Significance
Regressions of some blood cancer can follow infusion of patients’ own T cells gene-modified to express a chimeric antigen receptor (CAR). These regressions are associated with extensive proliferation and engraftment of CAR T cells. However, for most common solid cancers, CAR T cells encounter antigen in a microenvironment that is immunosuppressive and lacking T cell support, and significant engraftment does not always occur. Here, we deliver enterotoxins or bispecific antibody to provide CAR T cells with support signals from antigen-presenting cells in lymphoid tissue, away from the tumor microenvironment. We demonstrate that this enables CAR T cell activation and proliferation, leading to enhanced responses against solid tumors in mice. This approach could lead to effective treatments for many common cancers.
Keywords: CAR T cells, antigen-presenting cells, engraftment, superantigen, costimulation
Abstract
Responses of solid tumors to chimeric antigen receptor (CAR) T cell therapy are often minimal. This is potentially due to a lack of sustained activation and proliferation of CAR T cells when encountering antigen in a profoundly immunosuppressive tumor microenvironment. In this study, we investigate if inducing an interaction between CAR T cells and antigen-presenting cells (APCs) in lymphoid tissue, away from an immunosuppressive microenvironment, could enhance solid-tumor responses. We combined CAR T cell transfer with the bacterial enterotoxin staphylococcal enterotoxin-B (SEB), which naturally links a proportion of T cell receptor (TCR) Vβ subtypes to MHC-II, present on APCs. CAR T cell proliferation and function was significantly enhanced by SEB. Solid tumor-growth inhibition in mice was increased when CAR T cells were administered in combination with SEB. CAR T cell expansion in lymphoid tissue was demonstrated, and inhibition of lymphocyte egress from lymph nodes using FTY720 abrogated the benefit of SEB. We also demonstrate that a bispecific antibody, targeting a c-Myc tag on CAR T cells and cluster of differentiation 40 (CD40), could also enhance CAR T cell activity and mediate increased antitumor activity of CAR T cells. These model systems serve as proof-of-principle that facilitating the interaction of CAR T cells with APCs can enhance their ability to mediate antitumor activity.
While chimeric antigen receptor (CAR) T cell transfer can be highly effective against some leukemias and lymphomas (1–4), the adoptive transfer of CAR T cells alone against solid cancer is generally much less effective in patients and immunocompetent animal models (5, 6). Although the reasons for this are not entirely clear, a feature of adoptive transfer in liquid cancers such as leukemia/lymphoma is extensive expansion and persistence of CAR T cells, which is not usually seen to the same extent in the solid cancer setting (7, 8). CAR T cell expansion of 1,000-fold or greater has been demonstrated for CAR T cells in patients with hematological malignancies, with CAR T cell numbers often exceeding 20% of circulating lymphocytes (9, 10). In clinical studies of CAR T cells targeting cluster of differentiation 19 (CD19)-positive B cell malignancies, absolute counts of circulating CAR T cells can achieve levels up to several hundred thousand per milliliter, even though relatively few CAR T cells were initially transferred (1, 11, 12).
A potential contributor to CAR T cell expansion in liquid cancers is their engagement with a target antigen expressed on cancer cells with an antigen-presenting cell (APC)-like phenotype. APCs are capable of providing T cells with costimulation and cytokine support in lymphoid tissue (13, 14), in the absence of the profoundly immunosuppressive microenvironment often found in solid cancers (15). The classical interaction between T cells and APCs involves T cell receptor (TCR)-MHC ligation and a complex assortment of membrane-bound and soluble costimulatory signals. This interaction leads to a hyperproliferative state in T cells and initiation of activation and differentiation programs, which are conditions necessary for effective responses against disease and protection against disease recurrence. It is the initial interaction between TCR and MHC that both increases the dwell time of cellular interaction and initiates a 2-way biochemical response between T cells and APCs that leads to optimal T cell activity (16). However, most cell-surface antigens on solid tumors are not expressed by APCs, and therefore CAR T cells are unable to fully engage with APCs in most common solid cancers. Despite the inclusion of some costimulatory motifs in chimeric receptor design, synapse formation through CARs is nonclassical (17), and many costimulatory signals from defined and as-yet undefined nature, including cytokines, are absent (18).
In considering how an interaction between CAR T cells and APCs could be mediated, we drew on knowledge gained from infectious diseases involving enterotoxins, which can lead to toxic shock syndrome. In toxic shock syndrome, a systemic inflammatory response resulting from severe infection leads to fever, low blood pressure, and malaise that can progress to multiple organ failure and death. Infections are usually localized to mucous membranes or wound sites, and toxicity results from secretion of enterotoxins, which are typified by staphylococcal enterotoxin B (SEB) (19).
SEB simultaneously binds MHC-II (on APCs) and particular Vβ chains of TCRs (on T cells), chiefly Vβ 3, 7, 8, and 17 in mice (20). Binding of SEB to MHC-II occurs outside the peptide-binding groove independent of MHC haplotype. T cell stimulation can be mediated by SEB engagement with MHC-II on a variety of cells including dendritic cells, B cells, and monocytes/macrophages (21–23). A range of costimulatory molecules are present on MHC-II+ cells, which also provide signals important in enterotoxin-mediated T cell stimulation (24). Thus, while conventional antigens presented by MHC-II activate less than 0.001% of T cells, enterotoxins stimulate between 2 to 20% of T cells in an MHC haplotype-independent manner, encompassing both CD8+ and CD4+ T cells. Due to their ability to stimulate a large proportion of T cells, enterotoxins are often referred to as superantigens (19). Large doses of enterotoxins lead to APC-mediated proliferation of T cells and high systemic levels of cytokine production, which results in toxicity (25).
In this work, we hypothesized that providing engagement between CAR T cells and APCs may boost CAR T cell proliferation and activity. To test this hypothesis, we utilized mouse solid-tumor models to investigate proof-of-principle studies using subtoxic doses of enterotoxins, or a bispecific antibody, to determine the effect on CAR T cell therapy. Here, we demonstrate that coadministration of SEB and CAR T cells leads to enhanced proliferation and function of CAR T cells and an improved ability to inhibit the growth of solid tumors.
Results
CAR T Cells Proliferate and Acquire Effector Function in Response to Enterotoxin.
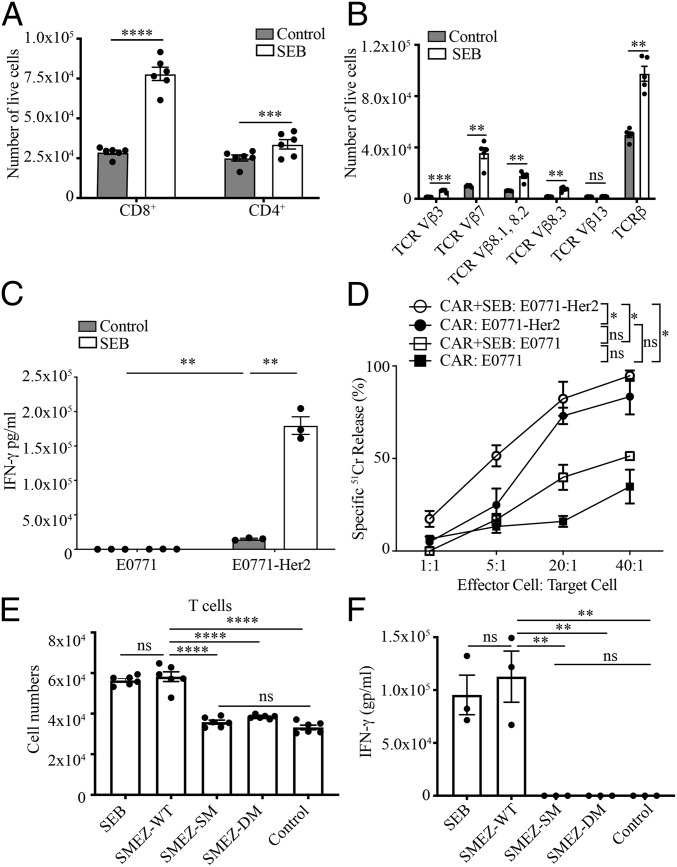
The CAR used in this study was specific for the human breast cancer-associated antigen Her2 and incorporated a second-generation cytoplasmic domain composed of CD3-ζ and CD28 signaling moieties (26). Central to our hypothesis that SEB could enhance CAR T cell antitumor activity was that interaction of CAR T cells with SEB and APCs would lead to T cell proliferation and effector activity. Coculture of mouse CAR T cells and SEB with splenocytes, as a source of MHC-II+ APCs, led to significantly increased proliferation of CAR T cells (Fig. 1A). Proliferation was largely restricted to T cells possessing Vβ subtypes engaged by SEB, chiefly Vβ3, 7, 8.1, 8.2, and 8.3, as demonstrated by enumeration of cells (Fig. 1B). Using flow-cytometric analysis of bromodeoxyuridine incorporation, proliferation was confirmed for both CD8+ T cells and CD4+ T cells (SI Appendix, Fig. S1A) and for appropriate Vβ subsets (SI Appendix, Fig. S1B). The role of an SEB-TCR interaction in proliferation was further supported by the observation that CAR T cells expressing a Vβ13 subtype that does not bind SEB did not proliferate significantly in response to SEB (Fig. 1B and SI Appendix, Fig. S1B). Enhanced effector function of SEB-stimulated CAR T cells was demonstrated by the observation that, following their coculture with Her2+ tumor cells, SEB-stimulated CAR T cells secreted IFN-γ to a greater degree than CAR T cells lacking SEB stimulation (Fig. 1C). In addition, SEB-stimulated CAR T cells were able to mediate increased levels of cytolysis of tumor cells (Fig. 1D).
Fig. 1.
Bacterial enterotoxins induce proliferation and function of CAR T cells. (A) CAR T cells proliferate in response to SEB. CAR T cells were incubated with splenocytes with or without SEB (25 µg/mL) for 72 h, and both CD8+ and CD4+ T cells were enumerated (±SEM). Data are representative of 3 experiments. (B) Specific TCR-Vβ subtypes proliferate in response to SEB. The number of CAR T cells of the indicated Vβ subtypes following coculture with splenocytes with or without SEB is as in A (±SEM). (C) CAR T cells with or without SEB secrete IFN-γ in response to Her2+ tumor cells. CAR T cells were incubated with splenocytes with or without SEB for 72 h before removing the SEB, followed by incubation with tumor cells for 24 h (±SEM). Data are representative of 2 independent experiments. (D) SEB-stimulated CAR T cells kill Her2+ tumor cells. CAR T cells were incubated with splenocytes with or without SEB for 48 h before adding the tumor cells. The cytotoxicity was determined after 20 h using a 51Cr-release assay. Data are representative of 3 independent experiments. (E) SMEZ induces proliferation of CAR T cells, which is dependent on MHC-II/TCR binding. CAR T cells and splenocytes were incubated with or without WT SMEZ or SMEZ lacking the ability to bind to either TCR or MHC/TCR. SMEZ-M1, do not bind to TCR (23); SMEZ-DM, do not bind to TCR or MHC-II (27, 60). Mean cell numbers ± SEM are presented. Data are from 2 experiments. (F) SMEZ-stimulated CAR T cells secrete IFN-γ; 1 × 106 CAR T cells were incubated with splenocytes in the presence of 1 µM superantigens for 72 h, and the supernatant was tested for IFN-γ concentration using enzyme-linked immunosorbent assay. Data are representative of 2 independent experiments. ns, not significant; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
SEB is known to mediate an interaction between T cells and MHC-II+ cells (20). Support for the ability of SEB to mediate an interaction between CAR T cells and MHC-II+ cells was derived from observations, using confocal microscopy, of an enhanced interaction between Vβ3, 7, 8.1, 8.2, and 8.3 T cells and MHCII+ cells after 2 h of cell coculture in the presence of SEB (SI Appendix, Fig. S2 and Movie S1 and S2).
To widen the investigation to additional bacterial superantigens and gain insight into the requirements of their binding to TCR and MHC-II, we utilized another superantigen, streptococcal mitogenic exotoxin-Z (SMEZ), for which we had alternate forms mutated at key amino acid residues to eliminate binding to TCR (SMEZ-SM) and MHC-II (SMEZ-DM) (27). Similar to our observations with SEB, wild-type (WT) SMEZ enabled significant proliferation and cytokine secretion from CAR T cells (Fig. 1 E and F). The requirement for binding to MHC-II and TCR was demonstrated by the loss of T cell proliferation and activation when using the mutated forms of SMEZ (Fig. 1 E and F and SI Appendix, Fig. S3). In addition, the requirement for SEB binding to MHC-II for proliferation and function of CAR T cells was demonstrated using an MHC-II–blocking antibody (SI Appendix, Fig. S4).
SEB Enhances CAR T Cell Activity against Established Solid Tumors.
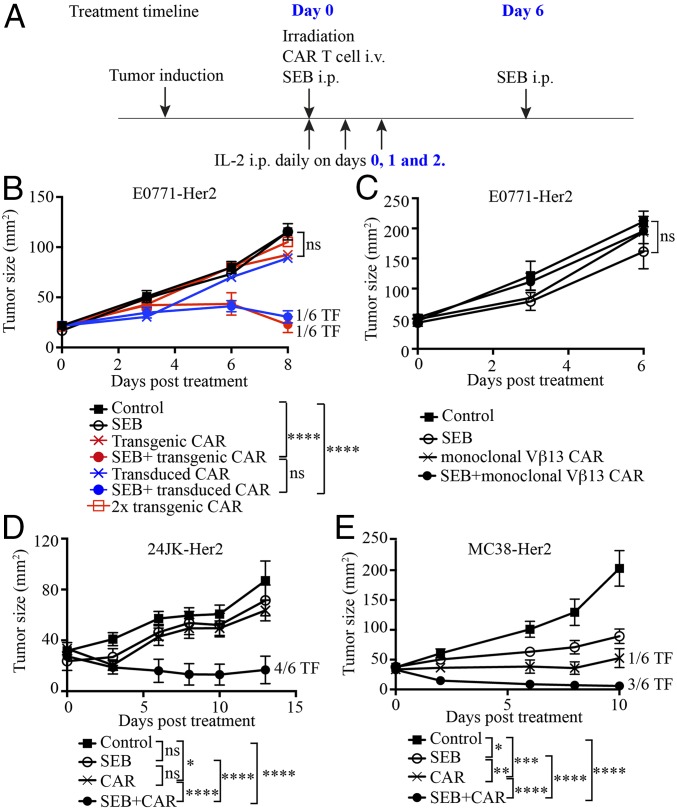
Mice bearing established E0771-Her2 tumors received a standard regimen of adoptive T cell transfer, consisting of CAR T cells together with whole-body irradiation preconditioning and T cell growth factor IL-2, to enhance T cell engraftment. Some mice also received intraperitoneal (i.p.) injections of SEB (Fig. 2A). Significantly enhanced tumor-growth inhibition was observed in mice receiving the combination of CAR T cells and SEB compared to mice receiving the CAR T cell regimen alone or SEB alone (Fig. 2B and SI Appendix, Fig. S5A). The importance of in vivo activation and expansion mediated by SEB was demonstrated by the lack of efficacy of CAR T cells alone, even at double the dose (Fig. 2B).
Fig. 2.
SEB enhances the antitumor activity of CAR T cells. (A) Treatment schema. (B) The antitumor effect of CAR T cells plus SEB for treating E0771-Her2 tumors. Some mice received CAR T cells derived from CAR transgenic mice, while others received CAR T cells generated by retroviral transduction (as listed). (C) SEB together with CAR T cells from TCR transgenic mice expressing a Vβ13 TCR (not engaged by SEB) were used to treat E0771-Her2 tumors. (D) CAR T cells and SEB were used to treat subcutaneous tumors formed from injection of 24JK-Her2 sarcoma cells. (E) CAR T cells and SEB were used to treat subcutaneous tumors formed by injection of MC38-Her2 colon carcinoma cells. Data are presented as means ± SEM and are derived from a single experiment (B and C) and 2 experiments ( D and E) (>5 mice per group). ns, not significant (2-way ANOVA); i.v., intravenous; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
A proportion of E0771-Her2 tumors were eradicated, and long-term surviving mice were resistant to rechallenge with E0771-Her2 (SI Appendix, Fig. S6), suggesting the establishment of immunological memory. Consistent with this interpretation, CAR T cells were shown to persist in tissues of mice greater than 100 d, and T cells were of a central memory phenotype (SI Appendix, Fig. S7).
In the above experiments, as a convenient source of CAR T cells expressing consistent levels of CAR in all T cells, we used T cells from transgenic mice expressing the CAR under the control of the vav promoter (28). To confirm the ability of SEB to enhance CAR T cell-mediated tumor inhibition, we also used CAR T cells generated by retroviral transduction, as used in clinical applications. We transduced mouse T cells with the anti-Her2 CAR (SI Appendix, Fig. S8) and demonstrated their capacity to inhibit tumors in combination with SEB to a similar degree as transgenic CAR T cells (Fig. 2B and SI Appendix, Fig. S5A). In subsequent experiments, we used T cells from transgenic CAR mice as a consistent source of CAR T cells.
The requirement for CAR T cells of the correct SEB-specific subtype was confirmed by the observation that CAR T cells expressing a transgenic Vβ13 TCR, that is unable to engage SEB, were ineffective against E0771-Her2 tumors (Fig. 2C and SI Appendix, Fig. S5B). The ability of SEB to enhance CAR T cell therapy was further supported by the demonstration of enhanced tumor responses in 2 further Her2+ mouse tumor models (Fig. 2 D and E and SI Appendix, Fig. S5 C and D). Enhanced tumor growth inhibition was also demonstrated using another superantigen, SMEZ, and the inhibition of tumors was reduced when using SMEZ mutants lacking the ability to bind MHC-II (SI Appendix, Fig. S9). The requirement for MHC-II was also supported by the observation that SEB did not enhance CAR T cell therapy in the NSG mice, which have a greatly reduced frequency of MHC-II–positive cells (29) (SI Appendix, Fig. S10). The requirement for CAR expression in optimal antitumor activity in NSG mice was indicated by the lack of tumor inhibition using control (non-CAR) T cells and SEB (SI Appendix, Fig. S10). Additional experiments in immunocompetent mice also demonstrated that CAR expression and a second dose of SEB were all necessary for optimal treatment efficacy (SI Appendix, Fig. S11).
Although toxicity has been reported previously following injection of mice with SEB, no overt toxicity was observed using the dose and treatment regimen implemented in the current study, as demonstrated by a lack of reduction in body weight (SI Appendix, Fig. S12A). In addition, there was no increase in the levels of a variety of cytokines in the serum (SI Appendix, Fig. S12 B–N), and no infiltration of T cells into the Her2-expressing regions of the brain (SI Appendix, Fig. S13). Taken together, these data indicate that there was no significant induction of autoimmunity following treatment of tumors.
SEB-Stimulated CAR T Cells Proliferate in Lymphoid Tissue and Migrate to Tumors.
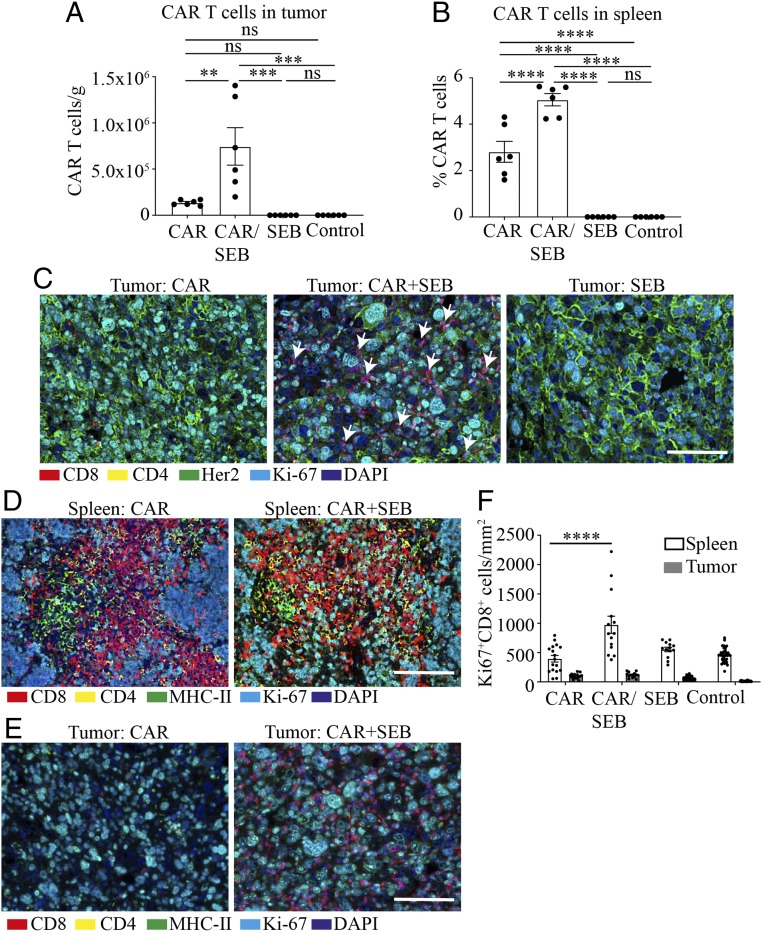
To gain insight into how SEB augmented CAR T cell antitumor activity, we determined the effect of SEB on CAR T cell proliferation in vivo. In combination with SEB, greater numbers and frequency of CAR T cells were observed in tumors using flow cytometry gated on a congenic marker for CAR T cells (Fig. 3A). A significant increase in frequency of CAR T cells was also demonstrated in spleen following SEB administration (Fig. 3B). An increase in tumor-infiltrating T cells following SEB administration was also shown using immunohistochemical staining for CD8 (Fig. 3C). Endogenous T cells were present in significant numbers among tumor-infiltrating T cells (SI Appendix, Fig. S14), and although their numbers did not increase significantly in the presence of SEB, it was still possible that these endogenous T cells contributed to the inhibition of tumor growth in the presence of CAR T cells. However, it seems clear that, in the absence of CAR T cells, endogenous T cells in combination with SEB were not sufficient to induce complete tumor regressions, despite their presence in tumors (SI Appendix, Fig. S11).
Fig. 3.
SEB-stimulated CAR T cells proliferate in lymphoid tissue. Mice bearing E0771-Her2 subcutaneous tumors were treated with CAR T cells with or without SEB, and tumors and spleens were removed 8 d later (2 d post the second SEB injection) and subjected to flow-cytometric analysis or immunohistochemistry (IHC). (A) Frequency of congenic CAR T cells in tumors of mice receiving the listed treatments. (B) Percentage of congenic CAR T cells in spleens. Data are presented as means ± SEM. (C) Representative multiplexed IHC (OPAL) stained with anti-CD8 in tumors 8 d after receiving treatment. White arrows point to CD8+ cells. (D) Multiplexed IHC staining of spleens from treated mice. (E) Multiplexed IHC staining of tumors from treated mice. (F) Quantitated data from D and E displaying the density of Ki67+ (proliferating) T cells in spleens and tumors (mean ± SEM). (A and B) Data from a single experiment. (C–E) Representative data from more than 3 independent experiments. (Scale bars: 50 μm.) ns, not significant; **P < 0.01, ***P < 0.001, ****P < 0.0001.
To investigate the relative role of anatomical site on CAR T cell expansion, we used multiplex immunohistochemistry to determine the location of T cells expressing the proliferation marker Ki67. Proliferating CD8+ T cells were observed at a high frequency in spleen when SEB was coadministered with CAR T cells (Fig. 3 D and F), whereas proliferating T cells were relatively rare in tumors (Fig. 3 E and F). Together with this information and observations that MHC-II+ cells were abundantly present in the spleen, but not tumors (Fig. 3 D and E), it is likely SEB enhanced CAR T cell interaction with MHC-II+ APCs in lymphoid organs.
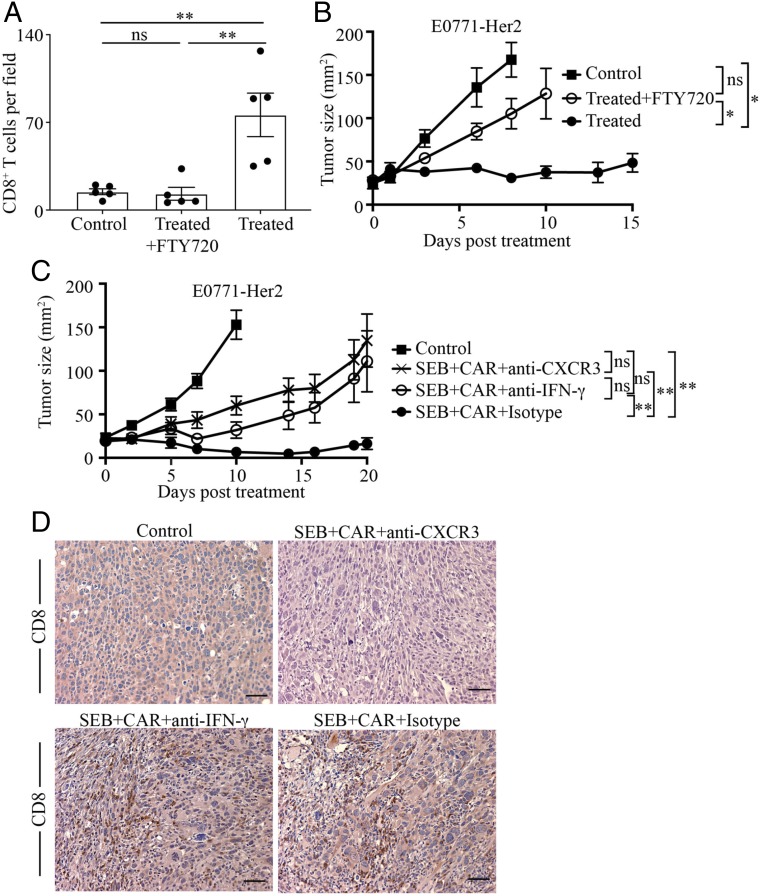
Having determined that lymphoid tissue was likely the main site of T cell proliferation in response to SEB, we investigated whether migration of CAR T cells from lymphoid tissue was necessary for their antitumor efficacy. To do this, we administered the immunomodulatory drug FTY720, which inhibits egress of T cells from lymphoid organs. We first confirmed that administration of FTY720 led to a significant reduction in the frequency of circulating CD4+ and CD8+ T cells in mice (SI Appendix, Fig. S15). We also determined that administration of FTY720 markedly reduced the infiltration of T cells into tumors (Fig. 4A). The importance of CAR T cell migration on antitumor efficacy was confirmed by a significant reduction in antitumor activity after FTY720 administration (Fig. 4B).
Fig. 4.
SEB-stimulated T cells migrate from lymphoid tissue to tumors to mediate inhibition of tumor growth. (A) Tumors were taken from untreated mice or those receiving CAR T cells and SEB (Treated) with or without the lymphoid tissue egress inhibitor FTY720. Sections of tumors were subjected to immunohistochemistry, and CD8+ cells were enumerated microscopically in 5 random fields at 200× magnification. Data are represented as means ± SEM. (B) Mice were injected subcutaneously with E0771-Her2 tumor cells, followed by CAR T cells plus SEB treatment (Treated) with or without FTY720 11 d after tumor inoculation. Data are presented as the mean tumor size ± SEM for 6 to 7 mice per group. (C) Cohorts of mice bearing established E0771-Her2 tumors were either left untreated or received CAR T cell therapy and SEB in the presence or absence of antibodies that blocked CXCR3 or IFN-γ. (D) Representative images of CD8 staining of tumors from mice receiving the listed treatments. (Scale bars: 50 μm.) ns, not significant; *P < 0.05, **P < 0.01.
To gain further insight into factors important in the migration and action of CAR T cells when coadministered with SEB, we used specific blocking monoclonal antibodies to inhibit the activity of the chemokine receptor CXCR3 and the cytokine IFN-γ. Blocking CXCR3 significantly inhibited the antitumor activity of CAR T cells (Fig. 4C) and reduced the infiltration of T cells into tumors (Fig. 4D), suggesting a role for one or more of the chemokines CXCL9 and CXCL10. A role for IFN-γ was also indicated by a significant reduction in tumor-growth inhibition upon blocking this cytokine (Fig. 4C), despite having no significant impact on T cell infiltration of tumors (Fig. 4D).
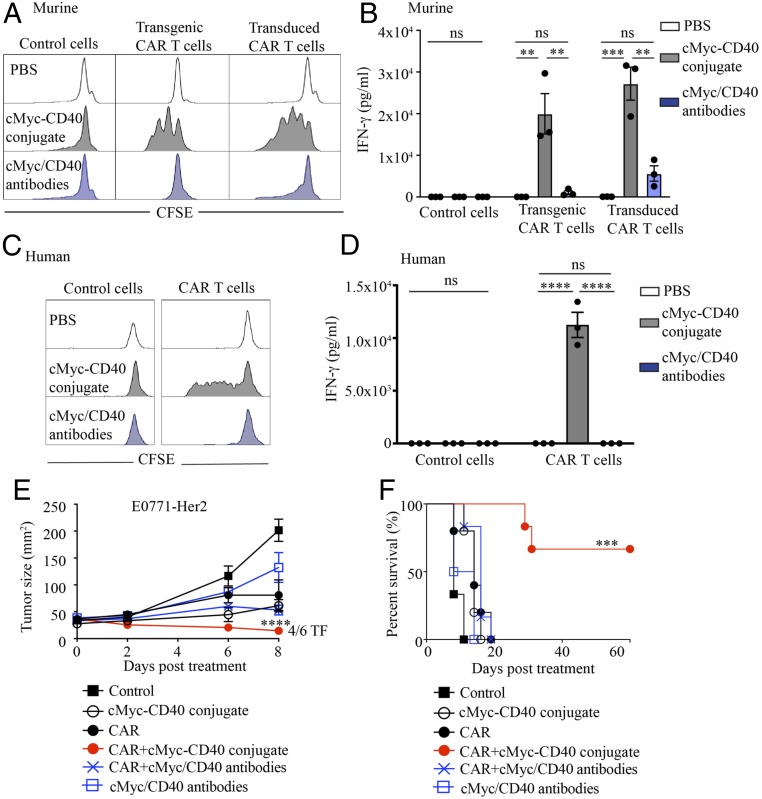
In this mouse model, we targeted MHC-II, whose expression is largely restricted to cells with antigen-presenting capabilities. However, in humans, MHC-II can be expressed by activated T cells, raising the possibility that T cells will engage in fratricide. Indeed, in the human, after initial activation and expansion of T cells by enterotoxins, a period of contraction and anergy follows, leading to reduced T cell function (30). Therefore, despite serving as proof-of-principle of the benefit of inducing CAR T cell activation and proliferation to enhance their efficacy against tumors, superantigens are unlikely to be suitable for use in the clinic. We therefore performed preliminary studies using a more targeted bispecific biologic composed on an antibody specific for the cMyc tag, present only in CAR T cells, conjugated to an antibody specific for CD40, expressed on a range of APCs. Addition of the cMyc-CD40 bispecific to CAR T cells greatly enhanced both murine and human CAR T cell proliferation and IFN-γ secretion in the presence of CD40+ APCs in vitro (Fig. 5 A–D). In addition, administration of the cMyc-CD40–bispecific and murine CAR T cells in tumor-bearing mice led to rejection of tumors in the majority of treated mice (Fig. 5 E and F), whereas a mixture of unconjugated antibodies was unable to enhance CAR T cell activity. These data suggest that a targeted bispecific could mediate an interaction between CAR T cells and CD40-expressing cells, leading to enhancement of CAR T cell function.
Fig. 5.
Bispecific anti-CD40/cMyc antibodies promote CAR T cell proliferation and antitumor function. (A) Carboxyfluorescein succinimidyl ester (CFSE)-labeled murine transgenic or transduced CAR T cells were incubated with an anti-murine CD40/anti-cMyc tag-bispecific conjugate, or unconjugated antibodies, for 72 h in the presence of irradiated murine splenocytes (non–CFSE-labeled). (B) Supernatant from A was analyzed for IFN-γ using an AlphaLISA immunoassay. (C) CFSE-labeled human CAR T cells, or empty vector-transduced T cells, were incubated with the anti-human CD40/anti-cMyc tag-bispecific conjugate, or unconjugated antibodies, for 72 h in the presence of cell trace violet-labeled monocyte-derived dendritic cells from the same donor. (D) Supernatant from C was analyzed for IFN-γ using an AlphaLISA immunoassay. (E) Tumor growth. (F) Survival curves of mice receiving the listed treatments. (A and B) Representative data from >5 experiments. (C and D) Representative data from over 3 repeats using peripheral blood mononuclear cells from different donors. (E and F) Representative data from 2 experiments (>5 mice per group). ns, not significant; **P < 0.01, ***P < 0.001, ****P < 0.0001.
Discussion
In a natural immune response against disease, the initial activation and extensive proliferation of T cells is mediated by APCs in lymphoid tissue away from the site of disease. Activated T cells then migrate to the disease site to deliver their effector functions of cytolysis and cytokine secretion. This method of immune protection has developed through evolution to provide an efficient means of antigen presentation to specific T cells and mediate the acquisition of optimal differentiation and trafficking phenotype, and induce proliferation in an immune-supportive environment. In this natural immune response, antigen presentation occurs through interaction of MHC on APCs with TCR on T cells.
The concept of CAR T cells was developed to direct T cells against tumor-associated antigens in a non–MHC-dependent manner (31–33). While this approach has the benefit of enabling redirection of patient T cells irrespective of their MHC haplotype, in many cases, it also foregoes an interaction of T cells with APCs, which possess a variety of T cell costimulatory activities. The need for T cell costimulation has been partially addressed by inclusion of costimulatory domains into CAR formats (34), but not all possible cellular and soluble costimulators are engaged in this manner (18). In a strategy to maintain the non-MHC dependency of the CAR approach while also providing CAR T cells with the opportunity to interact with APCs, we used forms of bacterial products, often termed superantigens, which link TCR-Vβ to MHC-II in a haplotype-independent manner.
In this study, we used an immunocompetent self-antigen mouse model to demonstrate enhanced CAR T cell responses against solid tumors when used in combination with superantigens. Although superantigens can elicit toxic immune reactions, their affinity for MHC-II and subsequent toxicity is lower in mice, thereby enabling us to investigate this principle to enhance CAR T cell proliferation and activity. Although these superantigen studies served as proof-of-principle, the potential toxicity of superantigens in humans renders them unlikely to be suitable for therapeutic application. Nevertheless, it is intriguing to consider whether better outcomes to CAR T cell therapy have been associated with enterotoxin-producing bacteria, present either subclinically in the microbiome or during infection. Certainly, enhanced tumor responses to immunotherapy have been linked to certain compositions of the microbiome, but factors contributing to this association are not well defined (35–37).
To extend our studies beyond the use of superantigens, we generated a bispecific antibody with specificity for CAR T cells and an APC-expressed molecule. We demonstrated that a bispecific, targeting a cMyc-tag and CD40, enhanced CAR T cell activity against tumors. CD40 is expressed on a range of cells with antigen-presenting capacity, including subsets of dendritic cells, monocytes, macrophages, and B cells (38). At present, it is unclear if stimulation of CAR T cells using the CD40 bispecific occurs predominantly within lymphoid tissue, as occurs using SEB. However, previous studies indicate that CTL can be activated and disseminate from lymph nodes following systemic delivery of anti-CD40 (39), but that CD40+ APCs can also be present within tumors (40, 41), suggesting that T cell activation may occur at both sites. Interestingly, CD40 agonists alone can stimulate T cells to inhibit some tumors (42, 43), and CD40 agonists combined with chemotherapy, vaccination or irradiation can induce enhanced T cell-dependent antitumor responses (44–47). CD40 ligation can stimulate APCs to secrete cytokines and up-regulate costimulatory and adhesion molecules, thereby licensing them to activate T cells (48), and it will be of interest in future studies to determine the response from APCs to ligation with the CD40 bispecific and the corresponding response of CAR T cells. The future development of this or similar bispecifics may provide an effective, yet safer option than superantigens.
Potential support for the concept of promoting CAR T cell engagement of APCs can be found in studies targeting CD19, which is expressed on cells of B cell origin that can have APC capabilities under some circumstances. In those studies, extensive expansion of CAR T cells, and remarkable antitumor responses, have been observed following adoptive transfer (1, 9, 11).
Further support for the potential of providing APC support for CAR T cells can be found in the use of artificial antigen-presenting cells (A-APCs). A-APCs have been developed in several formats, including cell-based and nanoparticle-based. In any case, a variety of surface-displayed T cell-activating and costimulatory molecules are included in the design. A major use of A-APCs is for in vitro production of antigen-specific T cells, but in vivo use is also feasible (49–51). Interestingly, A-APCs have been demonstrated to be better than conventional anti-CD3/CD28 beads in generating T cells with a stem cell-like memory phenotype and mediating superior antitumor activity (52).
In the current work, we performed proof-of-principle studies demonstrating that a soluble protein could be used to provide CAR T cells with activation and proliferative capacity to enhance their antitumor activity. The nature of a soluble protein may have pharmacokinetic and regulatory advantages over A-APCs for use in patients and provide support for CAR T cells to enhance antitumor responses.
In this study, we used a second-generation Her2-CAR incorporating CD3-ζ and CD28 cytoplasmic domains. However, other CAR formats exist that comprise alternate costimulatory domains, including 4-1BB (53–56). The stimulation of CAR T cells by SEB is mediated through their TCR (of certain well-defined Vβ subtypes), which would likely occur irrespective of the CAR, but it is possible that the subsequent activity of the CAR T cells may vary depending on the nature of the CAR. From the literature, T cells expressing CD28-ζ CARs have more rapid expansion and higher peak immunokinetics, while those expressing 4-1BB-ζ CARs preferentially express memory-associated genes and have more sustained antitumor activity (57). It would be of interest to investigate other CAR formats and antigen specificities in future studies, although the current study using a CD28-ζ CAR, similar in format to that used in a large proportion of clinical trials, serves as proof of a promising principle.
Materials and Methods
CAR T cells were derived from spleens of C57BL/6-CAR mice, which express a CAR (anti–Her2-CD28-CD3ζ) driven by the vav promoter (28). C57BL/6-pMEL mice express a transgenic Vβ13 TCR specific for human gp100 (58). Human T cells were derived using normal donor human buffy coats provided by the Australian Red Cross Blood Service. Transduction of T cells using a Her2-specific CAR retroviral vector or control empty vector was carried out as previously described (59). Full details of materials and methods are available in SI Appendix and the figure legends.
Supplementary Material
Acknowledgments
This work was supported by grants from Cure Cancer Australia (1100199), the Peter MacCallum Cancer Center Foundation, the National Health and Medical Research Council (NHMRC) of Australia (1103352 and 1132373), the National Breast Cancer Foundation (NBCF) of Australia (IIRS-18-064), and the Susan G. Komen Breast Cancer Foundation (16376637). P.K.D. and M.H.K. were supported by NHMRC Senior Research Fellowships. C.Y.S. was supported by a Postdoctoral Fellowship from the NBCF.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. E.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1904618116/-/DCSupplemental.
References
- 1.Kochenderfer J. N., et al. , Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J. Clin. Oncol. 35, 1803–1813 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park J. H., et al. , Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 378, 449–459 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster S. J., et al. , Chimeric antigen receptor T cells in refractory B-cell lymphomas. N. Engl. J. Med. 377, 2545–2554 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turtle C. J., et al. , Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J. Clin. Oncol. 35, 3010–3020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beatty G L., et al. , Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology 155, 29–32 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed N., et al. , Human epidermal growth factor receptor 2 (HER2) -specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J. Clin. Oncol. 33, 1688–1696 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kershaw M. H., et al. , A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 12, 6106–6115 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thistlethwaite F. C., et al. , The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol. Immunother. 66, 1425–1436 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grupp S. A., et al. , Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 368, 1509–1518 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller K. T., et al. , Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood 130, 2317–2325 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brentjens R. J., et al. , CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 5, 177ra38 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kochenderfer J. N., et al. , Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood 122, 4129–4139 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garzon-Muvdi T., et al. , Dendritic cell activation enhances anti-PD-1 mediated immunotherapy against glioblastoma. Oncotarget 9, 20681–20697 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heath W. R., et al. , Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol. Rev. 199, 9–26 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Binnewies M., et al. , Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mempel T. R., Henrickson S. E., Von Andrian U. H., T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Davenport A. J., et al. , Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proc. Natl. Acad. Sci. U.S.A. 115, E2068–E2076 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans D. E., Weinberg A. D., Boosting T cell costimulation in cancer: The possibilities seem endless. Int. Rev. Immunol. 22, 173–194 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Fraser J. D., Proft T., The bacterial superantigen and superantigen-like proteins. Immunol. Rev. 225, 226–243 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Herrmann T., Maryanski J. L., Romero P., Fleischer B., MacDonald H. R., Activation of MHC class I-restricted CD8+ CTL by microbial T cell mitogens. Dependence upon MHC class II expression of the target cells and V beta usage of the responder T cells. J. Immunol. 144, 1181–1186 (1990). [PubMed] [Google Scholar]
- 21.Carlsson R., Fischer H., Sjögren H. O., Binding of staphylococcal enterotoxin A to accessory cells is a requirement for its ability to activate human T cells. J. Immunol. 140, 2484–2488 (1988). [PubMed] [Google Scholar]
- 22.Yoon S., et al. , Analysis of the in vivo dendritic cell response to the bacterial superantigen staphylococcal enterotoxin B in the mouse spleen. Histol. Histopathol. 16, 1149–1159 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Dickgreber N., et al. , Targeting antigen to MHC class II molecules promotes efficient cross-presentation and enhances immunotherapy. J. Immunol. 182, 1260–1269 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Miethke T., Heeg K., Wahl C., Wagner H., Crosslinked staphylococcal enterotoxin B stimulates CD8+ T cells only in the presence of unlinked costimulator signals. Immunobiology 183, 433–450 (1991). [DOI] [PubMed] [Google Scholar]
- 25.Miethke T., et al. , T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: Critical role of tumor necrosis factor. J. Exp. Med. 175, 91–98 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slaney C. Y., et al. , Dual-specific chimeric antigen receptor T cells and an indirect vaccine eradicate a variety of large solid tumors in an immunocompetent, self-antigen setting. Clin. Cancer Res. 23, 2478–2490 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radcliff F. J., et al. , Antigen targeting to major histocompatibility complex class II with streptococcal mitogenic exotoxin Z-2 M1, a superantigen-based vaccine carrier. Clin. Vaccine Immunol. 19, 574–586 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yong C. S., et al. , Expression of a chimeric antigen receptor in multiple leukocyte lineages in transgenic mice. PLoS One 10, e0140543 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pino S., et al. , Development of novel major histocompatibility complex class I and class II-deficient NOD-SCID IL2R gamma chain knockout mice for modeling human xenogeneic graft-versus-host disease. Methods Mol. Biol. 602, 105–117 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hewitt C. R., et al. , Major histocompatibility complex independent clonal T cell anergy by direct interaction of Staphylococcus aureus enterotoxin B with the T cell antigen receptor. J. Exp. Med. 175, 1493–1499 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kershaw M. H., Westwood J. A., Darcy P. K., Gene-engineered T cells for cancer therapy. Nat. Rev. Cancer 13, 525–541 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Köhl U., Arsenieva S., Holzinger A., Abken H., CAR T cells in trials: Recent achievements and challenges that remain in the production of modified T cells for clinical applications. Hum. Gene Ther. 29, 559–568 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Gross G., Waks T., Eshhar Z., Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. U.S.A. 86, 10024–10028 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurton L. V., et al. , Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc. Natl. Acad. Sci. U.S.A. 113, E7788–E7797 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gopalakrishnan V., et al. , Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matson V., et al. , The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Routy B., et al. , Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018). [DOI] [PubMed] [Google Scholar]
- 38.van Kooten C., Banchereau J., CD40-CD40 ligand. J. Leukoc. Biol. 67, 2–17 (2000). [DOI] [PubMed] [Google Scholar]
- 39.Stumbles P. A., et al. , Cutting edge: Tumor-specific CTL are constitutively cross-armed in draining lymph nodes and transiently disseminate to mediate tumor regression following systemic CD40 activation. J. Immunol. 173, 5923–5928 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Winograd R., et al. , Induction of T-cell immunity overcomes complete resistance to PD-1 and CTLA-4 blockade and improves survival in pancreatic carcinoma. Cancer Immunol. Res. 3, 399–411 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bindea G., et al. , Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39, 782–795 (2013). [DOI] [PubMed] [Google Scholar]
- 42.van Mierlo G. J., et al. , CD40 stimulation leads to effective therapy of CD40(-) tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proc. Natl. Acad. Sci. U.S.A. 99, 5561–5566 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mangsbo S. M., et al. , The human agonistic CD40 antibody ADC-1013 eradicates bladder tumors and generates T-cell-dependent tumor immunity. Clin. Cancer Res. 21, 1115–1126 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Kedl R. M., et al. , CD40 stimulation accelerates deletion of tumor-specific CD8(+) T cells in the absence of tumor-antigen vaccination. Proc. Natl. Acad. Sci. U.S.A. 98, 10811–10816 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Byrne K. T., Vonderheide R. H., CD40 stimulation obviates innate sensors and drives T cell immunity in cancer. Cell Rep. 15, 2719–2732 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Honeychurch J., Glennie M. J., Johnson P. W., Illidge T. M., Anti-CD40 monoclonal antibody therapy in combination with irradiation results in a CD8 T-cell-dependent immunity to B-cell lymphoma. Blood 102, 1449–1457 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Vonderheide R. H., CD40 agonist antibodies in cancer immunotherapy. Annu Rev Med., 10.1146/annurev-med-062518-045435 (14 August 2019). [DOI] [PubMed] [Google Scholar]
- 48.Lanzavecchia A., Immunology. Licence to kill. Nature 393, 413–414 (1998). [DOI] [PubMed] [Google Scholar]
- 49.Ugel S., et al. , In vivo administration of artificial antigen-presenting cells activates low-avidity T cells for treatment of cancer. Cancer Res. 69, 9376–9384 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neal L. R., et al. , The basics of artificial antigen presenting cells in T cell-based cancer immunotherapies. J. Immunol. Res. Ther. 2, 68–79 (2017). [PMC free article] [PubMed] [Google Scholar]
- 51.Turtle C. J., Riddell S. R., Artificial antigen-presenting cells for use in adoptive immunotherapy. Cancer J. 16, 374–381 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kagoya Y., et al. , Transient stimulation expands superior antitumor T cells for adoptive therapy. JCI Insight 2, e89580 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weinkove R., George P., Dasyam N., McLellan A. D., Selecting costimulatory domains for chimeric antigen receptors: Functional and clinical considerations. Clin. Transl. Immunology 8, e1049 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zapata J. M., et al. , CD137 (4-1BB) signalosome: Complexity is a matter of TRAFs. Front. Immunol. 9, 2618 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Stegen S. J., Hamieh M., Sadelain M., The pharmacology of second-generation chimeric antigen receptors. Nat. Rev. Drug Discov. 14, 499–509 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campana D., Schwarz H., Imai C., 4-1BB chimeric antigen receptors. Cancer J. 20, 134–140 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Salter A. I., et al. , Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function. Sci. Signal. 11, eaat6753 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Overwijk W. W., et al. , Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J. Exp. Med. 198, 569–580 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ritchie D. S., et al. , Persistence and efficacy of second generation CAR T cell against the LeY antigen in acute myeloid leukemia. Mol. Ther. 21, 2122–2129 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slaney C. Y., Toker A., Fraser J. D., Harper J. L., Bäckström B. T., A modified superantigen rescues Ly6G- CD11b+ blood monocyte suppressor function and suppresses antigen-specific inflammation in EAE. Autoimmunity 46, 269–278 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.