While specific sleep disorders are known to precede Parkinson’s disease, it remains unclear how sleep disturbances in the general population affect risk. Lysen et al. report that poor sleep quality and short sleep duration, and their deterioration over time, are associated with increased risk of parkinsonism, including Parkinson’s disease.
Keywords: PSQI, epidemiology, cohort, sleep
Abstract
Sleep disturbances may signal presence of prodromal parkinsonism, including Parkinson’s disease. Whether general sleep quality or duration in otherwise healthy subjects is related to the risk of parkinsonism remains unclear. We hypothesized that both worse self-reported sleep quality and duration, as well as a longitudinal deterioration in these measures, are associated with the risk of parkinsonism, including Parkinson’s disease. In the prospective population-based Rotterdam Study, we assessed sleep quality and duration with the Pittsburgh Sleep Quality Index in 7726 subjects (mean age 65 years, 57% female) between 2002 and 2008, and again in 5450 subjects between 2009 and 2014. Participants were followed until 2015 for a diagnosis of parkinsonism and Parkinson’s disease. Outcomes were assessed using multiple modalities: interviews, physical examination, and continuous monitoring of pharmacy records and medical records of general practitioners. We used Cox regression to associate sleep, and changes in sleep over time, with incident parkinsonism and Parkinson’s disease, adjusting for age, sex, education and smoking status. Over 64 855 person-years in 13 years of follow-up (mean: 8.4 years), 75 participants developed parkinsonism, of whom 47 developed Parkinson’s disease. We showed that within the first 2 years of follow-up, worse sleep quality {hazard ratio (HR) 2.38 per standard deviation increase [95% confidence interval (CI 0.91–6.23)]} and shorter sleep duration [HR 0.61 per standard deviation increase (95% CI 0.31–1.21)] related to a higher risk of parkinsonism. Associations of worse sleep quality [HR 3.86 (95% CI 1.19–12.47)] and shorter sleep duration [HR 0.48 (95% CI 0.23–0.99)] with Parkinson’s disease were more pronounced, and statistically significant, compared to parkinsonism. This increased risk disappeared with longer follow-up duration. Worsening of sleep quality [HR 1.76 per standard deviation increase (95% CI 1.12–2.78)], as well as shortening of sleep duration [HR 1.72 per standard deviation decrease (95% CI 1.08–2.72)], were related to Parkinson’s disease risk in the subsequent 6 years. Therefore, we argue that in the general population, deterioration of sleep quality and duration are markers of the prodromal phase of parkinsonism, including Parkinson’s disease.
Introduction
Parkinson’s disease is primarily characterized by motor disturbances (Berg et al., 2015), but also includes non-motor features. Sleep-wake disturbances are a common non-motor feature of Parkinson’s disease (Zoccolella et al., 2011; Breen et al., 2014; Chen et al., 2015a; Zis et al., 2015; Zhu et al., 2016; Chahine et al., 2017; Zhang et al., 2017) and related synucleinopathies (Plazzi et al., 1997; Boeve et al., 2004; Plazzi, 2004). Sleep-wake disturbances are also reported to precede a diagnosis of parkinsonism in prodromal Parkinson’s disease (Al-Qassabi et al., 2017). Objectively measured increases in sleep fragmentation have also been related to increased Parkinson’s disease pathology at brain autopsy in older subjects without Parkinson’s disease (Sohail et al., 2017). Sleep-wake disturbances may be a risk factor for Parkinson’s disease, or indicate presence of disease in a prodromal phase (Hawkes, 2008; Hawkes et al., 2010).
Several sleep disorders have been reported to precede Parkinson’s disease or related synucleinopathies (Al-Qassabi et al., 2017), including rapid eye movement (REM) sleep behaviour disorder (RBD) (Iranzo et al., 2014; Berg et al., 2015; Postuma et al., 2015) and obstructive sleep apnoea (Chen et al., 2015b; Sheu et al., 2015; Yeh et al., 2016; Chou et al., 2017). These seem to represent, however, only the ‘tip of the iceberg’ of various sleep-wake disturbances in prodromal Parkinson’s disease (Abbott et al., 2005; Gaenslen et al., 2011; Gao et al., 2011; Wong et al., 2014; Yang et al., 2014; Schrag et al., 2015). Subclinical impairments in sleep, such as poor sleep quality and short sleep duration, are more common in the general population and may well capture aforementioned sleep-wake disturbances. These impairments are particularly important as they are often investigated and easily determinable aspects of sleep in any healthcare setting. To date, however, only few studies have investigated if sleep duration reflects prodromal Parkinson’s disease (Chen et al., 2006; Gao et al., 2011), and none studied sleep quality. Furthermore, it is unknown if long-term changes in sleep duration and quality relate to subsequent risk of parkinsonism, including Parkinson’s disease.
We studied the association of subjectively assessed sleep quality and duration with parkinsonism, including Parkinson’s disease. We hypothesized that (i) worse sleep quality, and shorter sleep duration, are associated with the risk of parkinsonism, including Parkinson’s disease; and (ii) deterioration in sleep quality and duration over time is associated with the subsequent risk of parkinsonism. We tested these hypotheses in a prospective, population-based study, using the Pittsburgh Sleep Quality Index (PSQI) to (repeatedly) measure sleep quality and duration, with up to 13 years of follow-up for incident parkinsonism.
Materials and methods
Study setting
The study was embedded in the Rotterdam Study, a large, prospective, population-based study in a suburban district in the city of Rotterdam, the Netherlands, details of which are described elsewhere (Ikram et al., 2017). The study was set up to investigate the frequency, risk factors and natural history of common chronic diseases in the elderly, including neurodegenerative diseases such as Parkinson’s disease. The first cohort was initiated in 1990 and included 7983 subjects aged ≥55 years, and was expanded with 3011 subjects aged ≥55 years in 2000, and 3932 subjects aged ≥45 years in 2006. Examination rounds consisted of a home interview and visits to our dedicated research centre, including a wide range of questionnaires and physical measurements. Visits are repeated every 4–5 years. Measurements are kept similar across inclusion rounds and time. In between examination rounds, incident disease is assessed with continuous linkage of the study database and medical records of general practitioners, which also hold summaries from all specialist and inpatient care.
The Rotterdam Study has been approved by the Medical Ethics Committee of the Erasmus MC (registration number MEC 02.1015) and by the Dutch Ministry of Health, Welfare and Sport (Population Screening Act WBO, license number 1071272–159521-PG). The Rotterdam Study Personal Registration Data collection is filed with the Erasmus MC Data Protection Officer under registration number EMC1712001. This study is registered with the Netherlands National Trial Register and WHO International Clinical Trials Registry Platform under the shared catalogue number NTR6831. All participants provided written informed consent to participate in the study and to have their information obtained from treating physicians.
Study population
We included participants from all three inclusion rounds when a sleep questionnaire, the PSQI, was first introduced. At this baseline visit (between 2002 and 2008), we included 7726 subjects who had valid data on sleep quality or sleep duration, did not have prevalent parkinsonism or Parkinson’s disease (de Rijk et al., 1995), and were not cognitively impaired based on a Mini-Mental State Examination (MMSE) score > 25. We followed the remaining participants until the first of: onset of parkinsonism or Parkinson’s disease, 1 January 2015, or death. Study follow-up for incident parkinsonism was nearly complete [64 855 person-years (98.1%)] (Clark et al., 2002).
For analyses of changes in sleep over time, we similarly included 5450 subjects at the follow-up visit (between 2009 and 2014) and started follow-up time for parkinsonism and Parkinson’s disease after this visit. See Supplementary Fig. 1 for a detailed flow chart of included participants for analyses at baseline and the follow-up visit.
Assessment of sleep
Subjective aspects of sleep were measured with a Dutch version of the PSQI, which assesses past month’s average sleep quality. The PSQI (Buysse et al., 1989) has a good test-retest reliability and validity in a non-clinical sample of older adults (Mollayeva et al., 2016). Answers can be categorized, scored and combined into seven component scores ranging from 0 (not problematic) to 3 (very problematic), labelled ‘quality’, ‘latency’, ‘duration’, ‘efficiency’, ‘disturbances’, ‘sleep medication’, and ‘daytime dysfunction’. These scores are summed to provide the global PSQI score (range: 0–21) of subjectively assessed sleep quality (hereafter: ‘sleep quality’). Higher scores indicate poorer sleep, and scores > 5 indicate a ‘poor’ sleep quality.
For participants with more than one PSQI component missing, the global PSQI score was not calculated (n = 156, 2%). We calculated weighted component scores for participants who missed one component score (n = 1,099, 13%) by multiplying the six-component sum scores by 7/6. Most of these participants missed information on sleep disturbances (n = 847) due to introducing a skip rule in PSQI items on disturbances [5a-5j (Buysse et al., 1989)] in a subset of participants, to limit participant burden. If answers to items 5a–5b were both negative (‘not in the last month’), items 5c–5j were skipped. Weighting scores minimized any effect of the skip rule on global PSQI scores, as in subjects who answered items 5a–5b negatively, weighted scores were not different between those who followed the skip rule versus those who did not (data not shown). Analogously, at follow-up we did not calculate the global PSQI score for 484 (8%) due to missing more than one PSQI component at the follow-up visit [and excluded participants who missed global PSQI score at the baseline visit so that changes could not be calculated (n = 203)]. We weighted scores for 252 participants (5%) who mostly missed data on efficiency (n = 190; see flow chart in Supplementary Fig. 1).
Assessment of parkinsonism and Parkinson’s disease
A detailed description of parkinsonism and Parkinson’s disease assessment methods used in this study has been published previously (Darweesh et al., 2016). In short, we used four overlapping modalities to collect information on parkinsonism and Parkinson’s disease: in-person interviews, examinations, use of antiparkinsonian medication, and continuous monitoring of medical records. Examinations included standardized screening assessments of parkinsonian signs (i.e. tremors, hypo- and bradykinesia, cogwheel rigidity, and postural reflex) by a trained research nurse during centre visits. If one or more signs were present, subjects were subsequently invited for a structured physical examination by a trained research physician.
Parkinsonism was defined by presence of hypo- or bradykinesia in combination with ≥1 cardinal sign (resting tremor, rigidity or postural imbalance) observed by any physician, or a clinical diagnosis of parkinsonism by a neurologist or geriatrician (if motor examination details were unavailable). Within those subjects, we diagnosed Parkinson’s disease in presence of a clinical Parkinson’s disease diagnosis by a neurologist or geriatrician, or a documented positive response to dopaminergic treatment in subjects who did not have evidence for a secondary cause (e.g. pre-existent dementia diagnosis, use of anti-dopaminergic drugs, cerebrovascular disease). We classified subjects with ‘unspecified parkinsonism’ if they had multiple possible causes or lacked a clear underlying cause of parkinsonism.
Potential confounders and effect-modifiers
Analyses were adjusted for potential confounders measured at baseline, selected based on relevant publications (Berg et al., 2015; Schrag et al., 2015; Ascherio and Schwarzschild, 2016): age, sex, education and smoking history. Educational attainment was assessed by interview and categorized as primary, secondary/lower vocational, intermediate vocational, and higher vocational or university. Smoking habits were assessed by interview and categorized as never, former or current smoking. We also examined potential effect-modification by depressive symptoms and anxiety disorders. Depressive symptoms were assessed with the validated Dutch version (Beekman et al., 1997) of the Centre for Epidemiological Studies Depression Scale (Radloff, 1977). Presence of an anxiety disorder was assessed by an adapted version of the Munich Composite International Diagnostic Interview (Wittchen et al., 1998).
Statistical analysis
A detailed explanation of our statistical methods is provided in the Supplementary material. In short, we first used Cox proportional hazards regression models to associate both sleep quality and duration at baseline with both incident parkinsonism and Parkinson’s disease. As we found that the Cox model assumption of proportionality was violated in some analyses, we also examined how aforementioned associations changed over follow-up time by performing analyses in incremental epochs of follow-up time from baseline (extending follow-up time e.g. baseline to 2 years, baseline to 4 years, etc.) (Hernan, 2010), or using a stratified Cox model to obtain period-specific hazards (e.g. baseline to 2 years, 2 to 4 years, etc.). We furthermore looked at the effect of other PSQI components separately. As sensitivity analyses, we restricted analyses to subjects without comorbid depression and anxiety. We also investigated potential effect-modification by age, sex, and presence versus absence of any of four parkinsonian signs. Second, we related changes in sleep quality and duration between the baseline and the follow-up visit with incident parkinsonism and Parkinson’s disease after the follow-up visit.
Variables were standardized and, when right-skewed, log-transformed before standardization. Missing values on covariates were imputed using five multiple imputations.
Data availability
Data can be obtained on request. Requests should be directed toward the management team of the Rotterdam Study (secretariat.epi@erasmusmc.nl), which has a protocol for approving data requests. Because of restrictions based on privacy regulations and informed consent of the participants, data cannot be made freely available in a public repository.
Results
Characteristics of the study sample at baseline are summarized in Table 1. Median global PSQI score was 3, and 2115 participants (27%) scored over 5 indicating poor sleep quality. Global PSQI score and sleep duration were moderately correlated (Spearman’s r = −0.69; P < 0.01). During 13.0 years of follow-up (mean 8.4 years), we observed 75 incident parkinsonism cases, of which 47 (63%) with Parkinson’s disease (Supplementary Table 1).
Table 1.
Characteristics of study population at baseline
| Characteristic, unit | Total sample n = 7726 | Incident PS n = 75 | No incident PS n = 7651 |
|---|---|---|---|
| Age at baseline, years | 65.4 ± 10.3 | 71.6 ± 8.4 | 65.4 ± 10.3 |
| Female | 4396 (57%) | 33 (44%) | 4365 (57%) |
| Educational level | |||
| Primary education | 708 (9%) | 8 (11%) | 700 (9%) |
| Lower/intermediate or lower vocational | 3088 (40%) | 29 (39%) | 3060 (40%) |
| Higher or intermediate vocational | 2371 (31%) | 24 (32%) | 2347 (31%) |
| Higher vocational or university | 1559 (20%) | 14 (19%) | 1545 (20%) |
| Smoking status | |||
| Never | 3416 (44%) | 34 (45%) | 3383 (44%) |
| Former | 3549 (46%) | 33 (44%) | 3516 (46%) |
| Current | 761 (10%) | 8 (11%) | 753 (10%) |
| Cognitive functioning, MMSE score | 28 (27–29) | 28 (27–29) | 28 (27–29) |
| Depressive symptoms, CES-D score | 3 (1–8) | 4 (1–8) | 3 (1–8) |
| Presence of any anxiety disorder | 588 (8%) | 8 (11%) | 580 (8%) |
| Presence of any parkinsonian signs | 807 (10%) | 16 (21%) | 792 (10%) |
| Sleep quality (global PSQI score) | 3 (2–6) | 3 (1–6) | 3 (2–6) |
| Missing | 46 (1%) | 0 (0%) | 46 (1%) |
| Sleep duration, h | 6.8 ± 1.2 | 7.1 ± 1.3 | 6.8 ± 1.2 |
Characteristics of study population at baseline. Values are expressed as frequency (%) for categorical variables and mean ± SD or median (interquartile range) for continuous variables, unless specified otherwise. Includes imputed values for covariates.
CES-D = Center for Epidemiological Studies – Depression Scale; MMSE = Mini-Mental State Examination; PS = parkinsonism.
Analyses using overall follow-up
Sleep quality was not associated with the risk of parkinsonism [hazard ratio (HR) per standard deviation (SD) increase in global PSQI score 0.95, 95% confidence interval (CI) 0.76–1.20] or Parkinson’s disease (HR per SD increase 0.87, 95% CI 0.65–1.16). We observed similar estimates when analysing categorized poor (versus good) sleep quality: HR 0.97, 95% CI 0.57–1.66 for parkinsonism, and HR 0.79, 95% CI 0.39–1.59 for Parkinson’s disease (Supplementary Table 2).
Longer sleep duration was not associated with the risk of parkinsonism (HR per SD increase 1.21, 95% CI 0.95–1.54) and Parkinson’s disease (HR 1.24, 95% CI 0.92–1.69). After categorizing sleep duration, we did not observe a significant increase in risk with increasing categories of sleep duration (Supplementary Table 2).
In the aforementioned analyses for Parkinson’s disease risk, but not for parkinsonism, the proportionality assumption for both sleep quality and duration was significantly violated.
Studying different epochs of follow-up time
We found that worse sleep quality related to an increased risk of parkinsonism (HR 2.38, 95% CI 0.91–6.23) in the first 2 years of follow-up, which attenuated when increasing follow-up time from baseline (Fig. 1A). In these 2 years, associations were more pronounced, and statistically significant, for Parkinson’s disease (HR 3.86, 95% CI 1.19–12.47) compared to parkinsonism. Results for sleep duration were analogous (Fig. 1B): short sleep duration was associated with an increased risk of parkinsonism (HR 0.61, 95% CI 0.31–1.21) and Parkinson’s disease (HR 0.48, 95% CI 0.23–0.99). Additionally, analysis of period-specific hazard ratios using a stratified Cox model suggested that associations of worse sleep quality, and shorter sleep duration, with an increased risk of parkinsonism and Parkinson’s disease were confined to the first 2 years of follow-up (Supplementary Fig. 2).
Figure 1.
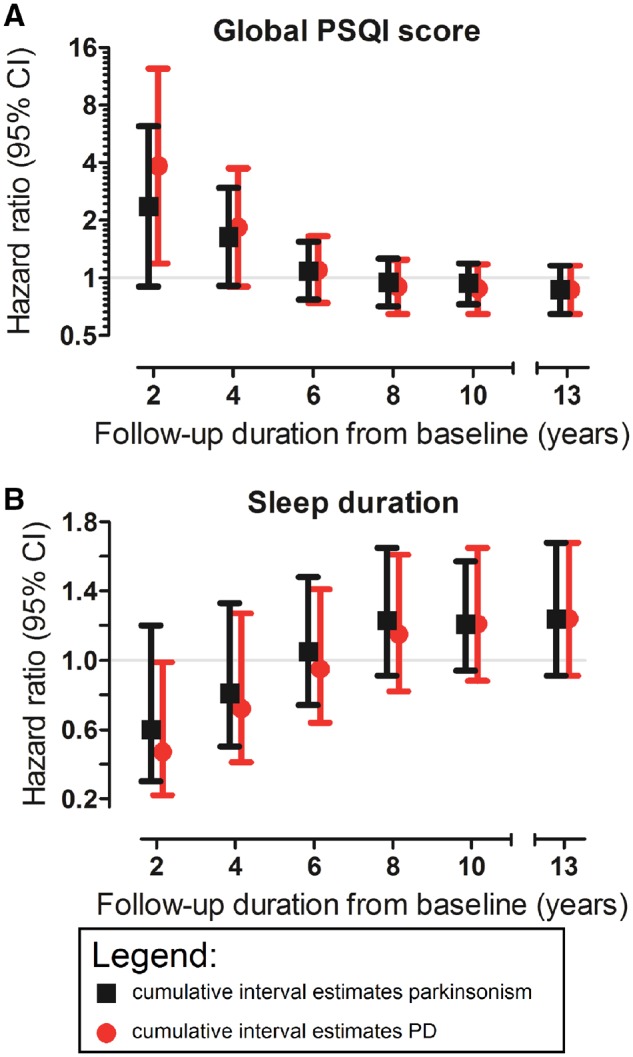
Associations of sleep quality and duration with risk of parkinsonism and Parkinson’s disease, per cumulatively increasing duration of follow-up. The associations of (A) sleep quality and (B) sleep duration with incident parkinsonism and Parkinson’s disease are shown for cumulatively increasing follow-up duration within the study timeframe. HR estimates were obtained from multivariate Firth’s penalized Cox regression models by censoring all participants still at risk at 2, 4, 6, 8 and 10 years after baseline, and after the total follow-up of 13 years. HR estimates were adjusted for age at baseline, sex, educational level and smoking status, are expressed per standard deviation increase of (A) worse sleep quality, or (B) longer sleep duration, and are plotted at a (A) logarithmic (base 2) scale and (B) a linear scale. PD = Parkinson’s disease.
Other PSQI components
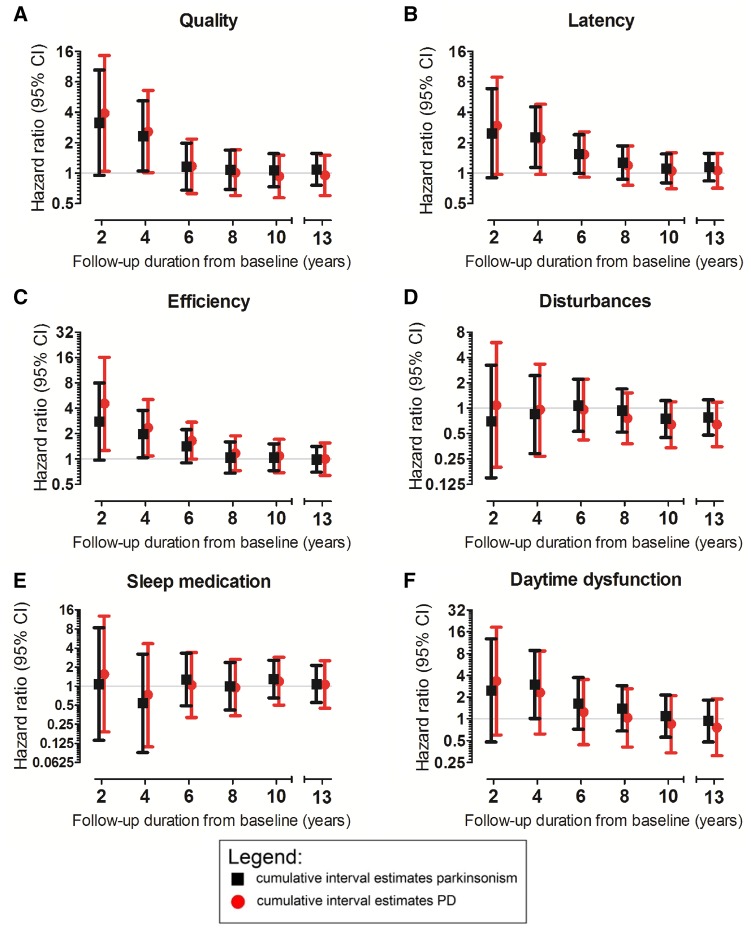
Most PSQI components showed a similar pattern of associations with cumulative increasing follow-up duration, except for sleep medication (Fig. 2A–F). We observed noteworthy changes in effect sizes from short to long follow-up for sleep efficiency, and to a lesser extent for sleep quality, latency and daytime dysfunction (Fig. 2A–C, F and Supplementary Table 3). Also, for daytime dysfunction, the direction of hazard ratio estimates changed over increasing epochs of follow-up (Supplementary Table 3).
Figure 2.
Associations of PSQI component scores with risk of parkinsonism and Parkinson’s disease, per cumulatively increasing duration of follow-up. The associations of the PSQI components (A) quality, (B) latency, (C) efficiency, (D) disturbances, (E) sleep medication, and (F) daytime dysfunction with incident parkinsonism and Parkinson’s disease are shown for cumulatively increasing follow-up duration within the study timeframe. HR estimates were obtained from multivariate Firth’s penalized Cox regression models by censoring all participants still at risk at 2, 4, 6, 8 and 10 years after baseline, and after the total follow-up of 13 years. Estimates are adjusted for age at baseline, sex, educational level, and smoking status, are expressed per category increase in component score, and are plotted at different logarithmic (base 2) scales per component. For parkinsonism analyses, we included following numbers of participants: (A) 7716, (B) 7718, (C) 7473, (D) 6840, (E) 7725, (F) 7689 (samples were five to seven participants smaller for analyses on Parkinson’s disease). To ensure sufficient (>10%) observations in each category, we combined scores 2 and 3 for components quality, latency and efficiency, and scores 1, 2 and 3 for components disturbances, medication and daytime dysfunction. PD = Parkinson’s disease.
Sensitivity analyses
We restricted the sample to subjects without clinically relevant depressive symptoms and without any anxiety disorder, leaving 6605 subjects, 61 of whom were cases of parkinsonism, including 39 cases with Parkinson’s disease. Associations over cumulatively increasing follow-up duration were similar to the total sample (Supplementary Fig. 3). For the association of sleep duration with Parkinson’s disease, all hazard ratios shifted to higher values. As a result, longer sleep duration was now associated with increased Parkinson’s disease risk in the overall follow-up (HR 1.47, 95% CI 1.02–2.11), for which proportionality was not violated.
Stratified analyses
Analyses stratified at median age did not reach statistical significance. We observed hazard ratio estimates suggesting associations of worse sleep quality with a lower risk of parkinsonism and Parkinson’s disease in younger subjects, while hazard ratios in older subjects were close to the null. Similarly, estimates also suggested associations of longer sleep duration with a higher risk of both outcomes in younger subjects. Case numbers in separate strata were small. Also, there were no significant interactions between age and sleep quality or duration on the risk of either outcome (Supplementary Table 4).
We observed a similar relation between sleep quality and duration and disease risk in subjects without parkinsonian signs at baseline. Statistically testing these interactions on a multiplicative scale showed significant interactions of sleep quality with presence of parkinsonian signs on the risk of both parkinsonism and Parkinson’s disease (Supplementary Table 4).
Change in sleep quality or duration
Characteristics of the study population at the follow-up visit are provided in Supplementary Table 5. Changes in sleep between the baseline and follow-up visit were measured over 10.9 years (on average 6.0 years) in all participants. In the subsequent 6.0 years (average follow-up: 2.9) after the follow-up visit, we observed 25 incident parkinsonism cases, of which 17 were with Parkinson’s disease.
Worsening of sleep quality was related to a subsequent increase in Parkinson’s disease risk (HR per SD increase 1.76, 95% CI 1.12–2.78), as was a shortening of sleep duration (HR per SD increase 1.72, 95% CI 1.08–2.72; Table 2). Results were independent of the absolute average level of sleep quality or duration (Table 2). Also, additional adjustment for depressive symptoms at baseline did not attenuate results. Associations of sleep quality (HR 1.23, 95% CI 0.83–1.83) and sleep duration (HR 1.45, 95% CI 0.99–2.13) with incident parkinsonism were less pronounced. When examining hazard ratios over increasing epochs of follow-up time measured from the follow-up visit, we found that worsening of sleep quality, and shortening of sleep duration, were associated with parkinsonism on the short term, but not the longer term (Supplementary Fig. 4). For both sleep parameters, risk of Parkinson’s disease was also slightly higher on the short than on the long term.
Table 2.
Association of changes in sleep quality and duration between the baseline and follow-up visit, and risk of parkinsonism and Parkinson’s disease
| Determinant (unit) | Parkinsonism | Parkinson’s disease | ||
|---|---|---|---|---|
| Cases/n | HR (95% CI) | Cases/n | HR (95% CI) | |
| Change in sleep quality (worse sleep) | 25/5206 | 1.23 (0.83–1.83) | 17/5244 | 1.76 (1.12–2.78) |
| Change in sleep duration (shorter sleep) | 25/5244 | 1.45 (0.99–2.13) | 17/5238 | 1.72 (1.08–2.72) |
Changes in sleep quality were modelled per standard deviation increase (‘worsening’) of global PSQI score, and changes for sleep duration were modeled as standard deviation decrease (‘shortening’) of sleep duration from the baseline visit to the follow-up visit. HR estimates are adjusted for age at baseline, sex, educational level, smoking status and time interval between measurements. Additional adjustment for depressive symptoms at baseline minimally changed point and interval estimates (data not shown). After additional adjustment for the average level of sleep quality or sleep duration of the two measurements, point and interval estimates for the relation with parkinsonism barely changed. Estimates for associations of change in sleep quality (HR 1.87, 95% CI 1.12–3.10) and change in sleep duration (HR 1.85, 95% CI 1.14–2.98) with risk of Parkinson’s disease increased. Estimates in bold indicate statistically significant results at P < 0.05.
Discussion
In the general population, baseline sleep quality and duration within the next 2 years relate to incident parkinsonism, and specifically to Parkinson’s disease. Similarly, deterioration over 6 years in these parameters is associated with incident parkinsonism and Parkinson’s disease.
Several methodological considerations should be mentioned. First, our study focused on subjectively measured sleep, which does not necessarily reflect similar constructs as objective measurements. While the first incorporates the experience of sleep, objective measurements indicate physiological sleep. Therefore, subjective measures cannot provide similar insight in underlying biological processes as objective measures (e.g. polysomnography). Second, we did not include subjects with cognitive impairment to minimize information bias of sleep quality (Choi and Pak, 2005; Krystal and Edinger, 2008) and duration (Choi and Pak, 2005; Lavie, 2009), but these subjects are at increased risk of having prodromal parkinsonism (Darweesh et al., 2017b) which could bias our associations. In addition, subjects with cognitive impairment are also predisposed to develop RBD (Swallow et al., 2016), which has been suggested to be associated to a longer sleep duration in the general population (Haba-Rubio et al., 2018). This could lead to an underestimation of associations of sleep duration with parkinsonism and Parkinson’s disease. Third, although the PSQI is used in patients with Parkinson’s disease (Hogl et al., 2010), it may miss Parkinson’s disease-specific sleep disturbances (Chaudhuri et al., 2002; Marinus et al., 2003). Patients with prodromal disease may thus under-report sleep problems, or overstate their sleep quality. If so, we have even underestimated especially short-term effect estimates of worse sleep quality with increased risk of parkinsonism and Parkinson’s disease risk. Fourth, despite strong indications otherwise, we cannot be definitely sure that missing values on the disturbances component did not inadvertently affect the global PSQI score. Fifth, the number of parkinsonism and Parkinson’s disease cases in our study is small, which may have unpowered us to detect small effects. Sixth, subjective assessment of sleep may be more prone to measurement error than objective methods. This lack of precision may have precluded us from detecting small effect sizes. Seventh, we cannot exclude any residual confounding of medication use beyond those questioned in the PSQI in our estimates.
We found associations of poor sleep quality and short sleep duration with increased risk of parkinsonism, and especially Parkinson’s disease, in the first 2 years of follow-up, attenuating with incremental follow-up. Our study adds to the previous findings by showing that associations evidently change with incremental follow-up time. This is in line with findings of large registry-based studies in general practice that show increases in insomnia diagnoses 2 years (Plouvier et al., 2014; Schrag et al., 2015), but not 5 and 10 years before diagnosis of Parkinson’s disease (Schrag et al., 2015). Such results suggest that sleep disturbances occur as prodromal features rather than as causes of Parkinson’s disease and related synucleinopathies, as sleep is measured closer to the diagnosis of an incident case when follow-up is short. Our measurements of sleep likely represent common, subclinical sleep problems as well as those severe enough to diagnose a sleep disorder, and therefore fit well with the variety of sleep disturbances preceding Parkinson’s disease (Al-Qassabi et al., 2017).
Mechanisms behind sleep disturbances marking prodromal Parkinson’s disease remain speculative. Sleep may be disturbed by early-stage dysfunction of serotonergic neurons in the dorsal raphe nuclei and sleep-promoting areas in the basal forebrain (Braak et al., 2003). Such dysfunction may also negatively impact switching between sleep and wake (Saper et al., 2010). Additionally, early spread of pathology to the coeruleus/subcoeruleus complex may disturb REM sleep independent of RBD (Hawkes et al., 2010). Sleep may also be impaired via circadian dysfunction occurring around the time of diagnosis (Breen et al., 2014), via hypothalamic neuron loss (Thannickal et al., 2007; Breen et al., 2016), or via the loss of dopaminergic modulation (Golombek and Rosenstein, 2010).
Of note, results do not exclude that sleep disturbances may cause Parkinson’s disease. An effect of sleep disturbance on neurodegenerative disease is plausible, as sleep deprivation has been shown to increase levels of amyloid-β, a pathological hallmark of Alzheimer’s disease. Mechanisms include decreased clearance (Xie et al., 2013), or activity-dependent increased production, of amyloid-β. The sleep wake cycle has also been shown to regulate tau levels, and sleep deprivation can increase extracellular levels of tau and, interestingly, alpha-synuclein (Holth et al., 2019). A recent study importantly showed that increased actigraphy-derived sleep fragmentation in old subjects without Parkinson’s disease was associated with an increased burden of Parkinson’s disease pathology at brain autopsy (Sohail et al., 2017). This indicates that objective disturbances, besides subjectively impaired sleep, relate to Parkinson’s disease pathology. Unfortunately, the cross-sectional design does not allow inference on temporality of the association. Authors speculate that potential pathways between sleep fragmentation and disease risk may include increased oxidative stress, or reduced clearance of metabolic waste including extracellular α-synuclein (Sohail et al., 2017).
Analyses of changes in sleep quality and duration suggest that sleep in prodromal Parkinson’s disease already deteriorates over 2 years prior to diagnosis in the general population, independent from baseline depressive symptoms, and the absolute levels over which the changes occurred. To our knowledge, the only study investigating changes in sleep has been performed in patients with RBD (Postuma et al., 2017). This study, however, reported opposite findings: improving insomnia symptoms and increasing self-reported sleep duration increased the risk for conversion to Parkinson’s disease and dementia with Lewy bodies. Differences in findings could results from their selection of patients prone to develop a severe, cognitively more impaired subtype of prodromal Parkinson’s disease (Fereshtehnejad et al., 2015), but differences may additionally be explained by non-recognition of sleep problems due to including subjects with subclinical cognitive deficits (Hancock and Larner, 2009; Most et al., 2012; Lysen et al., 2018). Their study not only had a high incidence (50%) of Lewy body dementia patients, but also showed under-reporting (reporting increased sleep duration and quality discrepant from objective decreases in total sleep time) in those developing neurodegenerative disease (Postuma et al., 2017).
If the aforementioned changes in sleep were driven by a specific sleep disorder, RBD may not be a likely candidate: subjects with RBD in a population-based polysomnography study had a similar sleep quality, and even longer sleep duration, than others (Haba-Rubio et al., 2018). RBD patients also did not perceive their sleep as worse, or shorter, than healthy controls (Postuma et al., 2017).
After excluding subjects with comorbid depressive symptoms or anxiety disorders, results remained mostly similar. Noteworthy was that hazard ratio estimates of the relation of sleep duration with Parkinson’s disease risk were all slightly higher. This resulted in an association of longer sleep duration with increased Parkinson’s disease risk in the overall follow-up. Given the number of associations investigated in our sensitivity analyses, and the small number of cases when restricting the sample, this result may be a spurious finding and should be interpreted with caution.
A methodological explanation is that in these sensitivity analyses subjects in a late prodromal phase of Parkinson’s disease may have been selectively excluded, as depression and anxiety are both part of the prodromal phase and considered predominantly late features (Alonso et al., 2009; Schrag et al., 2015; Darweesh et al., 2017a). This could have resulted in selective exclusion of susceptible subjects (Hernan, 2010) resulting in a decreased long-term risk of Parkinson’s disease in those remaining subjects with short sleep duration. It is also possible that short sleep duration is merely symptomatic of (prodromal emergence of) depression, which explains why exclusion of subjects with depression resulted in an inverse association of sleep duration with Parkinson’s disease. Nevertheless, we re-emphasize the small number of cases in our analyses, which may have compromised the robustness of these findings.
Analogous to the aforementioned sensitivity analysis, stratified analyses on the presence of parkinsonian signs might also select participants based on either a more advanced stage of an underlying neurodegenerative process, or its absence. A statistical interaction with sleep quality could guide future investigations of identifying high risk groups for parkinsonism or Parkinson’s disease risk.
Patterns of associations between separate PSQI components and Parkinson’s disease risk over time indicate that, aside from sleep duration, efficiency may mark prodromal disease. This applies to sleep quality, latency and daytime dysfunction to a lesser extent. Although these aspects of sleep may correlate well to known markers of prodromal Parkinson’s disease such as pain or autonomic failure (Schrag et al., 2015), or excessive daytime sleepiness (Iranzo, 2011; Al-Qassabi et al., 2017), results also warrant further investigation of these easily measured aspects of sleep in etiological or risk prediction efforts. Future studies on prodromal Parkinson’s disease are needed to investigate associations with objective measures of sleep, and to assess the predictive value of (perceived) shortening or worsening of sleep over known (sleep) markers of prodromal parkinsonism.
In conclusion, poor sleep quality and short sleep duration increased the risk of parkinsonism and Parkinson’s disease in the 2 years after baseline measurement. Moreover, sleep quality and duration change for the worse over 2 years prior to a diagnosis of parkinsonism, especially Parkinson’s disease. Both are congruent with presence of prodromal Parkinson’s disease progressively deteriorating sleep.
Supplementary Material
Acknowledgements
The authors are grateful to the study participants, the staff from the Rotterdam Study and the participating general practitioners and pharmacists.
Glossary
Abbreviations
- PSQI
Pittsburgh Sleep Quality Index
- RBD
REM sleep behaviour disorder
- REM
rapid eye movement
Funding
The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam. M.A.I. received support by a grant from the Dutch ParkinsonFonds foundation. S.K.L.D. received support by a grant from the Dutch ParkinsonFonds foundation.
Competing interests
The authors report no competing interests.
References
- Abbott RD, Ross GW, White LR, Tanner CM, Masaki KH, Nelson JS, et al. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology 2005; 65: 1442–6. [DOI] [PubMed] [Google Scholar]
- Al-Qassabi A, Fereshtehnejad SM, Postuma RB. Sleep disturbances in the prodromal stage of parkinson disease. Curr Treat Options Neurol 2017; 19: 22. [DOI] [PubMed] [Google Scholar]
- Alonso A, Rodriguez LA, Logroscino G, Hernan MA. Use of antidepressants and the risk of Parkinson’s disease: a prospective study. J Neurol Neurosurg Psychiatry 2009; 80: 671–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol 2016; 15: 1257–72. [DOI] [PubMed] [Google Scholar]
- Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol Med 1997; 27: 231–5. [DOI] [PubMed] [Google Scholar]
- Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord 2015; 30: 1600–11. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Silber MH, Ferman TJ. RBD in Parkinson’s disease and dementia with Lewy bodies. J Geriatr Psychiatry Neurol 2004; 17: 146–57. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003; 24: 197–211. [DOI] [PubMed] [Google Scholar]
- Breen DP, Nombela C, Vuono R, Jones PS, Fisher K, Burn DJ, et al. Hypothalamic volume loss is associated with reduced melatonin output in Parkinson’s disease. Mov Disord 2016; 31: 1062–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen DP, Vuono R, Nawarathna U, Fisher K, Shneerson JM, Reddy AB, et al. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol 2014; 71: 589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28: 193–213. [DOI] [PubMed] [Google Scholar]
- Chahine LM, Amara AW, Videnovic A. A systematic review of the literature on disorders of sleep and wakefulness in Parkinson’s disease from 2005 to 2015. Sleep Med Rev 2017; 35: 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri KR, Pal S, DiMarco A, Whately-Smith C, Bridgman K, Mathew R, et al. The Parkinson’s disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2002; 73: 629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Schernhammer E, Schwarzschild MA, Ascherio A. A prospective study of night shift work, sleep duration, and risk of Parkinson’s disease. Am J Epidemiol 2006; 163: 726–30. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhao E, Zhang W, Lu Y, Liu R, Huang X, et al. Meta-analyses on prevalence of selected Parkinson’s nonmotor symptoms before and after diagnosis. Transl Neurodegener 2015a; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Tsai TY, Li CY, Hwang JH. Obstructive sleep apnea and risk of Parkinson’s disease: a population-based cohort study. J Sleep Res 2015b; 24: 432–7. [DOI] [PubMed] [Google Scholar]
- Choi BC, Pak AW. A catalog of biases in questionnaires. Prev Chronic Dis 2005; 2: A13. [PMC free article] [PubMed] [Google Scholar]
- Chou PS, Lai CL, Chou YH, Chang WP. Sleep apnea and the subsequent risk of Parkinson’s disease: a 3-year nationwide population-based study. Neuropsychiatr Dis Treat 2017; 13: 959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet (Lond Engl) 2002; 359: 1309–10. [DOI] [PubMed] [Google Scholar]
- Darweesh SK, Koudstaal PJ, Stricker BH, Hofman A, Ikram MA. Trends in the incidence of parkinson disease in the general population: the Rotterdam study. Am J Epidemiol 2016; 183: 1018–26. [DOI] [PubMed] [Google Scholar]
- Darweesh SK, Verlinden VJ, Stricker BH, Hofman A, Koudstaal PJ, Ikram MA. Trajectories of prediagnostic functioning in Parkinson’s disease. Brain 2017a; 140: 429–41. [DOI] [PubMed] [Google Scholar]
- Darweesh SKL, Wolters FJ, Postuma RB, Stricker BH, Hofman A, Koudstaal PJ, et al. Association between poor cognitive functioning and risk of incident parkinsonism: the Rotterdam study. JAMA Neurol 2017b; 74: 1431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rijk MC, Breteler MM, Graveland GA, Ott A, Grobbee DE, van der Meche FG, et al. Prevalence of Parkinson’s disease in the elderly: the Rotterdam Study. Neurology 1995; 45: 2143–6. [DOI] [PubMed] [Google Scholar]
- Fereshtehnejad SM, Romenets SR, Anang JB, Latreille V, Gagnon JF, Postuma RB. New clinical subtypes of Parkinson disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. JAMA Neurol 2015; 72: 863–73. [DOI] [PubMed] [Google Scholar]
- Gaenslen A, Swid I, Liepelt-Scarfone I, Godau J, Berg D. The patients’ perception of prodromal symptoms before the initial diagnosis of Parkinson’s disease. Mov Disord 2011; 26: 653–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Huang X, Park Y, Hollenbeck A, Blair A, Schatzkin A, et al. Daytime napping, nighttime sleeping, and Parkinson disease. Am J Epidemiol 2011; 173: 1032–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev 2010; 90: 1063–102. [DOI] [PubMed] [Google Scholar]
- Haba-Rubio J, Frauscher B, Marques-Vidal P, Toriel J, Tobback N, Andries D, et al. Prevalence and determinants of REM sleep behavior disorder in the general population. Sleep 2018; 41: 1–8. [DOI] [PubMed] [Google Scholar]
- Hancock P, Larner AJ. Diagnostic utility of the Pittsburgh Sleep Quality Index in memory clinics. Int J Geriatr Psychiatry 2009; 24: 1237–41. [DOI] [PubMed] [Google Scholar]
- Hawkes CH. The prodromal phase of sporadic Parkinson’s disease: does it exist and if so how long is it? Mov Disord 2008; 23: 1799–807. [DOI] [PubMed] [Google Scholar]
- Hawkes CH, Del Tredici K, Braak H. A timeline for Parkinson’s disease. Parkinsonism Relat Disord 2010; 16: 79–84. [DOI] [PubMed] [Google Scholar]
- Hernan MA. The hazards of hazard ratios. Epidemiology 2010; 21: 13–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogl B, Arnulf I, Comella C, Ferreira J, Iranzo A, Tilley B, et al. Scales to assess sleep impairment in Parkinson’s disease: critique and recommendations. Mov Disord 2010; 25: 2704–16. [DOI] [PubMed] [Google Scholar]
- Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 2019; 363: 880–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikram MA, Brusselle GGO, Murad SD, van Duijn CM, Franco OH, Goedegebure A, et al. The Rotterdam Study: 2018 update on objectives, design and main results. Eur J Epidemiol 2017; 32: 807–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iranzo A. Sleep-wake changes in the premotor stage of Parkinson disease. J Neurol Sci 2011; 310: 283–5. [DOI] [PubMed] [Google Scholar]
- Iranzo A, Fernandez-Arcos A, Tolosa E, Serradell M, Molinuevo JL, Valldeoriola F, et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients. PLoS One 2014; 9: e89741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal AD, Edinger JD. Measuring sleep quality. Sleep Med 2008; 9 (Suppl 1): S10–7. [DOI] [PubMed] [Google Scholar]
- Lavie P. Self-reported sleep duration—what does it mean? J Sleep Res 2009; 18: 385–6. [DOI] [PubMed] [Google Scholar]
- Lysen TS, Wolters FJ, Luik AI, Ikram MK, Tiemeier H, Ikram MA. Subjective sleep quality is not associated with incident dementia: the Rotterdam study. J Alzheimers Dis 2018; 64: 239–47. [DOI] [PubMed] [Google Scholar]
- Marinus J, Visser M, van Hilten JJ, Lammers GJ, Stiggelbout AM. Assessment of sleep and sleepiness in Parkinson disease. Sleep 2003; 26: 1049–54. [DOI] [PubMed] [Google Scholar]
- Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev 2016; 25: 52–73. [DOI] [PubMed] [Google Scholar]
- Most EI, Aboudan S, Scheltens P, Van Someren EJ. Discrepancy between subjective and objective sleep disturbances in early- and moderate-stage Alzheimer disease. Am J Geriatr Psychiatry 2012; 20: 460–7. [DOI] [PubMed] [Google Scholar]
- Plazzi G. REM sleep behavior disorders in Parkinson’s disease and other Parkinsonian disorders. Sleep Med 2004; 5: 195–9. [DOI] [PubMed] [Google Scholar]
- Plazzi G, Corsini R, Provini F, Pierangeli G, Martinelli P, Montagna P, et al. REM sleep behavior disorders in multiple system atrophy. Neurology 1997; 48: 1094–7. [DOI] [PubMed] [Google Scholar]
- Plouvier AO, Hameleers RJ, van den Heuvel EA, Bor HH, Olde Hartman TC, Bloem BR, et al. Prodromal symptoms and early detection of Parkinson’s disease in general practice: a nested case-control study. Fam Pract 2014; 31: 373–8. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Gagnon JF, Pelletier A, Montplaisir JY. Insomnia and somnolence in idiopathic RBD: a prospective cohort study. NPJ Parkinsons Dis 2017; 3: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Iranzo A, Hogl B, Arnulf I, Ferini-Strambi L, Manni R, et al. Risk factors for neurodegeneration in idiopathic rapid eye movement sleep behavior disorder: a multicenter study. Ann Neurol 2015; 77: 830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977; 1: 385. [Google Scholar]
- Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron 2010; 68: 1023–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag A, Horsfall L, Walters K, Noyce A, Petersen I. Prediagnostic presentations of Parkinson’s disease in primary care: a case-control study. Lancet Neurol 2015; 14: 57–64. [DOI] [PubMed] [Google Scholar]
- Sheu JJ, Lee HC, Lin HC, Kao LT, Chung SD. A 5-year follow-up study on the relationship between obstructive sleep apnea and parkinson disease. J Clin Sleep Med 2015; 11: 1403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohail S, Yu L, Schneider JA, Bennett DA, Buchman AS, Lim ASP. Sleep fragmentation and Parkinson’s disease pathology in older adults without Parkinson’s disease. Mov Disord 2017; 32: 1729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow DM, Lawton MA, Grosset KA, Malek N, Smith CR, Bajaj NP, et al. Variation in recent onset Parkinson’s disease: implications for prodromal detection. J Parkinsons Dis 2016; 6: 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal TC, Lai YY, Siegel JM. Hypocretin (orexin) cell loss in Parkinson’s disease. Brain 2007; 130 (Pt 6): 1586–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen HU, Lachner G, Wunderlich U, Pfister H. Test-retest reliability of the computerized DSM-IV version of the Munich-Composite International Diagnostic Interview (M-CIDI). Soc Psychiatry Psychiatr Epidemiol 1998; 33: 568–78. [DOI] [PubMed] [Google Scholar]
- Wong JC, Li Y, Schwarzschild MA, Ascherio A, Gao X. Restless legs syndrome: an early clinical feature of Parkinson disease in men. Sleep 2014; 37: 369–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science 2013; 342: 373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YW, Hsieh TF, Yu CH, Huang YS, Lee CC, Tsai TH. Zolpidem and the risk of Parkinson’s disease: a nationwide population-based study. J Psychiatr Res 2014; 58: 84–8. [DOI] [PubMed] [Google Scholar]
- Yeh NC, Tien KJ, Yang CM, Wang JJ, Weng SF. Increased risk of Parkinson’s disease in patients with obstructive sleep apnea: a population-based, propensity score-matched, longitudinal follow-up study. Medicine (Baltimore) 2016; 95: e2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Xu CY, Liu J. Meta-analysis on the prevalence of REM sleep behavior disorder symptoms in Parkinson’s disease. BMC Neurol 2017; 17: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K, van Hilten JJ, Marinus J. The course of insomnia in Parkinson’s disease. Parkinsonism Relat Disord 2016; 33: 51–7. [DOI] [PubMed] [Google Scholar]
- Zis P, Erro R, Walton CC, Sauerbier A, Chaudhuri KR. The range and nature of non-motor symptoms in drug-naive Parkinson’s disease patients: a state-of-the-art systematic review. NPJ Parkinsons Dis 2015; 1: 15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccolella S, Savarese M, Lamberti P, Manni R, Pacchetti C, Logroscino G. Sleep disorders and the natural history of Parkinson’s disease: the contribution of epidemiological studies. Sleep Med Rev 2011; 15: 41–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be obtained on request. Requests should be directed toward the management team of the Rotterdam Study (secretariat.epi@erasmusmc.nl), which has a protocol for approving data requests. Because of restrictions based on privacy regulations and informed consent of the participants, data cannot be made freely available in a public repository.