Abstract
Background
In this study, we aimed to investigate the risk factors contributing to secondary vertebral compression fractures (SVCF) in patients undergoing percutaneous vertebroplasty (PVP) or kyphoplasty (PKP) due to osteoporotic vertebral compression fracture (OVCF).
Material/Methods
Between January 2010 and December 2017, 650 patients with regular follow-up were identified and retrospectively analyzed in this study. Of these patients, 410 patients underwent PVP and 240 patients underwent PKP surgery. Patients were followed for 24 months on average, ranging from 6 months to 36 months follow-up. Possible risk factors screened for were age, gender, regional distribution, outdoor activity (ODA), bone mineral density (BMD), surgical methods (unilateral or bilateral), bone cement dose, bone cement leakage, chronic disease history, postoperative anti-osteoporosis treatment, and level of preoperative OVCF. Logistic regression analysis was applied to determine potential risk factors.
Results
As a result, 102 patients (15.7%) suffered SVCF after PVP/PKP surgery at the last follow-up. Binary logistic regression model showed that older age increased the risk of developing SVCF [odds ratio (OR)=2.48, P=0.031] while high-level BMD (OR=0.31, P<0.001) and ODA (OR=0.38, P=0.001) decreased the risk. Binary logistic regression model showed the following: Logit (P)=1.03+0.91X1–1.18X2–0.97X3 (X1=age, OR=2.48, P=0.031; X2=BMD, OR=0.31, P<0.001; X3=ODA, OR=0.38, P=0.001).
Conclusions
In conclusion, older age and lower BMD were identified as risk factors of SVCF for OVCF patients following PVP/PKP surgery, whereas more ODA played a protective role in SVCF development.
MeSH Keywords: Fractures, Compression; Kyphoplasty; Osteoporosis; Risk Factors; Vertebroplasty
Background
Nowadays, we have to pay more attention to osteoporotic vertebral compression fracture (OVCF) which has become a severe medical issue as the global population has increasingly aged [1]. To our knowledge, OVCF is a common disorder which particularly affects elderly patients. Usually, OVCF can reduce life quality of the aged patients by causing long-time back pain, impairing their mobility, and thus influencing their daily activities [2]. Hence, research is urgently needed to seek methods to prevent osteoporosis, as well as OVCF [3,4].
Over the past years, we have frequently applied percutaneous vertebroplasty (PVP) and kyphoplasty (PKP) in clinical situation and have cured many patients who suffered from OVCF [5–9]. However, secondary vertebral compression fractures (SVCF) have been linked to PVP/PKP surgery for patients with OVCF [7,10–17]. It has been reported that long-time menopause and preoperative multi-level vertebral fractures were likely to increase the risk that the patients would suffer from SVCF after PVP surgery, while high bone mineral density (BMD) likely decreased that risk [7]. Bae et al. [16] indicated that SVCF that followed PVP surgery was associated with poor bone mineral content and poor distribution of bone cement. Besides, a higher incidence of SVCF may be caused by bone cement leakage into the disc, as compared to non-leakage. Similarly, another study [15] showed that the SVCF group had higher cement leakage, obviously when the cement volume fraction increased during the PKP surgery. However, others have indicated that PVP might reduce the incidence of SVCF [18]. Farrokhi et al. [10] reported that PVP group had statistically significant improvement in visual analogue scale and life quality scores, and fewer adjacent-level fractures compared with the optimal medical therapy group. Hence, it still remains elusive regarding the relation between the incidence of postoperative SVCF and PVP/PKP surgery.
Herein, we focused on the occurrence of SVCF that followed PVP/PKP surgery. In addition, we tried to investigate and identify the risk contributors which may be relevant to SVCF after PVP/PKP surgery in OVCF patients.
Material and Methods
Ethics statement
This study was approved by Ethics Committee of Orthopaedic Hospital of Xingtai. The approval number is K2018-03-001. And all informed consents of the patients were obtained.
Patients
Between January 2010 and December 2017, 650 OVCF patients underwent PVP surgery (410 patients) or PKP surgery (240 patients) and all had regular follow-up thereafter. All of their medical records were collected, including medical radiographic images, and were retrospectively analyzed in this study. Inclusion criteria were: OVCF patients who underwent PVP or PKP surgery and no trauma or systematic diseases occurred during the follow-up period. All patients returned to our hospital for regular follow-up every 6 months after surgery. Based on the occurrence of SVCF, patients were divided into the following group: the SVCF group and the non-SVCF group.
Evaluation of risk factors
We screened for the following possible risk factors: age, gender, regional distribution (RD), outdoor activity (ODA), surgical methods (unilateral or bilateral), bone cement dose, bone cement leakage (BCL), chronic disease history [including hypertension, heart disease (HD), diabetes mellitus (DM), and chronic obstructive pulmonary disease (COPD)], and postoperative anti-osteoporosis treatment (AOT), bone mineral density (BMD), and QCT measurement <80 mg/cm3 for osteoporosis [19].
Statistical analyses
Statistical analyses were performed using SPSS for Windows, version 18.0 (SPSS IBM, Chicago, IL, USA). Data are presented as the median (interquartile range, IQR) where appropriate. Chi-square tests were used for data analysis. Potential risk factors with P<0.10 were included in binary logistic regression analysis. Statistical significance was identified where P value was less than 0.05 with a 2-tailed test.
Results
Distribution of vertebral fractures
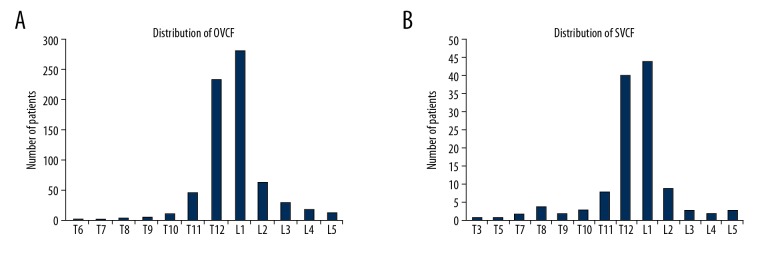
In this study, 650 OVCF patients underwent PVP surgery (410 patients) or PKP surgery (240 patients). There were 526 females and 124 males. The medium age was 73 (from 56 to 85 years). All patients were followed for 24 months on average (range, 6 months to 36 months). Totally, 701 fractured vertebras were identified in 650 OVCF patients. Figure 1A shows OVCF distribution. In addition, Figure 1B shows that 122 fractured vertebras were found in 102 patients (15.7%) who sustained SVCF after PVP/PKP. Notably, most fractures were located in L1 and T12 vertebrae, no matter OVCF or SVCF.
Figure 1.
The distribution of OVCF/SVCF in vertebral bodies. (A) OVCFs before PVP/PKP surgery are shown. (B) SVCFs after surgery are shown. OVCF – osteoporotic vertebral compression fracture; SVCF – secondary vertebral compression fracture; PVP – percutaneous vertebroplasty; PKP – percutaneous kyphoplasty.
Comparison regarding age and gender
As shown in Table 1, the medium age of the SVCF patients (n=102, 15.7%) was 75 years, and age in the non-SVCF group (n=548, 84.3%) was 69 years. It was apparent that more aged patients existed in the SVCF group (P=0.003). As Table 2 is displaying, no statistical difference was found regarding gender comparison between the two groups (P=0.689).
Table 1.
Comparison regarding age.
| Age <60 | 60≤ age <70 | 70≤ age <80 | Age ≥80 | |
|---|---|---|---|---|
| SVCF* (n=102) | 2 | 31 | 63 | 6 |
| Non-SVCF (n=548) | 15 | 266 | 254 | 13 |
P=0.003, compared with non-SVCF group, by chi-squared test.
SVCF – secondary vertebral compression fracture.
Table 2.
Comparison regarding gender.
| Female | Male | |
|---|---|---|
| SVCF* (n=102) | 84 | 18 |
| Non-SVCF (n=548) | 442 | 106 |
P=0.689, compared with non-SVCF group, by chi-squared test.
SVCF – secondary vertebral compression fracture.
Comparisons of BMD and regional distribution
As Table 3 shows, BMD in the SVCF group was lower than BMD in the non-SVCF group (P=0.004). Over half of SVCF patients suffered low BMD level with less than 60 mg/cm3, while only one-third of patients in the non-SVCF group did. As Table 4 shows, comparison of regional distribution found no difference between the SVCF group and the non-SVCF group (P=0.068).
Table 3.
Comparison regarding BMD.
| BMD (mg/cm3) | 60≤ BMD <80 | 40≤ BMD <60 | 20≤ BMD <40 | <20 |
|---|---|---|---|---|
| SVCF* (n=102) | 50 | 38 | 11 | 3 |
| Non-SVCF (n=548) | 364 | 145 | 34 | 5 |
P=0.004, compared with non-SVCF group, by chi-squared test.
SVCF – secondary vertebral compression fracture; BMD – bone mineral density.
Table 4.
Comparison regarding regional distribution.
| Rural area | Urban area | |
|---|---|---|
| SVCF* (n=102) | 50 | 52 |
| Non-SVCF (n=548) | 322 | 226 |
P=0.068, compared with non-SVCF group, by chi-squared test.
SVCF – secondary vertebral compression fracture.
Comparisons of outdoor activity and bone cement leakage
In this study, the patients were asked to give feedback regarding their outdoor activity (ODA) time. Outdoor activity was scored as 3 levels: low level (ODA <0.5 hour per day), medium level (0.5≤ ODA <2 hours per day), and high level (ODA ≥2 hours per day). As shown in Table 5, outdoor activity between the SVCF group and the non-SVCF group showed a significant difference (P<0.001). Most SVCF patients were observed with low-level outdoor activity, compared with the non-SVCF group. As shown in Table 6, there was a significant difference regarding bone cement leakage between the SVCF group and the non-SVCF group (P=0.042). The SVCF patients showed a higher percent of bone cement leakage than the non-SVCF patients.
Table 5.
Comparison regarding outdoor activity (ODA).
| ODA level | SVCF patients* (n=102) | Non-SVCF (n=548) |
|---|---|---|
| High | 15 | 133 |
| Modetare | 23 | 280 |
| Low | 64 | 135 |
P<0.001, compared with non-SVCF group, by chi-squared test.
SVCF – secondary vertebral compression fracture; low level, ODA <0.5 hour/day; medium level, 0.5≤ ODA <2 hour/day; high level, ODA ≥2 hour/day.
Table 6.
Comparison regarding bone cement leakage.
| Bone cement leakage | Yes | No |
|---|---|---|
| SVCF* (n=102) | 8 | 94 |
| Non-SVCF (n=548) | 19 | 529 |
P=0.042, compared with non-SVCF group, by chi-squared test.
SVCF – secondary vertebral compression fracture.
Comparisons of other parameters
As shown in Tables 7 and 8, there was no significant difference regarding anti-osteoporosis treatment or chronic disease history between the SVCF group and the non-SVCF group respectively (both P>0.05). As shown in Table 9, there was no significant difference regarding the level of preoperative OVCF (P=0.752). In addition, no significance was identified regarding surgical methods or bone cement dose (all P>0.05).
Table 7.
Comparison regarding postoperative anti-osteoporosis treatment.
| AOT | Yes | No |
|---|---|---|
| SVCF* (n=102) | 55 | 47 |
| Non-SVCF (n=548) | 347 | 201 |
P=0.073, compared with non-SVCF group, by chi-squared test.
SVCF – secondary vertebral compression fracture; AOT – anti-osteoporosis treatment.
Table 8.
Comparison regarding chronic disease history.
| SVCF patients (n=102) | Non-SVCF (n=548) | χ2-Value | p-Value | |
|---|---|---|---|---|
| HBD | 24 | 98 | 1.798 | 0.18 |
| DM | 16 | 72 | 0.477 | 0.49 |
| HD | 22 | 106 | 0.269 | 0.604 |
| COPD | 9 | 34 | 0.955 | 0.328 |
SVCF – secondary vertebral compression fracture; HBD – high blood pressure; DM – diabetes mellitus; HD – heart disease; COPD – chronic obstructive pulmonary disease.
Table 9.
Comparison regarding the level of preoperative OVCF between SVCF group and non-SVCF group.
| Level of OVCF | 1 | 2 | 3 |
|---|---|---|---|
| SVCF* (n=102) | 93 | 8 | 1 |
| Non-SVCF (n=548) | 510 | 35 | 3 |
P=0.752, compared with non-SVCF group, by chi-squared test.
OVCF – osteoporotic vertebral compression fracture; SVCF – secondary vertebral compression fracture.
Logistic regression analysis
The conditions of regression were set as follows: backward (LR), probability for stepwise (Entry 0.10, Removal 0.15). As Table 10 shows, the binary logistic regression analysis used the following model: Logit(P)=1.03+0.91X1–1.18X2–0.97X3 [X1=age: age <60 (value: 0), 60≤ age <70 (value: 1), 70≤ age <80 (value: 2), age ≥80 (value: 3); X2=BMD: <20 (value: 0), 20≤ BMD <40 (value: 1), 40≤ BMD <60 (value: 2), 60≤ BMD <80 (value: 3); X3=ODA: low level, ODA <0.5 hour/day (value: 0), medium level, 0.5≤ ODA <2 hour/day (value: 1), high level, ODA ≥2 hour/day (value: 2)]. Pearson chi-square test showed that the equation above was statistically significant (P<0.001).
Table 10.
Binary logistic regression analysis for SVCF.
| No. | Items | B | Exp(B) | P-value | 95% CI for Exp(B) |
|---|---|---|---|---|---|
| X1 | Age | 0.91 | 2.48 | 0.031 | (1.16, 3.80) |
| X2 | BMD | −1.18 | 0.31 | <0.001 | (0.06, 0.56) |
| X3 | ODA | −0.97 | 0.38 | 0.001 | (0.11, 0.65) |
| X4 | RD | 0.15 | 1.16 | 0.241 | (0.14, 2.18) |
| X5 | BCL | 0.22 | 1.25 | 0.506 | (0.31, 2.19) |
| X6 | AOT | −0.43 | 0.65 | 0.147 | (0.13, 1.17) |
| X0 | Constant | 1.03 | 2.80 | 0.000 | – |
SVCF – secondary vertebral compression fracture; BMD – bone mineral density; ODA – outdoor activity; RD – regional distribution; BCL – bone cement leakage; AOT – anti-osteoporosis treatment. Logit(P)=1.03+0.91X1–1.18X2–0.97X3 [X1=age: age <60 (value: 0), 60≤ age <70 (value: 1), 70≤ age <80 (value: 2), age ≥80 (value: 3); X2=BMD: <20 (value: 0), 20≤ BMD <40 (value: 1), 40≤ BMD <60 (value: 2), 60≤ BMD <80 (value: 3); X3=ODA: low level, <0.5 hour/day (value: 0), medium level, 0.5≤ ODA <2 hour/day (value: 1), high level, ODA ≥2 hour/day (value: 2)].
Discussion
As we know, lower BMD is a general character of osteoporosis, which may result in a high fracture risk [20,21]. Clinically, OVCF of the vertebrae is usually caused by osteoporosis, leading to low back pain, and even disability in the elderly [22]. Thus, how to treat such patients is very important. Conservative treatment, such as staying in bed and taking pain-killers, has proven to be effective for the relief of low back pain, but it may work for only a few weeks. Invasive surgery can be an option although may not be the best therapeutic option, because the body status of the elderly patients is usually always poor [23]. For the past few years, the minimally invasive methods of PVP and PKP have been widely used to treat OVCF in order to relieve low back pain and correct spinal deformity [1,24–26]. Both PVP and PKP are believed effective and safe to treat OVCF clinically. However, SVCF might occur after PVP and PKP [10–13,27], and it tends to form postoperatively, within 1 month, next to the surgically treated segment [28]. By contrast, some findings have reported the opposite conclusion [1,6,29]; others even have indicated that PVP can reduce the risk of new fractures [18]. To date, there has been a debate on whether PVP/PKP likely increases the incidence of postoperative SVCF, or whether it is only caused by the natural progression of osteoporosis.
In a recent retrospective study of 193 OVCF patients, Bae et al. [16] found that 14.6% OVCF patients sustained SVCF after PVP procedures. Compared with the patients who did not experience SVCF, SVCF patients were identified as having poorer bone mineral content and worse bone cement distribution, which can be predictive factors of SVCF. In our current study, univariable analysis showed that bone cement leakage was a likely risk factor in development of postoperative SVCF following PVP/PKP procedures (P<0.05), but not a risk factor in the logistic regression model (P>0.05). Thus, our findings showed that bone cement leakage was not a risk factor in development of postoperative SVCF following PVP/PKP procedures.
Older age and lower BMD were identified as significant risk factors of OVCF [30], as well as SVCF for patients following PVP/PKP surgery in some previous studies [7,28]. In our study, as independent risk factors, age and BMD were first analyzed (both P<0.05), and then they were analyzed by binary logistic regression. Both older age and BMD were identified as risk factors of postoperative SVCF after PVP/PKP surgery, and formed a logistic regression equation. It was obvious that advanced age patients usually have lower BMD, so that would be the reason why older age and lower BMD seemed to be bound together as a whole in the development of SVCF after PVP/PKP surgery.
Another study [30] indicated that the prevalence of vertebral fractures in postmenopausal women would increase from 13% for women younger than age 60 to over 50% for women by age 80 years. A model with 7 clinical risk factors with or without BMD is considered better than simple models and might guide the use of spine x-rays to identify women with vertebral fractures. Notably, it was found that more than half an hour of outdoor activity might correlate with lower risk of vertebral fracture in this population. The aforementioned study divided outdoor activity (ODA) into 3 levels: <0.5 hours per day, <1.0 (including <0.5 hours per day), <2.0 (including <1.0 hour per day). Having less than half an hour outdoor activities was significantly associated with the probability of having a vertebral fracture: the risk increased by 1.73 times for < 0.5 hours per day compared with ≥0.5 hours per day. In contrast, ODA was scored in 3 levels in our study: low level (ODA <0.5 hours per day), medium level (0.5≤ ODA <2 hours per day), and high level (ODA ≥2 hours per day). As our result showed, ODA between the SVCF group and the non-SVCF group showed a significant difference (P<0.001); most SVCF patients were observed with low-level outdoor activity, compared with the non-SVCF group. Therefore, appropriate ODA level would be more beneficial to less fracture events caused by osteoporosis. The reason could be that high-level ODA was more helpful to higher BMD level, which was a protective factor in postoperative SVCF development after PVP/PKP surgery.
This study has indicated some clinically significant findings. However, some study limitations still exist. To start with, the current study design may lack extensive representativeness due to the retrospective single-center study design. In addition, we did not apply blind methods in assessment of this study. Hence, future researches should overcome those study limitations and provide more reliable clinical research data. We believe it would be best to conduct a large-sample, prospective, multicenter, randomized, controlled study with blinded methodology applied.
Conclusions
In summary, this study indicated that older age and lower BMD were likely risk factors of SVCF for OVCF patients following PVP/PKP surgery, whereas more outdoor activity played a protective role in SVCF development.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Zhang H, Xu C, Zhang T, et al. Does percutaneous vertebroplasty or balloon kyphoplasty for osteoporotic vertebral compression fractures increase the incidence of new vertebral fractures? A meta-analysis. Pain Physician. 2017;20:E13–28. [PubMed] [Google Scholar]
- 2.Nishimura A, Akeda K, Kato K, et al. Osteoporosis, vertebral fractures and mortality in a Japanese rural community. Mod Rheumatol. 2014;24:840–43. doi: 10.3109/14397595.2013.866921. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki T, Yoshida H. Low bone mineral density at femoral neck is a predictor of increased mortality in elderly Japanese women. Osteoporos Int. 2010;21:71–79. doi: 10.1007/s00198-009-0970-6. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki N, Ogikubo O, Hansson T. Previous vertebral compression fractures add to the deterioration of the disability and quality of life after an acute compression fracture. Eur Spine J. 2010;19:567–74. doi: 10.1007/s00586-009-1162-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rousing R, Hansen KL, Andersen MO, et al. Twelve-months follow-up in forty-nine patients with acute/semiacute osteoporotic vertebral fractures treated conservatively or with percutaneous vertebroplasty: a clinical randomized study. Spine (Phila Pa 1976) 2010;35:478–82. doi: 10.1097/BRS.0b013e3181b71bd1. [DOI] [PubMed] [Google Scholar]
- 6.Klazen CA, Lohle PN, de Vries J, et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): An open-label randomised trial. Lancet. 2010;376:1085–92. doi: 10.1016/S0140-6736(10)60954-3. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Yang DL, Ma L, et al. Risk factors associated with adjacent vertebral compression fracture following percutaneous vertebroplasty after menopause: A retrospective study. Med Sci Monit. 2017;23:5271–76. doi: 10.12659/MSM.907364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan G, Li F, Zhou D, et al. Unilateral versus bilateral percutaneous balloon kyphoplasty for osteoporotic vertebral compression fractures: A systematic review of overlapping meta-analyses. Medicine (Baltimore) 2018;97:e11968. doi: 10.1097/MD.0000000000011968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang H, Chen L, Zheng Z, et al. Therapeutic effects analysis of percutaneous kyphoplasty for osteoporotic vertebral compression fractures: A multicentre study. J Orthop Translat. 2017;11:73–77. doi: 10.1016/j.jot.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrokhi MR, Alibai E, Maghami Z. Randomized controlled trial of percutaneous vertebroplasty versus optimal medical management for the relief of pain and disability in acute osteoporotic vertebral compression fractures. J Neurosurg Spine. 2011;14:561–69. doi: 10.3171/2010.12.SPINE10286. [DOI] [PubMed] [Google Scholar]
- 11.Pflugmacher R, Schroeder RJ, Klostermann CK. Incidence of adjacent vertebral fractures in patients treated with balloon kyphoplasty: two years’ prospective follow-up. Acta Radiol. 2006;47:830–40. doi: 10.1080/02841850600854928. [DOI] [PubMed] [Google Scholar]
- 12.Uppin AA, Hirsch JA, Centenera LV, et al. Occurrence of new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology. 2003;226:119–24. doi: 10.1148/radiol.2261011911. [DOI] [PubMed] [Google Scholar]
- 13.Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320–23. doi: 10.1001/jama.285.3.320. [DOI] [PubMed] [Google Scholar]
- 14.Zhan Y, Jiang J, Liao H, et al. Risk factors for cement leakage after vertebroplasty or kyphoplasty: A meta-analysis of published evidence. World Neurosurg. 2017;101:633–42. doi: 10.1016/j.wneu.2017.01.124. [DOI] [PubMed] [Google Scholar]
- 15.Lin D, Hao J, Li L, et al. Effect of bone cement volume fraction on adjacent vertebral fractures after unilateral percutaneous kyphoplasty. Clin Spine Surg. 2017;30:E270–75. doi: 10.1097/BSD.0000000000000204. [DOI] [PubMed] [Google Scholar]
- 16.Bae JS, Park JH, Kim KJ, et al. Analysis of risk factors for secondary new vertebral compression fracture following percutaneous vertebroplasty in patients with osteoporosis. World Neurosurg. 2017;99:387–94. doi: 10.1016/j.wneu.2016.12.038. [DOI] [PubMed] [Google Scholar]
- 17.Yang S, Chen C, Wang H, et al. A systematic review of unilateral versus bilateral percutaneous vertebroplasty/percutaneous kyphoplasty for osteoporotic vertebral compression fractures. Acta Orthop Traumatol Turc. 2017;51:290–97. doi: 10.1016/j.aott.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Movrin I. Adjacent level fracture after osteoporotic vertebral compression fracture: A nonrandomized prospective study comparing balloon kyphoplasty with conservative therapy. Wien Klin Wochenschr. 2012;124:304–11. doi: 10.1007/s00508-012-0167-4. [DOI] [PubMed] [Google Scholar]
- 19.Engelke K, Adams JE, Armbrecht G, et al. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: The 2007 ISCD Official Positions. J Clin Densitom. 2008;11:123–62. doi: 10.1016/j.jocd.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee S, Yeh J, Ellamushi H. Pain and functional outcomes following vertebroplasty for vertebral compression fractures – A tertiary centre experience. Br J Neurosurg. 2016;30:57–63. doi: 10.3109/02688697.2015.1096901. [DOI] [PubMed] [Google Scholar]
- 21.Nas ÖF, İnecikli MF, Hacıkurt K, et al. Effectiveness of percutaneous vertebroplasty in patients with multiple myeloma having vertebral pain. Diagn Interv Radiol. 2016;22:263–68. doi: 10.5152/dir.2016.15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun ZY, Li XF, Zhao H, et al. Percutaneous balloon kyphoplasty in treatment of painful osteoporotic occult vertebral fracture: A retrospective study of 89 cases. Med Sci Monit. 2017;23:1682–90. doi: 10.12659/MSM.903997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark W, Bird P, Gonski P, et al. Safety and efficacy of vertebroplasty for acute painful osteoporotic fractures (VAPOUR): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2016;388:1408–16. doi: 10.1016/S0140-6736(16)31341-1. [DOI] [PubMed] [Google Scholar]
- 24.Barr JD. Randomized controlled trial of vertebroplasty versus kyphoplasty in the treatment of vertebral compression fractures. J Neurointerv Surg. 2016;8:765–66. doi: 10.1136/neurintsurg-2016-012279. [DOI] [PubMed] [Google Scholar]
- 25.Awwad A, Le JI, Kumaran M, Sosin MD. A rock in a hard place: Cement pulmonary emboli after percutaneous vertebroplasty. Int J Cardiol. 2016;208:162–63. doi: 10.1016/j.ijcard.2016.01.176. [DOI] [PubMed] [Google Scholar]
- 26.Bornemann R, Jansen TR, Otten LA, et al. Comparison of radiofrequency kyphoplasty and balloon kyphoplasty in combination with posterior fixation for the treatment of vertebral fractures. J Back Musculoskelet Rehabil. 2017;30:591–96. doi: 10.3233/BMR-140224. [DOI] [PubMed] [Google Scholar]
- 27.Gao C, Zong M, Wang WT, et al. Analysis of risk factors causing short-term cement leakages and long-term complications after percutaneous kyphoplasty for osteoporotic vertebral compression fractures. Acta Radiol. 2018;59:577–85. doi: 10.1177/0284185117725368. [DOI] [PubMed] [Google Scholar]
- 28.Takahara K, Kamimura M, Moriya H, et al. Risk factors of adjacent vertebral collapse after percutaneous vertebroplasty for osteoporotic vertebral fracture in postmenopausal women. BMC Musculoskelet Disord. 2016;17:12. doi: 10.1186/s12891-016-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang HK, Lu K, Liang CL, et al. Comparing clinical outcomes following percutaneous vertebroplasty with conservative therapy for acute osteoporotic vertebral compression fractures. Pain Med. 2010;11:1659–65. doi: 10.1111/j.1526-4637.2010.00959.x. [DOI] [PubMed] [Google Scholar]
- 30.Cui L, Chen L, Xia W, et al. Vertebral fracture in postmenopausal Chinese women: A population-based study. Osteoporos Int. 2017;28:2583–90. doi: 10.1007/s00198-017-4085-1. [DOI] [PubMed] [Google Scholar]