Multiple sclerosis (MS) is a neuroinflammatory disease of the central nervous system (CNS) that affects over 2.5 million people worldwide. Most patients develop a relapsing−remitting disease in their second or third decade, although some have a progressive disease from onset. Many of those with relapsing−remitting disease eventually progress into a secondary progressive phase in which there is no improvement, and, like primary progressive MS, there is a gradual worsening of clinical disease (1). In early disease, an immune-mediated inflammatory attack in the CNS leads to myelin loss (demyelination) and the beginning of axon and neuronal loss (2–4). Inflammatory T cells play a key role in driving the disease in association with an innate immune response, and the activation of resident microglia. As myelin breaks down, macrophages infiltrate from the periphery. There are now many disease-modifying therapies that target the inflammatory and immune response and benefit many MS patients by reducing the relapse rate and severity of disease (1). However, none of these therapies have been clearly shown to promote restoration of myelin on demyelinated axons (remyelination), either in relapsing−remitting or progressive disease. Indeed, the greatest challenge in MS research at present is how to prevent or stop progression associated with axon loss in progressive disease (5). The most discussed approach to this issue is to develop therapies that promote remyelination. Remyelination will not only restore conduction and subsequent neurologic function (6), but will also protect axons from degenerating (7–10). Thus, enhancing remyelination is currently a much sought-after therapeutic strategy. In PNAS, McMurran et al. (11) investigate the previously unstudied connection between the microbiome and the CNS innate immune response, and, in particular, whether manipulation of the microbiome can promote remyelination (Fig. 1).
Fig. 1.
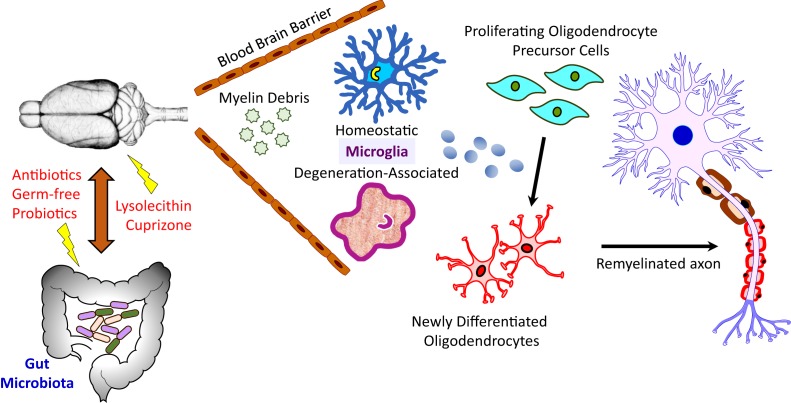
Remyelination is not enhanced by microbiome manipulation. A schematic of the gut microbiome−brain axis tested in the study by McMurran et al. (11). The authors used germ-free mice, or specific pathogen-free mice in which the intestinal microbiota were depleted with antibiotics or skewed with probiotics, in order to study the effects of the microbiome on remyelination. Two models of toxicity-induced demyelination were used: 1) focal demyelination in the ventral thoracic spinal cord induced by lysolecithin injection and 2) oral cuprizone-induced demyelination in the corpus callosum. The abundance of myelin debris within demyelinated lesions, the prevalence of homeostatic versus degeneration-associated microglia, the frequency of both OPCs and differentiated oligodendrocytes, and the density of remyelinated axons were assessed. The results show that, while microbiome manipulation alters the numbers of homeostatic and degeneration-associated microglia within the demyelinated lesion differently depending upon the model tested, neither OPC proliferation nor oligodendrocyte differentiation are impacted in any model, nor is remyelination enhanced.
Gut microbes play key roles in health and disease by regulating metabolism (12), pain (13), the pathophysiology of MS (14), and onset/progression of neurodegenerative (15) and neuropsychiatric disorders (16). Although mechanisms underlying gut−brain axis communication are still poorly understood, it is now well accepted that the gut microbiome impacts many aspects of CNS development and function, including neuroimmune cell maturation, formation of the blood−brain barrier, neurogenesis, and myelination (17). Microglia are CNS-resident innate immune cells whose homeostatic/surveillant activities play critical roles in many aspects of healthy brain development and function. Their phagocytic activities are critical for synaptic pruning and refining functional neural circuits during early postnatal brain development, and for clearing cell and myelin debris resulting from synaptic remodeling or neural injury (18). While microglial inflammatory and antigen presentation activities are involved in initiation of demyelination in MS, their phagocytosis of myelin debris and secretion of proregenerative factors are also necessary for permitting myelin repair (remyelination) (19). These degeneration-associated microglia acquire a different morphology with concurrent alterations in molecular phenotype. The microbiome is now recognized as an important modulator of the innate immune system, having significant impact on microglial activities throughout life (20). Intestinal microbes are necessary for a proper CNS immune response, since microglia from mice raised in a germ-free environment, or that are treated with antibiotics to ablate gut biota, are phenotypically immature and have an impaired immune response to bacterial or viral challenge (21).
To study the effect of manipulation of the microbiome by antibiotics, probiotics, or germ-free husbandry on remyelination, McMurran et al. (11) utilize 2 well-characterized models of demyelination in mice in which spontaneous remyelination occurs at various times after the initial insult (Fig. 1). Focal demyelination was induced by lysolecithin injection into the ventral column of the thoracic spinal cord, or by feeding germ-free mice for 5 wk with cuprizone, a drug that kills adult oligodendrocytes and results in demyelination of restricted areas of the brain, primarily the corpus callosum. Although altering the microbiome did successfully modify microglial inflammatory activities within the demyelinated lesions, the effects differed depending on the mode of biome modification. For example, antibiotic administration to lysolecithin-injected mice results in the development of highly proinflammatory microglia characterized by increased levels of Clec7a and low levels of P2RY12, indicative of an increase in degeneration-associated microglia and a reduction in homeostatic microglia in mice in which antibiotics were used to deplete the microbiome. Although these microglia are less phagocytic, remyelination in the spinal cord after focal lysolecithin injection is unaltered. In contrast, in germ-free mice in which cuprizone was used to induce demyelination, microglia with high levels of P2RY12 and low levels of Clec7a are increased, indicating increases in homeostatic microglia. Degeneration-associated microglia are less abundant, and, like the antibiotic treatment model, myelin phagocytosis is similarly reduced. While there is some variability in the division of oligodendrocyte progenitor cells (OPCs), there is a similar increase in these cells at 5 wk of treatment, consistent with prior studies (6). Critically, however, after cuprizone withdrawal, OPC numbers decrease and the number of mature oligodendrocytes increases, indicating that there is no effect of treatment on OPC differentiation and the generation of remyelinating oligodendrocytes. Likewise, microscopic studies on the corpus callosum show no differences in the rate or extent of remyelination.
Lastly, given that the microbiome is clearly important for modulating the microglial immune response, but not remyelination, the authors (11) did the converse experiment. They tested whether the microbiome could modify the neuroimmune response, subsequently influencing remyelination. To do this, they administered a probiotic (VSL#3) to aged mice with lysolecithin-induced demyelination, and studied the preponderance of degeneration-associated microglia. They find that degeneration-associated microglia are less abundant with probiotic administration, indicating a less severe inflammatory environment in the context of altered biota. Accordingly, myelin debris clearance is unchanged by probiotic administration, as are OPC number and oligodendrocyte differentiation; no changes in remyelination are seen either.
The rationale for performing these studies is abundantly clear. Prior studies have shown that the microbiome influences the innate immune response in models of other CNS disorders. The critical involvement of the innate immune response in MS, combined with prior studies showing that a probiotic can alter the microbiome and immunity in MS (14), strongly suggests that promoting such changes in the CNS might have multiple benefits. Likewise, altering the microbiome in experimental autoimmune encephalomyelitis (EAE), the animal model of MS, can delay the development of disease (22, 23). Hence, manipulating the micro
In PNAS, McMurran et al. investigate the previously unstudied connection between the microbiome and the CNS innate immune response, and, in particular, whether manipulation of the microbiome can promote remyelination.
biome with a simple and safe oral treatment that dampens the toxic aspect of an up-regulated innate immune response, while also enhancing its positive effects such as myelin debris removal and promoting remyelination, would be a major advance in the field. However, McMurran et al. (11) conclude, from their very thorough exploration of this hypothesis in toxic models of mouse demyelination, that remyelination is not promoted by this treatment, even though the innate immune response is altered. Nevertheless, there may be several factors that contribute to the observed lack of effect of microbiome manipulation on remyelination: 1) Since myelin debris was not effectively removed in either the antibiotics-treated or germ-free models, a neural environment conducive to remyelination was likely not created, regardless of alterations in the microglial phenotype. Thus, it may be that phagocytosis of myelin debris by other cells such as astrocytes (24, 25) must also be appropriately impacted by microbiome manipulation in order to effectively enhance remyelination. The consequences of microbiome modification on astrocyte function remain to be tested. 2) Although altering the microbiome successfully impacted the prevalence of homeostatic and degeneration-associated microglia following lysolecithin and cuprizone administration, it is not yet known whether that translates into requisite alterations in microglial release of factors necessary for promoting OPC proliferation or differentiation. 3) Lastly, it is possible that the remyelinating milieu created by these toxic models of demyelination does not completely recapitulate the microenvironment in MS and EAE, and therefore may not be applicable to demyelination in MS.
In summary, the idea that modifying the microbiome will be the “magic bullet” to enhance remyelination may be somewhat unrealistic. Indeed, it is probable that the complex process of functional CNS regeneration will require a combination therapy approach to impact multiple physiologic aspects, including, but not limited to, the microenvironment within a demyelinating lesion, microglial activities directly impacting myelin debris removal, and OPC proliferation and differentiation, as well as the function of other neuroglia. The comprehensive studies of McMurran et al. (11) discussed here strongly support the conclusion that microbiome manipulation is not yet a viable therapy on its own for enhancing remyelination.
Acknowledgments
This work was supported by National Multiple Sclerosis Society Grant RG1501-02876 (I.D.D.) and NIH Grant R01 NS085226 (J.J.W.).
Footnotes
The authors declare no competing interest.
See companion article on page 25311.
References
- 1.Reich D. S., Lucchinetti C. F., Calabresi P. A., Multiple sclerosis. N. Engl. J. Med. 378, 169–180 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trapp B. D., Nave K. A., Multiple sclerosis: An immune or neurodegenerative disorder? Annu. Rev. Neurosci. 31, 247–269 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Stadelmann C., Wegner C., Brück W., Inflammation, demyelination, and degeneration - Recent insights from MS pathology. Biochim. Biophys. Acta 1812, 275–282 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Lassmann H., Mechanisms of white matter damage in multiple sclerosis. Glia 62, 1816–1830 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Franklin R. J. M., Ffrench-Constant C., Regenerating CNS myelin—From mechanisms to experimental medicines. Nat. Rev. Neurosci. 18, 753–769 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Duncan I. D., Brower A., Kondo Y., Curlee J. F. Jr, Schultz R. D., Extensive remyelination of the CNS leads to functional recovery. Proc. Natl. Acad. Sci. U.S.A. 106, 6832–6836 (2009) Erratum in: Proc. Natl. Acad. Sci. U.S.A.106, 12208 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kornek B., et al. , Multiple sclerosis and chronic autoimmune encephalomyelitis: A comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am. J. Pathol. 157, 267–276 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irvine K. A., Blakemore W. F., Remyelination protects axons from demyelination-associated axon degeneration. Brain 131, 1464–1477 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Mei F., et al. , Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. eLife 5, e18246 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schultz V., et al. , Acutely damaged axons are remyelinated in multiple sclerosis and experimental models of demyelination. Glia 65, 1350–1360 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMurran C. E., et al. , The microbiota regulates murine inflammatory responses to toxin-induced CNS demyelination but has minimal impact on remyelination. Proc. Natl. Acad. Sci. U.S.A. 116, 25311–25321 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saiyasit N., et al. , Gut dysbiosis develops before metabolic disturbance and cognitive decline in high-fat diet-induced obese condition. Nutrition 69, 110576 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Guo R., Chen L. H., Xing C., Liu T., Pain regulation by gut microbiota: Molecular mechanisms and therapeutic potential. Br. J. Anaesth. 123, 637–654 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Camara-Lemarroy C. R., Metz L., Meddings J. B., Sharkey K. A., Wee Yong V., The intestinal barrier in multiple sclerosis: Implications for pathophysiology and therapeutics. Brain 141, 1900–1916 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hickman S., Izzy S., Sen P., Morsett L., El Khoury J., Microglia in neurodegeneration. Nat. Neurosci. 21, 1359–1369 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cryan J. F., et al. , The microbiota-gut-brain axis. Physiol. Rev. 99, 1877–2013 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Sharon G., Sampson T. R., Geschwind D. H., Mazmanian S. K., The central nervous system and the gut microbiome. Cell 167, 915–932 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galloway D. A., Phillips A. E. M., Owen D. R. J., Moore C. S., Phagocytosis in the brain: Homeostasis and disease. Front. Immunol. 10, 790 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miron V. E., et al. , M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 16, 1211–1218 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y., et al. , The gut-microglia connection: Implications for central nervous system diseases. Front. Immunol. 9, 2325 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erny D., et al. , Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yokote H., et al. , NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am. J. Pathol. 173, 1714–1723 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochoa-Repáraz J., et al. , Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol. 183, 6041–6050 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Ponath G., et al. , Myelin phagocytosis by astrocytes after myelin damage promotes lesion pathology. Brain 140, 399–413 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mills E. A., et al. , Astrocytes phagocytose focal dystrophies from shortening myelin segments in the optic nerve of Xenopus laevis at metamorphosis. Proc. Natl. Acad. Sci. U.S.A. 112, 10509–10514 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]