Abstract
Background
Most of the oral drugs have the properties of weak intestinal absorption and low bioavailability, which leads to little treatment to diseases. By nanotechnology, these drugs can be efficiently delivered to pass biological barriers and promote the cell uptake ability for the enhancement of the oral bioavailability.
Methods
The present work chose the prepared curcumin-loaded galactosylated albumin nanoparticles (Gal-BSA NPs) as the nano-drug samples to study the intestinal capacity and the oral bioavailability.
Results
The cell uptake assay showed that the Gal-BSA NPs could promote the internalization of more curcumin into the Caco-2 cells. Moreover, the cell uptake mechanism of Gal-BSA-Cur NPs depended on the clathrin-mediated endocytosis transport. The intestinal permeation assay using one Ussing chamber exhibited that the absorptive amounts of curcumin in Gal-BSA-Cur NPs group were 1.5-fold of pure curcumin group. Meanwhile, the permeation mechanism of Gal-BSA-Cur NPs across the intestine mainly depended on the passive transport. The pharmacokinetics study in vivo suggested that the oral bioavailability of Gal-BSA-Cur NPs was improved by 1.4-fold compared with pure curcumin.
Conclusion
All results demonstrated that Gal-BSA NPs could improve the intestinal absorption capacity and oral bioavailability of curcumin through the double absorption mechanisms of the clathrin-mediated endocytosis and the passive transport.
Keywords: intestinal absorption, galactosylated nanoparticles, oral bioavailability, absorption mechanism, curcumin
Introduction
Most of oral drugs usually enter the gastrointestinal tract of the body at the first for the treatments of diseases. Thus, intestinal absorption, as the first step of ADME (absorption, distribution, metabolism and excretion), is the vital precondition to play therapeutic effects of oral drugs. In the intestinal tract, the capacity of intestinal absorption of drugs is influenced by the three factors including the physicochemical property of drugs themselves, the uptake capacity of intestinal epithelial cells and the permeability of the intestines to drugs1. However, most of the drugs themselves have poor water-solubility, which leads to weak intestinal absorption and low bioavailability.
Thus, it is important to improve the intestinal absorption ability and oral bioavailability for these low water-soluble drugs. Nanotechnology, as one effective strategy, has been widely focused for the improvement of bioavailability of drugs. Because nanocarriers own various advantages to deliver drugs including the capacity to pass multiple biological barriers,2,3 the internalization of drugs into cells4 and the ability to change the physicochemical properties of drugs.5,6
Among these nanocarriers, bovine serum albumin nanoparticles (BSA NPs) are widely used for the effective drug delivery7,8 because of their non-toxicity, non-immunogenicity, biocompatibility and high water-solubility.9 Meanwhile, BSA with a long half-life can help the drugs to improve the blood drug concentrations for a relatively longer time.10 In addition, BSA has many functional carboxylic and amino groups, which can provide various binding sites to drugs. Therefore, the BSA NPs have high drug loadings11 and can be easily modified by other molecules.12–14 The BSA NPs show good effects as drug nanocarriers, but these abilities connected with the intestinal absorption of oral drugs have not been reported.
Curcumin (Cur) is a potential drug with multiple therapeutic effects in clinic trails.15–17 However, as a Biopharmaceutics Classification System (BCS) Class Ⅳ compound, its disadvantages of the weak water solubility and the poor bioavailability always hamper the clinical application. After oral administration of curcumin, a little portion can be absorbed in the gastrointestinal tract.18 Yang reported that the oral bioavailability of curcumin was only 1% in rats.19 Thus, curcumin was chosen as one presentative low water-soluble compound.
In the present work, the curcumin-loaded galactosylated bovine serum nanoparticles (Gal-BSA-Cur NPs), prepared in our previous work, were used as the nano-drugs. In addition, they showed higher water-solubility than pure curcumin.20 Therefore, we now focus on the intestinal absorption capacity and mechanism of the Gal-BSA-Cur NPs and expect that the NPs can improve oral bioavailability of curcumin through promoting the intestinal absorption. We hypothesize that the Gal-BSA-Cur NPs will interact with the cells of the intestinal tract and transport across the intestine barriers. After the absorption of intestines, the nano-drugs will enter into the blood circulation of the body and increase the blood concentrations to improve oral bioavailability.
Specifically, the cell uptake ability and mechanism of the Gal-BSA-Cur NPs in vitro were evaluated using caco-2 cells. Further, intestinal permeability property and mechanism of the Gal-BSA-Cur NPs was investigated by using the Ussing chamber equipped with intestinal tissues of rats. Finally, the pharmacokinetic study in vivo was conducted to demonstrate the oral bioavailability of the Gal-BSA-Cur NPs.
Materials and Methods
Materials
Gal-BSA-Cur NPs were prepared by our laboratory and sterilized by ultraviolet (UV). Curcumin was purchased from Adamas (Shanghai, China). Sodium carboxyl methyl cellulose (CMC-Na) was purchased from Aladdin (Shanghai, China). Methanol (HPLC grade) was obtained from Chengdu Kelong Chemical Co., Ltd. (Chengdu, China). Deionized water was obtained from Watsons. Tetrazolium salt (MTT), dimethyl sulfoxide (DMSO), trypsin solution (0.25%) and sucrose were all provided by Solarbio Science & Technology Co., Ltd. (Beijing, China). Fetal bovine serum (FBS) was purchased from Lonsa Science Srl Co., Ltd. (Shanghai, China). Dulbecco’s modified eagle’s medium (DMEM) with high glucose and penicillin-streptomycin solution and nonessential amino acids were supplied by Thermo Fisher Scientific Biological Chemical Co., Ltd. (Beijing, China). Heparin was purchased from Sigma (Shanghai, China). Capillary for blood collection was supplied by Taizhou Jiaojiang Glass Instrument Factory. (Taizhou, China). Anticoagulation tube was purchased from Jiangsu Datang Medical Devices Co., Ltd. (Jiangsu, China).
Cell Culture
The Caco-2 cell line was obtained from Chongqing Medical University, which was approved by the Animal Ethics Committee of Chongqing Medical University. The cells were cultured in a 5% CO2 humidified incubator at 37 °C using growth medium (DMEM, 20% FBS, 1% penicillin-streptomycin solution and 1% NEAA). No ethics statement was required from the institutional review board.
Cell Viability Using MTT Assay
Caco-2 cells were seeded (5 ×103 cells/well) in 96-well culture plates.21 The cells were treated with the Cur and Gal-BSA-Cur NPs using different concentrations of 3.125, 6.25, 12.5, 25, 50, 100 μM and incubated for 24 hrs. After incubation, 20 μL of MTT solution (5 mg/mL) was added to each well and further incubated for 4 hrs. Then, the medium was removed and 150 μL of DMSO was added to each well. The plate was shaken for 10 mins to fully solute formazan. The absorbance was measured using Microplate Reader (iMark; Bio-Rad Laboratories Inc., Hercules, CA, USA) at 490 nm. The percent of cell viability was measured from the equation as followed:
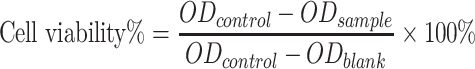 |
Cell Uptake Assay
The qualitative and quantitative evaluations of cellular internalization are the prerequisite for the intestinal absorption of drugs. Fluorescence imaging and HPLC analysis were carried out. The caco-2 cells (2×105 cell/well) were seeded in 6-well plates. After the adherence of these cells, Cur, Gal-BSA-Cur NPs and control solution were added and co-incubated for 4 hrs, respectively. Then, these cells were washed and fixed by 4% formaldehyde for 10 mins. 1 μg/mL DAPI was used for the stain of cell nucleus for 10 mins. Further, the DAPI solution was removed and the cells were washed with PBS. Finally, the cells were imaged by using fluorescence microscopy. For quantitative analysis, the cells were collected and re-frozen at −80°C three times. After centrifugation, 1.5 mL ethyl acetate was added and vortexed to extract curcumin. The solution was dried to remove reagents. Finally, the samples were redissolved and analyzed by HPLC.
Uptake Mechanism in the Caco-2 Cell Monolayer
The cell uptake mechanism of Gal-BSA-NPs as nanocarriers was evaluated by the pretreatment of different inhibitors. Caco-2 cells were seeded in the 6-well plate at the concentration of 2×105 cell/well and incubated for 48 hrs. After the cell adherence, the cells were pretreated with sodium azide (0.1%, w/v) and sucrose (0.45 M) for 1 hr, respectively.6,22 Then, Cur and Gal-BSA-Cur NPs were added into the cells for 4 hrs, respectively. Finally, the cells were extracted using ethyl acetate and analyzed by HPLC at 430 nm of wavelength.
Animal
Male Sprague-Dawley rats (250 g ±15 g each) were purchased by Chongqing Medical University Animal Experiment Center. All the experiments on animals were approved by the Animal Ethics Committee of Chongqing Medical University and performed in full compliance with international practices for animal care and use. The rats were kept in the environment of 12 light/dark at the temperature 20 ± 2 °C and the relative humidity remained at 55%-60%.
Transport Study and Permeation Assays in the Ussing Chambers
The Ussing chamber system,23 equipped with intestinal tissues from rats, was used to evaluate the permeability of Cur and Gal-BSA-Cur NPs. The jejunum portions from intestine of the sacrificed rats were excised, washed with cold physiological saline solution and cut into 1–2 cm length.
The jejunum segments were mounted in the Ussing chambers (intestinal surface of 0.25 cm2) and bathed with Krebs-Ringer solution (5 mL, pH 7.4), which was maintained at 37°C and continuously supplied with O2/CO2 (95%/5%). After the equilibration for 5 mins, the Krebs-Ringer solution was removed. For absorption transport study, the buffers of 5 mL containing Cur (1 mg/mL) or Gal-BSA-Cur NPs (equivalent to 1 mg/mL Cur) were added in the apical side (AP), and the fresh Krebs-Ringer solution (5 mL) was added in the basal side (BL). For secretive transport study (the permeation direction of BL-AP compartment), the buffers of 0.5mL with Cur or Gal-BSA-Cur NPs were added to the basal side and 0.5 mL of the receiving medium (fresh Krebs-Ringer solution) was added to the apical side. Samples (0.5 mL) were collected from the basal side at 15, 30, 60, 90 and 120 mins. All samples were dried and redissolved by methanol, and then analyzed by HPLC.
The cumulative amounts of Cur and Gal-BSA-Cur NPs permeating in the mucosal-to-serosal (M-S, absorptive) or serosal-to mucosal (S-M, secretory) direction were calculated and plotted with time. To compare Cur with Gal-BSA-Cur NPs, the apparent permeability coefficient (Papp) was calculated using the following equation:
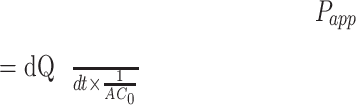 |
where dQ/dt was the transport rate (μg/s), C0 was the initial concentration of Cur or Gal-BSA-Cur NPs in the donor compartment (μg/mL), and A was the area of the membrane (cm2).
The cumulative absorption amounts were calculated as follows:
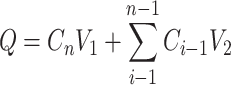 |
where Q was cumulative permeation amount (ng) of curcumin, Cn was the actual concentration (ng/mL) at n time point, and V1, V2 represented tank volume (5 mL) and sampling volume (0.5 mL), respectively.
The efflux ratio (ER) was determined by calculating the ratio of Papp (S-M) versus Papp (M-S) as the following equation:
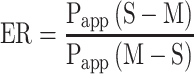 |
Pharmacokinetic Study of Oral Gal-BSA-Cur NPs and Cur
Male Sprague-Dawley rats (250 g ±15 g) were divided into three groups (6 rats per group). The rats were fasted for 12 hrs with free access to water. Cur and Gal-BSA-Cur NPs were dissolved in 0.5% CMC-Na for oral administration. Group 1 and Group 2 rats were orally treated with curcumin (40 mg/kg) and Gal-BSA-Cur (relative to the curcumin concentration of 40 mg/kg), respectively. Group 3 was treated as the blank group. Blood samples (approximately 0.5 mL) were collected in a heparinized tube at 3 mins, 5 mins, 15 mins, 30 mins, 1 hr, 2 hrs, 4 hrs, 8 hrs, 12 hrs, 24 hrs and 48 hrs after the drug administration. All the collected blood samples were centrifugation at 6000 rpm and 4°C for 10 mins, and then the supernatant plasma samples were obtained and frozen at −20 °C for further analysis.
The pharmacokinetic parameters were showed as follows: the area under the concentration-time curve (AUC); maximum peak concentration (Cmax); time of maximum peak concentration (Tmax) and mean residence time (MRT). These parameters were performed by DAS software version 2.0. All data were presented as mean and standard deviation (mean ± SD).
HPLC Quantitative Analysis
Sample Preparation
Liquid-liquid extraction method was used to extract plasma samples. 0.2 mL of the plasma sample was added into a 1.5 mL EP tube. 2 mL of ethyl acetate was added and vortexed for 3 mins to extract curcumin. After the centrifugation at 10000 r/min for 10 mins, the upper solution was dried and then redissolved by mobile phase solution of HPLC.
HPLC Conditions
The concentration of curcumin was analyzed by HPLC instrument (LC-20A, Shimadzu, Japan) with a InertSustain® C18 column (15 cm ×4.5 mm × 5 μm, GL Sciences Inc.). The mobile phase was composed of methanol and 5% acetic acid (70:30, v/v), and the flow rate was 1.0 mL/min. All the samples were detected at 430 nm with the temperature of 40 °C.
Method Validation
The method validation in blank plasma was carried out according to the Guidance for Industry Bioanalytical Method Validation from the US Food and Drug Administration. These tests included specificity, linearity and sensitivity, extraction recovery, precision and accuracy (detailed description in supporting information).
Statistical Analysis
All the results were expressed as the mean standard deviation (SD). Data were analyzed by the Student’s t-test at the significance level of P<0.05.
Results and Discussion
Assessment of Cell Viability
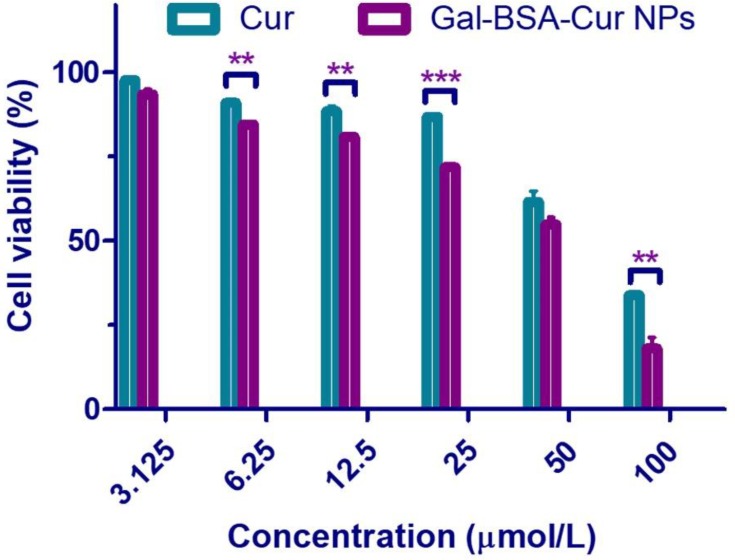
Caco-2 cell viability assay was carried out to select a suit concentration for the next cell uptake experiments. As shown in Figure 1, more than 80% of cell viability treated with Cur was observed at the 25 μmol/L concentration, while Gal-BSA-Cur NPs showed more than 80% cell viability at the concentration of 12.5 μmol/L. Moreover, the cell viability treated with Cur and Gal-BSA-Cur NPs for 24 hrs decreased in a concentration-dependent manner. The decrease trend of curcumin was similar with the results of Gavrilas et al24 Considering the incubation time of drug with the cells and the quantity of drugs in the cells, the concentration of 25 μmol/L was selected for the further cell uptake assay. In addition, the cytotoxicity of Gal-BSA-Cur NPs was significantly stronger than that of Cur, which may lead to more curcumin internalized into the cancer cells.20
Figure 1.
The cell viability of Cur and Gal-BSA-Cur NPs on Caco-2 cells.
Notes: Each point represents the mean ± standard deviation (n=6). **P < 0.01, ***P< 0.001, compared to Gal-BSA-Cur NPs.
Abbreviations: Cur, curcumin; Gal-BSA-Cur NPs, curcumin-loaded galactosylated BSA nanoparticles.
Cell Uptake Study
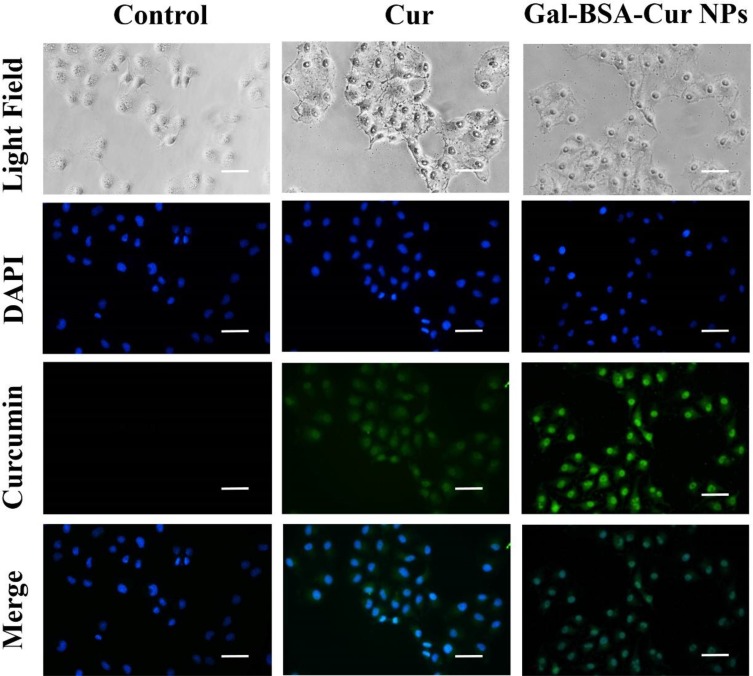
As Figure 2 shows, in the control group, there was no green fluorescence in Caco-2 cells. While the Cur group showed the weak green florescence because of the curcumin inside the cells. The poor aqueous solubility of curcumin influenced the internalization efficiency into the cells, which further reduced its therapeutic efficacy.25,26 After 4 hrs of incubation, the green fluorescence intensity in Gal-BSA-Cur NPs group was stronger than that in the Cur group, which indicated that Gal-BSA NPs could enhance the ability of internalization of curcumin into the cells and release more curcumin in the cytoplasm as well as in the nucleus. In addition, in the Gal-BSA-Cur NPs group, more fluorescence was observed in the nucleus of Caco-2 cells than that in the cytoplasm, which demonstrated that curcumin mainly enriched in the nucleus. This enrichment may increase the therapeutic effect of curcumin in the cell nucleus because curcumin itself usually interacts with nuclear receptors in the nucleus of cells.27,28
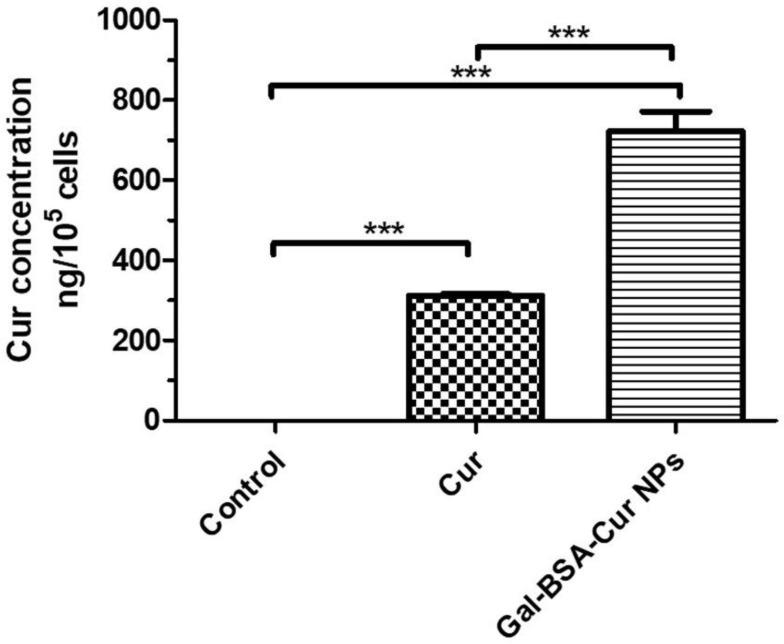
Figure 3.
The quantitative assay of cellular uptake of Cur and Gal-BSA-Cur NPs in Caco-2 cells using HPLC.
Notes: The curcumin concentration was detected by HPLC. ***P <0.001 compared with Gal-BSA-Cur NPs. The results were expressed as the mean ± standard deviation (n=3).
Abbreviations: Cur, curcumin; Gal-BSA-Cur NPs, curcumin-loaded galactosylated BSA nanoparticles; HPLC, high-performance liquid chromatography.
Figure 2.
Fluorescence microscopy images of cells incubated with 25 μmol/L of Cur and Gal-BSA-Cur NPs for 4 hrs.
Notes: Scale bar: 50μm, Magnification ×400. DAPI: cell nuclei (blue), merged: fluorescence overlaid image.
Abbreviations: Cur, curcumin; Gal-BSA-Cur NPs, curcumin-loaded galactosylated BSA nanoparticles.
In Figure 3, the quantitative results of cell uptake showed that the concentrations of curcumin in Cur and Gal-BSA-Cur NPs were 298.94 ± 22.26 and 722.79 ± 49.50 ng/105 cells, respectively, which indicated that the curcumin amounts of cell uptake in Gal-BSA-Cur NIPs were about twice more than that in Cur. The results were in accordance with that of the fluorescence analysis. For Gal-BSA-Cur NPs, more curcumin was obtained in the cells whatever the HepG2 cell line20 or the Caco-2 cell line were used, which indicated that the NPs did promote the internalization of curcumin into cells.
Elucidation of Uptake Mechanism
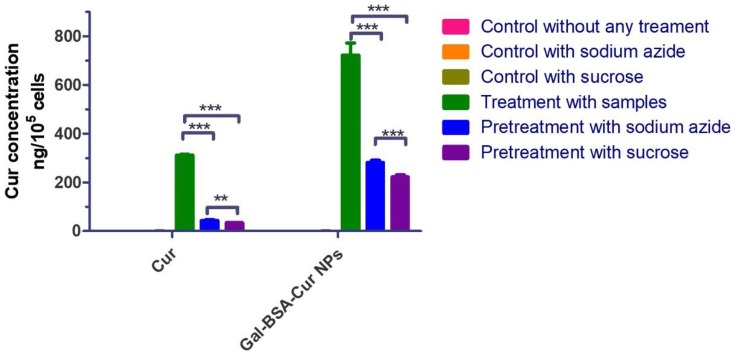
For better understanding, the uptake mechanism of Gal-BSA-Cur NPs as nanocarriers, Caco-2 cells were pretreated with different inhibitors for 1 hr. As shown in the green bars of Figure 4, the uptake concentrations of Cur and Gal-BSA-Cur NPs were 312.27 ± 3.01 and 722.79 ± 49.50 ng/105 cells, respectively. When the cells were pretreated with sodium azide as a known pharmaceutical endocytosis inhibitor22 (Figure 4 blue bars), the concentrations of Cur and Gal-BSA-Cur NPs decreased to 44.24 ± 3.34 and 281.58 ± 8.71 ng/105 cells, respectively, compared to the control group without any inhibitors (green bars). These results indicated that endocytosis was involved in the uptake of both Cur and Gal-BSA-Cur NPs. The result of the curcumin internalized into caco-2 cells was similar with Kim et al reported.29 Curcumin alone has been internalized by cells with 30 mins of drug exposure,30 and thus the incubation time of 4 hrs satisfied the request of the experiment.
Figure 4.
Intracellular uptake of Cur and Gal-BSA-Cur NPs in Caco-2 cells pretreated with different inhibitors.
Notes: ***P <0.001 compared with Cur or Gal-BSA-Cur NPs. **P <0.01, compared to the pretreatment with sodium azide or sucrose. The results were expressed as the mean ± standard deviation (n=3).
Abbreviations: Cur, curcumin; Gal-BSA-Cur NPs, curcumin-loaded galactosylated BSA nanoparticles.
To further understand the cell endocytosis process, sucrose (0.45M) was used to create hypertonic environment in the cells disrupting the formation of clathrin.22 The cellular curcumin concentrations in Cur and Gal-BSA-Cur NPs were reduced to 34.29 ± 1.65 and 222.54 ± 7.96 ng/105 cells, respectively, suggesting that clathrin-mediated endocytosis involved in the cell uptake of Cur and Gal-BSA-Cur NPs. Meanwhile, the absorption mechanism of Gal-BSA-Cur NPs is shown in Figure 5. The reduce degrees of curcumin concentrations of Gal-BSA-Cur NPs by using two inhibitors were much more than that of Cur, confirming that Cal-BSA NPs can accumulate more cellular curcumin and efficiently facilitate absorption of curcumin through cell endocytosis. The absorption mechanism of the NPs has not been reported, which may be an ideal material for oral drug delivery. In addition, other nanocarriers were internalized into Caco-2 cells by multiple endocytosis mechanisms,31 which reminds us that these nanocarriers may be also considered to improve the intestine absorption by oral administration.32–34
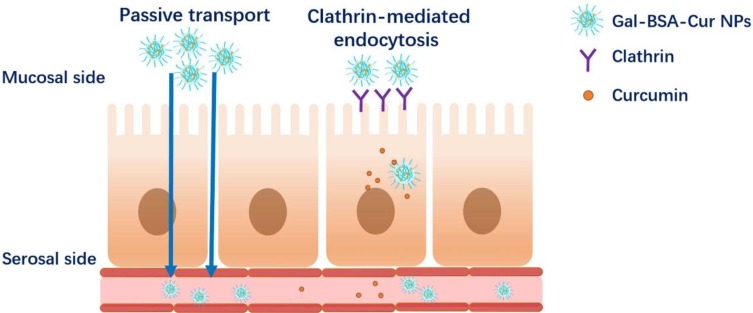
Figure 5.
Scheme of the absorption mechanism used by Cur or Gal-BSA-Cur NPs.
Abbreviations: Cur, curcumin; Gal-BSA-Cur NPs, curcumin-loaded galactosylated BSA nanoparticles.
Permeation Assay and Transport Study
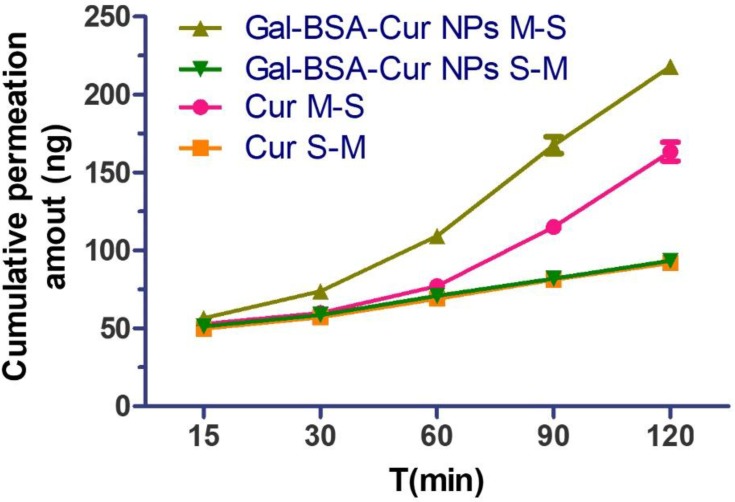
Permeation across the intestinal epithelia to the circulation system is significant to improve oral absorption of curcumin.31 The intestinal absorption profile of Cur and Gal-BSA-Cur NPs through in vitro intestinal permeability of Ussing chamber was showed in Figure 6 and Table 1. The cumulative permeation amount of Gal-BSA-Cur NPs was much more than that of Cur at any time point. Moreover, the Cur showed a Papp of (0.69±0.03) ×10−7 cm/s, while Gal-BSA-Cur NPs was a relatively higher Papp of (1.04±0.01) ×10−7 cm/s, which demonstrated that the absorptive transport (M-S) of curcumin in Gal-BSA-Cur NPs was enhanced by around 1.51 fold. This indicated that Gal-BSA NPs could facilitate the intestinal absorption of curcumin.
Table 2.
Pharmacokinetic Parameters of Cur and Gal-BSA-Cur NPs
| Parameter | Gal-BSA-Cur NPs | Cur |
|---|---|---|
| AUC (0–48 hrs)/(ng/mL*h). | 1047.6±41.2 | 775.4±35.7 |
| MRT(0–48 hrs)/h | 22.6±0.4 | 21.7±0.8 |
| Cmax(ng/mL) | 48.7±7.4 | 25.2±7.0 |
| Tmax/h | 0.5 | 2 |
Abbreviations: Cur, curcumin; Gal-BSA-Cur NPs, curcumin-loaded galactosylated BSA nanoparticles; AUC, area under the curve; MRT, mean retention time; Cmax, peak plasma concentration; Tmax, time at peak concentration.
Figure 6.
In vitro the Ussing chamber cumulative absorption of Cur and Gal-BSA-Cur NPs in rat jejunums (n=3).
Abbreviations: Cur, curcumin; Gal-BSA-Cur NPs, curcumin-loaded galactosylated BSA nanoparticles.
Table 1.
The Apparent Permeability Values (papp) of Cur and Gal-BSA-Cur NPs Determined from Ussing Chamber Assay in Mucosal-to-Serosal (M-S) and Serosal-to-Mucosal (S-M)
| Groups | Papp (×10−7, cm • s−1) |
|---|---|
| Cur M-S | 0.69±0.03 |
| Cur S-M | 0.27±0.01 |
| Gal-BSA-Cur NPs M-S | 1.04±0.01 |
| Gal-BSA-Cur NPs S-M | 0.26±0.00 |
Abbreviations: Cur, curcumin; Gal-BSA-Cur NPs, curcumin-loaded galactosylated BSA nanoparticles; M-S, mucosal-to-serosal; S-M, serosal-to-mucosal.
The bidirectional transport assay was to further determine the mechanism of intestinal transport. The efflux ratio (ER) of Cur and Gal-BSA-Cur NPs were 0.39 and 0.25, respectively. Usually, the transport amount of one compound with ER values >2 should be considered as an active efflux.35 Therefore, no active efflux involved in the transport process of Cur and Gal-BSA-Cur NPs, which was similar with the reported.21,31 While these studies reported the permeability mechanism of Cur was only on the cell level. Our work firstly focused on the real intestine transport mechanism by using Ussing chamber, which more closely mimicked the human body. The accumulation curves of Cur and Gal-BSA-Cur NPs (in the direction of M-S and S-M) showed the both linear in a concentration-dependent manner, suggesting that the main transport mechanism across the intestine of rats of Cur and Gal-BSA-Cur NPs was passive transport without active efflux. While the permeation and transport capacity of Gal-BSA-Cur NPs were higher than that of Cur, indicating the better intestinal absorption of Gal-BSA-Cur NPs would help more curcumin to enter into the blood circulation shown in Figure 5.
Thus, the absorption mechanism of Gal-BSA-Cur NPs not only showed the cell endocytosis but also the passive transport, which was described in Figure 5. To further demonstrate the oral bioavailability of Gal-BSA-Cur NPs after entering into the blood circulation, next pharmacokinetic study will be carried out.
Pharmacokinetic Study
The concentrations of curcumin in rat plasma were determined by HPLC method. The calibration curve (Y=20.2X-223.1) of curcumin in plasma showed good linearity (R2 > 0.999) with the concentration range from 12.5 to 400 ng/mL. The lower limit of quantification (LLOQ) and the lower limit of detection (LLOD) were 24.37 ng/mL and 7.31 ng/mL, respectively. The specificity demonstrated that the interfering substances were not disturbed at the retention time of the curcumin with the established chromatographic conditions (Figure S1). As shown in Tables S1 and S2, the precision (CV, from 1.2% to 4.5%), accuracy (RE, from −6.8% to 6.8%) and relative extraction recovery (from 88.3 ± 4.6% to 103.4 ± 3.5%) demonstrated that the analysis method was reliable.
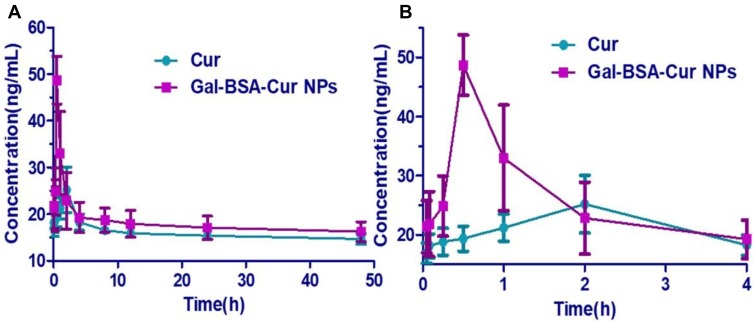
The plasma concentration-time curves of Cur and Gal-BSA-Cur NPs after oral administration are shown in Figure 7. Because of the poor solubility and low bioavailability of curcumin, the plasma concentration of curcumin was difficult to detect. The oral dosages of curcumin were selected at 40 mg/kg, which based on previous studies.36,37 After the oral administration, shown in Table 2, Gal-BSA-Cur NPs showed shorter time of the peak concentration at 0.5 hrs, while the Tmax value of Cur was 2 hrs, indicating rapid absorption of Gal-BSA-Cur NPs. Moreover, the Cmax value of Gal-BSA-Cur NPs was 48.7±7.4 ng/mL, which was nearly 1.9 folds higher than that of Cur. These results may due to that the nanoparticles size could easily transport the intestine barrier and let more curcumin accumulate in rats' plasma. The AUC value of Gal-BSA-Cur NPs was about 1.4 folds higher than that of Cur. Therefore, the Gal-BSA NPs could help curcumin maintain the higher concentration in plasma, which may improve the oral bioavailability of curcumin. In addition, the MRT value of Gal-BSA-Cur NPs was relatively higher than that of Cur, suggesting that the longer residence time of Gal-BSA-Cur NPs in plasma may be good for the enhancement of oral bioavailability. Generally, these mentioned parameters of Gal-BSA-Cur NPs were better than that of curcumin, indicating that Gal-BSA NPs can improve the oral bioavailability of curcumin.
Figure 7.
The plasma concentrations versus time curves of Cur and Gal-BSA-Cur NPs after oral administration in rats. (A) The time from 0 to 48 hrs. (B) The time from 0 to 4 hrs.
Notes: Rats were treated with Cur (40 mg/kg) or Gal-BSA-Cur NPs (equivalent to the Cur). Data were represented as mean ± SD (n=6).
Abbreviations: Cur, curcumin; Gal-BSA-Cur NPs, curcumin-loaded galactosylated BSA nanoparticles.
Conclusion
In this work, the intestinal absorption property and the oral bioavailability of the prepared curcumin-loaded Gal-BSA nanoparticles (Gal-BSA-Cur NPs) were the first studied. Compared to pure Cur, the intestinal absorption amounts of curcumin in Gal-BSA-Cur NPs were higher. Meanwhile, the Gal-BSA-Cur NPs showed the double absorption mechanisms that were the Clathrin-mediated endocytosis and the passive transport. Further, the pharmacokinetic parameters suggested that the Gal BSA-Cur NPs could enhance the oral bioavailability of poor water-soluble curcumin, which may owe to the improved intestinal absorption capacity. Thus, the Gal-BSA NPs can promote the intestinal absorption process to improve the oral bioavailability. In addition, the galactose on the Gal-BSA NPs may also facilitate curcumin absorption through the specific affinity with the galectins which are expressed abundantly in the intestine tract. Generally, the novel Gal-BSA NPs with better absorption ability can be a potential tool for the delivery of poor water-soluble drugs to enhance the oral bioavailability.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 21705012, 21675016).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lundquist P, Artursson P. Oral absorption of peptides and nanoparticles across the human intestine: opportunities, limitations and studies in human tissues. Adv Drug Deliv Rev. 2016;106:256–276. doi: 10.1016/j.addr.2016.07.007 [DOI] [PubMed] [Google Scholar]
- 2.Choudhury H, Gorain B, Pandey M, et al. Strategizing biodegradable polymeric nanoparticles to cross the biological barriers for cancer targeting. Int J Pharm. 2019;565:509–522. doi: 10.1016/j.ijpharm.2019.05.042 [DOI] [PubMed] [Google Scholar]
- 3.Lai J, Deng G, Sun Z, et al. Scaffolds biomimicking macrophages for a glioblastoma NIR-Ib imaging guided photothermal therapeutic strategy by crossing blood-brain barrier. Biomaterials. 2019;211:48–56. doi: 10.1016/j.biomaterials.2019.04.026 [DOI] [PubMed] [Google Scholar]
- 4.Kim JA, Aberg C, Salvati A, et al. Role of cell cycle on the cellular uptake and dilution of nanoparticles in a cell population. Nat Nanotechnol. 2012;7(1):62–68. doi: 10.1038/nnano.2011.191 [DOI] [PubMed] [Google Scholar]
- 5.Aftab S, Shah A, Nadhman A, et al. Nanomedicine: an effective tool in cancer therapy. Int J Pharm. 2018;540(1–2):132–149. doi: 10.1016/j.ijpharm.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 6.Beloqui A, Solinis MA, Gascon AR, et al. Mechanism of transport of saquinavir-loaded nanostructured lipid carriers across the intestinal barrier. J Control Release. 2013;166(2):115–123. doi: 10.1016/j.jconrel.2012.12.021 [DOI] [PubMed] [Google Scholar]
- 7.Salehiabar M, Nosrati H, Javani E, et al. Production of biological nanoparticles from bovine serum albumin as controlled release carrier for curcumin delivery. Int J Biol Macromol. 2018;115:83–89. doi: 10.1016/j.ijbiomac.2018.04.043 [DOI] [PubMed] [Google Scholar]
- 8.Elzoghby AO, Samy WM, Elgindy NA. Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release. 2012;157(2):168–182. doi: 10.1016/j.jconrel.2011.07.031 [DOI] [PubMed] [Google Scholar]
- 9.Yu Z, Yu M, Zhang Z, et al. Bovine serum albumin nanoparticles as controlled release carrier for local drug delivery to the inner ear. Nanoscale Res Lett. 2014;9(1):343. doi: 10.1186/1556-276X-9-343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasuyama T, Matsunaga H, Ando S, et al. A study of the strength of a template molecule – a functional monomer interaction that affects the performance of molecularly imprinted polymers and its application to chiral amplification. Chem Pharm Bull (Tokyo). 2017;65(4):396–402. doi: 10.1248/cpb.c16-00974 [DOI] [PubMed] [Google Scholar]
- 11.Fu L, Sun Y, Ding L, et al. Mechanism evaluation of the interactions between flavonoids and bovine serum albumin based on multi-spectroscopy, molecular docking and Q-TOF HR-MS analyses. Food Chem. 2016;203:150–157. doi: 10.1016/j.foodchem.2016.01.105 [DOI] [PubMed] [Google Scholar]
- 12.Li C, Zhang D, Guo H, et al. Preparation and characterization of galactosylated bovine serum albumin nanoparticles for liver-targeted delivery of oridonin. Int J Pharm. 2013;448(1):79–86. doi: 10.1016/j.ijpharm.2013.03.019 [DOI] [PubMed] [Google Scholar]
- 13.Spicer CD, Jumeaux C, Gupta B, et al. Peptide and protein nanoparticle conjugates: versatile platforms for biomedical applications. Chem Soc Rev. 2018;47(10):3574–3620. doi: 10.1039/c7cs00877e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nosrati H, Abhari F, Charmi J, et al. Multifunctional nanoparticles from albumin for stimuli-responsive efficient dual drug delivery. Bioorg Chem. 2019;88:102959–102959. doi: 10.1016/j.bioorg.2019.102959 [DOI] [PubMed] [Google Scholar]
- 15.Farhood B, Mortezaee K, Goradel NH, et al. Curcumin as an anti-inflammatory agent: implications to radiotherapy and chemotherapy. J Cell Physiol. 2019;234(5):5728–5740. doi: 10.1002/jcp.27442 [DOI] [PubMed] [Google Scholar]
- 16.Kunnumakkara AB, Bordoloi D, Padmavathi G, et al. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol. 2017;174(11):1325–1348. doi: 10.1111/bph.13621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallianou NG, Evangelopoulos A, Schizas N, et al. Potential anticancer properties and mechanisms of action of curcumin. Anticancer Res. 2015;35(2):645–651. [PubMed] [Google Scholar]
- 18.Liu W, Zhai Y, Heng X, et al. Oral bioavailability of curcumin: problems and advancements. J Drug Target. 2016;24(8):694–702. doi: 10.3109/1061186X.2016.1157883 [DOI] [PubMed] [Google Scholar]
- 19.Yang K-Y, Lin L-C, Tseng T-Y, et al. Oral bioavailabilit of curcurnin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J Chromatogr B Anal Technol Biomed Life Sci. 2007;853(1–2):183–189. doi: 10.1016/j.jchromb.2007.03.010 [DOI] [PubMed] [Google Scholar]
- 20.Huang Y, Hu L, Huang S, et al. Curcumin-loaded galactosylated BSA nanoparticles as targeted drug delivery carriers inhibit hepatocellular carcinoma cell proliferation and migration. Int J Nanomedicine. 2018;13:8309–8323. doi: 10.2147/IJN.S184379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng Z, Shen ZL, Zhai S, et al. Transport of curcumin derivatives in Caco-2 cell monolayers. Eur J Pharm Biopharm. 2017;117:123–131. doi: 10.1016/j.ejpb.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 22.Shah MK, Madan P, Lin S. Elucidation of intestinal absorption mechanism of carvedilol-loaded solid lipid nanoparticles using Caco-2 cell line as an in-vitro model. Pharm Dev Technol. 2015;20(7):877–885. doi: 10.3109/10837450.2014.938857 [DOI] [PubMed] [Google Scholar]
- 23.Miyake M, Koga T, Kondo S, et al. Prediction of drug intestinal absorption in human using the Ussing chamber system: a comparison of intestinal tissues from animals and humans. Eur J Pharm Sci. 2017;96:373–380. doi: 10.1016/j.ejps.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 24.Gavrilas LI, Cruceriu D, Ionescu C, et al. Pro-apoptotic genes as new targets for single and combinatorial treatments with resveratrol and curcumin in colorectal cancer. Food Funct. 2019;10(6):3717–3726. doi: 10.1039/C9FO01014A [DOI] [PubMed] [Google Scholar]
- 25.Khan S, Imran M, Butt TT, et al. Curcumin based nanomedicines as efficient nanoplatform for treatment of cancer: new developments in reversing cancer drug resistance, rapid internalization, and improved anticancer efficacy. Trends Food Sci Technol. 2018;80:8–22. doi: 10.1016/j.tifs.2018.07.026 [DOI] [Google Scholar]
- 26.Gurung RB, Gong SY, Dhakal D, et al. Synthesis of curcumin glycosides with enhanced anticancer properties using one-pot multienzyme glycosylation technique. J Microbiol Biotechnol. 2017;27(9):1639–1648. doi: 10.4014/jmb.1701.01054 [DOI] [PubMed] [Google Scholar]
- 27.Mortezaee K, Salehi E, Mirtavoos-mahyari H, et al. Mechanisms of apoptosis modulation by curcumin: implications for cancer therapy. J Cell Physiol. 2019;234(8):12537–12550. doi: 10.1002/jcp.28122 [DOI] [PubMed] [Google Scholar]
- 28.Shimizu K, Funamoto M, Sunagawa Y, et al. Anti-inflammatory action of curcumin and its use in the treatment of lifestyle-related diseases. Eur Cardiol. 2019;14(2):117–122. doi: 10.15420/ecr.2019.17.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S, Diab R, Joubert O, et al. Core-shell microcapsules of solid lipid nanoparticles and mesoporous silica for enhanced oral delivery of curcumin. Colloids Surf B Biointerfaces. 2016;140:161–168. doi: 10.1016/j.colsurfb.2015.12.040 [DOI] [PubMed] [Google Scholar]
- 30.Das RP, Gandhi VV, Singh BG, et al. Preparation of albumin nanoparticles: optimum size for cellular uptake of entrapped drug (Curcumin). Colloids Surf A. 2019;567:86–95. doi: 10.1016/j.colsurfa.2019.01.043 [DOI] [Google Scholar]
- 31.Wang J, Ma W, Tu P. The mechanism of self-assembled mixed micelles in improving curcumin oral absorption: in vitro and in vivo. Colloids Surf B Biointerfaces. 2015;133:108–119. doi: 10.1016/j.colsurfb.2015.05.056 [DOI] [PubMed] [Google Scholar]
- 32.Ishii M, Fukuoka Y, Deguchi S, et al. Energy-dependent endocytosis is involved in the absorption of indomethacin nanoparticles in the small intestine. Int J Mol Sci. 2019;20(3):476. doi: 10.3390/ijms20030476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel M, Mundada V, Sawant K. Enhanced intestinal absorption of asenapine maleate by fabricating solid lipid nanoparticles using TPGS: elucidation of transport mechanism, permeability across Caco-2 cell line and in vivo pharmacokinetic studies. Artif Cells Nanomed Biotechnol. 2019;47(1):144–153. doi: 10.1080/21691401.2018.1546186 [DOI] [PubMed] [Google Scholar]
- 34.Song Y, Shi Y, Zhang L, et al. Synthesis of CSK-DEX-PLGA nanoparticles for the oral delivery of exenatide to improve its mucus penetration and intestinal absorption. Mol Pharm. 2019;16(2):518–532. doi: 10.1021/acs.molpharmaceut.8b00809 [DOI] [PubMed] [Google Scholar]
- 35.Anand P, Sundaram C, Jhurani S, et al. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267(1):133–164. doi: 10.1016/j.canlet.2008.03.025 [DOI] [PubMed] [Google Scholar]
- 36.Gao Y, Chen G, Luan X, et al. Improved oral absorption of poorly soluble curcumin via the concomitant use of borneol. AAPS PharmSciTech. 2019;20(4):150. doi: 10.1208/s12249-019-1364-5 [DOI] [PubMed] [Google Scholar]
- 37.Bangphumi K, Kittiviriyakul C, Towiwat P, et al. Pharmacokinetics of curcumin diethyl disuccinate, a prodrug of curcumin, in Wistar rats. Eur J Drug Metab Pharmacokinet. 2016;41(6):777–785. doi: 10.1007/s13318-015-0308-z [DOI] [PubMed] [Google Scholar]