Abstract
The apoptotic protease activating factor 1 (APAF1) gene encodes a cytoplasmic protein that initiates apoptosis and is a crucial factor in the mitochondria-dependent death pathway. APAF1 is implicated in many pathways such as apoptosis, neurodegenerative diseases, and cancer. The purpose of this study was to predict deleterious/damaging SNPs in the APAF1 gene viain silicoanalysis. To this end, APAF1 missense SNPs were obtained from the NCBI dbSNP database. In silico analysis of the missense SNPs was carried out by using publicly available online software tools. The stabilization and three-dimensional modeling of mutant proteins were also determined by using the I-Mutant 2.0 and Project HOPE webservers, respectively. In total, 772 missense SNPs were found in the APAF1 gene from the NCBI dbSNP database, 18 SNPs of which were demonstrated to be deleterious or damaging. Of those, 13 SNPs had a decreasing effect on protein stability, while the other 5 SNPs had an increasing effect. Based on the modeling results, some dissimilarities of mutant type amino acids from wild-type amino acids such as size, charge, and hydrophobicity were revealed. The SNPs predicted to be deleterious in this study might be used in the selection of target SNPs for genotyping in disease association studies. Therefore, we could suggest that the present study could pave the way for future experimental studies.
Keywords: APAF1, apoptosis, neurodegenerative diseases, single nucleotide polymorphism (SNP), in silico
1. Introduction
Apoptosis is the programmed cell death process that is required for tissue homeostasis and development. It elicits a cellular response to stress and pathogens as well. The disruption of the apoptosis process can lead to human diseases such as cancer, neurodegenerative disorders, heart disease, autoimmunity, and immunodeficiencies (u1d6a). After Bax activation, cytochrome c is released from the mitochondrial intermembrane space. Cytochrome c and ATP/dATP then interact with APAF1, resulting in the formation of apoptosome, which binds and activates procaspase-9, which in turn activates caspase 3, thereby leading cells into apoptosis (Li et al., 1997; u1d6d; u1d6e). APAF1 plays a key role in the intrinsic or mitochondrial pathway of apoptosis. Therefore, APAF1 is related to regulation and function in cell death (Shakeri et al., 2017). The APAF1 gene is implicated in various pathophysiological pathways such as platinum drug resistance, apoptosis, and neurodegenerative diseases—e.g., Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), Parkinson’s disease, Huntington’s disease, legionellosis, tuberculosis, hepatitis B, and cancer (https://www.genome.jp/).
APAF1 protein contains 3 functional regions that are involved in protein–protein interactions. The caspase recruitment domain (CARD) is located at the N-terminus of the protein, and it is very important for the interaction of APAF1 with caspase-9 (Riedl and Salvesen, 2007). CARD is followed by a nucleotide-binding and oligomerization domain (NOD, also known as NB-ARC). NB-ARC is an ATPase domain that causes a conformational change in APAF1 during apoptosis (Bratton and Salvesen, 2010). WD repeats domains, located at the C-terminus of the protein, are responsible for cytochrome c binding (Reed et al., 2004; Riedl and Salvesen, 2007).
There are various functional classes of single nucleotide polymorphisms (SNPs) such as 3’ splice site, 3’ UTR (untranslated region), 5’ splice site, 5’ UTR, coding synonymous, frameshift, intron, missense, nonsense, and gained. Among these, missense SNPs cause amino acid substitutions. The effect of amino acid substitutions on protein structure and function is critical for understanding the complex mechanisms of human diseases caused by single amino acid mutations (Cargill et al., 1999; Teng et al., 2010). SNPs are known biomarkers for predicting disease susceptibility. Studies on SNPs in candidate genes associated with diseases have witnessed a remarkable increase in recent years. The association of SNPs in the APAF1 gene with diseases has also been investigated recently (Ester et al., 2007; Enjuanes et al., 2008; u1d71; u1d72; u1d73). According to published research studies, the damaging effects of missense SNPs in the APAF1 gene have not yet been studied using in silico methods.
In this study, we aimed to detect missense SNPs in the APAF1 gene to investigate the possible effects of SNPs on the physicochemical properties of the amino acid residues of APAF1 (such as size, charge, hydrophobicity, structure, domain, and conservation), as well as the APAF1 protein structure, by using bioinformatics methods.
2. Materials and methods
2.1. Data mining
The NCBI dbSNP database was used to access the SNPs in the APAF1 gene (NCBI Gene ID: 317) in July 2018 https://www.ncbi.nlm.nih.gov/snp. Missense SNPs cause changes in the amino acid sequence of the corresponding gene and may have an impact on the function of the given protein. For this reason, the missense SNPs in the APAF1 gene were selected for analysis. The primary sequence of the protein (Uniprot accession number: O14727) encoded by the APAF1 gene was obtained from the UniProt database u1fdb.
2.2 Bioinformatics data analysis
Online software tools were used to determine the effects of APAF1 SNPs on the APAF1 protein through the use of three-dimensional models of mutant proteins (Figure 1) (u1d74; u1d75; u1d76; u1d77; u1d78). The effects of the missense SNPs in the APAF1 gene were assessed using SIFT (Sorting Intolerant from Tolerant), PolyPhen-2 (Polymorphism Phenotyping v2), SNPs & GO, PROVEAN (Protein Variation Effect Analyzer), and PANTHER online software tools. SIFT estimates the effects of an amino acid replacement on the function of a protein based on the sequence similarity and physical features of amino acids u1fdd. PolyPhen-2 is a software tool that predicts the effects of an amino acid replacement on the structure and function of a given protein based on physical and comparative properties u1fdf. SNPs & GO predicts whether a single nucleotide polymorphism is disease-related using protein functional annotation u1fe1. PROVEAN is a software tool that estimates the possible effect of an amino acid replacement on the protein function u1fe3. PANTHER estimates the potential effect of a missense SNP on the function of a given protein u1fe5. The SNPs predicted to be deleterious by all of the software tools used were selected for further analysis. The effect of these SNPs on the protein stabilization was determined via I-Mutant 2.0 u1fe7. Three-dimensional modeling of mutant proteins was performed with the Project HOPE software tool u1fe9
Figure 1.

Usage of online software tools in SNP analyses (modified from Marwa Mohamed Osman et al., 2016).
2.3 Gene–gene interactions
Additionally, gene–gene networks including known and predicted gene interactions from different sources such as gene coexpression, colocalization, and shared protein domains were investigated by using the GeneMANIA software tool u1fec.
3. Results
3.1. Gene–gene interactions
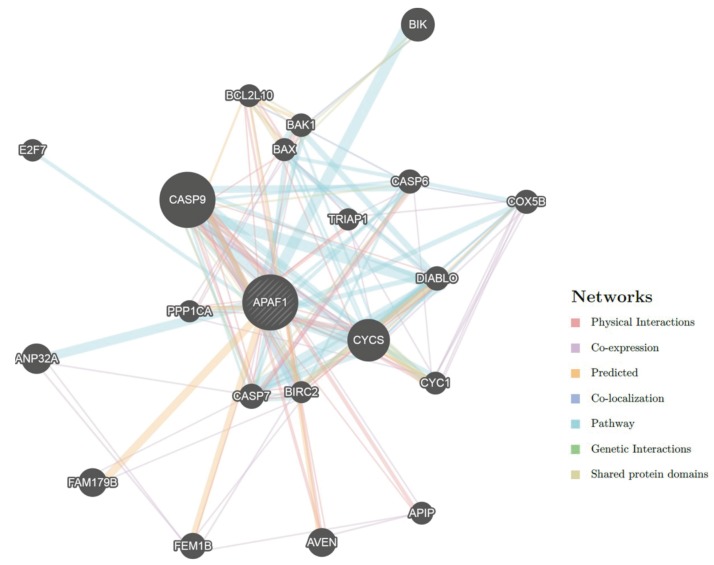
The gene–gene interaction network of the APAF1 gene is shown in Figure 2. The APAF1 gene is linked to 20 genes in a highly interconnected network, with 170 edges10.
Figure 2.
Gene–gene interactions of APAF1.
3.2. Prediction of the effect of APAF1 SNPs on APAF1 protein structure
According to the data obtained from the NCBI dbSNP database, there were a total of 18084 SNPs in the APAF1 gene. Of those, 772 SNPs were found to be missense. First, 772 missense SNPs were submitted to the SIFT software tool; 38 SNPs were found to be deleterious (Figure 3). These 38 SNPs were then submitted to the PolyPhen-2 software tool; 12 SNPs were detected as possibly damaging, while the other 26 SNPs were found to be probably damaging (Figure 3). The 38 SNPs determined by the SIFT software tool to be deleterious were uploaded to the PROVEAN, SNPs & GO, and PANTHER software tools, respectively. We obtained 26 SNPs that were determined by PROVEAN to have no effect on protein stability (Figure 3). According to the SNPs & GO results, 24 SNPs were found to be associated with different diseases, although the other 14 SNPs were not predicted to have any effect (Figure 3). Moreover, 30 SNPs were predicted as probably damaging, 3 SNPs were predicted as possibly damaging, and 5 SNPs were found to be probably benign via the PANTHER software tool (Figure 3). Finally, 18 SNPs were predicted to be deleterious/damaging or disease-related via all of the online software tools (Table 1). In addition, the I-Mutant 2.0 software tool was used to evaluate the effects of damaging/deleterious SNPs on protein stabilization. The results showed that 13 SNPs have a decreasing effect on the stability of APAF1, while the other 5 SNPs have an increasing effect (Table 1).
Figure 3.
Deleterious or damaging SNPs predicted by online software tools.
Table 1.
Results of SIFT, POLYPHEN-2, PROVEAN, SNPs & GO, PANTHER, and I- MUTANT software tools.
| SNP ID | Nucleotide Change | Amino Acid Change | SIFT Result | SIFT Score | POLYPHEN-2 Result | POLYPHEN-2 Score | PROVEAN Result | PROVEAN Score | SNPs & GO | PANTHER Result | I-Mutant Result | I-Mutant RI |
| rs11613534 | A/C (FWD) | A580D | Deleterious | 0 | Probably damaging | 1.000 | Deleterious | –4.46 | Disease | Probably damaging | Decrease | 6 |
| rs61758871 | A/C (FWD) | Y619S | Deleterious | 0.025 | Probably damaging | 0.997 | Deleterious | –3.57 | Disease | Possibly damaging | Decrease | 6 |
| rs143963802 | C/T (FWD) | A494V | Deleterious | 0 | Probably damaging | 1.000 | Deleterious | –3.75 | Disease | Probably damaging | Increase | 2 |
| rs144721573 | A/G (FWD) | D443N | Deleterious | 0 | Probably damaging | 1.000 | Deleterious | –3.17 | Disease | Probably damaging | Decrease | 6 |
| rs144787055 | G/T (FWD) | D170E | Deleterious | 0.013 | Possibly damaging | 0.892 | Deleterious | –2.61 | Disease | Probably damaging | Increase | 5 |
| rs145807191 | C/T (FWD) | S860L | Deleterious | 0.004 | Possibly damaging | 0.954 | Deleterious | –3.49 | Disease | Probably damaging | Decrease | 3 |
| rs146424586 | A/G (FWD) | H521R | Deleterious | 0.024 | Probably damaging | 1.000 | Deleterious | –3.93 | Disease | Probably damaging | Decrease | 4 |
| rs147932236 | C/T (FWD) | S161F | Deleterious | 0.02 | Probably damaging | 0.999 | Deleterious | –4.54 | Disease | Probably damaging | Increase | 3 |
| rs150457288 | A/G (FWD) | Y24C | Deleterious | 0 | Probably damaging | 1.000 | Deleterious | –4.42 | Disease | Probably damaging | Increase | 3 |
| rs200830441 | A/G (FWD) | H183R | Deleterious | 0.009 | Probably damaging | 0.999 | Deleterious | –3.83 | Disease | Probably damaging | Decrease | 3 |
| rs201122276 | A/G (FWD) | H1002R | Deleterious | 0.024 | Probably damaging | 0.997 | Deleterious | –5.58 | Disease | Probably damaging | Decrease | 4 |
| rs201911170 | A/G (FWD) | G951E | Deleterious | 0.005 | Probably damaging | 0.999 | Deleterious | –5.62 | Disease | Probably damaging | Decrease | 0 |
| rs202243082 | A/C (FWD) | P559T | Deleterious | 0.011 | Probably damaging | 1000 | Deleterious | –5.44 | Disease | Probably damaging | Decrease | 8 |
| rs371118366 | C/T (FWD) | C401R | Deleterious | 0.027 | Probably damaging | 1000 | Deleterious | –2.69 | Disease | Probably damaging | Increase | 0 |
| rs371897242 | C/T (FWD) | R612C | Deleterious | 0.001 | Probably damaging | 1000 | Deleterious | –4.79 | Disease | Probably damaging | Decrease | 5 |
| rs372400613 | A/T (FWD) | F544I | Deleterious | 0.007 | Probably damaging | 1.000 | Deleterious | –4.08 | Disease | Probably damaging | Decrease | 7 |
| rs373209321 | A/G (FWD) | D479G | Deleterious | 0 | Possibly damaging | 0.932 | Deleterious | –2.57 | Disease | Probably damaging | Decrease | 5 |
| rs376175905 | A/T (FWD) | F547I | Deleterious | 0 | Probably damaging | 1000 | Deleterious | –4.35 | Disease | Probably damaging | Decrease | 6 |
PolyPhen-2 score ≥0.5 = probably/possibly damaging; SIFT score ≤0.05 = deleterious , >0.05 = tolerated.
3.3. Modeling of amino acid substitutions caused by SNPs
Amino acids have physicochemical properties such as hydrophobicity value, charge, and size. Project HOPE showed that some of these features differed between wild-type and mutant-type amino acids, as shown in Table 2. In addition, 3-D APAF1 protein models generated based on the presence of APAF1 SNPs are given in Table 3. Further information obtained from the Project HOPE software on the structure, domain, and conservation of 18 deleterious SNPs is given in Table 4.
Table 2.
Results of wild-type and mutant-type amino acid properties obtained from Project Hope software.
| SNP ID | AMINO ACID CHANGE | WILD-TYPE AMINO ACIDS | MUTANT TYPE AMINO ACIDS | ||||
|---|---|---|---|---|---|---|---|
| Size | Charge | Hydrophobicity | Size | Charge | Hydrophobicity | ||
| rs11613534 | A580D | < | neutral | > | > | – charge | < |
| rs61758871 | Y619S | > | < | ||||
| rs143963802 | A494V | < | > | ||||
| rs144721573 | D443N | – charge | neutral | ||||
| rs144787055 | D170E | < | > | ||||
| rs145807191 | S860L | < | < | > | > | ||
| rs146424586 | H521R | < | neutral | > | + charge | ||
| rs147932236 | S161F | < | < | > | > | ||
| rs150457288 | Y24C | > | < | < | > | ||
| rs200830441 | H183R | < | neutral | > | + charge | ||
| rs201122276 | H1002R | < | neutral | > | + charge | ||
| rs201911170 | G951E | < | neutral | > | > | – charge | < |
| rs202243082 | P559T | > | < | ||||
| rs371118366 | C401R | < | neutral | > | > | + charge | < |
| rs371897242 | R612C | > | + charge | < | < | neutral | > |
| rs372400613 | F544I | > | < | ||||
| rs373209321 | D479G | > | – charge | < | < | neutral | > |
| rs376175905 | F547I | > | < | ||||
Table 4.
Results of the 3D models of the APAF1 protein via Project Hope.
Table 5.
Effects of amino acid changes on APAF1 protein from Project Hope.
| SNP ID | Amino acid change | Structure | Domain | Conservation |
| rs11613534 | A580D | Wild-type amino acid is found in an α-helix opposite the mutant amino acid. | Wild-type amino acid is embedded in the core of a domain, and the mutation can disrupt its structure. | The amino acid is 100% conserved in this location. Thus, the mutation is generally harmful to the protein. |
| rs61758871 | Y619S | The mutant residue is found in WD 1-1 repeated residues. | The domain in which the amino acid at this position is located has an effect on binding to other molecules and interacts with a domain that is also involved in binding. The mutation of this position can disrupt these interactions and the function ofthe protein. | The wild-type amino acid is very conserved; however, another amino acid type may be found at this location. Thus, the mutation is likely not harmful to the protein. |
| rs143963802 | A494V | Wild-type amino acid is found in an α-helix opposite to mutant amino acid. | The amino acid is embedded in the core of a domain which can be disrupted due to mutation at this position. | The amino acid is 100% conserved in this location. Thus, the mutation is generally harmful to the protein. |
| rs144721573 | D443N | The amino acid is embedded in the core of a domain which can be disrupted due to mutation at this position. | The amino acid is 100% conserved in this location. Thus, the mutation is generally harmful to the protein. | |
| rs144787055 | D170E | The mutation occurs within the NB-ARC (UniProt) domain. | The domain in which the amino acid at this position is located has an effect on binding to other molecules and interacts with a domain that is also involved in binding. The mutation of this position can disrupt these interactions and the function ofthe protein. | The wild-type amino acid is very conserved; however, another amino acid type may be found at this location. Thus, the mutation is likely not harmful to the protein. |
| rs145807191 | S860L | The mutant residue is found in WD 1-6 repeated residues. | The domain in which the amino acid at this position is located has an effect on binding to other molecules and interacts with a domain that is also involved in binding. The mutation of this position can disrupt these interactions and the function ofthe protein. | The amino acid is 100% conserved in this location. Thus, the mutation is generally harmful to the protein. |
| rs146424586 | H521R | The amino acid at this position is situated on the surface of a domain that has unknown function. | The wild-type amino acid is very conserved; however, another amino acid type may be found at this location. Thus, the mutation is likely not harmful to the protein. | |
| rs147932236 | S161F | The mutation occurs within the NB-ARC (UniProt) domain. | The domain in which the amino acid at this position is located has an effect on binding to other molecules and interacts with a domain that is also involved in binding. The mutation of this position can disrupt these interactions and the function ofthe protein. | The wild-type amino acid is very conserved; however, another amino acid type may be found at this location. Thus, the mutation is likely not harmful to the protein. |
| rs150457288 | Y24C | The mutation occurs within the CARD (UniProt) domain. | The amino acid is embedded in the core of a domain which can be disrupted due to mutation at this position. | The wild-type amino acid is very conserved; however, another amino acid type may be found at this location. Thus, the mutation is likely not harmful to the protein. |
| rs200830441 | H183R | The mutation occurs within the NB-ARC (UniProt) domain. | The domain in which the amino acid at this position is located has an effect on binding to other molecules and interacts with a domain that is also involved in binding. The mutation of this position can disrupt these interactions and the function ofthe protein. | The amino acid is 100% conserved in this location. Thus, the mutation is generally harmful to the protein. |
| rs201122276 | H1002R | The mutant residue is found in WD 2-3 repeated residues. | The domain in which the amino acid at this position is located has an effect on binding to other molecules and interacts with a domain that is also involved in binding. The mutation of this position can disrupt these interactions and the function ofthe protein. | The wild-type amino acid is very conserved; however, another amino acid type may be found at this location. Thus, the mutation is likely not harmful to the protein. |
| rs201911170 | G951E | The mutant residue is found in WD 2-1 repeated residues. Mutation of glycine can damage the function of protein becauseglycine is the most flexible amino acid. | The domain in which the amino acid at this position is located has an effect on binding to other molecules and interacts with a domain that is also involved in binding. The mutation of this position can disrupt these interactions and the function ofthe protein. | The amino acid is 100% conserved in this location. Thus, the mutation is generally harmful to the protein. |
| rs202243082 | P559T | The wild-type amino acid is proline, which is known to be very inelastic. The mutation of proline can disrupt the conformation of the protein in this position. | The amino acid at this position is situated on the surface of a domain that has unknown function. | The amino acid is 100% conserved in this location. Thus, the mutation is generally harmful to the protein. |
| rs371118366 | C401R | The mutation occurs within the NB-ARC (UniProt) domain. | The domain in which the amino acid at this position is located has an effect on binding to other molecules and interacts with a domain that is also involved in binding. The mutation of this position can disrupt these interactions and the function of the protein. | The mutation can be harmful to the protein because the amino acid is located near a substantially conserved position. |
| rs371897242 | R612C | The domain in which the amino acid at this position is located has an effect on binding to other molecules and interacts with a domain that is also involved in binding. The mutation of this position can disrupt these interactions and the function of the protein. | The mutant residue is located near a substantially conserved position but is probably not harmful for the protein due to some homolog sequences. | |
| rs372400613 | F544I | The amino acid is embedded in the core of a domain which can be disrupted due to mutation at this position. | The amino acid is 100% conserved in this location. Thus, the mutation is generally harmful to the protein. | |
| rs373209321 | D479G | The mutant amino acid is glycine, which is known to be very elastic. Therefore, the mutation can disrupt the rigidity of the protein at this position. | The amino acid is embedded in the core of a domain which can be disrupted due to mutation at this position. | The wild-type amino acid is frequently located at this position but another type of amino acid can be found here, too. However, the mutation is likely not harmful to the protein. |
| rs376175905 | F547I | The amino acid at this position is situated on the surface of a domain which has unknown function. | The amino acid is 100% conserved in this location. Thus, the mutation is generally harmful to the protein. | |
| rs147932236 | S161F | The mutation occurs within the NB-ARC (UniProt) domain. | The domain in which the amino acid at this position is located has an effect on binding to other molecules and interacts with a domain that is also involved in binding. The mutation of this position can disrupt these interactions and the function of the protein. | The wild-type amino acid is very conserved; however, another amino acid type may be found at this location. Thus, the mutation is likely not harmful to the protein. |
| rs150457288 | Y24C | The mutation occurs within the CARD (UniProt) domain. | The amino acid is embedded in the core of a domain which can be disrupted due to mutation at this position. | The wild-type amino acid is very conserved; however, another amino acid type may be found at this location. Thus, the mutation is likely not harmful to the protein. |
| rs200830441 | H183R | The mutation occurs within the NB-ARC (UniProt) domain. | The domain in which the amino acid at this position is located has an effect on binding to other molecules and interacts with a domain that is also involved in binding. The mutation of this position can disrupt these interactions and the function of the protein. | The amino acid is 100% conserved in this location. Thus, the mutation is generally harmful to the protein. |
| rs201122276 | H1002R | The mutant residue is found in WD 2-3 repeated residues. | The domain in which the amino acid at this position is located has an effect on binding to other molecules and interacts with a domain that is also involved in binding. The mutation of this position can disrupt these interactions and the function of the protein. | The wild-type amino acid is very conserved; however, another amino acid type may be found at this location. Thus, the mutation is likely not harmful to the protein. |
| rs201911170 | G951E | The mutant residue is found in WD 2-1 repeated residues. Mutation of glycine can damage the function of protein because glycine is the most flexible amino acid. | The domain in which the amino acid at this position is located has an effect on binding to other molecules and interacts with a domain that is also involved in binding. The mutation of this position can disrupt these interactions and the function of the protein. | The amino acid is 100% conserved in this location. Thus, the mutation is generally harmful to the protein. |
| rs202243082 | P559T | The wild-type amino acid is proline, which is known to be very inelastic. The mutation of proline can disrupt the conformation of the protein in this position. | The amino acid at this position is situated on the surface of a domain that has unknown function. | The amino acid is 100% conserved in this location. Thus, the mutation is generally harmful to the protein. |
| rs371118366 | C401R | The mutation occurs within the NB-ARC (UniProt) domain. | The domain in which the amino acid at this position is located has an effect on binding to other molecules and interacts with a domain that is also involved in binding. The mutation of this position can disrupt these interactions and the function of the protein | The mutation can be harmful to the protein because the amino acid is located near a substantially conserved position. |
| rs371897242 | R612C | The domain in which the aminoacid at this position is located has an effect on binding to other molecules and interacts with a domain that is also involved in binding. The mutation of this position can disrupt these interactions and the function of the protein. | The mutant residue is located near a substantially conserved position but is probably not harmful for the protein due to some homolog sequences. | |
| rs372400613 | F544I | The amino acid is embedded in the core of a domain which can be disrupted due to mutation at this position. The amino acid is 100% conserved in this location. Thus, the mutation is generally harmful to the protein. | ||
| rs373209321 | D479G | The mutant amino acid is glycine, which is known to be very elastic. Therefore, the mutation can disrupt the rigidity of the protein at this position. | The amino acid is embedded in the core of a domain which can be disrupted due to mutation at this position. | The wild-type amino acid is frequently located at this position but another type of amino acid can be found here, too. However, the mutation is likely not harmful to the protein. |
| rs376175905 | F547I | The amino acid at this position is situated on the surface of a domain which has unknown function. | The amino acid is 100% conserved in this location. Thus, the mutation is generally harmful to the protein. |
4. Discussion
Project Hope software results have provided important information about the possible effects of missense SNPs in the APAF1 gene. The APAF1 rs144787055, rs147932236, rs200830441, and rs371118366 polymorphisms resulting in D170E, S161F, H183R, and C401R amino acid substitutions, respectively, were investigated within the NB-ARC domain (Table 4, Figure 4). These substituted amino acids have different physicochemical properties which could possibly disrupt the NB-ARC domain and eliminate its function. In addition, the wild-type aspartic acid residue at position 170 forms a hydrogen bond with glutamine at position 137 and serine at position 172, as well as a salt bridge with glycine at position 105. The SNP rs144787055 results in D170E substitution. The difference in size between the wild type and the substituted residue caused the new amino acid not to be in the correct position to form the original hydrogen bonds (Table 2). Furthermore, the wild-type serine at position 161 is apparently involved in a metal-ion contact in the APAF1 protein structure. An S161F substitution due to rs147932236 could possibly disrupt the metal-ion interactions, because phenylalanine is larger compared to the wild-type amino acid. In contrast to serine, the mutant amino acid could not form a hydrogen bond with aspartic acid at position 243 due to size and hydrophobicity (Table 2). The Project HOPE results showed that the wild-type amino acid serine at position 161 interacted with a ligand, DTP (like deoxyadenosine triphosphate). Mutation of serine to phenylalanine at this position might disturb the protein’s function, because ligand binding is important for the function of proteins. Cysteine at position 401 can form a hydrogen bond with threonine at position 408 (Table 4). The mutation of cysteine into an arginine (C401R) because of rs371118366 could affect hydrogen bond formation due to size and hydrophobicity (Table 2). The SNP rs150457288 resulted in cysteine to tryptophan substitution (Y24C) within the CARD domain (Table 4, Figure 4). The mutation of tyrosine into a cysteine at position 24 could possibly affect the structure and function of the CARD domain. The polymorphisms rs61758871, rs145807191, rs201911170, and rs201122276 were found to result in Y619S, S860L, G951E, and H1002R amino acid substitutions, respectively, within the tryptophan–aspartic acid (WD) 1-1, WD 1-6, WD 2-1, and WD 2-3 repeats, respectively (Table 4, Figure 4). These mutations might have a possible effect on the function of the WD repeats. In addition, the D443N mutation due to rs144721573 showed that the difference in charge between the wild-type and mutant amino acids could result in the disruption of ionic contacts, such as a salt bridge formed by the aspartic acid with lysine at position 348 and lysine at position 391 (Table 2).
Figure 4.
Schematic diagram of the APAF1 proteins and association with deleterious SNPs in APAF1 gene.
According to the results of the GeneMANIA software tool, among the genes that APAF1 interacted most with were CASP9 (caspase 9), CYCS (cytochrome c, somatic), BIK (BCL2 interacting killer), ANP32A (acidic nuclear phosphoprotein 32 family member A), and AVEN (apoptosis and caspase activation inhibitor). Furthermore, GeneMANIA results showed that APAF1, CASP9, and CYCS genes have many important functional roles such as positive regulation of peptidase activity and the intrinsic apoptotic signaling pathway.
Thorough literature research shows that there have been a limited number of studies investigating the association of SNPs in the APAF1 gene with diseases. Zheng et al. (2016) examined the associations of SNPs in apoptotic pathway genes including APAF1 with chronic myeloid leukemia; they reported no significant results for the impact of rs1439123 and rs2288713 on the APAF1 gene (Zheng et al., 2016). Choi et al. (2015) investigated the relationship between the pathology determinants of colorectal cancer and the frameshift mutations of the proapoptotic APAF1, BAX, and FLASH genes. They suggested that the genes might play a synergistic role in tumorigenesis (Choi et al., 2015). Enjuanes et al. (2008) reported that the rs17028658 polymorphism located in the 3’ region of the APAF1 gene was significantly associated with a risk of chronic lymphocytic leukemia (Enjuanes et al., 2008).
5. Conclusions
The present study investigated the influence of functional SNPs associated with the APAF1 gene via in silico methods because APAF1 is related to many diseases such as cancer, neurodegenerative disorders, heart disease, autoimmunity, and immunodeficiencies. In a total of 18,084 SNPs in the APAF1 gene, 772 SNPs were found to be missense. Furthermore, 18 SNPs were found to be deleterious or damaging by several software tools. We believe that our results will lay the foundation for future experimental and in silico studies.
References
- AbdulAzeez S Borgio JF In-silico computing of the most deleterious nsSNPs in HBA1 gene. PLOS One. 2016;11:e0147702–e0147702. doi: 10.1371/journal.pone.0147702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acehan D Jiang X Morgan DG Heuser JE Wang X Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Molecular Cell. 2002;9:423. doi: 10.1016/s1097-2765(02)00442-2. [DOI] [PubMed] [Google Scholar]
- Arshad M Bhatti A John P Identification and in silico analysis of functional SNPs of human TAGAP protein: A comprehensive study. PLOS One. 2018;13:e0188143–e0188143. doi: 10.1371/journal.pone.0188143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A Salvesen G Regulated cell death: signaling and mechanisms. Annual Review of Cell and Developmental Biology. 2014;30:337. doi: 10.1146/annurev-cellbio-100913-013226. [DOI] [PubMed] [Google Scholar]
- Bao Q Lu W Rabinowitz JD Shi Y Calcium blocks formation of apoptosome by preventing nucleotide exchange in Apaf-1. Molecular Cell. 2007;25:181. doi: 10.1016/j.molcel.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Bhatnager R Dang AS Comprehensive in-silico prediction of damage associated SNPs in Human Prolidase gene. Scientific Reports. 2018;8:9430–9430. doi: 10.1038/s41598-018-27789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratton SB Salvesen GS Regulation of the Apaf-1–caspase-9 apoptosome. Journal of Cell Science. 2010;123:3209. doi: 10.1242/jcs.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargill M Altshuler D Ireland J Sklar P Ardlie K Characterization of single-nucleotide polymorphisms in coding regions of human genes. Nature Genetics. 1999;22:231–231. doi: 10.1038/10290. [DOI] [PubMed] [Google Scholar]
- Choi MR Gwak M Yoo NJ Lee SH Regional bias of intratumoral genetic heterogeneity of apoptosis-related genes BAX, APAF1, and FLASH in colon cancers with high microsatellite instability. Digestive Diseases and Sciences. 2015;60:1674. doi: 10.1007/s10620-014-3499-2. [DOI] [PubMed] [Google Scholar]
- Enjuanes A Benavente Y Bosch F Martín-Guerrero I Colomer D Genetic variants in apoptosis and immunoregulation-related genes are associated with risk of chronic lymphocytic leukemia. Cancer Research. 2008;68:10178. doi: 10.1158/0008-5472.CAN-08-2221. [DOI] [PubMed] [Google Scholar]
- Ester AR Tyerman G Wise CA Blanton SH Hecht JT Apoptotic gene analysis in idiopathic talipes equinovarus (clubfoot) Clinical Orthopaedics and Related Research. 2007;462:32. doi: 10.1097/BLO.0b013e318073c2d9. [DOI] [PubMed] [Google Scholar]
- Ferraro E Corvaro M Cecconi F Physiological and pathological roles of Apaf1 and the apoptosome. Journal of Cellular and Molecular Medicine. 2003;7:21. doi: 10.1111/j.1582-4934.2003.tb00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y-T Chen Meng Z-L Ge W-Y Research progress on osteoarthritis treatment mechanisms. Biomedicine & Pharmacotherapy. 2017;93:1246. doi: 10.1016/j.biopha.2017.07.034. [DOI] [PubMed] [Google Scholar]
- Hazra A Chanock S Giovannucci E Cox DG Niu T Large-scale evaluation of genetic variants in candidate genes for colorectal cancer risk in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Cancer Epidemiology and Prevention Biomarkers. 2008;17:311. doi: 10.1158/1055-9965.EPI-07-0195. [DOI] [PubMed] [Google Scholar]
- Li P Nijhawan D Budihardjo I Srinivasula SM Ahmad M Alnemri ES Wang X Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Marwa MO Ahmed SK Alaa EYM Mosab MG In silico analysis of single nucleotide polymorphisms (SNPs) in human FTO gene. JSM Bioinformatics, Genomics and Proteomics. 2016;1:1003–1003. [Google Scholar]
- Ou L Przybilla MJ Whitley CB Phenotype prediction for mucopolysaccharidosis type I by in silico analysis. Orphanet Journal of Rare Diseases. 2017;12:125–125. doi: 10.1186/s13023-017-0678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak A Wenzlaff AS Hyland PL Cote ML Keele GR Apoptosis-related single nucleotide polymorphisms and the risk of non-small cell lung cancer in women. Journal of Cancer Therapeutics & Research. 2014;3:1–1. doi: 10.7243/2049-7962-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira G Da Silva A Do Nascimento S De Mesquita J In silico analysis and molecular dynamics simulation of human superoxide dismutase 3 (SOD3) genetic variants. Journal of Cellular Biochemistry. 2019;120:3583. doi: 10.1002/jcb.27636. [DOI] [PubMed] [Google Scholar]
- Reed JC Doctor KS Godzik A The domains of apoptosis: a genomics perspective. Science Signaling. 2004;239:re9–re9. doi: 10.1126/stke.2392004re9. [DOI] [PubMed] [Google Scholar]
- Riedl SJ Salvesen GS The apoptosome: signalling platform of cell death. Nature reviews Molecular Cell Biology. 2007;8:405–405. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- Shakeri R Kheirollahi A Davoodi J Apaf-1: Regulation and function in cell death. Biochimie. 2017;135:111. doi: 10.1016/j.biochi.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Teng S Wang L Srivastava AK Schwartz CE Alexov E Structural assessment of the effects of amino acid substitutions on protein stability and protein-protein interaction. International Journal of Computational Biology and Drug Design. 2010;3:334–334. doi: 10.1504/IJCBDD.2010.038396. [DOI] [PMC free article] [PubMed] [Google Scholar]