMed] [Google Scholar]
- 69.Ray P. D., Huang B. W., Tsuji Y., Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 24, 981–990 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gladigau G., et al. , A role for Toll-like receptor mediated signals in neutrophils in the pathogenesis of the anti-phospholipid syndrome. PLoS One 7, e42176 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Underhill D. M., Rossnagle E., Lowell C. A., Simmons R. M., Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 106, 2543–2550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gantner B. N., Simmons R. M., Underhill D. M., Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 24, 1277–1286 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang C. S., et al. , ASK1-p38 MAPK-p47phox activation is essential for inflammatory responses during tuberculosis via TLR2-ROS signalling. Cell Microbiol. 10, 741–754 (2008). [DOI] [PubMed] [Google Scholar]
- 74.Romero M. M., et al. , Reactive oxygen species production by human dendritic cells involves TLR2 and dectin-1 and is essential for efficient immune response against Mycobacteria. Cell Microbiol. 18, 875–886 (2016). [DOI] [PubMed] [Google Scholar]
- 75.Pinke K. H., Lima H. G., Cunha F. Q., Lara V. S., Mast cells phagocyte Candida albicans and produce nitric oxide by mechanisms involving TLR2 and Dectin-1. Immunobiology 221, 220–227 (2016). [DOI] [PubMed] [Google Scholar]
- 76.Walachowski S., Tabouret G., Foucras G., Triggering dectin-1-pathway alone Is not sufficient to induce cytokine production by murine macrophages. PLoS One 11, e0148464 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cebecauer M., Spitaler M., Sergé A., Magee A. I., Signalling complexes and clusters: Functional advantages and methodological hurdles. J. Cell Sci. 123, 309–320 (2010). [DOI] [PubMed] [Google Scholar]
- 78.Hartman N. C., Groves J. T., Signaling clusters in the cell membrane. Curr. Opin. Cell Biol. 23, 370–376 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roh K. H., Lillemeier B. F., Wang F., Davis M. M., The coreceptor CD4 is expressed in distinct nanoclusters and does not colocalize with T-cell receptor and active protein tyrosine kinase p56lck. Proc. Natl. Acad. Sci. U.S.A. 112, E1604–E1613 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Min L., et al. , Synergism between curdlan and GM-CSF confers a strong inflammatory signature to dendritic cells. J. Immunol. 188, 1789–1798 (2012). [DOI] [PubMed] [Google Scholar]
- 81.Shah P., et al. , Toll-like receptor 2 ligands regulate monocyte Fcγ receptor expression and function. J. Biol. Chem. 288, 12345–12352 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rittirsch D., et al. , Cross-talk between TLR4 and FcgammaReceptorIII (CD16) pathways. PLoS Pathog. 5, e1000464 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vogelpoel L. T., et al. , FcγRIIa cross-talk with TLRs, IL-1R, and IFNγR selectively modulates cytokine production in human myeloid cells. Immunobiology 220, 193–199 (2015). [DOI] [PubMed] [Google Scholar]
- 84.Lee S. C., Srivastava R. M., López-Albaitero A., Ferrone S., Ferris R. L., Natural killer (NK): Dendritic cell (DC) cross talk induced by therapeutic monoclonal antibody triggers tumor antigen-specific T cell immunity. Immunol. Res. 50, 248–254 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moreno M., et al. , Toll-like receptor agonists and invariant natural killer T-cells enhance antibody-dependent cell-mediated cytotoxicity (ADCC). Cancer Lett. 272, 70–76 (2008). [DOI] [PubMed] [Google Scholar]
- 86.den Dunnen J., et al. , IgG opsonization of bacteria promotes Th17 responses via synergy between TLRs and FcγRIIa in human dendritic cells. Blood 120, 112–121 (2012). [DOI] [PubMed] [Google Scholar]
- 87.Suurmond J., Dorjée A. L., Knol E. F., Huizinga T. W., Toes R. E., Differential TLR-induced cytokine production by human mast cells is amplified by FcεRI triggering. Clin. Exp. Allergy 45, 788–796 (2015). [DOI] [PubMed] [Google Scholar]
- 88.Gao Y., Yu Y., How half-coated janus particles enter cells. J. Am. Chem. Soc. 135, 19091–19094 (2013). [DOI] [PubMed] [Google Scholar]
- 89.Li W., Data from: Geometrical reorganization of Dectin-1 and TLR2 on single phagosomes alters their synergistic immune signaling. Figshare. https://figshare.com/s/53dcafcdecac2c604094. Deposited 9 November 2019. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data that were used to generate Figs. 1 B and C, 2 A–E, 3 A and B, 4B, and 5 have been deposited on Figshare (89). These data include: Fig. 1B, raw fluorescence and BF images; Fig. 1C, raw SEM image; Fig. 2 A–E, raw BF and fluorescence images; Fig. 3, raw histogram workbook; Fig. 4B, raw data used for the plot; and Fig. 5, raw histogram workbook.