Significance
Industrial-scale production of crops through monocultures has resulted in “green deserts” of reduced biodiversity in many areas worldwide. Such simplified landscapes may impact ecosystem services such as pollination. Here, we present a large-scale, longitudinal study of managed honey bee colonies in the context of corn and soybean monocultures. Our results reveal a brief burst of colony growth during soybean bloom, followed by a longer period of forage dearth, resulting in decline in several aspects of honey bee health at both colony and individual levels. We demonstrate this decline is reversible when honey bees have access to native, perennial plants (i.e., prairie). Our results suggest sustainable pollinator management in landscapes dominated by monocultures can be achieved through reintegration of native biodiversity.
Keywords: honey bee, Apis mellifera, land use, agriculture, pollinators
Abstract
Intensive agriculture can contribute to pollinator decline, exemplified by alarmingly high annual losses of honey bee colonies in regions dominated by annual crops (e.g., midwestern United States). As more natural or seminatural landscapes are transformed into monocultures, there is growing concern over current and future impacts on pollinators. To forecast how landscape simplification can affect bees, we conducted a replicated, longitudinal assessment of honey bee colony growth and nutritional health in an intensively farmed region where much of the landscape is devoted to production of corn and soybeans. Surprisingly, colonies adjacent to soybean fields surrounded by more cultivated land grew more during midseason than those in areas of lower cultivation. Regardless of the landscape surrounding the colonies, all experienced a precipitous decline in colony weight beginning in August and ended the season with reduced fat stores in individual bees, both predictors of colony overwintering failure. Patterns of forage availability and colony nutritional state suggest that late-season declines were caused by food scarcity during a period of extremely limited forage. To test if habitat enhancements could ameliorate this response, we performed a separate experiment in which colonies provided access to native perennials (i.e., prairie) were rescued from both weight loss and reduced fat stores, suggesting the rapid decline observed in these agricultural landscapes is not inevitable. Overall, these results show that intensively farmed areas can provide a short-term feast that cannot sustain the long-term nutritional health of colonies; reintegration of biodiversity into such landscapes may provide relief from nutritional stress.
As human population grows (1), habitat loss from anthropogenic landscape changes threaten the health and existence of many species (2). An ever-increasing demand for food and biofuels following human population expansion requires more land be dedicated to agricultural production (3, 4). Global land use has shifted to meet this demand, with natural areas and smaller-scale agricultural enterprises transformed into high-yielding monocultures (5–7), but with some cost (8). Monocultures can have substantial negative environmental effects on soil, water, and air quality, and when coupled with the removal of native, noncrop habitat, this form of agriculture is associated with declines in pollinator populations (9–13). This conversion is provoking concerns for reduced pollination of crops and wild plants that could lead to reductions in agricultural production and ecosystem service delivery (14).
Worldwide, honey bees (Apis mellifera) are the most economically important pollinator of crops, with honey bee colonies in the United States alone responsible for over $15 billion per year (10, 15). Like other bee species, honey bees are challenged by environmental stresses that reduce colony survival, with statewide losses as high as 60% depending upon their location within the continental United States. This rate is higher than beekeepers consider sustainable (16–19), resulting in increased costs for contracted pollination services (15, 20). These losses are associated with multiple, potentially interacting, stressors, including pest/pathogen pressure, pesticide exposure, and nutritional shortages (9, 11, 21, 22), all associated with anthropogenic influence (23, 24).
How do honey bees respond to landscapes that become increasingly dominated by intensive agriculture, particularly of crops considered to have limited nutritional benefit? Nationwide surveys have shown some of the worst colony losses occur in the midwestern United States (16, 18, 25), a region of major agricultural production (5). Furthermore, agricultural land use has been associated with lower amounts of protein in stored pollen (26), lower honey production (27, 28), and decreased physiological health of honey bees (29, 30). Conversion of noncropped land to crops has been linked to a decline in suitability for productive apiaries (4, 7) and several key metrics of honey bee health and productivity (31–33) in the Northern Great Plains region of the United States, where agricultural intensification has recently increased (4, 24, 34).
While the popular press has evocatively described regions that are agriculturally productive but devoid of biodiversity as “green deserts” (35), corn and soybean fields can host dozens of pollinator species (36). Furthermore, increases in cropland can correlate with improvements in key honey bee growth metrics like food accumulation (37), as mass flowering crops or noncrop plants growing in field edges can provide forage for honey bees and wild bees (38–40). Thus, it remains unclear whether intensely farmed landscapes are overall net-positive or net-negative for managed pollinators such as honey bees. Studies of honey bees’ responses to crop production that do not explore seasonal exposure to landscape features may miss changes in phenology that can be significant for colony and individual honey bee health. Determining the net effects of agriculture upon honey bee survival requires multiseason, longitudinal studies of replicated, researcher-controlled colonies embedded in multiple types of agroecosystems.
Herein, we describe a comprehensive, longitudinal study of colony growth and bee nutrition in one of the most intensively farmed areas of the world, Iowa in the United States, a perennial leader in the production of corn and soybean (41), with 92.6% of the state dedicated to agriculture and 72.9% planted with annual crops (42). Despite this general lack of landscape diversity, variation in land use within the state can explain the abundance and diversity of key members of the insect community found within soybean fields (43–46). By placing bee colonies next to soybean fields and comprehensively studying their response to variation in land use surrounding these fields, we can understand how honey bees respond to a highly intensified agricultural landscape and begin to forecast the future of honey bee health in other regions undergoing similar agricultural intensification (4, 7, 24, 31, 32). Analogous longitudinal approaches can be used to assess intensification in other cropping systems.
We placed apiaries of 4 colonies adjacent to commercial soybean fields surrounded in a 1.6-km radius (7) by either a majority of cultivated cropland (average 83.9% ± 0.023 SEM corn and soybean; referred to as “high cultivation”) or minority of cropland (average 38.2% ± 0.053 SEM corn and soybean; “low cultivation”). The remaining portions of these landscapes were comprised of more perennial, uncultivated features (i.e., woodland, grassland/pasture, urban development). We selected these 2 categories of land use as extremes within a range shown to affect the diversity and abundance of insect communities within soybean fields of Iowa (39–42). By midseason, apiaries within high cultivation landscapes had the greatest populations and heaviest hives. By the end of August, all colonies, regardless of surrounding land use, declined precipitously, suggesting that—no matter the surroundings—intensively farmed landscapes can be poorly suited for sustainable, summer-long apiculture. We further demonstrate that this decline in colony health is mitigated by providing colonies access to more diverse, native forage (i.e., prairie), suggesting that the addition of flowering resources late in the growing season has the potential to reverse some negative effects arising from the current landscape.
Results
Apiaries Were Heavier in Landscapes with High Cultivation than Low Cultivation.
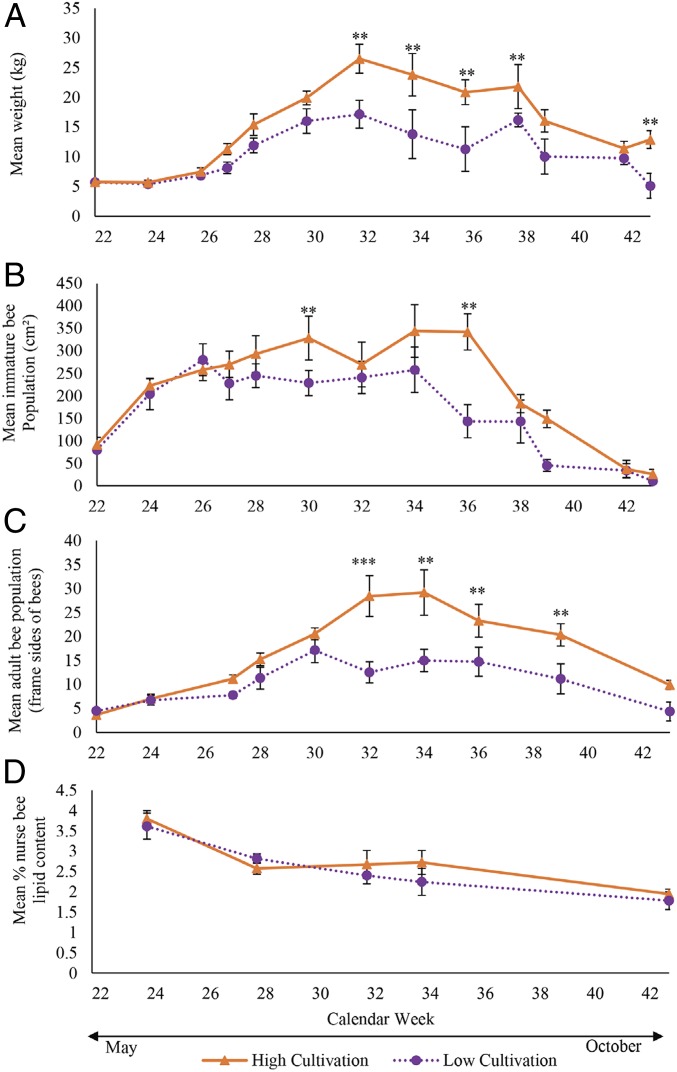
In both years, apiaries kept adjacent to soybean fields in high-cultivation landscapes were heavier (Fig. 1A) (F1, 17.38 = 5.66, P = 0.0291), with marginally higher immature bee populations (Fig. 1B) (F1, 17.33 = 3.63, P = 0.0737) and higher adult bee populations (Fig. 1C) (F1, 8 = 6.53, P = 0.0339) than those in low-cultivation landscapes. All metrics of colony growth varied significantly within a year (F12, 126.1 = 38.13, P ≤ 0.0001; F12, 116.3 = 16.72, P ≤ 0.0001; F9, 72 = 31.12, P ≤ 0.0001 for weight, immature population, and adult population, respectively). We also detected interactions between cultivation category and sampling week for apiary weight (F12, 127.4 = 3.22, P = 0.0005) and adult bee populations (F9, 72 = 5.74, P ≤ 0.0001), as discussed below. However, weight (F1, 20.25 = 0.70, P = 0.4121) and immature population (F1, 34.79 = 3.08, P = 0.0882) did not vary by year. We did not observe a significant difference in nurse bee nutritional state, as estimated by lipid content (i.e., fat stores), between cultivation categories (Fig. 1D) (F1, 69 = 1.28, P = 0.2625) or sampling years (F1, 69 = 1.22, P = 0.2735), and there was no interaction between landscape categories and sampling week (F4, 69 = 0.65, P = 0.6298).
Fig. 1.
High-cultivation landscapes result in better bee health metrics, but all experience late-season declines. Apiary-averaged hive weight (A), immature bee population (i.e., capped pupae) (B), adult bee population (C), and percent lipid content (D) of colonies kept in soybeans surrounded by high (n = 10; solid red lines and triangles) and low cultivation (n = 10; dotted purple lines and circles) landscapes over the growing season, mean ± SEM. Weight and adult bee population were significantly higher overall in high-cultivation landscapes while immature bee population was only marginally higher. Results based on repeated mixed-model ANOVA with post hoc Tukey comparisons. For lipid content all weeks include 1 colony from each site from each year (totaling 10 per cultivation category), with the exception of week 32, which includes only 2015 (n = 5 per category) and week 34, which includes only 2016 (n = 5 per category), while weight, immature population, and adult population include 4 colonies per location (totaling 40 colonies per cultivation category) on each week. Results based on mixed-model ANOVA with post hoc Tukey comparisons; **P < 0.05, ***P < 0.0001 for statistical difference between cultivation category at a specific week (SI Appendix, Tables S5–S7 for weight, immature population, and adult population respectively).
Apiaries and Individual Bee Health Declined Drastically in Late Summer.
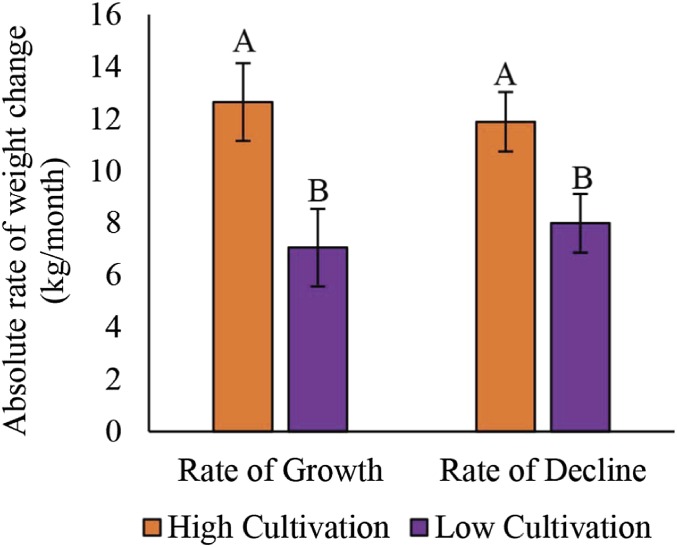
To further understand the temporal dynamics of colony growth and decline in light of the interaction between cultivation category and weeks when weight was estimated, we calculated rates at which apiaries gained weight (from initial weight to the seasonal maximum), and lost weight (seasonal maximum to the end of our observations) (Fig. 2). Apiaries surrounded by high cultivation gained and lost weight at greater rates than those in low-cultivation landscapes (Fig. 2). The rates of gain and loss were nearly identical within a cultivation category (Fig. 2). Apiaries in both cultivation categories began to lose weight after 10 wk at rates that were similar to the rates at which they gained weight, such that by mid-October (week 43) all apiaries returned to their initial weight. Similar patterns of gains and declines were observed in immature and adult bee populations (SI Appendix, Table S11). These declines began nearly 2 mo before central Iowa normally experiences subfreezing temperatures (47) that terminate all flowering resources; therefore, the significantly faster rate at which colonies lost weight in high-cultivation landscapes may put them at an increased risk for nutritional deficit and overwinter starvation.
Fig. 2.
Apiaries in high-cultivation landscapes grow and decline at a faster rate. Apiary-averaged absolute rate of weight growth and decline in colonies surrounded by high- (red solid bars) and low-cultivation (purple and pattern bars) landscapes in 2015 and 2016, mean ± SEM. Rate of growth includes all time points from weeks 22 to 30 calculated by months (May, June, July). Rate of decline includes all time points from weeks 32 to 43 calculated by months (August, September, October). Results based on mixed-model ANOVA with post hoc contrasts within and across treatments; letters represent significance between cultivation categories P < 0.05 (SI Appendix, Table S11).
However, despite the differences in weight decline, lipid concentration of worker bees did not differ by cultivation category, but only by date (F4, 32.2 = 21.38, P ≤ 0.0001). Regardless of where apiaries were located, lipid content of nurse bees was highest at the initiation of the experiment (week 26) and declined throughout the 22 wk of our monitoring (Fig. 1D and SI Appendix, Table S10). This is noteworthy as the final sampling period occurred in mid-October, when honey bee colonies in temperate regions such as Iowa enter a preoverwintering stage commonly associated with increased lipid stores (48).
The Type of Forage Used by Apiaries Did Not Vary by Location, but Varied during the Season.
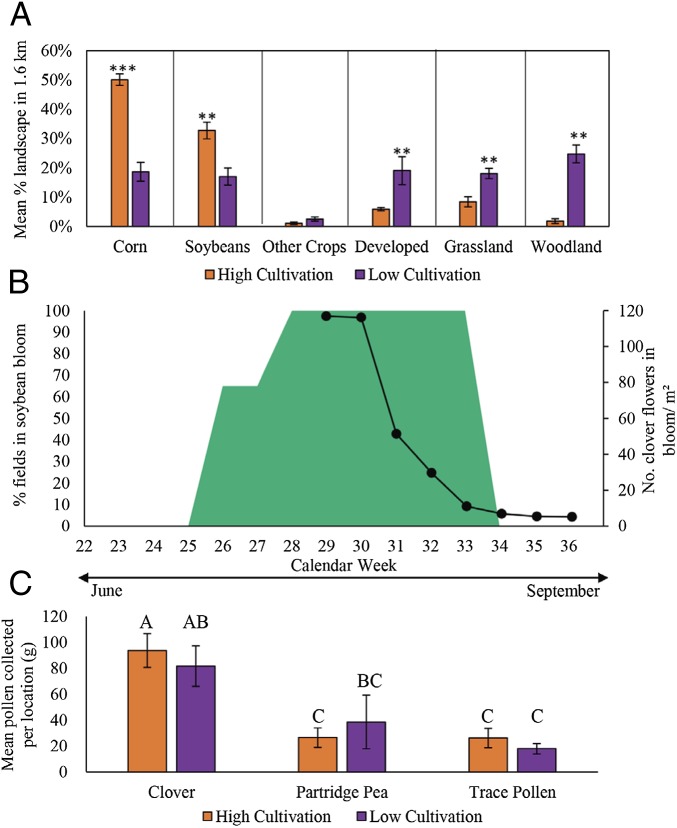
Apiaries at every location began our experiment with the same average weight but reached different seasonal maximums, suggesting that variation in land use between the cultivation categories contributed to available forage. Honey stores are the greatest contributor to hive weight, derived from foragers focused on collecting nectar over other material (e.g., pollen, water, propolis) (49, 50). The design of this experiment does not allow us to determine how much a specific plant contributed to honey production, but there is indirect evidence suggesting several plants were nectar sources when colonies were gaining weight. Colonies in high-cultivation landscapes were surrounded by significantly more soybean (and thus field edges) than those in low-cultivation landscapes (Fig. 3A) (T17.993 = 3.88, P = 0.0011). Field edges are likely to contain a higher abundance of clover, a resource that has previously been identified as a significant source of nectar for honey production (37, 51). During our experiment, the period of greatest colony weight gain (Fig. 1A) occurred when clover was in bloom (Fig. 3B). However, this period also occurred when the majority of soybean fields adjacent to our apiaries were blooming (Fig. 3B). Although soybeans have been bred for self-pollination (52, 53), the flowers sometimes produce nectar used by honey bees for honey production (38, 54–56), although nectar production varies by cultivar and growing conditions (38, 57, 58). Nectar foragers incidentally come in contact with pollen during foraging [i.e., sticking to hairs (59)], and observations from stored honey within our colonies revealed traces of both soybean and clover pollen (SI Appendix, Table S13). Although traces of both plants’ pollens were present in honey, these observations do not allow us to determine when and to what degree a single plant contributed to overall honey production. Overall, these observations suggest that hives in the high-cultivation landscapes may have grown heavier and at a faster rate because more nectar forage was available.
Fig. 3.
Even though land use differs significantly, pollen collection is driven by few plants. (A) Site averages of percent landscape features within 1.6-km radius of apiaries in high- (red solid bars) and low-cultivation (purple pattern bars) landscapes in 2015 and 2016, mean ± SEM. There was significantly more corn and soybean in high-cultivation landscapes. Low-cultivation landscapes consisted of significantly more developed land, low forb diversity grasslands, and woodland. Results based on t tests; **P < 0.05, ***P < 0.0001 for statistical difference between cultivation categories for each landscape feature. (B) Percentage of fields in soybean bloom (green and filled area) across the season in 2015 and 2016 (represented in left axis). Number of clover blooms present per meter square (solid lines and circles) in 2016 transect (represented in right axis). (C) Mean colony collected pollen grams in high- (red solid bars) and low-cultivation (purple pattern bars) landscapes in 2015 and 2016, mean ± SEM. There were no significant differences in total pollen collected by cultivation category; however, significantly more clover pollen was collected in both landscapes compared to partridge pea (T36 = 4.68, P = 0.0001) and trace pollens (T36 = 5.57, P ≤ 0.0001). Partridge pea and trace pollen amounts did not differ (T36 = 1.97, P = 0.6513). Results based on mixed-model ANOVA with post hoc Tukey comparisons; no significance was detected between cultivation categories for each pollen type collected (SI Appendix, Table S12).
Conversely, apiaries in the low-cultivation landscapes may have had more alternative sources of forage available later in the season such that their weight loss occurred at a slower rate than those in the high-cultivation landscapes. We tracked the collection of pollen by colonies in these apiaries to determine if this type of forage provided insight into whether flowering resources varied by cultivation category. We did not observe differences in the amount of pollen collected between the landscape categories (Fig. 3C) (F1, 17 = 0.06, P = 0.8121), nor the pollen types collected (F2, 36 = 0.60, P = 0.5525). No soybean pollen was detected in pollen traps at any apiary. Pollen was collected primarily from clover (Trifolium spp.) (61.9%) and secondarily from partridge pea (Chamaecrista fasciculata) (20.9%), with the remaining 17.2% comprised of 25 species (i.e., trace pollens).
Although our analysis of pollen collected by honey bees did not help explain potential differences in forage availability between the 2 landscape categories, they provided insight into why apiaries in both categories lost weight at the same time. Clover (Trifolium spp.) was the most common pollen source for our apiaries and is also a common nectar source for honey bees in the United States (51), and is likely to have contributed substantially to differences in colony weight. Flower production of both clover and soybean declined dramatically by week 33 (Fig. 3B). Without a substantial source of flowering resources during late August and September (weeks 33 to 38), honey bees would be left with only their stored honey and pollen as a food source. The larger colonies in the highly cultivated landscapes may have lost weight at a faster rate than those in the lower cultivated landscapes simply because their greater populations consumed their honey stores at a faster rate than smaller colonies.
Providing Colonies Access to Prairie Reverses Late-Summer Declines in Weight and Lipids.
We conducted a separate experiment in summer 2016 to determine if declines in honey bee weight and health could be prevented by providing access to prairie habitat. We selected prairie because it is comprised of flowering plants that bloom during the late summer to early fall and are not commonly found in purely agricultural landscapes. Many prairie plant species are attractive to pollinators, and a subset bloom when we observed colony decline in our first experiment (60).
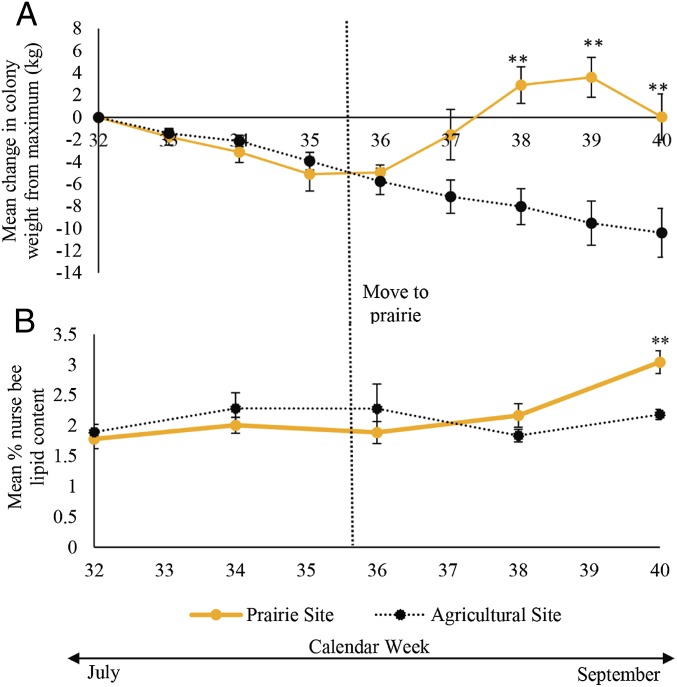
For this experiment, we focused on honey bee colonies as the experimental unit, and used 10 colonies of similar population and weight, established at an agricultural location (Bee and Wasp Research Facility, Iowa State University Horticulture Station, Ames, IA; 44% cropland in surrounding landscape). After 3 consecutive weeks of weight loss after the midsummer mass peak, a random selection of 5 colonies were moved to a reconstructed tallgrass prairie, with the remaining colonies kept at the agricultural site. After the move to prairie, these colonies not only ceased losing weight (Fig. 4A), but became heavier than those remaining at the agricultural site on weeks 38 (T72 = 2.87, P = 0.0054), 39 (T72 = 3.18, P = 0.0022), and 40 (T72 = 2.56, P = 0.0127) (SI Appendix, Table S15). Colonies remaining at the agricultural site continued to decrease in weight and ended the season significantly lower than their summer maxima (Fig. 4A) (average 34.81 kg ± 5.096 SEM, T76 = 3.76, P = 0.0209). In contrast, colonies with access to prairie ended the season with a weight that reached their summer maxima (Fig. 4A and SI Appendix, Table S16) (average 50.08 kg ± 5.327 SEM, T76 = 0.74, P = 1.000). In addition, colonies placed in prairie contained nurse bees with significantly higher lipid content at week 40 than those that remained at the agricultural site (Fig. 4B) (T30 = 3.01, P = 0.0053). While we cannot definitively tell what plants bees foraged on in the prairie, we report a qualitative list of flowering forbs observed at this site and their approximate bloom times in SI Appendix, Table S17.
Fig. 4.
Access to prairie arrests and reverses late-season declines in hive weight and body quality. Change in weight of colonies from maximum summer mass (A) and colony percent lipid content of nurse bees (B) moved to a prairie or remaining in an agricultural site from July to September of 2016, mean ± SEM. Results based on repeated-measures mixed-model ANOVA with post hoc Tukey comparisons; **P < 0.05 for significance between landscape at a specific week.
Discussion
Overall, our results demonstrate that some highly cultivated landscapes can provide short-term gains in colony growth but can also fail to support colony health across the entire growing season, especially in the critical preoverwintering period. This longitudinal perspective on honey bee health greatly helps to clarify the dynamics of honey bee responses to landscape and forage availability, especially given previous, sometimes conflicting reports suggesting both positive and negative impacts of intensive farming on honey bee health (7, 33–36).
In the midwestern United States corn and soybean system, we found that apiaries located in landscapes with higher cultivation accrued greater weight, higher immature bee populations during peak season, and higher adult bee populations than those kept in areas with less cultivation (Fig. 1A). The rate at which apiaries added weight was greatest in landscapes with more soybean (Figs. 2 and 3A), and occurred during the bloom period of soybean and clover (Fig. 3B), suggesting that soybean and clover are sources of nectar for honey bees in central Iowa. Our observations of soybean and clover pollen in samples of honey collected at all locations provide further evidence that honey bees utilize both as nectar resources (59), although it is unclear what their relative contributions are (SI Appendix, Table S13). It is also notable that no corbicular soybean pollen was ever detected in pollen traps, suggesting that any foraging on soybean was only for nectar; that is, trace amounts of pollen are known to fall into nectar or incidentally attach to nectar foragers, where it then is incorporated into honey at low levels (59). Furthermore we observed the greatest weight loss in apiaries within high cultivation landscapes (Fig. 2) after cessation of soybean and clover bloom. Although apiaries in low-cultivation landscapes had access to more grassland, woodland, and developed land (Fig. 3A), which is more likely to contain alternative sources of forage (i.e., something other than soybean or clover), this did not prevent a late-season weight loss. These data therefore demonstrate a “critical period” of limited forage availability in late summer and early fall that is present in areas of both high and low cultivation.
In both high- and low-cultivation landscapes, colonies relied upon a startlingly limited number of plants, primarily clover (Fig. 3C), for pollen, suggesting agricultural landscapes as a whole do not provide a diverse pollen resource for bees. Bees use pollen as their primary source of proteins, lipids, and micronutrients (50); furthermore, honey bees are generalist pollinators and prefer mixed-pollen diets (61). Polyfloral pollen diets are associated with longer honey bee lifespan (62, 63), increased resilience against pathogens (64–66), and can interact with their response to pesticide exposure (67). Colony reliance on a limited pollen diet may contribute to honey bee stress in agroecosystems; first, access to pollen only occurs for part of the season, and second, even when pollen is most abundant, the lack of diversity may produce colonies that are less tolerant of other stressors (21).
While we report evidence consistent with studies that reveal a positive response between annual crop production and colony health (25, 35–38), the uniform decline late in the growing season supports the findings of other studies suggesting that agricultural lands are detrimental to bee health (24, 27–32). While honey bees can survive long periods of forage dearth, like winters in temperate climates (68), the responses we observed are not consistent with healthy colonies. By October, before the overwintering period has begun for central Iowa, colonies had lost on average 53% of their total maximum weight, bringing their food stores to a dangerously low level unlikely to allow survival during the winter in a temperate climate (69), let alone produce a harvestable honey crop. Furthermore, the lipid content of nurse bees at the end of the season was reduced (Fig. 1D), suggesting that individual bees were not transitioning to a physiological state for successful overwintering. By the end of the growing season, adult bees in an overwintering state should have high fat stores (68, 70); for example, experimentally stimulated winter bees exhibit 43 to 59% higher lipid stores than summer controls (48). In contrast, the lipid concentration for bees kept in both of our cultivation categories changed in similar magnitude, but in the opposite direction, declining by 49% from June to October. Even if colonies were able to reduce populations to a level that could survive on the existing stored resources, or if supplemental food source (e.g., sugar solution) were added, the remaining bees may not be physiologically capable of surviving. To what extent the colonies we tracked in these experiments capture the physiological state of commercially managed honey bees is not clear, as we did not provide a supplemental food source, a common practice for managed colonies experiencing a lack of forage.
Is decline during this period inevitable, or can land management practices be implemented to arrest or ameliorate the reduction in colony food stores and physiological health? Our colony relocation experiment revealed that providing declining colonies with access to tallgrass prairie reversed this trajectory. Although much of the upper Midwest was covered in tallgrass prairie before settlement by Europeans, very little currently remains. Before European settlement, Iowa was ∼80% prairie, but is now only 0.1%, with most of this lost by the 20th century (71, 72). Plants native to prairies are highly attractive to bee pollinators (60), and when grown in a mixture, attract a more abundant and diverse community of pollinators than cultivated features of an agricultural landscape (73). Small patches of prairie (1 to 4 ha) embedded within annual crop fields increase pollinator abundance along with improvement to other agriculturally related ecosystem services (74). Previously, it was unclear how beneficial native plants are to the health of the exotic honey bee (75). Our results confirm that a habitat comprised of native plants can be used as forage by honey bees at least during this late-summer dearth period to counteract colony weight loss (Fig. 4A) and reverse the changes in physiological health of putative nurse bees (Fig. 4B).
Pesticide exposure is a significant stressor experienced by bees in agricultural landscapes (9), and since 2000, insecticide use on soybean has increased, due in part to the invasive soybean aphid (76, 77). Although we did not control for insecticide use within our experiments, we did not observe evidence of direct, lethal exposure to insecticides in any of our colonies. In contrast, colonies performed better in areas of higher cultivation, particularly during a period when insecticide use to prevent aphid outbreaks is recommended [i.e., the flowering period of soybeans (76)]. Furthermore, no foliar insecticides were applied to any of the adjacent soybean fields, although applications could have occurred in the surrounding landscape, possibly leading to sublethal exposure. Thus, we cannot rule out a possible interaction between sublethal exposure to insecticides and forage availability contributing to the nutritional deficiencies in nurse bees. Future experimental work is needed to better understand the interaction between nutritional stress and sublethal pesticide exposure in a field setting.
Conclusions
In 2016, Iowa was planted with 5.62 million ha of corn and 3.84 million ha of soybean (78), making it the top producer of both crops by dedicating the highest percentage of its landscape (72.9%) to their production compared to any other US state (79). This extreme example of crop production represents a worst-case scenario for studying how landscape transformation can affect the food supply for bees, with a majority of the landscape taken up by crops that provide limited floral resources. This transformation is occurring elsewhere, with important beekeeping states like North and South Dakota increasing their production of both corn and soybean in the last decade (4). While our focus is on honey bees, many other insect fauna are likely impacted by the conversion of perennial grassland for annual crop production (80).
With a loss of floral resources and increased risk of insecticide exposure associated with the production of many annual crops, is such a landscape no longer tenable for honey bees or other pollinators? Our results show that agricultural intensification can result in honey bee colonies experiencing poor nutritional conditions, particularly in the late summer and autumn, and dependency on a limited number of floral resources that grow primarily in field edges around agricultural farms. We addressed these deficiencies by providing honey bees access to prairie; exposure to a diverse, late-summer forage successfully reversed the sharp decline in weight and improved bee lipid stores, a key to successful overwintering (48, 68, 81, 82), suggesting that even in the most extreme landscapes, colony decline is not inevitable if patches of suitable habitat are available (24).
Our data do not allow us to determine the relative contribution of overall forage availability or increased forage diversity to these benefits. While honey bees can survive on low-diversity diets, they perform best on mixed plant sources (50, 62, 83, 84), and more diverse pollen may improve resilience to pathogens (64, 65) and pesticides (67, 85). Thus, providing honey bees late-summer forage in the form of prairie could improve the food accumulation of colonies (as witnessed by increased colony weight), the physiological health of their bees (increased lipids), and potentially increase their resilience to other stressors. Efforts to apply these findings should address to what extent the amount and diversity of forage, independent of each other, affect honey bee productivity and health. Further elucidation of nutritional deficiencies within agricultural landscapes can help inform efforts to efficiently improve pollinator habitats within intensively farmed regions. Moreover, moving honey bee colonies to the limited patches of prairie available in these systems is likely not a sustainable remedy; however, efforts to integrate native vegetation into and around agricultural fields may improve honey bee health while providing other benefits (24, 74).
Methods
Site Selection.
To determine how honey bees respond to varying levels of crop cultivation (high vs. low), we conducted a landscape-level study in which apiaries of 4 colonies were assigned to soybean fields embedded within landscapes of varying amounts of crop production. For this experiment, each site was an experimental unit. Because soybean is annually rotated with corn, new sites had to be selected each year, for a total of 10 site-years for each cultivation category. In 2015 and 2016, we screened Iowa State University (ISU) and privately owned commercial soybean farms in Story, Boone, Marshall, and Hardin counties, Iowa to locate farms that were greater than 20 ha and did not have honey bee colonies within 1.6 km (SI Appendix, Table S1). Land use surrounding each farm was quantified in ArcGIS, ArcMap 10.3.1 using a 1.6-km radius centered on the apiary location. Land use features were based on the US Department of Agriculture–National Agricultureal Statistics Service cropland data layer for 2015 and 2016 at a 30-m × 30-m resolution (https://nassgeodata.gmu.edu/CropScape/). Using the “isecpolyrst” function in Geospatial Modeling Environment (v0.7.4.0), the proportion of all landscape feature classes were identified by counting pixels associated with each land category (SI Appendix, Table S2). Land-use types were categorized into 4 groups (cropland, developed, grassland, and woodland) (SI Appendix, Table S3). On average, corn or soybean was 95.4% of crop cover in the cropland category. We selected a subset of farms classified into 2 distinct categories, high cultivation and low cultivation, defined by the percentage of land in the 1.6-km radius dedicated to corn and soybean production. Farms surrounded by >73% (average 83.9% ± 0.023 SEM) corn and soybean were considered in a landscape of high cultivation, while those surrounded by <53% (average 38.2% ± 0.053 SEM) corn and soybean production were considered in a landscape of low cultivation. For comparison, 91% of the land in Story County is dedicated to farm use with 73.2% planted with crops (86); Iowa as a whole dedicates ∼92.6% to farm use with 72.9% planted with crops (42). Therefore, our low-cultivation sites were surrounded by substantially less crop production than the region as a whole. Additionally, the proportions of developed land, grassland, and woodland were all significantly higher in low-cultivation sites compared to high-cultivation sites (Fig. 3A). Each year we randomly chose fields that fit both categories, and then we randomly selected a subset of 5 fields per category, to serve as sites for our experimental apiaries. Each site was separated by at least 3.2 km to help ensure that honey bees foraged only within the landscape defined by that category, allowing use to assume each site was an independent experimental unit.
All soybean fields managed weeds with glyphosate and were planted with seed-applied pesticides; ISU-managed fields were planted with a fungicide only (Fluopyram, ILeVO, Bayer), while privately managed fields were planted with an insecticide and fungicide (Imidicloprid and Fluopyram, respectively; Acceleron seed treatment, Bayer). We transported apiaries to farms in June after 90% of the corn and soybean had been planted in Iowa, which would reduce honey bee exposure to dust contaminated with neonicotinoids originating from seed treatments. No insecticides were applied to foliate at any of the soybean farms or farms directly surrounding our apiaries. All fields were in a corn and soybean rotation with corn planted in the previous year.
Hive Source and Apiary Management.
In 2015, all colonies were started from packages sourced from C.F. Koehnen & Sons LLC, and purchased via a local honey bee broker. All packages contained 0.9 kg of adult bees and an Italian (A. mellifera ligustica) queen from the same source, and were started on bare plastic foundation in a 10-frame Langstroth hive, on April 24, 2015. All packages were installed at the same day at the Bee and Wasp Research Apiary, ISU Horticulture Station, Ames, IA. Each colony was kept on a 4-colony pallet, similar to those used in the migratory beekeeping industry. Each pallet represents an apiary that could be moved to a given soybean farm. After 4 wk, colonies were inspected for growth, and then equalized such that each apiary received the same quantity of hives, bees, drawn frame, and pupae, and that every colony had a healthy laying queen. In the first week of June, each pallet was randomly assigned and transported to a site within a single day. The day before transportation each colony was inspected to determine starting metrics (see below). All apiaries were placed 3 m from the edge of a soybean field. In 2016, this protocol was repeated with the exception that colonies were derived from the overwintered colonies from the 2015 experiment and colonies were started on fully drawn comb from the previous year rather than bare foundation. In 2016, apiaries were fully equalized to the same size as 2015 and each colony was provided with a new A. mellifera ligustica queen purchased from the same provider used in 2015.
Apiary Inspection Regime.
At each site, apiaries were inspected on a biweekly basis from June to October in 2016, and in 2015, biweekly during June to August and monthly during August to October. During each inspection, each colony within an apiary was weighed and additional hive bodies were added when those present reached ∼75% capacity. The mass of these additional hive boxes was weighed before inspection, allowing the calculation of weight added by bee-forage only. Immature bee population was estimated by capped pupae area (cm2) in each colony via photography in 2015 and with a Plexiglas grid screen in 2016 (87). In 2016, adult bee populations were estimated based on fractional estimates of sides of a frame covered in bees (i.e., “frame sides”) (87). At each inspection, queen presence was determined by observation of the queen or eggs in a colony; if the queen was determined to be absent, a new queen from the same source was provided within 1 wk. Monthly quantification of Varroa desctructor mites was performed via alcohol wash (88). At the beginning of all experiments, mite load (mites per 300 bees) for every colony was 0. Mite levels remained below this threshold throughout the season, but thymol (Apilife Var; Mann Lake, LTD) was applied beginning in the last week of August to prevent mite infestation from confounding the effects of our experiment (30). During each inspection, a 15-mL tube was filled with worker bees collected from frames of exposed larvae (i.e., putative nurse bees), placed on ice and transported back to the laboratory, and frozen at −80 °C until further processing. In addition to assessing each colony at an apiary, the adjacent soybean field was assessed for its growth and development using methods developed by Hodgson et al. (89) (SI Appendix, Fig. S1), to determine when and to what extent the crop was blooming.
Lipid Content Quantification.
To measure colony lipid levels of nurse bees, sampled bees from each date were processed via the protocol of Toth and Robinson (90) as modified in Dolezal et al. (30). Approximately 50 nurse bees, by mass, were homogenized in liquid nitrogen, and ∼0.25 g of homogenate was subsampled and weighed. Lipid content was quantified via phosphor-vanilin spectrophotometric assay and lipid calculated as milligram lipid/milligram bee mass.
Pollen Collection and Quantification.
To quantify pollen collected by honey bees in each cultivation category, a colony was randomly chosen within each apiary to receive a pollen trap (Brushy Mountain Bee Supply). This trap was attached to the front of the hive and requires foraging bees to pass through a plastic plate that releases pollen from the bees and is collected in a pan. Although pollen collection may vary by colony (32), pollen traps were only added to 1 colony per apiary to reduce overall stress to colony growth at an apiary. Each trap was open for 24 h each week during June to October.
A subsample of 2 g was extracted from each pollen sample collected on each day and sorted by pellet color. The sorted pellets were weighed, dissolved in Caberla’s solution with fuschin dye and mounted onto glass slides (91). Pollen was identified to the lowest possible taxonomic unit or morphospecies using light microscopy to observe morphological features. To validate pollen identification, pollen was also collected from all flowering plants found near each site during collection days and compared to mounted specimens.
In 2015, we found clover pollen to be the most abundant pollen collected by honey bees (60.4%) in the pollen traps. To assess when clover was blooming, we created 2 10-m2 plots around a patch of white clover (Trifolium repens) at the ISU Bee and Wasp Research Apiary. We sampled blooms per square meter once per week starting July 12 (week 29), when clover blooms were at maximum abundance, and continued through September 6 (week 37).
Prairie Access Rescue Experiment.
To evaluate if the decline in honey bee health metrics (Results) could be prevented or reversed, we kept a separate set of colonies (n = 10) at an independent agricultural site (ISU Bee and Wasp Research Apiary) in 2016 and monitored changes in weight beginning July 15. Unlike our first experiment, in which the site was the experimental unit, herein the colony was the experimental unit, with the treatment being the availability of late-summer forage. Colonies were sourced and maintained as described above with the exception that inspections occurred weekly and did not include brood or bee assessments. A sample of putative nurse bees was collected (see apiary inspection above) biweekly to assess individual lipid content. Three weeks after colonies reached their peak weight (July 28), half (n = 5) were randomly selected and moved to a reconstructed tallgrass prairie located in the Chichaqua Bottoms Greenbelt, Polk County, IA, ∼48 km from the ISU Bee and Wasp Research Apiary. This location was selected because it is 55.8 ha of contiguous prairie that contain species that bloom during August and September, with several species considered highly attractive to pollinators, including honey bees (60). This location was not insulated from crop production, as 36% of the land within 1.6 km from the colonies was comprised of corn and soybean. Colonies were inspected weekly until September 29, when all were moved back to the research apiary in preparation for overwintering. Although it is not quantifiably comparable to pollen trap data from the other experiments, we qualitatively assessed the presence and blooming status of flowering forbs present along a 60-m linear transect at this site on a weekly basis from July 26 to September 19, 2017. A blooming forb was considered any plant with at least 1 stem in anthesis within 10 m on either side of the transect.
Statistical Analysis.
Apiary growth and lipid content in cultivated landscapes.
All statistical analyses were performed using SAS 9.4 to investigate how apiaries responded to the 2 levels of cultivation, by performing repeated-measures analysis within a linear mixed-effect model using the “proc glimmix” function. To include site-years in 1 analysis, we binned all inspection days by calendar week across the 2 y because measurements taken during 2015 and 2016 did not always take place on the same calendar date and frequency. We avoided pseudoreplication in estimating the impact of cultivation category on honey bees by averaging individual colony metrics for each apiary, creating a single site-level metric (92) as site was the unit of replication.
We created models to estimate the response of weight, immature bee population, and adult bee population (2016 only) with cultivation category, week, year, and cultivation category/week interaction as predictor variables and site as a random variable. Due to crop rotation, we selected new sites each year within the 2 cultivation categories preventing us from exploring year by site interactions. To ensure that neither mite load nor queen loss during the season had an effect on colony growth metrics, we first ran mixed models including mite values and a binary measure of queen presence along with the predictor variables listed above. We did not observe an effect of mite load or queen presence on any growth metrics (SI Appendix, Table S4); therefore, we removed them from the models to avoid rank deficiency. The effect of year was removed from all analyses of adult bee population because bee population was measured in 2016 only. Colony weight, immature bee population, and adult bee population were analyzed within a linear mixed models to identify which dates differed using a simple effects comparisons of least-square means (weight in SI Appendix, Table S5; immature bee population in SI Appendix, Table S6; adult bee population in SI Appendix, Table S7). We performed additional repeated-measures analyses within a linear mixed-effect model investigating the response of apiary weight, immature bee population, and adult bee population (2016 only) using percent landscape categories (e.g., cropland, grassland, woodland, developed) as continuous variables. Due to collinearity, not all features could be examined in the same model. Cropland was most correlated with other features of the landscape; therefore, we ran a model with cropland, week, year, and cropland/week interaction as predictor variables and site as a random variable. We performed a separate model with grassland, woodland, developed land, week, year, and all interactions of landscape type and week as predictor variables and site as a random variable. Results from this analysis were analogous to cultivation category, with percent cultivation in the landscape being positively correlated with weight, immature bee, and adult bee populations (SI Appendix, Table S8).
To explore the effects of high and low cultivation on colony level lipid content of nurse honey bees, we created a similar model as above with cultivation category, week, year, and the cultivation category/week interaction as predictor variables and site as a random factor. From this model, least-squares means were used to make multiple comparisons with Tukey honest significant difference (HSD) adjustment to identify which weeks and cultivation categories exhibited significantly different colony nurse bee lipid levels (SI Appendix, Table S9). Lipids from every date were not evaluated in the model; rather, key dates were chosen to represent starting level, 2 midseason time points, and end of season lipid content. One colony from each apiary was randomly chosen to analyze lipid content. Lipids from the month of June (starting level) were sampled from week 24, July (early season) lipids were sampled from week 28, August (midseason) lipids were sampled from weeks 32 and 34, and October (end of season) lipids were sampled from week 43 for 2015 and 2016. Due to the fact that sampling periods across years did not always line up, samples from the month of August did not occur on the same weeks; rather, week 32 lipids are from 2015 and week 34 lipids are from 2016. All lipid content is represented as percent lipid (milligram of lipid per milligram of bee mass).
Honey bee colony growth and lipid content over time.
To identify when colony growth began to decline for both high- and low-cultivation categories, post hoc multiple comparisons of least-squares means from cultivation category and week were performed on the respective linear mixed-effects models as described above with a Tukey HSD adjustment (SI Appendix, Tables S5–S8). In addition to post hoc comparisons, we were also interested in the overall rate of weight growth and decline from the initiation of the experiment to peak growth and from peak growth to preoverwintering at the end of the season. To investigate the difference in these rates over the season in colonies in high- and low-cultivation landscapes, we adjusted our linear mixed-effects model from above to include month as the binning system as a replacement for week; that is, all weeks within a month were combined for 1 month average of colony weight. Using estimates from the respective model, we calculated rates of growth as the slopes of the linear trend from May to July and rates of decline as the slopes of the linear trends from August to October. We used absolute values of slopes to perform multiple contrasts to compare growth rates vs. the decline rates within and across cultivation categories (SI Appendix, Table S11). To explore changes in colony lipid content over time regardless of cultivation category, we made multiple comparisons of least-squares means of weeks from the mixed linear model above (SI Appendix, Table S10).
Forage resources used by honey bees (pollen availability and prairie access rescue experiment).
To confirm that apiaries placed at sites of either high- and low-cultivation landscapes have access to different amounts of landscape features, we performed t tests comparing each of the land use types (corn, soybean, grassland, woodland, developed). To test the effects of cultivation category on total pollen and type of pollen collected, a linear mixed-effect model was performed with cultivation category, pollen type (clover, partridge pea, trace pollens), year, and the cultivation category/pollen type interaction as fixed effects with site as a random factor.
To test the effects that prairie had on colony weight and on lipid content in the late season, linear mixed models were performed using the “proc glimmix” function as listed above with landscape (prairie vs. agricultural site) and week as predictor variables and with change in weight from maximum summer weight as the response variable.
Data Availability.
Data are contained in SI Appendix, Tables S1–S17. Any other details are available upon request.
Supplementary Material
Acknowledgments
We thank the many farmers who allowed us to conduct experiments in their fields, and to Iowa State University and Blomgren Seed Company for helping us connect with them; F. Kate Hunter, Edward Hsieh, David Stein, Zoe Pritchard, and members of the A.L.T. and M.E.O. laboratories for helping with field and laboratory assistance; Dr. Richard Joost for encouragement to initiate this project; Drs. Matthew Smart and Kathleen Ryan for reviewing an earlier version; and Kelly Dolezal for editing. This research was supported with funding from United Soybean Board Grant 1520-732-7225 (to A.G.D., A.L.T., and M.E.O.) and US Department of Agriculture (USDA)/National Institute of Food and Agriculture (NIFA) Grant 2016-07965 (to A.G.D., A.L.T., and M.E.O.); this article is a product of the Iowa Agriculture and Home Economics Experiment Station, Ames, IA Project No. 5351 and sponsored by Hatch Act and State of Iowa funds (to M.E.O.). Funding sources were not involved in the design, collection, interpretation, or writing of this paper.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1912801116/-/DCSupplemental.
References
- 1.Godfray H. C. J., et al. , Food security: The challenge of feeding 9 billion people. Science 327, 812–818 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Crist E., Mora C., Engelman R., The interaction of human population, food production, and biodiversity protection. Science 356, 260–264 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Meyer W. B., Turner B. L., Human-population growth and global land-use cover change. Annu. Rev. Ecol. Syst. 23, 39–61 (1992). [Google Scholar]
- 4.Otto C. R. V., Roth C. L., Carlson B. L., Smart M. D., Land-use change reduces habitat suitability for supporting managed honey bee colonies in the Northern Great Plains. Proc. Natl. Acad. Sci. U.S.A. 113, 10430–10435 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plourde J. D., Pijanowski B. C., Pekin B. K., Evidence for increased monoculture cropping in the central United States. Agric. Ecosyst. Environ. 165, 50–59 (2013). [Google Scholar]
- 6.Aizen M. A., Garibaldi L. A., Cunningham S. A., Klein A. M., Long-term global trends in crop yield and production reveal no current pollination shortage but increasing pollinator dependency. Curr. Biol. 18, 1572–1575 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Otto C. R. V., et al. , Past role and future outlook of the Conservation Reserve Program for supporting honey bees in the Great Plains. Proc. Natl. Acad. Sci. U.S.A. 115, E7651 (2018) Erratum in: Proc. Natl. Acad. Sci. U.S.A.115 7629–7634 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Najafi E., Devineni N., Khanbilvardi R. M., Kogan F., Understanding the changes in global crop yields through changes in climate and technology. Earths Future 6, 410–427 (2018). [Google Scholar]
- 9.Goulson D., Nicholls E., Botías C., Rotheray E. L., Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Potts S. G., et al. , Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Spivak M., Mader E., Vaughan M., Euliss N. H. Jr, The plight of the bees. Environ. Sci. Technol. 45, 34–38 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Kremen C., Williams N. M., Thorp R. W., Crop pollination from native bees at risk from agricultural intensification. Proc. Natl. Acad. Sci. U.S.A. 99, 16812–16816 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winfree R., Aguilar R., Vázquez D. P., LeBuhn G., Aizen M. A., A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 90, 2068–2076 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Kovács-Hostyánszki A., et al. , Ecological intensification to mitigate impacts of conventional intensive land use on pollinators and pollination. Ecol. Lett. 20, 673–689 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calderone N. W., Insect pollinated crops, insect pollinators and US agriculture: Trend analysis of aggregate data for the period 1992–2009. PLoS One 7, e37235 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulhanek K., et al. , A national survey of managed honey bee 2015–2016 annual colony losses in the USA. J. Apic. Res. 56, 328–340 (2017). [Google Scholar]
- 17.Kleijn D., et al. , Delivery of crop pollination services is an insufficient argument for wild pollinator conservation. Nat. Commun. 6, 7414 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seitz N., et al. , A national survey of managed honey bee 2014–2015 annual colony losses in the USA. J. Apic. Res. 54, 292–304 (2016). [Google Scholar]
- 19.Smart M. D., et al. , A comparison of honey bee-collected pollen from working agricultural lands using light microscopy and ITS metabarcoding. Environ. Entomol. 46, 38–49 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Sumner D. A., Borriss H., Bee-conomics and the leap in pollination fees. ARE Update 9, 9–11 (2006). [Google Scholar]
- 21.Dolezal A. G., Toth A. L., Feedbacks between nutrition and disease in honey bee health. Curr. Opin. Insect Sci. 26, 114–119 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Naug D., Nutritional stress due to habitat loss may explain recent honeybee colony collapses. Biol. Conserv. 142, 2369–2372 (2009). [Google Scholar]
- 23.Owen R., Role of human action in the spread of honey bee (Hymenoptera: Apidae) pathogens. J. Econ. Entomol. 110, 797–801 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Durant J. L., Otto C. R. V., Feeling the sting? Addressing land-use changes can mitigate bee declines. Land Use Policy 87, 104005 (2019). [Google Scholar]
- 25.Vanengelsdorp D., Hayes J., Underwood R. M., Pettis J., A survey of honey bee colony losses in the US, fall 2007 to spring 2008. PLoS One 3, e4071 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donkersley P., Rhodes G., Pickup R. W., Jones K. C., Wilson K., Honeybee nutrition is linked to landscape composition. Ecol. Evol. 4, 4195–4206 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odoux J. F., et al. , ECOBEE: A tool for long-term honey bee colony monitoring at the landscape scale in West European intensive agroecosystems. J. Apic. Res. 53, 57–66 (2014). [Google Scholar]
- 28.Sande S. O., Crewe R. M., Raina S. K., Nicolson S. W., Gordon I., Proximity to a forest leads to higher honey yield: Another reason to conserve. Biol. Conserv. 142, 2703–2709 (2009). [Google Scholar]
- 29.Alaux C., et al. , A ‘Landscape physiology’ approach for assessing bee health highlights the benefits of floral landscape enrichment and semi-natural habitats. Sci Rep 7, 40568 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dolezal A. G., Carrillo-Tripp J., Miller W. A., Bonning B. C., Toth A. L., Intensively cultivated landscape and Varroa mite infestation are associated with reduced honey bee nutritional state. PLoS One 11, e0153531 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smart M. D., Otto C. R. V., Carlson B. L., Roth C. L., The influence of spatiotemporally decoupled land use on honey bee colony health and pollination service delivery. Environ. Res. Lett. 13, 084016 (2018). [Google Scholar]
- 32.Smart M. D., Pettis J. S., Euliss N., Spivak M. S., Land use in the Northern Great Plains region of the US influences the survival and productivity of honey bee colonies. Agric. Ecosyst. Environ. 230, 139–149 (2016). [Google Scholar]
- 33.Smart M., Pettis J., Rice N., Browning Z., Spivak M., Linking measures of colony and individual honey bee health to survival among apiaries exposed to varying agricultural land use. PLoS One 11, e0152685 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rashford B. S., Walker J. A., Bastian C. T., Economics of grassland conversion to cropland in the prairie pothole region. Conserv. Biol. 25, 276–284 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Marcotty J., Bees at the brink: Fields of green are a desert for bees. Star Tribune, 27 September 2014. http://www.startribune.com/part-3-fields-of-green-are-a-desert-for-bees/274225251/. Accessed 1 July 2019.
- 36.Wheelock M. J., Rey K. P., O’Neal M. E., Defining the insect pollinator community found in Iowa corn and soybean fields: Implications for pollinator conservation. Environ. Entomol. 45, 1099–1106 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Sponsler D. B., Johnson R. M., Honey bee success predicted by landscape composition in Ohio, USA. PeerJ 3, e838 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alburaki M., et al. , Agricultural landscape and pesticide effects on honey bee (Hymenoptera: Apidae) biological traits. J. Econ. Entomol. 110, 835–847 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Requier F., Odoux J. F., Henry M., Bretagnolle V., The carry-over effects of pollen shortage decrease the survival of honeybee colonies in farmlands. J. Appl. Ecol. 54, 1161–1170 (2017). [Google Scholar]
- 40.Westphal C., Steffan-Dewenter I., Tscharntke T., Mass flowering crops enhance pollinator densities at a landscape scale. Ecol. Lett. 6, 961–965 (2003). [Google Scholar]
- 41.US Department of Agriculture, National Agriclutural Statistics , State agricultural review. https://www.nass.usda.gov/Quick_Stats/Ag_Overview/stateOverview.php?state=IOWA. Accessed 21 March 2019.
- 42.Iowa State University Extension and Outreach , “Crop and Land Use: Statewide Data.” https://www.extension.iastate.edu/soils/crop-and-land-use-statewide-data. Accessed 21 March 2019.
- 43.Gardiner M. M., et al. , Landscape diversity enhances biological control of an introduced crop pest in the north-central USA. Ecol. Appl. 19, 143–154 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Gardiner M. M., et al. , Landscape composition influences the activity density of Carabidae and Arachnida in soybean fields. Biol. Control 55, 11–19 (2010). [Google Scholar]
- 45.Gardiner M. M., et al. , Landscape composition influences patterns of native and exotic lady beetle abundance. Divers. Distrib. 15, 554–564 (2009). [Google Scholar]
- 46.Noma T., et al. , Relationship of soybean aphid (Hemiptera: Aphididae) to soybean plant nutrients, landscape structure, and natural enemies. Environ. Entomol. 39, 31–41 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Jauron R., Iowa State University Extension and Outreach , “Last and first frost dates in Iowa.” https://hortnews.extension.iastate.edu/2005/5-4-2005/iowafrostdates.html. Accessed 21 March 2019.
- 48.Fluri P., Bogdanov S., Effects of artificial shortening of the photoperiod on honeybee (Apis-Mellifera) polyethism. J. Apic. Res. 26, 83–89 (1987). [Google Scholar]
- 49.Brodschneider R., Crailsheim K., Nutrition and health in honey bees. Apidologie 41, 278–294 (2010). [Google Scholar]
- 50.Wright G. A., Nicolson S. W., Shafir S., Nutritional physiology and ecology of honey bees. Annu. Rev. Entomol. 63, 327–344 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Weaver N., Foraging behavior of honeybees on white clover. Insectes Soc. 12, 231–240 (1965). [Google Scholar]
- 52.McGregor S. E., Insect Pollination of Cultivated Crop Plants (Agricultural Research Service, US Department of Agriculture, Washington, DC, 1976). [Google Scholar]
- 53.Morse M. J., Cartter J. L., “Improvement in soybeans” in Yearbook of Agriculture for the Year 1937 (US Department of Agriculture, Washington, DC, 1937), pp. 1154–1189. [Google Scholar]
- 54.Alburaki M., et al. , Honey bee survival and pathogen prevalence: From the perspective of landscape and exposure to pesticides. Insects 9, E65 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erickson E. H., Garment M. B., Soybean flowers—Nectary ultrastructure, nectar guides, and orientation on the flower by foraging honeybees. J. Apic. Res. 18, 3–11 (1979). [Google Scholar]
- 56.Villanueva-Gutierrez R., Echazarreta-Gonzalez C., Roubik D. W., Moguel-Ordonez Y. B., Transgenic soybean pollen (Glycine max L.) in honey from the Yucatan peninsula, Mexico. Sci Rep 4, 4022 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Erickson E. H., Effect of honey bees on yield of 3 soybean cultivars. Crop Sci. 15, 84–86 (1975). [Google Scholar]
- 58.Erickson E. H., Variability of floral characteristics influences honey bee visitation to soybean blossoms. Crop Sci. 15, 767–771 (1975). [Google Scholar]
- 59.Bryant V. M., Jones G. D., The R-values of honey: Pollen coefficients. Palynology 25, 11–28 (2001). [Google Scholar]
- 60.Tuell J. K., Fiedler A. K., Landis D., Isaacs R., Visitation by wild and managed bees (Hymenoptera: Apoidea) to eastern U.S. native plants for use in conservation programs. Environ. Entomol. 37, 707–718 (2008). [DOI] [PubMed] [Google Scholar]
- 61.Schmidt J. O., Feeding preferences of Apis-mellifera L (Hymenoptera, Apidae)—Individual versus mixed pollen species. J. Kans. Entomol. Soc. 57, 323–327 (1984). [Google Scholar]
- 62.Schmidt J. O., Thoenes S. C., Levin M. D., Survival of honey-bees, Apis-mellifera (Hymenoptera, Apidae), fed various pollen sources. Ann. Entomol. Soc. Am. 80, 176–183 (1987). [Google Scholar]
- 63.Schmidt L. S., Schmidt J. O., Rao H., Wang W. Y., Xu L. G., Feeding preference and survival of young worker honey-bees (Hymenoptera, Apidae) fed rape, sesame, and sunflower pollen. J. Econ. Entomol. 88, 1591–1595 (1995). [Google Scholar]
- 64.Di Pasquale G., et al. , Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS One 8, e72016 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dolezal A. G., et al. , Interacting stressors matter: Diet quality and virus infection in honeybee health. R. Soc. Open Sci. 6, 181803 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rutter L., et al. , Transcriptomic responses to diet quality and viral infection in Apis mellifera. BMC Genomics 20, 412 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmehl D. R., Teal P. E. A., Frazier J. L., Grozinger C. M., Genomic analysis of the interaction between pesticide exposure and nutrition in honey bees (Apis mellifera). J. Insect Physiol. 71, 177–190 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Döke M. A., Frazier M., Grozinger C. M., Overwintering honey bees: Biology and management. Curr. Opin. Insect Sci. 10, 185–193 (2015). [DOI] [PubMed] [Google Scholar]
- 69.Caron D. M., Conner L. J., Honey Bee Biology and Beekeeping (Wicwas Press, 2013). [Google Scholar]
- 70.Fluri P., Luscher M., Wille H., Gerig L., Changes in weight of the pharyngeal gland and hemolymph titers of juvenile-hormone, protein and vitellogenin in worker honey bees. J. Insect Physiol. 28, 61–68 (1982). [Google Scholar]
- 71.Samson F., Knopf F., Prairie conservation in North-America. Bioscience 44, 418–421 (1994). [Google Scholar]
- 72.Smith D. D., Iowa prairie: Original extent and loss, preservation and recovery attempts. J. Iowa Acad. Sci. 105, 4 (1998). [Google Scholar]
- 73.Gill K. A., Cox R., O’Neal M. E., Quality over quantity: Buffer strips can be improved with select native plant species. Environ. Entomol. 43, 298–311 (2014). [DOI] [PubMed] [Google Scholar]
- 74.Schulte L. A., et al. , Prairie strips improve biodiversity and the delivery of multiple ecosystem services from corn-soybean croplands. Proc. Natl. Acad. Sci. U.S.A. 114, 11247–11252 (2017). Erratum in: Proc. Natl. Acad. Sci. U.S.A.114, E108251 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Otto C. R. V., et al. , Using publicly available data to quantify plant-pollinator interactions and evaluate conservation seeding mixes in the Northern Great Plains. Environ. Entomol. 46, 565–578 (2017). [DOI] [PubMed] [Google Scholar]
- 76.Ragsdale D. W., Landis D. A., Brodeur J., Heimpel G. E., Desneux N., Ecology and management of the soybean aphid in North America. Annu. Rev. Entomol. 56, 375–399 (2011). [DOI] [PubMed] [Google Scholar]
- 77.Yang Y., Suh S., Changes in environmental impacts of major crops in the US. Environ. Res. Lett. 10, 094016 (2015). [Google Scholar]
- 78.Thessen G., “Iowa Ag News–Acreage” (US Department of Agriculture-National Agricultureal Statistical Service, 2017). https://www.nass.usda.gov/Statistics_by_State/Iowa/Publications/Crop_Report/2017/IA_Acreage06_17.pdf. Accessed 21 March 2019.
- 79.US Department of Agriculture, Economic Research Service , “Cash recipets by commodity, state ranking, 2017” (2017). https://data.ers.usda.gov/reports.aspx?ID=17844. Accessed 22 March 2019.
- 80.Shackelford N., et al. , Primed for change: Developing ecological restoration for the 21st century. Restor. Ecol. 21, 297–304 (2013). [Google Scholar]
- 81.Kunert K., Crailsheim K., Seasonal-changes in carbohydrate, lipid and protein-content in emerging worker honeybees and their mortality. J. Apic. Res. 27, 13–21 (1988). [Google Scholar]
- 82.Münch D., Amdam G. V., The curious case of aging plasticity in honey bees. FEBS Lett. 584, 2496–2503 (2010). [DOI] [PubMed] [Google Scholar]
- 83.Keller I., Fluri P., Imdorf A., Pollen nutrition and colony development in honey bees—Part II. Bee World 86, 27–34 (2005). [Google Scholar]
- 84.Keller I., Fluri P., Imdorf A., Pollen nutrition and colony development in honey bees: Part I. Bee World 86, 3–10 (2005). [Google Scholar]
- 85.Tosi S., Nieh J. C., Sgolastra F., Cabbri R., Medrzycki P., Neonicotinoid pesticides and nutritional stress synergistically reduce survival in honey bees. Proc. Biol. Sci. 284, 20171711 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.US Department of Agriculture, National Agriclutural Statistics , Census of Agriculture County Profile: Story County, Iowa. https://www.nass.usda.gov/Publications/AgCensus/2012/Online_Resources/County_Profiles/Iowa/cp19169.pdf. Accessed 21 March 2019.
- 87.Delaplane K. S., van der Steen J., Guzman-Novoa E., Standard methods for estimating strength parameters of Apis mellifera colonies. J. Apic. Res. 52, (2013). [Google Scholar]
- 88.Dietemann V., et al. , Standard methods for varroa research. J. Apic. Res. 52, 1–54 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hodgson E. W., McCornack B. P., Tilmon K., Knodel J. J. et al. , Management recommendations for soybean aphid (Hemiptera: Aphididae) in the United States. J. Integr. Pest Manag. 3, E1–E10 (2012). [Google Scholar]
- 90.Toth A. L., Robinson G. E., Worker nutrition and division of labour in honeybees. Anim. Behav. 69, 427–435 (2005). [Google Scholar]
- 91.Weber R. W., Pollen identification. Ann. Allergy Asthma Immunol. 80, 141–145, quiz 146–147 (1998). [DOI] [PubMed] [Google Scholar]
- 92.Pirk C. W. W., et al. , Statistical guidelines for Apis mellifera research. J. Apic. Res. 52, 1–24 (2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained in SI Appendix, Tables S1–S17. Any other details are available upon request.