Significance
Obesity is considered a strong factor in many chronic diseases, including heart disease, diabetes, and cancer. Along with genetics, consumption of excessive fat and cholesterol-rich food are the primary causes of obesity. We have developed an ionic liquid that interacts with fat to form micrometer-sized particles. The ionic liquid prevents the fat from penetrating through the intestinal membrane. We have observed that a regular dose of 10 μL of CAGE (equivalent to a 500-mg human dose) resulted in 12% less body weight gain compared with the rats treated with CAGE. The results from the animal studies support oral CAGE as a promising approach for treating obesity.
Keywords: obesity, ionic liquid, fat uptake, high-fat diet
Abstract
More than 70% of American adults are overweight or obese, a precondition leading to chronic diseases, including diabetes and hypertension. Among other factors, diets with high fat and carbohydrate content have been implicated in obesity. In this study, we hypothesize that the choline and geranate (CAGE) ionic liquid can reduce body weight by decreasing fat absorption through the intestine. In vitro studies performed using docosahexaenoic acid (DHA), a model fat molecule, show that CAGE forms particles 2 to 4 μm in diameter in the presence of fat molecules. Ex vivo permeation studies in rat intestine showed that formation of such large particles reduces intestinal fat absorption. In vivo, CAGE reduces DHA absorption by 60% to 70% compared with controls. DHA administered with CAGE was retained in the intestine even after 6 h. Rats fed with a high-fat diet (HFD) and 10 μL of daily oral CAGE exhibited 12% less body weight gain compared with rats fed with an HFD without CAGE for 30 d. Rats that were given CAGE also ate less food than the control groups. Serum biochemistry and histology results indicated that CAGE was well tolerated by the rats. Collectively, our data support the hypothesis that CAGE interacts with fat molecules to prevent their absorption through intestinal tissue and potentially providing a feeling of satiety. We conclude that CAGE offers an effective means to control body weight and a promising tool to tackle the obesity epidemic.
One in every 3 Americans are living with obesity, a precondition that accompanies several diseases, including hypertension, diabetes, asthma, stroke, chronic back pain, and congestive heart failure. Each year, there are more than 3 million deaths globally from complications associated with obesity and comorbidities (1). A key reason for this epidemic is the intake of high-caloric foods, the consumption of which is exacerbated by a lack of affordable access to fresh foods (2). Obesity also affects different demographic groups disproportionately, with people facing financial instability at greatest risk (3).
Obesity is a significant risk factor for decreased life expectancy of future generations (1). In addition to physical issues, studies emphasize the high incidence of discrimination associated with obesity, particularly relating to employment and healthcare, which leads to poor mental health (4). Studies have clearly illustrated a relationship between weight and the risk of low self-esteem, eating disorders, and poor health habits (1). Despite its severity, this epidemic has continued to advance. If the trend continues, >80% of US adults will be overweight or obese by 2030, and that number will reach 100% by 2048 (5). This is expected to impose a serious burden on healthcare costs, doubling every decade and ballooning to account for roughly 16% of overall US healthcare costs by 2030 (5).
Several therapeutic approaches have been proposed to tackle the epidemic of obesity (6–10). Over the last several decades, the Food and Drug Administration has approved various weight loss drugs, including orlistat and lorcaserin (11, 12). These medications have shown to reduce body weight by ∼10% compared with controls (13). However, these drugs carry significant side effects, including headaches, stomach pain, diarrhea, severe liver injury, constipation, birth defects, sleep apnea, suicidal thoughts, and pancreatitis (13, 14). As an alternate approach, linagliptin and GLP-1, when given together with a standard diet, resulted in a 10% body weight loss compared with controls (5, 15). The loss of body weight was accompanied by decreased appetite in the treated group, which is believed to be a consequence of changes in the cognitive elements of the brain (16). In another approach, efforts have been directed toward adipocytes that store unneeded fat (17). Specifically, Won et al. (18) used an adipocyte-targeting fusion-oligopeptide gene carrier with an adipocyte-targeting sequence and 9-arginine to prevent expression in fatty acid-binding protein 4, a key protein in fatty acid storage, using a short-hairpin RNA. This approach led to a body weight reduction of >20%.
Here we report an approach for the reduction of weight gain and food intake based on an ionic liquid, choline and geranate (CAGE). CAGE is a relatively new material with properties that fit the classical definitions of both ionic liquids and deep eutectic solvents (19, 20) and has been previously shown to enhance oral and transdermal delivery of drugs (21, 22). In this study, a daily 10 μL oral dose of CAGE (corresponding to a human equivalent dose of ∼500 mg) led to a reduction in body weight of up to 12% in rats (23, 24). CAGE reduced intestinal uptake of fat as well as overall food intake. Safety studies based on serum biochemistry and histology showed that CAGE was well-tolerated by rats after 1-mo daily dosing. This approach provides a potential treatment for people living with obesity and its secondary complications.
Results
Fat Forms Microparticles in the Presence of CAGE and Exhibits Reduced Intestinal Transport.
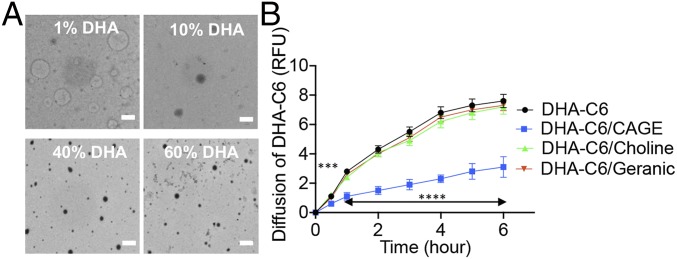
Docosahexaenoic acid (DHA) is an omega-3 fat that is highly bioavailable after ingestion and has been used a model fat molecule (25, 26). DHA (1%, 10%, 40%, and 60% by vol/vol) is soluble in CAGE owing to its hydrophobicity. On addition of water, the DHA-CAGE mixture forms a microemulsion (SI Appendix, Fig. S1). This emulsion was characterized by transmission electron microscopy (TEM) and dynamic light scattering (DLS), which showed that DHA formed large 3- to 4-μm particles in the presence of CAGE and water (Fig. 1A). This large size is clearly due to the combined presence of DHA, CAGE, and water, since CAGE alone in water (10% vol/vol) forms particles ∼100 nm in diameter and DHA alone in water forms particles in the range of 50 to 400 nm, depending on the concentration (Fig. 1A and SI Appendix, Fig. S2). The formation of larger CAGE-DHA particles is likely a result of intermolecular interactions, especially ionic and hydrophobic interactions, between CAGE and DHA. The effect of CAGE on permeation of DHA across the intestine was measured ex vivo. DHA was chemically labeled with Coumarin 6 to quantify its permeation. CAGE significantly reduced the permeation of DHA compared with controls (no CAGE) (Fig. 1B). Interestingly, neither choline nor geranic acid by itself reduced the transport of DHA, thus confirming that the ability of CAGE to reduce DHA uptake is unique to its composition.
Fig. 1.
CAGE forms large micelles in the presence of water and fat, resulting in reduction of diffusion. (A) TEM images showing the formation of particles when CAGE is mixed with different concentrations of DHA with water. (Scale bar: 5 μm.) (B) Ex vivo permeation profiles showing that CAGE significantly reduces the permeation rate of DHA across the intestine, whereas both choline bicarbonate and geranic acid have no effect on DHA-C6 permeation. All data are presented as mean ± SE (n = 5). ***P < 0.001; ****P < 0.0001.
In Vivo Assessment of the Effect of CAGE on Fat Uptake.
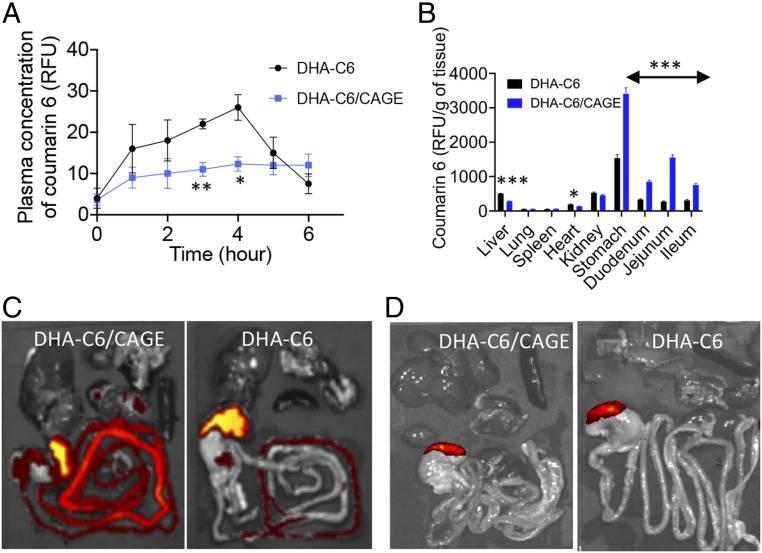
To investigate the effect of CAGE on oral absorption of fat, we prepared capsules (size 9) containing 2 mg of DHA-coumarin 6 (C6) with and without 10 μL of CAGE. Capsules were administered orally to rats. Based on the area under the curve values for plasma concentrations of DHA over a period of 6 h after administration, the amount of DHA absorbed in the presence of CAGE was approximately one-half that in the absence of CAGE (Fig. 2A). Organs were harvested at 6 h to further investigate the location and distribution of DHA in the gastrointestinal (GI) tract. Biodistribution studies on tissues collected at 6 h after administration indicated that CAGE increased localization of DHA in the stomach and intestine, whereas it reduced the concentration in the liver (Fig. 2B). Fluorescence images clearly indicated that the intestines of rats in the CAGE+DHA group contained significantly higher amounts of DHA-C6 compared with the non-CAGE group at 6 h (Fig. 2C). At 12 h after administration, no significant DHA-C6 was observed in either group (Fig. 2D).
Fig. 2.
CAGE reduces the intestinal absorption of DHA-C6 conjugates in vivo. (A) The 6-h pharmacokinetics profile shows that the serum concentration of DHA-C6 is less for a DHA-C6/CAGE-dosed rat compared with those dosed with DHA-C6 alone. The results demonstrate that CAGE reduces uptake of fat through the intestinal membrane. (B) After 6 h of oral administration, the biodistribution profile shows greater accumulation of DHA-C6 in the GI tract for the rats administered with a DHA-C6/CAGE capsule. In contrast, higher accumulation of DHA-C6 was observed in the liver and kidney for the rats administered with a DHA-C6 capsule. (C and D) Optical ex vivo imaging of the harvested organs show localization of DHA after (C) 6 h or (D) 12 h of oral administration in CAGE-fed animals. All data are presented as mean ± SE (n = 5). *P < 0.05; **P < 0.01; ***P < 0.001.
Orally Administered CAGE Significantly Slows Body Weight Gain with a High-Fat Diet.
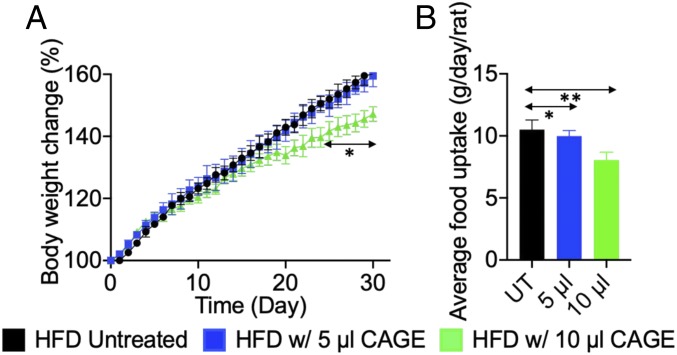
The effect of CAGE on the weight gain of rats after a high fat diet was studied. Three groups of rats were fed a high-fat diet (HFD), which contains 20% more fat than a regular diet, for 30 d. Two of the groups were dosed for 30 d with a daily CAGE capsule containing 5 or 10 μL. These doses correspond to human equivalent doses of ∼250 and ∼500 mg, respectively (24). The third group of rats was not treated with CAGE but had access to the same HFD. Body weights were monitored daily. Daily 10 μL CAGE significantly reduced weight gain compared with the untreated controls (Fig. 3A). Specifically, rats given 10 μL od CAGE gained 12% less weight compared with both the untreated and 5 μL-dosed rats (Fig. 3A). Each group was presented with the same amount of food for consumption each day; however, the rats dosed with 10 μL of CAGE daily ate less food than the untreated rats, observed over a period of 15 d (Fig. 3B). The untreated rats usually ate approximately 10 g of food every day, compared with 9 g in the 5 μL CAGE group and 8 g in the 10 μL CAGE group.
Fig. 3.
Oral administration of CAGE significantly reduces the rate of body weight gain of rats given an HFD and also reduces food uptake. (A) Rats orally administered 10 µL of CAGE gained about 12% less body weight compared with rats without CAGE administration. (B) Rats given an oral CAGE capsule daily ate less food than untreated rats. Rats from the treatment groups ate 5 to 10% less food when observed for 15 d (UT). All data are presented as mean ± SD (n = 5). *P < 0.05; **P < 0.01 compared with the untreated group.
Since food uptake has been correlated with inhibition of the DPP-IV enzyme, we further investigated the ability of CAGE to inhibit DPP-IV. CAGE induced a dose-dependent inhibition of DPP-IV (SI Appendix, Fig. S4). Specifically, 20% CAGE in water induced a nearly 100% inhibition of DPP-IV, similar to the effect of sitagliptin (0.1%), a clinical therapeutic agent for DPP-IV inhibition (13).
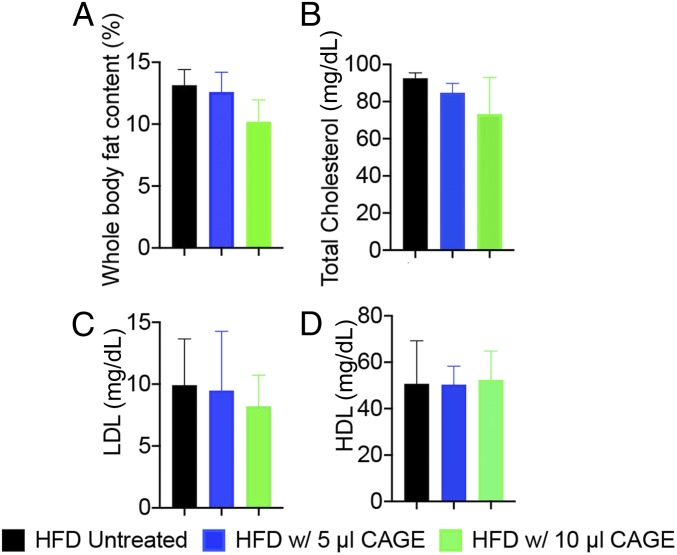
To further understand whether the body weight gain was directly related to the fat uptake through food, we measured the whole-body fat content in rats treated with CAGE. EchoMRI measurements of whole-body fat content showed a 26% lower body fat content in the rats treated with 10 μL of CAGE, although the difference was not statistically significant (Fig. 4A). No significant differences in lean muscle content were observed between the treated and control groups (SI Appendix, Fig. S4). Somewhat lower, but statistically nonsignificantly different, low-density lipoprotein (LDL) levels were noted in treated rats compared with untreated rats. No differences in serum high-density lipoprotein (HDL) levels were seen between CAGE-treated rats and untreated rats (Fig. 4 B–D).
Fig. 4.
Effect of oral administration of CAGE on whole-body fat content and serum total cholesterol and LDL compared with untreated rats. (A) Whole-body fat content in both treated and untreated rats was measured using EchoMRI. (B) Effect of CAGE on total cholesterol level. (C) Effect of CAGE on LDL level. (D) Effect of CAGE on HDL level. All data are presented as mean ± SE (n = 5). P > 0.05 for both 5 and 10 µL CAGE-fed compared with control (untreated).
The toxicity of CAGE was investigated for rats treated with oral CAGE for 30 d. No difference was found in cell counts of treated and control rats in terms of red blood cells and platelets (SI Appendix, Fig. S5). The following biomarkers of serum biochemistry were also measured: albumin, alkaline phosphatase, globulin, alanine aminotransferase/glutamic pyruvic transaminase, total bilirubin, total protein, blood urea nitrogen, calcium, and creatinine. No differences in these biomarkers were observed between controls and CAGE-treated rats (SI Appendix, Fig. S6). Histological analysis of tissues was also performed for liver, lung, kidney, spleen, heart, kidney, stomach, duodenum, jejunum, and ileum after hematoxylin and eosin staining. No morphological abnormalities were observed, and tissue morphology was identical in the treated and untreated animals (SI Appendix, Fig. S7). We measured the concentrations of the components of CAGE (choline and geranic acid) in tissues by liquid chromatography-mass spectroscopy (LC-MS). The measured amounts of choline were comparable in the treated and untreated rats (SI Appendix, Fig. S8). Note that choline is a naturally occurring molecule in living organisms. No geranic acid was found in the organs of either group of rats.
Discussion
The results presented here demonstrate the ability of CAGE to reduce fat uptake and lower weight gain. CAGE interferes with absorption of fat in the GI tract. Studies performed using DHA as a model fat species show that DHA forms micelles/microstructures upon contact with CAGE and water. DLS and TEM data indicate that the size of the self-assembled structures was several microns, which is likely to reduce their transport across the epithelium. The large particle formation may result from self-assembly of CAGE and DHA in water due to molecular interactions driven by hydrophobic and ionic forces. CAGE is an amphiphilic entity owing to the combination of hydrophobic/anionic geranic acid with hydrophilic/cationic choline. By itself, CAGE has been shown to undergo self-assembly in water leading to the formation of micelles (27). Indeed, DLS studies indicated the presence of nanoscale structures with a size of ∼100 nm. By itself, DHA also forms aggregates in the size range of 50 to 400 nm in diameter when diluted in water. In contrast to nanoscale self-assembled structures of CAGE and DHA each alone in water, the combination of CAGE and DHA led to micrometer-sized aggregates in water. The microscopic size of the aggregates was evident from DLS as well as the milky appearance of the emulsion. Xia et al. (28) demonstrated that smaller nanoemulsions (∼100 nm) distribute to enterocytes and basolateral tissues and larger nanoemulsions (∼1,000 nm) adhered to villi. Larger particles were also shown to exhibit 2-fold lower bioavailability than smaller particles. Jenkins et al. (29) also reported that bioavailability of 3-μm polystyrene particles is 2.5-fold less than that of 150-nm particles in rats after oral administration. The ex vivo permeation results confirm that CAGE reduces the uptake of DHA. The kinetics as well as the magnitude of DHA permeation were reduced in the presence of CAGE.
Of note, the retarding effect of CAGE on DHA transport was not seen for the individual components. This likely originates from the unique amphiphilic and solubilization properties of CAGE compared with its components. The slower rate of transepithelial DHA permeation in the presence of CAGE is likely a result of the formation of large micelles that prevent the DHA from penetrating through the intestinal membrane ex vivo.
The in vivo pharmacokinetics results are correlated with the ex vivo permeation results. CAGE significantly reduced the absorption of DHA in rats. The maximum serum concentration of DHA was reduced by 2-fold after coadministration of CAGE. The absorption profile of DHA was also altered by CAGE. Specifically, in the absence of CAGE, DHA was absorbed quickly, reaching the peak concentration at 4 h. CAGE slowed the absorption of DHA and also reduced the peak concentrations. CAGE increased the retention of DHA in the intestine even at 6 h after oral administration. In the absence of CAGE, at 6 h after administration, there was little fat left in the stomach or intestines. In fact, no detectable signal could be found in the intestine. Fat would have been metabolized after oral delivery and absorbed by the body. This was not the case for the animals given CAGE, however. Significant DHA remained in both the stomach and the intestines, in agreement with the hypothesis that CAGE interacts with hydrophobic DHA, trapping it in aggregates and preventing its absorption into the body. Based on the fluorescence intensity, the amount of DHA left in the stomach was 2-fold higher in the CAGE- treated rats compared with the untreated rats. Similar results were seen in the duodenum and ileum of the small intestine. In the jejunum, CAGE-fed rats retained 3-fold more fat in the jejunum compared with the rats not given CAGE. At 12 h, no significant DHA-C6 could be observed in the GI tract of CAGE-fed and non–CAGE-fed rats. The pathways of fat absorption in the presence of CAGE and its subsequent disposition require further research.
The effect of CAGE on fat uptake translated into changes in the body weight. Both CAGE-treated and untreated rats were fed an HFD. We selected 2 dosages of CAGE, 5 and 10 μL. The 10 μL dose for a 200 g rat is equivalent to an ∼500 mg dose in humans. A 10 μL daily dose of CAGE for 30 d to rats fed HFD resulted in 12% less body weight gain compared with the rats not given CAGE. The in vitro data on micellization, ex vivo data on intestinal penetration, and in vivo data on weight gain collectively suggest that CAGE forms micelles/aggregates in the presence of fat/water in the intestine, which prevents its permeation across the intestinal membrane. Fat retained in the intestine likely leaves the body as stool, although further research is needed to understand the metabolism of fat in the presence of CAGE. We also observed that the rats given CAGE orally ate less compared with the untreated rats. This may be a result of both inhibition of DPP-IV (SI Appendix, Fig. S3) and the retention of fat/CAGE micelles in the intestine and stomach, which could increase satiety. DPP-IV inhibition is known to reduce appetite (30). At the same time, the retention of fat/CAGE in the intestine causes a feeling of fullness, which results in less food uptake.
Daily CAGE administration was well tolerated by the rats. An analysis based on complete blood count, serum biochemistry, LDL, HDL, and histology of multiple organ systems indicated no differences between controls and CAGE-treated rats. A multiplex analysis is warranted as a part of further development to fully assess the compatibility of CAGE. LC-MS analysis confirmed that no geranic acid was detected in organs. LC-MS analysis of choline content in the organs also indicated no absorption of CAGE itself, likely due to the formation of large micelles. Choline and geranic acid have a previous history of use in humans; choline is a commonly used dietary supplement, and geranic acid is a common food flavorant. The amount of choline delivered through CAGE is lower than that used in the dietary supplement; thus, we do not anticipate any notable biological effects of choline from CAGE, although additional research is needed to fully understand the potential effects of choline.
CAGE offers a simple and promising alternative to treat individuals living with obesity and its complications. While lifestyle intervention and bariatric surgery are the current standard therapies for obesity, the development of pharmacotherapies and removable devices has begun to bridge the gap between these extremes of care. In addition, the manipulation of thermogenic fat cells found in brown adipose tissue as a means to combat obesity is drawing significant interest (31). In this active and varied landscape of current and investigational approaches, CAGE provides several unique advantages. Compared with surgical intervention, oral CAGE offers a noninvasive alternative with reduced adverse effects. Compared with drugs that act by inhibiting GI tract enzymes or altering brain chemistry, CAGE acts directly on fats in the intestine, reducing the potential for side effects. CAGE represents a simple and near-term treatment compared with manipulating the mechanisms that control the activity or amount of brown adipose tissue.
CAGE facilitated a 12% percent reduction in fat absorption in rats on an HFD compared with untreated controls. CAGE also appears to affect appetite by keeping fats in the GI tract longer and could potentially inhibit endogenous DPP-IV, extending the circulation of appetite-regulating GLP-1. It is possible that DPP-4 inhibition makes a biological contribution to the observed effect, potentially through some interaction with leptin signaling. Such interactions will be explored in future studies. Future studies also should assess potential roles of biological mechanisms in the effect of CAGE to fully understand the (sub)types of obesity that can be treated by CAGE. After additional research focused on understanding the molecular mechanisms of fat–CAGE interactions and efficacy with a real-life HFD, CAGE offers an exciting potential treatment option for those living with obesity.
Materials and Methods
Materials.
Geranic acid, choline bicarbonate, DMSO, and C6 were purchased from Sigma-Aldrich, and bovine trypsin was obtained from MP Biomedicals. Paraformaldehyde (10% wt/vol) was purchased from Spectrum Chemicals, and DHA was obtained from BioGem. Male Sprague–Dawley rats were purchased from Charles River Laboratories. Size 9 capsules were obtained from Torpac. Hematoxylin and eosin solutions were purchased from Sigma-Aldrich. The DPP-IV inhibition ELISA kit was purchased from Cayman Chemical. All other reagents used were of analytical grade.
Preparation of CAGE.
CAGE was synthesized as described in our previous study (16, 21, 22). In brief, 2 equivalents of neat geranic acid (20 g, 0.119 mol) that had been recrystallized at least 5 times in acetone at <−70 °C to remove impurities were added to 1 equivalent of choline bicarbonate (80 wt% solution, 12.275 g, 0.059 mol) in a 500-mL round-bottom flask. The mixture was stirred at 40 °C overnight, and the water was removed by rotary evaporation at 60 °C for 2 h, followed by drying in a vacuum oven for 48 h at 60 °C. Physical characterization at 25 °C showed good agreement with previous values. The NMR spectra (collected using a 500-MHz Varian instrument) was also in good agreement with previous preparations: 1H NMR (DMSO-d6), δ5.60 (s, 2H), 5.07 (t, J = 6.1, 2H), 3.86 (t, J = 6.6, 2H), 3.42 (t, J = 6.6, 2H), 3.12 (s, 9H), 2.57 (m, 4H), 2.01 (m, 4H), 1.97 (s, 6H), 1.73 (s, 2H), 1.64 (s, 6H), and 1.57 (s, 6H); 13C NMR (DMSO-d6), δ170.1, 150–1, 131.5, 124.1, 122, 67.6, 55.5, 53.6, 53.5, 32.8, 25.9, and 17.9.
Formation of Particles and Size and Morphology Analysis.
Different amounts of DHA were added to 200 μL of CAGE as 1, 10, 20, 40, and 60% of DHA (vol/vol) and vortexed until the mixture was uniform. Then 10% water was added to the CAGE/DHA mixture. The addition of water to the mixture resulted in the immediate formation of micelles. We took a portion of the mixture for DLS analysis on dilution with adequate water by particle size analysis (zen3600; Malvern Instrument). Morphologies of the micelles were imaged by TEM (JEM-1400; JEOL) after the liquid sample was placed on a TEM grid and dried overnight.
Ex Vivo Diffusion Study.
To investigate ex vivo diffusion, intestines were harvested from healthy rats, and jejunum sections were used for this study (32). DHA (0.1 mol) and C6 (0.1 mol) were added to a round-bottom flask and dissolved into dichloromethane (5 mL). 3-(1,3-benzothiazol-2-yl)-7-[ethyl(2-hydroxyethyl)amino]-2H-chromen-2-one (0.5 mol; Chess Fine Organics) was added to the reaction and stirred overnight. The reactant was collected through precipitation of the conjugates by adding an excess amount of water. The DHA-C6 conjugate was collected and lyophilized overnight and characterized by NMR to confirm chemical conjugation. The DHA-C6 conjugate was injected into the intestinal lumen with and without CAGE. The intestines were then knotted and submerged in a beaker containing saline. Samples (200 μL) were collected from both the controls and experimental groups every 30 min for 6 h. Coumarin was measured by excitation and emission of the dye using a microplate reader (Neo2; BioTek Instruments), which was a proxy for the diffusion of fat throughout the respective samples.
Pharmacokinetics and Biodistribution.
In order to investigate the pharmacokinetics and biodistribution of DHA-C6, we made capsules of the CAGE/DHA-C6 and DHA-C6, loading the powder or liquid into size 9 capsules. The Sprague–Dawley rats were fed with AIN-93M food for 3–7 d (Scott Pharma) before dosing the capsule. Blood was drawn from the tail and collected into heparin-coated microtubes. The collected blood was centrifuged at 1,800 rpm for 15 min, after which the C6 content in plasma was analyzed with a microplate reader (Neo2; BioTek Instruments) with an excitation wavelength of 428 nm and an emission wavelength of 536 nm. The harvested organs were imaged by an in vivo imaging instrument (IVIS Spectrum; PerkinElmer) at excitation and emission wavelengths of 465 and 520 nm, respectively. Finally, a portion of the tissue from individual organs was collected and added to water, followed by mechanical homogenization. The homogenized tissue was centrifuged at 2,000 rpm for 15 min, and supernatant was collected to measure the content of C6 based on fluorescence intensity measured by a microplate reader (Neo2; BioTek Instruments) with excitation and emission wavelengths of 428 and 536 nm, respectively.
Body Weight and Food Uptake Monitoring.
The rats were divided into 3 groups (5 rats/group) and housed in separate cages during the observation period. While all of the rats were given an HFD, 2 groups were given regular doses of CAGE (5 μL in I group and 10 μL in the other group) in a capsule that was prepared by inserting an adequate amount of CAGE into the size 9 capsule and coating with 10% Eudragit L 100. The capsules were administered orally daily for 30 d, and body weight was measured in each rat during the observation period. To determine food uptake, we weighed the food added to the cage and the remaining food the next day before the addition of additional food. The HFD was purchased from Research Diet (catalog no. D12492). The composition of the HFD was as follows: protein (casein, lactic), 200 g; protein (l-cystine), 3 g; carbohydrate (Lodex 10), 125 g; carbohydrate (sucrose), 72.8 g; fiber (Solka Floc FCC200; International Fiber), 50 g; fat (lard), 245 g; fat (soybean oil), 25 g; minerals, 50 g; vitamin, 2 g; and dye, 0.05 g in every 1,000 g of food. Choline content, in the form of choline bitartrate, was 0.25% in both diets.
Whole-Body Fat and Lean Tissue Analysis.
After 30 d of dosing with CAGE (5 and 10 μL), whole-body fat content and lean tissue content were measured in both treated and untreated rats by EchoMRI (E26-283-MT) (33). Because the EchoMRI is capable of measuring only 100 g of tissue at a time, the rats were harvested, and tissues were split into small portions to measure. The scan was repeated 3 times for each tissue sample, and the average contents of fat and lean tissue were calculated manually.
Cholesterol Analysis.
After 30 d of administration, blood samples (200 μL) were collected from the portal vein and centrifuged (1,800 rpm, 4 °C, 15 min), and total cholesterol, HDL, and LDL in the plasma were quantified using assay kits from Abcam (ab65390, ab65390, and ab65390, respectively) (34).
DPP-IV Inhibition Activity Measurement.
DPP-IV inhibition assay (DPP-IV ELISA kit 700210-96; Cayman Chemical) was conducted according the manufacturer’s instructions. In brief, the CAGE was diluted with water to 20, 10, 5, 1 and 0.5% by adding the appropriate amounts of water. Sitagliptin served as a positive control. Inhibition activity was measured based on the absorbance value read with a microplate reader (Neo2; BioTek Instruments).
Complete Blood Count and Serum Biochemistry.
Blood was collected from the portal vein after sacrificing the rats. Approximately 50 μL of blood was kept in a heparinized tube to prevent the blood from clotting. White blood cells, red blood cells, and platelets were quantified using an Element HT5 hematology analyzer (Heska). To facilitate a serum biochemistry analysis, ∼1 mL of blood was collected in a separate tube and centrifuged at 1,800 rpm for 15 min to separate plasma and serum. Serum albumin, alkaline phosphatase, globulin, alanine aminotransferase/glutamic pyruvic transaminase, total bilirubin, total protein, blood urea nitrogen, calcium, and creatinine were measured with a DRI-CHEM 7000 chemistry analyzer (Heska) as described previously (35).
Tissue Histology.
The histological analysis methodology was adopted from the literature (36). In brief, tissues from the harvested organs were fixed in 10% buffered formalin, dehydrated in ethanol, and embedded in OCT. Cross-sections of each tissue (20 μm) were washed, rehydrated, and stained with hematoxylin and eosin. Histological morphology was examined using an upright compound microscope with 10× magnification (AxioScan Z1; Carl Zeiss).
Choline and Geranic Acid Analysis in Tissue.
Tissues from the harvested organs were weighed and added to 10× water volume (wt/vol), followed by homogenization with a mechanical homogenizer. The supernatant was collected after centrifugation by a benchtop centrifuge at 2,000 rpm for 15 min. The supernatant was analyzed by LC-MS (6140; Agilent Technologies) and run on a C-18 column to detect the analytes choline and geranic acid based on mass. Mobile phases consisted of 20 mM heptafluorobutyric acid in water or acetonitrile. Retention times were 2.7 min for choline and 7.6 min for geranic acid, and MS peaks were 104 for choline and 169 for geranic acid. Calibration standards were created using choline and geranic acid. Internal standards of d-choline and d-hexanoic acid were added to samples and calibration standards to correct for loss of the analyte during sample preparation.
Data Analysis.
All data are presented as mean ± SE or mean ± SD. Statistical analyses were performed using Student’s t test.
Data Availability.
Material characterization, enzyme inhibition, serum chemistry and histology data are provided in SI Appendix.
Supplementary Material
Acknowledgments
Support for this work was provided by the John A. Paulson School of Engineering and analytical facilities of the Wyss Institute.
Footnotes
Competing interest statement: S.M. is a shareholder of and board member/consultant to Liquideon, CAGE Bio, and i2O Therapeutics.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1914426116/-/DCSupplemental.
References
- 1.Djalalinia S., Qorbani M., Peykari N., Kelishadi R., Health impacts of obesity. Pak. J. Med. Sci. 31, 239–242 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkelstein E. A., Ruhm C. J., Kosa K. M., Economic causes and consequences of obesity. Annu. Rev. Public Health 26, 239–257 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Wang Y., Beydoun M. A., The obesity epidemic in the United States—Gender, age, socioeconomic, racial/ethnic, and geographic characteristics: A systematic review and meta-regression analysis. Epidemiol. Rev. 29, 6–28 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Hesselberg J. O., Wahl A., The effect of a new dietary mineral product on body composition and weight in overweight and obese people. The results from a comparative randomized 30-day study. J. Obes. Eat. Disord. 2, 1–4 (2016). [Google Scholar]
- 5.Wang Y., Beydoun M. A., Liang L., Caballero B., Kumanyika S. K., Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 16, 2323–2330 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Quaresma P. G. F., et al. , Cdc2-like kinase 2 in the hypothalamus is necessary to maintain energy homeostasis. Int. J. Obes. 41, 268–278 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Sharma M. K., Murumkar P. R., Kanhed A. M., Giridhar R., Yadav M. R., Prospective therapeutic agents for obesity: Molecular modification approaches of centrally and peripherally acting selective cannabinoid 1 receptor antagonists. Eur. J. Med. Chem. 79, 298–339 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Chen J., He X., Huang J., Diet effects in gut microbiome and obesity. J. Food Sci. 79, R442–R451 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Saltiel A. R., New therapeutic approaches for the treatment of obesity. Sci. Transl. Med. 8, 323rv2 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Tsou Y. H., et al. , Nanotechnology-mediated drug delivery for the treatment of obesity and its related comorbidities. Adv. Healthc. Mater. 8, e1801184 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Bays H. E., Lorcaserin: Drug profile and illustrative model of the regulatory challenges of weight-loss drug development. Expert Rev. Cardiovasc. Ther. 9, 265–277 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Chao A. M., et al. , A randomized controlled trial of lorcaserin and lifestyle counselling for weight loss maintenance: Changes in emotion- and stress-related eating, food cravings and appetite. Clin. Obes. 8, 383–390 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanovski S. Z., Yanovski J. A., Long-term drug treatment for obesity: A systematic and clinical review. JAMA 311, 74–86 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins G. A., et al. , Evaluation of chemically diverse 5-HT2c receptor agonists on behaviours motivated by food and nicotine and on side effect profiles. Psychopharmacology (Berl.) 226, 475–490 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Nurunnabi M., et al. , Oral delivery of a therapeutic gene encoding glucagon-like peptide 1 to treat high fat diet-induced diabetes. J. Control. Release 268, 305–313 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Garaulet M., Ordovás J. M., Madrid J. A., The chronobiology, etiology and pathophysiology of obesity. Int. J. Obes. 34, 1667–1683 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi J. H., Yun J. W., Chrysin induces brown fat-like phenotype and enhances lipid metabolism in 3T3-L1 adipocytes. Nutrition 32, 1002–1010 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Won Y. W., et al. , Oligopeptide complex for targeted non-viral gene delivery to adipocytes. Nat. Mater. 13, 1157–1164 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Rogers R. D., Gurau G., Is “choline and geranate” an ionic liquid or deep eutectic solvent system? Proc. Natl. Acad. Sci. U.S.A. 115, E10999 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banerjee A., et al. , Reply to Rogers and Gurau: Definitions of ionic liquids and deep eutectic solvents. Proc. Natl. Acad. Sci. U.S.A. 115, E11000–E11001 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banerjee A., et al. , Ionic liquids for oral insulin delivery. Proc. Natl. Acad. Sci. U.S.A. 115, 7296–7301 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zakrewsky M., et al. , Ionic liquids as a class of materials for transdermal delivery and pathogen neutralization. Proc. Natl. Acad. Sci. U.S.A. 111, 13313–13318 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin J.-W., Seol I.-C., Son C.-G., Interpretation of animal dose and human equivalent dose for drug development. J. Korean Med. 31, 1–7 (2010). [Google Scholar]
- 24.Nair A. B., Jacob S., A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 7, 27–31 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puri R., et al. , Self-nanoemulsifying drug delivery system of docosahexanoic acid: Development, in vitro, in vivo characterization. Drug Dev. Ind. Pharm. 42, 1032–1041 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Rusca A., Di Stefano A. F. D., Doig M. V., Scarsi C., Perucca E., Relative bioavailability and pharmacokinetics of two oral formulations of docosahexaenoic acid/eicosapentaenoic acid after multiple-dose administration in healthy volunteers. Eur. J. Clin. Pharmacol. 65, 503–510 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Tanner E. E. L., et al. , The influence of water on choline-based ionic liquids. ACS Biomater. Sci. Eng. 5, 3645–3653 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Xia F., et al. , Size-dependent translocation of nanoemulsions via oral delivery. ACS Appl. Mater. Interfaces 9, 21660–21672 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Jenkins P. G., et al. , Microparticulate absorption from the rat intestine. J. Control. Release 29, 339–350 (1994). [Google Scholar]
- 30.Hansen H. H., et al. , The DPP-IV inhibitor linagliptin and GLP-1 induce synergistic effects on body weight loss and appetite suppression in the diet-induced obese rat. Eur. J. Pharmacol. 741, 254–263 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Birerdinc A., Jarrar M., Stotish T., Randhawa M., Baranova A., Manipulating molecular switches in brown adipocytes and their precursors: A therapeutic potential. Prog. Lipid Res. 52, 51–61 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Pretorius E., Bouic P. J. D., Permeation of four oral drugs through human intestinal mucosa. AAPS PharmSciTech 10, 270–275 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taicher G. Z., Tinsley F. C., Reiderman A., Heiman M. L., Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Anal. Bioanal. Chem. 377, 990–1002 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Suzuki K., Kim K. S., Bae Y. H., Long-term oral administration of Exendin-4 to control type 2 diabetes in a rat model. J. Control. Release 294, 259–267 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nurunnabi M., et al. , In vivo biodistribution and toxicology of carboxylated graphene quantum dots. ACS Nano 7, 6858–6867 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Khatun Z., et al. , Optical imaging, biodistribution and toxicity of orally administered quantum dots loaded heparin-deoxycholic acid. Macromol. Res. 23, 686–695 (2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Material characterization, enzyme inhibition, serum chemistry and histology data are provided in SI Appendix.